Highlights
-
•
An optimal bladder volume and filling protocol is proposed.
-
•
The current hydration protocol was well-accepted and tolerated.
Keywords: Prostate radiotherapy, Full bladder hydration protocol, Intra-fraction motion, 4D TPUS clarity, Real-time tracking
Abstract
Background and purpose
Inconsistent bladder and rectal volumes have been associated with motion uncertainties during prostate radiotherapy. This study investigates the impact of these volumes to determine the optimal bladder volume.
Materials and methods
60 patients from two Asian hospitals were recruited prospectively. 1887 daily cone-beam computed tomography (CBCT) images were analysed. Intra-fraction motion of the prostate was monitored real-time using a four-dimension transperineal ultrasound (4D TPUS) Clarity® system. The impact of planned bladder volume, adequacy of daily bladder filling, and rectum volume on mean intra-fraction motion of the prostate was analysed. Patients’ ability to comply with the full bladder hydration protocol and level of frustration was assessed using a questionaire. Acute side effects were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and quality of life (QoL) assessed using the International Prostate Symptom Score (IPSS).
Results
The mean (SD) bladder and rectum volumes achieved during daily treatment were 139.7 cm3 (82.4 cm3) and 53.3 cm3 (18 cm3) respectively. Mean (SD) percentage change from planned CT volumes in bladder volume was reduced by 8.2% (48.7%) and rectum volume was increased by 12.4% (42.2%). Linear Mixed effect model analysis revealed a reduction in intra-fraction motion in both the Sup/Inf (p = 0.008) and Ant/Post (p = 0.0001) directions when the daily bladder was filled between 82 and 113% (3rd Quartiles) of the planned CT volumes. A reduction in intra-fraction motion of the prostate in the Ant/Post direction (z-plane) (p = 0.03) was observed when the planned bladder volume was greater than 200 ml. Patients complied well with the hydration protocol with minimal frustration (mean (SD) scores of 2.1 (1.4) and 1.8 (1.2) respectively). There was a moderate positive correlation (0.496) between mean bladder volume and IPSS reported post-treatment urinary straining (p = 0.001).
Conclusions
A planned bladder volume >200 cm3 and daily filling between 82 and 113%, reduced intra-fraction motion of the prostate. The hydration protocol was well tolerated.
Introduction
Prostate radiotherapy accuracy is impacted by the uncertainties of proximal organs at risk (OARs) such as the filling rate and volumes of the bladder and rectum [1], [2]. Since 2014, majority of prostate patients at our centre have been treated using Volumetric Arc Therapy (VMAT) to reduce treatment time and thus intra-fraction motion. In contrast to the static gantry angle and dose rates used in Intensity Modulated Radiation Therapy (IMRT), VMAT is highly sensitive to intra-fractional temporal and anatomical changes due to the intense modulation of treatment parameters such as multi-leaf collimators (MLCs), dose rate and gantry rotation [3], [4]. Hence, maintaining consistent bladder and rectum volumes with daily image-guided setup correction is pertinent in reducing delivery errors.
Previous studies [5], [6] have investigated ways of ensuring bladder volume consistency at the start of each treatment fraction through the adoption of different standardised hydration protocols. Historically at our centre, an empty bladder protocol was practiced based on the assumption it resulted in a consistent bladder volume during prostate treatment resulting in a more stable target volume [7], [8], [9]. However, more recently a full bladder hydration protocol was adopted, given the availability of CBCT and rationale of allowing the displacement of small bowel from the treatment field [6], especially in cases with pelvic nodal irradiation [9].
A full bladder has been shown to reduce inter-fraction setup error when compared to an empty bladder [10], although difficulty maintaining a consistent bladder volume has been reported in the literature. Nakamura et al. [11] and O’Doherty et al. [12] both observed inconsistent bladder volume during the course of radiotherapy when using a full bladder protocol, whilst irregular prostate motion has been attributed to both bladder filling and patient movement [13]. Previous studies have also investigated the impact of rectal diameter on prostate motion [14], [15], [16]. However, in these studies either real-time imaging or bladder volumes was missing in the study design. A desire to quantify these variables in our department triggered our research on the consistency of daily bladder volumes, their impact on prostate motion, and associated treatment related toxicities.
To our knowledge, this is the first Asian study that investigates the relationship between planned/daily bladder and rectum volumes and mean intra-fraction motion of the prostate using the 4D transperienal ultrasound (TPUS) system (Clarity® system, Elekta AB Stockholm, Sweden). Based on the published literature discussed previously [10], [11], [12], we hypothesized that a large planned bladder volume (>200 ml) and consistent bladder filling would be associated with reduced intra-fraction motion. Data was analysed to provide an optimum filling range based on the initial planned bladder volume that can be applied to each daily treatment fraction. Bladder volume consistency was reviewed over the course of treatment, and patient reported satisfaction of the hydration protocol was assessed. The relationships between OAR doses, IPSS, acute gastrointestinal (GI) and genitourinary (GU) toxicities during and after radical radiotherapy were also reported.
Material and methods
This was a two-institutional prospective study consisting of 60 patients who were part of a study to evaluate of the use of a non-invasive 4D ultrasound Clarity® system in real-time tracking of the target volume in prostate IMRT/VMAT therapy. Our evaluation of the TPUS system has already been published [17], and this paper focusses on the impact of bladder volume on prostate motion. Ethics approval was obtained through the local ethics committee in November 2014 and informed consent was obtained from each patient. The study is registered on the National Institutes of Health (NIH) clinical trial registry (ID: NCT02408497).
Patient characteristics
All patients underwent routine CT simulation where they were scanned in the supine position using a 4D TPUS Clarity® knee rest. 55 patients from the National Cancer Centre Singapore (NCCS) were treated using a Trilogy® linear accelerator (Varian Medical System, PA) and five (5) patients from Tuen Mun Hospital (TMH), Hong Kong, were treated with an Elekta Synergy® system (Elekta system, USA). CT images slice thickness was acquired at 2.5–3 mm with 60 cm field-of-view (FOV). Four-point tattoos on skin were used for setup and positioning of each patient. All patients received volumetric modulated arc therapy (VMAT) with a prescription of 74–78 Gy to the prostate delivered in 2 Gy per fraction. Dosimetric parameters of the bladder (V60 (non-constrained), V65 ≤ 30%, V70 ≤ 50%) and rectum (V50 ≤ 60%, V60 ≤ 50%, V65 ≤ 35%, V70 ≤ 25%) were used. Treatment was delivered five times a week for 37 (NCCS)-39 (TMH) fractions using 10 megavoltage (MV) x-rays at a dose rate of 400–600 monitor units per minute (MU/min). Intra-fraction motion of the prostate was monitored daily using the 4D TPUS system. At NCCS, 34 out of 55 patients underwent whole pelvis radiotherapy and 21 received prostate-only (± seminal vesicles) radiotherapy. At TMH, all patients received prostate-only (± seminal vesicles) radiotherapy.
Bladder and rectal preparation
At NCCS, all patients undergoing radiotherapy to the prostate were educated on a hydration protocol. Patients were educated on the need to aim for a reasonably full, yet comfortable, bladder volume that they felt they could consistently reproduce during the course of their treatment. As a guide to help them achieve this, at their planning appointment patients were instructed to empty their bladder, and then drink between 2 and 3 cups of water (400–600 ml) 30–60 min before the procedure. Thereafter, each patient was instructed to drink this same amount of water within the same time duration before each of their daily treatments. At TMH, the same hydration protocol was implemented, but an additional ultrasound bladder scan was used to evaluate bladder volume during the initial CT scan and each subsequent treatment. No specific rectal emptying or dietary advice was given, but patients were encouraged to empty their bowels prior to each daily treatment.
Image registration and CBCT analysis
Daily rigid image registration was performed using the bony match algorithm on the Varian on-board imager (OBI) (NCCS) and Elekta X-rays volume imaging (XVI) (TMH) consoles followed by manual registration through visualisation of the soft tissues. A total of 1887 cone-beam computed tomography (CBCT) were contoured and analysed (37–39 CBCTs acquired for each patient) to assess bladder and rectal volumes. According to the Radiation Therapy Oncology Group (RTOG) contouring guidelines, the bladder was contoured from the base to dome and the rectum was delineated from the level of recto-sigmoid flexure superiorly to the ischial tuberosities inferiorly [18]. In order to eliminate inter-observer variations, all the bladder and rectal volumes from both institutions were contoured by an independent observer (Radiation Oncologist). The contouring process was performed on each of the CBCT on the Varian Eclipse treatment planning system (NCCS) to reflect the daily bladder and rectal volumes at the start of each fraction. Changes in daily bladder and rectum volumes were recorded and compared against the planned CT volumes.
Daily 4D TPUS monitoring of prostate motion
Intra-fraction monitoring in this study was observed real-time using an autoscanning TPUS probe at a frame rate of 3–4 data points per second depending on the depth and scan angle for each patient. The acquisition process of TPUS images was described by the authors in an earlier paper [17]. Intra-fraction motion was recorded from the beginning of treatment (after couch position was applied based on CBCT) until the beam-off time. For the analysis, motion data was normalised at the beginning of the treatment to reflect the true motion occurred during the treatment.
Hydration survey, treatment toxicities and IPSS
In order to investigate the acceptance rate of the hydration protocol by the patients, a visual analogue scale (VAS) of 1–10 (i.e. 1 being the most tolerable and 10 being very intolerable) was used [5]. This was administered on a weekly basis (seven-time points) throughout the course of treatment to assess the patient’s perception on the hydration protocol (Appendix A). The survey was designed to assess the ability of patient to comply with the bladder hydration protocol and the associated level of frustration.
Patients were reviewed at three-time points (CT simulation, mid-treatment and the first post-treatment review) to assess the onset of acute GI and GU side effects. This scoring criterion was designed based on the guidelines from CTCAE version 3.0 [19]. In addition, IPSS was reported at three time points (CT simulation, mid-treatment and the first post-treatment review) to elicit patient’s overall perceived quality-of-life (QoL). The IPSS questionnaire consisted of 8 questions (7 symptom questions and 1 QoL question) [20]. This IPSS is a validated questionnaire, also employed in a recent clinical trial ICORG 05-04 conducted by Saint Luke’s Hospital, Ireland to compare two sets of bladder-filling protocols [5].
Statistical analysis
The mean and range of the daily bladder and rectal volumes (cm3) were reported with reference to the planned volumes at CT simulation. The bladder and rectum were contoured on the daily pre-treatment CBCT images and the consistency of OAR volumes were analysed. A Linear Mixed Effect model (performed using the nlme package in R software v.3.2.5.) was used to analyse the impact of bladder and rectum volumes (covariates include planned bladder volume <200 cm3 and >200 cm3, percentage change in daily bladder volume and rectum volume) on intra-fraction motion of the prostate. 200 cm3 was selected as the threshold as it represented 80 percentile of the daily bladder volume in the cohort and is consistent with volumes that have been previously reported as reasonably reproducible [6].
Severity of acute radiation-induced GI/GU toxicities and overall IPSS were correlated to the dosimetric parameters of the bladder and rectum. Differences in the sub-score of IPSS and acute side effects across the 3-time points were analysed using Kruskal Wallis and a Mann-Whitney U Test was used as Post-Hoc to determine the pair-wise difference between three groups. Spearman’s correlation was used to investigate the relationship between mean bladder and rectum volumes and observed acute side effects and sub-categories within the IPSS. These statistical analyses were performed at 95% significance level using PASW for Windows, version 20.0 (SPSS Inc, Chicago, IL).
Results
Patient characteristics, and consistency of bladder and rectal volume
A summary of the patient characteristics can be found in Table 1. A total of 1887 CBCT were analysed and the mean (SD) daily bladder and rectum volume calculated as 139.7 cm3 (82.4 cm3) and 53.3 cm3 (18 cm3) respectively. The mean (SD) bladder volume reduced by 8.2% (48.7%) and rectum volume increased by 12.4% (42.2%) with respect to the CT simulation scan respectively. 20 out of 60 patients had achieved a planned bladder volume of the selected threshold >200 cm3.
Table 1.
Patient characteristics.
| Characteristic | Value | % |
|---|---|---|
| Total | 60 | 100 |
| Age at diagnosis (years) | ||
| Median | 71 | |
| Range | 56–80 | |
| Prostate specific antigen (ng/dL) | ||
| Median | 14.95 | |
| Range | 3.2–1032 | |
| Gleason score | ||
| ≤6 | 5 | 8.4 |
| 7 | 26 | 43.3 |
| 8 and above | 29 | 48.3 |
| Median | 7 | |
| Range | 6–9 | |
| Tumor stage | ||
| T1 | 23 | 38.3 |
| T2 | 16 | 26.7 |
| T3 | 19 | 31.7 |
| T4 | 2 | 3.3 |
| NCCN risk group | ||
| Low | 0 | 0 |
| Intermediate | 16 | 26.7 |
| High | 44 | 73.3 |
| Radiation field | ||
| Prostate only (+/−SV) | 26 | 43.3 |
| Prostate and pelvis | 34 | 57.7 |
| Dose/fraction (Gy/#) | ||
| 74 Gy/37# | 55 | 91.7 |
| 78 Gy/39# | 5 | 8.3 |
| Androgen deprivation therapy | ||
| No | 1 | 1.6 |
| Short term (3–6 months) | 16 | 26.7 |
| Long term (2–3 years) | 43 | 71.7 |
Impact of bladder and rectum volumes on intra-fraction motion of the prostate
To facilitate an analysis of the effect of bladder filling on intra-fraction motion, the percentage of the daily bladder volume obtained with reference to the planned volume at CT was categorised into 4 groups based on the calculated interquartile range (<58%, 58–82%, 82–113% and >113%). The Linear Mixed Effect model revealed an inverse relationship between planned bladder volume greater than 200 cm3 and intra-fraction motion of the prostate in the Anterior/Posterior direction (z-plane) (p = 0.03) (Fig. 1). There was a reduction in intra-fraction motion in both Sup/Inf (p = 0.008) and Ant/Post (p = 0.0001) directions when the daily bladder was filled between 82 and 113% (3rd Quartiles) (Fig. 2), independent of the planned bladder volume. This effect of rectal volume on all directions of intra-fraction motion was not statistically significant (p > 0.05).
Fig. 1.
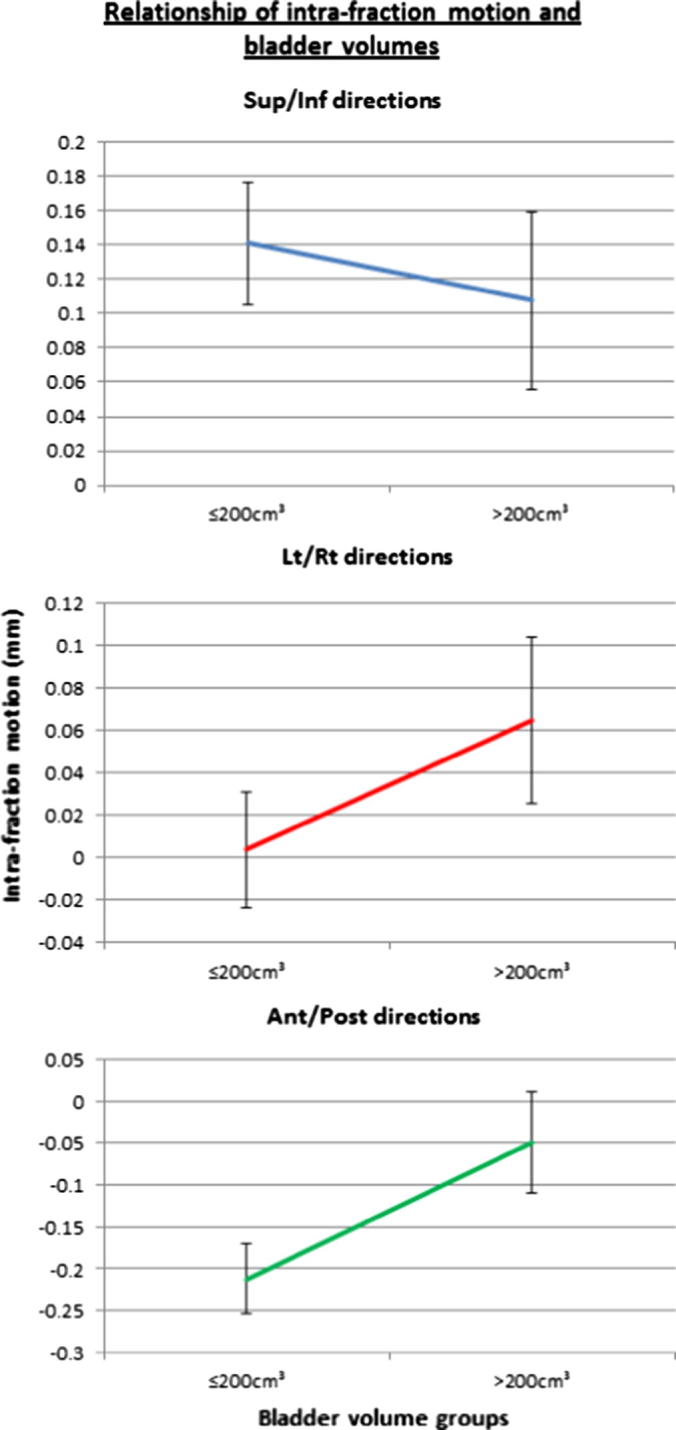
Convergence of lines (Sup/Inf and Ant/Post) illustrates the tendency of intra-fraction shifts towards zero in the group with bladder volume above 200 cm3.
Fig. 2.
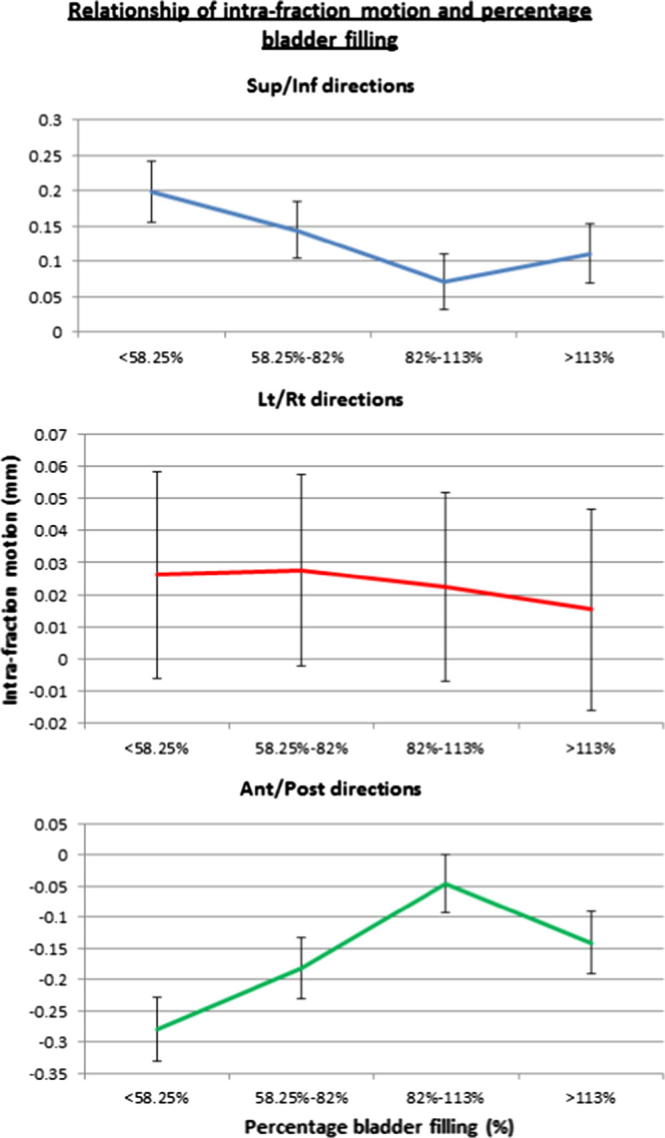
Convergence of lines (Sup/Inf and Ant/Post) illustrates the reduction of intra-fraction shifts towards zero when daily bladder volume falls within the range of 82–113%.
Patient satisfaction survey, IPSS and acute side effects
The population reported a relatively low overall mean (SD) score of 2.1 (1.4) and 1.8 (1.2) on the ability to comply with the hydration protocol (where 1 = Very tolerable and 10 = Very intolerable) and the level of frustration respectively (where 1 = No frustration and 10 = Very high). The mean (SD) score for each event on IPSS and acute GI and GU toxicity is summarised in Table B1 (Appendix B). For IPSS and QOL scores, only ‘incomplete emptying’ has demonstrated a statistical significant difference in IPSS sub-scores between Pre vs treatment and Pre vs post-treatment time points (p < 0.05). Mann-Whitney U Test was used as Post-Hoc to determine the pair-wise difference between the 3-timepoints (Table 2). The overall incidence and severity of acute toxicity data is summarised in Table 3.
Table 2.
Illustrate the results from Post-hoc Mann-Whitney U Test to determine the pair-wise difference for the IPSS and acute GI and GU toxicity subscale at 3-timepoints (displaying only statistically significant pairwise comparison).
| Test statisticsa |
||||||||
|---|---|---|---|---|---|---|---|---|
| Incomplete emptying |
Diarrhoea |
Urinary_retention |
GI |
|||||
| Pre vs tx | Pre vs Post-tx | Pre vs tx | Pre vs Post-tx | Pre vs tx | Pre vs Post-tx | Pre vs tx | Pre vs Post-tx | |
| Mann-Whitney U | 1504.500 | 1563.500 | 1504.500 | 1563.500 | 1559.500 | 1487.500 | 1470.000 | 1530.000 |
| Wilcoxon W | 3274.500 | 3333.500 | 3274.500 | 3333.500 | 3389.500 | 3317.500 | 3300.000 | 3360.000 |
| Z | −3.080 | −2.692 | −3.080 | −2.692 | −1.633 | −2.312 | −3.463 | −3.105 |
| Asymp. Sig. (2-tailed) | 0.002 | 0.007 | 0.002 | 0.007 | 0.103 | 0.021 | 0.001 | 0.002 |
Grouping variable: timepoints.
Table 3.
Summary of incidence and severity of acute GI and GU toxicities assessed using CTCAE.
| Toxicities | Grade | Time points |
||
|---|---|---|---|---|
| Pre-treatment# | Mid-treatment | Post-treatment | ||
| Diarrhoea | 0 | 59 (100%) | 51 (85.0%) | 53 (88.3%) |
| 1 | 0 | 8 (13.3%) | 6 (10.0%) | |
| 2 | 0 | 1 (1.7%) | 1 (1.7%) | |
| 3 | 0 | 0 | 0 | |
| Bladder Spasms | 0 | 56 (94.9%) | 56 (93.3%) | 56 (93.3%) |
| 1 | 2 (3.4%) | 3 (5.0%) | 3 (5.0%) | |
| 2 | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | |
| 3 | 0 | 0 | 0 | |
| Incontinence, urinary | 0 | 50 (84.7%) | 53 (88.3%) | 52 (86.7%) |
| 1 | 9 (15.3%) | 7 (11.7%) | 8 (13.3%) | |
| 2 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | |
| Urinary frequency | 0 | 26 (44.1%) | 18 (30.0%) | 29 (48.3%) |
| 1 | 20 (33.9%) | 30 (50.0%) | 26 (43.3%) | |
| 2 | 13 (22.0%) | 10 (16.7%) | 5 (8.3%) | |
| 3 | 0 | 2 (3.3%) | 0 | |
| Urinary retention | 0 | 44 (74.6%) | 52 (86.7%) | 55 (91.7%) |
| 1 | 14 (23.7%) | 7 (11.7%) | 2 (3.3%) | |
| 2 | 1 (1.7%) | 1 (1.7%) | 3 (5.0%) | |
| 3 | 0 | 0 | 0 | |
Pre-treatment data for one patient was unavailable.
Association of bladder and rectum volume on side effects and IPSS
The Spearman’s rho coefficient revealed no correlations between the mean bladder or rectum volumes and observed GI/GU side effects. Likewise, no correlation was found between the mean bladder or rectum volumes against IPSS. However, there was a moderate positive correlation (0.496) between mean bladder volume and post-treatment straining to commence urination in IPSS (p = 0.001). Kruskal Wallis analysis demonstrated statistical significant difference in the 3-time points for Diarrhoea, Urinary retention and total GI score (p = 0.003–0.045) (Table B1). The Post-hoc Mann-Whitney U test illustrated the pair-wise difference between the 3-time points for each sub-score (p = 0.001–0.021) (Table 2).
Impact of OAR dose parameters on GI/GU toxicities and IPSS
11 out of 60 patients exceeded the V70 dose constrains and 5 out of those had their bladder dose constrains exceeding 50% for V65. All 11 patients had planned bladder volume <200 cm3. All the patients’ rectal dose constrains were within limits except 3 out of 60 patients had exceeded 60% for V50. Spearman’s rho correlation coefficient reveals no significant correlation between mid- or post-treatment GU side effects with all bladder dose parameters. A weak-to-moderate negative correlation (−0.254 to −0.451) was found with IPSS sub-score on incomplete bladder emptying (during and post), urinary frequency (during), intermittency (post) and nocturia (during) for all bladder dose parameters (p < 0.05). A moderate negative correlation was also found with the mean bladder volume (−0.529 to −0.574) (p = 0.0001). No significant correlation existed between mid- or post-treatment GI side effects with rectum dose parameters. However, a moderate negative correlation was also found with the mean rectum volume (−0.3 to −0.389) (p = 0.002–0.02).
Discussion
Effect of bladder and rectum volume on mean intra-fraction motion of the prostate
We found that a planned bladder volume greater than 200 cm3 was associated with a small reduction in the mean intra-fraction prostate motion in the Ant/Post direction (p = 0.03). Fig. 1 illustrated a reduction in the mean intra-fraction motion in Sup/Inf direction as well; however, it was not statistically significant. The mean duration of a VMAT treatment in this study was 3.37 min (pre-treatment imaging and verification was 4.21 min). These results are consistent with the planned bladder volume range (150–300 cm3) recommended by Eminowicz et al. [21] for cervix radiotherapy. Fujioka et al. [6] recently reported that patients who did not require hydration interventions (i.e. readjust the timing of last void or timing and amount of water to drink) had a mean planned bladder volume of 196 cm3. A minimum 100 cm3 bladder volume was recommended in view of dosimetric advantages when the small bowel is displaced from the prostate [6]. More importantly, they reported the optimal bladder volume to be between 100 and 250 cm3 in terms of volume consistency between treatment planning and daily treatment [6]. This is congruent with our study suggesting a minimum planned bladder volume of 200 cm3. The minimum bladder volume could be assessed prior to CT simulation using a ultrasound bladder scanner.
In terms of bladder filling, there was a reduction in mean intra-fraction motion in the Sup/Inf (p = 0.0084) and Ant/Post directions (p = 0.0001) when the bladder volume was reproduced within a range of 82–113% regardless of the initial planned bladder volume. In general, patients tolerated the hydration protocol well. Setting an optimal bladder volume range is consistent with previous recommendations based on evidence that once bladder volumes exceed the upper bound of the optimal range organ motion increases [21]. Hence, a comfortably filled bladder that is reproduced with minimum of 80% and maximum of 113% may have added benefit in reducing mean intra-fraction motion. Although the effect of rectal volume on intra-fraction motion of prostate was not statistically significant (p > 0.05 in all directions), there may be a need to investigate its impact on the seminal vesicles motion in future.
Impact of bladder and rectum volume and dose parameters on side effects and IPSS
Patients tolerated the treatment well with reported rates of 1.7% and 5–8.3% for acute Grade 2 GI and GU toxicities at post-treatment respectively. Acute Grade 2 urinary frequency symptom improved from 22% at pre-treatment to 8.3% at post-treatment (Table 3). Mean bladder and rectum volumes did not correlate with either acute GI/GU side effects or IPSS scores. Larger bladder volumes were associated with the onset of post-treatment urinary straining (p = 0.001), however, this does not necessarily extrapolate to support a conclusion that a smaller bladder volume (<200 ml) is optimal. Smaller bladder and rectum volumes would naturally result in an inverse relationship with the respective dose parameters since a higher percentage of these volumes would be within the high dose region, and as such, could lead to higher IPSS scores in other areas. Interestingly, increased V70, V65 and V60 of the bladder in our study were associated with reduced IPSS sub-scores on incomplete bladder emptying, urinary frequency, intermittency and nocturia.
In our study, the mean (SD) bladder volume obtained during treatment was only 137.9 cm3 (82.4 cm3) slightly lower than the volume of 162 cm3 (98 cm3) reported by Mullaney et al. [5]. However, we observed the same inconsistent bladder volumes during treatment despite the use of hydration protocols. Bladder volume inconsistency during treatment could be attributed to the incomplete emptying symptom as reflected in the slight elevated IPSS score (p = 0.002) before and during treatment (Table 2). In general, there was some improvement of the side effects and IPSS (except weak stream) at post-treatment time points compared to pre-treatment (Table B1). Mullaney et al. [5] previously reported the associated side effects for patients treated with different hydration protocols (1080 ml versus 540 ml) and concluded that 540 ml was the preferred hydration protocol in consideration of the associated side effects, patient acceptance and QoL. This water consumption supports our existing hydration protocol which ranged from 400 to 600 ml.
Limitations and future directions
Although acute toxicities were assessed, this study was limited to a short follow up and did not account for the impact of pre-treatment clinical procedures which may have been a contributing factor. For instance, previous transurethral resection of the prostate (TURP) is one of the main independent predictors for late urinary incontinence elicited from a multivariate- analysis by Cozarini et al. [22]. Moving forward, this study has suggested a minimum threshold of bladder-filling and volume to minimise intra-fraction motion. However, diet and bowel preparation was not strictly regulated and could explain for the increase in the mean rectum volume by 12.4% from the planned volume. Lastly, the growing interest in the use of a hydrogel-based spacer [23], [24] to reduce rectal toxicities may also affect the optimal hydration policy.
Conclusion
A planned bladder volume greater than 200 cm3 with adequate daily filling between 82 and 113%, resulted in an immobilisation effect and reduction in intra-fraction motion of the prostate. The current hydration protocol of 2–3 cups of water 30–60 mins prior to treatment was well-accepted and tolerated.
Conflict of interest
The authors do not have any potential conflicts of interest.
Acknowledgements
The authors express their appreciation to Elekta Pte Ltd for providing the 4D TPUS Clarity equipment and in-house training. We thank Mr David Cooper, Clinical Researcher from Elekta Pte Ltd for the technical advice and validation of the output data from the Clarity US system. We would like to thank the Radiation Oncologists for facilitating the consent process and assessment of the acute side effects. We would like to thank our Physics team (Dr James Lee, Mr Ang Khong Wei and Ms Wendy Chow) for the technical support on installation and commission of the equipment. We also thank the radiation therapists involved in the treatment process. We are thankful for the IT support provided by Mr Gan Soon Ann and Mr Peter Huang, from the Department of Cancer Informatics, National Cancer Centre Singapore. The authors extend appreciation of clinical trial facilitation by Ms Ma Than Than and Ms Yeo Sook Kwan. We thank Tuen Mun Hospital, Hong Kong, for their collaborative work; Mr Matthew Wong and Ms Tong Man for the administration support required for data collection and transfer.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tipsro.2018.01.003.
Appendix A. Supplementary material
References
- 1.Gill S., Thomas J., Fox C. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat Oncol. 2011;6:145. doi: 10.1186/1748-717X-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggio A., Gabriele D., Garibaldi E. Impact of a rectal and bladder preparation protocol on prostate cancer outcome in patients treated with external beam radiotherapy. Strahlenther Onkol. 2017;193:722–732. doi: 10.1007/s00066-017-1163-4. [DOI] [PubMed] [Google Scholar]
- 3.Ravkilde T., Keall P.J., Grau C., Hoyer M., Poulsen P.R. Time-resolved dose distributions to moving targets during volumetric modulated arc therapy with and without dynamic MLC tracking. Med Phys. 2013;40:111723. doi: 10.1118/1.4826161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyagi N., Yang K., Gersten D., Yan D. A real time dose monitoring and dose reconstruction tool for patient specific VMAT QA and delivery. Med Phys. 2012;39:7194–7204. doi: 10.1118/1.4764482. [DOI] [PubMed] [Google Scholar]
- 5.Mullaney L.M., O’Shea E., Dunne M.T. A randomized trial comparing bladder volume consistency during fractionated prostate radiation therapy. Pract Radiat Oncol. 2014;4:e203–e212. doi: 10.1016/j.prro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka C., Ishii K., Yamanaga T. Optimal bladder volume at treatment planning for prostate cancer patients receiving volumetric modulated arc therapy. Pract Radiat Oncol. 2016;6:395–401. doi: 10.1016/j.prro.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Miralbell R., Ozsoy O., Pugliesi A. Dosimetric implications of changes in patient repositioning and organ motion in conformal radiotherapy for prostate cancer. Radiother Oncol. 2003;66:197–202. doi: 10.1016/s0167-8140(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 8.Siow T.R., Ngoi C.L., Tan W.K. Inter-fraction prostate motion during intensity-modulated radiotherapy for prostate cancer. Singapore Med J. 2011;52:405–409. [PubMed] [Google Scholar]
- 9.Pinkawa M., Asadpour B., Siluschek J. Bladder extension variability during pelvic external beam radiotherapy with a full or empty bladder. Radiother Oncol. 2007;83:163–167. doi: 10.1016/j.radonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Poh S., Pang E., Simmons K. EP-1304: comparing magnitude of inter-fraction movement in prostate cancer patients treated with full versus empty bladder. Radiother Oncol. 2015;115:S703. [Google Scholar]
- 11.Nakamura N., Shikama N., Takahashi O. Variability in bladder volumes of full bladders in definitive radiotherapy for cases of localized prostate cancer. Strahlenther Onkol. 2010;186:637–642. doi: 10.1007/s00066-010-2105-6. [DOI] [PubMed] [Google Scholar]
- 12.O'Doherty Ú.M., McNair H.A., Norman A.R. Variability of bladder filling in patients receiving radical radiotherapy to the prostate. Radiother Oncol. 2006;79:335–340. doi: 10.1016/j.radonc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Butler W.M., Merrick G.S., Reed J.L., Murray B.C., Kurko B.S. Intrafraction displacement of prone versus supine prostate positioning monitored by real-time electromagnetic tracking. J Appl Clin Med Phys. 2013;14:4141. doi: 10.1120/jacmp.v14i2.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oates R., Gill S., Foroudi F. What benefit could be derived from on-line adaptive prostate radiotherapy using rectal diameter as a predictor of motion? J Med Phys. 2015;40:18–23. doi: 10.4103/0971-6203.152237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oates R., Brown A., Tan A. Real-time image-guided adaptive-predictive prostate radiotherapy using rectal diameter as a predictor of motion. Clin Oncol. 2017;29:180–187. doi: 10.1016/j.clon.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Wang K.K., Vapiwala N., Bui V. The impact of stool and gas volume on intrafraction prostate motion in patients undergoing radiotherapy with daily endorectal balloon. Radiother Oncol. 2014;112:89–94. doi: 10.1016/j.radonc.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Pang E.P.P., Knight K., Baird M., Tuan J.K.L. Inter- and intra-observer variation of patient setup shifts derived using the 4D TPUS Clarity system for prostate radiotherapy. Biomed Phys Eng Express. 2017;3:025014. [Google Scholar]
- 18.Gay H.A., Barthold H.J., O'Meara E. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–62. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Cancer Therapy Evaluation Program: common terminology criteria for adverse events, version 3.0, <https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf>; 2006 [accessed 15 Jan 2015].
- 20.Barry M.J., JrFJ Fowler., O'Leary M.P. The American Urological Association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 21.Eminowicz G., Rompokos V., Stacey C., Hall L., McCormack M. Understanding the impact of pelvic organ motion on dose delivered to target volumes during IMRT for cervical cancer. Radiother Oncol. 2016;122:116–121. doi: 10.1016/j.radonc.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Cozzarini C., Rancati T., Palorini F. Patient-reported urinary incontinence after radiotherapy for prostate cancer: quantifying the dose-effect. Radiother Oncol. 2017;125:101–106. doi: 10.1016/j.radonc.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Muller A.C., Mischinger J., Klotz T. Interdisciplinary consensus statement on indication and application of a hydrogel spacer for prostate radiotherapy based on experience in more than 250 patients. Radiol Oncol. 2016;50:329–336. doi: 10.1515/raon-2016-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamstra D.A., Mariados N., Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: final results of a Phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


