Abstract
Background
Patients with cancer and severe aortic stenosis are often ineligible for surgical aortic valve replacement (SAVR). Patients with cancer may likely benefit from emerging transcatheter aortic valve replacement (TAVR), given its minimally invasive nature.
Methods and Results
The US‐based National Inpatient Sample was queried between 2012 and 2015 using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), codes to identify all hospitalized adults (aged ≥50 years), who had a primary diagnosis of aortic stenosis. We examined the effect modification of cancer on the relative use rate, outcomes, and dispositions associated with propensity‐matched cohort TAVR versus SAVR. Overall, 47 295 TAVRs (22.6% comorbid cancer) and 113 405 SAVRs (15.2% comorbid cancer) were performed among admissions with aortic stenosis between 2012 and 2015. In the year 2015, patients with cancer saw relatively higher rates of TAVR use compared with SAVR (relative use rateTAVR versus relative use rateSAVR, 67.8% versus 57.2%; P<0.0001). Among patients with cancer, TAVR was associated with lower odds of acute kidney injury (odds ratio, 0.64; 95% CI, 0.54–0.75) and major bleeding (odds ratio, 0.44; 95% CI, 0.38–0.51]), with no differences in in‐hospital mortality and stroke compared with SAVR. In addition, TAVR was associated with higher odds of home discharge (odds ratio, 1.92; 95% CI, 1.68–2.19) compared with SAVR among patients with cancer. Lower risk of acute kidney injury was noted in cancer versus noncancer (P<0.001) undergoing TAVR versus SAVR in effect modification analysis.
Conclusions
TAVR use has increased irrespective of cancer status, with a greater increase in cancer versus noncancer. In patients with cancer, there was an association of TAVR with lower periprocedural complications and better disposition when compared with patients undergoing SAVR.
Keywords: aortic valve replacement, epidemiology, oncology, transcatheter aortic valve
Subject Categories: Epidemiology, Catheter-Based Coronary and Valvular Interventions, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Quality and Outcomes,
Clinical Perspective
What Is New?
Compared with patients without cancer, the use of transcatheter aortic valve replacement in patients with cancer has steadily increased.
In patients with cancer, there was an association of transcatheter aortic valve replacement with lower risk of acute kidney injury, lower length of stay, and higher likelihood of discharge to home when compared with patients undergoing surgical aortic valve replacement.
What Are the Clinical Implications?
These findings suggest that transcatheter therapies have improved access to aortic valve replacement in a population at high risk for surgery and offered an additional option for patients who may not have been previously eligible for therapy.
Cardiovascular disease and cancer represent 2 of the largest contributors to mortality worldwide.1 Aortic stenosis (AS), with an incidence of 2% to 7% in elderly individuals, affects almost half of all patients with valvular heart disease.2, 3 Among those with cancer, the prevalence of AS is even higher than that observed in the general population, and this is only expected to continue to increase with time.4, 5, 6, 7, 8 Much of this increase coincides with the increasing life expectancy of patients with cancer in the era of rapid proliferation of novel anticancer therapies, many of which have been linked with cardiotoxic adverse effects on the valvular structure and function. Compounding this is the progressively increasing use of radiotherapy, an intervention known to accelerate the disruption of cardiac valve architecture and function.4, 5, 6, 7, 8 Novel cancer therapies as well as increasing use of radiotherapy over time have led to a sharp increase in cancer survival7; thus, treating AS has become even more relevant, especially because most patients with AS die within a few years of onset of symptoms.8 The prognosis of nonsurgically treated AS remains poor.8 In fact, aortic valve replacement (AVR) is currently the only widely available definitive treatment known to improve hemodynamic and functional status, in addition to clinical survival among those with AS.9
Available data suggest that patients with AS and a concurrent cancer, irrespective of prognosis, may not receive the same contemporary management strategies seen among populations without cancer.8 Despite a 1‐year mortality of 50% in those not undergoing AVR for severe AS, those with severe AS with concurrent cancer are often denied surgical AVR (SAVR) because of concern for surgical risk. Approved in 2011, transcatheter AVR (TAVR) has changed the landscape in the treatment of severe AS, as patients in the past considered to be inoperable or high risk for SAVR can now be treated with minimal risk with this new invention.10, 11, 12, 13, 14, 15 Among patients with severe, symptomatic AS who were at low surgical risk, TAVR compared with conventional surgery significantly reduced the primary end point of death, stroke, and rehospitalizations by 46% at 1 year.16 Yet, it is currently unknown whether patients with cancer have shared the same benefits of TAVR.
Methods
Data Source
The data that support the findings of this study are available from the corresponding author on reasonable request. The National Inpatient Sample (NIS)17 is an inpatient database in the United States developed by the Agency for Healthcare Research and Quality. NIS is a self‐weighted, stratified, systematic, random sample of 20% discharges from all hospitals (100%) in the sampling frame, after sorting discharges by diagnosis‐related group, hospital, and admission month. The sample is stratified on hospital characteristics. This form of clustering tends to induce dependence among discharges within hospitals; hence, variance analysis of subsets in line with NIS methods17 was performed. In the present study, we used data from January 1, 2012, through September 30, 2015. The study design used methodological standards laid out in Khera et al.18 Because this study included deidentified data, per the data use agreement with the Agency for Healthcare Research and Quality, the institutional review board requirement was waived.
Study Population and Variables
We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), codes to identify all hospitalized adults (aged ≥50 years), who had a primary diagnosis (diagnosis 1 of NIS) of AS (ICD‐9‐CM code 424.1).10 Patients diagnosed with congenital aortic disorders (code 746.3), rheumatic AS (codes 395.0–395.9), or hypertrophic obstructive cardiomyopathy (code 425.11) or who underwent additional vascular procedures (codes 00.61–00.69 and 36.00–36.99), such as coronary artery bypass grafting, were excluded.10 The discharge diagnoses and procedures were recoded using the clinical classification of diseases software into broad categories, available as separate variables within the NIS data set. In this cohort, we then identified patients with cancer using DXCCS (diagnosis [dx] clinical classification software [ccs]) codes (DXCCS1‐DXCCS30) 11 to 45 (Table S1). NIS provides 29 comorbidities (also known as Elixhauser comorbidity measures) based on ICD‐9 CM diagnoses and the diagnosis‐related group in effect on the date of discharge. These comorbidities are not directly related to the principal diagnosis or the main reason for admission and are likely to have originated before the hospital stay.19 Hospitalizations with the comorbidities of cancer were included in the cancer cohort. All patients who did not have either the DXCCS codes listed above or the listed specific comorbidities were considered noncancer patients. A subanalysis showed that there were no TAVRs reported in the cancer cohort with metastatic disease.
NIS variables included in the study were demographic characteristics (age, sex, and race), income quartile, insurance status, hospital‐level characteristics, comorbidities, and procedures. The procedures of interest were TAVR (codes 35.05 and 35.06) or SAVR (codes 35.21 and 35.22).10 In 2015, the Healthcare Cost and Utilization Project State Inpatient Database was used to create 2 indexes based on 29 comorbidity measures designed to predict in‐hospital mortality (or score) and 30‐day readmission.20 Those indexes were calculated for our cohort as well. In addition to NIS‐provided comorbidities, other comorbidities and outcomes that have been highlighted in other TAVR studies were recoded using ICD‐9‐CM and DXCCS fields (Table S1).
Outcomes
NIS provided data on specific outcomes of interest, including hospitalization charges, length of stay, in‐hospital mortality, and discharge disposition, by cancer status. The actual cost of hospitalization was obtained by multiplying each hospital's charges with its cost/charge ratios21 and wage index for a given year. The wage index helps correct for geographic variations in costs among hospitals.21 Charges and costs were inflation adjusted to 2015.22
Statistical Analysis
Survey‐specific statements (SURVEYMEANS and SURVEYFREQ) with hospital‐ and patient‐level weights were used to obtain national estimates. The Rao‐Scott χ2 test was used to compare categorical variables, and a survey‐specific t test (SURVEYREG) was used for continuous variables. We used the Cochrane Armitage test of trend for categorical variables and survey‐specific linear regression for continuous variables. Hospital charges and length of stay were log transformed because they were not normally distributed, and the geometric mean was presented.23, 24 For a length of stay of 0 days, a value of 0.0001 was imputed to avoid negative log values. Relative use rate was calculated by dividing the number of TAVRs estimated by total estimated AVR for cancer and noncancer, and the proportions were compared using Rao‐Scott χ2 test.
To study the difference in outcomes among patients with and without cancer undergoing TAVR versus SAVR, we used a method presented by Arora et al.10 Briefly, the propensity score25 (ie, the probability) of each patient for undergoing TAVR versus SAVR was estimated using sex, age, race/ethnicity, primary insurance type, income, presence of congestive heart failure, coronary artery disease, prior myocardial infarction, prior coronary artery bypass grafting, atrial fibrillation, hypertension, diabetes mellitus, renal disease, chronic lung disease, coagulopathy, smoking, Elixhauser comorbidity (0, 1, 2, and >2), hospital region, hospital type, hospital size, and discharge weight (Data S1). Trimming was performed at the first percentile of TAVR propensity scores and the 99th percentile of SAVR propensity scores to remove nonoverlapping regions of the propensity score distributions. This step was necessary because those patients represent either TAVR patients who always undergo TAVR or SAVR patients who always undergo SAVR, and neither group is at risk for undergoing the other procedure. A standardized morbidity ratio (SMR) weight was then calculated for each patient, where patients undergoing TAVR were assigned a weight of 1, and patients undergoing SAVR were weighed using PS (propensity score)/(1−PS). SMR weights standardize the distribution. Furthermore, SMR weights were selected to ensure that the effect of TAVR was assessed only among high‐risk patients (ie, those eligible to undergo TAVR during the time period), without needing to measure risk level directly.26 SMR‐weighted generalized logistic regression, accounting for NIS sampling and clustering structure, was conducted to estimate the average effect of TAVR compared with SAVR. Furthermore, SMR‐weighted effect modification of cancer comparing outcomes of TAVR versus SAVR was tested.26
Another subgroup analysis using SMR‐weighted generated logistic regression was performed to compare some of the outcomes presented above in specific cancer subgroups. The cancer subgroups selected for this analysis were breast cancer, lung cancer, colon cancer, prostate cancer, and another group that did not include these National Cancer Institute top 4 cancers.27
All analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC); and the description of the method is presented in graphical form in Figure 1.
Figure 1.
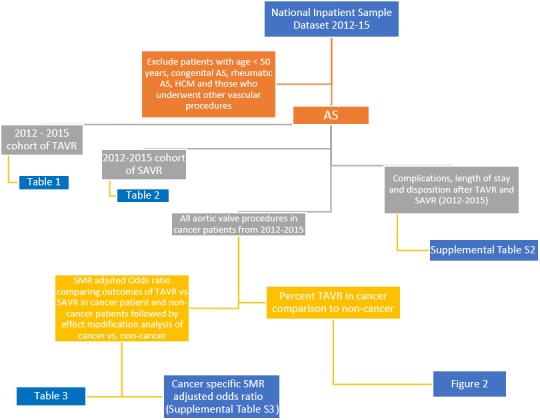
Recruitment scheme. Flowchart showing methods. AS indicates aortic stenosis; HCM, hypertrophic cardiomyopathy; SAVR, surgical aortic valve replacement; SMR, standardized morbidity ratio; TAVR, transcatheter aortic valve replacement.
Results
A total of 47 295 TAVRs and 113 405 SAVRs were performed among admissions with AS between 2012 and 2015 from NIS. Among the TAVRs and SAVRs, 10 670 (22.6%) and 17 290 (15.2%) had comorbid cancer, respectively.
Over time, the proportion of patients undergoing TAVR compared with SAVR has increased in the 4 years, considering overall higher use of TAVR in patients with cancer compared with patients without cancer (P‐trends2012–2015<0.0001; Figure 2). In the year 2015, patients with cancer saw relatively higher rates of TAVR use compared with SAVR (relative use rateTAVR versus relative use rateSAVR, 67.8% versus 57.2%; P<0.0001), considering all AVRs performed that year. Up to 21.8% and 19.6% of total TAVR procedures were performed in patients with prostate or breast cancer, respectively, in 2015 (Figure 2).
Figure 2.
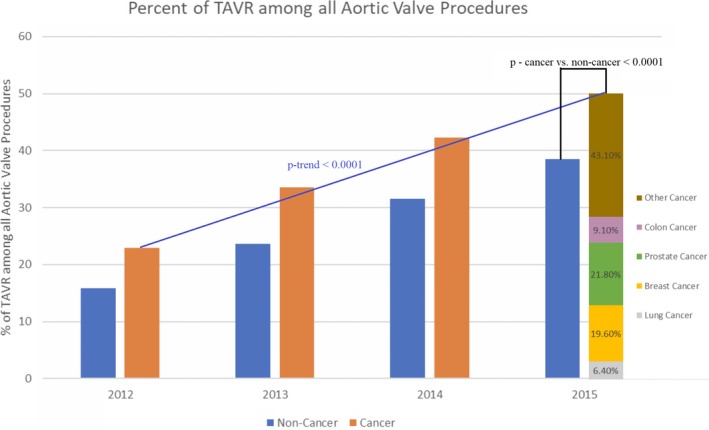
Relative use of transcatheter aortic valve replacement (TAVR) in cancer vs noncancer. On comparing the cancer and noncancer groups, TAVR use was more evident in the cancer group (P<0.0001 for cancer vs noncancer, year 2015). TAVR use in cancer subtypes in 2015 included 21.8% prostate cancer, 19.6% breast cancer, 9.1% colon cancer, 6.4% lung cancer, and 43.1% other cancers.
Patient Characteristics Undergoing TAVR and SAVR in Patients With Cancer Versus Patients Without Cancer
Patients with cancer undergoing TAVR were older and more commonly women. Patients undergoing TAVR with concomitant cancer also had lower rates of traditional cardiovascular disease risk factors, including hypertension, diabetes mellitus, and obesity, although the prevalence of dyslipidemia and tobacco use was higher (Table 1). Similarly, patients with cancer undergoing SAVR were older and had lower rates of diabetes mellitus. However, patients undergoing SAVR with concomitant cancer were less commonly women and had higher rates of other cardiovascular disease risk factors, like hypertension, dyslipidemia, and smoking (Table 2). Notably, average Elixhauser mortality scores of patients undergoing TAVR versus SAVR were 8.9 versus 8.5 and 8.5 versus 7.1 for patients with cancer and patients without cancer, respectively (P<0.0001).
Table 1.
TAVR Demographics (Patient, Financial, and Hospital Levels) From 2012 to 2015 in Patients With Cancer Versus Patients Without Cancer
| Variables | Patients With Cancer (n=10 670) | Patients Without Cancer (n=36 625) | P Value |
|---|---|---|---|
| Patient characteristics | |||
| Age, mean±SE, y | 81.1±0.2 | 80.8±0.1 | 0.14 |
| Women, % | 42.8 | 47.4 | 0.0002 |
| Race, % | 0.11 | ||
| White | 88.8 | 87.4 | |
| Black | 3.4 | 3.9 | |
| Hispanic | 3.1 | 4.2 | |
| Asian or Pacific Islander | 0.9 | 1.1 | |
| Native American | 0.1 | 0.2 | |
| Other | 3.6 | 3.2 | |
| Payment source, % | 0.41 | ||
| Medicare | 88.9 | 90.9 | |
| Medicaid | 0.9 | 1.0 | |
| Private | 7.3 | 6.5 | |
| Self‐pay | 0.5 | 0.5 | |
| No charge | 0 | 0.03 | |
| Others | 1.5 | 1.1 | |
| Comorbidities, % | |||
| Traditional cardiovascular | |||
| Cardiomyopathy | 15.4 | 15.3 | 0.87 |
| Known coronary artery disease | 67.8 | 68.8 | 0.43 |
| Prior myocardial infarction | 14.0 | 13.4 | 0.39 |
| Prior percutaneous coronary intervention | 22.6 | 19.7 | 0.004 |
| Prior coronary bypass grafting | 21.9 | 23.4 | 0.16 |
| Carotid disease | 7.7 | 7.1 | 0.30 |
| Peripheral vascular disease | 28.2 | 29.0 | 0.46 |
| Prior TIA/stroke | 14.0 | 13.3 | 0.43 |
| Atrial fibrillation | 41.4 | 43.4 | 0.09 |
| Hypertension | 83.5 | 83.8 | 0.72 |
| Diabetes mellitus | 38.0 | 41.5 | 0.006 |
| Obesity | 12.4 | 16.3 | <0.0001 |
| Dyslipidemia | 68.8 | 65.7 | 0.009 |
| Nontraditional | |||
| Weight loss | 4.0 | 4.5 | 0.34 |
| Anemia | 26.4 | 25.4 | 0.34 |
| Arthritis and collagen vascular disease | 4.7 | 4.9 | 0.76 |
| Chronic liver disease | 2.6 | 2.6 | 0.97 |
| Chronic renal disease | 36.9 | 37.9 | 0.39 |
| Chronic lung disease | 34.6 | 32.9 | 0.14 |
| Hypothyroidism | 22.0 | 19.9 | 0.02 |
| Neurologic | 6.6 | 8.0 | 0.03 |
| Psychiatric | 9.5 | 9.1 | 0.55 |
| Fluid/electrolyte disorder | 21.9 | 24.8 | 0.006 |
| Coagulation disorder | 23.1 | 22.0 | 0.25 |
| Substance abuse | 1.6 | 1.2 | 0.13 |
| Smoker | 34.5 | 28.9 | <0.0001 |
| Total Elixhauser comorbidities | 0.12 | ||
| 0 | 1.0 | 1.7 | |
| 1 | 8.1 | 8.3 | |
| 2 | 16.6 | 17.4 | |
| ≥3 | 74.4 | 72.6 | |
| Elixhauser readmission score, mean±SE | 21.0±0.3 | 19.2±0.2 | <0.0001 |
| Elixhauser mortality score, mean±SE | 8.9±0.2 | 8.2±0.2 | 0.003 |
| Teaching hospital, % | 89.6 | 89.7 | 0.82 |
| Bed size, % | 0.32 | ||
| Small | 4.4 | 4.8 | |
| Medium | 16.8 | 17.9 | |
| Large | 78.9 | 77.3 | |
| Region, % | <0.0001 | ||
| Northeast | 25.4 | 24.8 | |
| Midwest | 25.4 | 21.5 | |
| South | 29.9 | 35.7 | |
| West | 19.3 | 18.1 | |
| Hospital in urban location, % | 99.2 | 99.2 | 0.99 |
| Weekend admission, % | 5.1 | 5.9 | 0.15 |
| Elective admission, % | 81.4 | 78.5 | 0.005 |
TAVR indicates transcatheter aortic valve replacement; TIA, transient ischemic attack.
Table 2.
SAVR Demographics (Patient, Financial, and Hospital Levels) From 2012 to 2015 in Patients With Cancer Versus Patients Without Cancer
| Variables | Patients With Cancer (n=17 290) | Patients Without Cancer (n=96 115) | P Value |
|---|---|---|---|
| Patient characteristics | |||
| Age, mean±SE, y | 73.0±0.2 | 68.8±0.1 | <0.0001 |
| 50–65 y, % | 15.7 | 32.1 | <0.0001 |
| ≥65 y, % | 84.3 | 67.9 | |
| Women, % | 37.8 | 39.9 | 0.03 |
| Race, % | <0.0001 | ||
| White | 88.3 | 83.0 | |
| Black | 3.5 | 5.3 | |
| Hispanic | 3.9 | 6.6 | |
| Asian or Pacific Islander | 0.9 | 1.5 | |
| Native American | 0.2 | 0.4 | |
| Other | 3.2 | 3.2 | |
| Income quartiles | <0.0001 | ||
| 0–25 | 17.7 | 21.9 | |
| 26–50 | 24.6 | 25.5 | |
| 51–75 | 27.2 | 26.2 | |
| 76–100 | 30.5 | 26.3 | |
| Payment source, % | <0.0001 | ||
| Medicare | 78.7 | 64.2 | |
| Medicaid | 1.7 | 3.8 | |
| Private | 17.6 | 28.3 | |
| Self‐pay | 0.6 | 1.5 | |
| No charge | 0.1 | 0.2 | |
| Others | 1.3 | 1.9 | |
| Comorbidities, % | |||
| Traditional cardiovascular | |||
| Cardiomyopathy | 7.1 | 8.6 | 0.003 |
| Known coronary artery disease | 41.9 | 38.1 | <0.0001 |
| Prior myocardial infarction | 4.9 | 5.3 | 0.35 |
| Prior percutaneous coronary intervention | 9.1 | 7.3 | 0.0003 |
| Prior coronary bypass grafting | 6.1 | 5.5 | 0.15 |
| Carotid disease | 4.7 | 4.1 | 0.12 |
| Peripheral vascular disease | 18.8 | 19.7 | 0.21 |
| Prior TIA/stroke | 9.2 | 7.6 | 0.002 |
| Atrial fibrillation | 51.7 | 45.6 | <0.0001 |
| Hypertension | 80.0 | 77.4 | 0.0007 |
| Diabetes mellitus | 42.7 | 43.4 | 0.47 |
| Obesity | 19.9 | 23.4 | <0.0001 |
| Dyslipidemia | 64.8 | 61.2 | <0.0001 |
| Nontraditional | |||
| Weight loss | 3.7 | 3.7 | 0.99 |
| Anemia | 19.1 | 17.5 | 0.04 |
| Arthritis and collagen vascular disease | 3.5 | 3.4 | 0.63 |
| Chronic liver disease | 1.6 | 1.8 | 0.47 |
| Chronic renal disease | 18.0 | 14.6 | <0.0001 |
| Chronic lung disease | 22.6 | 21.3 | 0.08 |
| Hypothyroidism | 16.7 | 13.5 | <0.0001 |
| Neurologic | 6.1 | 6.0 | 0.94 |
| Psychiatric | 10.3 | 10.2 | 0.75 |
| Fluid/electrolyte disorder | 33.1 | 33.8 | 0.42 |
| Coagulation disorder | 35.2 | 31.7 | <0.0001 |
| Substance abuse | 2.6 | 3.3 | 0.02 |
| Smoker | 37.9 | 32.9 | <0.0001 |
| Total Elixhauser comorbidities | <0.0001 | ||
| 0 | 2.7 | 3.6 | |
| 1 | 10.9 | 13.7 | |
| 2 | 21.6 | 22.0 | |
| ≥3 | 64.8 | 60.7 | |
| Elixhauser readmission score, mean±SE | 16.2±0.3 | 13.7±0.3 | <0.0001 |
| Elixhauser mortality score, mean±SE | 8.5±0.2 | 7.1±0.1 | <0.0001 |
| Teaching hospital, % | 76.4 | 75.1 | 0.10 |
| Bed size, % | 0.10 | ||
| Small | 6.7 | 7.4 | |
| Medium | 20.2 | 21.4 | |
| Large | 73.0 | 71.2 | |
| Region, % | <0.0001 | ||
| Northeast | 24.3 | 22.2 | |
| Midwest | 24.8 | 24.2 | |
| South | 29.1 | 33.3 | |
| West | 21.8 | 20.3 | |
| Hospital in urban location, % | 98.0 | 97.6 | 0.09 |
| Weekend admission, % | 4.2 | 4.4 | 0.44 |
| Elective admission, % | 81.7 | 78.3 | <0.0001 |
SAVR indicates surgical aortic valve replacement; TIA, transient ischemic attack.
Complications, Outcomes, and Disposition of TAVR Versus SAVR in Cancer Versus Noncancer
Among patients with cancer, TAVR was associated with lower odds of acute kidney injury (AKI; odds ratio [OR], 0.64; 95% CI, 0.54–0.75), cardiogenic shock (OR, 0.55; 95% CI, 0.36–0.84), and major bleeding (OR, 0.44; 95% CI, 0.38–0.51), with no differences in in‐hospital mortality and stroke compared with SAVR. In addition, TAVR was associated with higher odds of home discharge (OR, 1.92; 95% CI, 1.68–2.19) compared with SAVR among patients with cancer (Table 3).
Table 3.
Standardized Associations Between Hospitalizations for TAVR, Compared With SAVR, on In‐Hospital Complications, Discharge Disposition, and Length of Stay After Valve Replacement, Among Patients With and Without Cancer Who Underwent TAVR, Compared Using Effect Modification Odds Ratio
| Variables | Patients With Cancer | Patients Without Cancer | P Valuea |
|---|---|---|---|
| In‐hospital complications | |||
| Permanent pacemaker implantation | 2.16 (1.70–2.73) | 2.26 (1.97–2.59) | 0.33 |
| Transient ischemic attack/stroke | 0.88 (0.60–1.30) | 1.00 (0.81–1.24) | 0.82 |
| Cardiogenic shock | 0.55 (0.36–0.84) | 0.83 (0.68–1.02) | 0.31 |
| Cardiac arrest | 1.13 (0.80–1.60) | 1.17 (0.97–1.42) | 0.99 |
| Acute kidney injury | 0.64 (0.54–0.75) | 0.74 (0.68–0.81) | <0.0001 |
| Blood transfusion | 0.44 (0.38–0.51) | 0.45 (0.42–0.49) | 0.12 |
| Vascular complications | 0.61 (0.20–1.89) | 1.46 (0.88–2.41) | 0.23 |
| Discharge disposition and outcomes | |||
| Home discharge | 1.92 (1.68–2.19) | 1.50 (1.39–1.62) | <0.0001 |
| Transfer to SNF or acute care hospital | 0.73 (0.63–0.83) | 0.78 (0.72–0.84) | 0.0001 |
| Home health care | 0.71 (0.62–0.80) | 0.85 (0.79–0.91) | 0.04 |
| In‐hospital mortality | 1.10 (0.73–1.66) | 1.15 (0.94–1.40) | 0.77 |
Data are given as odds ratio (95% CI). Standardized morbidity ratio weights were calculated using sex, age, race/ethnicity, primary insurance type, income, presence of congestive heart failure, coronary artery disease, prior myocardial infarction, prior coronary artery bypass grafting, atrial fibrillation, hypertension, diabetes mellitus, renal disease, chronic lung disease, coagulopathy, smoking, Elixhauser comorbidity (0, 1, 2, and >2), hospital region, hospital type, hospital size, and discharge weight; propensity scores were trimmed using 1% and 99% cut points. The change in estimated length of stay (95% CI) for length of stay after aortic valve replacement was −1.65 (−1.96 to −1.34) days in patients with cancer and −1.37 (−1.56 to −1.17) days in patients without cancer (P<0.0001). SAVR indicates surgical aortic valve replacement; SNF, skilled nursing facility; TAVR, transcatheter aortic valve replacement.
Standardized morbidity ratio weighted effect modification analysis of cancer on the outcomes was performed.
Similar findings were seen in the noncancer arm, such as patients with cancer undergoing TAVR were more likely to be discharged home when compared with SAVR (OR, 1.50; 95% CI, 1.39–1.62) but less likely to be transferred to nursing facility transfer (OR, 0.73; 95% CI, 0.63–0.83) or use of home health care (OR, 0.71; 95% CI, 0.62–0.80) when compared with SAVR (Table 3). Absolute numbers of complications, outcomes, and disposition in TAVR and SAVR in cancer versus noncancer are presented in Table S2. Finally, it was noticed that cancer was an effect modifier for the association of TAVR with lower risk of AKI in patients with cancer compared with patients without cancer undergoing the procedure (P<0.001).
Complications, Outcomes, and Disposition of TAVR/SAVR in Specific Cancers
Among patients with cancer undergoing TAVR from 2012 to 2015, 19.3%, 22.8%, 6.6%, and 9.8% had underlying breast, prostate, lung, and colon cancers, respectively. The remaining patients had other cancers (44.6%). In patients with breast cancer, TAVR was associated with lower odds of major bleeding (OR, 0.45; 95% CI, 0.32–0.62), but no difference in in‐hospital mortality and stroke compared with SAVR. Similarly, for prostate cancer, TAVR was associated with lower odds of AKI (OR, 0.62; 95% CI, 0.44–0.87) and major bleeding (OR, 0.48; 95% CI, 0.36–0.66), but no differences in in‐hospital mortality and stroke compared with SAVR. Additional information about in‐hospital complications, discharge dispositions, and in‐hospital outcomes by respective cancer type is shown in Table S3.
Discussion
In this large contemporary population, we found a higher proportion of patients with cancer undergoing TAVR versus SAVR compared with patients without cancer. Furthermore, TAVR had similar outcomes in patients with and without cancer and was, in fact, associated with lower rates of AKI, cardiogenic shock, and major bleeding but higher likelihood of discharge to home and lower inpatient length of stay when compared with SAVR. Moreover, a positive effect modification of cancer was noticed with respect to AKI and posthospitalization disposition outcome in patients with cancer. This is important, given the expected continued increase in patients with cancer presenting with significant AS.
Valvular disease has been long acknowledged as a serious adverse effect of cancer therapy caused by the effects of exposure to radiotherapy and chemotherapy.4, 28 Radiation therapy is associated with heightened valvular endothelial dysfunction, in part because of accelerated thickening and calcification of the aortic valve, thus leading to AS.6, 28, 29 In fact, valvular heart disease can potentially occur in >75% of patients who receive prior radiotherapy.4, 30, 31 This number is ever increasing because of increased cancer survivorship.32 Despite an increasing need for AVR in those experiencing cancer, patients often were not offered surgery.33 These trends have changed in the recent years with the inception of TAVR. Our study provides novel insights into current practice patterns associated with AVR in cancer. AVR use for AS in patients with cancer has risen more in the TAVR era, compared with those without cancer, and is largely attributable to TAVR, suggesting that patients with a history of cancer seem to have particularly benefited from the TAVR with regard to candidacy for AVR. This is likely because TAVR is less invasive in nature compared with SAVR.34 In addition, TAVR has been shown to be associated with a lower incidence of periprocedural myocardial infarction, major bleeding, AKI, and new‐onset atrial fibrillation when compared with SAVR.35, 36
Similar mortality rates in patients with cancer compared with patients without cancer who underwent TAVR at 1‐year follow‐up have been previously observed; however, the generalizability of these findings was limited because of small study and a general focus on lower‐risk cohorts.37 Another study of 350 patients with cancer in single‐center setting found the short‐term, 30‐day outcomes of TAVR to be as similar in those with cancer, compared with those without cancer. However, within the same cohort, 1‐year mortality was higher in those patients with cancer.38 To our knowledge, previous data comparing TAVR versus SAVR in those with cancer versus those without cancer currently do not exist. A recent study evaluated the factors specifically associated with a poor outcome (mortality or lack of functional status improvement, as evaluated by New York Heart Association functional classification at 6‐month follow‐up) after TAVR in patients with chronic lung disease at 6‐month follow‐up.39 New York Heart Association status or symptom in patients with cancer versus patients without cancer might be a useful tool for selecting patients with cancer undergoing TAVR or SAVR compared with patients without cancer. Given the relative safety of TAVR and improving cancer outcomes, the presence of concurrent nonadvanced cancer may not significantly affect candidacy for TAVR; however, randomized study is needed to fully elucidate this claim. Moreover, relatively healthier patients with active cancer could potentially resume cancer treatment sooner because of shorter recovery time afforded by TAVR. With the expanding prevalence of AS among cancer survivors, consideration of outcomes obtained from this study may help in a patient‐centered optimal management strategy for both active and prior patients with cancer. Several studies have indicated that surgical stress suppresses the immune system and that the use of cardiopulmonary bypass contributes to this, resulting in increasing tumor recurrence. However, cardiopulmonary bypass has a modest association with cancer progression and relating cardiopulmonary bypass and cancer dissemination is logical but probably an unlikely association.40
Moreover, a positive effect modification of cancer was noticed with respect to AKI and posthospitalization disposition outcome in patients with cancer. This is difficult to interpret as the data are cross‐sectional and do not allow us to track AKI over time in patients with cancer compared with patients without cancer after TAVR or SAVR.
There are several limitations of this study that warrant consideration. Because of reliance on ICD‐9‐CM codes, we were unable to determine the physician‐perceived indication for hospital admission by specific cancer type. We also could not determine the clinical severity of AS or other concomitant disease, like mitral disease or coronary artery disease, duration of a cancer diagnosis, cancer stage (active versus prior), life expectancy, or concomitant cancer treatments (radiation versus chemotherapy versus recent cancer surgery). Furthermore, there were no patients who underwent TAVR in the metastatic cancer arm, making this study inapplicable to that population. Although we used a matched propensity score design to account for indication bias, important unmeasured clinical characteristics that may be predictors of outcomes were not available, including standardized surgical risk score (eg, STS [society of thoracic surgeons]) factors, like “last hematocrit” and “unresponsive state,” and therefore these findings may be subject to confounding. In addition, because of the administrative nature of data, we were unable to distinguish comorbidities from complications of hospitalization. Moreover, as the NIS is a deidentified database, long‐term outcomes and complications occurring after the initial TAVR hospitalization could not be assessed. We were also not able to determine if this study includes repeated observations from patients who underwent >1 AVR (TAVR or SAVR) during the study period. There was also the potential for coding errors and differences in coding practices across the hospitals included in the database. However, we suspect that these differences were random and would not be expected to differ between patients undergoing TAVR and SAVR. Moreover, ICD‐9‐CM coding prevented us from being able to differentiate between different endovascular approaches and, therefore, prevented us from comparing outcomes between different endovascular approaches, such as transfemoral, direct aortic, or transaxillary approaches. In addition, the type of valve used (stented versus stentless) was unavailable. The use of administrative data also precluded the role of valve/heart team, whereby contraindications to either TAVR/SAVR could be studied. Although we used a matched propensity score design to account for indication bias, important unmeasured clinical characteristics that may be predictors of outcomes were not available, and therefore these findings may be subject to confounding.
Conclusions
Compared with patients without cancer, the use of TAVR in patients with cancer has steadily increased. In patients with cancer, there was an association of TAVR with lower risk of AKI, lower length of stay, and higher likelihood of discharge to home when compared with patients undergoing SAVR. Moreover, a positive effect modification of cancer was noticed with respect to AKI and posthospitalization disposition outcome in patients with cancer. This is difficult to interpret as the data are cross‐sectional and do not allow us to track AKI over time in patients with cancer compared with patients without cancer after TAVR or SAVR. These findings suggest that transcatheter therapies have improved access to AVR in a population at high risk for surgery and offered an additional option for patients who may not have been previously eligible for therapy. Further research into the factors related to these differences along with longitudinal follow‐ups of these patients to study outcomes over time are needed.
Sources of Funding
Dr Addison is supported by National Institutes of Health grant number K12‐CA133250. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Data S1. Propensity model.
Table S1. Diagnosis Codes Used in the Study
Table S2. Complications, Length of Stay and Disposition After TAVR and SAVR, Among Cancer and Non‐Cancer Patients From 2012 to 2015
Table S3. Standardized Associations Between Hospitalizations for TAVR, Compared to SAVR, on In‐Hospital Complications, Discharge Disposition, and Length of Stay After Valve Replacement, Among Cancer and Non‐Cancer Patients Who Underwent TAVR
(J Am Heart Assoc. 2020;9:e014248 DOI: 10.1161/JAHA.119.014248.)
This article was handled independently by Isabella Grumbach, MD, PhD, as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et al. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke‐Bärwolf C, Levang OW, Tornos P, Vanoverschelde J‐L, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 3. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 4. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 5. Lenneman Carrie G, Sawyer Douglas B. Cardio‐oncology. Circ Res. 2016;118:1008–1020. [DOI] [PubMed] [Google Scholar]
- 6. Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L, Murthy VL, Hearn JWD, Kong FM, Kalemkerian GP, Hayman JA, Ten Haken RK, Lawrence TS, Schipper MJ, Jolly S. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2017;35:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guha A, Armanious M, Fradley MG. Update on cardio‐oncology: novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med. 2019;29:29–39. [DOI] [PubMed] [Google Scholar]
- 8. Yusuf SW, Sarfaraz A, Durand J‐B, Swafford J, Daher IN. Management and outcomes of severe aortic stenosis in cancer patients. Am Heart J. 2011;161:1125–1132. [DOI] [PubMed] [Google Scholar]
- 9. Carabello BA. Aortic stenosis: a fatal disease with but a single cure. JACC Cardiovasc Interv. 2008;1:127–128. [DOI] [PubMed] [Google Scholar]
- 10. Arora S, Strassle PD, Kolte D, Ramm CJ, Falk K, Jack G, Caranasos TG, Cavender MA, Rossi JS, Vavalle JP. Length of stay and discharge disposition after transcatheter versus surgical aortic valve replacement in the United States. Circ Cardiovasc Interv. 2018;11:e006929. [DOI] [PubMed] [Google Scholar]
- 11. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 12. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 13. Arora S, Ramm CJ, Strassle PD, Vaidya SR, Caranasos TG, Vavalle JP. Review of major registries and clinical trials of late outcomes after transcatheter aortic valve replacement. Am J Cardiol. 2017;120:331–336. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura Rick A, Otto Catherine M, Bonow Robert O, Carabello Blase A, Erwin John P, Fleisher Lee A, Jneid H, Mack Michael J, McLeod Christopher J, O'Gara Patrick T, Rigolin Vera H, Sundt Thoralf M, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 15. Arora S, Strassle PD, Qamar A, Kolte D, Pandey A, Paladugu MB, Borhade MB, Ramm CJ, Bhatt DL, Vavalle JP. Trends in inpatient complications after transcatheter and surgical aortic valve replacement in the transcatheter aortic valve replacement era. Circ Cardiovasc Interv. 2018;11:e007517. [DOI] [PubMed] [Google Scholar]
- 16. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 17. Method series report 2015‐09. Healthcare Cost and Utilization Project (HCUP) 2016. Accessed September 15, 2019. [Google Scholar]
- 18. Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Overview of disease severity measures disseminated with the nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID). 2005;2018. Accessed September 15, 2019.
- 20. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 21. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 22. CPI inflation calculator. 2018;2018. Accessed September 15, 2019.
- 23. Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbert BD, Horstman JM. Captain's LOG: taking command of SAS® logarithm functions. 2014;2018. Accessed September 15, 2019.
- 25. Guha A, Dey AK, Armanious M, Dodd K, Bonsu J, Jneid H, Abraham W, Fradley MG, Addison D. Health care utilization and mortality associated with heart failure‐related admissions among cancer patients. ESC Heart Fail. 2019;6:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect‐measure modification. Pharmacoepidemiol Drug Saf. 2006;15:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donnellan E, Griffin BP, Johnston DR, Popovic ZB, Alashi A, Kapadia SR, Tuzcu EM, Krishnaswamy A, Mick S, Svensson LG, Desai MY. Rate of progression of aortic stenosis and its impact on outcomes in patients with radiation‐associated cardiac disease. JACC Cardiovasc Imaging. 2018;11:1072. [DOI] [PubMed] [Google Scholar]
- 29. Darby SC, Ewertz M, McGale P, Bennet AM, Blom‐Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 30. Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation‐induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg. 2007;55:53–56. [DOI] [PubMed] [Google Scholar]
- 31. Crawford MH. Chemotherapy‐induced valvular heart disease. JACC Cardiovasc Imaging. 2016;9:240. [DOI] [PubMed] [Google Scholar]
- 32. Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379:2438–2450. [DOI] [PubMed] [Google Scholar]
- 33. Landes U, Iakobishvili Z, Vronsky D, Zusman O, Barsheshet A, Jaffe R, Jubran A, Yoon S‐H, Makkar RR, Taramasso M, Russo M, Maisano F, Sinning J‐M, Shamekhi J, Biasco L, Pedrazzini G, Moccetti M, Latib A, Pagnesi M, Colombo A, Tamburino C, D’ Arrigo P, Windecker S, Pilgrim T, Tchetche D, De Biase C, Guerrero M, Iftikhar O, Bosmans J, Bedzra E, Dvir D, Mylotte D, Sievert H, Watanabe Y, Søndergaard L, Dagnegård H, Codner P, Kodali S, Leon M, Kornowski R. Transcatheter aortic valve replacement in oncology patients with severe aortic stenosis. JACC Cardiovasc Interv. 2019;12:78–86. [DOI] [PubMed] [Google Scholar]
- 34. Mack MC, Szerlip M, Herbert MA, Akram S, Worley C, Kim RJ, Prince BA, Harrington KB, Mack MJ, Holper EM. Outcomes of treatment of nonagenarians with severe aortic stenosis. Ann Thorac Surg. 2015;100:74–80. [DOI] [PubMed] [Google Scholar]
- 35. Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochelliere R, Doyle D, Masson JB, Gutierrez MJ, Clavel MA, Bertrand OF, Pibarot P, Rodes‐Cabau J. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huded CP, Tuzcu EM, Krishnaswamy A, Mick SL, Kleiman NS, Svensson LG, Carroll J, Thourani VH, Kirtane AJ, Manandhar P, Kosinski AS, Vemulapalli S, Kapadia SR. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA. 2019;321:2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe Y, Kozuma K, Hioki H, Kawashima H, Nara Y, Kataoka A, Shirai S, Tada N, Araki M, Takagi K, Yamanaka F, Yamamoto M, Hayashida K. Comparison of results of transcatheter aortic valve implantation in patients with versus without active cancer. Am J Cardiol. 2016;118:572–577. [DOI] [PubMed] [Google Scholar]
- 38. Mangner N, Woitek FJ, Haussig S, Holzhey D, Stachel G, Schlotter F, Hollriegel R, Mohr FW, Schuler G, Linke A. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. 2018;31:188–196. [DOI] [PubMed] [Google Scholar]
- 39. Mok M, Nombela‐Franco L, Dumont E, Urena M, DeLarochelliere R, Doyle D, Villeneuve J, Cote M, Ribeiro HB, Allende R, Laflamme J, DeLarochelliere H, Laflamme L, Amat‐Santos I, Pibarot P, Maltais F, Rodes‐Cabau J. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. 2013;6:1072–1084. [DOI] [PubMed] [Google Scholar]
- 40. Braile DM, Évora PRB. Cardiopulmonary bypass and cancer dissemination: a logical but unlikely association. Braz J Cardiovasc Surg. 2018;33:I–II. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Propensity model.
Table S1. Diagnosis Codes Used in the Study
Table S2. Complications, Length of Stay and Disposition After TAVR and SAVR, Among Cancer and Non‐Cancer Patients From 2012 to 2015
Table S3. Standardized Associations Between Hospitalizations for TAVR, Compared to SAVR, on In‐Hospital Complications, Discharge Disposition, and Length of Stay After Valve Replacement, Among Cancer and Non‐Cancer Patients Who Underwent TAVR


