Graphical abstract
Keywords: Total body irradiation, IMRT, Imaging, In-vivo dosimetry, Bone marrow transplant
Highlights
-
•
Implementable in a standard linac bunker with no need for additional equipment.
-
•
Mean doses to the brain, lungs and kidneys 11.1–11.8 Gy.
-
•
V(12 Gy) was below 5% for the brain, 2% for the lungs and 0% for the kidneys.
-
•
Mean TLD dose difference was 1.9%, st. dev. 4.5%.
Abstract
Introduction
Total body irradiation (TBI) is a part of the conditioning regimen for bone marrow transplant.
At the Royal Marsden (Sutton, UK) and Rigshospitalet (Copenhagen, Denmark), we introduced a step and shoot IMRT (SS IMRT) technique for TBI. This technique requires no equipment other than that used to deliver other external beam radiation. In this paper, we describe this technique and report on data from the two clinics.
Materials and methods
The patients were positioned supine, supported by vacuum bag(s). The entire body of the patients were CT scanned with 5 mm slices. Multiple multi-leaf collimator (MLC) defined fields were used.
In-vivo dosimetry was performed at the Royal Marsden for 113 patients.
Calculated doses for 18 adult and 4 paediatric patients from Rigshospitalet were extracted.
Results
The in-vivo data from the Royal Marsden showed that the mean TLD measured dose difference was −1.9% with a standard deviation of 4.5%.
SS IMRT plans for 22 patients from Rigshospitalet resulted in mean doses to the brain, lungs and kidneys all within the range of 11.1–11.8 Gy, while the V(12 Gy) was below 5% for the brain, 2% for the lungs and 0% for the kidneys.
Discussion
SS IMRT is feasible for TBI and can deliver targeted doses to the organs at risk.
Introduction
Total body irradiation (TBI) is a part of the conditioning regimen for bone marrow transplant (BMT). The target in TBI is the entire patient body, although the lung dose is often reduced [1]. Some centres also reduce the dose to the kidneys [2], [3].
Toxicity after BMT includes interstitial pneumonitis (IP), veno-occlusive disease (VOD) [4], [5], [7], [9], radiation-induced cancer [8], renal failure [9] and cataracts [10], [11]. Severe interstitial pneumonitis is of particular concern. Although the incidence has been reduced by infection prophylaxis, when contracted, the fatality rate has remained roughly constant at 67% [12].
Although most of these toxicities are multifactorial, correlations with severity and radiation dose or fractionation have been shown for IP [13], [14], VOD [13], renal complications [2] and cataracts [10]. Thus, it is pertinent to ensure that the organs at risk are not unnecessarily overdosed.
TBI delivery techniques vary substantially across centres, but the most commonly used technique is large open static fields (the open fields (OF) technique). Intensity modulated TBI with a translating couch [15], [16] and VMAT techniques [17] have also been used.
At the Royal Marsden (Sutton, UK) and Rigshospitalet (Copenhagen, Denmark), we introduced a step and shoot IMRT (SS IMRT) technique for TBI. This technique requires no equipment other than that used to deliver other external beam radiation. This paper describes the SS IMRT technique, and presents dosimetric data for 113 consecutive patients treated at our clinics.
It is our hope that this description will allow other clinics to implement this technique with their existing equipment.
The SS IMRT technique for TBI described in this paper was created at the Royal Marsden, where the treatment planning system (TPS) is Pinnacle and the linacs are Elekta, MLCi or Agility. This technique was adopted and further developed at Rigshospitalet, where the TPS is Eclipse and the linacs are Varian iX.
Materials and method
Dosimetric verification
Prior to the clinical implementation, measurements were carried out to investigate the agreement between the calculated and measured doses. This was especially pertinent since the TPS was commissioned for isocentric data and the TBI treatments are done with 350 cm from the radiation source to the patient midline.
Measurements were carried out using a NACP pancake chamber and a Unidos electrometer in a 40 × 40 × 20 cm solid water phantom for 6 and 18 MV at SSDs of 340, 360, 380, 400, 420 and 440 cm (Fig. 1); at depths of 0.1, 10, 19 and 19.9 cm in the phantom; with and without a 1.5 cm thick plastic build-up screen; and with a 40 × 40 cm jaw field and an MLC field limiting the field to 5 cm greater than the phantom (Fig. 2).
Fig. 1.
The set-up used to investigate the agreement between the TPS doses and measurements – a 20 × 40 × 40 cm solid water phantom positioned at SSDs of 340–440 cm, with a NACP pancake chamber.
Fig. 2.
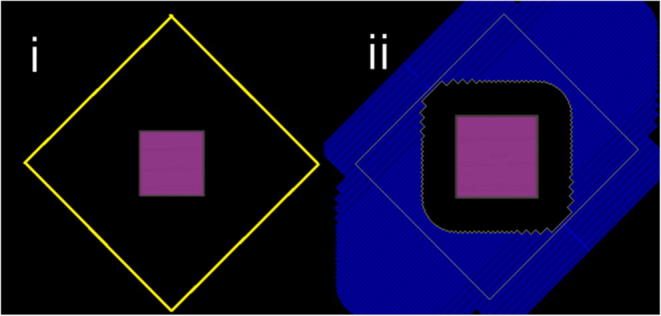
The 40 × 40 cm jaw field and MLC defined field (limiting the field size to 5 cm greater than the phantom at the extended SSD).
The doses were calculated using the same algorithm as that is used for the clinical TBI plans (AAA v. 13.6), and a calculation grid of 1 mm.
Patient selection
All patients who received TBI at both clinics were treated with the SS IMRT technique described in this paper.
Fixation and scanning
The patients were positioned supine, supported by two vacuum bags (Rigshospitalet) or in a whole body vacuum bag (the Royal Marsden). The legs were bent such that the maximum patient length was 165 cm, to ensure that the patients fit in the radiation field. Care was also taken to ensure that the knees fit within the field of view of the CT scanner Phillips BigBore (the Royal Marsden) and a Siemens Somatom Definition (Rigshospitalet). The entire bodies of the patients were CT scanned with 5 mm slices.
Planning
The planning target volume (PTV) was defined as the body excluding the outer 5 mm of body and excluding the lungs. The brain, lungs and kidneys were contoured, and autocontouring was used for the brain and lungs (both clinics) and kidneys (the Royal Marsden).
The distance from the radiation source to the patient’s midline was 350 cm (Rigshospitalet) and 355 cm (the Royal Marsden). The accelerator dose rate was 100 MU/min. Beam spoilers of 15 mm thickness were used, and were included in the dose calculation through an attenuation factor (the Royal Marsden) or by drawing them in the TPS and assigning them a density of 1.09 g/cm3 (Rigshospitalet).
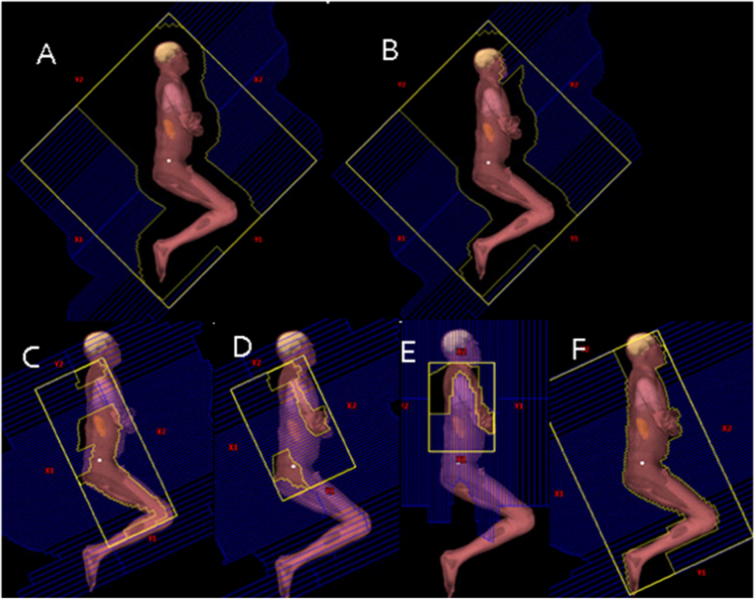
Multiple multileaf collimator (MLC) defined fields ere used. The energy was selected (6 or 18 MV) to produce the best plan possible. A plan template was used as a starting point for the plan. The template fields used at Rigshospitalet are shown in Fig. 3. The MLC positions and field weights were manually altered to produce an acceptable plan. The planning aims are shown in Table 1.
Fig. 3.
Fields of one of the two lateral beams from the plan template used at Rigshospitalert. Field A targeted the entire body. In field B the dose to the oral cavity was reduced. Fields C and D boosted the thicker body parts. Field E boosted the anatomy anterior, posterior and cranial to the lung while sparing the lung. Field F was used exclusively for patient positioning. A similar set of fields would be used to irradiate the patient from the other side.
Table 1.
Planning aims at the Royal Marsden and Rigshospitalet for high-dose TBI. Dmin is minimum dose, Dmax maximum dose.
| Planning aims | Rigshospitalet | The Royal Marsden |
|---|---|---|
| Prescribed dose for high-dose TBI | 12 Gy (2 Gy × 6) | 14.4 Gy (1.8 Gy × 8) |
| Lungs | V_100% < 2% | V_100% < 2% |
| Lungs | V_10.2 Gy > 95% | |
| Lungs | Mean dose as low as possible – it usually end up around 93% (11.3 Gy) | Mean lung dose between 12 and 12.5 Gy, lower preferred Usually 85% (12.24 Gy) |
| Brain | V_12 Gy < 5% | Dmin > 13 Gy (90%) Dmean ∼ prescription dose Dmax < 15.8 Gy (110%) |
| Kidneys | Should receive between 10.2 and 12 Gy | D5% < prescription dose Mean kidney dose between 12 and 12.5 Gy, lower preferred |
| PTV (the body excluding the outer 5 mm of body and excluding the lungs) | Dmin > 90% (10.8 Gy) | |
| PTV (the body excluding the outer 5 mm of body and excluding the lungs) | Dmax < 110% (13.2 Gy) | |
| Body | Dmin > 80% | Dmin > 80% |
| Body | The volume receiving more than 120% should be as small as possible | No part of the patient should receive > 17.3 Gy (120%) |
During planning, the gantry rotation was set to 87° for the fields that irradiated the patient from the right, and 273° for the fields from the left for Rigshospitalet, and 85° and 275° for the Royal Marsden. These angles were chosen so that the patient would be vertically positioned below the isocentre for ease of set-up with a hospital bed. The lungs were shielded by the MLC fields rather than separate lung shields.
When the patient was treated, however, the gantry rotation was the same throughout the treatment (273° or 275°). To achieve irradiation from both sides, the patient was rotated half way through the treatment so that the patients’ right side faced the gantry for the first half of the fields, and his or her left side faced the gantry for the second half.
This was taken into account in a final planning step. After the plan was approved by a physician, a “deliverable plan” was made, which was a copy of the plan but with all gantry angles set to 273° For the fields where the gantry rotation was changed the collimator was rotated by 180°.
Fractionation
At the Royal Marsden, the standard TBI fractionation was 14.4 Gy in 8 fractions over 4 days, or 13.2 Gy in 8 fractions for acute lymphomacytic lymphoma (ALL), if the patient was undergoing myeloablative double umbilical cord transplant; 12 Gy in 6 fractions over 3 days was used for relapsed anaplastic large cell lymphoma (ALCL), a single fraction of 2–4 Gy is used for patients undergoing reduced intensity conditioning transplant.
At Rigshospitalet, high-dose TBI for myeloablative conditioning transplants was 12 Gy in 6 fractions of 2 Gy over 3 days with a minimum of 6 hours in-between fractions. For non-myeloablative conditioning transplants treatments of 1 × 2 Gy, 1 × 3 Gy or 2 × 2 Gy were also used.
Treatment
At both clinics the patient was positioned for treatment using an extra room laser indicating the mid-line of the patient. The extra laser was placed at 355 and 350 cm from the radiation source respectively in the Royal Marsden and Rigshospitalet.
At Rigshospitalet, lines on the two vacuum bags used for fixation (Fig. 4) indicated their relative placement to each other. Finally, patient positioning was fine-tuned using the set-up field (Fig. 3F). This allowed for the patient’s head tilt, arm position and knee position to be adjusted.
Fig. 4.
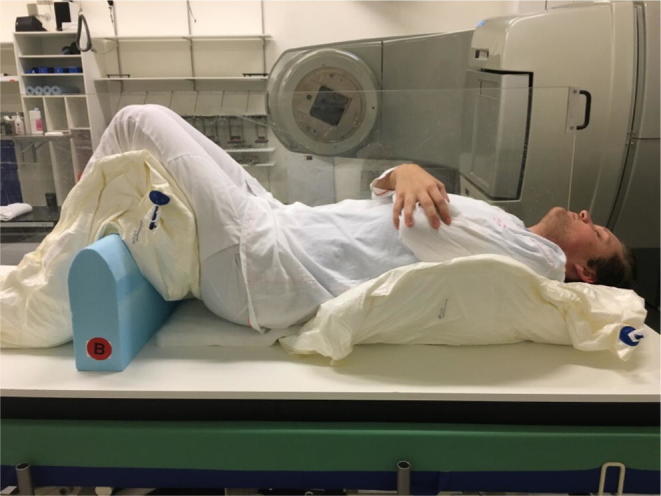
Patient positioning for SS TBI at Rigshospitalet.
In-vivo dosimetry at the Royal Marsden
In-vivo dosimetry was carried out at the Royal Marsden using TLD, but not at Rigshospitalet. TLD measurements were completed on the first fraction for 113 consecutive fractionated patients, and repeated for the second fraction if the measured doses were outside tolerance. The TLDs had an individual calibration factor and a batch calibration was done post annealing at Dmax using 10 MV, as was used for treatment. The TLDs were placed in a plastic holder with a 2 mm Cu build-up layer to ensure measurement at Dmax, and 2TLDs were placed in each plastic holder. Each of the positions of the TLDs was shown with a radiopaque marker on the planning CT scan of the patient, and individual marks were made on the vacuum bag for the positions of the head, upper and lower lungs and the left and right pelvis. The TLD measurements were compared with predicted doses from the TPS at Dmax, and the differences in dose were reported.
Treatment slots of one hour were booked for each fraction.
Clinical doses at Rigshospitalet
Dosimetric data from the TPS for 22 consecutive patients (4 paediatric, 18 adult) treated at Rigshospitalet with SS IMRT were analysed. Specifically, the volume receiving 95% of the prescription dose (D95) and 5% of the prescription dose (D5) for the PTV, and the mean doses and V(12 Gy) to the brain, lungs and kidneys were extracted.
Imaging
Daily imaging was carried out at Rigshospitalet but not at the Royal Marsden.
The imaging was performed with the Theraview™ imaging system.
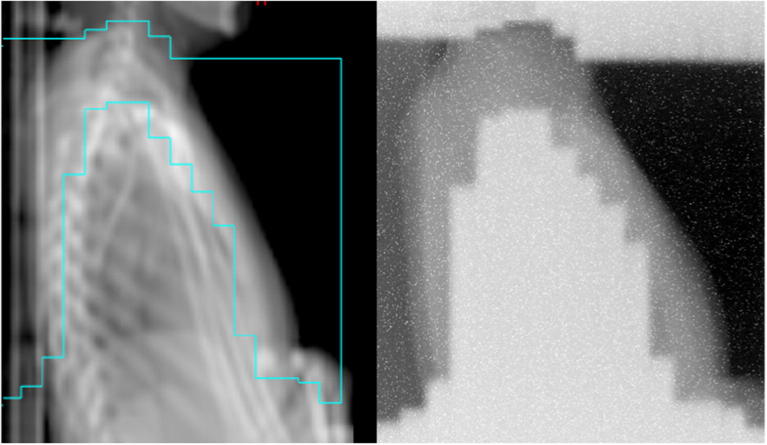
The Theraview detector was placed by the patients’ thorax and images were acquired with the treatment fields. A clinical image is shown in Fig. 5. The primary aim of the imaging was to ensure that the MLC fields were delivered at the correct collimator angle, and the secondary aim was to ensure that the patient was positioned correctly with respect to the treatment field.
Fig. 5.
A digitally reconstructed radiograph from a field treating the patients thoracic while shielding the lungs, and the treatment field image from Theraview™.
Results
Dosimetric verification
Without a build-up screen, the differences between the measured and calculated doses were less than 7% for all measurements except those 0.1 cm from the entrance and exit surfaces of the phantom. At the point 0.1 cm from the entrance surface, the measured dose was greater than the calculated by up to 30% for 6 MV and 21% for 18 MV. At the point 0.1 cm from the exit surface, the measured dose was smaller than the calculated by up to 11% for 6 MV and 6% for 18 MV. The differences between measured and calculated doses increased with SSD for all setups.
The use of MLCs to limit the field reduced the entrance surface dose by 28% for 6MV and 44% for 18 MV.
With a build-up screen, the only measurement was made 0.1 cm from the phantoms entrance surface. At 340 cm SSD, the difference between measured and calculated doses were 3.0% (jaw field, 6MV); 9.1% (jaw field, 18MV), 4.2% (MLC field, 6MV) and 12.5% (MLC field, 18MV).
In-vivo dosimetry
The mean TLD measured dose difference from all points (see position of points in Fig. 6) was −1.9% with a standard deviation of 4.5%.
Fig. 6.
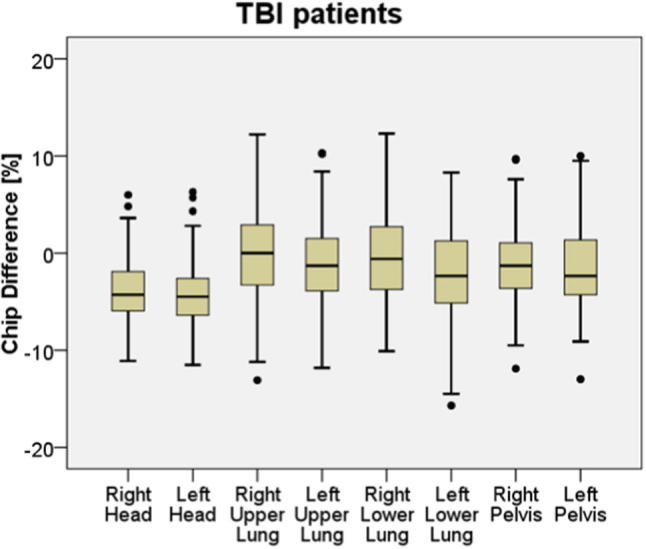
TLD measurement results based on 113 patients, where 3 TLDs were placed at each measuring point in a capsule with build-up. The black horizontal lines indicate median values; the boxes the 25th to the 75th percentiles, the black dots beyond the black bars are considered outliers.
Clinical doses at Rigshospitalet
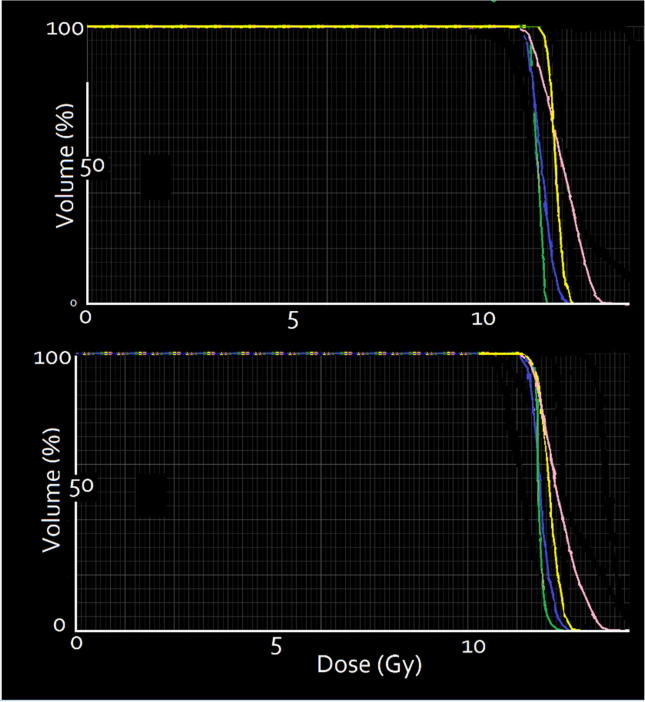
Dose volume histograms for a typical adult (top and paediatric (bottom) patient in Fig. 7.
Fig. 7.
Dose volume histograms for a typical adult (top) and paediatric (bottom) patient, showing data for brain (yellow), lungs (blue), kidneys (green) and PTV (pink). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
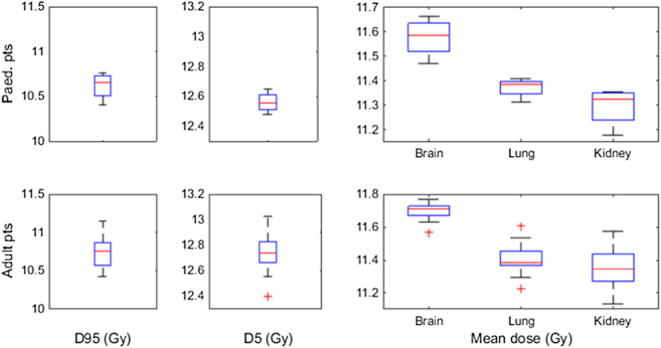
The D95 for the body ranged from 10.4 to 10.9 for the adult patients and 10.4 to 10.8 Gy for the paediatric patients (Fig. 8). D5 ranged from 12.4 to 12.8 Gy for the adult patients and 12.5 to 12.7 Gy for the paediatric patients. Thus, the dose volume histogram (DVH) curve fell off quite sharply.)
Fig. 8.
Distribution of D95 and D5 (the dose delivered to 95% and 5% of the body volume) and the mean doses to the brain, lungs and kidneys. Data is shown for paediatric patients (4 patients) and for adult patients (18 patients). The red lines indicate median values, the blue boxes the 25th to 75th percentiles, the black lines the region outside which data points are considered outliers and the red crosses are outliers. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The mean doses to the brain, lungs and kidneys ranged from 11.1 to 11.8 Gy.
The mean doses to the brain, lungs and kidneys were all in the range of 11.1–11.8 Gy, while the V(12 Gy) was kept below 5% for the brain, below 2% for the lungs and 0% for the kidneys.
Discussion
Current global TBI practice is vary, with the most commonly used technique of TBI delivery being the patient standing or lying, in an extended SSD setup, treating the entire patient with a single field. Compensators are used in some clinics to reduce the dose to the thinner body parts.
Increasing numbers of centers have begun to use CT based 3-D planning, intensity-modulated radiation therapy, and inverse planning in an effort to improve dose uniformity and organ sparing [3].
The SS IMRT technique for TBI is feasible in a clinical setting. The two clinics prioritised different on–line verification of the TBI treatment technique: the Royal Marsden prioritised in-vivo dosimetry, and Rigshospitalet prioritised treatment imaging. In general, in-vivo dosimetry could be performed with various available systems (TLDs, diodes, film or MOSFETs), and imaging could be performed with films or in-house modified portal vision imagers (this was used at RHC prior to the purchase of Theraview).
Crucially, no additional equipment purchases would be strictly necessary in a clinic implementing SS IMRT TBI.
In this paper we focused on the technical aspects of SS IMRT TBI rather than clinical outcomes.
Limitations of this work include that we have only demonstrated that SS IMRT TBI can be done using two of the available treatment planning systems. Ideally, a clinic doing TBI would have in-vivo dosimetry and treatment imaging available, however we cannot correlate the results of imaging and in-vivo dosimetry. Finally, since this paper focusses on technique, it does not include clinical outcomes.
The phantom measurements showed considerable discrepancies between measured and calculated doses 1 mm from the phantom surface, but less than 7% difference deeper in the phantom. It appears the TPS does not accurately model surface doses at extended SSDs, possibly due to scatter from the treatment room. One approach to this is to exclude the outer few mm of the patients anatomy when evaluating the DVHs for the TBI plans.
The technique of total marrow irradiation (TMI) has gained clinical implementation in the past decade. While TMI is used as the main radiation therapy technique in trials [18], TMI is typically used as a boost to TBI treatment rather than a treatment modality on its own.
TMI techniques have been investigated using IMRT [19], IMRT with couch rotations [20] and tomotherapy [21], however to our knowledge this is the first report of IMRT delivering TBI.
Conclusion
The SS IMRT TBI technique is readily implementable in a standard linac bunker of a size that allows the 350–355 cm radiation source to patient mid-line with no need for additional equipment.
Disclaimers
None.
Funding disclosure
HM and VNH acknowledges the NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2019.05.002.
Contributor Information
Lotte S. Fog, Email: lottesfog@yahoo.co.uk.
Vibeke N. Hansen, Email: Vibeke.Nordmark.Hansen@rsyd.dk.
Flemming Kjær-Kristoffersen, Email: Flemming.Kjaer-Kristoffersen@regionh.dk.
Tim Egholm Berlon, Email: tim.egholm.berlon@regionh.dk.
Peter Meidahl Petersen, Email: Peter.Meidahl.Petersen@regionh.dk.
Henry Mandeville, Email: Henry.Mandeville@rmh.nhs.uk.
Lena Specht, Email: Lena.specht@regionh.dk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Beyzadeoglu M., Dirican B., Oysul K., Arpaci F., Pak Y. Evaluation of fractionated total body irradiation and dose rate on cataractogenesis in bone marrow transplantation. Haematologia. 2002;32(1):25–30. doi: 10.1163/156855902760262736. [DOI] [PubMed] [Google Scholar]
- 2.Igaki H., Karasawa K., Sakamaki H., Saito H., Nakagawa K., Ohtomo K. Renal dysfunction after total-body irradiation: Significance of selective renal shielding blocks. Strahlenther Onkol. 2005;181(11):704–708. doi: 10.1007/s00066-005-1405-8. [DOI] [PubMed] [Google Scholar]
- 3.Wong J., Fillippi A.R., Dabaja B.S., Yahalom J., Specht L. Total body irradiation: guidelines from the international lymphoma radiation oncology group (ILROG) Int J/Radia Onc Biol Phys. 2018;101(3) doi: 10.1016/j.ijrobp.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 4.Ozsahin M., Pene F., Cosset J., Laugier A. Morbidity after total body irradiation. Semin Radiat Oncol. 1994 Apr;4(2):95–102. doi: 10.1053/SRAO00400095. [DOI] [PubMed] [Google Scholar]
- 5.Hartman A.R., Williams S.F., Dillon J.J. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transpl. 1998;22(April):439–443. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 7.Chiang Y., Tsai C.-H., Kuo S.-H., Liu C.-Y., Yao M., Li C.-C. Reduced incidence of interstitial pneumonitis after allogeneic hematopoietic stem cell transplantation using a modified technique of total body irradiation. Sci Rep. 2016;6:36730. doi: 10.1038/srep36730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broerse J.J., Bartstra R.W., van Bekkum D.W., van der Hage M.H., Zurcher C., van Zwieten M.J., Hollander C.F. The carcinogenic risk of high dose total body irradiation in non-human primates. Radiotherapy Oncol 2000: J Europ Soc Therap Radiol Oncol. 2000;54(3):247–253. doi: 10.1016/s0167-8140(00)00147-x. [DOI] [PubMed] [Google Scholar]
- 9.Borg M., Hughes T., Horvath N., Rice M., Thomas A.C. Renal toxicity after total body irradiation. Int J Radiat Oncol Biol Phys. 2002;54(4):1165–1173. doi: 10.1016/s0360-3016(02)03039-0. [DOI] [PubMed] [Google Scholar]
- 10.Belkacémi Y., Ozsahin M., Pène F., Rio B., Laporte J.P., Leblond V., Laugier A. Cataractogenesis after total body irradiation. Int J Radiat Oncol, Biol, Phys. 1996;35(1):53–60. doi: 10.1016/s0360-3016(96)85011-5. http://www.ncbi.nlm.nih.gov/pubmed/8641927 [DOI] [PubMed] [Google Scholar]
- 11.Henslee-Downey P.J., Abhyankar S.H., Parrish R.S., Pati A.R., Godder K.T., Neglia W.J. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood. 1997;89(10):3864–3872. http://www.ncbi.nlm.nih.gov/pubmed/9160695 [PubMed] [Google Scholar]
- 12.Aristei C., Aversa F., Chionne F., Martelli M.F., Latini P. Interstitial pneumonitis in acute leukemia patients submitted to T-depleted matched and mismatched bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1998;41(3):651–657. doi: 10.1016/s0360-3016(98)00068-6. [DOI] [PubMed] [Google Scholar]
- 13.Cosset J.M., Socie G., Dubray B., Girinsky T., Fourquet A., Gluckman E. Single dose versus fractionated total body irradiation before bone marrow transplantation: Radiobiological and clinical considerations. Int J Radiat Oncol, Biol, Phys. 1994;30(2):477–492. doi: 10.1016/0360-3016(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 14.Weiner R.S., Bortin M.M., Gale R.P., Gluckman E., Kay H.E., Kolb H.J. Interstitial pneumonitis after bone marrow transplantation. Assessment of risk factors. Ann Intern Med. 1986;104(2):168–175. doi: 10.7326/0003-4819-104-2-168. [DOI] [PubMed] [Google Scholar]
- 15.Gerig L.H., Szanto J., Bichay T., Genest P. A translating-bed technique for total-body irradiation. Phys Med Biol. 1994;39(1):19–35. doi: 10.1088/0031-9155/39/1/002. [DOI] [PubMed] [Google Scholar]
- 16.Chrétien M., Côté C., Blais R., Brouard L., Roy-Lacroix L., Larochelle M. A variable speed translating couch technique for total body irradiation. Med Phys. 2000;27:1127–1130. doi: 10.1118/1.598978. [DOI] [PubMed] [Google Scholar]
- 17.Springer A., Hammer J., Winkler E., Track C., Huppert R., Böhm A. Total body irradiation with volumetric modulated arc therapy: Dosimetric data and first clinical experience. Radiat Oncol. 2016:1–9. doi: 10.1186/s13014-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong J.Y.C., Rosenthal J., Liu A., Schultheiss T., Forman S., Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73(1):273–279. doi: 10.1016/j.ijrobp.2008.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydogan B., Mundt A.J., Roeske J.C. Linac-based intensity modulated total marrow irradiation (IM-TMI) Technol Cancer Res Treat. 2006;5(5):513–519. doi: 10.1177/153303460600500508. [DOI] [PubMed] [Google Scholar]
- 20.Jiang B., Dai J., Zhang Y., Zhang K. Feasibility study of a novel rotational and translational method for linac-based intensity modulated total marrow irradiation. Technol Cancer Res Treat. 2012;11(3):237–247. doi: 10.7785/tcrt.2012.500292. http://www.scopus.com/inward/record.url?eid=2-s2.0-84859172974&partnerID=tZOtx3y1 [DOI] [PubMed] [Google Scholar]
- 21.Hui S.K., Kapatoes J., Fowler J., Henderson D., Olivera G., Manon R.R. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(October):3214–3224. doi: 10.1118/1.2044428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.