Highlights
-
•
Selection of MRI sequences for use in MRI-guided radiotherapy by end-users.
-
•
T2w images were preferred in the pelvis, abdomen and head & neck regions.
-
•
T1w images were preferred in the thorax.
Keywords: MR Linac, Adaptive Radiotherapy, Sequence Selection, MRI-guided Radiotherapy
Abstract
Objective(s)
To systematically identify the preferred magnetic resonance imaging (MRI) sequences following volunteer imaging on a 1.5 Tesla (T) MR-Linear Accelerator (MR Linac) for future protocol development.
Methods
Non-patient volunteers were recruited to a Research and Ethics committee approved prospective MR-only imaging study on a 1.5T MR Linac system. Volunteers attended 1–3 imaging sessions that included a combination of mDixon, T1w, T2w sequences using 2-dimensional (2D) and 3-dimensional (3D) acquisitions. Each sequence was acquired over 2–7 minutes and reviewed by a panel of 3 observers to evaluate image quality using a visual grading analysis based on a 4-point Likert scale. Sequences were acquired and modified iteratively until deemed fit for purpose (online image matching or re-planning) and all observers agreed they were suitable in 3 volunteers.
Results
26 volunteers underwent 31 imaging sessions of six general anatomical regions. Images were acquired in one or two of six general anatomical regions: male pelvis (n = 9), female pelvis (n = 4), chestwall/breast (n = 5), lung/oesophagus (n = 5), abdomen (n = 3) and head and neck (n = 5). Images were acquired using a pre-defined exam-card that on average, included six sequences (range 2–10), with a maximum scan time of approximately one hour. The majority of observers preferred T2-weighted sequences. The thorax teams were the only groups to prefer T1-weighted imaging.
Conclusions
An iterative process identified sequence agreement in all anatomical regions. These sequences will now be evaluated in patient volunteers.
Advances in knowledge
This manuscript is the first publication sharing the results of the first systematic selection of MRI sequences for use in on-board MRI-guided radiotherapy by end-users (therapeutic radiographers and clinical oncologists) in healthy volunteers.
Introduction
Of the advances in multimodality imaging contributing to the improved accuracy of the irradiated target volume and organs at risk, perhaps MRI (magnetic resonance imaging) is the most significant through the provision of soft-tissue contrasts superior to that of computed tomography (CT), the ability to monitor and quantify motion, and capacity for non-invasive functional imaging. The integration of magnetic resonance imaging (MRI) into radiotherapy treatment planning was first reported in the mid-1980s [1]. Yet despite the benefits of MRI, which include superior soft tissue characterisation compared to computed tomography (CT), multi-planar capabilities, absence of ionizing radiation and capability for non-invasive functional imaging [2], [3], [4], [5], the optimal use of MRI in the radiotherapy pathway has not been realised. With the clinical release of MRI-only planning platforms [6], [7], [8] and integrated MRI-radiotherapy systems, [9], [10], [11], [12] interest in the seamless integration of MRI in the radiotherapy pathway has generated momentum. Recently, guidance on the use of MRI in external beam radiotherapy planning (EBRT), has been published and highlights essential technical MRI requirements to enable MR integration into the radiotherapy pathway [13], [14]. These requirements include (1) the ability to acquire images in the radiotherapy treatment position, (2) the ability to resolve geometric distortions over a large field of view and (3) rapid sequence acquisition and reconstructions [13], [14]. However, agreement on the most appropriate type of acquisition and weightings for use in radiotherapy planning, in most tumour types, remains unaddressed. Without consensus on image interpretation the potential for the introduction of systematic errors in inter-observer contouring variations for traditional EBRT planning pathways remains.
When MRI is used for on-board image guidance or as a sole imaging modality throughout the entire radiotherapy pathway, agreement in overcoming these challenges is even more critical, as clinical teams are required to make image registration and adaptive planning decisions within the timeframe of a single radiotherapy session whilst the patient remains in the treatment position. In addition to challenges found in MR-Simulation (e.g., optimal sequence and protocol determination for target and normal tissue definition, quantification of geometric distortion, reproducibility of patient positioning), sequence selection on the MR Linac (1) must be able to be acquired and reconstructed for immediate use within the radiotherapy pathway (for image registration and plan adaptation) whilst the patient is still on the treatment bed awaiting treatment delivery (2) should undergo minimal (if any) online optimisation or manipulation to avoid invalidating geometric distortion quantifications and (3) using novel coil technology that permits the delivery of radiotherapy beams without damaging the electronics but may incur standoff reducing the signal-to-noise ratio (SNR) at regions within the imaged anatomy. As such it is important that systematic evaluations of the sequences to be used are undertaken, to achieve the priorities of MR imaging on the MR Linac. To this end, this work reports on the preliminary evaluation of MR sequences following volunteer imaging on a 1.5 Tesla (T) MR Linac imaging platform using a visual grading assessment (VGA), an established method for evaluating image quality in radiography [15] which has been translated into radiotherapy [16]. Sequences were evaluated for suitability for real-time MR-CT image registration to determine gross positional changes, as well as for use in online re-contouring for adaptive radiotherapy by clinical oncologist and therapeutic radiographer members of the MR Linac team at our institution.
Materials and Methods
A Research and Ethics committee approved, four-stage non-comparative prospective feasibility study for the development of online MRI for magnetic resonance image guided radiotherapy (MRIgRT) was opened at our centre in 2017. The primary aims of the study were to develop generalised MR Linac based image sequences and imaging suitable for normal tissue and target visualisation and to be used for MRIgRT and adaptive radiotherapy (ART).
To evaluate the MRI sequences, the first stage of this study invited non-patient volunteers, with no known medical issues or MRI contraindications, to undergo up to 12 imaging sessions on the 1.5 T MR Linac investigational device (Elekta ATL 1, Elekta AB, Stockholm). The purpose of this stage was to review overall MRI quality using the not yet clinical system, with a primary focus on organs at risk within 6 anatomical regions. Normal tissue visualisation and the ability to contour them, is a very important part of on-line adaptive radiotherapy, as organs at risk (OARs) may well be the dose limiting structures in the delivery of radiotherapy. Further stages of the study (subject of on-going work) will include patient volunteers and focus on treatment targets.
Study information sheets were sent to volunteers and after providing written informed consent, volunteers completed an MR safety screening form that was reviewed by a member of the study team. The total number of volunteers was designed to be dependent on the ability to optimise imaging protocols and the number of repeat imaging sessions volunteers were willing to undergo. As imaging would be concluded for each body region, acceptable protocols were developed for a cohort of three volunteers within that region, the total number ranged from 18-54 volunteers (minimum of 3 and maximum of 9 volunteers per anatomical region), for 6 anatomical regions. For volunteers undergoing more than one imaging session, MR Safety was re-confirmed by a study team member at each session.
Participant recruitment was staggered into cohorts of 3 patients per anatomical region which included the male pelvis, female pelvis, thorax, abdomen and head and neck. The thorax region was further sub-divided into lung/oesophagus and breast/chest wall due to the specific imaging needs of these particular tumour sites for a later stage of the study.
All images were acquired with the volunteers positioned on patient immobilisation devices, to mimic treatment positions for the body region being imaged. A maximum of 60 minutes on-table time was allowed and volunteers were invited to complete an experience questionnaire at the end of each session. The questionnaire asked for feedback on the tolerability of the MR Linac scan, namely how anxious was the volunteer before and after the scan, and did the volunteer experience any dizziness, discomfort, tingling or heating. Volunteers were imaged using a pre-defined set of sequences making up a protocol (or ‘examcard’) best thought to demonstrate normal tissues within the anatomical region being reviewed. Each sequence was acquired over 2–7 minutes using vendor-specific geometric distortion correction. The protocols included a combination of mDixon, 2D, 3D, T1w and T2w sequences (Table 1). The motivation for the initial sequence selection was based on:
-
1.
Manufacturers provided proposed T1 and T2 sequences on the pre-clinical system. These were the first sequences used.
-
2.
Sequences used as part of MR Simulation (using a different manufacturer’s magnet), translated for evaluation to MR Linac system.
Table 1.
Most commonly acquired/evaluated sequences across all body regions.
| Sequence | Orientation | echo | TE (ms) | TR (ms) | FA (⁰) | FOV (mm) | Voxel size (mm) | Recon voxel (mm) | Time (mins) | NSA |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 3D TFE mDixon | Axial | 2 | shortest | Shortest | 12 | 420 * 420 * 300 | 1.1 * 1.1 * 1.1 | 0.5 | 5.11 | 2 |
| T2 3D TSE | Axial | 1 | shortest | 1300 | 90 | 420 * 420 * 220 | 1.2 * 1.2 * 0.6 | 0.5 | 6.05 | 2 |
| T2 TSE MV | Axial | 1 | 60 | 1800–6000 | 90 | 500 * 500 * 120 | 1.3 * 1.3 * 3.0 | 0.7 | 5.26 | 1 |
| T1 3D | Axial | 1 | 4.5 | Shortest | 30 | 320 * 452 * 300 | 1.8 * 1.8 * 1.0 | 0.9 | 3.42 | 3 |
| T2 3D | Axial | 1 | 82 | 1300 | 70 | 400 * 400 * 250 | 1.2 * 1.2 * 0.6 | 0.6 | 6.01 | 2 |
| T2 TSE MV SPIR | Axial | 1 | 100 | 3121 | 90 | 500 * 500 * 120 | 1.25 * 1.25 * 3.5 | 0.89 | 5.18 | 1 |
| T2 MS mDixon TSE | Axial | 1 | 90 | 3712 | 90 | 300 * 457 * 151 | 0.9 * 1.0 * 4.0 | 0.6 | 4.50 | 2 |
TFE = Turbo Field Echo, TSE = Turbo Spin Echo, MS = Multi-slice, MV = Multi-vane, FA = flip angle, NSA = number of signal averages, FOV = field of view, TE = time to echo, TR = time to recovery, Recon = reconstruction, mm = millimetres, mins = minutes.
Images were acquired and modified iteratively whenever sequences were determined to be unsuitable (i.e. the majority of relevant tissues were deemed unclear or not visible by a majority of observers). Recruitment continued until imaging from a minimum of three consecutive non-patient volunteers using the same protocol were deemed acceptable for normal tissue visualisation for each anatomical region and to be used for the next stage of the study, with patient volunteers.
The structures reviewed related to tumour sites anticipated to be treated (Table 2). The images from each session were reviewed by a panel of 3 observers (a clinical oncologist, a therapeutic radiographer and one other MR Linac-based researcher) with the view to being used within a particular patient population so therefore, some sequences were reviewed by multiple panels. For example, images acquired of the male pelvis were reviewed by prostate, bladder, and rectal cancer observer panels; the abdominal images were reviewed by observers with a view to use in hepatobiliary-pancreatic (HBP) and paediatric radiotherapy and images of the female pelvis were reviewed with a view to treating cervix, bladder and rectal cancers. In total, there were 10 ‘review panels’, each formed of 3 observers. The multi-disciplinary teams provided consensus to determine the ‘preferred’ sequences, as it was important that all end-users were in agreement moving forward.
Table 2.
Anatomy evaluated using VGA within each anatomical region imaged and associated treatment site.
| Body Region | Treatment Site | Anatomy Evaluated |
|---|---|---|
| Pelvis - female | Cervix | Cervix, bladder, rectum, femoral heads, large bowel, small bowel, psoas, lymph nodes, sigmoid colon |
| Pelvis - male | Prostate | Prostate, bladder, rectum, seminal vesicles, bowel, femoral heads |
| Pelvis -male/female | Rectum/Bladder | Rectum, bladder, prostate/cervix, large bowel, small bowel, femoral heads, iliac vessels, meso-rectum |
| Thorax | Breast | Breast, chest wall (muscle), lungs, heart, supra clavicular nodes, internal mammary nodes, nodal regions I, II, III, brachial plexus |
| Lung/oesophagus | Lungs, spinal cord, heart, oesophagus, brachial plexus | |
| Abdomen | Adult hepatobiliary pancreatic tumours | Liver, kidneys, aorta, vertebral bodies, pancreas, duodenum, stomach, small bowel |
| Paediatric hepatobiliary pancreatic tumours | Liver, kidneys, pancreas, spleen, spinal cord, heart, trachea, brachial tree | |
| Head and neck | Oropharynx, brain | Brain stem, optic nerves, optic chiasm, spinal cord, parotid gland, submandibular gland, oropharynx |
Images were reviewed in isolation and with no reference image therefore individual structures were reviewed using an absolute VGA [17]. The VGA scale had four grades which were “very clear”, “clear”, “unclear” and “not visible”. For each image a list of structures was provided by the clinical oncologist, which would typically be contoured as a target volume or organ at risk in radiotherapy planning e.g. prostate, seminal vesicles, rectum, bladder, bowel.
Results
Between October 2017 and February 2018, 31 imaging sessions were undertaken in 26 volunteers. Images were acquired each session in one or two of six general anatomical regions: male pelvis (n = 9), female pelvis (n = 4), chestwall/breast (n = 5), lung/oesophagus (n = 5), abdomen (n = 3) and head and neck (n = 5). Each imaging session included an average of six sequences (range 2–10), with a maximum scan time of approximately one hour.
Volunteers ranged in age from 22-61 years and included 11 men and 15 women. A validated experience questionnaire assessing tolerability of the MR Linac scan, namely how anxious was the volunteer before and after the scan, and did the volunteer experience any dizziness, discomfort, tingling or heating, was provided. Imaging was reported to be well tolerated, with only three sessions requiring early termination due to positional discomfort. Full results of the experience study will be presented elsewhere. To address the reported discomfort, shorter scan times and position optimisation are being considered.
Overall, 23 observers participated in the sequence selection, including five therapeutic radiographers, ten consultant clinical oncologists and eight clinical oncology research fellows.
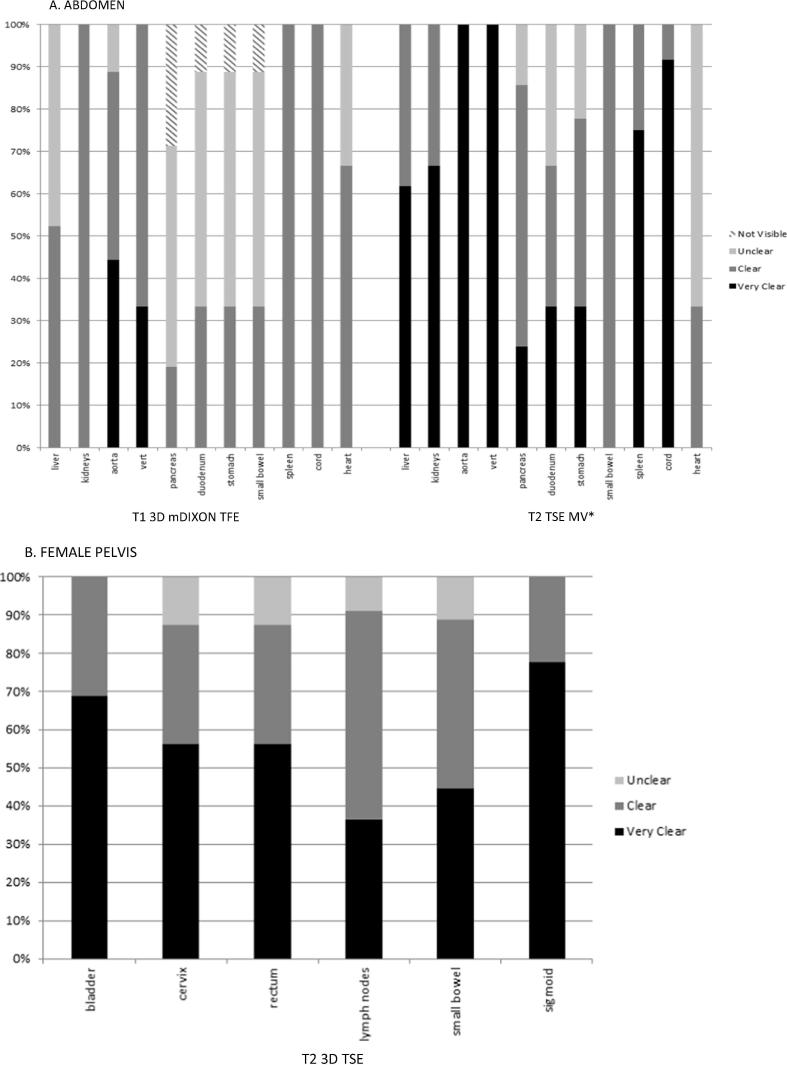
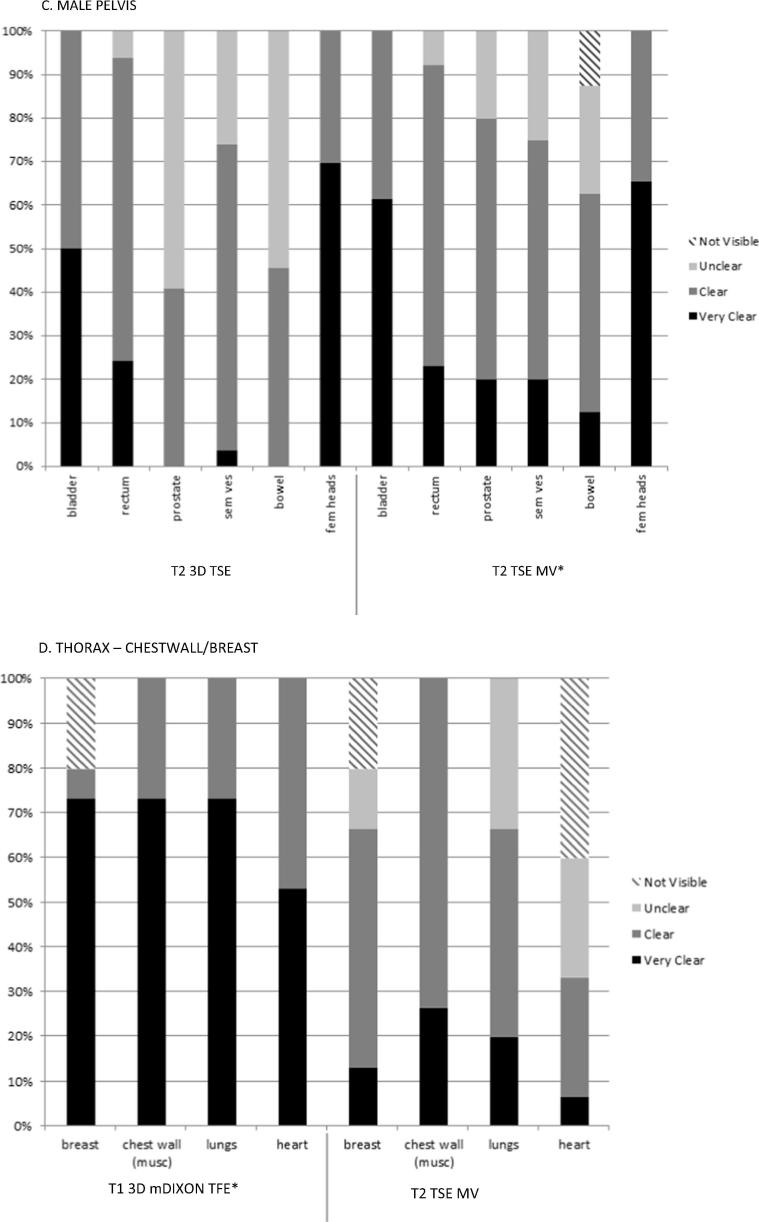
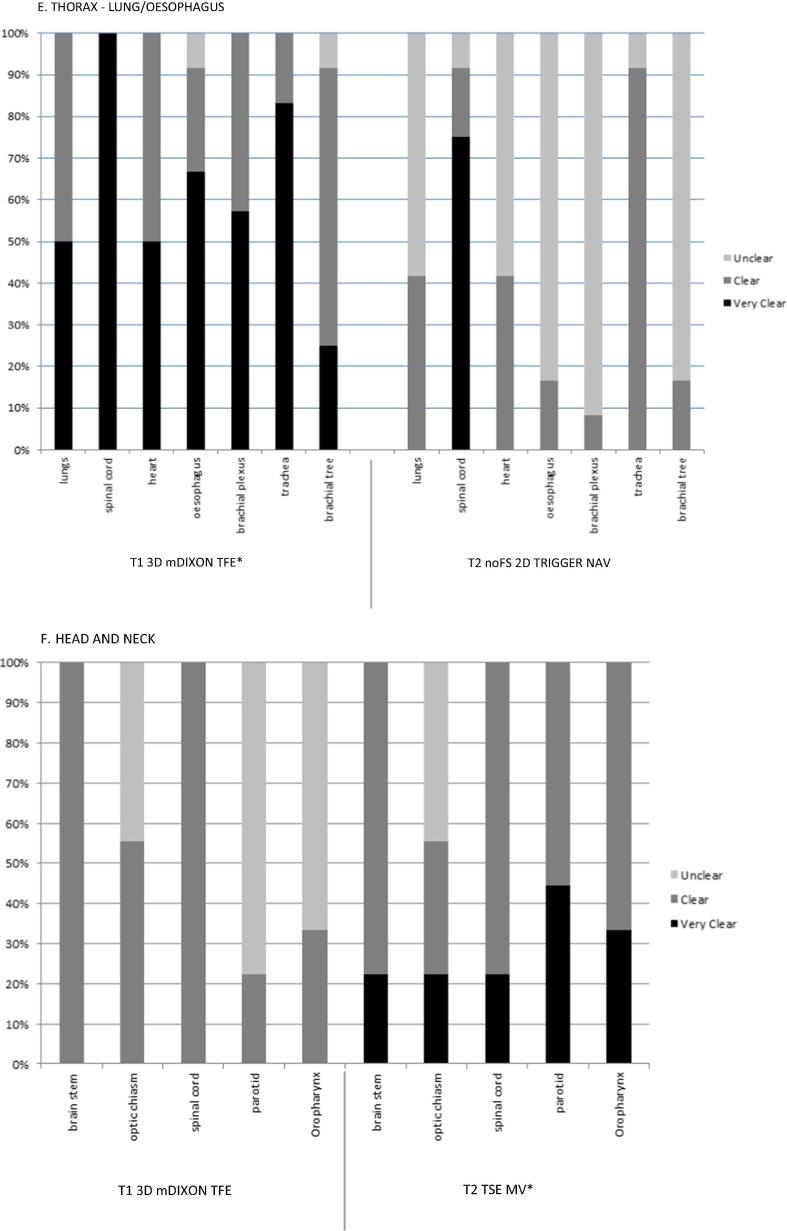
The preferred sequences for each treatment site were determined based on the frequency with which all (or most) of the organs at risk were graded as ‘clear’ or ‘very clear’. Fig. 1, Fig. 2 are a representative example of the results of sequence selection to demonstrate the proportion of observers selecting ‘clear’ or ‘very clear’ versus ‘unclear’ or ‘not visible’ for anatomy reviewed.
Fig. 1.
Example plot of anatomy evaluation for the most frequently acquired sequences reviewed for all patients by three observers for each volunteer, showing proportion of evaluation results as very clear, clear, unclear or not visible. *preferred sequence.
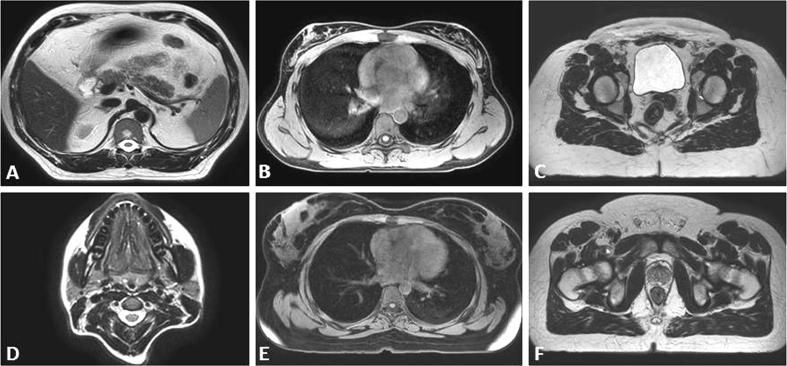
Fig. 2.
Representative single slice (axial) views of the preferred sequences for each anatomical region. Legend: A T2 TSE MV Abdomen, B T1 3D mDIXON TFE Lung/Oesophagus, C T2 3D TSE Female Pelvis, D T2 TSE MV Head and Neck, E T1 3D mDIXON TFE BREAST/thorax F. T2 TSE MV Male Pelvis.
T2w images were selected as the preferred weighting in most sites except the chestwall/breast and lung/oesophagus (Table 3). The T1 3D mDIXON TFE was the preferred sequence in the thorax region. Three teams (lung/oesophagus, female pelvis, chestwall/breast) selected the volumetrically acquired sequences as their preferred images, and the remaining three teams selected the T2 TSE MV (multivane). By the end of this process, treatment sites had agreed on at least one suitable sequence to be evaluated in patient volunteers.
Table 3.
Preferred imaging sequence details by anatomical region.
| Anatomical Region | Preferred Sequence |
|---|---|
| Male Pelvis | T2 TSE MV |
| Female Pelvis | T2 3D TSE |
| Abdomen | T2 TSE MV |
| Chestwall/Breast | T1 3D mDIXON TFE |
| Lung/Oesophagus | T1 3D mDIXON TFE |
| Head and Neck | T2 TSE MV |
Discussion
To our knowledge this is the first report of a systematic selection of sequences for the use of on-board MRI guided radiotherapy undertaken in non-patient volunteers. A strength of this study is that all observers were end-users, (i.e., clinical oncologists and therapeutic radiographers) with requirement for image interpretation in their respective roles to deliver MRIgRT.
T2w images were preferred in the abdomen, pelvis and head and neck anatomical regions. T1w sequences were preferred in the thorax. All of the preferred sequences in this study demonstrated qualities desirable for on-treatment position verification and re-planning MRIgRT, specifically, sufficient signal to noise ratios (SNR) providing contrast suitable for rapid image interpretation [18], [19], [13]. Volumetric image acquisitions offer advantages in this area as they are based on the excitation of the entire imaging volume, with additional phase encoding along the slice directions to generate 3D images without gaps, as often found in 2D acquisitions acquired in diagnostic imaging. Another advantage of 3D image acquisitions is the improved SNR because this is related to the number of phase encoding steps, and the signal is acquired over the entire volume [20].
With the exception of the thoracic anatomical regions (lung/oesophagus and chestwall/breast), the majority of preferred sequences were T2w, which is consistent with the generalisation that T1w images are considered best suited for visualisation of gross structural information (i.e., anatomy) and T2w images for biological characteristics that may aid identifying pathological information [14], [21]. MRI acquisition is slow when compared to CT, and T2w spin echo sequence acquisitions are inherently slower the T1w gradient echo acquisitions due in part to their long repetition times (TR). In the context of on-board imaging for radiotherapy patient positioning and adaptive treatment planning, it is important to exploit any and all techniques to achieve the fastest acquisition possible with minimal geometric distortion. Turbo spin echo (TSE) or fast spin echo (FSE) used in this work are known to be able to speed up T2 weighted image acquisitions significantly, reducing image acquisition times. The ability of the TSE sequence to use the interval time after the first echo to receive the echo train, filling k-space quickly also makes TSE image acquisitions less sensitive to motion. Another preferred T2w acquisition was the multi-vane (MV) sequence. This is a fast-imaging tool that uses a radial sampling technique to reduce motion sensitivity and aliasing artifacts, by leveraging vendor-specific parallel imaging techniques [22].
In the thorax, observers preferred T1w mDIXON sequences. The mDIXON is a multi-echo sequence that provides images in and out of phase, as well as water and fat saturated weightings. The in-phase T1w component of the mDIXON sequence looks most similar to the planning CT in terms of image contrast and therefore makes image interpretation for registration with the planning CT straight-forward. Another advantage is that this is the same type of sequence that is used for MRI-only planning products by commercial partners (Koninklijke Philips NV) [23], [24]. In future, it is anticipated that using consistent imaging contrasts across radiotherapy planning, in-room positional imaging and adaptation will not only minimise inter-observer image registration variation, but also facilitate rapid online registrations.
This early experience with MRI sequence evaluation on the MR Linac has demonstrated the feasibility of multi-disciplinary observer image interpretation and agreement in sequence selection for MRIgRT and ART. It is anticipated that when imaging patients the use of functional imaging will result not only in different weighting preferences, but also in additional challenges, for example reduced geometric fidelity when using parallel imaging techniques. As such it would be recommended that the implementation of more sophisticated imaging techniques adhere to the recommended characteristics by Paulson et al [13] and undergo a similar systematic review for selection.
This study did not evaluate inter-observer registration or segmentation using these images however, work in those areas is on-going for example in the prostate [25]. This study is currently being opened at a second institution, the results of which will be compared to these preliminary outcomes.
The results presented here are from the first stage (Stage A) of a four-stage trial (PRIMER) which aims to optimise protocols (examcards) on the MR Linac for normal tissue and tumour visualisation during radiotherapy. The purpose of Stage A was to evaluate whether normal anatomical structures could be visualised on this novel technology, having not been used in the clinical setting at the time of recruitment. The technology differs from a standard diagnostic MRI by having a magnet modified to allow the insertion of a rotational linear accelerator gantry [26]. Image acquisition on non-patient volunteers allowed the researchers to build confidence with using the system as preparatory work for moving onto patient volunteer image evaluation in Stage B of the PRIMER trial. Stage B will focus on target and OAR visualisation for specific tumour types using sequences selected as optimal in Stage A for normal tissue. These sequences will be acquired in patient volunteers and follow the same VGA as Stage A. Sequences may then be adapted to enhance visualisation of the treatment target if necessary.
Conclusion
This work has facilitated the selection of agreed exam cards for evaluation in patients both from the perspective of MRIgRT appropriateness (i.e., selecting from high resolution, geometrically robust images) and interdisciplinary agreement on the sequences that will meet the requirements of MRIgRT and ART at our institution.
Conflicts of interest and funding statements
The Institute of Cancer Research is supported by Cancer Research UK Programme (C33589/A19727 and C33589/A19908) Grants (C33589/A19727 and C33589/A19908) and the CRUK ART-NET Network Accelerator Award (A21993); MRC Grant MR/M009068/1. We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research and financial and non-financial support from Elekta AB, Stockholm, Sweden. This research was undertaken under a research agreement between ICR/RMH and Philips Best, Netherlands.
Clinical trial information
The work presented in this data includes images acquired on volunteers who have provided written informed consent to PRIMER: development of daily online magnetic resonance imaging for magnetic resonance image guided radiotherapy. Clinicaltrials.gov NCT02973828 at the Royal Marsden NHS Foundation Trust.
Acknowledgments
Acknowledgements
The authors submit this manuscript on behalf of the PRIMER Trial Management team and gratefully acknowledge input and support from all co-investigators and collaborators, without whom this work would not have been possible: Katharine Aitken*, Hannah Bainbridge*, Shreerang Bhide*, David Collins, Helen Creasey*, Nandita de Souza, Oliver Gurney-Champion, Kevin J Harrington, Brian Hin*, Emma Hall, Anna Kirby*, Dow-Mu Koh, Susan Lalondrelle*, Naomi Lavan*, Megan Llewelyn*, Henry Mandeville*, Fiona McDonald*, Angela Pathmanathan*, Anna-Maria Shiarli*, Maria Schmidt, Erica Scurr, Alison Tree*, Liam Welsh*, Andreas Wetscherek, and Ingrid White* in their respective areas of expertise.
*acted as observer in this study.
This work was presented in part at the 2018 ASTRO Annual Meeting supported by a Society and College of Radiographers Travel Grant, and the Christie NHS Foundation Trust.
References
- 1.Curran W.J., Bossi A., Molteni M., Richetti A., Tordiglione M. The value of magnetic resonance imaging in treatment planning of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1986:2189–2196. doi: 10.1016/0360-3016(86)90019-2. [DOI] [PubMed] [Google Scholar]
- 2.Dirix P., Haustermans K., Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24:151–159. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Khoo VS, Joon DL New developments in MRI for target volume delineation in radiotherapy, Br J Radiol 2006; 79 (S1):S2-15. [DOI] [PubMed]
- 4.Kupelian P. Sonke JJMagnetic resonance-guided adaptive radiotherapy; a solution to the future. Semin Radiat Oncol. 2014:227–232. doi: 10.1016/j.semradonc.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Maingon P. Argumentaire clinique pour la radiothérapie guidée par imagerie par résonance magnétique. CancerRadiother. 2016;20:6–7. doi: 10.1016/j.canrad.2016.07.070. [DOI] [PubMed] [Google Scholar]
- 6.[6] Kohler M, Vaara T, Van Grootel M, Hoogeveen R, Kemppainen R, Renisch S. White paper: Philips MRCAT for prostate dose calculations using on MRI data. S.L.: Koninklijke Philips N.V., May 2015.
- 7.Siversson C., Nordstrom F., Nilsson T., Nyholm T., Jonsson J., Gunnlaugsson A. Technical Note: MRI only prostate radiotherapy planning using the statistical decomposition algorithm. Med Phys. 2015;442:6090–6097. doi: 10.1118/1.4931417. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi N., Fontenla S., Zhang J., Cloutier M., Kadbi M., Mechalakos J. Dosimetric and workflow evaluation first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62(8):2961–2975. doi: 10.1088/1361-6560/aa5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ViewRay. World's first MRIdian center celebrates five years of MRI-guided radation treatments. Cleavland : PRNewswire, https://viewray.com/newsroom/ 2019 [accesssed 5 November 2019].
- 10.Liney G.P., Whelan B., Oborn B., Barton M., Keall P. MRI-Linear accelerator radiotherapy systems. Clin Oncol. 2018;30(11):711–719. doi: 10.1016/j.clon.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Ménard C, van der Heide U (editors). Magnetic Resonance Imaging in Radiation Oncology. Seminars in Radiation Oncology, Special Issue 2014;24(3): 149-232. [DOI] [PubMed]
- 12.[12] 2019 Elekta AB (pub). Elekta.com. Elekta Unity. [Online] https://www.elekta.com/radiotherapy/treatment-delivery-systems/unity/?utm_source=unity&utm_medium=redirect&utm_campaign=redirects. [accessed 5 November 2019].
- 13.Paulson E.S., Crijns S.P., Keller B.M., Wang J., Schmidt M.A., Coutts G. Consensus opinion on MRI simulation for external beam radiation treatment planning. Radiother Oncol. 2016;121:187–9292. doi: 10.1016/j.radonc.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M.A., Payne G. Radiotherapy planning using MRI. Phys Med Biol. 2015;60:R323–R361. doi: 10.1088/0031-9155/60/22/R323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bath M., Mansson L.G. Visual grading characteristic (VGC) anlaysis: a non-parametric rank-inariant satistical method for image quality evaluation. Br J Radiol. 2007;80:169–176. doi: 10.1259/bjr/35012658. [DOI] [PubMed] [Google Scholar]
- 16.Kember S.A., Hansen V.H., Fast M.F., Nill S., McDonald F., Ahmed M. Evaluation of three presets for four-dimensional cone beam CT in lung radiotherapy verification by visual grading analysis. Br J Radiol. 2016;89(1063) doi: 10.1259/bjr.20150933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Precht H., Hansson J., Outzen C., Hogg P., Tingberg A. Radiographers' perspectives' on Visual Grading Analysis as a scientific method to evaluate image quality. Radiography. 2019;25:S14–S18. doi: 10.1016/j.radi.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 18.McWillain A., Rowland B., van Herk M. The challenges of using mri during radiotherapy. Clin Oncol. 2018;30:680–685. doi: 10.1016/j.clon.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Chandarana H., Wang H., Tijssen RHN Das I.J. Emerging role of MRI in radiation therapy. J Magn Reson Imaging. 2018;48:148–178. doi: 10.1002/jmri.26271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser C., D'Anatasi M., Theisen D., Notohamiprodjo M., Horger W., Dominik P. Understanding 3D TSE sequences: advantages, disadvantages, and appliation in msk imaging. Semin Muscoloskelet Radiol. 2015;19:321–327. doi: 10.1055/s-0035-1563732. [DOI] [PubMed] [Google Scholar]
- 21.Metcalfe P., Liney G.P., Holloway L., Walker A., Barton M., Delaney G.P. SThe potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013:429–446. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philips Healthcare. ds-SENSE propels Ingenia's imaging speed. S.L.: Koninklijke Philips N.V., 2012.
- 23.Kohler M, Vaara T, Van Grootel M, Hoogeveen R, Kemppainen R, Renisch S. White paper: Philips MRCAT for prostate dose calculations using on MRI data. s.l.: Koninklijke Philips N.V., May 2015. http://incenter.medical.philips.com/doclib/enc/fetch/2000/4504/577242/577251/587787/White_Paper_MR-only_sim_LR.pdf%3Fnodeid%3D11147198%26vernum%3D-2 [accessed 5 November 2019].
- 24.Schubert G, Vaara T, Lindstrom M, Kemppainen R, van Grootel-Rensen Marieke. Commissioning of MR-only simulation for radiotherapy planning. s.l. : 2017 Koninklijke Philips N.V., Aug 2017. https://www.philips.co.uk/healthcare/education-resources/publications/fieldstrength/mri-in-prostate-cancer [accessed 5 November 2019].
- 25.Pathmanathan A., McNair H.A., Schmidt M.A., Brand D.H., Delacroix L., Eccles C.L. Comparison of prostate delineation on multi-modality imaging for MR-guided radiotherapy. Br J Radiol. 2019;92(1095):20180948. doi: 10.1259/bjr.20180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagendijk J.J., Raaymakers B.W., Raaijmakers A.J., Overweg J., Brown K.J., Kerkhof E.M. MRI/linac integration. Radiother Oncol. 2008;86(1):25–29. doi: 10.1016/j.radonc.2007.10.034. [DOI] [PubMed] [Google Scholar]