Highlights
-
•
In pre-treatment ESAS geriatric patients had significantly less pain at rest and depression.
-
•
No strong trends towards higher symptom burden in older patients emerged for any of the items.
-
•
More geriatric patients failed to complete RT, despite lower rates of prescription of >10 fractions.
-
•
Overall survival was not associated with old age.
Keywords: Palliative radiotherapy, Edmonton symptom assessment system, Age, Geriatric patients, Octogenarians, Prognostic factors
Abstract
Purpose
To evaluate differences in baseline parameters including performance status and self-reported symptom burden between geriatric and non-geriatric cancer patients, and to assess the hypothesis that these factors might predispose older patients to incomplete radiotherapy and short survival.
Patients and methods
Retrospective comparison of geriatric and non-geriatric patients treated with palliative radiotherapy (age ⩾80 years and <80 years, respectively). Between 2013 and 2015, 26 geriatric and 76 non-geriatric patients were treated. The Edmonton symptom assessment system (ESAS) was employed to document baseline symptoms.
Results
Most patients received radiotherapy for bone metastases, commonly 5–10 fractions. Geriatric patients had significantly less pain at rest and depression. No strong trends towards higher symptom burden in older patients emerged for any of the items. Overall survival was similar in the two subgroups with different age and also in a separate age-stratified analysis of patients with performance status >2. Relatively few patients were irradiated in the terminal stage of disease, defined as final 30 days of life (8% in geriatric and 12% in other patients, p = 0.73). A higher number of geriatric patients failed to complete their prescribed course of radiotherapy (14 vs. 3%, p = 0.08), despite lower rates of prescription of more than 10 fractions in this group (15 vs. 23%, p > 0.2).
Conclusions
These data support utilization of palliative radiotherapy irrespective of age. However, care should be taken in assigning the right fractionation regimen in order to avoid lengthy treatment courses when survival is limited, such as in patients with performance status >2.
1. Introduction
Geriatric cancer patients contribute substantially to the workload of radiation oncology facilities [1], [2]. In principle, longer time slots for consultation and treatment might be needed as a result of impaired mobility, vision and hearing. It has also been reported that these patients are less likely to receive systemic therapy [3], possibly leading to higher symptom burden and demand for treatment of multiple target volumes when referred for palliative irradiation. Given that most developed countries are facing ageing populations and increasing numbers of newly diagnosed patients with cancer [4], [5], it is important to perform dedicated studies addressing the unique challenges associated with geriatric oncology. It has been realized that treatment decisions should not simply rely on biological age [6], [7], [8]. Rather, comprehensive assessments of organ function, comorbidity and patients’ ability to function independently are needed to provide individualized care [9], [10], [11]. Studies focusing on palliative radiotherapy in geriatric patients are scarce. Important questions include 1) are these patients at increased risk of dropout from fractionated regimens and 2) do they survive long enough to experience the benefits from symptom palliation? We hypothesized that reduced performance status and worse patient-reported baseline symptoms might be more common in geriatric patients, and that these factors might predispose them to incomplete radiotherapy and short survival. As in our previous study [3], we continued to use an arbitrary cut-off of 80 years when comparing geriatric and non-geriatric patients, although other definitions can be found in the literature. Studies focusing on octogenarians have been performed by several groups [7], [12], [13], [14], [15], [16], [17], [18] and are urgently needed to better understand the special challenges around treatment of the oldest patients, both in early and advanced stages of different types of cancer. In contrast to previous analyses, patient-reported baseline data were included in the present study.
2. Patients and methods
We performed a retrospective chart review in 102 unselected, consecutive cancer patients who received palliative radiotherapy at a single academic teaching hospital during the time period 2013–2015. In 2013, our pre-treatment work-up changed towards routine inclusion of the Edmonton symptom assessment system (ESAS) [19], administered by a registered oncology nurse immediately before physician consultation and imaging for treatment planning, i.e. approximately one week before radiotherapy. The ESAS is a short, one-sheet questionnaire addressing major symptoms and wellbeing on a numeric scale of 0–10, which can easily be integrated into routine workflow in radiation oncology facilities [20], [21]. The questionnaire had been part of routine assessment of palliative cancer patients in our Department of Oncology and Palliative Medicine for more than 10 years. However, due to lack of registered oncology nurses in our radiation oncology facility before 2013, it was not used in conjunction with this particular treatment modality.
We analyzed two different subgroups, patients <80 and ⩾80 years of age. Typical fractionation regimes were 8 Gy single fraction, five fractions of 4 Gy or ten fractions of 3 Gy for painful bone metastases, five fractions of 4 Gy or ten fractions of 3 Gy for brain metastases, and two fractions of 8.5 Gy, ten fractions of 3 Gy or fifteen fractions of 2.8 Gy for lung cancer. However, higher doses and other fractionations were also prescribed in some patients. Stereotactic radiotherapy was not included in the present study. The treating physician recorded the patients' medical history and ECOG performance status (PS) at pre-treatment consultation. Comorbidity was retrospectively scored by use of the Charlson comorbidity index, a validated and widely used tool [22]. All medical records, treatment details and information on date of death or last contact were available in the hospital’s electronic patient record (EPR) system (DIPS®, DIPS ASA, Bodø, Norway). At the time of analysis with IBM SPSS Statistics 22 in Spring 2016, 85 patients had died and 17 were still alive. Median follow-up for all living patients was 17.5 months, range 6.9–34.6. Survival time was measured from start of radiotherapy. Actuarial survival curves were generated by Kaplan–Meier method and compared by log-rank test. The prognostic impact of all baseline variables included in Table 1 was analyzed. For multivariate analysis of survival, Cox regression analysis was used (backward stepwise method). Associations between different variables of interest were assessed with the chi-square test (when appropriate Fisher exact probability test or t-test). A p-value ⩽0.05 was considered statistically significant. Two-tailed tests were performed. The study was performed as a retrospective analysis of palliative radiotherapy in geriatric patients. As a quality of care analysis, no approval from the Regional Committee for Medical and Health Research Ethics (REK) was necessary.
Table 1.
Baseline characteristics before palliative radiotherapy.
| Characteristic | Age < 80 years, n = 76 | Age ⩾ 80 years, n = 26 | p-value |
|---|---|---|---|
| No (%) | No (%) | ||
| ECOG performance status | |||
| 0 | 14 (18) | 1 (4) | |
| 1 | 18 (24) | 5 (19) | |
| 2 | 24 (32) | 10 (38) | |
| ⩾3 | 20 (26) | 10 (38) | 0.24 |
| Family1 | |||
| Single | 13 (17) | 15 (58) | |
| Married | 55 (72) | 11 (42) | |
| Partner | 7 (9) | 0 | 0.0001 |
| Gender | |||
| Male | 56 (74) | 19 (73) | |
| Female | 20 (26) | 7 (27) | 1 |
| Primary tumor site | |||
| Prostate | 19 (25) | 12 (46) | |
| Breast | 10 (13) | 2 (8) | |
| Lung (small cell) | 1 (1) | 1 (4) | |
| Lung (non-small cell) | 23 (30) | 3 (12) | |
| Colorectal | 5 (7) | 0 | |
| Bladder | 1 (1) | 4 (15) | |
| Malignant melanoma | 4 (5) | 0 | |
| Kidney | 4 (5) | 0 | |
| Multiple myeloma | 2 (3) | 1 (4) | |
| Other | 7 (9) | 3 (12) | 0.03 |
| More than 1 cancer diagnosis | |||
| No | 70 (92) | 23 (88) | |
| Yes | 6 (8) | 3 (12) | 0.69 |
| Total no of TV in RT course | |||
| 1 | 48 (63) | 17 (65) | |
| 2 | 21 (28) | 7 (27) | |
| ⩾3 | 7 (9) | 2 (8) | 0.95 |
| RT target types2 | |||
| Bone metastases | 47 (55) | 16 (57) | |
| Brain metastases | 13 (15) | 0 | |
| Lymph node metastases | 5 (6) | 1 (4) | |
| Lung | 9 (11) | 3 (11) | |
| Prostate | 2 (2) | 2 (7) | |
| Bladder | 1 (1) | 4 (14) | |
| Others | 8 (9) | 2 (7) | 0.29 |
| Selected RT regimens, ITT | |||
| 1–4 fractions | 6 (8) | 4 (15) | |
| 5–9 fractions | 23 (30) | 7 (27) | |
| 10 fractions | 30 (39) | 11 (42) | |
| 11–15 fractions | 15 (20) | 4 (15) | |
| >15 fractions | 2 (3) | 0 | 0.84 |
| Incomplete fractionated RT* | |||
| No | 70 (97) | 19 (86) | |
| Yes | 2 (3) | 3 (14) | 0.08 |
| Patients without metastatic disease | 3 (4) | 7 (27) | |
| One organ system with metastases | 30 (39) | 11 (42) | |
| Two organ systems with metastases | 25 (33) | 7 (27) | |
| >2 organ systems with metastases | 18 (24) | 1 (4) | 0.003 |
| Progressive disease outside TV | |||
| No | 40 (53) | 14 (54) | |
| Yes | 36 (47) | 12 (46) | 1 |
| Systemic cancer treatment | |||
| No | 37 (49) | 12 (46) | |
| Before RT | 39 (51) | 14 (54) | 1 |
| Charlson comorbidity index3 | |||
| 0–2 | 49 (64) | 8 (31) | |
| >2 | 27 (36) | 18 (69) | 0.003 |
RT: Radiotherapy, ITT: intention to treat, TV: Target volume.
Missing information in some cases.
More than one could be present in the same patient.
Excluding currently treated cancer.
Excluding 8 patients treated with 8-Gy single fraction.
3. Results
The study included 26 patients (25%) who were 80 years or older and 76 patients (75%) who were younger than 80 years. Their baseline characteristics are shown in Table 1. Median age was 84 (range 80–91) and 68 years (range 49–79), respectively. Median interval from tumor diagnosis to radiotherapy was 33 (range 1–177; older patients) and 26 months (range 1–236; younger patients), respectively (p = 0.65). Older patients were more likely to be single (58 vs. 17%, p = 0.0001). They also had a higher proportion of prostate cancer (46 vs. 25%) and a lower proportion of lung cancer (16 vs. 31%), p = 0.03. Apart from this, the Charlson comorbidity index was significantly higher in geriatric patients, p = 0.003. They received radiotherapy for non-metastatic disease more often than their younger counterparts (27 vs. 4%, p = 0.003).
Regarding the ESAS (Table 2), self-rated pain while moving and tiredness were the symptoms associated with the highest scores, followed by a lack of appetite. Geriatric patients had significantly less pain at rest and depression. No strong trends towards higher symptom burden in older patients emerged for any of the items.
Table 2.
Edmonton symptom assessment system (ESAS) before palliative radiotherapy.
| Item | Age < 80 years, n = 76 | Age ⩾ 80 years, n = 26 | p-value, t-test |
|---|---|---|---|
| Mean, standard deviation | Mean, standard deviation | ||
| Pain at rest | 3.1; 2.7 | 1.9; 2.3 | 0.03 |
| Pain while moving | 4.7; 3.3 | 3.8; 2.8 | 0.21 |
| Tiredness | 4.3; 3.0 | 4.6; 2.7 | 0.61 |
| Nausea | 1.3; 2.0 | 0.7; 1.6 | 0.10 |
| Dry mouth | 2.8; 2.8 | 3.3; 2.9 | 0.40 |
| Shortness of breath | 2.6; 2.8 | 2.8; 2.8 | 0.65 |
| Appetite | 3.8; 3.3 | 3.7; 3.3 | 0.91 |
| Anxiety | 2.8; 3.1 | 2.2; 2.7 | 0.37 |
| Depressed | 2.4; 3.0 | 1.3; 1.9 | 0.04 |
| Obstipation | 2.5; 3.1 | 2.8; 3.4 | 0.70 |
| Sleep | 2.8; 2.8 | 2.0; 2.5 | 0.17 |
| Overall wellbeing | 3.7; 2.7 | 3.2; 2.2 | 0.35 |
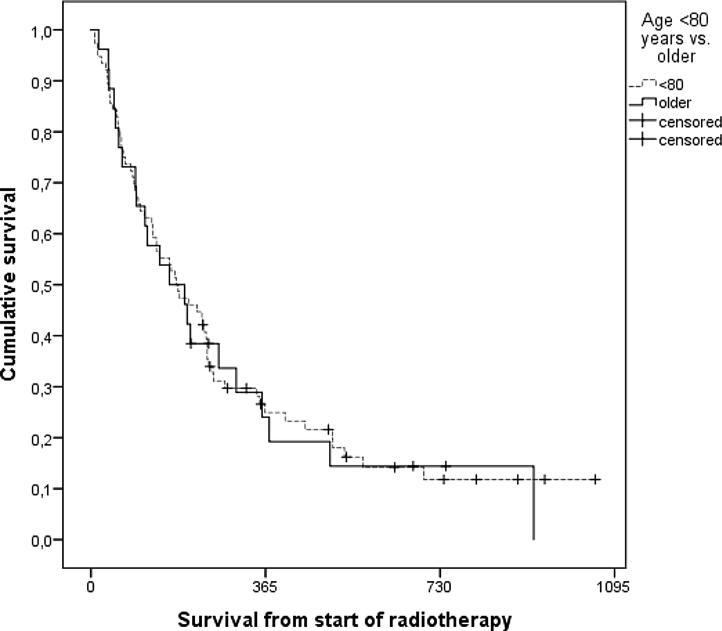
As shown in Fig. 1, overall survival was similar in the two subgroups with different age. Relatively few patients were irradiated in the terminal stage of disease, defined as final 30 days of life (8% in geriatric, 12% in younger patients, p = 0.73). After excluding single fraction treatment, a higher number of geriatric patients failed to complete their prescribed course of radiotherapy (14 vs. 3%, p = 0.08), despite lower rates of prescription of more than 10 fractions in this group (15 vs. 23%).
Fig. 1.
Actuarial overall survival after palliative radiotherapy (Kaplan-Meier estimates, log-rank test, p = 0.86). The study included 26 patients who were 80 years or older and 76 younger patients. Median survival was 164 days in older and 179 days in younger patients.
Even geriatric patients with PS > 2 (n = 10) had survival similar to their younger counterparts (n = 20), median 61 and 51 days, respectively (p = 0.14). However, two of these geriatric patients (20%) failed to complete fractionated radiotherapy.
The baseline parameters included in Table 1 were analyzed as potential prognostic factors for overall survival. Univariately, PS, progressive disease outside of the target volume(s), lack of systemic therapy and primary tumor type were significantly associated with survival. Prognosis was better in patients with prostate cancer, breast cancer and multiple myeloma. In the multivariate regression model, these baseline features remained significant (Table 3).
Table 3.
Results of the multivariate Cox regression analysis, endpoint overall survival.
| Baseline parameter predicting survival | B | SE | Wald | df | Exp (B) | p-value |
|---|---|---|---|---|---|---|
| ECOG performance status, 4 strata | 0.546 | 0.119 | 20.965 | 1 | 1.727 | 0.000 |
| Systemic therapy, yes/no | −0.791 | 0.235 | 11.278 | 1 | 0.454 | 0.001 |
| Primary tumor, favorable*/unfavorable | 0.623 | 0.249 | 6.277 | 1 | 1.864 | 0.012 |
| Progressive disease outside TV, yes/no | 0.593 | 0.237 | 6.259 | 1 | 1.810 | 0.012 |
TV target volume.
Prostate and breast cancer, multiple myeloma.
4. Discussion
Palliative radiotherapy is a well-established, widely used means of providing symptom improvement and, in selected patients with less advanced disease, increased overall survival. Common indications include bone and brain metastases, thoracic and pelvic tumors, as well as hematologic malignancies [23], [24], [25], [26], [27]. This treatment is often associated with improved quality of life and functional independence. Age should not be a barrier when deciding about referrals to palliative radiotherapy [1], [3], [12]. Our previous study of patients treated before 2013 suggested that geriatric patients (⩾80 years old, n = 94) comprised 17% of all patients and were unlikely to receive systemic therapy (8%). Since systemic therapy also plays a role in symptom control, we hypothesized that geriatric patients might experience a higher symptom burden when starting palliative radiotherapy, and a need for more extensive treatment, e.g. of multiple sites of painful metastases. Because our department introduced symptom assessment by ESAS prior to radiotherapy in 2013, we had the opportunity to study these questions in a different patient population.
When interpreting our results, the following drawbacks must be taken into account. Statistical power was limited because only 26 patients were ⩾80 years old. In a retrospective study, selection bias cannot be excluded, e.g., the fact that geriatric patients were not referred or failed to start therapy as a result of rapid clinical deterioration, preventing them from being identifiable in our database. ESAS is not a tool for geriatric or frailty screening. The vulnerable elders survey (VES-13) and G8 are often employed in the latter context [28], [29]. However, comprehensive geriatric assessment was not part of our clinical routine work-up. Waiting times were mostly in the range of 1–2 weeks, depending on indication (absent for emergency situations). In the publicly-funded Norwegian health care system, out-of-pocket costs do not prevent access to radiotherapy. However, in our rural health care region, travel distances are often bothersome (100–300 km), which could influence referral patterns, especially in vulnerable geriatric subgroups. Interestingly, the proportion of geriatric patients increased from 17% in the previous study to 25%. A tremendous increase was seen in prescription of systemic therapy, from only 8% to 54%. A possible explanation is the availability of new oral medications for patients with prostate cancer [30] and a reduced reluctance to treat, resulting from steadily increasing clinical experience and data. As in the previous study, older patients were more likely to be single. None of the geriatric patients received whole-brain radiotherapy for brain metastases, likely because our previous study showed poor survival in this subgroup [3]. Consequently, we tended to recommend radiosurgery (performed at other hospitals) or best supportive care for geriatric patients with brain metastases.
As might be expected intuitively, significantly higher comorbidity scores were present in the geriatric group. The trend towards worse PS was not statistically significant. Unexpectedly, geriatric patients had significantly less self-rated pain at rest and depression. No strong trends towards higher symptom burden in older patients emerged for any of the ESAS items. A possible explanation is the imbalance in disease stage, i.e., patients ⩾80 years of age were significantly less likely to harbor metastatic tumors. The geriatric patients did not receive treatment to a higher number of target volumes.
A higher number of geriatric patients failed to complete their prescribed course of radiotherapy (14 vs. 3%, p = 0.08), despite lower rates of prescription of more than 10 fractions in this group (15 vs. 23%). Especially in geriatric patients with PS > 2, incomplete radiotherapy might compromise outcomes. Nevertheless, survival outcomes were very similar regardless of age. We did not collect data on symptom improvement and toxicity, endpoints that should be investigated in future studies. For many patients, palliative radiotherapy is not expected to prolong survival [31]. The risk of incomplete radiotherapy might be higher in geriatric patients scheduled for curative treatment, especially in vulnerable subgroups [32].
Conclusions
In line with previous studies [12], [32], [33], our data support utilization of palliative radiotherapy irrespective of age. However, care should be taken in assigning the right fractionation regimen in order to avoid lengthy treatment courses when survival is limited, such as in patients with PS > 2. Self-reported baseline symptoms in geriatric patients were largely comparable to those in patients <80 years of age, except for significantly less pain at rest and depression.
Conflict of interest statement
On behalf of all Authors, the corresponding Author states that there are no conflicts of interest.
References
- 1.Rodrigues G., Sanatani M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin Radiat Oncol. 2012;22:277–283. doi: 10.1016/j.semradonc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Van Oorschot B., Schuler M., Simon A., Schleicher U., Geinitz H. Patterns of care and course of symptoms in palliative radiotherapy: a multicenter pilot study analysis. Strahlenther Onkol. 2011;187:461–466. doi: 10.1007/s00066-011-2231-9. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C., Angelo K., Haukland E., Pawinski A. Survival after palliative radiotherapy in geriatric cancer patients. Anticancer Res. 2014;34:6641–6645. [PubMed] [Google Scholar]
- 4.Vineis P., Wild C.P. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 5.Borras J.M., Lievens Y., Barton M., Corral J., Ferlay J., Bray F. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother Oncol. 2016;119:5–11. doi: 10.1016/j.radonc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Joerger M., Thürlimann B., Savidan A., Frick H., Rageth C., Lütolf U. Treatment of breast cancer in the elderly: a prospective, population-based Swiss study. J Geriatr Oncol. 2013;4:39–47. doi: 10.1016/j.jgo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Baimatova I., Smith C., Beckert L., Singh H. Treatment of octogenarians with lung cancer: A single centre audit of treatments and outcomes. N Z Med J. 2015;128:29–34. [PubMed] [Google Scholar]
- 8.Evers J.N., Schild S.E., Segedin B., Nagy V., Khoa M.T., Trang N.T. A new score predicting survival prognosis after whole-brain radiotherapy alone for brain metastases in elderly patients. Anticancer Res. 2014;34:2455–2458. [PubMed] [Google Scholar]
- 9.Bouzereau V., Le Caer F., Guardiola E., Scavennec C., Barriere J.R., Chaix L. Experience of multidisciplinary assessment of elderly patients with cancer in a French general hospital during 1 year: A new model care study. J Geriatr Oncol. 2013;4:394–401. doi: 10.1016/j.jgo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Kenis C., Bron D., Libert Y., Decoster L., Van Puyvelde K., Scalliet P. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24:1306–1312. doi: 10.1093/annonc/mds619. [DOI] [PubMed] [Google Scholar]
- 11.Mishra M.V., Showalter T.N., Dicker A.P. Biomarkers of aging and radiation therapy tailored to the elderly: future of the field. Semin Radiat Oncol. 2012;22:334–338. doi: 10.1016/j.semradonc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Zachariah B., Balducci L., Venkattaramanabalaji G.V., Casey L., Greenberg H.M., DelRegato J.A. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39:1125–1129. doi: 10.1016/s0360-3016(97)00552-x. [DOI] [PubMed] [Google Scholar]
- 13.Mamtani A., Gonzalez J.J., Neo D., Slanetz P.J., Houlihan M.J., Herold C.I. Early-stage breast cancer in the octogenarian: Tumor characteristics, treatment choices, and clinical outcomes. Ann Surg Oncol. 2016;23:3371–3378. doi: 10.1245/s10434-016-5368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen K.Y., Chen J.H., Shih J.Y., Yang C.H., Yu C.J., Yang P.C. Octogenarians with advanced non-small cell lung cancer: treatment modalities, survival, and prognostic factors. J Thorac Oncol. 2010;5:82–89. doi: 10.1097/JTO.0b013e3181c09b28. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A., Sanuki N., Eriguchi T., Kaneko T., Morita S., Handa H. Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;8:257–263. doi: 10.1016/j.ijrobp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Cannon N.A., Iyengar P., Choy H., Timmerman R., Meyer J. Stereotactic ablative body radiation therapy for tumors in the lung in octogenarians: a retrospective single institution study. BMC Cancer. 2014;14:971. doi: 10.1186/1471-2407-14-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy N., Yu J., Fakih M.G. Toxicities and survival among octogenarians and nonagenarians with colorectal cancer treated with chemotherapy or concurrent chemoradiation therapy. Clin Colorectal Cancer. 2007;6:362–366. doi: 10.3816/CCC.2007.n.005. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima M., Kagami Y., Toita T., Uno T., Sugiyama M., Tamura Y. Prospective trial of radiotherapy for patients 80 years of age or older with squamous cell carcinoma of the thoracic esophagus. Int J Radiat Oncol Biol Phys. 2006;64:1112–1121. doi: 10.1016/j.ijrobp.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Bruera E., Kuehn N., Miller M.J., Selmser P., Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 20.Fan G., Hadi S., Chow E. Symptom clusters in patients with advanced-stage cancer referred for palliative radiation therapy in an outpatient setting. Support Cancer Ther. 2007;4:157–162. doi: 10.3816/SCT.2007.n.010. [DOI] [PubMed] [Google Scholar]
- 21.Zeng L., Koo K., Zhang L., Jon F., Dennis K., Holden L. Fatigue in advanced cancer patients attending an outpatient palliative radiotherapy clinic as screened by the Edmonton Symptom Assessment System. Support Care Cancer. 2012;20:1037–1042. doi: 10.1007/s00520-011-1179-8. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Matuschek C., Ochtrop T.A., Bölke E., Ganswindt U., Fenk R., Gripp S. Effects of radiotherapy in the treatment of multiple myeloma: a retrospective analysis of a single institution. Radiat Oncol. 2015;10:71. doi: 10.1186/s13014-015-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvold N.D., Lee E.Q., Mehta M.P., Margolin K., Alexander B.M., Lin N.U. Updates in the management of brain metastases. Neuro Oncol. 2016;18:1043–1065. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Oorschot B., Assenbrunner B., Schuler M., Beckmann G., Flentje M. Survival and prognostic factors after moderately hypofractionated palliative thoracic radiotherapy for non-small cell lung cancer. Strahlenther Onkol. 2014;190:270–275. doi: 10.1007/s00066-013-0507-y. [DOI] [PubMed] [Google Scholar]
- 26.Nieder C., Pawinski A., Dalhaug A. Continuous controversy about radiation oncologists' choice of treatment regimens for bone metastases: should we blame doctors, cancer-related features, or design of previous clinical studies? Radiat Oncol. 2013;8:85. doi: 10.1186/1748-717X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron M.G., Kersten C., Vistad I., van Helvoirt R., Weyde K., Undseth C. Palliative pelvic radiotherapy for symptomatic incurable prostate cancer – A prospective multicenter study. Radiother Oncol. 2015;115:314–320. doi: 10.1016/j.radonc.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Baitar A., Van Fraeyenhove F., Vandebroek A., De Droogh E., Galdermans D., Mebis J. Geriatric screening results and the association with severe treatment toxicity after the first cycle of (radio)chemotherapy. J Geriatr Oncol. 2014;5:179–184. doi: 10.1016/j.jgo.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Denkinger M.D., Hasch M., Gerstmayer A., Kreienberg R., Nikolaus T., Hancke K. Predicting fatigue in older breast cancer patients receiving radiotherapy. A head-to-head comparison of established assessments. Z Gerontol Geriatr. 2015;48:128–134. doi: 10.1007/s00391-014-0840-5. [DOI] [PubMed] [Google Scholar]
- 30.Nieder C., Haukland E., Mannsåker B., Norum J. Impact of intense systemic therapy and improved survival on the use of palliative radiotherapy in patients with bone metastases from prostate cancer. Oncol Lett. 2016;12:2930–2935. doi: 10.3892/ol.2016.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger B., Ankele H., Bamberg M., Zips D. Patients who die during palliative radiotherapy. Status survey. Strahlenther Onkol. 2014;190:217–220. doi: 10.1007/s00066-013-0471-6. [DOI] [PubMed] [Google Scholar]
- 32.Spyropoulou D., Pallis A.G., Leotsinidis M., Kardamakis D. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5:20–25. doi: 10.1016/j.jgo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Laurent M., Paillaud E., Tournigand C., Caillet P., Le Thuaut A., Lagrange J.L. Canouï-Poitrine F; ELCAPA Study Group. Assessment of solid cancer treatment feasibility in older patients: a prospective cohort study. Oncologist. 2014;19:275–282. doi: 10.1634/theoncologist.2013-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]