Highlights
-
•
RTTs report substantial anatomic variances during cone-beam CT guided radiotherapy.
-
•
These variances led to unscheduled adaptive re-planning in 21% of cases.
-
•
Suspected tumor progression was a frequent cause of re-planning.
-
•
Understanding clinical scenarios for re-planning will enable more formal adaptive strategies to be developed.
Keywords: Cone-beam CT, Adaptive radiation therapy, Image registration, Inter-fraction motion
Abstract
Purpose
Substantial, unanticipated anatomic variances during cone-beam CT (CBCT)-guided radiotherapy can potentially impact treatment accuracy and clinical outcomes. This study assessed patterns of practice of CBCT variances reported by RTTs and subsequent interventions for multiple-disease sites.
Methods
A chart review was conducted at a large cancer centre for patients treated with daily online CBCT-guided radiotherapy. Patients selected for review were identified via RTT-reported variances that then triggered offline multi-disciplinary assessment. Cases were categorized by the type of anatomic variance observed on CBCT and any further interventions recorded such as un-scheduled adaptive re-planning.
Results
Over a 1-year period, 287 variances from 261 patients were identified (6.2% of the 4207 patients treated with daily CBCT-guided radiotherapy), most often occurring within the first 5 fractions of the treatment course. Of these variances, 21% (59/287) were re-planned and 3.5% (10/287) discontinued treatment altogether. Lung was the most frequent disease-site (27% of 287 variances) reported with IGRT-related variances although head and neck and sarcoma were most frequently re-planned (19% of 59 re-plans for each site). Technical or clinical rationales for re-planning were not routinely documented in patient medical records. All disease-sites had numerous categories of variances. Three of the four most frequent categories were for tumor-related changes on CBCT, and the re-planning rate was highest for tumor progression at 25%. Normal tissue variances were the second most frequency category, and re-planned in 14% of those cases.
Conclusion
RTTs identified a wide range of anatomic variances during CBCT-guided radiotherapy. In a minority of cases, these substantially altered the care plan including ad hoc adaptive re-planning or treatment discontinuation. Improved understanding of the clinical decisions in these cases would aid in developing more routine, systematic adaptive strategies.
Introduction
Image-guided radiation therapy (IGRT) using cone-beam CT (CBCT) enables soft-tissue anatomy visualization, quantification and correction of daily patient setup errors and monitoring of patient changes [1], [2]. This level of accuracy enables smaller planning target volumes and normal tissues sparing [3]. There is clinical evidence that IGRT improves outcomes when coupled with conformal planning and delivery techniques [4]. However, some patients exhibit large unexpected spatial and temporal anatomic variations that are difficult to correct with standard IGRT practices.
Adaptive radiation therapy is ideally a closed loop process where patient-specific variations are monitored and incorporated into a re-optimized plan [5]. Despite being introduced over 20 years ago, the increased workload per patient associated with adaptation has been a barrier to widespread implementation. A few simple and effective adaptive strategies have been successfully implemented for cancers of the pelvis. These include using initial CBCT images to redefine patient-specific margins and re-plan offline for later fractions [6], or online application of different plans from a pre-generated library [7], [8]. Specialized magnetic resonance-based IGRT systems have been recently used by limited institutions for daily online re-planning, making adaptation vastly more feasible [9], [10]. Even with advanced technical solutions however, understanding the ideal conditions under which to adapt are not well understood and decisions are largely based on clinical judgement.
IGRT often relies on CBCT assessment by radiation therapists (RTT) as an essential competency aided by in-house training [11], [12], [13], and standardized protocols [14], [15]. Without systematic adaptation strategies in place, RTTs may be required to identify and report IGRT-related variances (e.g. motion beyond tolerances, large anatomic changes) that require further assessment or interventions such as ad hoc adaptive re-planning [16]. At our institution, IGRT-related variances reported by RTTs are reviewed offline by a multi-disciplinary team with further interventions ordered at the discretion of the radiation oncologist. These activities are resource intensive so studying the outcomes of IGRT workflows may improve consistency of practices and help define relevant clinical scenarios. Increasing knowledge of re-planning decisions may allow RTTs to participate more independently in adaptive strategies thereby facilitating their application in clinic. The purpose of this study is to assess the outcomes and patterns of practices for IGRT-related variances reported by RTTs across multiple disease-sites.
Materials and methods
The research ethics board approved this retrospective review of IGRT practices and adaptive re-planning. Patients treated with CBCT-guided external-beam radiotherapy between January 1 and December 31, 2015 were eligible for inclusion. Techniques not utilizing daily CBCT at our institution such as breast tangents, whole-brain or total-body radiation were excluded.
Clinical environment and practices
Data was retrieved from the radiotherapy clinic in a large, academic comprehensive cancer centre with 15 CBCT-equipped linear accelerators in clinical operation during the time period and a capacity of approximately 400 patients/day. Units are organized by anatomic site for staff specialization of disease management and techniques.
Daily, online CBCT-guidance was standard care for most patients. Mature IGRT processes include standardized RTT training [11], [12], [13] and well-defined CBCT workflows [14], consistent with best-practice guidelines [17], [18]. RTTs independently performed image assessment and correction with a 0 mm action level, except for some stereotactic techniques requiring physician presence.
Each disease-specific workflow described the approximate conditions of an IGRT-related variance requiring further offline assessment by the multi-disciplinary team. The most common reporting mechanism was treatment RTTs e-mailing a description with a representative screen capture of the CT-CBCT fusion. Outcomes of these variances were determined case-by-case and ranged from no action to ad hoc adaptive re-planning. Systematic adaptation strategies were rarely used and relied on pre-scheduled re-planning in anticipation of soft-tissue changes during treatment [6].
Data collection
For this study, IGRT-related variances reported by RTTs were compiled by searching archived e-mails sent from each linac/treatment units’ accounts for keywords “CBCT”, “cone beam”, “image” or “screenshot”. The resulting threads were reviewed for cases of RTTs notifying the multi-disciplinary team of variances observed on CBCT. Additional variances for which there was no email thread were identified for patients who underwent repeat CT-simulation and/or re-planning by compiling related billing codes captured in the record and verify system (MOSAIQ, Elekta, Crawley, UK) or identified in a custom, in-house web-based treatment plan review and approval database. All clinical notes were reviewed to confirm ad hoc repeat CT-simulation and/or adaptive re-planning was due to unanticipated anatomic variations on CBCT. Re-planning that was pre-scheduled (e.g. for multiple phases), or required following QA procedures or peer-review rounds was excluded.
Analysis
Each reported IGRT-related variance was summarized and categories were created to reflect the range of observations (Table 1). All cases were then categorized by single observer (an RTT trainee). Ambiguous cases were discussed with a 2nd experienced RTT for categorization. Outcomes and any interventions were noted (e.g. continue treatment, re-planning etc.). To assess patterns of practice, descriptive statistics summarized results by disease site, treatment intent and timing when first observed. Treatment intent (radical or palliative) was defined as documented in the record and verify system. Treatment timing was defined as the absolute fraction number or relative treatment quarter to account for the wide range of dose/fractionations.
Table 1.
Categories of IGRT-related variances reported by RTTs.
| Main Category | Sub-Category | Description |
|---|---|---|
| Tumor-related | Tumor motion | Target displacement, motion or deformation with stable volume |
| Tumor regression | Suspected decrease in target volume | |
| Tumor progression | Suspected increase in target volume | |
| Non tumor-related | Normal tissue motion | Normal tissue displacement, motion or deformation |
| Large shift/rotation | A translation/rotation exceeding the maximum action level | |
| Bone mismatch | Bony anatomy deformation or rotation | |
| Bladder/rectum filling | Increase or decrease in bladder/rectum volume | |
| Weight gain | Overall expansion of external body contour | |
| Weight loss | Overall decrease of external body contour | |
| Local swelling | Localized expansion of external body contour | |
| Abdominal gas | Substantial increase in gas (excluding rectum) | |
| Fluids | Increase of fluid in lungs or sinuses | |
The number of events was also compared to the total number of patients treated with CBCT-guidance (N = 4207) over the same period to quantify the overall rate of rate of variances and re-planning.
Results
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tipsro.2019.10.003.
E-mail accounts for 12 of 15 operational treatment units were reviewed, identifying 272 IGRT-related variances. Three units did not have archived e-mails available for review. A search of the treatment planning and record-and-verify systems identified 15 additional instances of repeat scanning and/or planning. In total 287 IGRT-related variances in 261 patients were identified over one year. Patient demographics are in Supplementary Table S1. Re-planning resulted in 21% (59 variances in 59 patients) of these 287 variances, with treatment continuation with/without modified IGRT instructions in the remaining 79% (Table 2). Descriptions of the technical or clinical rationale for re-planning or continuing treatment were rarely documented in the patients’ medical record, and therefore not available for analysis.
Supplementary Table S1.
Table 2.
Cases of IGRT-related variances over 1-year and their outcomes following offline multi-disciplinary assessment.
| Intervention | Variances reported by RTTs1 (N = 272) | Additional variances identified2 (N = 15) | Total (N = 287) | |
|---|---|---|---|---|
| No adaptive re-planning performed | ||||
| Continue treatment3 | 207 | 0 | 207 | |
| Repeat CT-sim only, then continue treatment | 17 | 4 | 21 | |
| Adaptive re-planning performed | ||||
| Re-plan using original CT-sim | 2 | 1 | 3 | |
| Repeat CT-sim and re-plan4 | 46 | 10 | 56 | |
Notes:
Reported by unit e-mail.
Identified in the treatment planning and record-and-verify systems.
Treatment later discontinued in 9 cases.
Treatment later discontinued in 1 case.
Compared to the total number of patients treated with CBCT-guidance over the study period, the overall rate of IGRT-related variances and re-planning was 6.2% and 1.4% respectively.
Twenty-two patients had 2 variances reported and 2 patients had 3. For these 24 patients with multiple reports, 60% of the variances were in different categories with a median interval of 9 fractions. Five patients were re-planned after the 1st report (and subsequently had a 2nd report even after re-planning), and five re-planned after the final report.
Treatment timing
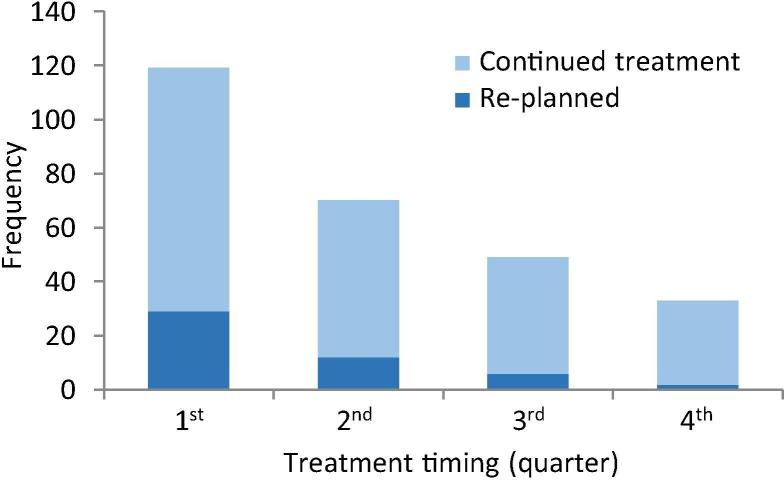
Overall, most variances reported by RTTs and re-planning occurred early and declined steadily over the course of treatment (Fig. 1). The median absolute time when first reported was at fraction 3 for short courses (≤10 fractions) and fraction 8 for long courses (>10 fractions). The absolute timing distribution is shown in Supplementary Fig. S1.
Fig. 1.
Relative timing of IGRT-related variances reported by RTTs during the treatment course including all dose/fraction schedules.
Supplementary Fig. S1.
Disease site distribution
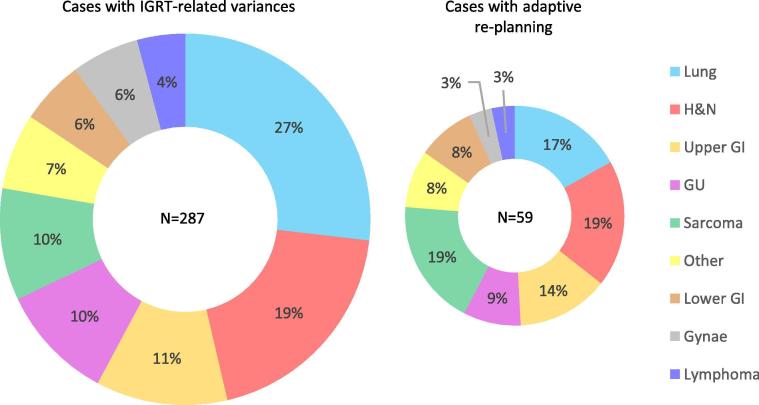
The distribution by disease site varied between those identified with IGRT-related variances and those re-planned (Fig. 2). Lung was most frequently reported (27% of variances) although head-and-neck and sarcoma were most frequently re-planned (19% of re-plans). The positive predictive value (reported variances/re-plans) of the RTT-reporting mechanism in correctly identifying the need for a re-plan, assuming one was truly needed, varied by site. The positive predictive values were: sarcoma-48%, lower GI-31%, other-29%, upper GI-24%, head-and-neck-21%, GU-19%, lymphoma-18%, gyane-13% and lung-13%.
Fig. 2.
Distribution by disease site for all IGRT-related variances identified (left) and those subsequently re-planned (right).
For each site, the proportion of re-planned cases to the total number treated the same year ranged from 1% re-planning for all gynae cases treated to 4% each for sarcoma, upper and lower GI.
Categories of variances
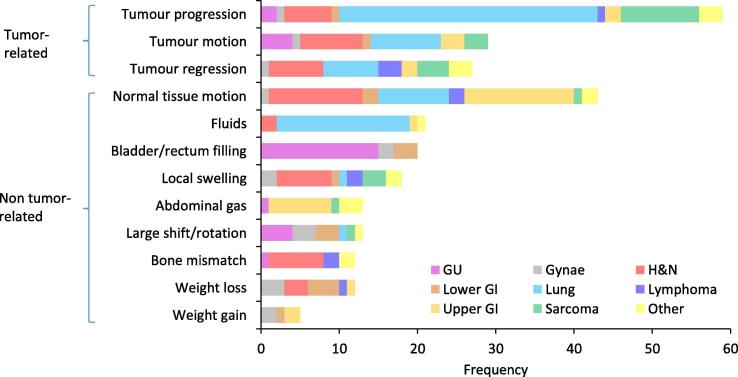
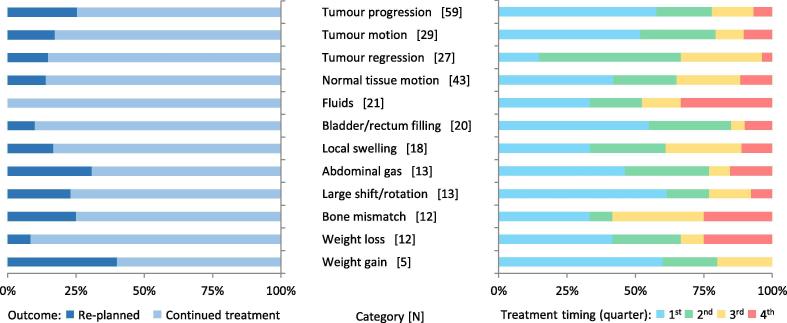
Of the IGRT-related variances, 47% were tumor-related and 53% were non-tumor related, with substantial variations in frequency by sub-category (Fig. 3). The re-planning rate for tumor-related and non tumor-related variances was 21% and 16% respectively. Fig. 4 shows the variations in re-planning rate by sub-category (ranging from 0% for fluid observed in the sinuses/lungs [n = 21] to 40% of suspected weight gain [N = 5]), and variations in timing.
Fig. 3.
Frequency of RTT-reported variances by category of findings on CBCT.
Fig. 4.
Re-planning outcomes (left) and timing (right) of IGRT-related variances by category.
Patterns of practice by disease site
The distribution of IGRT-related variances by each disease site is shown in Supplementary Fig. S2. The five most common sites accounting for over ¾ of all IGRT-related variances identified are summarized below.
Supplementary Fig. S2.
Lung: The majority of lung variances (63%) were tumor related (‘tumor progression’ being the most common sub-category), and these were re-planned at a rate of 16%. The remaining 37% of non-tumor related variances were re-planned at a rate of 4%. Notably lung also had the largest absolute and relative (22% of lung variances) proportion of non-tumor related ‘fluid’ variances for any site, and none were re-planned.
Sarcoma: The majority of sarcoma variances (74%) were tumor related (‘tumor progression’ being the most common sub-category), and these were re-planned at a rate of 29%. The remaining 26% of tumor-related variances were re-planned at rate of 67%.
Head-and-neck: The majority of head-and-neck variances (60%) were non-tumor related (‘normal tissue motion’ being the most common sub-category), and these were re-planned at a rate of 3%. The remaining 40% of tumor-related variances were re-planned at rate of 24%.
Upper GI: The majority of upper GI variances (79%) were non-tumor related (‘normal tissue motion’ being the most common sub-category), and these were re-planned at a rate of 24%. Of the remaining 40% of tumor-related variances, none was re-planned.
GU: The majority of GU variances (78%) were non-tumor related (‘bladder/rectum filling’ being the most common sub-category), and these were re-planned at a rate of 14%. Of the remaining 22% of tumor-related variances, none was re-planned.
Radical vs. palliative intent
For the 287 variances reported, 71 (25%) were for patients treated with palliative intent. The proportion re-planned was 20% and 17% for palliative and radical cases respectively.
Compared to all patients (Fig. 2), variances from the 71 palliative patients had a higher proportion of lung (45%) and upper GI (20%) cases and only rare head-and-neck and GU cases (1% each). Most palliative cases were treated with short courses (≤10 fractions), and the median absolute time when first reported was at fraction 3, similar to radical patients treated with short courses (Fig. S1).
The proportion of tumor-related variances was similar within each group (37% for palliative, 44% radical). The distribution of variance sub-categories varied by treatment intent (Supplementary Fig. S3). A lower proportion of ‘tumor regression’, ‘bone mismatch’ and ‘bladder/rectum filling’ and higher proportion of ‘fluids’ were observed with palliative cases (absolute difference in proportions ≥10%). These sub-category differences correlate with more lung/upper GI and fewer head-and-neck/GU cases in the palliative distribution.
Supplementary Fig. S3.
RTTs reported IGRT-related variances for 10 patients (4 radical, 6 palliative intent) who ultimately discontinued radiotherapy. For 7/10 cases the variances described tumor or normal tissue changes on CBCT consistent with deteriorating clinical status and the time to discontinuation time ranged from same-day to 2 days.
Discussion
In this study, RTT-reported IGRT variances in a minority of cases led to substantial changes in the care plan ranging from ad hoc adaptive re-planning to treatment discontinuation. Anatomic changes were often identified within the first few fractions of the treatment course, potentially leaving adequate time for the clinical team to perform ad hoc adaptive re-planning. However, only one fifth were actually re-planned overall. Patterns of practice in terms frequency and type of the variances (tumor or non-tumor related) and the re-planning rate varied by disease site. The clinical decision to re-plan is likely a function of clinical significance (e.g. fast growing sarcoma tumors vs. prostate), specific technique (e.g. margin size) and ability to detect changes on CBCT quality (e.g. lungs having superior soft tissue contrast). Notably, clinical notes describing the potential dosimetric or clinical impact of reacting or not, were not routinely documented. This is a barrier to understanding which patients may benefit the most from interventions. The reliability of many adaptive strategies, including sophisticated online MR-based re-planning, is limited by inter-observer variations in decision making which are larger for visual-only assessment of images [19]. The results from this study capture the baseline variances during CBCT-guided radiation therapy and will inform future process changes and workflows for re-planning.
The language and detail in IGRT-related variance reports were observed to be inconsistent within the categories. Tumor volume increases for example, ranged from objective radiographic description (e.g. “increased intensity in target area”) to subjective response description (e.g. “tumor progression”). RTTs must ensure reporting remains within their scope-of-practice, therefore, standardized language is recommended at our institution going forward for clear and consistent communication with the multidisciplinary team.
Increasingly complex IGRT practices places greater demands on RTT training to ensure accurate and precise treatment delivery, including calls for advanced practice RTTs to enable adaptive radiotherapy [20], [21]. However, several studies have demonstrated that specialized training enables adaptive radiotherapy decision making (e.g. plan-of-the-day selection) to be performed as part of standard RTT practice [22], [23].
Patterns of practice differed across disease-sites, as expected given that IGRT practices are highly site specific. These results will aid in refinement of the site-specific CBCT workflows at our institution. This is particularly true for lung cases that represented over a quarter of all IGRT-reported variances yet were re-planned less frequently, especially for causes such as fluid in lungs. The positive predictive value for identifying re-planned cases were higher for head-and-neck and sarcoma. Notably these sites are unique by specifying objective thresholds for soft tissue changes (i.e. <1 cm of change) within their existing CBCT workflows [16], [24]. Proposed ‘traffic light protocols’ for triaging CBCT review by radiation oncologists [25], combined with the results of this study may reduce unnecessary investigations and increase efficiency of adaptive re-planning. For example, because reports of observed sinus/fluid were relatively common yet never re-planned, they can potentially be disregarded in this context to reduce workload.
Most reports were in the 1st quarter of treatment for suspected tumor progression and motion, or issues related to normal tissues, with sufficient lead-time for re-planning. This also suggests systematic differences in patient geometry between planning and treatment. Further study is warranted to improve the robustness of CT-simulation (e.g. reduce planning to treatment time, improve patient preparation etc.). The exception was suspected tumor regression which occurred progressively later in treatment. This was re-planned less frequently than other tumor-related issues, possibly due to the lower risk of geographic miss or uncertainties in residual microscopic disease.
Patterns of practice were generally similar between patients treated with radical or palliative intent, with both cohorts having similar re-planning rates. Although palliation with radiotherapy often evokes simple and efficient techniques, this result is consistent with the overall increasing technical complexity of techniques used in the palliative setting [26], [27].
The overall prevalence of cases with IGRT-related variances and subsequent re-planning was low (6.2 and 1.4% respectively). Radiation oncologists may be a reluctant to order re-planning given the additional resources required, and currently limited evidence that adaptation directly improves outcomes. This is particularly true for well-studied sites such as head-and-neck, where weight-loss is common [28]. Most re-planning in this study occurred when there was a risk of tumor geographic miss.
Several patients discontinued radiotherapy shortly after RTTs reported variances. Although not diagnostic-quality, radiographic findings on CBCT triggered clinical evaluation by radiation oncologists and led to alteration in the patient’s care plan. This reinforces the key dual RTT responsibilities of patient care and technical specialist, and the importance of coordinating care with other team members.
A limitation of this study is that the number of IGRT-related variances may have been under-estimated. Archived e-mail reports were not available for 3 of 15 treatment units and in some urgent cases RTTs contacted the radiation oncologist for consultation in real-time using other methods. However, additional cases were identified in the record and verify and planning systems and the re-planning frequency was consistent with our prior studies [16], [24]. An unexpected finding was that the clinical impact of anatomic changes on CBCT were rarely described in the patients’ medical records. Therefore, the significance of each variance can only be inferred by its outcome and it is not possible to review consistency across cases or diseases sites.
Our institution recently implemented more structured IGRT-variance reporting and offline 3D assessment of CBCT in both the record and verify and treatment planning systems [29]. Additionally, quarterly RTT peer-review rounds for challenging IGRT cases has been implemented for continuous practice improvements. Processes to reconstruct delivered radiotherapy doses are also being developed, using dose re-calculation on CBCT and deformable dose accumulation, to quantify the dosimetric impact of anatomic variations [30]. These efforts, combined with standardized reporting language by RTTs, will streamline clinical decision-making regarding adaptation for routine CBCT-guided radiotherapy.
Conclusions
RTTs identified a wide range of anatomic variances during CBCT-guided radiotherapy. In a minority of cases, these substantially altered the care plan including ad hoc adaptive re-planning or treatment discontinuation. Improved understanding of the clinical decisions in these cases would aid in developing routine, systematic adaptive strategies.
Declaration of Competing Interest
None.
Acknowledgements
The authors thank Lucy Lu and Jerry Roussos for help with data collection and the multi-disciplinary disease site group and imaging leads for their work.
References
- 1.Jaffray D.A. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–699. doi: 10.1038/nrclinonc.2012.194. [DOI] [PubMed] [Google Scholar]
- 2.Nabavizadeh N., Elliott D.A., Chen Y., Kusano A.S., Mitin T., Thomas C.R. Image guided radiation therapy (IGRT) practice patterns and IGRT's impact on workflow and treatment planning: results from a national survey of American Society for Radiation Oncology members. Int J Radiat Oncol Biol Phys. 2016;94:850–857. doi: 10.1016/j.ijrobp.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 3.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Bujold A., Craig T., Jaffray D., Dawson L.A. Image-guided radiotherapy: has it influenced patient outcomes? Semin Radiat Oncol. 2012;22:50–61. doi: 10.1016/j.semradonc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Yan D., Vicini F., Wong J., Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 6.Tolan S., Kong V., Rosewall T., Craig T., Bristow R., Milosevic M. Patient-specific PTV margins in radiotherapy for bladder cancer - a feasibility study using cone beam CT. Radiother Oncol. 2011;99:131–136. doi: 10.1016/j.radonc.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Murthy V., Master Z., Adurkar P., Mallick I., Mahantshetty U., Bakshi G. 'Plan of the day' adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol. 2011;99:55–60. doi: 10.1016/j.radonc.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Heijkoop S.T., Langerak T.R., Quint S., Bondar L., Mens J.W.M., Heijmen B.J.M. Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT. Int J Radiat Oncol Biol Phys. 2014;90:673–679. doi: 10.1016/j.ijrobp.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Ménard C., van der Heide U. Introduction: Systems for magnetic resonance image guided radiation therapy. Semin Radiat Oncol. 2014;24(3):192–232. doi: 10.1016/j.semradonc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White E., Kane G. Radiation medicine practice in the image-guided radiation therapy era: new roles and new opportunities. Semin Radiat Oncol. 2007;17:298–305. doi: 10.1016/j.semradonc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Harnett N., Moseley D.J., Higgins J., Chan K., Jaffray D.A. Investigating user perspective on training and clinical implementation of volumetric imaging. J Med Imag Radiat Sci. 2010;41:57–65. doi: 10.1016/j.jmir.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Li W., Cashell A., Jaffray D.A., Moseley D. Development and implementation of an electronic learning module for volumetric image-guided radiation therapy. J Med Imag Radiat Sci. 2016;47:43–48. doi: 10.1016/j.jmir.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Jaffray D.A., Wilson G., Moseley D. How long does it take? An analysis of volumetric image assessment time. Radiother Oncol. 2016;119:150–153. doi: 10.1016/j.radonc.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Li W., Appiah S., Hill C., Becker N., Catton C., Chung P. Evidence-based region of interest matching guidelines for sarcoma volumetric image-guided radiation therapy. Tech Innov Patient Support Radiat Oncol. 2018;5:3–8. doi: 10.1016/j.tipsro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerase C., Loudon J., O'Sullivan B. The incidence of treatment modification based on daily cone beam CT assessment for head and neck cancer patients. Radiother Oncol. 2012;104:S41–S42. [Google Scholar]
- 17.Bissonnette J.P., Balter P.A., Dong L., Langen K.M., Lovelock D.M., Miften M. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys. 2012;39:1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 18.Jaffray David A., Langen Katja M., Mageras Gikas, Dawson Laura A., Yan Di, EdD Robert Adams, Mundt Arno J., Fraass Benedick. Safety considerations for IGRT: executive summary. Pract Radiat Oncol. 2013;3(3):167–170. doi: 10.1016/j.prro.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyran M., Jiang N., Cao M., Raldow A., Lamb J.M., Low D. Retrospective evaluation of decision-making for pancreatic stereotactic MR-guided adaptive radiotherapy. Radiother Oncol. 2018;129:319–325. doi: 10.1016/j.radonc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Coffey M., Leech M., Poortmans P. Benchmarking Radiation TherapisT (RTT) education for safe practice: the time is now. Radiother Oncol. 2016;119:12–13. doi: 10.1016/j.radonc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Duffton A., Devlin L., Tsang Y., Mast M., Leech M. on behalf of the RTTC. Advanced practice: an ESTRO RTTC position paper. Tech Innovat Patient Supp Radiat Oncol. 2019;10:16–19. doi: 10.1016/j.tipsro.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foroudi F., Wong J., Kron T., Roxby P., Haworth A., Bailey A. Development and evaluation of a training program for therapeutic radiographers as a basis for online adaptive radiation therapy for bladder carcinoma. Radiography. 2010;16:14–20. [Google Scholar]
- 23.McNair H.A., Hafeez S., Taylor H., Lalondrelle S., McDonald F., Hansen V.N. Radiographer-led plan selection for bladder cancer radiotherapy: initiating a training programme and maintaining competency. Br J Radiol. 2015;88(1048) doi: 10.1259/bjr.20140690. [DOI] [PubMed] [Google Scholar]
- 24.Dickie C., Parent A., Griffin A.M., Wunder J., Ferguson P., Chung P.W. The value of adaptive preoperative radiotherapy in management of soft tissue sarcoma. Radiother Oncol. 2017;122:458–463. doi: 10.1016/j.radonc.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Kwint M., Conijn S., Schaake E., Knegjens J., Rossi M., Remeijer P. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol. 2014;113:392–397. doi: 10.1016/j.radonc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza N., Holden L., Robson S., Mah K., Di Prospero L., Wong C.S. Modern palliative radiation treatment: do complexity and workload contribute to medical errors? Int J Radiat Oncol Biol Phys. 2012;84:e43–e48. doi: 10.1016/j.ijrobp.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S., Hertan L., Jones J. Palliative radiotherapy: current status and future directions. Semin Oncol. 2014;41:751–763. doi: 10.1053/j.seminoncol.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz D.L., Garden A.S., Thomas J., Chen Y., Zhang Y., Lewin J. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–993. doi: 10.1016/j.ijrobp.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naccarato N., Ellis C., Jang H., Kim J., Li W. A picture is not worth a thousand words: implementation of offline volumetric image review. J Med Imag Radiat Sci. 2018;49:S14. [Google Scholar]
- 30.Zhang B., Lee S.-W., Chen S., Zhou J., Prado K., D'Souza W. Action levels on dose and anatomic variation for adaptive radiation therapy using daily offline plan evaluation: preliminary results. Pract Radiat Oncol. 2019;9:49–54. doi: 10.1016/j.prro.2018.08.006. [DOI] [PubMed] [Google Scholar]