Highlights
-
•
Different registration methods for position verification of oesophageal cancer.
-
•
Clipbox around PTV and vertebrae with soft tissue match was best suited.
-
•
Tumour location influences the required setup margins.
-
•
A large variety of anatomical changes is revealed when using kV-CBCT.
-
•
GTV Reduction and diaphragm position changes were most common anatomical changes.
Abbreviations: CBCT, Cone Beam Computed Tomography; EPID, Electronic Portal Imaging Device; GTV, Gross Tumor Volume; CTV, Clinical Target Volume; PTV, Planning Target Volume; OAR, Organs at Risk; IGRT, Image Guided RadioTherapy; IMRT, Intensity Modulated Radiotherapy; AC, Adenocarcinoma; SCC, Squamous Cell Carcinoma; PET/CT, Positron Emission Tomography/Computed Tomography; EUS, Endoscopic UltraSound; TPS, Treatment Planning System; SAL, Shrinking Action Level
Keywords: Oesophageal cancer, Cone-Beam CT, Setup variations, Setup margins, Anatomical changes
Abstract
Purpose
To evaluate different registration methods, setup margins and number of corrections for CBCT-based position verification for oesophageal cancer and to evaluate anatomical changes during the course of radiotherapy treatment.
Methods
From 50 patients, 440 CBCT-scans were registered automatically using a soft tissue or bone registration algorithm and compared to the clinical match. Moreover, relevant anatomical changes were monitored. A sub-analysis was performed to evaluate if tumour location influenced setup variations. Margin calculation was performed and the number of setup corrections was estimated. Results were compared to a patient group previously treated with MV-EPID based position verification.
Results
CBCT-based setup variations were smaller than EPID-based setup variations, resulting in smaller setup margins of 5.9 mm (RL), 7.5 mm (CC) and 4.7 mm (AP) versus 6.0 mm, 7.8 mm and 5.5 mm, respectively. A reduction in average number of setup corrections per patient was found from 0.75 to 0.36. From all automatically registered CBCT-scans, a clipbox around PTV and vertebras combined with soft tissue registration resulted in the smallest setup margins of 5.9 mm (RL), 7.7 mm (CC), 4.8 mm (AP) and smallest average number of corrections of 0.38. For distally located tumours, a setup margin of 7.7 mm (CC) was required compared to 5.6 mm for proximal tumours. Reduction of GTV volume, heart volume and change in diaphragm position were observed in 16, 10 and 15 patients, respectively.
Conclusions
CBCT-based set-up variations are smaller than EPID-based variations and vary according to tumour location. When using kV-CBCT a large variety of anatomical changes is revealed, which cannot be observed with MV-EPID.
Introduction
The incidence of oesophageal cancer is increasing rapidly. Oesophageal cancer is the eight most common cancer worldwide and the sixth most common cause of death from cancer [1].
Radiotherapy is frequently used as part of the multimodality treatment for oesophageal cancer. For patients with potentially curable oesophageal or oesophagogastric junction cancer, the preferred treatment is neoadjuvant chemoradiotherapy in combination with surgery [2], [3]. For inoperable oesophageal or oesophagogastric cancer, definitive chemoradiotherapy is the treatment of choice [4]. The toxicity of chemoradiotherapy depends, among others, on the size of the treated volume and dose to the organs at risk (OAR) [5].
Accurate delineating of radiotherapy target volumes is needed to improve local tumour control and reduce toxicity [6]. The Gross Tumour Volume (GTV) is expanded with anisotropic margins to a Clinical Target Volume (CTV), mainly in cranio-caudal (CC) direction. Subsequently, the CTV is expanded to a Planning Target Volume (PTV) with an additional margin. This margin is used to account for uncertainties in delineation and variation in tumour position [7]. Smaller margins will result in less toxicity but will increase the risk of under-dosage of the clinical target volume [5]. Therefore Image Guided Radiotherapy (IGRT) using sophisticated imaging methods is crucial during treatment to improve the precision and accuracy of treatment delivery. For position verification, different methods are available, such as MV-EPID and kV-ConeBeam CT (CBCT). In 2013 the Catharina Hospital in Eindhoven introduced CBCT-based position verification for oesophageal and oesophagogastric junction cancer. kV-CBCT based imaging provides additional anatomical information compared to using EPID imaging. The aim of this study was to evaluate different registration methods, resulting setup margins and number of corrections for CBCT-based position verification of oesophageal cancer. Moreover, it was aimed to monitor anatomical changes during the course of the radiotherapy treatment and evaluate its influence on the position verification image registration process.
Materials and methods
Patients
Between January 2013 and August 2013, 50 consecutive patients treated in combination with CBCT-based position verification were included and data of these patients were analysed retrospectively. Patients had a histologically confirmed adenocarcinoma (AC) or Squamous Cell Carcinoma (SCC) of the oesophagus or oesophagogastric junction and no clinical evidence of distant metastatic spread.
PET/CT and treatment planning
Before treatment, a 18F-FDG PET/CT without intravenous contrast was made (Philips Gemini PET/CT, Best, The Netherlands). For this scan, patients were immobilised using an arm support and knee support (Sinmed Radiotherapy Products, Reeuwijk, The Netherlands). All patients with a tumour in the proximal oesophagus were immobilised in a mask (Orfit Industries, Wijnegem, Belgium). Scan area was from cricoid to kidneys and slice thickness was 3 mm. Delineation of GTV and CTV was performed by the radiation oncologist. GTV was defined by primary tumour and suspect regional lymph nodes. All available information was used to delineate the GTV: physical examination, endoscopy, Endoscopic Ultrasound (EUS) and PET/CT. CTV was defined by the GTV plus the area of the regional lymph nodes up to at least 3 cm in cranial and caudal extension of the oesophagus from the GTV. For distal tumours, the caudal margin should follow the oesophageal and cardia wall and the margin in direction of stomach wall was limited to 2 cm. In case of pathological lymph nodes, the CTV was extended up to the level of the pathologic nodes in all directions. PTV consisted of CTV with an isotropic margin of 1 cm. OAR consisted of lungs, heart, spinal cord and kidneys.
A seven beam Intensity Modulated Radiotherapy plan (IMRT) was created using the Pinnacle Treatment Planning System (Pinnacle 3 TPS, version 9.8), with a photon energy of 6 MV for all beams. The prescribed dose was 41.4 Gy or 50.4 Gy in 23 or 28 fractions. Patients were treated in combination with weekly carboplatin and paclitaxel.
Radiotherapy treatment and position verification
Before each treatment patients were positioned using tattoos and skin marks. During the treatment course, position verification was performed using CBCT (Elekta Synergy XVI system, version 4.5). CBCT was performed with a full 360° rotation and a M20 collimator resulting in a Field of View (FOV) of 41 cm and a scan length of 28 cm. Standardly, setup corrections were applied using an offline shrinking action level (SAL) protocol with an initial action level of 10 mm and a maximum number of three measurements (n = 3, α = 10) [8]. For patients with large setup variations in the first few fractions, a switch was made to an online correction protocol. For patients with relatively small setup variations, an offline correction protocol accommodated the setup variations adequately within the applied margins.
Data acquisition
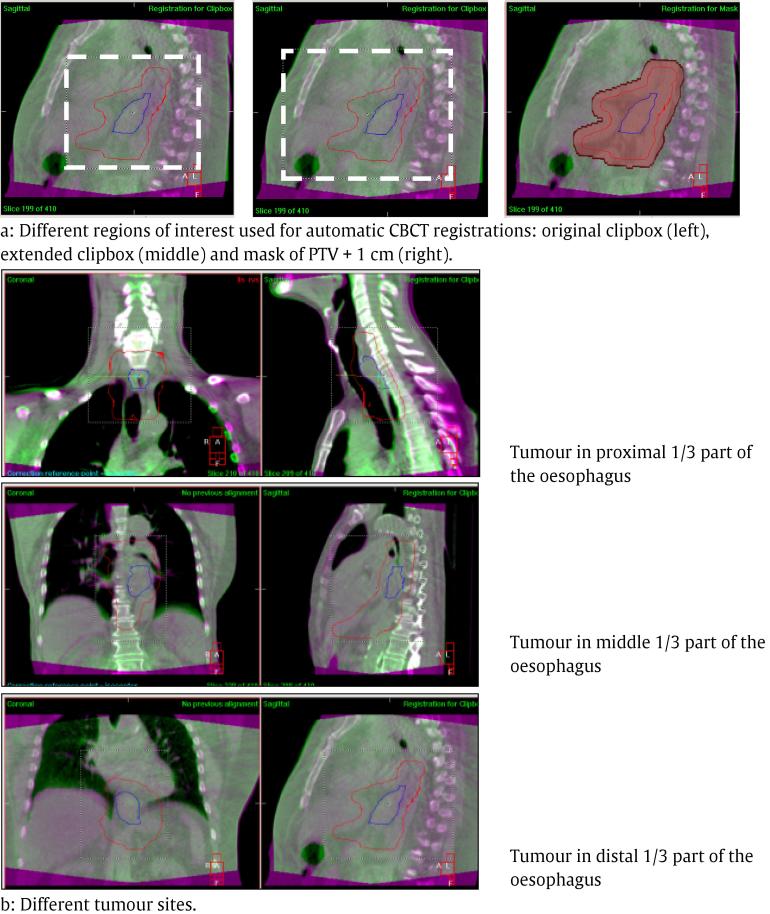
440 CBCT-scans were analysed retrospective and reviewed systematically. For evaluating the consistency of CBCT and the required setup margins, different registration methods were used (Fig. 1a). A clipbox around PTV and vertebras was used for clinical registration, which is referred to as the ‘original clipbox’. Clinically, the registration was made such that the best correspondence between CBCT-scan and reference CT-scan was obtained, with respect to all visible anatomical structures: tumour, oesophagus, trachea and tracheal bifurcation, heart, mediastinum, diaphragm and vertebras. This was obtained by soft tissue registration and if necessary manual adjustments were applied. Obviously not always all structures were equally visible, but in general it was sufficient to assess the accuracy of the registration. The longitudinal displacement depended on the tumour location which visible structure was best suited to assess the accuracy in this direction. In addition, the consistency of a registration method using an extended clipbox around PTV, vertebras and sternum and a registration method using a mask of PTV + 1 cm was investigated. All CBCT-scans were registered automatically using a soft tissue or a bone registration algorithm. In contrast to the clinical registration, no manual adjustments were allowed in the automatic registrations.
Fig. 1.
Different regions of interest and tumour sites. (Blue line: GTV, red line: PTV, CTV: not shown).
Data analysis
Using the setup results obtained with the different registration methods and algorithms, required setup margins for each direction were calculated according to the Van Herk Formula: margin [mm] = 2.5∑ + 0.7σ [9]. In this formula, ∑ indicates the systematic error and σ the random error for the analysed group of patients. Furthermore, the clinically applied SAL correction protocol was simulated for each registration method to estimate the number of setup corrections for each individual patient. Before simulation of the SAL correction protocol, the clinically performed corrections were made undone. For comparison, the results of the required setup margins and number of setup corrections were compared with data of 196 patients with oesophageal or oesophagogastric junction cancer previously treated with MV EPID-based position verification in our hospital. For this, manual registration based on bony anatomy was performed using two orthogonal EPID images.
To investigate if tumour location influenced the set-up variation and thus the required setup margins, a sub-analysis was performed by dividing the current patient group in three categories: tumours in the proximal 1/3 part of the oesophagus, tumours in the middle 1/3 part of the oesophagus and tumours in the distal 1/3 part of the oesophagus including oesophagogastric junction (Fig. 1b). For these different tumour locations, a margin calculation was performed for results based on a bone and a soft tissue registration when using the original clipbox.
Anatomical changes during radiotherapy treatment
Since all CBCT-scans were reviewed systematically, anatomical changes during radiotherapy treatment were described and monitored. Subsequently, it was evaluated as to what extent anatomical changes influenced the location of the GTV with respect to applied PTV margins. All findings were discussed with a radiation oncologist and a clinical physicist, in order to assess possible implications for treatment adaptation.
Results
Patient characteristics are summarised in Table 1. Setup results, required setup margins and estimated number of corrections for different registration strategies are summarised in Table 2. These results were adjusted for possible setup corrections. Clinically, CBCT-based setup variations were somewhat smaller than EPID-based setup variation, resulting in smaller setup margins of 5.86 mm (RL), 7.54 mm (CC) and 4.67 mm (AP) versus 6.04 mm (RL), 7.80 mm (CC) and 5.37 mm (AP), respectively. There was a reduction in average number of setup corrections per patient from 0.75 with EPID (i.e. a total of 147 corrections in 196 patients) to 0.36 with CBCT (i.e. a total of 16 corrections in 45 patients).
Table 1.
Patient characteristics.
| Number of patients | 50 |
| Gender (male/ female) | 38/12 |
| Mean age in years (range) | 69 (49–85) |
| Tumour characteristics | |
| Pathology (AC/SCC) | 34/16 |
| Tumour location (proximal/middle/distal) | 8/7/35 |
| Treatment | |
| Chemoradiotherapy + Surgery | 27 |
| Chemoradiotherapy | 23 |
| Position verification protocol (online/offline) | 5/45 |
Table 2.
Setup results, required setup margins and estimated number of setup corrections.
| EPID | CBCT | CBCT (original clipbox) |
CBCT (extended clipbox) |
CBCT (mask = PTV+1 cm) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Bone match | Clinical match | Soft tissue match |
Bone match | Soft tissue match |
Bone match | Soft tissue match |
Bone match | ||
| Group mean [mm] | Lateral (RL) | −0.40 | 0.13 | 0.11 | 0.20 | 0.10 | −0.08 | 0.03 | −0.01 |
| Longitudinal (CC) | −0.03 | 0.33 | 0.27 | 0.28 | 0.01 | 0.33 | 0.58 | 0.46 | |
| Vertical (AP) | 0.33 | 0.28 | 0.25 | 0.31 | 0.15 | 0.33 | 0.03 | −0.11 | |
| Σ [mm] | Lateral | 1.44 | 1.78 | 1.78 | 1.90 | 1.69 | 1.89 | 1.82 | 2.47 |
| Longitudinal | 2.01 | 2.07 | 2.14 | 2.63 | 1.93 | 2.33 | 3.12 | 2.68 | |
| Vertical | 1.49 | 1.42 | 1.46 | 1.64 | 1.88 | 1.73 | 1.77 | 1.51 | |
| σ [mm] | Lateral | 3.47 | 2.03 | 2.04 | 2.10 | 1.97 | 2.05 | 2.11 | 2.27 |
| Longitudinal | 3.97 | 3.38 | 3.38 | 3.40 | 3.21 | 3.34 | 3.53 | 3.09 | |
| Vertical | 2.35 | 1.59 | 1.62 | 1.45 | 1.68 | 1.46 | 1.92 | 1.37 | |
| Setup margin [mm] | Lateral | 6.04 | 5.86 | 5.88 | 6.23 | 5.62 | 6.17 | 6.02 | 7.78 |
| Longitudinal | 7.80 | 7.54 | 7.73 | 8.94 | 7.07 | 8.17 | 10.27 | 8.87 | |
| Vertical | 5.37 | 4.67 | 4.79 | 5.13 | 5.88 | 5.36 | 5.76 | 4.72 | |
| Average number of corrections using offline SAL protocol | 0.75 | 0.36 | 0.38 | 0.69 | 0.49 | 0.56 | 0.64 | 0.71 | |
Using the original clipbox, setup variations, required margins and estimated setup corrections increased slightly when applying a soft tissue algorithm compared to the clinical registration. There was a setup margin increase when applying a bone match algorithm from 5.86 mm to 6.23 mm (RL), 7.54 mm to 8.94 mm (CC) and 4.67 mm to 5.13 mm (AP). Using an extended clipbox, setup variations and margins were comparable to original clipbox registrations for both registration algorithms; the number of corrections increased slightly when applying a soft tissue algorithm (from 0.38 to 0.49), and decreased slightly when applying a bone match algorithm (from 0.69 to 0.56). Using a mask of PTV + 1 cm, setup variations, margins and corrections increased for both soft tissue and bone registration algorithms.
Setup results and required setup margins for different tumour locations are summarised in Table 3. All results were obtained using the original clipbox because this registration method resulted in the least variation and smallest number of setup corrections in the entire group of patients (Table 2).
Table 3.
Analysis of setup results and margins for different tumour sites.
| CBCT | CBCT (original clipbox) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Clinical match n = 50 |
Proximal n = 8 |
Middle n = 7 |
Distal n = 35 |
|||||
| Soft tissue match |
Bone match | Soft tissue match |
Bone match | Soft tissue match |
Bone match | |||
| Σ [mm] | Lateral | 1.78 | 1.52 | 2.00 | 1.21 | 1.13 | 1.78 | 1.90 |
| Longitudinal | 2.07 | 1.56 | 1.48 | 2.61 | 1.82 | 2.05 | 2.83 | |
| Vertical | 1.42 | 1.41 | 2.23 | 1.70 | 1.82 | 1.42 | 1.49 | |
| σ [mm] | Lateral | 2.03 | 1.76 | 1.78 | 2.18 | 2.10 | 2.05 | 2.14 |
| Longitudinal | 3.38 | 2.42 | 1.51 | 2.51 | 2.47 | 3.62 | 3.72 | |
| Vertical | 1.59 | 1.73 | 1.62 | 1.19 | 1.18 | 1.67 | 1.47 | |
| Setup margin [mm] |
Lateral | 5.86 | 5.03 | 6.24 | 4.54 | 4.29 | 5.88 | 6.24 |
| Longitudinal | 7.54 | 5.61 | 4.74 | 8.29 | 6.29 | 7.66 | 9.69 | |
| Vertical | 4.67 | 4.73 | 6.71 | 5.09 | 5.37 | 4.71 | 4.75 | |
When applying a soft tissue registration for tumours in the proximal part of the oesophagus, the required margins decreased slightly compared to the clinical registration for the whole patient group. The main decrease was in cranio-caudal (CC) direction from 7.54 mm to 5.61 mm, i.e. longitudinal direction. When applying a bone registration for this tumour site there was a margin increase in lateral (RL) direction from 5.86 mm to 6.24 mm and in vertical (AP) direction from 4.67 mm to 6.71 mm. In longitudinal (CC) direction a margin decrease was found from 7.54 mm to 4.74 mm.
For tumours in the middle part of the oesophagus, the required setup margin was comparable in RL and AP direction when applying a soft tissue or a bone match algorithm. In contrast, setup margin in CC direction increased from 7.54 mm to 8.29 mm when applying a soft tissue match algorithm and decreased from 7.54 mm to 6.29 mm when applying a bone match algorithm. For distally located tumours, the largest required setup margin was also in CC direction, 7.66 mm with soft tissue registration and 9.69 mm with bone registration. In contrast to tumours in proximal or middle part of the oesophagus, the required setup margins increased when applying a bone registration compared to a soft tissue registration from 5.88 mm to 6.24 mm (RL), 7.66 mm to 9.69 mm (CC) and 4.71 mm to 4.75 mm (AP).
The absolute difference (mean ± SD) between soft tissue and bone registration was on average small for tumours in proximal part of the oesophagus, 0.43 ± 0.22 mm (RL), 0.29 ± 0.25 mm (CC), 0.62 ± 0.60 mm (AP). The absolute differences were larger for tumours in distal part or at the oesophagogastric junction, 0.79 ± 0.63 mm (RL), 1.82 ± 1.29 mm (CC), 0.57 ± 0.44 mm (AP) (Table 4).
Table 4.
Analysis of differences between registration algorithms (mean ± SD).
| CBCT (original clipbox) |
||||
|---|---|---|---|---|
| Proximal n = 8 |
Middle n = 7 |
Distal n = 35 |
||
| Absolute difference between soft tissue and bone match [mm] | Lateral | 0.43 ± 0.22 | 0.68 ± 0.31 | 0.79 ± 0.63 |
| Longitudinal | 0.29 ± 0.25 | 1.03 ± 0.74 | 1.82 ± 1.29 | |
| Vertical | 0.62 ± 0.60 | 0.41 ± 0.49 | 0.57 ± 0.44 | |
An overview of observed anatomical changes is summarised in Table 5. Frequent anatomical changes were GTV and heart volume reduction and changes in diaphragm position in 16, 10 and 15 patients, respectively. The average heart volume reduction was 6%. All findings were discussed with a radiation oncologist and a clinical physicist to estimate the impact on CTV coverage and dose in OAR.
Table 5.
Overview of anatomical changes during radiotherapy treatment.
| Frequent changes | Cause (most likely) | Number of patients | Consequence |
|---|---|---|---|
| Reduction of GTV volume | Treatment-related | 16 9 (SCC) 7 (AC) |
Displacement of other mediastinal structures/organs, e.g. trachea, with respect to the reference CT |
| Change in diaphragm position | Different breathing pattern | 15 | Difference between bone match and soft tissue match |
| Large reduction in heart volume (>10%) | Unknown | 10 | Displacement of other mediastinal structures/organs, e.g. oesophagus, with respect to the reference CT |
| Rare changes | Possible explanation | Number of patients | Consequence |
| Air in oesophagus | Obstruction, air swallowing | 2 | Difficulties in soft tissue match |
| Air in stomach / bowel | Air swallowing, (carbonated) drinks | 2 | Difficulties in soft tissue match |
| Lateral displacement of oesophagus | Difference in lung filling left vs right | 1 | Difficulties in soft tissue match |
| Anatomical changes in oesophagus outside the radiation field | Treatment-related | 1 | No direct consequences |
| Changes in fatty tissue around the oesophagus | Treatment-related | 1 | No direct consequences |
| Displacement of tumour marker clips | Tumour regression/migration of clips | 1 | Clips not usable to determine accuracy of the match |
Discussion
In this study different registration methods and algorithms for oesophageal or oesophagogastric junction cancer patients and the resulting setup variations, setup margins and number of setup corrections were analysed. It is important to emphasize that the reported margins are only setup margins. It is not suggested that these margins are sufficient to accommodate all other uncertainties such as rotations, and should therefore not be used clinically. Furthermore, anatomical changes during the course of treatment were monitored and evaluated.
The required setup margins decreased slightly and a large reduction of setup corrections was found when using position verification based on kV-CBCT compared to MV-EPID [10]. Smaller setup margins, reduction of setup corrections and additional anatomical information suggested that performing kV-CBCT instead of MV-EPID could lead to a more consistent way of image registration and verification between different professionals.
Hawkins et al. [11] investigated in 20 oesophageal cancer patients which method was more accurate for position verification. They used both EPID and CBCT prior to treatment for each patient and made a comparison for both methods. Concerning CBCT, Hawkins et al. only applied the automatic soft tissue registration algorithm of XVI using a clipbox comparable to the original clipbox in the present study. They concluded that CBCT provides more accurate 3D volumetric imaging. Their results were comparable to the present results for MV-EPID and CBCT using the original clipbox and soft tissue registration. However, in the present study several additional registration methods and algorithms were investigated.
The required margins were calculated based on interfraction setup variation. This margin calculation did not account for rotations, intrafraction variation, delineation and treatment uncertainties. Clinical margins have to be larger to account for these uncertainties. The required margins based on setup results from CBCT were 5.86 mm in RL direction, 7.54 mm in CC direction and 4.67 mm in AP direction for all patients. These margins were slightly smaller compared to position verification with MV-EPID. The current margin of 10 mm seems adequate but should not be reduced when applying an offline correction protocol. Yamashita et al. [12] investigated CBCT-based setup errors for oesophageal cancer patients, resulting in required setup margins of 8 mm in all directions. These setup margins are slightly larger compared to the current study. Hawkins et al. [8] evaluated systematic and random errors. For CBCT systematic errors (mm) were 1.3, 1.7, 1.4 (RL, CC and AP direction) and random errors were respectively 2.6, 3.9, 2.0 mm, which is very similar to the current study. Hawkins et al. suggested that results for CBCT and EPID were similar, but there was no correlation between two modalities. In the current study the systematic and random errors were somewhat smaller when using CBCT for position verification compared to position verification with MV-EPID. By performing position verification with kV-CBCT, setup corrections reduced compared to position verification with MV-EPID. This reduction is possibly caused by larger random variations and possible erroneous registrations when using MV-EPID. In addition, improved visualisation of anatomy is available when using CBCT which highlights that patient setup was as planned and therefore corrections were not required. A smaller number of setup corrections implies that the original CT-scan is more representative for the actual patient setup during treatment and the original treatment plan better resembles the actually delivered dose to the patient. When using an online correction protocol, the required margins for setup variation may theoretically be reduced to 0 mm. However, other uncertainties such as rotations, intrafraction variation, delineation and treatment uncertainties still exist, therefore a CTV-PTV margin is still required. Even when using an online correction protocol, it is important to select the most appropriate registration method.
Registration with original clipbox and a soft tissue algorithm resulted in smallest setup margins and number of corrections. Hence, the original clipbox was used for sub-analysis. The differences between a soft tissue and bone match were small for the included patients in this study when using a certain registration method e.g. the original clipbox. It is difficult to determine which registration (bone or soft tissue) is correct, since there is no gold standard. This way, the automatic registration algorithms can merely be seen as a tool to limit the amount of variation between different observers. When applying clinically, it remains important to review the registration and not only rely on the automatic algorithm, and adjust the registration manually if necessary.
Performing the registration method with an extended clipbox resulted in larger setup variations. The position of oesophagus and vertebras is obviously not related to the sternum position. Motion due to breathing could be a possible explanation. When performing registration with an extended clipbox, sometimes the automatic registration focuses more on the sternum. In case this was seen, a mismatch between relevant visible structures such as tumour, oesophagus, trachea and tracheal bifurcation, heart, mediastinum and vertebras could be observed, leading to inaccurate registrations.
When applying the registration with a mask of PTV + 1 cm, it resulted in the most setup variation possibly caused by limited soft tissue contrast in the registration volume.
For tumours in the proximal and middle part of the oesophagus, setup margins increased when applying a soft tissue algorithm. For tumours in distal part, setup margins increased when applying a bone match algorithm. The position of the skin marks and tattoos in relation to the anatomy could be a possible explanation. However, evidence of these results is limited due to a small patient group with proximal or middle oesophagus tumours (n = 15).
It may be advisable to use region specific margins. The largest setup variations and required setup margins were found for patients with distally located tumours. The absolute difference between soft tissue and bone registration were predominantly determined by patients with distal oesophageal tumours, where variations in breathing pattern and diaphragm position resulted in large differences, mainly in longitudinal direction. One frequently observed anatomical change was a change in diaphragm position. The possible cause for this change is a different breathing pattern. Jin et al. [13] recently investigated the respiration-induced oesophageal tumour motion and concluded that the largest tumour motion was in the distal part of the oesophagus, that tumour motion in the proximal part was limited and that there is some need for individualised internal margins. Nowadays the Catharina Hospital standardly uses four-dimensional CT-scans for patients with a tumour of the distal part of the oesophagus or oesophagogastric junction and the individualised margins are based on tumour motion.
Another frequent anatomical change was reduction of GTV volume. Patients with different types of tumours, SCC and AC were included. Reduction of GTV volume was frequently observed for patients with a SCC. It is likely that this reduction is a result of tumour regression, but it is difficult to distinguish tumour from normal oesophagus on CBCT. Jin et al. [14] investigated the use of markers for setup verification. They concluded that markers can be useful to determine whether the coverage of the tumour is adequate or not, but the use of markers for setup verification is not feasible due to large tissue deformations during the treatment period.
The third frequent change observed was a reduction of heart volume. Lutkenhaus et al. [15] and Mohammad et al. [16] evaluated this reduction and found a significant reduction of the median heart volume of 8% over the radiotherapy treatment course. In the current study the reduction of heart volume was estimated in a similar way as Lutkenhaus et al. and Mohammad et al., and was found to be 6%.
Despite all possible anatomical variations, CBCT registration was in all cases considered to be accurate enough in order to confidently and accurately deliver the prescribed dose.
If reduction of GTV volume was observed, plan adaptation was considered not necessary because the CTV remained roughly unchanged. Only in a few cases, a new CT-scan was made to estimate the impact of the anatomical change on the treatment plan, but it never resulted in an actual adjustment or adaptation of the delivered plan. Nyeng et al. [17] investigated the dosimetric consequences of anatomical changes during treatment. An extra CT-scan was acquired during the treatment period at (median) fraction 10. These scans were deformably registered to the original planning-CT. A plan adaptation was performed when CTV coverage decreased >1% or PTV coverage decreased >3%. For nine out of twenty-nine patients, a plan adaptation was made. Main causes of plan adaptation were change in diaphragm position, mediastinal changes and bowel filling changes. Applied CTV-PTV margins in the study of Nyeng et al. were 5 mm in RL and AP direction and 8 mm in CC direction. In the current study isotropic margins of 1 cm were applied. A CTV coverage reduction is observed earlier when applying smaller margins. Moreover, Nyeng et al. performed registration on bony structures but in the current study soft tissue registration was performed. These differences could explain the need for plan adaptation in Nyeng’s study.
Another anatomical change in the current study and the study of Nyeng et al. was change in bowel filling and air in the oesophagus for some patients. All patients received concurrent chemotherapy, after which patients often drink carbonated drinks, which could explain the change in bowel filling and air in the oesophagus. Now patients are recommended to consume carbonated drinks only after the radiotherapy session.
In our study, the impact of the observed anatomical changes on the CTV coverage and dose in OAR was only a rough estimate based on clinical experience. For a more detailed analysis of the actually delivered dose to targets and OAR, further investigation is needed. A sophisticated method for this would be to apply dose calculations on the CBCT scans. This is currently not yet implemented in our hospital, and needs further refinement to accurately assess the impact of anatomical changes on the accuracy of the delivery of the prescribed dose.
Conclusions
CBCT-based position verification reduces setup variations and corrections, resulting in a more consistent IGRT method for oesophageal cancer. Registration with a clipbox around PTV and vertebras and a soft tissue algorithm resulted in the smallest setup margins and number of corrections. It is difficult to determine which registration (bone or soft tissue) is correct, since there is no gold standard.
Moreover, tumour location influences the required setup margins. For tumours of distal oesophagus or oesophagogastric junction, the required setup margin should be larger, especially in longitudinal direction, in comparison with tumours of more proximal parts of the oesophagus.
A large variety of anatomical changes is revealed when using kV-CBCT for oesophageal cancer patients, which cannot be observed using MV-EPID images. The most common anatomical changes were reduction of GTV volume, heart volume reduction and change in diaphragm position. The influence on tumour position was limited and applied PTV margins were still accurate. However, monitoring the influence of anatomical changes may become more important e.g. when applying smaller margins or plan adaptation.
Contributor Information
A. van Nunen, Email: Aniek.v.Nunen@Catharinaziekenhuis.nl.
M.J.C. van der Sangen, Email: maurice.vd.sangen@catharinaziekenhuis.nl.
M. van Boxtel, Email: marleen.v.boxtel@catharinaziekenhuis.nl.
P.M.A. van Haaren, Email: paul.v.haaren@catharinaziekenhuis.nl.
References
- 1.Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P.L. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Jin H.-L., Zhu H., Ling T.-S., Zhang H.-J., Shi R.-H. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5983–5991. doi: 10.3748/wjg.15.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev 2006:CD002092. [DOI] [PubMed]
- 5.Lesueur P., Servagi-Vernat S. Definition of accurate planning target volume margins for esophageal cancer radiotherapy. Cancer/Radiothérapie. 2016;20:651–656. doi: 10.1016/j.canrad.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 6.Gao X.-S., Qiao X., Wu F., Cao L., Meng X., Dong Z. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:389–396. doi: 10.1016/j.ijrobp.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Bel A., van Herk M., Bartelink H., Lebesque J.V. A verification procedure to improve patient set-up accuracy using portal images. Radiother Oncol. 1993;29:253–260. doi: 10.1016/0167-8140(93)90255-7. [DOI] [PubMed] [Google Scholar]
- 9.Van Herk M., Remeijer P., Rasch C., Lebesque J.V. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 10.Martins L., Couto J.G., Barbosa B. Use of planar kV vs. CBCT in evaluation of setup errors in oesophagus carcinoma radiotherapy. Rep Pract Oncol Radiother J Gt Cancer Cent Pozn Polish Soc Radiat Oncol. 2016;21:57–62. doi: 10.1016/j.rpor.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins M.A., Aitken A., Hansen V.N., McNair H.A., Tait D.M. Set-up errors in radiotherapy for oesophageal cancers–is electronic portal imaging or conebeam more accurate? Radiother Oncol. 2011;98:249–254. doi: 10.1016/j.radonc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H., Haga A., Hayakawa Y., Okuma K., Yoda K., Okano Y. Patient setup error and day-to-day esophageal motion error analyzed by cone-beam computed tomography in radiation therapy. Acta Oncol (Madr) 2010;49:485–490. doi: 10.3109/02841861003652574. [DOI] [PubMed] [Google Scholar]
- 13.Jin P., Hulshof M.C.C.M., de Jong R., van Hooft J.E., Bel A., Alderliesten T. Quantification of respiration-induced esophageal tumor motion using fiducial markers and four-dimensional computed tomography. Radiother Oncol. 2016;118:492–497. doi: 10.1016/j.radonc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Jin P., van der Horst A., de Jong R., van Hooft J.E., Kamphuis M., van Wieringen N. Marker-based quantification of interfractional tumor position variation and the use of markers for setup verification in radiation therapy for esophageal cancer. Radiother Oncol. 2015;117:412–418. doi: 10.1016/j.radonc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Lutkenhaus L.J., Kamphuis M., van Wieringen N., Hulshof M.C.C.M., Bel A. Reduction in cardiac volume during chemoradiotherapy for patients with esophageal cancer. Radiother Oncol. 2013;109:200–203. doi: 10.1016/j.radonc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Haj Mohammad N., Kamphuis M., Hulshof M.C.C.M., Lutkenhaus L.J., Gisbertz S.S., Bergman J.J.G.H.M. Reduction of heart volume during neoadjuvant chemoradiation in patients with resectable esophageal cancer. Radiother Oncol. 2015;114:91–95. doi: 10.1016/j.radonc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Nyeng T.B., Nordsmark M., Hoffmann L. Dosimetric evaluation of anatomical changes during treatment to identify criteria for adaptive radiotherapy in oesophageal cancer patients. Acta Oncol. 2015;54:1467–1473. doi: 10.3109/0284186X.2015.1068449. [DOI] [PubMed] [Google Scholar]