Highlights
-
•
The workflow of inspiration breath-hold SBRT for liver metastases is described.
-
•
Inspiration breath-hold in liver SBRT is feasible for 95% of the patients.
-
•
An individual margin recipe for inspiration breath-hold liver SBRT is explained.
-
•
Margin reduction of 10 mm using inspiration breath-hold compared to free breathing.
Keywords: Liver SBRT, Inspiration breath-hold, Margins
Introduction
Several investigators have shown that Stereotactic Body Radiation Therapy (SBRT) for liver metastases is an ablative treatment. A high dose (biological effective dose ≥ 100 Gy) results in high local control and overall survival [1], [2]. However, treating the target volume is challenged by several factors [3]. Movement of the liver due to breathing is the most prominent one. Van den Begin et al. reported that motion management is essential in treating liver metastases to avoid geographical misses [4]. To compensate for breathing motion, several options can be used [5], [6]. One of these options is the breath-hold technique, using the Active Breathing Coordinator™ (ABC) system (Elekta, Crawley, United Kingdom) [7], [8], [9]. This technique enables a minimization of the Clinical Target Volume (CTV) to Planning Target Volume (PTV) margins [10]. Eccles et al. described an average mean intrafraction difference in the liver surface with a standard deviation of 1.5 mm in all directions and an average interfraction reproducibility of 3.4 mm. A proper position verification procedure is necessary to correct for these interfraction differences [11].
The aim of this short communication is to describe our SBRT procedure for treating patients with liver metastases using a breath-hold technique without invasive fiducial markers. For each patient we calculated a personalized CTV-PTV margin. Also, we compared the personalized margins in breath-hold and free breathing.
Materials and methods
Patient inclusion and preparation.
The eligibility criteria for treating patients with liver metastases are:
-
–
Karnofsky score ≥ 70%;
-
–
Adequate remaining liver function (usually ≤ 3 liver metastases);
-
–
Diameter metastasis < 6 cm;
-
–
Adequate kidney function for intravenous contrast;
-
–
No extensive extra hepatic metastases
The patient is instructed to fast two hours prior to CT-scanning and all radiation treatments, in order to obtain comparable stomach filling.
CT simulation
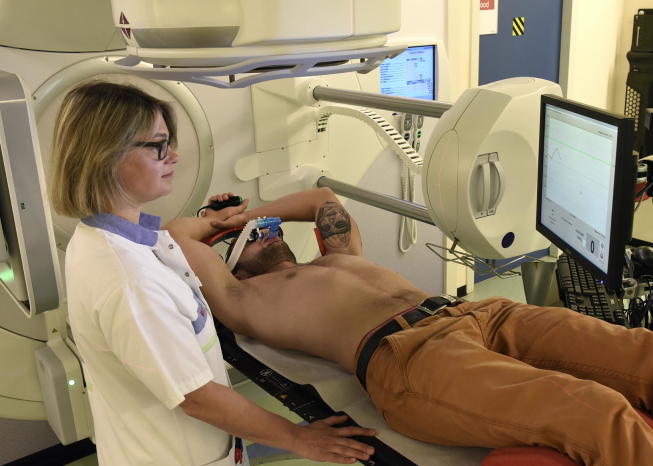
Patients are positioned with their arms above their head on the Wingstep (IT-V, Austria) and are trained to perform the breath-hold procedure (Active Breathing CoordinatorTM, ABC, Elekta, UK), Fig. 1.
Fig. 1.
The radiation therapist (RTT) is coaching the patient on the linear accelerator. The patient is breathing through a mouth piece. A nose clip is used to avoid air leakage. With the prism glasses the patient is able to see the threshold on the screen. A button is used by the patient to indicate whenever ready for the next breath-hold.
Liver SBRT delivery requires multiple breath-holds. Patients are instructed to hold their breath for 20 s. Ten consecutive CT scans of the liver are made to measure the individual inter breath-hold variation, which is included in the calculation of the individual CTV-PTV margin.
Also, a 3 mm slice thickness CT scan (breath-hold of 30 s) is made using intravenous contrast. This scan is used for delineating the Gross Tumour Volume (GTV); the treatment planning; and the position verification on the linear accelerator.
In the first year when we started with SBRT of liver metastases we also acquired a 4DCT scan for each patient to compare the CTV-PTV margins in breath-hold and in free breathing. In order to find out which method would result in the smallest CTV-PTV margins, and use these individual margins clinically.
Delineation procedure
The CTV was contoured in the Pinnacle3 planning system (version 9.10, Philips Medical Systems, US) by using the diagnostic liver MRI scan with PrimovistTM contrast, the CTV was defined by generously contouring the GTV in collaboration with a dedicated radiologist. The delineated Organs At Risk (OARs) are: kidneys; spinal cord; heart; oesophagus; stomach; bowel; chest wall/abdominal wall; biliary tract; liver volume excluding the gall bladder, metastases and scars of earlier treated metastases.
Margin definition
To obtain an individual CTV-PTV margin, all known uncertainties where included in the margin recipe. The more extended recipe to calculate the margin M was used [12]. All uncertainties are included in this equation and are either applicable for all patients, Table 1, or patient-specific. The individual inter breath-hold reproducibility (using ABC) is an example of the latter category and is treated as a random uncertainty in this equation. Finally, we adapted the equation to take a smaller number of fractions into account [13]. This resulted in the following equation:
Table 1.
Factors used in the calculation of the margin in both breath-hold and free breathing. Components are shown that apply for all patients.
| Margin contributions | Random (R)/Systematic (S) | Cran-Caud [mm] | Vent-Dors [mm] | Med-Lat [mm] |
|---|---|---|---|---|
| Delineation uncertainty: caused by interpretation differences and contrast limitations | S | 2.0 | 2.0 | 2.0 |
| Accuracy of pre-treatment imaging: half of the voxel size | S | 0.9 | 0.4 | 0.4 |
| Accuracy of the dose calculation: determined by resolution | S | 1.0 | 1.0 | 1.0 |
| Inter-fraction movement: analysed from our patient group using the breath-hold procedure | S | 0.2 | 0.4 | 0.2 |
| Inter-fraction movement: analysed from our patient group using the breath-hold procedure | R | 0.3 | 0.3 | 0.3 |
| Deformation [14] | S | 1.2 | 1.0 | 0.9 |
| Match accuracy [14] | R | 0.2 | 0.6 | 0.0 |
| Stability during breath-hold (intra breath-hold stability; used for breath-hold only) | R | 1.2 | 1.0 | 1.0 |
| Accuracy of the cone beam CT system | S | 0.5 | 0.5 | 0.5 |
| Isocenter accuracy: derived from measurements in our department for an Elekta linac with Agility MLC | S | 0.4 | 0.4 | 0.4 |
| MLC accuracy | R | 0.3 | 0.3 | 0.3 |
where
Σ = the root of the sum of the squares of all systematic errors.
σ = the root of the sum of the squares of all random errors.
σp = the standard deviation for the penumbra.
N = the number of fractions.
Breathing motion is taken into account both in breath-hold, and in free breathing. For breath-hold the individual inter breath-hold variability is added as a component for the CTV-PTV margin calculation. This variability is obtained using 10 breath-hold CT scans, and measured from the variation (quantified by its standard deviation) in diaphragm location. In free breathing the Internal Target Volume (ITV) concept is used. We expanded the ITV from the CTV by adding a margin of one half of the peak-to-peak breathing amplitude.
The margin recipe is applied to construct a PTV. Here, we only report the results for the variability in the cranio-caudal direction, the predominant direction for the breathing motion. Clinically, the complete CTV-PTV margins are used, and a minimal CTV – PTV margin of 13 mm was used in all directions [14].
To compare the margin differences in CC direction for breath-hold and free breathing we performed a Wilcoxon Signed Rank test. Using SPSS Statistics version 22.0 (IBM SPSS Statistics for Windows. USA.). P-values ≤ 0.05 (two-sided) were considered statistically significant.
Treatment planning
The prescribed total dose was 60 Gy. Depending on the dose to the OARs, the number of fractions was chosen to be 3, 5, 8 or 12. Dose limits were defined according to a consensus of all SBRT liver centres in the Netherlands, Table 2, and were also described in literature [15].
Table 2.
Dose limits defined according to a consensus of all SBRT liver centres in the Netherlands.
| # fractions | 3 | 5 | 8 | 12 | |
|---|---|---|---|---|---|
| Total dose [Gy] | 60 | 60 | 60 | 60 | |
| Spinal cord* | D max [Gy] | 18 | 22 | 27 | 31 |
| Oesophagus | D max [Gy] | 27 | 33 | 40 | 47 |
| Liver | D(700 cc) [Gy] | 15 | 18 | 21 | 24 |
| Duodenum/stomach | D max [Gy] | 30 | 37 | 45 | 53 |
| Duodenum/stomach | D(5cc) [Gy] | 23 | 28 | 33 | 38 |
| Kidney | D(66%) [Gy] | 15 | 18 | 21 | 24 |
| Heart** | Mean Dose** | 30 | 37 | 45 | 53 |
Spinal cord α/β = 2; other OAR’s α/β = 3.
No absolute constraint, but used in Haaglanden Medical Center as a planning objective to minimise dose in the heart.
All plans were calculated with a convolution-superposition algorithm in the Pinnacle3 planning system (version 9.10). We used a 6 MV Flattening Filter Free (FFF, 1400 MU/min) VMAT technique, to keep the treatment time as short as possible, and thus, to reduce the required number of breath-holds. If dose objectives could be met, one arc per lesion was used. If not, two arcs per lesion were applied. Dosimetric pre-treatment quality assurance was performed using the ArcCheck® device (Sun Nuclear Corporation, Australia).
Treatment
Automated gated breath-hold was performed on a Versa HDTM (Elekta) with a gating interface (ResponseTM, Elekta), resulting in an efficient workflow during treatment.
Position verification procedure
The positioning verification was performed with X-ray volume imaging (XVI) using Cone Beam CTs (CBCTs). In breath-hold a clearly defined liver contour is produced by the CBCT. The position verification procedure consisted of three CBCTs per fraction: one after initial positioning, one after correction and the third after treatment. A stop-and-go procedure was used [8]. In this procedure 3–4 breath-holds were needed for each CBCT. The position verification consisted of a match between the CBCT and the reference CT for the bony anatomy, followed by a soft tissue mask match. The mask was contoured around the diaphragm excluding bony structures. The reason for this two-step approach is, that it allows for determining the position of the liver relative to the bony anatomy. This way we could assess the variability of the liver position during treatment. Position correction was performed based on the mask match. Since liver deformation can occur a part of the liver may be used as a surrogate for the tumour instead of the whole liver.
Results
The Radiotherapy Department of Haaglanden Medical Center (HMC) is the first radiotherapy center in the Netherlands that performs liver SBRT in combination with inspiration breath-hold by using the ABC method, without fiducial markers. From January 2016 to November 2017 we treated 22 patients (1–4 metastases per patient).
Of the 22 patients 95% of the patients (n = 21 patients) were able to successfully perform the breath-hold procedure during the training. One patient was not able to undergo the ABC method because of claustrophobia. Another patient appeared to have a paralysis of the right diaphragm, thus the breath-hold procedure offered no advantage. This patient was able to perform the breath-hold technique but was treated in free-breathing.
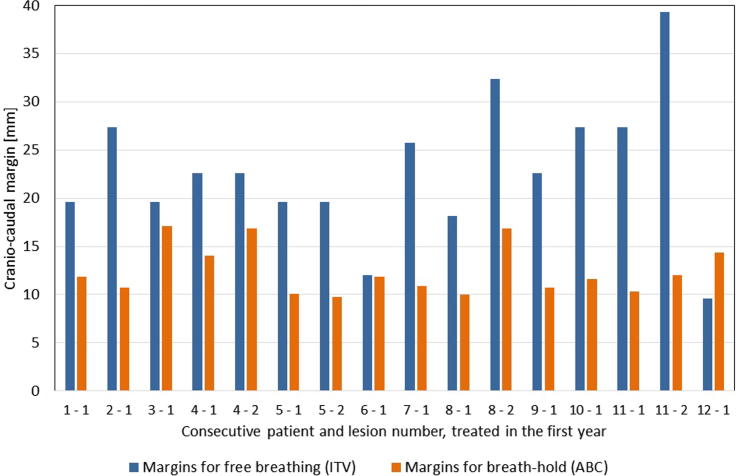
In the first year 12 patients were treated. In order to find out which method would result in the smallest CTV-PTV margins we also performed a 4DCT scan in free breathing in each patient treated in first year. The CTV-PTV margins in breath-hold were more than 10 mm smaller than those in free breathing (p < 0.01), Fig. 2.
Fig. 2.
Cranio-caudal margins for each consecutive patient (first number: patient number and second number: lesion number) in free breathing (ITV) and in breath-hold (ABC).
The delivery of the FFF VMAT technique was successful in all patients.
Beam-on time for a treatment fraction will maximally take 7 min, for a fraction dose of 20 Gy or in patients with multiple lesions treated. In these 7 min the multiple breath-holds and recovery time after every breath-hold are included. The total treatment time per fraction varied between 30–45 min mainly because of repeated position verifications. The number of breath-holds during treatment varied from 2 to 10. In two patients we reduced the breath-hold duration to 10 s instead of 20 s after the first fraction. It appeared that the breath-hold was more stable when using a shorter breath-hold duration in these two patients.
Discussion
We showed that our inspiration breath-hold procedure, using several breath-holds, is a feasible method. Inspiration breath-hold resulted in a significant CTV-PTV margin reduction of more than 10 mm compared to free breathing. Therefore, after one year (n = 12 patients) we stopped making the additional 4DCT scan.
In our cohort of patients 95% was able to perform the inspiration breath-hold procedure. This compares favourably to the results in the literature, where the reported compliance using expiration breath-hold was 62% [16].
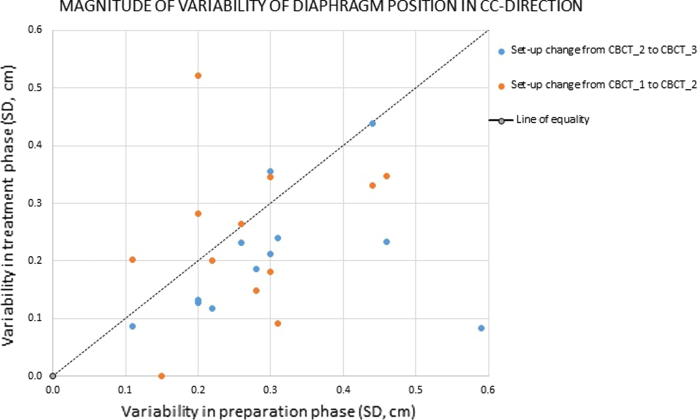
An assumption in the procedure is that the variability in diaphragm position during treatment, used as a surrogate for the liver dome position, is the same as measured during the CT simulation. To verify this assumption we compared the variability of diaphragm position during the CT simulation (horizontal axis) to the variability in the treatment phase obtained from CBCT imaging (vertical axis) for the patients treated in the first year, as shown in Fig. 3. Although the methodology was not ideal (CBCT’s were acquired during repeated breath-holds; variability in treatment phase was obtained using only three CBCT’s) it was found that the order of magnitude of variability’s was comparable, and for the majority of patients the variability during treatment was smaller than during preparation, as most bullets are situated below the line of equality, Fig. 3.
Fig. 3.
Variability of positioning of diaphragm due to repeated breath-holds for patients treated in the first year. Data are given in SD's and are obtained from repeated CT's (preparation phase; i.e. CT simulation) and from CBCT (treatment phase). Three CBCT's are acquired for each fraction, before and after position correction (CBCT_1 and CBCT_2), and after treatment (CBCT_3). This allows monitoring changes in diaphragm positioning twice for each fraction.
A considerable breath-hold variation affected the position of the target volume [17]. The approach we described is based on tailored, individual CTV-PTV margins, taking the inter breath-hold reproducibility into account in order to treat the target volume accurately.
With regard to a free breathing approach, it was demonstrated that the breathing pattern may vary and the 4DCT may not always be representative for the mean motion amplitude during treatment [18]. The same may be true for breath-hold, but our measurements comparing repeated breath-hold CT’s with CBCT’s show no major differences.
Free breathing and breath-hold show different kinds of artefacts on CBCT images. These may compromise the matching procedure with the reference CT. For free breathing, the breathing motion results in blurring. For breath-hold, the stop-and-go CBCT consisted of 3–4 breath-holds [8]. This may result in image artefacts mainly due to differences in position of the diaphragm for the different breath-holds. These were included in our margin recipe.
Previous studies showed that the diaphragm movement in cranio-caudal direction is a representative surrogate for the target volume movement in the liver [19]. The 3D position of the diaphragm dome appears to be the second best predictor, showing no dependence on the distance between liver lesion and diaphragm [20]. Consequently, there was no need to place radio-opaque invasive markers in the liver, which requires analgesics and can result in complications (bleeding, infections) and was described by Valentine et al. as a necessary evil [21]. Therefore, treating patients without these markers is a major advantage of performing breath-hold in liver SBRT.
Finally, we have chosen the robust inspiration breath-hold technique for liver SBRT since we have a wide experience with this method. The ABC method is part of our routine for all left-sided breast cancer patients since 2010 and we have found that 98% of our breast cancer patients were able to undergo the ABC technique successfully [22].
Conclusion
Inspiration breath-hold in liver SBRT is a feasible method, with 95% of the patients being able to perform this procedure. Also, our breath-hold technique results in a significant margin reduction of more than 10 mm compared to free breathing.
Conflict of interest
All authors state that actual or potential conflicts of interest do not exist.
References
- 1.Lee J., Lee J., Jang H. Hypofractionated radiotherapy with Tomotherapy for patients with hepatic oligometastases: retrospective analysis of two institutions. Clin Exp Metastasis. 2013;30:643–650. doi: 10.1007/s10585-013-9568-7. [DOI] [PubMed] [Google Scholar]
- 2.Chang D., Swaminath A., Kozak M. Stereotactic body radiotherapy for colorectal liver metastases. A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot J., Klein E. Technical challenges in liver stereotactic body radiation therapy: reflecting on the progress. Int J Radiat Oncol Biol Phys. 2013;87:869–870. doi: 10.1016/j.ijrobp.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Van den Begin R., Engels B., Gevaert T. Impact of inadequate respiratory motion management in SBRT for oligometastatic colorectal cancer. Radiother Oncol. 2014;113:235–239. doi: 10.1016/j.radonc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Brock K., Dawson L. Adaptive management of liver cancer. Semin Radiat Oncol. 2010;20:107–115. doi: 10.1016/j.semradonc.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lock M., Klein J., Chung H. Strategies to tackle the challenges of external beam radiotherapy for liver tumors. World J Hepatol. 2017;9:645–656. doi: 10.4254/wjh.v9.i14.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mageras G., Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol. 2004;14:65–75. doi: 10.1053/j.semradonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Boda-Heggemann J., Walter C., Mai S. Frameless stereotactic radiosurgery of a solitary liver metastasis using active breathing control and stereotactic ultrasound. Strahlenther Onkol. 2006;182:216–221. doi: 10.1007/s00066-006-1453-8. [DOI] [PubMed] [Google Scholar]
- 9.Boda-Heggemann J., Dinter D., Weiss C. Hypofractionated image-guided breath-hold SABR (Stereotactic Ablative Body Radiotherapy) of liver metastases – clinical results. Radiat Oncol. 2012;7:92. doi: 10.1186/1748-717X-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson T., Grills I.S., Hong Y., Entwistle A., Teahan M., Letts N. Six-year experience routinely using moderate deep inspiration breath-hold for the reduction of cardiac dose in left-sided breast irradiation for patients with early-stage or locally advanced breast cancer. Am J Clin Oncol. 2013;36:24–30. doi: 10.1097/COC.0b013e31823fe481. 10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eccles C., Brock K.K., Bissonnnette J.P. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Rad Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 12.Van Herk M., Remeijer P., Rasch C., Lebesque J.V. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Rad Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 13.De Boer J.C.J., Heijmen B.J.M. A new approach to off-line setup corrections: combining safety with minimum workload. Med Phys. 2002;29:1998–2012. doi: 10.1118/1.1500399. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins M.A., Brock K.K., Eccles C. Assessment of residual error in liver position using kV Cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Rad Oncol Biol Phys. 2006;66:610–619. doi: 10.1016/j.ijrobp.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Joo J.H., Park J.H., Kim J.C. Local control outcomes using Stereotactic Body Radiation Therapy for liver metastases from Colorectal Cancer. Int J Rad Oncol Biol Phys. 2017;99:876–883. doi: 10.1016/j.ijrobp.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Dawson L.A., Eccles C., Bissonnette J. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Rad Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 17.Lens E., van der Horst A., Versteijne E. Considerable pancreatic tumor motion during breath-holding. Acta Oncol. 2016;55:1360–1368. doi: 10.1080/0284186X.2016.1221532. [DOI] [PubMed] [Google Scholar]
- 18.Worm E.S., Hoyer M., Fledelius W. Variations in magnitude and directionality of respiratory target motion throughout full treatment courses of stereotactic body radiotherapy for tumors in the liver. Acta Oncol. 2013;52:1437–1444. doi: 10.3109/0284186X.2013.813638. [DOI] [PubMed] [Google Scholar]
- 19.Wunderink W., Mendez Romero A., Seppenwoolde Y. Potentials and limitations of guiding liver stereotactic body radiation therapy set-up on liver-implanted fiducial markers. Int J Rad Oncol Biol Phys. 2010;77:1573–1583. doi: 10.1016/j.ijrobp.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Seppenwoolde Y., Wunderink W., van Veen Wunderink. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker–tumour distance. Phys Med Biol. 2011;56:5445–5468. doi: 10.1088/0031-9155/56/17/001. [DOI] [PubMed] [Google Scholar]
- 21.Valentine K., Cabrera T., Roberge D. Implanting metal fiducials to guide stereotactic liver radiation: McGill experience and review of current devices, techniques and complications. Technol Cancer Res Treat. 2014;13:253–258. doi: 10.7785/tcrt.2012.500378. [DOI] [PubMed] [Google Scholar]
- 22.Mast M., van der Klein J., van Geen S. Een hartsparende bestralingstechniek bij vrouwen met linkszijdige borstkanker. De resultaten van vier jaar ervaring in Radiotherapiecentrum West. Ned Tijdschr Oncol. 2012;9:270–276. [Google Scholar]