Highlights
-
•
A total body irradiation technique based on CT simulation was newly introduced.
-
•
This technique succeeded in reducing the length of the overall treatment session.
-
•
This new technique reduced patient discomfort while ensuring accurate shielding of the lungs.
Keywords: Total body irradiation, Shielding blocks, CT simulation
Abstract
Purpose
During total body irradiation (TBI), customized shielding blocks are positioned in front of the lungs to reduce radiation dose. The difficulty is to accurately position the blocks to cover the entire lungs. A new technique based on Computed Tomography (CT) simulation was developed to determine the exact position of lung blocks prior to treatment in order to decrease overall treatment time and reduce patient discomfort.
Material/Methods
Patients were CT simulated and lungs were contoured using a treatment planning system. Anteroposterior/posteroanterior (AP/PA) fields were designed with MLC aperture conforming to lung contours. The fields were used to represent the extent of the lungs, which was subsequently marked on the patient’s skin. The lung blocks were positioned with their shadow matching the lungs’ marks. Their position was radiographically verified prior to the delivery of each beam. To evaluate the efficiency of this technique, the treatment session time and the number of repeated attempts to correctly position the shielding blocks was recorded for each beam. Exact treatment times for patients treated with the old technique were not available and were hence approximated based on previous experience.
Results
We succeeded in positioning the shielding blocks from the first attempt in 10/12 beams. The position of the shielding blocks was adjusted only one time prior to treatment in 2/12 beams. These results are compared to an average of 3 attempts per beam for each patient using the conventional technique of trial and error. The average time of a treatment session was 29 min with a maximum of 41 min versus approximately 60 min in past treatments and a maximum of 120 min.
Conclusion
This new technique succeeded in reducing the length of the overall treatment session of the conventional TBI procedure and hence reduced patient discomfort while ensuring accurate shielding of the lungs.
Introduction
Total Body Irradiation (TBI) is a treatment technique that consists of irradiating the entire body using very large mega-voltage photon beams. It is used in the treatment of several diseases including lymphoma, leukemia and aplastic anemia [1]. More specifically, TBI is most commonly used in the treatment of acute leukemia prior to hematopoietic stem cell transplantation. By irradiating the whole body, not only cancerous cells are killed but the patient’s immune system is also suppressed to prevent any immunologic rejection of the transplanted stem cells [1], [2].
The main aim of radiation therapy, and hence TBI, is to achieve the greatest possible therapeutic ratio by improving tumor control probability and reducing the risk of normal tissue complication [1], [2]. In a TBI treatment, the target is the whole body in which all organs are irradiated, however some organs in the body are more radiosensitive than others and can tolerate less radiation dose. Lungs are particularly a major concern during TBI treatments; a radiation beam passing through the lungs (a region of low electron density) is less attenuated compared to the rest of the body and the absorbed dose is consequently higher than the tolerable dose-limit [1], [3]. It is necessary to reduce the dose received by the lungs in order to prevent undesirable consequences such as pulmonary toxicity: one of the major causes of mortality after TBI [1], [4]. This is done with the use of a blocking material positioned over the lungs during treatment [1].
At our center, the most common TBI prescription for acute lymphoblastic leukemia is a fractionated schedule of 12 Gy given two times daily over three consecutive days with a gap of at least six hours in between daily fractions while limiting the total dose to the lungs to 8 Gy. Customized lung blocks consisting of high-density material such as Cerrobend should be accurately positioned as close as possible to the patient’s body to reduce the dose to the lungs. The exact positioning of the blocks is normally verified radiographically with portal films prior to the delivery of each beam [1], [5]. Any error in the positioning of the shielding blocks can either give higher doses to the lungs than intended or can lead to excessive shielding of the target volume [6], [7].
One of the downsides of a TBI procedure is the long treatment session time [8]. Beam-on time is usually in the order of ten minutes, however the position of the shielding blocks can be very difficult and can extend the time spent by patients in the treatment unit significantly [2]. The common technique nowadays is to approximately position the shielding blocks in front of the lungs, verify the position with portal films, and then correct it accordingly. This trial-and-error technique is time-consuming because it requires multiple sets of adjustments. Most TBI patients are pediatric patients and complain of discomfort being immobilized for a prolonged period of time. A solution for this time-consuming technique would be a different process where the intended position of the blocks is known prior to treatment for each specific patient. The purpose of this paper is to describe a new technique based on Computed Tomography (CT) simulation for accurate shielding to reduce lung toxicities and also reduce patient’s discomfort by minimizing the overall treatment session time.
Materials and methods
Three patients participated in this study and the characteristics of each patient are described in Table 1. As this is a proof of concept study, with no statistical analysis, additional patients are not needed.
Table 1.
Patients characteristics.
| Patient # | Gender | Age | Patient thickness | Diagnosis | Treatment prescription |
|---|---|---|---|---|---|
| 1 | M | 4 | 18 cm | Acute lymphoblastic leukemia | 12 Gy |
| 2 | M | 12 | 20 cm | Acute lymphoblastic leukemia | 12 Gy |
| 3 | F | 15 | 20 cm | Acute lymphoblastic leukemia | 12 Gy |
Patient and treatment set up without shielding blocks
On the first day, patients were prepared for CT simulation and were immobilized in their lateral recumbent position with the purpose of being treated with opposing anteroposterior/posteroanterior (AP/PA) fields [1]. Patients were lying on a comfortable mattress that can be later carried on a stretcher. The dimensions of the patient separation along the beam direction were measured with a caliper and monitor units (MU) were subsequently calculated based on the patient’s largest separation determined at the level of the umbilicus [5]. The irregular shape of the body causes a significant non-uniformity of the dose distribution. To account for these irregularities and hence deliver a more homogeneous dose distribution to the body, tissue-equivalent bolus materials were placed directly on the patient’s skin to decrease the dose delivered to thin body parts and to result in an even separation along the body [2], [3].
A surface dose of at least 90% of the prescribed TBI dose is normally required to be delivered to the skin, the superficial lymphatic and the marrow in the ribs, skull and clavicles. However, because of the build-up effect characteristics of a 6 MV photon beams, surface dose is less compared to the absorbed dose at greater depth [9], [10]. For this reason, a beam spoiler was employed to ensure that the dose absorbed at the skin and subcutaneous tissue levels is improved. The beam spoiler consists of a Perspex screen of 1 cm thickness and is large enough to encompass the entire patient’s body when placed at the stretcher’s edge closest to the patient (approximately 30 cm away from the patient). When irradiated, the beam spoiler will produce secondary electrons that will change the build-up characteristics of the X-ray beam by shifting the dose toward the surface and boosting the skin dose [3], [5], [9].
The patient lying on the stretcher in the desired position was placed at one side of the treatment room and the gantry of the linear accelerator (Artiste, Siemens Medical Systems, Concord, CA, USA) was rotated so that the source irradiates horizontally [4]. The largest possible field size of 40 × 40 cm was used and the collimator angle was set to 45° so that the diagonal of the square field is aligned with the patient’s axis [11]. In order for the entire body to fit in one radiation field, TBI treatments are usually performed at extended source to surface distance (SSD) [4]. The patient’s legs were bent at the knees if the field did not cover their full body [3]. According to international protocols, the central axis of the X-ray field should pass through the patient’s reference point, which was defined at the level of the umbilicus [5], [6]. An example of the patient’s positioning is shown in Fig. 1.
Fig. 1.
An example of a patient in position for a TBI treatment at our institution.
CT simulation
The patient was transferred to the CT simulator (Somatom, Siemens Healthcare GmbH, Erlangen, Germany) and CT scans of 3 mm slice thickness were acquired for the chest in the exact same treatment position (Fig. 2, left). For localization purposes, three fiducial markers were attached and marked with ink on the skin in one axial plane at the thorax level to ensure that patients will be positioned for treatment as previously simulated and to limit any axial rotation during future fractions with lung blocks (Fig. 2, right).
Fig. 2.
An example of a patient being CT simulated in the treatment position (left). Fiducial markers attached on the patient’s skin to be aligned with lasers for positioning purposes (right).
Contouring and lungs delineation
A treatment planning system (TPS) (Panther, Prowess, Concord, CA, USA) was used for contouring purposes. CT images were imported into the TPS and lungs were contoured on the axial slices. AP/PA beams were designed with multi-leaf collimator (MLC) aperture conforming to lung contours as shown in Fig. 3. These MLC fields were created to determine the shielding shape and not used for treatment delivery. MLC fields were verified on the axial cuts and transferred to the machine to be later projected on the patient’s skin.
Fig. 3.
Beam’s Eye View (BEV) of the AP and PA fields created by the TPS and conforming to lung contours to be later projected on the patient’s skin.
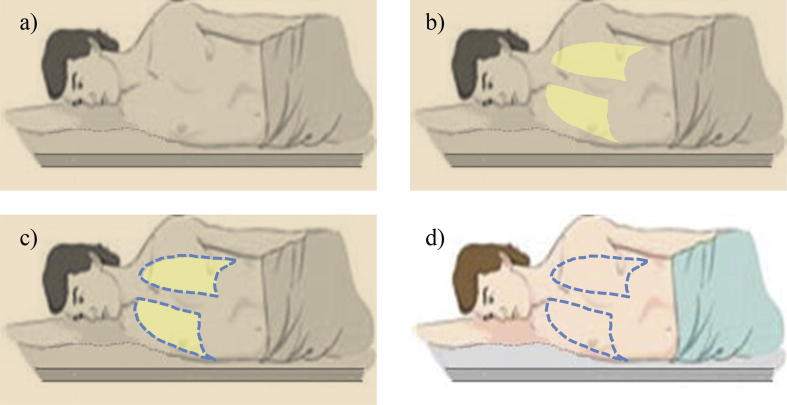
The last step was to have the patient back in the treatment room and on the treatment couch. The fiducial markers previously attached on the patient’s body were aligned with the laser in the treatment room to ensure that the patient was properly positioned. The MLC fields were used to project a light field on the patient’s skin representing the extent of the lungs, which was subsequently marked on the patient’s anterior and posterior skin (Fig. 4). The steps required to mark the lungs on the patient’s skin are illustrated in Fig. 5.
Fig. 4.
An example of a light field projected on the patient’s skin to mark the extent of the lungs.
Fig. 5.
Illustration of the steps needed for marking the projection of the lungs on the patient’s skin: Room lights are turned off (a), a light field is projected on the patient’s skin (b), physician marks the light field (c) and marks are kept on the patient for the remaining treatment days (d).
Shielding blocks
Since the prescribed dose for a TBI treatment is higher than the lungs tolerance, the dose to the lungs must be reduced [2]. Customized and patient specific blocks made of low-melting point metal alloy (Cerrobend) were placed near the patient’s body at the beam entrance to shield the lungs during the time of treatment. Studies recommend using Cerrobend shields of 7–8 cm thickness to completely attenuate the X-ray beam and protect the lungs from future complications [12].
Shielding blocks are individualized for each patient. In previous practice, the blocks dimensions were determined by taking a portal film when the patient is positioned for treatment and having the physician estimate the extent of the lungs and mark them on the developed film (Fig. 6). This imprecise procedure was not needed in our novel technique, since the blocks dimensions were determined from the beam’s eye view (BEV) obtained from the TPS at patient skin level (Fig. 3). Because the blocks were placed very close to the patient’s body and divergence was neglected due to the extended treatment distance, there was no need for any magnification or reduction of the block size. The acquired blocks were fixed on a tray and positioned on the table between the patient and the spoiler to be used for treatment as shown in Fig. 7.
Fig. 6.

A radiographic film acquired to delineate the lungs and to be later reproduced on a Styrofoam.
Fig. 7.
Cerrobend shielding blocks fixed on a tray to be used for treatment.
Positioning of shielding blocks and verification
For the lungs to receive a total shielded dose of 8 Gy, shielding blocks were used in two out of six fractions for the three patients. During treatment with lung blocks, the patient was placed on the treatment gurney with shields positioned at a close distance from the skin. After turning off the room lights and projecting the light field of the 40 × 40 cm treatment beam on the patient’s body, the shadow formed behind the blocks was superimposed to the lung marks drawn earlier on the patient’s skin. At the time of block positioning, the patient was kept in position and the fiducial markers attached on the patient’s body were always aligned with the lasers.
The position of the blocks is usually verified radiographically prior to the delivery of each beam [2], [8]. A digital cassette was placed at the beam exit behind the patient and 1.5 MU were delivered by Siemens’ imaging beam line which uses a carbon target for X-ray production. The position of the blocks was checked on the film directly developed in the Diagnostic Radiology department. If the film showed a full coverage of the lungs and hence, the placement of the blocks was accepted by the physician, the treatment of the corresponding beam was initiated. However, if the lungs could still be seen on the film, adjustments of the position of the blocks were made and portal films were repeated until position is accepted.
To evaluate the efficiency of this technique, the number of repeated attempts to correctly position the shielding blocks for each beam and the overall treatment time were recorded. Treatment time for patients treated with the old technique was not documented in the patients’ records and an approximate estimation was made based on staff experience.
Results
Films were reviewed for proper shielding of the lungs. If a shielding block did not cover the entire lung or, in other words, if part of the lung was still seen on the films, the films were characterized as unsuccessful. Adjustments of the position of lung blocks and another attempt for a successful film were then made.
As indicated in Table 2, all shielding blocks were positioned accurately from the first attempt for two out of three patients. Nevertheless, for one patient, two out of four films were considered unsuccessful and hence an adjustment of the position of the shielding blocks was made only one time and films were repeated after the first attempt. These results are compared to an average of three attempts per beam for one patient with the conventional technique of trial and error where the maximum number of film attempts could go up to 5 films per beam in difficult cases.
Table 2.
Number of attempts needed to accurately position the blocks.
| Patient # | 1st fraction |
2nd fraction |
||
|---|---|---|---|---|
| AP | PA | AP | PA | |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 2 | 1 | 1 | 2 |
| 3 | 1 | 1 | 1 | 1 |
The comparison included the time needed for patient set up and preparation, blocks positioning and verification, in addition to the irradiation time. Time was recorded for each patient and results are shown in Fig. 8. The procedure for patient set up and positioning in addition to the treatment session without blocks remain unchanged. With the new technique, an average of 20 min was added for the determination of lung marks. This latter phase involved a CT simulation, importing the CT images into the TPS, lung contouring in the TPS and the creation of the MLC fields, in addition to projecting the light field on the patient’s skin and marking the extent of the lungs. Lastly, with the new technique, the average time of a treatment session with shielded lungs including the positioning of blocks and film verification was 29 min with a maximum time of 41 min. This compares to an average of approximately 60 min in past treatments with the conventional technique and a maximum time of 120 min.
Fig. 8.
A comparison of the overall time of the conventional vs. new technique.
Discussion
TBI is a dosimetrically and practically complicated procedure that requires time, effort, and a lot of cooperation from the patient [4], [8]. The stringent immobilization that patients have to endure for a long time is often unbearable and causes elevated levels of stress and discomfort especially in children [8]. The purpose of this study was to introduce into clinical practice a simple technique for positioning of lung blocks to reduce the overall treatment time and to alleviate patient discomfort.
Pneumonitis is considered to be the most dangerous side effect from TBI [1], [4]. To protect the lungs from full prescription dose, Cerrobend blocks were placed on a tray between the source of radiation and the patient. [3], [7]. These individualized shielding blocks were shaped to accurately conform to the patient’s lungs [3]. Due to the challenging treatment setup and the toxicity caused by localization errors of the shielding blocks, the accuracy of the positioning of lung blocks in TBI treatments is a very important criterion for a successful treatment [7]. If the placement of the shielding blocks was not accurate, the delivered dose is either lower or higher than the prescribed dose, which may lead to an underdosage of the target volume or increased lung toxicities. For this reason, the verification of block positioning is an essential aspect of a TBI treatment quality control [7].
The drawback of the conventional technique is that the positioning of lung blocks is usually estimated and is based on a trial and error process. Portal films are normally acquired and analyzed for accuracy of block positioning prior to each fraction [7]. If several adjustments are needed, then multiple portal films are also required. This time-consuming procedure prolongs the length of the treatment session time and generally distresses patients, which sometimes disrupts the treatment workflow.
Films are processed in the Diagnostic Radiology department that is often far from the treatment area. Another disadvantage of the increased number of portal films is the time needed to go back and forth to the Radiology department to process the films [7].
The new technique described in this paper solves the problem of the prolonged treatment time for TBI patients. The accurate positioning of lung blocks is easier because radiation therapists start by placing the lung blocks at the reference position defined by the lung marks drawn on the patient’s skin. This definitely reduces the number of trials needed and hence the number of portal films acquired. In our case, only one adjustment was needed for two out of twelve beams (Table 2). The reason was that the patient was anxious and was unable to remain stable during those sessions. For the other patients, all portal films were successful from the first attempt and no adjustments were needed for all shielded beams. These results are compared to an average of three films per beam with the conventional technique. Therefore, the new technique was able to reduce the overall treatment time by reducing the number of portal films acquired.
In addition, the new technique is very effective and guarantees a precise and reproducible TBI treatment. The fiducial markers attached on the patient’s skin on the day of CT simulation are always aligned with the lasers of the treatment room. Consequently, the patient is ought to be oriented everyday as the first day of CT simulation. This ensures that patient inter-fraction motion is avoided and that the patient is always well positioned. Moreover, the lung marks drawn on the patient’s skin are an accurate representation of the extent of the lungs. Therefore, if the patient did not move and lung blocks are seen to be perfectly matching the marks, then this results that shielding blocks stay in perfect position. Portal films are subsequently acquired for quality control and documentation purposes.
Furthermore, with the conventional technique, the shielding blocks dimensions are based on a portal film taken in the treatment room when the patient is ready for treatment. The lungs are then marked by hand on the films as shown in Fig. 6; however, with the new technique, lung marks on patient skin were derived from the CT scan, and projected with an MLC field created in the TPS conforming to the lung contours. Shielding blocks were reproduced from the BEV acquired from the TPS and printed at skin level. Therefore, this technique also guarantees a more accurate block shape and size.
The only inconvenience of the new technique compared to the conventional one is the additional time required for CT simulation. This step can be performed on a separate day as a pre-treatment preparation. However, this added procedure avoids the need of an additional film to cut the blocks. Lastly, our results show that an additional 20 min of CT simulation are better tolerated by the patient compared to lengthy treatment sessions that may last up to two hours in a single session.
In this study, time comparison between the old and new technique was not evaluated accurately because treatment time was not recorded in the patients’ records for patients treated earlier with the old technique. However, the high success rate of block positioning from the first attempt (10 out of 12) with the new technique is in support of our conclusion that we are saving our patients prolonged treatment session time. From previous experience, it is known that such a high success rate is not attainable with the old trial and error technique.
In conclusion, we believe that the above described simple technique provides a shorter and more accurate method for TBI treatments.
Acknowledgments
We would like to thank the radiation therapists at our institution, especially Ali Sakr for their valuable assistance during the treatment of the patients.
Acknowledgments
Conflict of interest
This statement is to certify that all authors have no conflict of interest to declare.
Contributor Information
Hana Mekdash, Email: hanamekdash@gmail.com.
Bilal Shahine, Email: bs39@aub.edu.lb.
Wassim Jalbout, Email: wj01@aub.edu.lb.
Chirine Chehab, Email: cc07@aub.edu.lb.
Helena Abdel Khalek, Email: ha170@aub.edu.lb.
Bassem Youssef, Email: by04@aub.edu.lb, aubmc@aub.edu.lb.
References
- 1.Baldwin Z. Commissioning of a new total body irradiation protocol, Master of Science - Research thesis. Faculty of Engineering, University of Wollongong <http://ro.uow.edu.au/theses/3756> [accessed …].
- 2.Van Dyk J, Galvin JM, Glasgow GP, Podgorsak EB. The physical aspects of total and half body photon irradiation. American Association of Physicists in Medicine by the American Institute of Physics; 1986 [AAPM Report No. 17].
- 3.Galvin JM. Total body irradiation dosimetry and practical considerations. In: American association of physicists in medicine annual meeting 2001. Med Phys 2001;28(8):1820.
- 4.Oncolex oncology encyclopedia. Total body irradiation <http://oncolex.org/Prosedyrer/TREATMENT/RadiationTherapy/Lymphoma_TBI?lg=procedure> [accessed …].
- 5.Quast U. Whole body radiotherapy: a TBI-guideline. J Med Phys. 2006;31(1):5–12. doi: 10.4103/0971-6203.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravichandran R., Binukumar J.P., Davis C.A., Zahid A.M., Rajan B. Simple technique for fabrication of shielding blocks for total body irradiation at extended treatment distances. J Med Phys. 2009;34(4):223–225. doi: 10.4103/0971-6203.56084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurette C. Eburon; Creutzberg: 1998. Treatment verification in radiation oncology. [Google Scholar]
- 8.Gruen A., Ebell W., Wlodarczyk W. Total Body Irradiation (TBI) using Helical Tomotherapy in children and young adults undergoing stem cell transplantation. Radiat Oncol. 2013;8(1):92. doi: 10.1186/1748-717X-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butson M., Pope D., Haque M., Chen T., Song G., Whitaker M. Build-up material requirements in clinical dosimetry during total body irradiation treatments. J Med Phys. 2016;41(2):149–152. doi: 10.4103/0971-6203.181632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan F.M. Lippincott Williams Wilkins; Philadelphia: 2010. The physics of radiation therapy, 4th ed. [Google Scholar]
- 11.Galvin J.M., D’angio G.J., Walsh G. Use of tissue compensators to improve the dose uniformity for total body irradiation. Int J Radiat Oncol Biol Phys. 1980;6(6):767–771. doi: 10.1016/0360-3016(80)90238-2. [DOI] [PubMed] [Google Scholar]
- 12.Taherkhani A., Mohammadi M., Saboori M.S., Changizi V. Evaluation of the physical characteristic of Cerrobend blocks used for radiation therapy. Iran J Radiat Res. 2010;8(2):93–101. [Google Scholar]