Highlights
-
•
Region-of-interest (ROI) guidelines for soft tissue sarcoma CBCT-guidance were developed.
-
•
ROI guidelines were developed for four anatomic sites using the PDSA cycle.
-
•
ROI guidelines are intended to increase image registration reproducibility.
-
•
Results include improved image guidance decision making and workflow efficiencies.
Keywords: Soft tissue sarcoma, CBCT, PDSA
Abstract
Purpose
Region-of-interest (ROI) guidelines for online cone-beam computed tomography (CBCT) radiotherapy may improve matching reproducibility and reduce inter-user variability of soft tissue sarcoma (STS) image guidance. The purpose of this work is to standardize ROI STS CBCT image registration guidelines using the plan-do-study-act (PDSA) cycle for the lower extremity, retroperitoneal, pelvis, and thorax.
Methods
Based on anatomic bony surrogates, initial ROI matching guidelines for STS were developed by a team of radiation therapists, physicists and oncologists (Plan). Retrospective, qualitative evaluation of the guidelines was completed by the designated sarcoma lead therapist to determine clinical feasibility (Do). Validation of the ROI guidelines was performed through independent evaluation by radiation therapy CBCT imaging experts on a cohort of 10 patients per anatomic region (Study).
Results
Draft ROI guidelines were evaluated by 2 independent observers who registered weekly CBCT images to test their validity. Each observer assessed 5 patients per anatomic site, testing ROI options for accuracy of image registration and feasibility, while some ROI borders were adjusted based on algorithm matching performance. Validated ROI guidelines were presented to the sarcoma multidisciplinary site group, and an inter-professional committee of imaging experts for approval prior to clinical implementation (Act).
Conclusion
ROI matching guidelines for STS IGRT were standardized for 4 anatomic sites using the PDSA cycle for change testing and implementation. IGRT guidelines are intended to improve STS image registration reproducibility, and in turn, are expected to improve the confidence of IGRT decision making and workflow efficiencies for a rare disease with diverse presentation.
Introduction
Soft tissue sarcomas (STS) are rare and present many challenges for the standardization of patient positioning, image guidance, and accurate radiotherapy delivery [1], [2]. Approximately 200 STS patients are treated with radiation therapy annually in our institution. Confounding factors include variability in anatomic presentation, and changes in tumor size and shape during the treatment course [3].
Image Guided Radiation Therapy (IGRT) assures the accuracy of patient positioning prior to treatment delivery by reducing geometric uncertainties [4], [5]. Cone-beam computed tomography (CBCT) guidance systems allow for online volumetric visualization of patient anatomy, enabling daily setup variations to be quantified and corrected prior to treatment delivery, while monitoring daily patient changes and deformations [5], [6]. Efficient incorporation of daily CBCT application and decision making into the clinical workflow is influenced by the confidence in image assessment by end users, and their experience and comfort with volumetric image visualization and literacy [7], [8].
IGRT has had a significant impact on the clinical role of the Radiation Therapist and in some institutions, IGRT decisions rely on front-line therapists [9]. An institutional training program was designed to familiarize therapists with CBCT technology and consolidate image guidance concepts/knowledge at the onset of clinical implementation [10]. This previous work highlighted training, education and continuous clinical support are required for successful CBCT implementation [10], [11]. Specifically for sarcoma IG training, a specialized sarcoma radiation oncologist and lead sarcoma experts in radiation therapy and medical physics were involved in developing the curriculum and highlighting anatomical cross sectional anatomy considerations.
Safety considerations, education and consensus guidelines for IGRT have been identified as key issues to complement an expanding image guidance culture [12]. In particular, many have emphasized the importance of the quality paradigm in the era of IGRT to ensure that practice keeps pace with technology, and considerations are made for how to handle clinical information previously unavailable [4], [8]. Specifically, a lack of literature exists on optimal regions of interest (ROI) for CBCT image registration to facilitate consistent practice for radiation therapists, ensuring reliable registration results and subsequent treatment targeting. As such, the validation of standardized ROIs for image registration is required to ensure safe and efficient clinical practice. At the time of this analysis, IGRT guidelines for the STS sites were not optimized through standardized reference procedures.
The aim of this study was to develop standardized STS IGRT guidelines for four anatomic sites including lower extremity, retroperitoneal, pelvis and thorax using the plan-do-study-act (PDSA) cycle to facilitate change [13]. The PDSA cycle is a widely used framework for systematic improvement of a process. These guidelines are intended to improve matching reproducibility and reduce inter-user variability for a rare disease.
Methods
This work was performed under institutional ethics approval.
Plan
Based on anatomic bony surrogates, initial ROI matching guidelines for STS were developed by a team of radiation therapists, physicists and oncologists. The defined imaging region of interest (ROI) affects the reproducibility of image registration between the daily CBCT and reference planning computed tomography (CT) scan, as this area is used by the volumetric software for automatic image registration. ROI definition often involves a tumor surrogate as visualization of the actual tumor may be suboptimal, and in the case of large STSs, may not be fully encompassed by the imaging field-of-view. Bone was identified as a surrogate for STS CBCT-IGRT, as it remains stable in situations where the tumor volume may change in size and shape significantly. In addition, bone has also been identified in previous work as an organ-at-risk (OAR) that if protected may result in reduced bone fracture risk for patients [14]. Choosing bone as a priority in image guidance and matching ensures its protection despite soft tissue changes which may occur throughout the course of radiotherapy. Appropriate bony surrogates were considered in the development and initial drafted ROI guidelines for the four STS anatomic locations by an imaging working group formed for this purpose.
Do
Retrospective, qualitative evaluation of drafted ROI guidelines was completed by the radiation therapist sarcoma lead (S.A.) to determine their clinical feasibility. Patients (5 per anatomic site; n = 20) who received CBCT-guided intensity modulated radiation therapy were randomly selected between January 2013 and April 2014. The image registration process began with an automatic bone match based on the recommended ROI, with subsequent evaluation of registration accuracy and assessment of STS target coverage within the planning target volume (PTV) contour. Draft ROI guidelines were adjusted accordingly.
Study
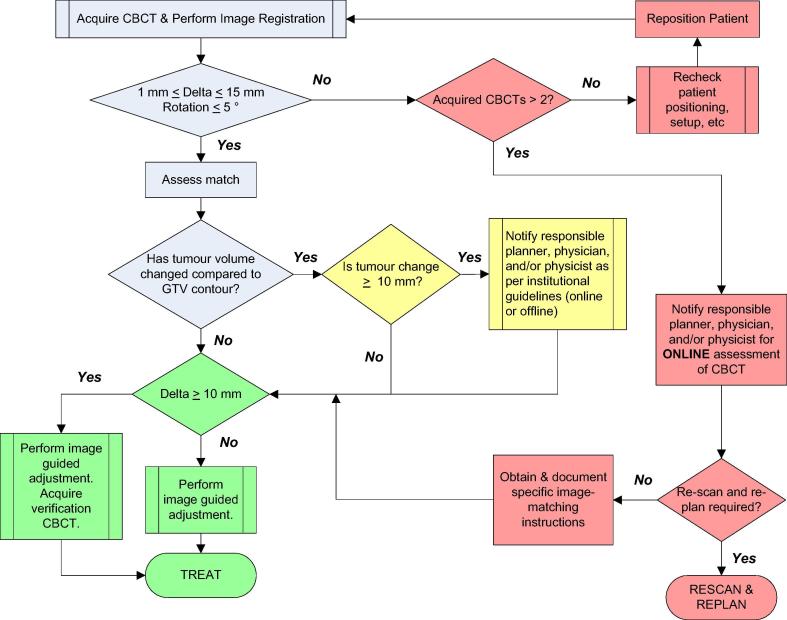
The objectives of the study phase were: to ensure optimal algorithm matching performance using the drafted guidelines throughout a treatment course; to assess quantitative translational and rotational offsets within institutional thresholds to identify a mismatch or failed attempt (see Fig. 1); to evaluate draft ROI options; and to adjust, revise and document changes to ROI borders if required to improve matching consistency.
Fig. 1.
Sarcoma cone-beam computed tomography image guidance workflow.
The draft ROI guidelines were independently evaluated by two institutional radiation therapy imaging experts (C.H., W.L.). Data validation was performed through retrospective image registration of 10 identified STS patients (different from those evaluated in the “Do” phase) for each sub-site that had clinically stable set ups and positioning to allow for assessment of the image registration without a significant set up variation bias (n = 40). There was no overlap in data validation patients between the two imaging experts.
Each imaging expert independently performed registrations by delineating ROI borders on the reference CT images outlined in draft guidelines. For each patient validation, 4–6 weekly CBCT images were reviewed depending on patient dose-fractionation to evaluate ROI consistency and reliability throughout a course of RT. Automatic image registration was performed to assess the matching success of proposed borders. Registration results were qualitatively evaluated by the validators to ensure a successful match, indicated by a visual spatial assessment that the correct bony anatomy was registered. Quantitatively, the translational and rotational offsets provided by the rigid registration were reviewed to see if they met current institutional guidelines and thresholds of 1.0 cm and 5°, realizing that this was a patient cohort that received radiotherapy with stable standardized positioning. Specifically, a custom extremity immobilization device (T-Form Extremity Immobilizer System, Bionix RT, Toledo, OH) was utilized for the lower limb, a polystyrene bead vacuum cradle (VacLok®, Civo Medical Solutions, Kalona, IA) was used for retroperitoneal patients, thorax patients were immobilized on a chestboard (MedTech, Orange City, IA) while pelvis patients were positioned supine with pillows under their head, and a standard immobilizer (Contour Fabricators Inc. Medical Solutions, Denton, Michigan) under their legs.
Act
Analysis from the ‘study’ phase contributed to amendment of the ROI borders. Finalized guidelines were approved by the multidisciplinary sarcoma site group consisting of therapy, oncology and physics with STS expertise. The final ROI guidelines were presented to an inter-professional image-guidance institutional committee for review and approval before clinical implementation.
Results
Plan
Based on anatomic bony surrogates in the 4 STS locations, ROI matching guidelines were drafted by the inter-professional team of STS experts.
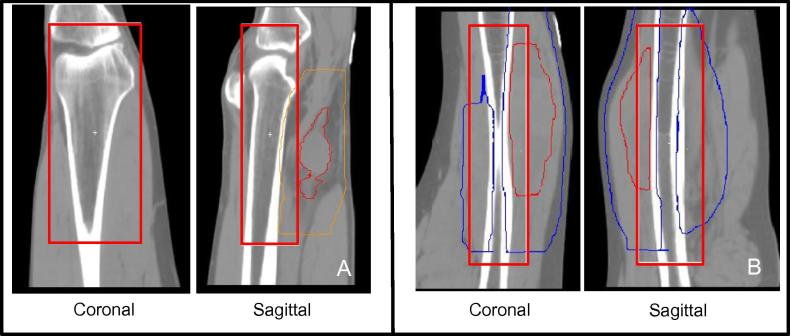
For the lower extremity, the proposed region included the long bone adjacent or encompassed by the PTV contour. The superior/inferior border was outlined as 2 cm from the edge of the PTV, anterior/poster and left/right border 1 cm from the bone, including the joint where possible. Where no joint was available in the imaging field-of-view, the protocol specified use of adjacent muscle compartments for image analysis (see Fig. 2).
Fig. 2.
Region of interest definition for lower limb sarcoma. (A) Limb with visible joint. Red contour = GTV, Orange contour = PTV. (B) Limb without visible joint. Red contour = GTV, Blue contour = PTV. The region of interest is outlined in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
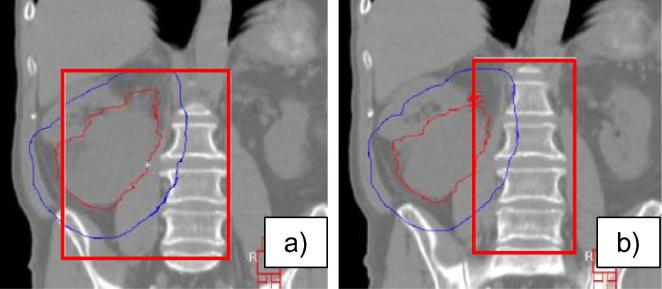
For the retroperitoneal site, two different guidelines were proposed for testing: one included the vertebral column adjacent to the treatment target, and one included the pelvic crest laterally as well as the vertebral column (see Fig. 3).
Fig. 3.
Proposed options for region of interest definition for retroperitoneal sarcoma: variations in the lateral borders. (a) Option 1 includes the pelvic crest laterally and the vertebral column. (b) Option 2 includes the vertebral column adjacent to the treatment target. Red contour = GTV, Blue contour = PTV. The region of interest is outlined in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The pelvis ROI guidelines concentrated on unilateral disease. The guidelines indicated to include bony anatomy adjacent to the PTV (see Fig. 4).
Fig. 4.
Proposed region of interest definition guidelines for unilateral pelvic sarcoma. Red contour = GTV, Blue contour = PTV. The region of interest is outlined in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
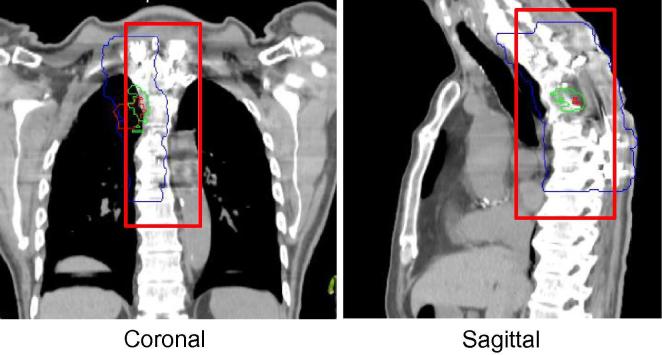
For the thoracic site, ROI borders from previously established guidelines for radical lung image guidance was used [15]. The ROI borders, defined on bony anatomy adjacent to PTV location including a minimum of three vertebral bodies, were tested (see Fig. 5).
Fig. 5.
Proposed region of interest definition guidelines for thoracic sarcoma. Red contour = GTV, Blue contour = PTV. The region of interest is outlined in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Do
This testing phase of the PDSA cycle ensured clinical feasibility, and reliability the automatic matching algorithm to reduce the need for manual adjustment. Manual adjustments were performed to correct for sub-optimal automatic image registration. This occurred more frequently in the absence of standardized guidelines. Following testing, these adjusted guidelines were reviewed by an inter-professional STS specialized team of therapists, oncologists and physicists. Minor amendments were made by the radiation therapist sarcoma lead prior to the ‘study’ phase according to the group’s recommendations.
Study
Based on quantitative assessment of the automatic registrations of the lower extremity and thorax sites, no further modifications to the draft guidelines for these two STS sites were required. The ROI borders passed automatic algorithm assessment, with registrations consistently resulting in high quality, accurate anatomic alignment of bony and soft tissue anatomy.
Two sets of ROI guidelines were proposed for the retroperitoneal site. One proposal included the pelvic crest, and the other did not. Both ROI recommendations were tested on weekly CBCT images for 10 patients, 5 per imaging expert. Image registration results for both approaches were similar, with the automatic algorithm matching the correct anatomy within our clinical translational and rotational thresholds. It was observed that the ROI guideline including the pelvic crest increased the registration time (i.e. 5–10 s per match) and was less consistently defined between observers (i.e. amount of pelvic crest to include). Therefore, inclusion of the pelvic crest was removed and the final recommendation included the vertebral bodies alone.
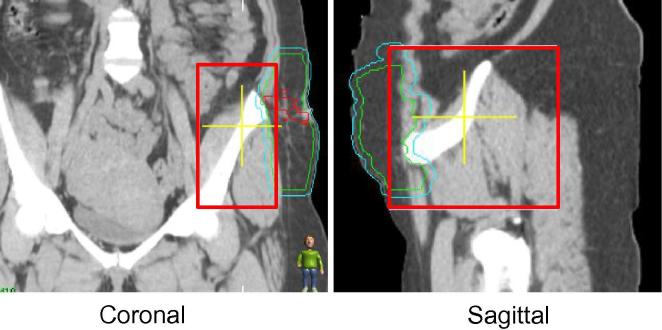
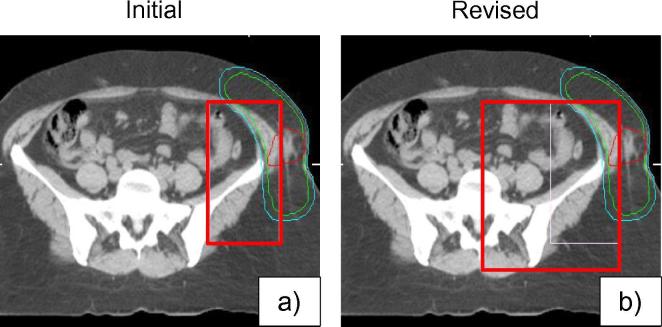
In the draft guideline for the unilateral pelvis location, only pelvic bones adjacent to the PTV were included in the ROI borders. During the study phase, issues were encountered with this ROI definition including an inconsistent match with large rotational offsets (>5°) as an insufficient amount of bony anatomy was included to perform the automatic registration. As a result, revisions were made to the recommended ROI guidelines to adjust the boundaries to include the hemi-pelvis which increased matching reliability by meeting clinical thresholds (see Fig. 6). Specific lateral and anterior/posterior anatomy was determined for ROI definition to reduce ambiguity and inter-observer variation.
Fig. 6.
(a) Initial and (b) revised region of interest definition guidelines for unilateral pelvic sarcoma. Adjustments were made to the lateral and posterior borders. Red contour = GTV, Blue contour = PTV. The region of interest is outlined in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Act
Final guidelines were presented to an institutional image guidance committee for approval prior to clinical implementation. Guidelines for lower limb and thoracic STS remained as initially drafted (Fig. 2, Fig. 5). Guidelines for retroperitoneal and pelvis STS followed the revised borders tested during the study phase (Figs. 3a and 6b). Following approval, STS-ROI guidelines were posted on the institutional intranet as reference for staff to ensure transparency, availability, and consistent clinical practice. Departmental training and education was conducted. Quality assurance activities were amended to ensure guidelines were realized clinically.
Discussion
The rare and variable presentation of STS presents considerable challenges for standardization of ROI matching guidelines. Standardizing STS ROI matching guidelines for IGRT improves reproducibility and efficiency of image registration and reduces inter-user variability. For STS, changing tumor volumes during radiotherapy [16] and large tumor volumes at presentation that are often larger than the available imaging field of view advocate the need for a stable surrogate such as bony anatomy.
This PDSA quality assurance study illustrates the process of developing a plan to test the standardization of STS ROI image matching guidelines, validating the test, observing and learning from the plan and finally, determining the final modifications to the guidelines before clinical implementation through presentation to a committee of imaging experts. The validated ROI borders demonstrated accurate and consistent image registration with an automatic registration algorithm, empowering radiation therapists to deliver IGRT with confidence using standardized image guidance policies and procedures. Robust IGRT guidelines limit inter-observer variability and streamline treatment processes by eliminating the uncertainty of ROI definition. Therapists can focus on patient related issues such as the assessment of changes in soft tissues (i.e. growers and shrinkers) [17] and patient care rather than treatment process considerations.
Benefits of standardizing guidelines in an image guidance era include ensuring the safety and effectiveness of IGRT [12]. As online IGRT increases volumetric information at the front line, radiation therapists are increasingly involved in image analysis and decision making that affects the accuracy and precision of patient treatment [8], [11], [12]. Dissemination of standardized documentation facilitates clinical implementation, ensures consistency in clinical practice, and aids in the education and professional development of radiation therapists [12], [18]. Additionally, standardized departmental ROI guidelines reduces decision making time for online image registration [19].
Existing ROI guidelines for other anatomic sites including lung, paraspinal, and head and neck have been developed through research activities [15], [20], [21]. It is important to note that each anatomic site has different considerations and priorities for online IGRT. Factors identified through these studies that influence online IGRT include identification of appropriate surrogates for matching, treatment intent, consideration of surrounding OARs, immobilization techniques and other personalized clinical considerations.
In this study, ROI definition and validation was guided by rigid registrations with six degrees-of-freedom, and isocenter discrepancies corrected by couch translations in the clinical setting. As patient anatomy may deform or change over a course of treatment, encompassing mobile anatomy in the ROI may result in an erroneous and inconsistent match, whereas a ROI with limited anatomical information may result in a registration that does not accurately represent the patient’s actual setup. From our experience, ROI definition should involve discussion of the stability and reliability of the target volume, organs at risk and bony anatomy to achieve a balance between included relevant anatomy and ROI size. The availability of shaped, non-rectangular ROI matching or non-rigid deformable registration will add complexities that require additional considerations. Additionally, once clinically available, the use of multiple ROIs for online IGRT will also require complex decision making such as identifying priority regions for matching, and defining thresholds between the various regions delineated for registration [22].
The upper extremity and axilla ROI guidelines were not developed in this study. This challenging region for patient positioning is associated with multiple points of rotation and angular flexion, heightening concerns regarding positioning reproducibility. An immobilization device innovation is currently underway with ROI guidelines under development.
Conclusion
Standardized ROI guidelines for STS have been developed and independently validated by radiation medicine experts in sarcoma treatment using the PDSA cycle for testing and implementation. These guidelines are expected to improve the workflow efficiency and image matching reproducibility for STS IGRT. The methods used for standardizing STS ROI guidelines may be applicable to other anatomic sites. Moreover, the experience with PDSA development of these CBCT matching guidelines could potentially form the foundation for development with other imaging modalities that may be used for RT guidance.
Conflict of interest
None.
Footnotes
This work was presented in part at the 11th Annual Radiation Therapy Conference, March 6th–7th 2015, Toronto, Ontario, Canada.
References
- 1.Dickie C.I., Parent A., Griffin A. A device and procedure for immobilization of patients receiving limb-preserving radiotherapy for soft tissue sarcoma. Med Dosim. 2009;34:243–249. doi: 10.1016/j.meddos.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Dickie C.I., Parent A.L., Chung P.W.M. Measuring interfractional and intrafractional motion with cone beam computed tomography and an optical localization system for lower extremity soft tissue sarcoma patients treated with preoperative intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78:1437–1444. doi: 10.1016/j.ijrobp.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Biau D.J., Ferguson P.C., Chung P. Local recurrence of localized soft tissue sarcoma. Cancer. 2012;118:5867–5877. doi: 10.1002/cncr.27639. [DOI] [PubMed] [Google Scholar]
- 4.Jaffray D.A. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–699. doi: 10.1038/nrclinonc.2012.194. [DOI] [PubMed] [Google Scholar]
- 5.Jaffray D.A. Emergent technologies for 3-dimensional image-guided radiation delivery. Semin Radiat Oncol. 2005;15:208–216. doi: 10.1016/j.semradonc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Jaffray D.A., Siewerdsen J.H., Wong J.W. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:1337–1349. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 7.Robb D., Plank A., Middleton M. Assessing the efficiency and consistency of daily image-guided radiation therapy in a modern radiotherapy centre. J Med Im Rad Sci. 2014;45:72–78. doi: 10.1016/j.jmir.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gillan C., Li W., Harnett N. Radiation therapist perspectives on cone-beam computed tomography practices and response to information. J Radiother Pract. 2013;12:237–244. [Google Scholar]
- 9.Odle T., Rosier N. American Society for Radiological Technologists; Albuquerque, NM: 2012. Radiation therapy safety: the critical role of the radiation therapist. [Google Scholar]
- 10.Li W., Harnett N., Moseley D.J. Investigating user perspective on training and clinical implementation of volumetric imaging. J Med Im Rad Sci. 2010;41:57–65. doi: 10.1016/j.jmir.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 11.White E., Kane G. Radiation medicine practice in the image-guided radiation therapy era: new roles and new opportunities. Semin Radiat Oncol. 2007;17:298–305. doi: 10.1016/j.semradonc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Jaffray D.A., Langen K.M., Mageras G. Safety considerations for IGRT: executive summary. Pract Radiat Oncol. 2013;3:167–170. doi: 10.1016/j.prro.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor M.J., McNicholas C., Nicolay C. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Safety. 2014;23:290–298. doi: 10.1136/bmjqs-2013-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickie C.I., Parent A.L., Griffin A.M. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: Relationship to irradiated bone length, volume, tumor location and dose. Int J Radiat Oncol Biol Phys. 2009;75:1119–1124. doi: 10.1016/j.ijrobp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Li W., Moseley D.J., Bissonnette J.-P. Setup reproducibility for thoracic and upper gastrointestinal radiation therapy: influence of immobilization method and on-line cone-beam ct guidance. Med Dosim. 2009;35:287–296. doi: 10.1016/j.meddos.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Dickie C., Parent A., Griffin A.M. The value of adaptive preoperative radiotherapy in management of soft tissue sarcoma. Radiother Oncol. 2017;122:458–463. doi: 10.1016/j.radonc.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Dickie C.I., Griffin A.M., Parent A.L. The relationship between local recurrence and radiotherapy treatment volume for soft tissue sarcomas treated with external beam radiotherapy and function preservation surgery. Int J Radiat Oncol Biol Phys. 2012;82:1528–1534. doi: 10.1016/j.ijrobp.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 18.Coffey M., Leech M., Poortmans P. Benchmarking radiation therapist education for safe practice: the time is now. Radiother Oncol. 2016;119:12–13. doi: 10.1016/j.radonc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Li W., Jaffray D.A., Wilson G. How long does it take? An analysis of volumetric image assessment time. Radiother Oncol. 2016;119:150–153. doi: 10.1016/j.radonc.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Sahgal A., Foote M. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2012;84:520–526. doi: 10.1016/j.ijrobp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Morley L., Waldron J., Dawson L. The effect of registration volume extent on residual errors assessed using cone-beam computed tomography in radiation treatment of head and neck cancer. J Med Im Rad Sci. 2012;43:95–102. doi: 10.1016/j.jmir.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 22.van Beek S., van Kranen S., Mencarelli A. First clinical experience with a multiple region of interest registration and correction method in radiotherapy of head-and-neck cancer patients. Radiother Oncol. 2010;94:213–217. doi: 10.1016/j.radonc.2009.12.017. [DOI] [PubMed] [Google Scholar]