Highlights
-
•
Tri-Co-60 MRI radiotherapy (RT) is feasible in locally advanced rectal cancer.
-
•
Larger volumes of normal tissue receive low-moderate doses in Tri-Co-60 MRI RT.
-
•
Further studies on functional imaging applications and LinacMRI approach are needed.
-
•
Tri-Co-60 MRI might represent a safe irradiation technique for pelvic tumors.
Abstract
Introduction
Aim of this paper is to investigate the plan quality of a tri-Co-60 MRI-Hybrid system for intensity-modulated radiation therapy (IMRT) in patients affected by locally advanced rectal cancer (LARC) undergoing neo-adjuvant radiotherapy.
Materials and methods
Ten consecutive LARC patients were selected. Tri-Co-60 step and shoot IMRT plans were generated simulating the presence of the magnetic field (Bon) or not (Boff) with the dedicated treatment planning system (TPS).
The total planned dose was 45 Gy in 25 fractions to the mesorectum and the pelvic nodes (planning target volume 2, PTV2) and 55 Gy to the tumor and correspondent mesorectum (PTV1) through simultaneous integrated boost (SIB). Tri-Co-60 IMRT plans were compared with Volumetric Modulated Arc Therapy (VMAT) and IMRT plans for Linear Accelerator (Linac).
Results
Bon and Boff tri-Co-60 IMRT plans showed no relevant differences. Mean values of PTV1 and PTV2 receiving at least 95% of the Dp (V95%) were higher than 95% in all treatment plans. All plans met the V105% constraint for the PTV1. Mean values of V105% for the PTV2 were 14.8, 5.0, and 7.3% respectively for tri-Co-60, VMAT and IMRT. Mean Wu’s HI values were similar in all plans (7.4–7.8%). All plans met the V45Gy constraint for small bowel, but mean V45Gy value was higher with tri-Co-60.
Bladder irradiation was comparable and always lower than the chosen D max 65 Gy constraint.
Mean values of V5Gy and V20Gy to the body and median skin doses were higher with tri-Co-60 plans.
Discussion
Treatment plans with Tri-Co-60 step and shoot IMRT met the dose-volume objectives in patients with LARC. Nevertheless, a larger volume of normal tissue received low-moderate doses when compared with Linac based VMAT and IMRT.
Introduction
Radiation therapy (RT) has a crucial role in the multidisciplinary management of locally advanced rectal cancer (LARC), particularly in the neoadjuvant setting, where it reaches significant tumour downsizing, allowing more conservative surgical approaches and assuring significantly better clinical outcomes to the best responder patients who may avoid postoperative treatment intensification [1], [2].
The technologies developed in the last decades, such as intensity modulated radiation therapy (IMRT) and volumetric arch therapy (VMAT) allow to tailor dose distributions with steep gradients on the target, potentially reducing the radiation damage to surrounding organs at risk (OaRs) and ensuring a safe dose delivery [3].
Previous studies about pelvic irradiation demonstrated that modern conformal techniques (such as the aforementioned IMRT and VMAT applications) can successfully reduce small bowel irradiation with promising gastrointestinal toxicity restraint results [4], [5], [6], [7], [8].
Nevertheless, the evidence about the real benefits of the use of these advanced irradiation techniques in rectal cancer is scarce and their real impact on toxicity reduction, when compared to 3D conformal radiotherapy (3DCR) techniques, is still topic of debate [9], [10], [11].
However, pelvic organs are mobile structures and without the addition of appropriate geometrical margins, the benefits of these advanced techniques may be lost or even become detrimental.
In order to take into account such geometric uncertainty, the consensus Radiation Therapy Oncology Group (RTOG) guidelines for CTV delineation in rectal cancer suggest an isotropic margin of 0.7–1.0 cm for PTV definition [12].
In this frame, image-guidance techniques may reduce geometrical uncertainty potentially leading to an improvement of local control, minimising the geographical missing risk and the potential radiation damage to organs at risk, enabling in the meanwhile a margin reduction on the target volumes that could allow dose escalation protocols [13].
To date, image guidance based on electronic portal imaging device (EPID) is widely available, even if it relies only on bony anatomy while significant inter and intrafraction variations may occur in the position of the target and OaRs secondary to rectal and bladder filling [14], [15].
The cone beam computed tomography (CBCT) better visualizes soft tissues, however, due to its inherent poor contrast resolution, difficulties in mesorectum delineation have been reported in literature also with this approach [15].
Magnetic resonance-imaging (MRI) appears therefore to be the image modality that offers and the best soft-tissue contrast and highest level of anatomical detail, discriminating tumours from their surrounding normal anatomy.
In addition, MRI is non-invasive and has no unnecessary radiation-exposure for patients.
The recently released MRIdian® system (ViewRay Inc., Cleveland, Ohio, USA) is a hybrid machine that consists in two main components: a 0.35 T whole body MRI scanner and a delivery system composed by three Cobalt-60 (tri-Co-60) sources with a dose rate of 550 cGy/minute, each connected to independent doubly focused multileaf collimators (leaf width 1.05 cm). The built-in MRI scanner allows the planar visualization and tracking of tumour and OaRs movements, assuring a more reliable visualization of the therapy volumes and achieving real time MRI based adaptive RT [16], [17].
However, the treatment beam delivery system of this machine is relatively inferior to conventional linear accelerators (Linacs) due to the larger penumbra of the Co-60 sources, the lower penetrating power of gamma ray of the Co-60 photon beam and larger leaf width of the multileaf collimators [18].
Various studies on the tri-Co-60 IMRT plan quality have already been performed, describing its performance in several anatomical sites [19], [20], [21], [22]; however, no investigations have been performed about rectal cancer.
Aim of this study is to perform an in silico evaluation of a tri-Co-60 IMRT planning system in LARC patients treated preoperatively.
This planning analysis represents a first proof of concept to verify the possibility to safely introduce MR guided hybrid treatments in clinical practice and could represent a dosimetric benchmark for the recently released hybrid MRIdian 6 MV linear accelerators (MRIdian Linac) that will shortly update and substitute the tri-Co-60 units.
Materials and methods
Patient selection
Ten consecutive patients affected by LARC who underwent neoadjuvant radio-chemotherapy in our Institution were retrospectively selected.
Five of them were men and five women; mean age was 62 years (range 55–70).
Patients’ diseases were staged as cT3-T4 cN0-1 according to TNM 2009 version [23].
Simulation imaging acquisition, target volume definition and treatment planning
Simulation and imaging acquisition
A helical CT scanner (GE HiSpeed DX/i Spiral) was used for simulation CT imaging acquisition (slice thickness was 2.5 mm). Patients were scanned in supine position on a flat table-top, without specific bowel preparation and following a bladder filling protocol. No intravenous contrast has been used.
Target volume definition
The simulation imaging was manually segmented on Eclipse treatment planning system (TPS) (Eclipse®, Varian Medical Systems, Palo Alto, California, USA).
Target volumes were identified through the co-registration of the staging diagnostic MRI T2 weighted and 18-FDG PET-CT imaging with the CT simulation.
The clinical target volume (CTV) 1 included the visible tumour and the correspondent mesorectum, while the CTV2 was represented by the whole mesorectum and the regional drainage pelvic nodes. The planning target volumes 1 and 2 (PTV1-2) were generated adding a 0.7 cm isotropic margin to the CTVs.
The OaRs considered for this analysis included: body, skin, small bowel (contoured as intestinal cavity) and bladder.
Treatment planning: prescribed dose
The total prescribed dose was: 45 Gy (1.8 Gy per fraction) to the PTV2 and 55 Gy (2.2 Gy per fraction) to the PTV1 through simultaneous integrated boost (SIB) technique, according to our internal clinical protocol.
Treatment planning – Linac
Sliding windows IMRT and VMAT plans were calculated using 6 or 15MV photons.
Sliding windows IMRT plans were optimized using five coplanar beams (180°, 108°, 36°, 324° and 252°) with a dose rate of 400 MU/min and beam energy of 6 or 15 MV photons.
VMAT plans were performed with two full coplanar arcs sharing the same isocentre and delivered with opposite rotation (clockwise and counter-clockwise) with collimator of 45° and 315° respectively.
Sliding windows IMRT plans and VMAT plans were optimized through the Eclipse progressive resolution optimizer 3 algorithm (PRO3, ver. 10, Varian Medical Systems, Palo Alto, CA, USA).
The treatment plan optimization process aimed at the following dose-volume objectives: more than 95% of the PTVs should receive more than 95% of the prescription dose (Dp), and no more than 5% of the PTV1 should receive doses higher than 105% of the Dp.
A dose-volume constraint was set also to limit the V105% of the PTV2.
On the OARs side, the following dose-volume constraints have been defined: V45 Gy less than 195 cc for small bowel (SB) and Dmax less than 65 Gy for the bladder, while no specific dose constraints has been applied for body and skin.
After optimization, dose distribution was calculated with the anisotropic analytic algorithm (AAA, ver. 11, Varian Medical Systems, Palo Alto, CA, USA) with a dose calculation grid of 2.5 mm for both techniques [24].
Treatment planning – MRIdian
The patients CT images and structure sets were imported to the MRIdian® TPS and tri-Co-60 IMRT step and shoot plans were generated.
The plans were computed with 21 equally-spaced non opposing beams divided into 7 groups of 3 fields each (triplets) simultaneously delivered. Two different scenarios of delivery were simulated: with (tri-Co-60 Bon) and without (tri-Co-60 Boff) the presence of the static 0.35 T magnetic field.
Coils were not considered in the calculation, as not present in CT simulation imaging. Monte Carlo algorithm was applied for dose calculation [25].
The values of the level and dose calculation grid were set to 15 and 0.3 cm, respectively. The optimization was performed with the same objectives as for the VMAT and IMRT planning.
Plan comparison
For target volumes, the endpoints compared among the different types of plans included: the percentage of PTVs receiving at least 95%, 100% and 105% of the Dp (V95%, V100% and V105%); and the homogeneity index (HI).
The HI was calculated according to Wu and colleagues, as follows [26]:
where D2% and D98% represent the minimum delivered doses to 2% and 98% of the PTV respectively, and Dp is the prescription dose. The ideal value of the Wu’s HI should be 0 and it increases as the dose distribution becomes less homogeneous.
For OARs, the endpoints compared among different type of plans included the V45Gy of the SB and the maximum dose to the bladder.
For the body, V5Gy and V20Gy were recorded to quantify normal tissue irradiation.
Furthermore, in order to investigate how much the dose delivered to the skin and subcutaneous tissues reflected the use of Co sources, the median dose in a 5 mm wide ring dummy volume contoured at a 3 mm depth from the body surface was calculated.
The outer 1 mm portion of the body was excluded from the analysis to avoid inconsistencies related to the uncertainty of dose calculation at air-body interface, as different dose calculation algorithms were used.
Statistical analysis
Mean values and standard deviations of dose-volumetric parameters were evaluated for each treatment technique.
Tukey multiple comparison of means test has also been calculated to compare VMAT to IMRT, tri-Co-60 to IMRT and tri-Co-60 to VMAT plans.
Differences characterized by p values less than 0.05 were considered statistically significant.
Data analysis was conducted with an in-house software named MODDICOM, realized with R platform (R software, version 3.1.2) [27].
Results
Four plans (Tri-Co-60 IMRT Bon; Tri-Co-60 IMRT Boff; IMRT; VMAT) were generated for each of the 10 evaluated patients (totaling 40 plans).
All plans met the dose-volumetric objectives for both target volumes and OARs.
Bon and Boff tri-Co-60 plans showed no relevant dosimetric differences and since the magnetic field is operating during treatment delivery with the MRIdian machine, only results of tri-Co-60 Bon were compared with VMAT and IMRT plans.
All the parameters took into account for the purposes of this analysis are shown in detail in Table 1 (target volumes) and Table 2 (OARs).
Table 1.
Target volumes dosimetric comparison mean values.
| V95%PTV1 | V105%PTV1 | V95%PTV2 | V100%PTV2 | V105%PTV2 | HI PTV1 | HI PTV2 | |
|---|---|---|---|---|---|---|---|
| Tri-Co-60 Bon | 98.9 | 0.14 | 98.0 | 55.8 | 14.7 | 7.0% | 20% |
| Range (97.4–99.6) | Range (0–0.4) | Range (97.4–98.5) | Range (55.83–64.86) | Range (7.12–21) | Range (5–9) | Range (16–21) | |
| SD ± 0.78% | SD ± 0.15% | SD ± 0.4% | SD ± 3.33% | SD ± 5.47% | SD ± 1% | SD ± 2% | |
| Tri-Co-60 Boff | 99.2 | 0.0 | 98.2 | 62.0 | 14.8 | 7.4% | 20.04% |
| Range (98.7–99.7) | Range (0.0) | Range (97.6–98.8) | Range (59.1–66) | Range (6.76–20.9) | Range (6.8–8) | Range (16.07–22.09) | |
| SD ± 0.39% | SD 0.0% | SD ± 0.46% | SD ± 2.7% | SD ± 5.9% | SD ± 0.41% | SD ± 2.07% | |
| VMAT | 97.8 | 0.0 | 98.4 | 47.3 | 5.0 | 7.82% | 13.26% |
| Range (95.7–99) | Range (0.0) | Range (95.8–99.5) | Range (42.9–57.6) | Range (1.36–8.6) | Range (6.8–8.7) | Range (8.29–17.30) | |
| SD ± 1.15% | SD 0.0% | SD ± 1.29% | SD ± 6.44% | SD ± 2.8% | SD ± 0.72% | SD ± 3.29% | |
| IMRT | 98.2 | 0.1 | 97.7 | 54.8 | 7.3 | 7.72% | 14.69% |
| Range (97–99) | Range (0–0.96) | Range (95.3–99.9) | Range (47.6–68.5) | Range (1.31–10.43) | Range (6.7–9.3) | Range (5.35–19.80) | |
| SD ± 0.76% | SD ± 0.35% | SD ± 1.58% | SD ± 7.1% | SD ± 3.31% | SD ± 0.96% | SD ± 4.76% | |
Legend: VMAT: Volumetric Modulated Arc Therapy; IMRT: Intensity Modulated Radiation Therapy; Tri-Co-60 IMRT-MRI: 60 Cobalt-Magnetic Resonance Imaging; Bon: with magnetic field presence; Boff: without magnetic field presence; HI: Homogeneity index; PTV: Planning Target Volume; Gy: Gray; SD: standard deviation.
Table 2.
Organs at risk dosimetric comparison.
| V5 Body (cm3) | V20 Body (cm3) | V45 Bowel (cm3) | Max dose bladder (mean values, Gy) | Median skin dose (Gy) | |
|---|---|---|---|---|---|
| Tri-Co-60 Bon | 12,678 | 6589 | 70.1 | 48.7 | 1.95 |
| Range (10969–14581) | Range (5893–7688) | Range (5.3–128) | Range (44.5–56.0) | Range (1.03–2.53) | |
| SD ± 1906.6 | SD ± 613.9 | SD ± 43.6 | SD ± 3.9 | SD ± 0.45 | |
| Tri-Co-60 Boff | 12,809 | 6871 | 59.4 | 50.2 | 1.97 |
| Range (11090–15907) | Range (6126–7491) | Range (6.84–114.7) | Range (46.6–55.7) | Range (1.1–2.97) | |
| SD ± 1847.8 | SD ± 566.6 | SD ± 36.0 | SD ± 3.5 | SD ± 0.54 | |
| VMAT | 10,826 | 4559 | 10.6 | 48.2 | 1.0 |
| Range (8986–13677) | Range (3792–5787) | Range (3–71.3) | Range (44.3–55.2) | Range (0.52–1.65) | |
| SD ± 1729.2 | SD ± 730.7 | SD ± 23.3 | SD ± 4 | SD ± 0.35 | |
| IMRT | 10,039 | 5901 | 48.7 | 50.4 | 0.98 |
| Range (8451–12410) | Range (4591–7738) | Range (9.6–272.2) | Range (45.4–54.6) | Range (0.57–1.48) | |
| SD ± 1487 | SD ± 1071 | SD ± 93.4 | SD ± 3.85 | SD ± 0.27 | |
Legend: VMAT: Volumetric Modulated Arc Therapy; IMRT: Intensity Modulated Radiation Therapy; tri-Co-60 IMRT-MRI: 60 Cobalt-Magnetic Resonance Imaging; Bon: with magnetic field presence; Boff: without magnetic field presence; Gy: Gray; SD: standard deviation.
Results of the Tukey multiple comparison of means test are reported in Table 3.
Table 3.
p values of the Tukey multiple comparison of means test.
| V5 Body (cm3) | V20 Body (cm3) | V45 Bowel (cm3) | Max dose bladder (max values, Gy) |
Median skin dose (Gy) | HI PTV1 | HI PTV2 | |
|---|---|---|---|---|---|---|---|
| VMAT-IMRT | 0.66 | 0.01 (S) | 0.34 | 0.51 | 0.99 | 0.96 | 0.73 |
| Tri-Co-60 –VMAT | 0.10 | 0.0001 (S) | 0.52 | 0.88 | 0.0006 (S) | 0.69 | 0.02 (S) |
| Tri-Co-60 – IMRT | 0.01 (S) | 0.09 | 0.94 | 0.80 | 0.0006 (S) | 0.54 | 0.005 (S) |
Legend: VMAT: Volumetric Modulated Arc Therapy; IMRT: Intensity Modulated Radiation Therapy; Tri-Co-60: 60 Cobalt; HI: Homogeneity index; PTV: Planning Target Volume; Gy: Gray; (S): significant (p ≤ 0.05).
Target volumes dose distribution comparison
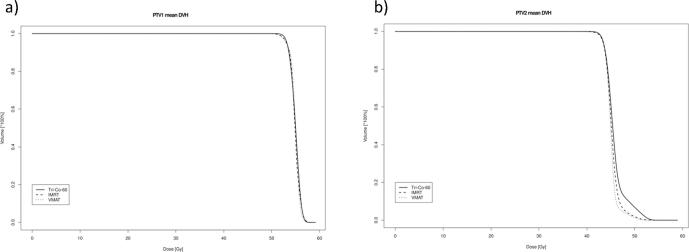
PTV1 coverage and mean PTV1 V95% were slightly higher with tri-Co-60 plans (98.9% for Tri-Co-60, 97.8% for VMAT and 98.2% for IMRT).
Comparable values were registered also for HI for the different techniques (7.0% for Tri-Co-60, 7.8% for VMAT and 7.7% for IMRT) and this difference appeared not to be statistically significant.
PTV1 V105% was around zero for all techniques (0.1% for Tri-Co-60, 0.0% for VMAT and 0.1% for IMRT).
Mean V95% values for the PTV2 were similar in all the different techniques (98% for Tri-Co-60, 98.4% for VMAT and 97.7% for IMRT), while a slight worse performance of tri-Co-60 plans has been observed for the V105% (14.7% for Tri-Co-60, 5% for VMAT and 7.3% for IMRT) and HI index (20% for Tri-Co-60, 13.26% for VMAT and 14.69% for IMRT).
Average DHVs for PTV1 and PTV2 are shown in Fig. 1.
Fig. 1.
Average DVHs for target structures: PTV1 (a) and PTV2 (b).
OARs dose distribution comparison
The analysis of OARs irradiation showed higher mean V45Gy values to the SB with tri-Co-60 (70.1 cc for Tri-Co-60, 10.6 cc for VMAT and 48.7 for IMRT), while comparable values have been registered for the bladder in terms of Dmax (48.7 Gy for Tri-Co-60, 48.2 Gy for VMAT and 50.4 Gy for IMRT).
Although the median skin dose had small absolute values (less than 2 Gy in all treatment plans), it nearly doubled in the tri-Co-60 plans (1.95 Gy for Tri-Co-60, 1.0 Gy for VMAT and 0.98 Gy for IMRT).
Similarly, mean V5Gy and V20Gy values of the body were higher in tri-Co-60 plan.
Fig. 2 provides an example of the dose distribution calculated with the different techniques, showing the larger portion of body encompassed by the 20 Gy isodose line in the tri-Co-60 plan.
Fig. 2.
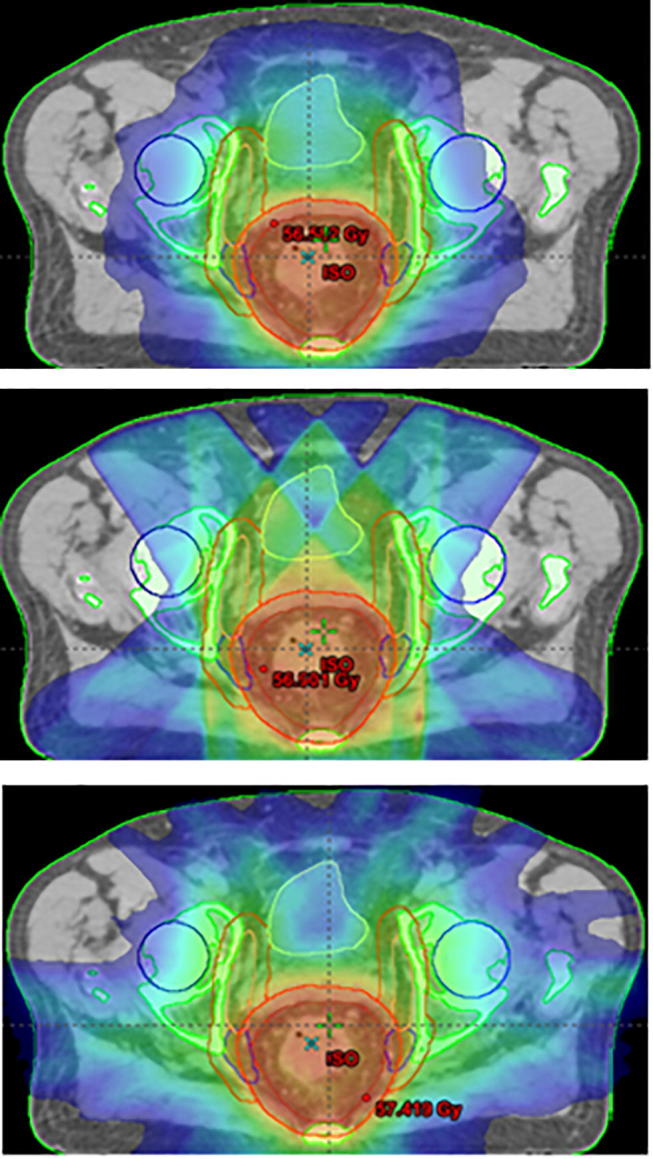
Dose color-wash distribution with a 20 Gy threshold obtained with VMAT (top), IMRT (middle) and tri-Co-60 Boff (down).
Discussion
This study is an in silico evaluation of the treatment plan quality reached by a tri-Co-60 treatment unit in patients undergoing long course pre-operative chemoradiotherapy for LARC.
In all cases, the tri-Co-60 step and shoot IMRT plans met the dose-volumetric objectives for both the target and the OARs, however plan quality was slightly worse as compared to Linac VMAT and IMRT plans, especially on the low dose OARs side.
The majority of the few previously published tri-Co-60 RT studies have dealt with small target volumes treated with hypofractionated high dose stereotactic radiotherapy. As an example, Kishan and colleagues compared eleven SBRT plans for liver lesions (prescription doses ranging between 36 and 60 Gy, in 3–5 fractions) and observed good target coverage with the exception of residual liver and skin which appeared to be exposed to low radiation doses [28].
Merna et al. analysed 20 SBRT plans for lung lesions (prescription doses of 50 Gy in 4 fractions) and observed comparable target coverage with worse OARs sparing, as compared with linac plans (especially for the low dose to the lung) [29].
Few studies investigated the use the tri-Co-60 MRI technology for long course treatments with standard fractionation and larger therapy volumes: Kishan et al. evaluated the dosimetric performance of a tri-Co-60 treatment unit in soft tissue sarcomas of the extremities (prescription dose of 50 Gy in 25 fractions) and observed that plan quality was comparable with Linac plans [30].
Saenz and colleagues compared in silico tri-Co-60 treatments with standard Linac plans for long course treatments in various sites, including pelvic tumors, and came to the conclusion that this new technology can achieve Linac-quality treatments with minor increase of target coverage heterogeneity and generally higher low-dose OARs irradiation [31].
In agreement with the aforementioned colleagues, also in the present series of LARC patients, the tri-Co-60 IMRT plans achieved almost equivalent target coverage and dose homogeneity when compared to Linac plans, while the doses to OARs were slightly higher.
The tri-Co-60 PTV2 V105% resulted to be higher as compared to Linac techniques: this suboptimal result is probably related both to a reduced possibility of the tri-Co-60 unit to generate steep dose gradients because of the large width of the MLC leaves (1.05 cm) and to the low priority usually given during the treatment plan optimization process to such dose-volumetric object.
The increased median skin dose and body irradiation with the tri-Co-60 technique represent one of the major limits of this technology and can be easily explained by the lower energy of the Co-60 photons as compared to Linac ones.
On the other hand, its benefits mainly consist of the possibility to use new and more precise IGRT protocols, that can also take advantage of the on board MR functional imaging techniques.
As an example, the use of Diffusion Weighted imaging (DWI) could allow fully personalized radiation treatment delivery for LARC, both in terms of dose escalation or treatment de-intensification.
The variation of the apparent diffusion coefficient (ADC) during treatment showed promising results in the assessment of early response and even as predictor of treatment’s outcome [32].
Furthermore, the use of such advanced imaging techniques, that provide a reliable morphological and biological visualization of therapy volumes, can successfully be integrated in hybrid MR guided dose escalation protocols able to reduce unintended irradiation of the organs at risk, while maximizing in the same time the dose to the target [33], [34], [35], [36].
Park et al. compared tri-Co-60 IMRT plans with standard practice VMAT ones for cervical cancer long course treatments (prescription dose 50.4 Gy in 28 fractions) and, interestingly, they observed a better overall quality of the tri-Co-60 IMRT plans, with several advantages with regards to normal tissue irradiation (statistically significant advantages were reported in the tri-Co-60 IMRT plans comparison with the VMAT ones with p values lower than 0.001).
These significant advantages are mainly related to the reduced margin used in the tri-Co-60 IMRT plans, confirming the promising performances of hybrid MRI guided radiotherapy in terms of target volume margin reduction, as an isotropic CTV to PTV margin of 1 mm has been used instead of the expansion of 0.7 cm in all directions and of 1 cm in the anterior-posterior axis, routinely added to CTV in the VMAT plans [21].
As reported by the mentioned planning experiences, the actual advantages of the hybrid MRI guided radiotherapy technology should be explored in further in vivo studies that should take into account the possibility to significantly reduce target margins thanks to the high-quality setup MR imaging.
These imaging advances, coupled with the fully online adaptive delivery workflow and the promising dosimetrical improvement of the MRIdian Linac update, will further enhance the clinical outcome of hybrid MR-RT treatments in LARC, especially in those cases in which high dose escalation protocols could avoid radical surgery approaches and preserve patients’ quality of life.
In conclusion, this dosimetric comparison showed that tri-Co-60 MRI systems can provide acceptable plans for neoadjuvant RT delivered with SIB technique in LARC patients, even if larger volumes of normal tissue receive higher low-moderated doses, when compared with standard Linac VMAT and sliding window IMRT plans.
In this context of innovation and rapid progress, the creation of dedicated users consortium is strongly advocated to foster clinical research in this field through the definition of a common lexicon which could be of great help to face this new challenge and understand its real advantages and pitfalls [37].
Conflict of interest
The authors declared that there is no conflict of interest.
References
- 1.Rahbari N.N., Elbers H., Askoxylakis V. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. doi: 10.1245/s10434-013-3198-9. [DOI] [PubMed] [Google Scholar]
- 2.Valentini V., van Stiphout R.G.P.M., Lammering G. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of european randomized clinical trials. J Clin Oncol. 2011;29(23):3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 3.Appelt A.L., Sebag-Montefiore D. Technological advances in radiotherapy of rectal cancer: opportunities and challenges. Curr Opin Oncol. 2016;28:353–358. doi: 10.1097/CCO.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 4.Nutting C.M., Convery D.J., Cosgrove V.P. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:649–656. doi: 10.1016/s0360-3016(00)00653-2. [DOI] [PubMed] [Google Scholar]
- 5.Portelance L., Chao K.S., Grigsby P.W. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and paraaortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51:261–266. doi: 10.1016/s0360-3016(01)01664-9. [DOI] [PubMed] [Google Scholar]
- 6.Roeske J.C., Lujan A., Rotmensch J. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48:1613–1621. doi: 10.1016/s0360-3016(00)00771-9. [DOI] [PubMed] [Google Scholar]
- 7.Duthoy W., De Gersem W., Vergote K. Clinical implementation of intensity-modulated arc therapy (IMAT) for rectal cancer. Int J Radiat Oncol Biol Phys. 2004;60:794–806. doi: 10.1016/j.ijrobp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Urbano M.T.G., Henrys A.J., Adams E.J. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys. 2006;65:907–916. doi: 10.1016/j.ijrobp.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 9.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 10.Mak A.C., Rich T.A., Schultheiss T.E. Late complications of postoperative radiation therapy for cancer of the rectum and rectosigmoid. Int J Radiat Oncol Biol Phys. 1994;28:597–603. doi: 10.1016/0360-3016(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 11.Lawrie T.A., Green J.T., Beresford M. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;1:CD012529. doi: 10.1002/14651858.CD012529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myerson R.J., Garofalo M.C., El Naqa I. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824e830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwynne S., Webster R., Adams R. Image-guided radiotherapy for rectal cancer: a systematic review. Clin Oncol (R Coll Radiol) 2012;24(4):250–260. doi: 10.1016/j.clon.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Ippolito E., Mertens I., Haustermans K. IGRT in rectal cancer. Acta Oncol. 2008;47(1317e):1324. doi: 10.1080/02841860802256459. [DOI] [PubMed] [Google Scholar]
- 15.Nijkamp J., de Jong R., Sonke J.J. Target volume shape variation during hypo-fractionated preoperative irradiation of rectal cancer patients. Radiother Oncol. 2009;92:202e209. doi: 10.1016/j.radonc.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Mutic S., Dempsey J. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24(3):196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Dempsey J., Dionne B., Fitzsimmons J. A real-time MRI guided external beam radiotherapy delivery system. Med Phys. 2006;33:2254. [Google Scholar]
- 18.Wooten H.O., Rodriguez V., Green O. Benchmark IMRT evaluation of a Co-60 MRI-guided radiation therapy system. Radiother Oncol. 2015;114:402–405. doi: 10.1016/j.radonc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Kishan A.U., Cao M., Wang P.C. Feasibility of magnetic resonance imaging-guided liver stereotactic body radiation therapy: a comparison between modulated tri-cobalt-60 teletherapy and linear accelerator-based intensity modulated radiation therapy. Pract Radiat Oncol. 2015;5:330–337. doi: 10.1016/j.prro.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Choi C.H., Park S.Y., Kim J.I. Quality of tri-Co-60 MRIGRT treatment plans in comparison with VMAT treatment plans for spine SABR. Br J Radiol. 2017 Feb;90(1070):20160652. doi: 10.1259/bjr.20160652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.M., Park S.Y., Kim J.I. A comparison of treatment plan quality between Tri-Co-60 intensity modulated radiation therapy and volumetric modulated arc therapy for cervical cancer. Phys Med. 2017 Aug;40:11–16. doi: 10.1016/j.ejmp.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Park J.M., Park S.Y., Kim H.J. A comparative planning study for lung SABR between tri-Co-60 magnetic resonance image guided radiation therapy system and volumetric modulated arc therapy. Radiother Oncol. 2016;120:279–285. doi: 10.1016/j.radonc.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Sobin L.H., Gospodarowicz M.K., Wittekind C., editors. TNM classification of malignant tumors. 7th ed. Wiley-Blackwell; Oxford: 2009. [Google Scholar]
- 24.Ulmer W., Pyyry J., Kaissl W. A 3D photon superposition/convolution algorithm and its foundation on results of Monte Carlo calculations. Phys Med Biol. 2005;50(8):1767–1790. doi: 10.1088/0031-9155/50/8/010. [DOI] [PubMed] [Google Scholar]
- 25.Sempau J., Wilderman S.J., Bielajew A.F. DPM, a fast, accurate Monte Carlo code optimized for photon and electron radiotherapy treatment planning dose calculations. Phys Med Biol. 2000;45:2263–2291. doi: 10.1088/0031-9155/45/8/315. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q., Mohan R., Morris M. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: dosimetric results. Int J Radiat Oncol Biol Phys. 2003;26:573–585. doi: 10.1016/s0360-3016(02)04617-5. [DOI] [PubMed] [Google Scholar]
- 27.Dinapoli N, Alitto AR, Vallati M, et al. Moddicom: a complete and easily accessible library for prognostic evaluations relying on image features. In: Conf proc IEEE eng med biol soc 2015;2015:771–4. [DOI] [PubMed]
- 28.Kishan A.U., Cao M., Wang P.C. Feasibility of magnetic resonance imaging-guided liver stereotactic body radiation therapy: a comparison between modulated tri-cobalt-60 teletherapy and linear accelerator-based intensity modulated radiation therapy. Pract Radiat Oncol. 2015;5(5):330–337. doi: 10.1016/j.prro.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Merna C., Rwigema J.C.M., Cao M. A treatment planning comparison between modulated tri-cobalt-60 teletherapy and linear accelerator-based stereotactic body radiotherapy for central early-stage non-small cell lung cancer. Med Dosim. 2016;41(1):87–91. doi: 10.1016/j.meddos.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Kishan A.U., Cao M., Mikaeilian A.G. Dosimetric feasibility of magnetic resonance imaging-guided tri-cobalt 60 preoperative intensity modulated radiation therapy for soft tissue sarcomas of the extremity. Pract Radiat Oncol. 2015;5(5):350–356. doi: 10.1016/j.prro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Saenz D.L., Bhudatt R.P., Bayouth J.E. A dose homogeneity and conformity evaluation between ViewRay and pinnacle-based linear accelerator IMRT treatment plans. J Med Phys. 2014;39(2):64–70. doi: 10.4103/0971-6203.131277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaverdian N., Yang Y., Hu P. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol. 2017 Mar;90(1071):20160739. doi: 10.1259/bjr.20160739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas C., Martinez A., Kestin L.L. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005 Aug 1;62(5):1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 34.de Jong R., Lutkenhaus L., van Wieringen N. Plan selection strategy for rectum cancer patients: an interobserver study to assess clinical feasibility. Radiother Oncol. 2016 Aug;120(2):207–211. doi: 10.1016/j.radonc.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Oates R., Brown A., Tan A. Real-time Image-guided Adaptive-predictive Prostate Radiotherapy using Rectal Diameter as a Predictor of Motion. Clin Oncol (R Coll Radiol) 2017 Mar;29(3):180–187. doi: 10.1016/j.clon.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Bohoudi O., Bruynzeel A.M.E., Senan S. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017 Dec;125(3):439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y., Cao M., Sheng K. Longitudinal diffusion MRI for treatment response assessment: preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys. 2016;43(3):1369–1373. doi: 10.1118/1.4942381. [DOI] [PMC free article] [PubMed] [Google Scholar]