Abstract
Background
Recently, direct oral anticoagulants have been introduced for venous thromboembolism (VTE), which might change the management strategies of VTE. However, there have been limited data on the current real‐world practice pattern for VTE in Asian countries.
Methods and Results
The JROAD‐DPC (Japanese Registry of All Cardiac and Vascular Diseases\xF6Diagnosis Procedure Combination) is a nationwide claim database from 1022 hospitals in Japan between April 2012 and March 2017. We identified 54 369 patients who were hospitalized with a diagnosis of VTE at admission based on the International Classification of Diseases, Tenth Revision (ICD‐10) code. The mean age was 69.1±15.6 years, 59% were women, and mean body mass index was 23.5±5.0 kg/m2. The proportion of patients with deep vein thrombosis decreased over time from 72% in 2012 to 38% in 2017. After the release of direct oral anticoagulants, the proportion of patients receiving direct oral anticoagulants increased dramatically among patients with anticoagulation therapy at discharge with the use of edoxaban, rivaroxaban, and apixaban in 35%, 22%, and 27% of patients, respectively, in 2017. On the other hand, the proportion of patients receiving warfarin decreased from 94% in 2012 to 15% in 2017. The median length of a hospital stay decreased over time from 20 days in 2012 to 13 days in 2017 in patients with pulmonary embolism, and from 14 days in 2012 to 12 days in 2017 in patients with deep vein thrombosis. The median cost of hospitalization for pulmonary embolism moderately decreased over time, whereas that for deep vein thrombosis slightly decreased over time.
Conclusions
A nationwide claim‐based database provided the current practice pattern for VTE in Japan, which revealed dynamic changes after the release of direct oral anticoagulants.
Clinical Trial Registration
URL: http://www.umin.ac.jp. Unique identifier: UMIN000037868.
Keywords: deep vein thrombosis, direct oral anticoagulant, pulmonary embolism, venous thromboembolism
Subject Categories: Thrombosis, Epidemiology, Quality and Outcomes
Clinical Perspective
What Is New?
After the release of direct oral anticoagulants in Japan, the proportions of direct oral anticoagulants increased dramatically, and that of warfarin decreased gradually.
Among patients hospitalized with venous thromboembolism, the proportion of patients with deep vein thrombosis decreased over time, and the median length of hospital stay and cost of hospitalization decreased over time, which was more prominent in cases of pulmonary embolism compared with deep vein thrombosis.
What Are the Clinical Implications?
Direct oral anticoagulants could be good alternatives for patients with venous thromboembolism, which have dynamically changed practice patterns after their release in Japan.
Direct oral anticoagulants would enable home treatment and early hospital discharge for patients with venous thromboembolism, which could provide not only medical benefits but also medical‐economic benefits.
Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is a major health problem in the world and the third most frequent cardiovascular disease in Western countries.1 The risk of development of VTE in Asian countries has been thought to be generally lower than that in Western countries,2 although a recent study reported that the incidence of VTE in Asian countries might be increasing over time.3, 4, 5 Actually, VTE is becoming a common issue in daily clinical practice in Asian countries including Japan.6
The standard treatment for VTE and prevention of its recurrence is anticoagulation therapy. Historically, VTE has been treated with heparin followed by a vitamin K antagonist such as warfarin, which was the only available oral anticoagulant. Recently, direct oral anticoagulants (DOAC; dabigatran, rivaroxaban, apixaban, and edoxaban) have been introduced for VTE. DOACs have predictable pharmacokinetics without the need for dose adjustment based on blood tests in individual patients, and some of the DOACs have been used as a single‐drug approach without administeration of intravenous anticoagulants, which would enable early hospital discharge or home treatment.7, 8, 9 Thus, these profiles of DOACs might change the management strategies of VTE. However, there have been limited data on the current practice pattern for VTE across Japan, which would be important for understanding the current issues and unmet needs of optimal management of VTE. Therefore, we sought to evaluate the temporal change in the practice pattern for VTE over time using a nationwide claim database in Japan.
Methods
Data Source
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The JROAD‐DPC (Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination) is a nationwide claim database, launched by the Japanese Circulation Society (JCS), which includes patients with hospitalizations.10, 11 The JROAD‐DPC database was created by combining JROAD data derived from a JCS national survey to assess the clinical activity of each Japanese institution with cardiovascular beds12 and the DPC, which is a mixed‐case patient classification system launched in 2002 by the Ministry of Health, Labor, and Welfare of Japan.13 The JROAD database did not include all hospitals in Japan but nearly all teaching hospitals with cardiovascular beds except for stroke, because this survey is mandatory at JCS‐certified teaching hospitals that provide cardiology training for physicians who wish to be JCS board‐certified cardiologists and undertake the JCS board test. The DPC database contains patient demographics and several disease‐specific data for each patient. An attending physician is responsible for clinical data entry for each patient, and drugs and procedures are recorded based on receipt data of medical care.14
The DPC database contains 6 categories of diagnoses, each with a limited number of recordable diseases. One diagnosis each is coded for “main diagnosis,” “admission‐precipitating diagnosis,” “most resource‐consuming diagnosis,” and “second most resource‐consuming diagnosis.” A maximum of 4 diagnoses (10 diagnoses from 2016) each can be coded for “comorbidities present at time of admission” and “conditions arising after admission.” All procedures performed during hospitalization are recorded according to the Japanese fee schedule for reimbursement.15
Study Population
The JROAD‐DPC database included 5 106 151 health records from 1022 certificated hospitals between April 2012 and March 2017. In the current study we identified 55 582 patients who were hospitalized with “main diagnosis” and/or “admission‐precipitating diagnosis” and/or “most resource‐consuming diagnosis” of VTE at admission based on the International Classification of Diseases, Tenth Revision (ICD‐10). A combination of PE codes and DVT codes was used to identify VTE: PE I26.0, I26.9; and DVT I80.0, I80.1, I80.2, I80.3, I80.9, I82.2, I82.3, I82.9, O22.2, O22.3, O22.9, O87.0, O87.1, O87.9. The accuracy of ICD‐10 to identify symptomatic VTE has been previously validated with high specificity and acceptable sensitivity.16 After exclusion of 257 patients aged <20 years and 956 patients with pregnancy, the current study population consisted of 54 369 VTE patients (Figure 1). We excluded pediatric patients aged <20 years in the current analysis because DOACs are not recommended for pediatric patients in the current Japanese practice, and practice patterns of pediatric patients could be different from those of adult patients.
Figure 1.
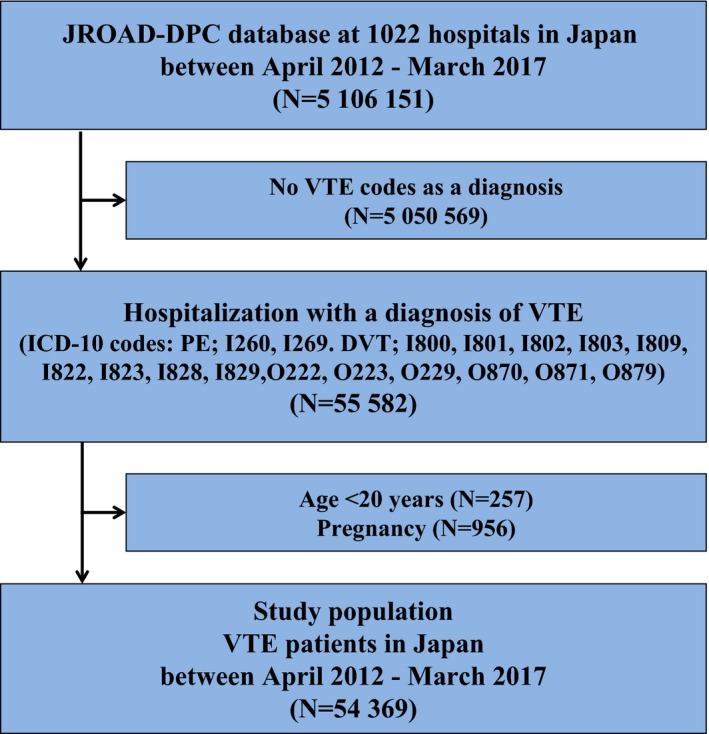
Study flow chart. VTE includes PE and/or DVT. DVT indicates deep vein thrombosis; ICD‐10, International Classification of Diseases, Revision 10; JROAD‐DPC, Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination; PE, pulmonary embolism; VTE, venous thromboembolism.
Data Collection and Definitions of Patient Characteristics
Patient characteristics were extracted from the claim data, including age, sex, initial diagnoses, and comorbidities at the time of admission. Comorbidities were determined primarily from the ICD‐10 codes but were also checked against the medications and procedures that each patient was receiving/undergoing to determine if these were compatible with the code data. When patients had a diagnosis of PE at admission, we classified them as PE patients irrespective of presence or absence of DVT.17 In‐hospital medications and procedures were also extracted from the claim data, including heparin, thrombolysis with tissue plasminogen activator or urokinase, inferior vena cava filter, ventilator support, and mechanical circulation support (intra‐aortic balloon pumping or percutaneous cardiopulmonary support). In addition, medications at discharge were extracted from the claim data, including detailed anticoagulation therapy. In Japan, edoxaban, rivaroxaban, and apixaban for treatment and prevention of recurrence of VTE became available in September 2014, September 2015, and December 2015, respectively. Dabigatran for VTE is not covered by Japanese national insurance.
Outcomes Measures
Outcomes measures in the present analysis were in‐hospital all‐cause death, length of hospitalization, and cost of hospitalization, which included charges related to drugs, medical materials, food, and personnel. All charges were converted into US dollars according to the current exchange rate (1 US dollar=110.0 Japanese yen).
Ethics Statement
This research plan was designed by the authors and approved by both Kyoto University Hospital Ethics Committee and the Institutional Review Board of the National Cerebral and Cardiovascular Center, which waived the requirement for individual informed consent according to the “opt‐out” principle. Each hospital anonymized patients’ ID by the code change equations made by each hospital in the original DPC data, which were sent to the Ministry of Health, Labor, and Welfare. Each hospital notified the patients through homepages or posters in each hospital that their information was being collected by this study. Patients could opt out from having their information in the database if they wished to exclude it.
Statistical Analyses
Categorical variables are presented as numbers and percentages, and continuous variables are presented as the mean and SD for normally distributed continuous variables or the median and interquartile range for nonnormally distributed continuous variables. We evaluated patient characteristics, treatment strategies, and outcomes. In‐hospital all‐cause death was calculated by dividing the number of deaths by the number of hospitalizations. In addition, we evaluated changes of proportions of PE and DVT, treatment strategies, and outcomes over time. Changes of categorical variables were evaluated using the Cochran‐Armitage test for trend,18 and changes of continuous variables were evaluated using the Jonckheere‐Terpstra test for trend.19 All statistical analyses were conducted using JMP version 10.0.2 (SAS Institute Inc, Cary, NC) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). All statistical analyses were 2‐tailed, and P<0.05 was considered statistically significant.
Results
Patient Characteristics
The mean age was 69.1±15.6 years, 59% were women, and body weight and body mass index were 55.8±20.0 kg and 23.5±5.0 kg/m2, respectively (Table 1). Patients with cancer accounted for 10 096 (19%), and 25% of them were those with metastasis. Patients with PE at presentation accounted for 27 887 (51%), and 5.7% of them showed cardiac arrest or collapse at presentation. The proportion of patients with DVT only decreased over time from 72% in 2012 to 38% in 2017 (P for trend <0.001) (Figure 2).
Table 1.
Patient Characteristics
| JROAD‐DPC Data (N=54 369) | |
|---|---|
| Baseline characteristics | |
| Age, y | 69.1±15.6 |
| Women | 31 894 (59%) |
| Body weight, kg | 55.8±20.0 |
| Body mass index, kg/m2 | 23.5±5.0 |
| Body mass index ≥30 kg/m2 | 3795 (7.0%) |
| Comorbidities | |
| Hypertension | 15 738 (29%) |
| Diabetes mellitus | 7239 (13%) |
| Dyslipidemia | 7680 (14%) |
| Cancer | 10 096 (19%) |
| Metastatic cancer | 2478/10 096 (25%) |
| Chronic lung disease | 2558 (4.7%) |
| Heart failure | 8629 (16%) |
| History of stroke | 758 (1.4%) |
| Connective tissue disease | 1738 (3.2%) |
| Presentation | |
| PE with or without DVT | 27 887 (51%) |
| Cardiac arrest/collapse | 1579/27 887 (5.7%) |
| DVT only | 26 482 (49%) |
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the mean and standard deviation. DVT indicates deep vein thrombosis; JROAD‐DPC, Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination; PE, pulmonary embolism.
Figure 2.
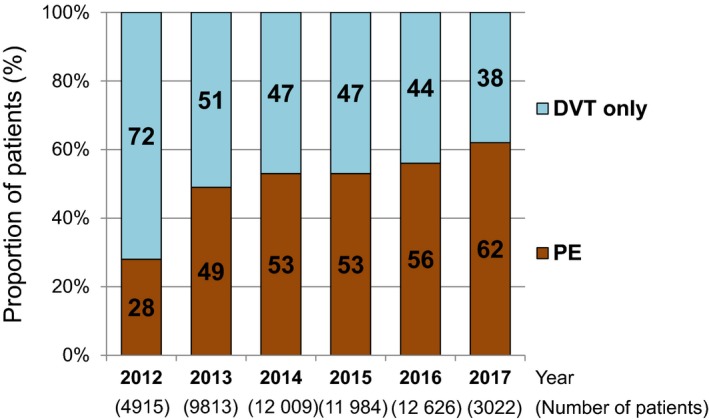
Annual changes in the proportion of PE and DVT among patients with VTE diagnosis from 2012 to 2017. DVT indicates deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Treatment Strategies
Parenteral anticoagulation therapy with unfractionated heparin was administered in 78% of patients during hospitalization (Table 2). The prevalence of thrombolysis and inferior vena cava filter use was 8.6% and 20%, respectively. The prevalence of thrombolysis use decreased over time from 11% in 2012 to 7% in 2017 (P for trend <0.001) (Figure 3A), and the prevalence of inferior vena cava filter use substantially decreased over time from 32% in 2012 to 14% in 2017 (P for trend <0.001) (Figure 3B).
Table 2.
Treatment Strategies
| JROAD‐DPC Data (N=54 369) | |
|---|---|
| Treatment during hospitalization | |
| Parenteral anticoagulation therapy with unfractionated heparin | 42 495 (78%) |
| Thrombolysisa | 4667 (8.6%) |
| Inferior vena cava filter use | 10 816 (20%) |
| Ventilator support | 1595 (6.5%) |
| Mechanical circulation supportb | 341 (1.4%) |
| Medications at discharge alive (N=51 169) | |
| Statins | 5375/51 169 (11%) |
| Antiplatelet drugsc | 3712/51 169 (7.3%) |
| Anticoagulation therapy | 38 198/51 169 (75%) |
| Warfarin | 23 191/51 169 (45%) |
| Direct oral anticoagulants (Xa inhibitors) | 14 252/51 169 (28%) |
| Edoxaban | 8260/51 169 (16%) |
| Rivaroxaban | 3373/51 169 (6.6%) |
| Apixaban | 2600/51 169 (5.1%) |
| Heparin | 504/51 169 (1.0%) |
| Others | 270/51 169 (0.5%) |
Categorical variables are presented as numbers and percentages. JROAD‐DPC indicates Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination.
Thrombolysis included tissue plasminogen activator and urokinase.
Mechanical circulation support included intra‐aortic balloon pumping and percutaneous cardiopulmonary support.
Antiplatelet drugs included aspirin, clopidogrel, ticlopidine, cilostazol, prasugrel, ticagrelor, sarpogrelate, and ozagrel.
Figure 3.
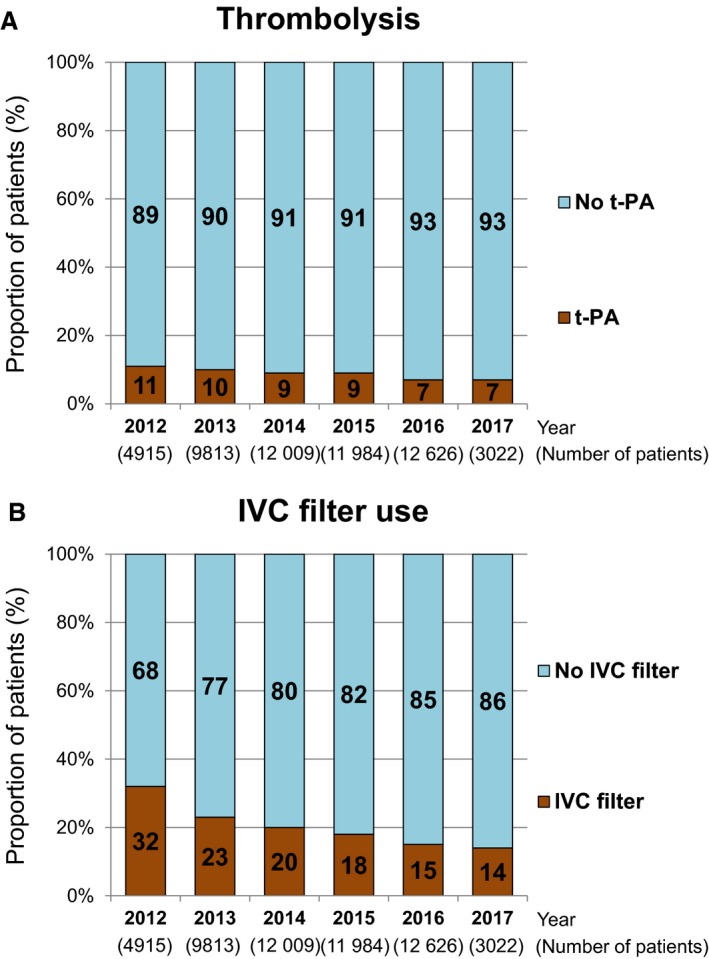
Annual changes in the prevalence of (A) thrombolysis and (B) IVC filter use from 2012 to 2017. IVC indicates inferior vena cava; t‐PA, tissue plasminogen activator.
Anticoagulation therapy was prescribed in 38 198 patients (75%) out of 51 169 patients who were alive at discharge (Table 2). Only few patients received heparin (1.0%), and the vast majority of patents received warfarin (45%) or DOACs (Xa inhibitors) at discharge (28%). After the release of the first DOAC (edoxaban) for VTE in September 2014 in Japan, the proportion of patients receiving DOACs increased dramatically among patients with anticoagulation therapy at discharge with use of edoxaban, rivaroxaban, and apixaban in 35%, 22%, and 27% of patients, respectively, in 2017 (Figure 4). On the other hand, the proportion of patients receiving warfarin decreased from 94% in 2012 to 15% in 2017.
Figure 4.
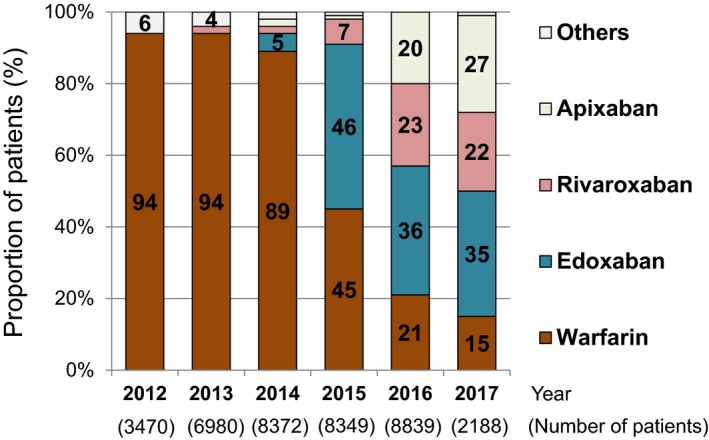
Annual changes in the proportions of the types of anticoagulation therapy (warfarin, edoxaban, rivaroxaban, apixaban, and others) among patients with anticoagulation therapy at discharge from 2012 to 2017.
In‐Hospital All‐Cause Death, and Length and Cost of Hospitalization
During hospitalization, 3200 (5.9%) patients died, and 736 (1.4%) patients died at 7 days (Table 3). The median length of hospital stay was 15 days, and those for PE and DVT were 16 and 13 days, respectively. The median cost of hospitalization was 685 813 Japanese yen (US$6235), and the costs for patients with PE and DVT were 846 379 Japanese yen (US$7694) and 540 487 Japanese yen (US$4914), respectively. The median length of hospital stay decreased over time from 16 days in 2012 to 13 days in 2017 (P for trend <0.001), which was more prominent in patients with PE (from 20 days in 2012 to 13 days in 2017, P for trend <0.001) than in patients with DVT (from 14 days in 2012 to 12 days in 2017, P for trend <0.001) (Table 4). Similarly, the median cost of hospitalization for PE moderately decreased over time from 1 271 128 Japanese yen (US$11 556) in 2012 to 715 340 Japanese yen (US$6503) in 2017 (P for trend <0.001), whereas that for DVT slightly decreased over time from 550 804 Japanese yen (US$5008) in 2012 to 524 662 Japanese yen (US$4770) in 2017 (P for trend <0.001). The median length of hospital stay was shorter and charge of hospitalization was lower in patients with DOACs than in patients with warfarin (Table 5).
Table 3.
In‐Hospital All‐Cause Death, and Length and Cost of Hospitalization
| JROAD‐DPC Data (N=54 369) | |
|---|---|
| In‐hospital all‐cause death | 3200 (5.9%) |
| Death at 1 d | 736 (1.4%) |
| Death at 7 d | 1670 (3.1%) |
| In‐hospital all‐cause death in PE patients (N=27 887) | 2679/27 887 (9.6%) |
| In‐hospital all‐cause death in DVT patients (N=26 482) | 521/26 482 (2.0%) |
| Length of hospital stay, d | 15 (9‐22) |
| Length of hospital stay in PE patients, d | 16 (10‐24) |
| Length of hospital stay in DVT patients, d | 13 (8‐21) |
| Cost of hospitalization (Japanese yen) | 685 813 (415 583‐1 136 967) |
| Cost of hospitalization in PE patients (Japanese yen) | 846 379 (527 045‐1 322 467) |
| Cost of hospitalization in DVT patients (Japanese yen) | 540 487 (355 999‐918 488) |
| Cost of hospitalization (US dollarsa) | 6235 (3778‐10 336) |
| Cost of hospitalization in PE patients (US dollarsa) | 7694 (4791‐12 022) |
| Cost of hospitalization in DVT patients (US dollarsa) | 4914 (3236‐8350) |
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the median and interquartile range. DVT indicates deep vein thrombosis; JROAD‐DPC, Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination; PE, pulmonary embolism.
1 dollar=110 Japanese yen.
Table 4.
In‐Hospital All‐Cause Death, and Length and Cost of Hospitalization by Year
| 2012 (N=4915) | 2013 (N=9813) | 2014 (N=12 009) | 2015 (N=11 984) | 2016 (N=12 626) | 2017 (N=3022) | P Values | |
|---|---|---|---|---|---|---|---|
| PE patients/DVT patients | 1384/3531 | 4821/4992 | 6321/5688 | 6404//5580 | 7073/5553 | 1884/1138 | <0.001a |
| In‐hospital all‐cause death | 213 (4.3%) | 527 (5.4%) | 781 (6.5%) | 722 (6.0%) | 757 (6.0%) | 200 (6.6%) | <0.001a |
| In‐hospital all‐cause death in PE patients | 143/1384 (10.3%) | 443/4821 (9.2%) | 665/6321 (10.5%) | 606/6404 (9.5%) | 650/7073 (9.2%) | 172/1884 (9.1%) | 0.15a |
| In‐hospital all‐cause death in DVT patients | 70/3531 (2.0%) | 84/4992 (1.7%) | 116/5688 (2.0%) | 116/5580 (2.1%) | 107/5553 (1.9%) | 28/1138 (2.5%) | 0.33a |
| Length of hospital stay, d | 16 (9‐25) | 16 (10‐24) | 15 (9‐23) | 14 (8‐22) | 14 (8‐21) | 13 (8‐19) | <0.001b |
| Length of hospital stay in PE patients, d | 20 (13‐31) | 17 (11‐26) | 16 (10‐24) | 15 (9‐23) | 15 (9‐22) | 13 (8‐20) | <0.001b |
| Length of hospital stay in DVT patients, d | 14 (9‐23) | 14 (9‐21) | 14 (8‐21) | 13 (8‐20) | 12 (7‐20) | 12 (7‐18) | <0.001b |
| Cost of hospitalization (Japanese yen) | 709 821 (407 223‐1 265 617) | 724 879 (432 995‐1 223 130) | 705 839 (428 489‐1 142 785) | 678 703 (410 659‐1 115 117) | 653 318 (400 483‐1 074 754) | 639 843 (398 974‐1 014 805) | <0.001b |
| Cost of hospitalization in PE patients (Japanese yen) | 1 271 128 (856 237‐1 953 864) | 947 670 (578 064‐1 477 492) | 861 313 (547 431‐1 324 174) | 822 105 (515 383‐1 267 296) | 764 853 (481 503‐1 190 960) | 715 340 (458 336‐1 079 578) | <0.001b |
| Cost of hospitalization in DVT patients (Japanese yen) | 550 804 (366 367‐953 045) | 559 040 (363 521‐942 797) | 540 365 (362 608‐922 006) | 536 711 (349 154‐899 444) | 525 192 (342 920‐886 537) | 524 662 (342 574‐881 519) | <0.001b |
| Cost of hospitalization (US dollarsc) | 6453 (3702‐11 506) | 6590 (3936‐11 120) | 6417 (3896‐10 389) | 6170 (3734‐10 137) | 5939 (3641‐9771) | 5817 (3628‐9226) | <0.001b |
| Cost of hospitalization in PE patients (US dollarsc) | 11 556 (7784‐17 763) | 8615 (5255‐13 432) | 7830 (4977‐12 038) | 7474 (4686‐11 521) | 6954 (4378‐10 827) | 6503 (4166‐9814) | <0.001b |
| Cost of hospitalization in DVT patients (US dollarsc) | 5008 (3331‐8664) | 5082 (3305‐8571) | 4913 (3296‐8382) | 4879 (3174‐8177) | 4774 (3117‐8059) | 4770 (3115‐8014) | <0.001b |
Continuous variables are presented as the median and interquartile range. DVT indicates deep vein thrombosis; JROAD‐DPC, Japanese Registry of All Cardiac and Vascular Diseases—Diagnosis Procedure Combination; PE, pulmonary embolism.
Changes of categorical variables were evaluated using the Cochran‐Armitage test for trend.
Changes of continuous variables were evaluated using Jonckheere‐Terpstra test for trend. The current analysis in the JROAD‐DPC database included between April 2012 and March 2017, and 2012 and 2017 were partial years.
1 dollar=110 Japanese yen.
Table 5.
Patient Characteristics, Treatment Strategies, and Outcomes According to Anticoagulation Therapy at Discharge
| Warfarin (N=23 191) | Edoxaban (N=8260) | Rivaroxaban (N=3373) | Apixaban (N=2600) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | 67.8±15.6 | 68.7±15.3 | 66.0±15.1 | 69.3±15.1 |
| Women | 13 156 (57%) | 4901 (59%) | 1789 (53%) | 1516 (58%) |
| Body weight, kg | 57.8±18.2 | 57.5±17.9 | 59.7±18.4 | 58.3±17.7 |
| Cancer | 4070 (18%) | 1728 (21%) | 507 (15%) | 419 (16%) |
| PE with or without DVT | 10 942 (47%) | 4726 (57%) | 1994 (59%) | 1545 (59%) |
| Treatment strategies | ||||
| Parenteral anticoagulation therapy with unfractionated heparin | 20 710 (89%) | 6765 (82%) | 2369 (70%) | 1778 (68%) |
| Thrombolysis | 2342 (10%) | 698 (8.5%) | 344 (10%) | 200 (7.7%) |
| Inferior vena cava filter use | 5566 (24%) | 1582 (19%) | 667 (20%) | 481 (19%) |
| Outcomes | ||||
| Length of hospital stay, d | 16 (11‐24) | 13 (9‐19) | 13 (9‐20) | 13 (9‐19) |
| Length of hospital stay in PE patients, d | 18 (13‐26) | 14 (10‐20) | 14 (10‐20) | 14 (10‐20) |
| Length of hospital stay in DVT patients, d | 15 (10‐22) | 12 (7‐18) | 12 (8‐18) | 12 (8‐17) |
| Cost of hospitalization (Japanese yen) | 750 422 (481 691‐1 176 026) | 648 740 (426 355‐1 021 018) | 681 706 (441 262‐1 065 280) | 655 103 (435 890‐985 990) |
| Cost of hospitalization in PE patients (Japanese yen) | 957 239 (648 433‐1 401 474) | 757 008 (514 060‐1 115 912) | 766 302 (524 554‐1 133 127) | 724 741 (505 599‐1 045 703) |
| Cost of hospitalization in DVT patients (Japanese yen) | 580 384 (401 938‐946 000) | 511 824 (353 912‐860 228) | 557 630 (354 136‐928 708) | 530 698 (366 654‐881 156) |
| Cost of hospitalization (US dollarsa) | 6822 (4379‐10 691) | 5898 (3876‐9282) | 6197 (4011‐9684) | 5956 (3963‐8964) |
| Cost of hospitalization in PE patients (US dollarsa) | 8702 (5895‐12 741) | 6882 (4673‐10 145) | 6966 (4769‐10 302) | 6589 (4596‐9506) |
| Cost of hospitalization in DVT patients (US dollarsa) | 5277 (3654‐8600) | 4653 (3217‐7820) | 5069 (3220‐8443) | 4825 (3333‐8011) |
Categorical variables are presented as numbers and percentages. Continuous variables are presented as the median and interquartile range. DVT indicates deep vein thrombosis; PE, pulmonary embolism.
1 dollar=110 Japanese yen.
Discussion
The main findings of the present report from the current nationwide database were these: (1) among patients hospitalized with VTE, the proportion of patients with DVT decreased over time; (2) after the release of DOACs, the proportions of DOACs increased dramatically, and that of warfarin decreased gradually; (3) the median length of hospital stay and cost of hospitalization decreased over time, and this effect was greater for PE than for DVT.
Now, it is a matter of active debate whether VTE patients should be treated at an inpatient department or an outpatient department. In the era of warfarin, patients with acute VTE needed to be treated with intravenous heparin followed by warfarin in hospital. However, in the era of DOACs for VTE, intravenous heparin in hospital can be replaced by the use of some DOACs, and patients with VTE can be treated entirely at home without administration of intravenous anticoagulant.7, 8, 9 The risk for mortality in DVT patients is thought to be relatively low if they have no concomitant PE, and most DVT patients could be treated without hospitalization using DOACs. The current study showed that the proportion of patients with DVT decreased over time among patients hospitalized with VTE, suggesting that some DVT patients were treated entirely at home. However, there might still be high‐risk DVT patients who should be treated in hospital with close monitoring. Thus, the identification of high‐risk DVT patients could be clinically relevant.
Due to these randomized clinical trial results and ease of use in daily clinical practice, the use of DOACs for VTE has prevailed worldwide. After the release of DOACs, several national guidelines for the management of VTE were updated and now recommend DOACs as broadly preferable to warfarin for most patients with VTE.20, 21 Consistent with these recommendations, the current study showed the dramatically increased use of DOACs and the gradually decreased use of warfarin. However, clinicians should be cautious in the use of DOACs for specific high‐risk patients such as patients with active cancer. Historically, low‐molecular‐weight heparin has been recommended over warfarin for VTE patients with active cancer. Recently, DOACs have been reported to have comparable efficacy and safety compared with low‐molecular‐weight heparin in VTE patients with active cancer.22, 23 DOACs could be good alternatives for VTE patients with active cancer. However, the Hokusai VTE Cancer study also showed that VTE patients with gastrointestinal cancer had an increased risk for major bleeding with edoxaban, mainly due to upper gastrointestinal bleeding.22 The guidance from the International Society on Thrombosis and Haemostasis suggested the use of low‐molecular‐weight heparins over DOACs for cancer patients with an acute diagnosis of VTE and a high risk of bleeding, including patients with gastrointestinal cancers, patients with cancers at risk of bleeding from the genitourinary tract, bladder, or nephrostomy tubes, and patients with active gastrointestinal mucosal abnormalities.24 Because of the higher risk for bleeding in VTE patients with specific cancers, clinicians should still be cautious about the use of DOACs for such patients.
DOACs could provide not only medical benefits but also medical‐economic benefits. If patients with VTE can be treated entirely at home, there was no cost associated with hospitalization. DVT patients could be treated entirely at home, and patients with low‐risk PE could also be treated with DOACs without hospitalization.25, 26, 27 Furthermore, DOACs have no need for dose adjustment based on blood tests, and some DOACs have been used as a single‐drug approach without administeration of intravenous anticoagulants, which would enable early hospital discharge. Although the duration of hospital stay could vary widely depending on both medical system and environment in various countries,28, 29 a recent study has reported that the mean length of hospital stay significantly decreased over time.30 Consistent with the previous report, the current study showed a decrease of the median length of hospital stay over time that was more prominent in PE patients. Accordingly, the median charge of hospitalization decreased over time, which was partly because patients with DOACs had a shorter duration of hospitalization compared with those with warfarin. Thus, the identification of low‐risk patients who would be more suitable for early hospital discharge or home treatment is becoming clinically more relevant.
Study Limitations
The current study has several limitations. First, although DPC data must be confirmed by a physician and are highly reliable, some of the data are based on medical claims. Therefore, there is a possibility that these data may contain certain errors, and some data may be underestimated because they are not reflected in claims. Especially, the data showing a relatively low proportion of anticoagulation at discharge should be viewed cautiously. Second, although this research was conducted using nationwide databases and very closely represents the current whole situation in Japan, there is a possibility that actual conditions in nonspecialized facilities are not reflected. Third, because the annual survey of the DPC database in Japan is conducted from April to March in each year, numbers in the database for first year and last year are small, which should indicate caution in interpreting the study results. Fourth, the prevalence of inferior vena cava filter use was high in Japan compared with that in the United States, which could have some influence on outcomes.28 Fifth, because the current analysis did not include patients without hospitalization, we could not evaluate VTE patients treated at outpatient departments. Sixth, we could not investigate the anticoagulation therapy status before admission.
Conclusions
A nationwide claim‐based database provided the current practice pattern for VTE in Japan, which revealed dynamic changes after the release of DOACs.
Sources of Funding
The current study is supported by a research grant from the Pfizer Health Research Foundation (Tokyo, Japan) to Dr Yamashita. The research funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosures
Dr Yamashita received lecture fees from Daiichi‐Sankyo, Pfizer, Bristol‐Myers Squibb, and Bayer Healthcare. Dr Morimoto received lecture fees from Mitsubishi Tanabe Pharma and Pfizer Japan and consultant fees from Asahi Kasei, Bristol‐Myers Squibb, and Boston Scientific. Dr Kimura serves as an advisory board member for Abbott Vascular and Terumo Company. The remaining authors have no disclosures to report.
Acknowledgments
We appreciate the contributions of all the investigators, clinical research coordinators, and data managers involved in the JROAD‐DPC study.
(J Am Heart Assoc. 2020;9:e014582 DOI: 10.1161/JAHA.119.014582.)
References
- 1. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M; VTE Impact Assessment Group in Europe . Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. [DOI] [PubMed] [Google Scholar]
- 2. Lee LH, Gallus A, Jindal R, Wang C, Wu CC. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117:2243–2260. [DOI] [PubMed] [Google Scholar]
- 3. Ota S, Matsuda A, Ogihara Y, Yamada N, Nakamura M, Mori T, Hamada M, Kobayashi T, Ito M. Incidence, characteristics and management of venous thromboembolism in Japan during 2011. Circ J. 2018;82:555–560. [DOI] [PubMed] [Google Scholar]
- 4. Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9:85–91. [DOI] [PubMed] [Google Scholar]
- 5. Lee CH, Lin LJ, Cheng CL, Kao Yang YH, Chen JY, Tsai LM. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost. 2010;8:1515–1523. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Konishi T, Akao M, Kobayashi Y, Inoue T, Oi M, Izumi T, Takahashi K, Tada T, Chen PM, Murata K, Tsuyuki Y, Sakai H, Saga S, Sasa T, Sakamoto J, Yamada C, Kinoshita M, Togi K, Ikeda T, Ishii K, Kaneda K, Mabuchi H, Otani H, Takabayashi K, Takahashi M, Shiomi H, Makiyama T, Ono K, Kimura T; COMMAND VTE Registry Investigators . Anticoagulation therapy for venous thromboembolism in the real world‐ from the COMMAND VTE Registry. Circ J. 2018;82:1262–1270. [DOI] [PubMed] [Google Scholar]
- 7. EINSTEIN Investigators , Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 8. EINSTEIN‐EP Investigators , Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. [DOI] [PubMed] [Google Scholar]
- 9. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI; AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 10. Yasuda S, Nakao K, Nishimura K, Miyamoto Y, Sumita Y, Shishido T, Anzai T, Tsutsui H, Ito H, Komuro I, Saito Y, Ogawa H; on the behalf of JROAD INVESTIGATORS . The current status of cardiovascular medicine in Japan—analysis of a large number of health records from a Nationwide Claim‐Based Database, JROAD‐DPC. Circ J. 2016;80:2327–2335. [DOI] [PubMed] [Google Scholar]
- 11. Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the aging society of Japan. Circulation. 2018;138:965–967. [DOI] [PubMed] [Google Scholar]
- 12. Tomoike H, Yokoyama H, Sumita Y, Hanai S, Kada A, Okamura T, Yoshikawa J, Doi Y, Hori M, Tei C; Scientific Committee of the JCS . Nationwide distribution of cardiovascular practice in Japan—results of Japanese Circulation Society 2010 annual survey. Circ J. 2015;79:1058–1067. [DOI] [PubMed] [Google Scholar]
- 13. Yasunaga H, Ide H, Imamura T, Ohe K. Impact of the Japanese Diagnosis Procedure Combination‐based payment system on cardiovascular medicine‐related costs. Int Heart J. 2005;46:855–866. [DOI] [PubMed] [Google Scholar]
- 14. Wada T, Yasunaga H, Horiguchi H, Matsubara T, Fushimi K, Nakajima S, Yahagi N. Outcomes of argatroban treatment in patients with atherothrombotic stroke: observational nationwide study in Japan. Stroke. 2016;47:471–476. [DOI] [PubMed] [Google Scholar]
- 15. Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc Med. 2015;20:364–368. [DOI] [PubMed] [Google Scholar]
- 17. Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002;88:407–414. [PubMed] [Google Scholar]
- 18. Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 19. Jonckheere AR. A distribution‐free kappa‐sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 20. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology . 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069, 3069a‐3069k. [DOI] [PubMed] [Google Scholar]
- 21. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 22. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Buller HR; Hokusai VTE Cancer Investigators . Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
- 23. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36:2017–2023. [DOI] [PubMed] [Google Scholar]
- 24. Khorana AA, Noble S, Lee AYY, Soff G, Meyer G, O'Connell C, Carrier M. Role of direct oral anticoagulants in the treatment of cancer‐associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891–1894. [DOI] [PubMed] [Google Scholar]
- 25. Barco S, Schmidtmann I, Ageno W, Bauersachs RM, Becattini C, Bernardi E, Beyer‐Westendorf J, Bonacchini L, Brachmann J, Christ M, Czihal M, Duerschmied D, Empen K, Espinola‐Klein C, Ficker JH, Fonseca C, Genth‐Zotz S, Jimenez D, Harjola VP, Held M, Iogna Prat L, Lange TJ, Manolis A, Meyer A, Mustonen P, Rauch‐Kroehnert U, Ruiz‐Artacho P, Schellong S, Schwaiblmair M, Stahrenberg R, Westerweel PE, Wild PS, Konstantinides SV, Lankeit M; HoT‐PE Investigators . Early discharge and home treatment of patients with low‐risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single‐arm clinical trial. Eur Heart J. 2019. DOI: 10.1093/eurheartj/ehz367. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Bledsoe JR, Woller SC, Stevens SM, Aston V, Patten R, Allen T, Horne BD, Dong L, Lloyd J, Snow G, Madsen T, Elliott CG. Management of low‐risk pulmonary embolism patients without hospitalization: the Low‐Risk Pulmonary Embolism Prospective Management Study. Chest. 2018;154:249–256. [DOI] [PubMed] [Google Scholar]
- 27. Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Saga S, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Shiomi H, Kato T, Makiyama T, Ono K, Kimura T; COMMAND VTE Registry Investigators . Validation of simplified PESI score for identification of low‐risk patients with pulmonary embolism: from the COMMAND VTE Registry. Eur Heart J Acute Cardiovasc Care. 2018. DOI: 10.1177/2048872618799993. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. Nathan AS, Geng Z, Dayoub EJ, Khatana SAM, Eberly LA, Kobayashi T, Pugliese SC, Adusumalli S, Giri J, Groeneveld PW. Racial, ethnic, and socioeconomic inequities in the prescription of direct oral anticoagulants in patients with venous thromboembolism in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB‐VTE population‐based study. Am J Med. 2016;129:879.e19–e25. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Lei J, Shao X, Dong F, Wang J, Wang D, Wu S, Xie W, Wan J, Chen H, Ji Y, Yi Q, Xu X, Yang Y, Zhai Z, Wang C; China Venous Thromboembolism Study Group . Trends in hospitalization and in‐hospital mortality from VTE, 2007 to 2016, in China. Chest. 2019;155:342–353. [DOI] [PubMed] [Google Scholar]


