Highlights
-
•
HFPV maybe a tool for immobilizing thoracic targets in radiotherapy.
-
•
The procedure itself was well tolerated and well complied.
-
•
Chest wall motion was significantly reduced by greater than 60%.
-
•
HFPV can be greatly advantageous, particularly for SBRT and PBS proton therapy.
-
•
Duty cycle under HFPV was significantly higher than conventional methods.
-
•
The appropriate interface can lead to extensive HFPV prolonged times.
Keywords: Percussive ventilation, Lung cancer radiotherapy, Motion reduction, Pencil beam scanning proton
Abstract
High frequency percussive ventilation (HFPV) employs high frequency low tidal volumes (100–400 bursts/min) to provide respiration in awake patients while simultaneously reducing respiratory motion. The purpose of this study is to evaluate HFPV as a technique for respiratory motion immobilization in radiotherapy. In this study fifteen healthy volunteers (age 30–75 y) underwent HFPV using three different oral interfaces. We evaluated each HFPV oral interface device for compliance, ease of use, comfort, geometric interference, minimal chest wall motion, duty cycle and prolonged percussive time. Their chest wall motion was monitored using an external respiratory motion laser system. The percussive ventilations were delivered via an air driven pneumatic system. All volunteers were monitored for PO2 and tc-CO2 with a pulse oximeter and CO2 Monitoring System. A total of N = 62 percussive sessions were analyzed from the external respiratory motion laser system. Chest-wall motion was well tolerated and drastically reduced using HFPV in each volunteer evaluated. As a result, we believe HFPV may provide thoracic immobilization during radiotherapy, particularly for SBRT and pencil beam scanning proton therapy.
Introduction
Improving immobilization and delivery techniques for thoracic tumors continues to be essential in radiotherapy; particularly for patients undergoing stereotactic body radiotherapy (SBRT), hypo-fractionated regimens and pencil beam scanning proton therapy. Unlike photon radiotherapy, pencil beam scanning proton radiotherapy consists of a 2–3 mm spot that is “raster” scanned across the target one slice at a time. The pencil beam is moved across the field with the aide of fast and slow magnets. The depth is achieved by a range modulator that alters the proton beam energy, shifting the range and Bragg Peak [1].
For stationary targets, the pencil beam scanning proton therapy scans across each slice and creates a highly conformal dose deposition throughout the target. However, significant inhomogeneities have been noted for targets that move during pencil beam scanning proton therapy due to interplay effects [2], [3], [4], [5]. Bert et al., reported that the homogeneity on their radiographic films study was less than 80% for motion amplitudes of about 15 mm. The homogeneity for their treatment-planning study, based on patient data, was on an average 71% for 95% of the target volume. Such drastic interplay effects for targets that move during pencil beam scanning particle radiotherapy, have prompted institutions to employ techniques that would allow for reduction or monitor of target motion.
Undoubtedly the goal in radiotherapy planning continues to be its accurate delivery of radiation to the target while maintaining good sparing of nearby healthy tissue. However, acceptable distribution of absorbed dose within the target, could become compromised when they are mobile. As a result, the International Commission on Radiation Units and measurements (ICRU) Report 50 [6] and the Supplement Report 62 [7] describe the current recommendation for incorporation of the tumor motion into radiation therapy planning. Subsequently, the American Association of Physicist in Medicine (AAPM) Task Group 76a report on the management of respiratory motion in radiation oncology [8] recommends margins around the treatment target be sufficient to ensure coverage of the target. Lastly, in a landmark SBRT trial for early stage inoperable lung cancer, Timmerman et al. [9] used planning target volume (PTV) expansions from the gross tumor volume (GTV) of up to 5 mm in the axial dimension and 10 mm in cranial-caudal direction. Several papers have been published on ways to mitigate motion in order to achieve optimal dose distribution and comply with the above recommendations. Abdominal compression can be inconsistent and in cases increase target motion [10]. Respiratory gating must rely on accurate predictive filters and has low duty cycle [11], [12], [13]. Respiratory tracking can be invasive if fiducial markers are used, but most importantly the correlation of the external surrogate (infrared tracking cubes or body surface tracking via stereo cameras) to internal tumor may not be accurate [14], [4]. Breath hold relies on the ability of the patient to hold their breath during simulation and treatment. As a result, such technique is often reserved for patients who are physiological capable.
The obvious solution to the above challenges would be to immobilize the target itself. High Frequency Percussive Ventilation (HFPV) employs high frequency low tidal volume ventilation to generate endotracheal percussion. The patient is connected to the Intrapulmonary Percussive Ventilation/Impulsator (IPV) device (Percussionaire Corp., Sagle, Idaho) through the phasitron and mouth piece interface (Fig. 1). Initiation of the IPV device will allow the phasitron to deliver small bursts of air into the patients’ trachea. The frequency of the air bursts can be 100–400 bursts per minute. The phasitron is attached to a water reservoir which reduces dry throat, in addition to providing a path for carbon dioxide (CO2) to escape the lungs while continually oxygenating (Fig. 1b).
Fig. 1.
HFPV equipment.
While HFPV is often used in bronchial hygiene therapy and other acute respiratory diseases that allow for sustained improvement of oxygenation and ventilation for such patients [15], recently it was reported as a technique to immobilize thoracic tumors for imaging purposes [16] and stereotactic body radiotherapy [17].
In this study, we recruited fifteen healthy volunteers (age 30–75 y) and investigated various types of interfaces. The aim for this part of our study was to evaluate each interface device for compliance, ease of use, comfort, geometric interference, minimal chest wall motion, duty cycle and prolonged percussive time.
Material and methods
Fifteen healthy volunteers between October 2, 2017 and February 1, 2018 were enrolled in this Beaumont Research Institute Institutional Review Board approved observational study (IRB # 2017-046). Each volunteer signed an informed consent and were entered into the clinical research database prior to the study entry. Volunteers that were unable to tolerate HFPV or required supplemental oxygen, in addition to other medical concerns or pregnancy, were excluded from the study. All volunteers were evaluated lying supine with their arms above their head and a knee sponge for comfort.
Each volunteer was asked to undergo trial periods of HFPV using three different types of oral interface devices: Amici Tru-Fit Mouthpiece Kit with tubing, elbow and nose clips, Fisher & Paykel Oracle 452 mask with straps and nose clips and Phillips Respironics Oro-Nasal mask with head gear (Fig. 2). Each interface connected to the Percussionaire IPV-2C via the phasitron and single use tubing kit. The chest wall motion was monitored using Anzai respiratory monitor laser system (Anzai Medical Co., Tokyo, Japan). The Anzai, is an FDA approved system that utilizes a low power red laser which projects onto the patients’ surface. Each volunteer underwent at least one HFPV session per interface, but multiple sessions were enabled. A typical example of two (Ns = 2) HFPV sessions per interface is denoted in (Fig. 3).
Fig. 2.

HFPV interfaces.
Fig. 3.
An example showing what a typical signal would look like for patient undergoing HFPV via one of the interfaces. Each volunteer undergoing HFPV could’ve resulted in multiple HFPV sessions as indicated in this figure (Ns = 2).
Another important parameter evaluated was the duty cycle. Duty cycle is the ratio of the amount of time that radiation delivery device is on relative to the total treatment time. A threshold band (2 mm and 5 mm), highlighted in (Fig. 7) was created for each of the Ns = 62 HFPV sessions and duty cycle was calculated for each threshold band. A small tcCO2 sensor from SenTec (SenTec AG, Therwil Switzerland) was placed onto the volunteers’ forehead. It is a non-invasive, transcutaneous device that uses thin adhesive membrane attached onto the transducer to gather real time data CO2, partial pressure (tc-PCO2), functional oxygen saturation (SpO2), pulse rate (PR) and pulsation index (PI). All volunteers in this study were monitored via SenTec device.
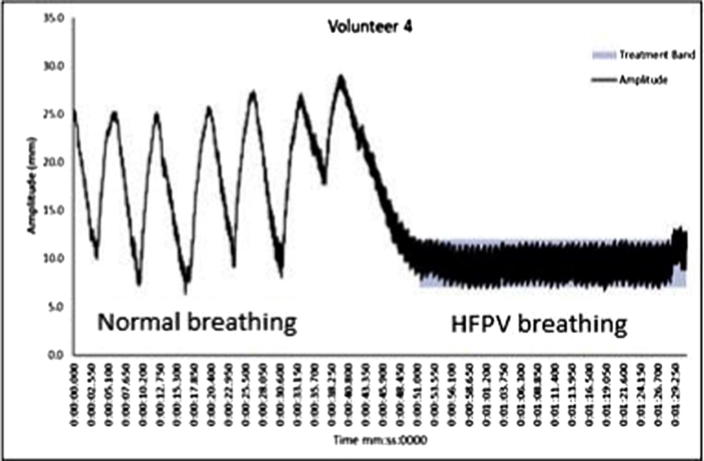
Fig. 7.
Typical chest wall motion recorded by Anzai detector for Volunteer #4. Highlighted section indicates a 5 mm band. The initial section indicates normal breathing followed by HFPV.
We utilized the Percussionaire IPV-2C (Percussionaire Corp., Sagle, Idaho) to provide HFPV bursts. The Percussionaire is pneumatic air driven device (Fig. 1a) that delivers continues positive airway pressure (CPAP) and mini bursts of air (100–400 bursts/minute). The phasitron is connected to the Percussionaire via single use tubing kit. Distilled water was used to provide moisturized air and prevent dry throat.
All volunteers (Nv = 15) completed a Likert scale (1 strongly disagree – 5 strongly agree) subjective survey after each interface. Each subjective self evaluating form constituted of eight linked questions separated into three main domains (Table 1). First domain (Q1,2,5) assessed for comfort, geometric interference and ease of use. Second domain (Q3,4,6,8) assessed for compliance. Third domain assessed for explanation of procedure. All volunteers were also allowed to provide written comments on each survey form. The duration and the number of HFPV sessions for each interface, was left at the discretion of the volunteer. Trained and authorized medical personnel operated all equipment.
Table 1.
Results from the subjective survey/questionnaire.
| Amici TruFit | Fischer Paykel | Phillips Respironics | |
|---|---|---|---|
| Domain 1 - Comfort (Least Square Means) | 3.81 | 3.74 | 4.01 |
| 1. Procedure was comfortable? | 3.63 | 3.66 | 4.03 |
| 2. Procedure was easy? | 4.09 | 4.09 | 4.44 |
| 5. Procedure was easy to tolerate? | 3.72 | 3.47 | 3.56 |
| Domain 2 - Compliance (Least Square Means) | 2.48 | 2.38 | 2.35 |
| 3. I felt shortness of breath? | 2.75 | 2.63 | 2.53 |
| 4. I felt panicked? | 1.63 | 1.44 | 1.38 |
| 6. Position was easy to hold? | 4.53 | 4.44 | 4.38 |
| 8. I felt pain? | 1.00 | 1.00 | 1.13 |
| Domain 3 - Explanation (Least Square Means) | 5.00 | 5.00 | 5.00 |
| 7. Procedure thoroughly explained? | 5.00 | 5.00 | 5.00 |
∗∗∗1 Strongly Disagree and ∗∗∗5 Strongly Agree (Nv = 15).
Several statistical analysis were performed. A one-way Analysis of Variance (ANOVA) test was used to compare mask and different scores on the Likert scales. Additionally, a two-way ANOVA was performed to rule out interaction between questions and mask. A paired t test was used to compare the difference after. Kruskal Wallis test was performed to analyze duty cycle. A Friedman test was utilized to rank the prolonged time for each interface.
Results
All volunteers complied with the instructions and successfully completed multiple sessions of HFPV for each of the three interfaces. All fifteen subjects were able to participate for an hour long session and hence were included in the analysis. The amount of time each person were under HFPV sessions using various masks were left to the discretion and tolerance of the subject. Fig. 4 shows a typical setup of the Fischer Paykel Oracle 452 interface. We evaluated the signals from the Anzai monitoring system for all fifteen volunteers. A total of Ns = 62 of HFPV sessions were analyzed.
Fig. 4.
Typical HFPV setup for Fischer & Paykel Oracle 452 interface with nose clips and CO2 skin monitor.
Significant maturation effects were noted during the first five volunteers with both investigative team and patients learning the techniques involved in using the interface and HFPV. Ns = 25 sessions were recorded for the first five volunteers, but fifteen of them were under sixty seconds. Subsequently, (Ns = 37) sessions were recorded for the last ten volunteers and only four of them were under sixty seconds. Due to these maturation effects in the first five volunteers, only sessions from the last 10 volunteers were used to analyze aim two and four. For aim two we analyzed one session per interface, per volunteer. Totaling Ns = 30. However, one session was excluded as a deviance, which we believe was due to the geometrical fit of interface for the particular volunteer in neutral position. For aim four we elected all sessions from the last ten volunteers Ns = 37 short of the sessions that were under sixty seconds.
The least square mean for Amici Tru-Fit, Fischer & Paykel Oracle 452 and Phillips Respironics Oro-Nasal were 3.81, 3.74 and 4.01 respectively. The second domain, represented by questions 3, 4, 6 & 8 on the survey, evaluated for compliance. The least square mean for Amici Tru-Fit, Fischer & Paykel Oracle 452 and Phillips Respironics Oro-Nasal were 2.48, 2.38 and 2.35 respectively. All three interfaces were reported favorably for comfort with Phillips Respironics Oro-Nasal interface average score higher than the other two. On the other hand, there was no statistical difference between the three interfaces for compliance. The third domain, represented by question 7 on the survey, evaluated for explanation of procedure. All three interfaces were scored very high (5 out of 5), therefore excluded from analysis. Our next aim was to investigate the chest wall motion reduction while under HFPV for all Nv = 10 volunteers. Ns = 29 HFPV sessions were analyzed. A temporal local mean reduction of 65.97% (Ns = 29, median: 71.43%, range: 14.29–87.50%) was observed for the ripple magnitude (peak to peak) between normal breath vs. percussive (Fig. 5). Which constituted to a chest wall motion reduction from an average of 8.14 mm (median: 6.90 mm, range: 2.70–18.6 mm) for normal breathing to an average of 2.52 mm (median: 1.80 mm, range: 0.60–8.40 mm) for percussive breathing. The mean difference before and after HFPV was 6.59 (95% CI 5.09–3.06, p < 0.001). A multi variance regression with significance for reduction in ripple percentage was significant for type of interfaces and baseline ripple value.
Fig. 5.
Amplitude distribution (a) range of % ripple (peak to peak) reduction while in HFPV, (b) chest wall amplitude (peak to peak) for Normal Breath vs. HFPV as measured by Anzai.
Out of Ns = 62 HFPV sessions analyzed, the mean duty cycle for 2 mm threshold band was 55.67% (range: 14.00–100.00%, median: 52.09%). Similarly, the mean duty cycle for 5 mm threshold band was 87.24% (range: 31.85–100.00%, median: 93.03%). No statistical difference was noted between the interfaces with both ANOVA and Kruskal Wallis test. A histogram of both threshold bands is provided in (Fig. 6).
Fig. 6.
Duty cycle distribution for each threshold band Ns = 62 (a) 5 mm, (b) 2 mm.
Lastly, we investigated prolonged percussive time for each volunteer. Out of Ns = 62, the average time in which the volunteers were under HFPV was 199.4 s (range:9 s–16.83 min, Upper 95% CI of mean: 258.8 s, Lower 95% CI of mean: 139.9 s). Additionally, we investigated whether the prolonged percussive time was statistically significant between the three interfaces. A total of Ns = 33 HFPV sessions from the last ten of the fifteen volunteers were analyzed, Ns = 11 for Amici Tru-Fit, Ns = 11 for Paykel Oracle 452 and Ns = 11 for Phillips Respironics Oro-Nasal. Any percussive times less than sixty seconds were omitted from the set calculations. A Friedman test resulted in statistical significance between the three interfaces (p < 0.001). Mean for Amici Tru-Fit was 210.73 s (median: 174.00 s, range: 60–570 s, Upper 95%CI of mean: 311.78 s), mean for Fischer Paykel Oracle 452 was 360.00 s (median: 273.00 s, range: 107.00–897.00 s, upper 95%CI of mean: 524.05 s), mean for Phillips Respironics Oro-Nasal was 400.73 s (median: 258.00 s, range: 68–1010.00 s, Upper 95%CI of mean: 629.29 s) (Table 2).
Table 2.
Duty Cycle (DC) and prolonged HFPV time for all three interfaces.
| Interface/#sessions in HFPV | Mean time in HFPV (s) | Mean DC 5 mm band (%) | Mean DC 2 mm band (%) |
|---|---|---|---|
| Amici TruFit (Np = 10, Ns = 11) | 210.73 | 82.20 | 43.93 |
| Fischer Paykel (Np = 10, Ns = 11) | 360.00 | 79.09 | 39.24 |
| Phillips Respironics (Np = 10, Ns = 11) | 400.73 | 78.96 | 48.75 |
| Total mean time in HFPV for Np = 15, Ns = 62 (s) | 199.37 s | ||
Discussion
All of the results obtained during this study were performed on healthy volunteers. As a result, the reduction of chest wall motion and prolonged HFPV times may not reflect that of a compromised patient.
We selected three different types of oral interfaces, that have been previously used in patients to mobilize and clear their pulmonary secretions. An average of all HFPV sessions (Ns = 62 from all Nv = 15), from all three interfaces, showed that the volunteers could tolerate percussions for an approximate average of 3 min to a maximum of 17 min. These times are promising and allow us to further investigate HFPV as a tool for motion reduction in a typical 15-min radiotherapy appointment.
The CO2 levels for the first five volunteers were normal therefore, we believe that providing detailed training to subjects and team will play an important role in maintaining long, consistent and comfortable HFPV sessions. Particularly for individuals that have no prior experience with aided breathing.
Our first aim for this study was whether or not volunteers would be able to comply with the procedure and subsequently provide feedback with regards to ease of use, comfort and geometric interference. As reported, all three interfaces were favorable for comfort with Phillips Respironics Oro-nasal interface mean score higher than the other two. No volunteer indicated pain or panic during the procedure. Almost all volunteers reported on the comment section of each subjective survey, that Amici tru-Fit mouthpiece was the hardest to hold in place during percussive ventilation due to lack of strap. Majority of the volunteers commented that Respironics Oro-nasal rested well on their face and introduced less dry throat than Fishcer Paykel Oracle 452. However, they indicated that they felt higher leakage for Respironics Oro-nasal than Fischer Paykel.
Motion reduction while in HFPV breathing compared to that of normal breathing, is an important parameter of this study. Particularly, if such technique is implemented for use in pencil beam scanning radiotherapy of mobile targets, which may result in drastic heterogeneous dose distributions [3], [14], [5], [18], etc. Although, during this part of our study we only recorded chest wall motion, we report statistically significant reduction in ripple magnitude. These results are promising and require further research. We noticed that most volunteers experienced a drift in motion amplitude of the chest wall. We believe that such drifts were caused due to the air leaks around the interface, in addition to the wave pulses losing pressure in the buccal space/oral cavity as opposed to directly going into the oropharynx for proper ventilation.
Duty cycle for respiratory gated radiotherapy is typically 30–50% for 3DCRT treatments [19], less than 30% for IMRT treatments [18] and of course 100% for non-gated treatments. The ability to deliver the dose quick after image verification is critical. Murphy et al. [12] estimated that if the imaging interval was approximately 2 min, then the percentage of dose that is misdirected by more than 1 mm was in the order of 11%, whereas 2% of the dose was misdirected by more than 1.5 mm and less than 1% was off target by more than 2 mm. Furthermore, they noticed that if the imaging interval was reduced by 1 min, than the mis-targeting was halved. High frequency percussive ventilation allows us to keep the duty cycle considerably higher than the current means for IMRT and pencil beam scanning proton radiotherapy. A median of 93% duty cycle was observed for 5 mm threshold band and a median of 52% for 2 mm. Since no smoothing filters were applied when collecting the Anzai data (raw data) and the system, like many, is known to have an inherent noise component [20], [21], we believe that the duty cycle for the 2 mm and 5 mm threshold band could be slightly higher than reported here. Example of the noise peaks are denoted in (Fig. 8). Additionally, it is believed that leakage around the interface allowed the volunteers to not be fully ventilated, resulting in the chest wall amplitude drifts noted above. Consequently, resulting in low duty cycle for both 2 mm and 5 mm threshold band.
Fig. 8.
Sample signal of Amplitude drift & noise from Anzai.
Our last aim for this study was to examine the prolonged time under HFPV and whether a particular interface prolonged percussive time more than the other. Recalling that each volunteer underwent at least one percussive session (Fig. 3) per interface (number of sessions was left at the discretion of the volunteer), we recorded a total of Ns = 33 (Nv = 10), 11 sessions per interface that were greater than sixty seconds. Statistical differences were noted between the three interfaces (p < 0.0001). The mean prolonged time for Phillips Respironics oro-nasal was longer than the other two interfaces. We believe that this is due the straps and the ability of the interface to rest comfortably around the patients’ oral space as indicated in the comment section of the subjective survey. However, it is important to note that the duty cycle for the 5 mm threshold band was lower for Phillips Respironics than the other two interfaces. We hypothesis this is due to the indirect path of the percussive bursts, different from Fischer & Paykel Oracle 452. The Amici TruFit allowed for similar direct path, however the lack of straps around the face made it difficult to keep the interface in place for long periods of time.
Conclusions
In this part of the study, we showed that the Fischer & Paykel Oracle 452 interface can prolong the percussive sessions when compared to the other two. Chest-wall motion was well tolerated and drastically reduced using HFPV in each volunteer evaluated. Average chest wall motion during normal breathing and HFPV was measured at 8.14 mm and 2.52 mm respectively. Even though tumor motion may or may not correlate well with that of the chest wall, we expect that the reduction in motion, in addition to prolonged time under HFPV, may translate to tumor immobilization. As a result, we believe HFPV may provide thoracic immobilization during radiotherapy, particularly in the SBRT and scanning pencil beam proton therapy setting.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Acknowledgments
This work was supported by the Radiation Oncology Department at William Beaumont Hospital in Royal Oak, MI. Special thanks to the research nursing staff: Melissa Wienczewski, RN and Joanne Gondert, RN for their dedication during volunteer recruitment. Additionally, this work would not be possible without the efficiency of Monica Abbate.
References
- 1.Newhauser W., Zhang R. The physics of proton therapy. Inst Phys Eng Med. 2015:R155–R209. doi: 10.1088/0031-9155/60/8/R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langen K., Jones D. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 3.Shirato H., Seppenwoolde Y., Kitamura K., Onimura R., Shimizu S. Intrafractional tumor motion: lung and liver. Seminars Radiat Oncol. 2004;14:10–18. doi: 10.1053/j.semradonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Seppenwoolde Y., Shirato H., Kitamura K., Shimizu S., Herk V., Lebesque J. Precise and real time measurement of 3d tumor motion in lung due to breathing and heartbeat measured during radiotherapy. Int J Radiat Oncol. 2002:822–834. doi: 10.1016/s0360-3016(02)02803-1. [DOI] [PubMed] [Google Scholar]
- 5.Bert C., Grozinger S., Rietzel E. Quantification of interplay effects of scanned particle beams and moving targets. Phys Med Biol. 2008;53:2253–2265. doi: 10.1088/0031-9155/53/9/003. [DOI] [PubMed] [Google Scholar]
- 6.ICRUReport50. Prescribing, recording, and reporting photon beam therapy. Internal commission on radiation units and measurements, vol. 1; 1993.
- 7.ICRUReport62. Prescribing, recording, and reporting photon beam therapy supplement to icru report 50. Internal commission on radiation units and measurements, vol. 1; 1999.
- 8.Keall P. Aapmreport76a. Am Assoc Phys Med. 2006;76a:3874–3900. [Google Scholar]
- 9.Timmerman R. Stereotactic body radiation therapy for inoperable early stage lung cancer; 2010. pp. 1070–6. [DOI] [PMC free article] [PubMed]
- 10.Bouilhol G., Ayadi M., Rit S., Thengumpallil S., Schaerer J., Vandemeulebroucke J. Is abdominal compression useful in lung stereotactic body radiation therapy? A 4dct and dosimetric lobe-dependent study. Euro J Med Phys. 2013:333–340. doi: 10.1016/j.ejmp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Verellen D., Depuydt T., Gevaert T., Linthout N., Tournel K., Duchateau M. Gating and tracking, 4d in thoracic tumors. Radiotherapie. 2010:446–454. doi: 10.1016/j.canrad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Murphy M.J. Tracking moving organs in real time. Seminars Radiat Oncol. 2004;91 doi: 10.1053/j.semradonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Keall P., Vedam S., George R., Williamson J. Respiratory regularity gated 4dct acquisition: concepts and proof of principle. Austral Phys Eng Sci Med. 2007;30 doi: 10.1007/BF03178428. [DOI] [PubMed] [Google Scholar]
- 14.Ionascu D., Jiang S., Nishioka S., Shirato H., Berbeco R. Internal and external correlation investigations of respiratory induced motion of lung tumors. Med Phys. 2007;34:3893–3903. doi: 10.1118/1.2779941. [DOI] [PubMed] [Google Scholar]
- 15.Spapen H., Boremans M., Diltoer M., Gorp W., Nguyen D., Honroe P. High frequency percussive ventilation in sever acute respiratory distress syndrome a single center experience. J Anesthesiol Clin Pharmacol. 2014:65–70. doi: 10.4103/0970-9185.125706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prior J., Peguret J., Pomoni A., Pappon M., Zeverino M., Belmondo B. Reduction of respiratory motion during petct by pulsatile flow ventilation a first clinical evaluation. J Nucl Med. 2016:416–419. doi: 10.2967/jnumed.115.163386. [DOI] [PubMed] [Google Scholar]
- 17.Peguret N., Ozsahin M., Zeverino M., Belmondo B., Durham A., Lovis AD. Apnea like suppression of respiratory motion first evaluation in radiotherapy. Radiother Oncol. 2016:220–226. doi: 10.1016/j.radonc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S. Technical aspects of image-guided respiratoion-gated radiation therapy. Med Dosimetry. 2006;31(2):141–151. doi: 10.1016/j.meddos.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Guang-wen L., Xiao-wu D., Zhen-yu Q., Wen-zhao S., Wu-fei C. Investigation on the impact to beam characteristics of a linear accelerator related to duty cycle of respiratory gating. Radiat Meas. 2011;46:1996–1999. [Google Scholar]
- 20.McNamara J. Investigation of two respiratory monitoring systems used for 4dct and respiratory gating; 2008.
- 21.Heinz C, Reiner M, Belka C, Walter F, Sohn M. Technical evaluation of different respiratory monitoring systems used for 4dct acquisition under free breathing 2015; 16(2). [DOI] [PMC free article] [PubMed]