Highlights
-
•
Oral mucositis (OM), the most common toxicity in patients with head and neck cancer.
-
•
Polideoxyribonucleotide (PDRN) is highly regenerative and anti-inflammatory drug.
-
•
OM lesions were attenuated to Grade 2 within the first week of PDRN treatment.
-
•
Pain was relieved about 2–3 days after starting treatment with PDRN spray.
-
•
All the patients completed Radiotherapy and chemotherapy without any interruptions.
Keywords: Radiotherapy, Chemotherapy, Oral mucositis, Head and neck cancer, Polydeoxyribonucleotide
Abstract
Introduction
Oral mucositis, the most common adverse effect of radiotherapy (RT) and/or chemotherapy is observed in almost 97% of patients with head and neck cancer. Although several agents like corticosteroids, lidocaine and vitamins are available for its prevention or management, results are often disappointing. Here we report on the effects of a topically applied, highly purified natural deoxyribonucleic acid from sturgeon gonads on three cases of moderate to severe oral mucositis in patients with head and neck cancer.
Case Description
Three patients who had undergone RT and/or chemotherapy received an oral spray containing sodium salt-based natural deoxyribonucleic acid (PDRN) for Grade 3 oral mucositis. Treatment continued for one month after the end of RT. No patient reported any allergic reactions. RT and chemotherapy were not interrupted and opioid therapy was not given to any patient. Pain was relieved about 2–3 days after starting treatment and oral mucositis was reduced to G2 within one week.
Conclusions
Outcomes in all 3 cases showed topical use of the sodium salt-based PDRN derived from sturgeon gonads was acceptable and safe when used topically for therapeutic and regenerative purposes.
Present results are encouraging and suggest a more in-depth study is warranted on its use in a larger patient cohort with RT-induced oral mucositis.
Introduction
Oral mucositis, the most common adverse effect of radiotherapy (RT) and/or chemotherapy (CT) has been reported in nearly 97% of patients with head and neck cancer [1]. Radiation-induced free radicals and DNA damage modify intra- and inter-cellular signalling pathways, which regulate epithelial and immune cell proliferation, differentiation and death [2].
The response cascade causes inflammation and activates apoptosis and epithelial hypoplasia. Erythema, the first sign of oral mucositis, appears when the cumulative RT dose is escalated to over 20 Gy (i.e. the threshold for mucosal tolerance) and worsens to ulceration as the cumulative dose reaches 30 Gy [3]. Lesions usually resolve completely, within 4 weeks of completing RT [4]. Erythema, edema, ulceration and pseudo-membrane formation in the oral cavity are often associated with pain [1], [5] which strongly affects quality of life (QoL). Patients may suffer from xerostomia, dysphagia and allodynia, all of which result in weight loss, nutritional deficits, recurrent infections and increased use of narcotic analgesics, prolonged hospitalization, total parenteral nutrition and ultimately RT suspension. Outcomes and cure of head and neck cancer patients are clearly negatively impacted [6], [7].
The oral mucosal response intensity depends on several factors. RT-related factors include the tumour site and treatment volume, total RT dose, the fractionation schedule as accelerated fractionation increases the risk. Associated CT administration may contribute to earlier mucositis development and severity. Patient-related factors are comorbidities, poor oral hygiene, dental status, alcohol intake and tobacco use, diet, local microbial environment [8], [9].
Although MASCC/ISOO guidelines [10] indicate treatment for RT with or without CT-related mucositis in patients with head and neck cancer, the level of evidence is low. Agents include, for example, corticosteroids, sucralfate, lidocaine, vitamins, anti-oxidants, growth factors and prostaglandins, with disappointing results in some instances. One innovative approach is a sodium salt-based product (Polideoxyribonucleotide-PDRN), Sanaryn from Veritas S.R.L. (Brescia, Italy) which has not yet been tested in a pilot study. Derived from sturgeon gonads, its biologically active component is highly purified natural deoxyribonucleic acid. Like other fish sources, such as salmon and trout, sturgeon is easily farmed and therefore plentiful. Other sources of deoxyribonucleic acid include calves, pigs and plants. PDRN is usually extracted from the gonads of the source animal under a patented process and purified at high temperature, yielding a > 95% pure active substance with inactivated proteins and peptides. This latter guarantees the safety of the product and no immunological side effects [11].
PDRN is reported to have highly regenerative and anti-inflammatory properties and is indicated in the treatment of burns, skin lesions, ulcers and diabetic necrosis. Consequently, in a proof of concept investigation, we tested acceptability of its use in three patients with head and neck cancer who developed oral mucositis after RT which was associated with CT in 2 of them. Outcome parameters were adverse reactions to PDRN, mucositis control or resolution, time required and pain control. All patients were advised about routine care to prevent oral mucositis according to current MASCC/ISOO guidelines. Nutrition was monitored weekly in each. Acute toxicity was evaluated according to the CTCAE 4.0 scale.
Case description
Case 1
After stage cT4N0M0 hard palate carcinoma was diagnosed, a 73-year-old man with ischemic heart disease and hearing loss was treated with intensity-modulated RT (IMRT) of the oral cavity 66 Gy/2.2 Gy/30 fractions (5 weekly for 6 weeks) and bilateral lymph nodes levels IA, IB, II, III 54 Gy/1.8 Gy/30 fractions. RT was associated with weekly cisplatinum-based CT (30 mg/m2). In the 3rd week of RT, after receiving 24.2 Gy the patient developed Grade 3 oral mucositis, glossitis, edema and pain leading to weight loss and depression. PDRN spray was started and continued.
Case 2
A 79-year-old female underwent left partial glossectomy and level I-III ipsilateral neck dissection for stage pT2N0M0 cheratizing G2 squamous carcinoma, that had infiltrated the intrinsic lingual muscle bundles. Radial margins were positive and all excised lymph nodes were negative. Comorbidities included hypertension, psoriasis, bilateral glaucoma and gout. The patient had a history of invasive ductal carcinoma of the breast.
She received adjuvant IMRT. The clinical target volume 1 (CTV1) was the left half of the tongue which received 66 Gy/2.2 Gy/30 fractions, the CTV 2 was the oral cavity which received 60 Gy/2Gy/30 fractions and the CTV 3 was the bilateral level IA, IB, II, III lymph nodes which received 54 Gy/1,8 Gy/30 fractions. A simultaneous integrated boost (IMRT-SIB) technique was used and treatment was administered in 5 weekly fractions for 6 weeks.
In the second week of RT after receiving 19.8 Gy the patient developed Grade 1 oral mucositis, associated with mycosis which was treated with an anti-fungal oral solution. In the third week of RT, after receiving 30.8 Gy oral mucositis worsened to Grade 3. Moist desquamation developed on the lower lip, associated with severe pain that resulted in weight loss and reduced QoL. PDRN spray was started.
Case 3
A 74-year-old female was diagnosed with Stage pT4aN1 G2 squamous carcinoma of the upper right retromolar trigone, infiltrating the jaw and adjacent tissues. Co-morbidities included high blood pressure, osteoporosis, depression and deep vein thrombosis. She underwent surgery with partial removal of the mandible, and excision of the mouth floor, the gingival mucosa, the peri-mandibular, masseter and pterygoid muscles and posterior tongue muscle fascia. Level I-II-II-IV-V omolateral neck dissection and level I-II-III contralateral neck dissection were performed. The face was reconstructed with a musculo-cutaneous flap of the pectoralis major muscle. Two months after surgery magnetic resonance imaging showed local relapse. She received IMRT-SIB. CTV 1 was the local relapse area (66 Gy/2.2 Gy/30 fractions, 5 weekly for 6 weeks). CTV 2 was the tumour bed (60 Gy/2.0 Gy/30 fractions, 5 weekly for 6 weeks). CTV3 was level I-V bilateral lymph nodes which received (54 Gy/1.8 Gy/30 fractions, 5 weekly for 6 weeks). RT was associated with weekly cisplatinum-based CT (40 mg/m2). At the beginning of the 3rd week of RT (22 Gy) the patient developed Grade 2 oral mucositis with candidosis which was treated with an anti-fungal agent. One week later (33 Gy) as the oral mucositis had worsened to Grade 3, PDRN spray was started.
In patients 1 and 2 PDRN was sprayed twice a day on areas of mucositis. Patient 3 started with 3 applications daily, rising to 6. All patients continued PDRN for at least a month after RT ended. PDRN was applied in hospital by nursing staff and at home by patients or carers. Patients were followed up weekly until one month after RT ended. Each check-up included a clinical examination and a photograph of oral lesions.
Outcomes
No patient reported any allergic reactions to Sanaryn and all benefitted from it. RT and CT were not interrupted and opioid therapy was not given to any patient. In patients 1 and 2 pain was relieved about 2–3 days after starting treatment and erythema and desquamation was reduced to Grade 2 within one week. No lesion worsened and no new ones developed. Although all lesions completely resolved two weeks after RT ended, use of the PDNR spray was prolonged to ensure mucosal hydration. In patient 3, pain always recurred 40 min after using the spray but a transient improvement was observed for five days, with mucositis reversing to Grade 2. Then 2 ulcers developed on the lower lip mucosa and tongue. The patient’s status worsened with weight loss and dehydration. She was treated with PDRN spray (6 times daily), a lidocaine and cortisone mouthwash, dexamethasone 4 mg twice a day and an anti-fungal agent until the end of RT. She continued with PDRN (6 times daily) and dexamethasone 4 mg once a day for another month until mucositis resolved and then with PDRN alone for another two weeks.
Fig. 1, Fig. 2 show patient 1 before and after PDRN treatment.
Fig. 1.
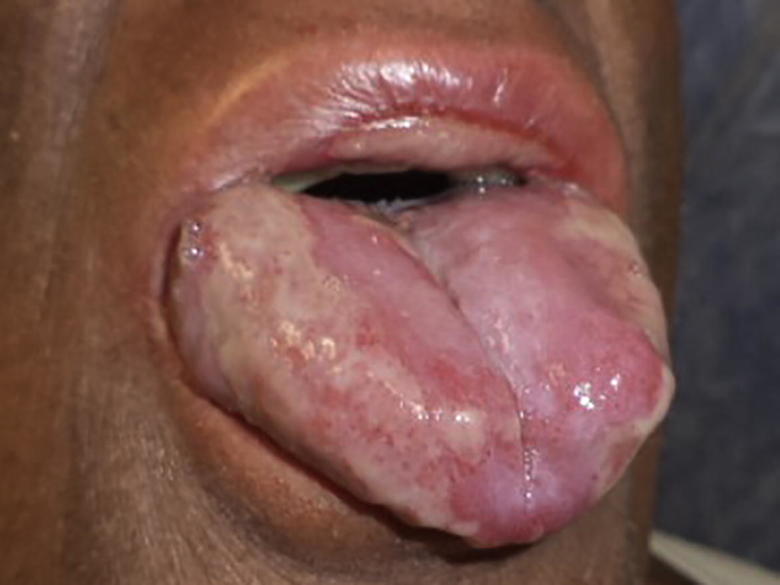
Patient 1 before PDRN treatment: Confluent Grade 3 oral mucositis in the 3rd week of RT and CT.
Fig. 2.
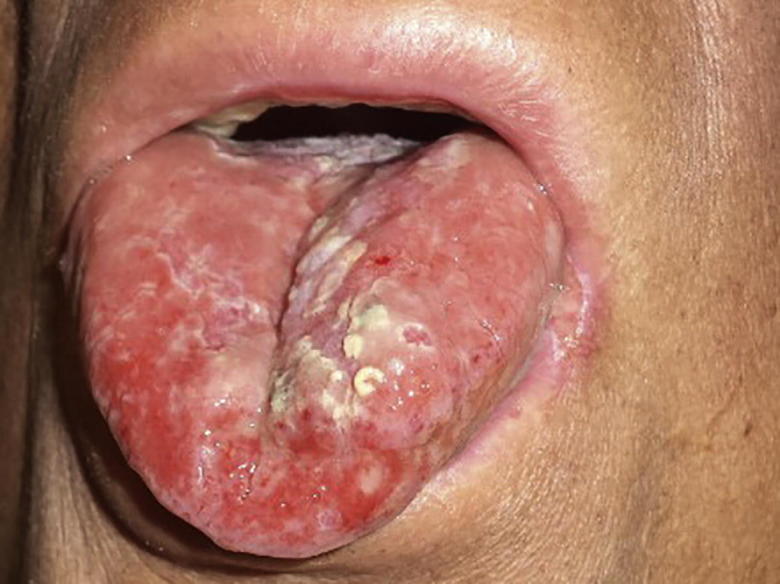
Patient 1 during PDRN treatment: Grade 1 mucositis a few days after the end of RT.
Conclusions
Outcomes in all 3 cases showed topical use of the sodium salt-based PDRN from sturgeon gonads was acceptable and safe when used topically for therapeutic and regenerative purposes. PDRN appears to exert its beneficial effects through two main mechanisms of action. It may act preferentially on the adenosine A2A receptor which, in the setting of patients with oral mucositis, plays a central role in modulating inflammation, oxygen consumption, ischemia, cell growth, and angiogenesis. On the other hand, its mechanism of action may include the salvage pathway. In fact, PDRN was reported to generate nucleotides and nucleosides that contributed to DNA formation, thus reactivating normal cell proliferation and growth patterns, as confirmed in vitro in human osteoblasts [11]. When added to cell cultures immediately after irradiation PDRN activated the p53 protein and enhanced DNA repair [11]. It also eliminated infections, counteracted inflammation and aided in scar repair by activating the monocyte-macrophage system and cytokine cascade and reduced the risk of cheloid scarring by increasing fibronectin and collagen production.
In the present case series a PDRN-containing spray was associated with marked pain relief in two patients within a very short space of time, allowing eating and drinking thus improving their QoL. Lesions were attenuated to Grade 2 status within the first week and even though therapy continued for another 2 weeks and patients reached their maximum RT dose, oral mucositis did not progress and gradually disappeared within two weeks after the end of RT. In the 3rd patient, whose clinical condition was much worse than the other two, the spray relieved pain for brief periods of time with weaker control of oral mucositis, even when associated with other agents. Despite this, the patient completed RT and CT without any interruptions and mucositis eventually resolved. Delayed resolution may have been due to demolitive surgery creating a more complex pre-treatment condition in this patient, which may have determined a greater CT impact than was observed in Patient 1 who had received a slightly lower dose.
According to current MASSC/ISOO guidelines [10] in patients with head and neck cancer, undergoing RT with or without CT recommendations for oral mucositis include oral care and hygiene as well as 2% morphine or 0.5% doxepin mouthwashes for pain relief.
No recommendations are available for agents with regenerative and stimulating functions like growth factors, cytokines or other agents like PDRN. The good outcomes in the present proof of concept and acceptability study encourage proceeding with research in a feasibility study on the use of PDRN in a larger patient cohort with RT and/or CT induced oral mucositis [12].
In addition, the role of PDRN as prophylaxis for RT and/or CT induced oral mucositis and/or dermatitis is also worth investigating.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tipsro.2018.05.003.
Appendix A. Supplementary material
References
- 1.Trotti A., Bellm L.A., Epstein J.B. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 2.Gruber S., Dörr W. Tissue reactions to ionizing radiation – oral mucosa. Mutat Res. 2016;770:292–298. doi: 10.1016/j.mrrev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Caballero A., Torres-Lagares D., Robles-García M., Pachon-Ibanez J., Gonzalez-Padilla D., Gutierrez-Perez J.L. Cancer treatment-induced oral mucositis: a critical review. Int J Oral Maxillofac Surg. 2012;41:225–238. doi: 10.1016/j.ijom.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Mucositis Sonis S.T. The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009;45:1015–1020. doi: 10.1016/j.oraloncology.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Villa A., Sonis S.T. Mucositis: pathobiology and management. Curr Opin Oncol. 2015;27:159–164. doi: 10.1097/CCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 6.González Ferreira J.A., Jaén Olasolo J., Azinovic I., Jeremic B. Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: review of the literature. Rep Pract Oncol Radiother. 2015;20:328–339. doi: 10.1016/j.rpor.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyfman S.A., Adelstein D.J. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: a critical review. Int J Radiat Oncol Biol Phys. 2012;84:581–589. doi: 10.1016/j.ijrobp.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Dörr W. Radiobiology of tissue reactions. Ann ICRP. 2015;44:58–68. doi: 10.1177/0146645314560686. [DOI] [PubMed] [Google Scholar]
- 9.De Sanctis V., Bossi P., Sanguineti G. Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: literature rewiev and consensus statement. Crit Rev Oncol Hematol. 2016;100:147–166. doi: 10.1016/j.critrevonc.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Lalla R.V., Bowen J., Barasch A. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Squadrito F., Bitto A., Irrera N. Pharmacological activity and clinical use of PDRN. Fornt Pharmacol. 2017;8:224. doi: 10.3389/fphar.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arain M., Campbell J.M., Cooper C.L., Lancaster G.A. What is a pilot or feasibility study? A rewiev of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


