Abstract
Background
The prevalence of hypertension in low‐ and middle‐income countries is rapidly increasing, with most cases undiagnosed and many poorly controlled among those diagnosed. Medication reconciliation studies from high‐income countries have demonstrated a high occurrence of antihypertensive medication errors and a strong association between medication errors and inadequate blood pressure control, but data from low‐ and middle‐income countries are lacking.
Methods and Results
We conducted a cross‐sectional study from April to October 2018 of adult patients on pharmacologic management for known hypertension at 7 public health facilities in Kweneng East District, Botswana. Our aims included to evaluate the frequency of uncontrolled hypertension, the frequency and type of medication errors causing discrepancies between patient‐reported and prescribed antihypertensive medications, and the association between medication errors and uncontrolled hypertension. Descriptive analyses and multivariable logistic regression were used. The prevalence of uncontrolled hypertension was 55% among 280 enrolled adult patients, and 95 (34%) had ≥1 medication error. The most common errors included patients taking medications incorrectly (11.1%; 31/280), patients omitting medications (7.9%; 22/280), and unfilled prescriptions caused by pharmacy stock outs (7.5%%; 21/280). Uncontrolled hypertension was significantly associated with having ≥1 medication error compared with no errors (adjusted odds ratio, 3.26; 95% CI, 1.75–6.06; P<0.001).
Conclusions
Medication errors are strongly associated with poor blood pressure control in this setting. Further research is warranted to assess whether medication reconciliation and other low‐cost interventions addressing root causes of medication errors can improve the control of hypertension and other chronic conditions in low‐ and middle‐income countries.
Keywords: hypertension, low‐ and middle‐income countries, medication errors, medication reconciliation
Subject Categories: High Blood Pressure, Hypertension, Quality and Outcomes, Health Services
Clinical Perspective
What Is New?
This is the first study to date to document an association between medication errors and blood pressure control among patients on pharmacologic management for hypertension in low‐ and middle‐income countries.
What Are the Clinical Implications?
Medication errors may be a major modifiable risk factor for uncontrolled hypertension, and interventions targeting the root causes of the most common errors may have the potential to significantly improve blood pressure control.
Reduction in errors will require multicomponent interventions targeting errors generated by patients, prescribers, and medication dispensers, including guideline‐driven hypertension management, patient education, and standard documentation and dispensing.
Hypertension is estimated to account for 14% of all global mortality and significant morbidity.1, 2 Although the prevalence of hypertension in high‐income countries is stable or decreasing, the prevalence is rapidly increasing in low‐ and middle‐income countries (LMICs).2, 3, 4 The efficacy of blood pressure–lowering medications for control of hypertension and prevention of cardiovascular complications is well demonstrated.5, 6, 7 However, disease control is poor worldwide, particularly in LMICs where many cases are undiagnosed and <50% of the affected population has controlled disease.8, 9, 10, 11, 12
In Botswana, a middle‐income country in southern Africa, the prevalence of hypertension is estimated to be 29% to 33% among 15‐ to 64‐year‐old adults.8, 13 In a large population‐based cross‐sectional household survey of 10% of the population, over half of those with hypertension were undiagnosed.8 Botswana, where adult HIV prevalence is 22%14 has long been considered a world leader in the fight against HIV/AIDS and has been successful in implementing universally accessible antiretroviral treatment and reducing HIV‐related morbidity and mortality. However, in Botswana as in other LMICs, healthcare gains in the field of infectious disease are now being challenged by epidemiological and lifestyle transitions, leading to an increasing burden of noncommunicable diseases, such as hypertension. As with other LMICs, the medical management of hypertension in the public healthcare setting in Botswana presents particular challenges caused by multiple barriers, including frequent medication stock outs, inadequate follow‐up and continuity of care, low provider/patient ratio, and suboptimal training and support of medical providers.15, 16, 17 In such settings, identifying opportunities for low‐cost, high‐quality interventions may be essential to achieve adequate disease control.
One proposed intervention is the use of medication reconciliation. Medication reconciliation is a formal process of obtaining the most complete and accurate list of a patient's current medications and comparing this list with that in the patient's medical record with the goal of identifying errors and reconciling discrepancies.18 Studies in high‐income countries have documented frequent discrepancies, ranging from 14.1% to 87%, between medications patients report taking and those listed in their medical record.19, 20, 21, 22, 23 Accurate medication reconciliation has been shown to decrease hospital readmissions and improve medication adherence.21, 22, 23, 24, 25 Evidence on similar discrepancies and their consequences in LMICs is limited.25, 26 Frequent drug changes and multidrug regimens make patients with hypertension particularly susceptible to medication errors, and subsequent discrepancies in antihypertensive medications have been strongly associated with poor blood pressure control in high‐income countries.18 The extent to which medication discrepancies affect hypertension control in LMICs is not well understood.
We sought to describe the frequency and causes of antihypertensive medication discrepancies between patient‐reported medications and medications listed in the outpatient record and to evaluate the association between medication errors and hypertension control in ambulatory healthcare facilities in Botswana.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Setting and Participants
Kweneng East is a rural district in Botswana with a population of 190 000.27
The district's public health system consists of a 300‐bed district referral hospital, a 25‐bed primary hospital, 13 primary care clinics, and 15 rural health posts. Consultations and medications in all these facilities are available free of charge to citizens of Botswana. The district's outpatient care services are staffed by 6 nonspecialized general physicians serving adults and children at the community clinics and health posts. An additional 11 nonspecialist physicians staff outpatient clinics and emergency departments at the primary hospital and district referral hospital. Two internal medicine specialists are available at the district referral hospital. Subspecialty services are only available at a tertiary referral facility located in an adjacent district. Given the relative shortage of physicians, many primary care services in the district health system are provided primarily by nurses. Patients diagnosed with hypertension were recruited from 7 public healthcare facilities serving semiurban (n=4) and rural (n=3) communities in the district. Facilities were chosen in discussion with the 6 community physicians working in the district and selected on the basis of volume of patients seen with hypertension. The facilities chosen included 2 hospital‐based outpatient departments, 2 community clinics, and 3 rural health posts. All facilities were staffed primarily by nurses. Family nurse practitioners were available in the community clinics. Physicians staffed hospital‐based outpatient departments daily, community clinics twice a week, and rural health posts once a month. Eligible participants were aged ≥18 years, already diagnosed with hypertension, and managed with antihypertensive medications. Patients were ineligible if they did not meet inclusion criteria or were unable to give informed consent.
Data Collection
From April 2018 to October 2018, trained nurses at each of the 7 sites consented patients with hypertension presenting for routine care visits and scheduled them for study interviews with a physician. All study interviews were conducted in Setswana with a trained nurse translator. Each patient was scheduled for a study visit within a month of enrollment and asked to bring his/her home medications to the study visit. Patients were reimbursed for their transportation costs to attend the study visit but were not otherwise compensated. Blood pressure measurements were obtained and recorded by health facility staff at enrollment and on patients’ return for the study visit using automatic blood pressure cuffs. In addition, during the study visit, all other blood pressure measurements recorded in patients’ charts during the 12 months preceding the study visit were collected. Blood pressures taken in the emergency department or inpatient setting were excluded. Sociodemographic and clinical data, including years of formal education, employment status, number of household members, household income, comorbidities, tobacco and alcohol use, and physical activity, were collected through patient interviews. Data on household income were collected on the basis of income quartiles that included the following: (1) below poverty line (<$60 US dollars [USD]); (2) between the poverty line and the rural average monthly salary ($60–$259.9 USD); (3) between the rural average monthly salary and the urban average monthly salary ($260–$524.9 USD); and (4) above the urban average monthly salary (>$525 USD).26 Self‐reported adherence was assessed using a modified Morisky 8‐Item Medication Adherence Scale.28 Health literacy was assessed using a 6‐question health literacy questionnaire developed in collaboration with local healthcare practitioners. This included questions on awareness of hypertension and its complications, related lifestyle issues, blood pressure goals, and benefits of treatment. The medications prescribed in the medical chart at patients’ most recent follow‐up visit were compared with the medications patients brought or reported taking. Prescribed medication dosages and total pill number for hypertension, diabetes mellitus, and cardiovascular risk management (cardiovascular disease [CVD] pill burden) were recorded. Patients who failed to bring their medications and were unable to name them were shown sample pills to test for recognition. When identified, medication discrepancies were categorized by cause, referred to as medication error, and recorded. At the end of the study visit, medication errors causing discrepancies were corrected, active medical issues were addressed, and necessary follow‐up, including routine follow‐up clinic appointments, was scheduled by the study physicians.
Classification of Causes of Medication Discrepancies
Medication discrepancies were classified by underlying cause (medication error) as resulting from patient errors, dispenser errors, or prescriber errors. Errors were further categorized by error type as follows:
Patient errors
Type I: Omission of a prescribed medication (did not refill prescription or missing medication not explained by medication out of stock error).
Type II: Taking a medication not prescribed.
Type III: Taking medication at a different frequency or dose from that prescribed.
Dispenser errors
Type I: Discrepancy between prescribed medication or instruction and dispensed medication or instruction.
Type II: Patient not taking prescribed medication because of medication stock out and no referral back to prescriber for alternative medication option.
Prescriber errors
Type I: Dose or frequency inconsistent with guidelines or manufacturer's recommendations.
Type II: Prescription of ≥2 forms of the same medication or medication class.
Type III: Changes in antihypertensive medication prescription without adequate indication or explanation.
Type IV: Incomplete prescription (omission of dose, frequency, or duration).
Definition of Blood Pressure Control
Blood pressure cutoffs for the definition of uncontrolled hypertension were selected on the basis of 2016 Botswana National Primary Care guidelines.29 These included a systolic or diastolic blood pressure of ≥140 and ≥90 mm Hg, respectively, in nondiabetic patients and ≥130 and ≥80 mm Hg, respectively, in patients with diabetes mellitus. Primary data analysis was performed using an average of 2 blood pressure readings taken within 1 month of each other (at initial screening and at a scheduled study visit). The use of long‐term cumulative blood pressure measurements has been shown to more accurately predict cardiovascular risk.30 We, therefore, also recorded all blood pressure measurements documented in the patient‐held paper medical record over the course of the preceding 12 months and calculated the percentage of uncontrolled readings.
Statistical Analysis
Analyses were conducted using Stata, version Stata/MP 15.0 (StataCorp).31
χ2 and t tests were used to compare patient characteristics between those with medication discrepancies and those without discrepancies. A 2‐tailed P<0.05 was considered statistically significant. Multivariable logistic regression was used to compute adjusted odds ratios (aORs) for uncontrolled blood pressure. The final model controlled for sex, age, education (total number of years), monthly household income, tobacco use status, CVD pill burden, number of comorbidities, clinic type (health post, community clinic, or hospital outpatient clinic), suboptimal medication reconciliation, and self‐reported adherence.
Ethics
The study protocol and all study materials were approved by the institutional review boards of the Botswana Health Development and Research Council, Gaborone, Botswana; Beth Israel Deaconess Medical Center, Boston, MA; and Kweneng East District Health Management Team, Molepolole, Botswana.
Results
Patient Demographics and Comorbidities
A total of 73.5% of patients who consented to the study and were scheduled for a study visit returned to complete the study interview (Figure). A total of 280 patients with hypertension were enrolled in the study and completed a study interview, of which 233 (83.2%) were women. All participants were citizens of Botswana, with a homogeneous Motswana ethnic origin. A total of 128 patients (45.7%) were enrolled at hospital clinics, 89 (31.8%) at community clinics, and 63 (22.5%) at health posts. Patients’ median age was 59 years (interquartile range, 49–67 years), with 24 (8.6%) aged <40 years and 125 (44.6%) aged >60 years. A total of 80 patients (28.6%) had never attended school, and 207 (74.0%) had <8 years of formal education. The median household size was 5 (interquartile range, 3–7). A total of 134 patients (47.9%) had a monthly household income below the poverty line of <$2 USD/day (600 Botswana pula/month). A total of 154 patients (55.0%) reported being unemployed. A total of 140 patients (50.0%) had ≥1 comorbidity not including HIV, 60 (21.4%) had diabetes mellitus, and 39 (13.9%) were HIV positive, lower than the national average of 22%27 (Table 1). A total of 239 patients (85.4%) self‐reported daily adherence to their antihypertensive regimens. The median number of pills a patient was prescribed to take daily for hypertension, diabetes mellitus, CVD, or CVD prevention (CVD pill burden) was 3 (interquartile range, 2–5). Patients were prescribed an average of 2.01 (SD, 0.80) blood pressure medications. Patients with uncontrolled blood pressure were prescribed significantly more medications for blood pressure control than those with controlled blood pressure (average, 2.12 versus 1.87; P=0009). There was no significant difference in the number of medications prescribed between patients who had ≥1 episode of hypotension and those with no recorded hypotension (1.78 versus 2.03; P=0.12). Over the course of a year, 124 (44.3%) of participants received all their clinical outpatient care at a single facility, 108 (38.6%) at 2 different facilities, and 48 (17.1%) at ≥3 different facilities.
Figure 1.
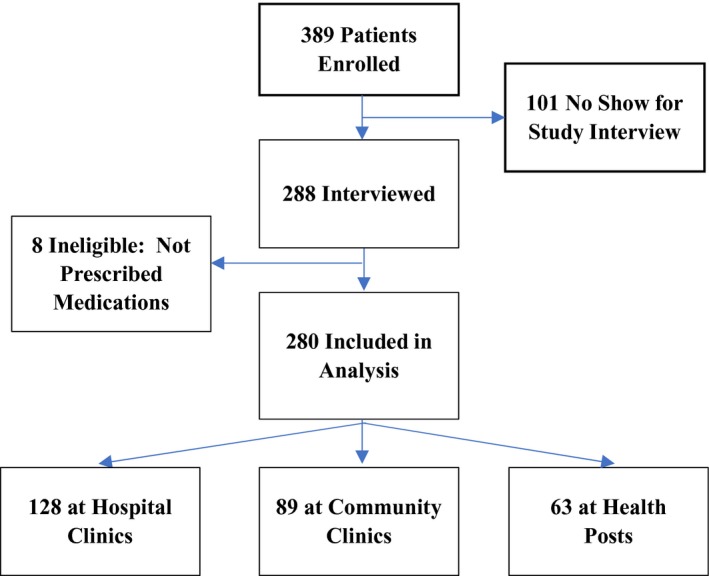
Summary of population enrolled, interviewed, and included in analysis.
Table 1.
Patient Characteristics by Presence of ≥1 Medication Error
| Variable | No Medication Errors (n=185) | ≥1 Medication Error (n=95) | Total Participants (n=280) | P Value |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 57.9 (13.2) | 60.1 (14.3) | 58.7 (13.6) | 0.199 |
| <40 y, % | 9.2 | 7.4 | 8.6 | 0.606 |
| >60 y, % | 43.8 | 46.3 | 44.6 | 0.687 |
| Sex | ||||
| Women, % | 82.2 | 85.3 | 83.2 | 0.511 |
| Body mass index, mean (SD), kg/m2 a | 29.2 (6.4) | 31.4 (7.4) | 30.0 (6.8) | 0.714 |
| Employment status | ||||
| Unemployed, % | 53.0 | 58.9 | 55 | 0.341 |
| Education | ||||
| Years of school, mean (SD) | 5.1 (4.5) | 4.6 (4.0) | 4.9 (4.4) | 0.359 |
| No education, % | 30.3 | 25.3 | 28.6 | 0.380 |
| Household size, mean (SD) | 5.3 (3.6) | 5.5 (3.2) | 5.4 (3.4) | 0.583 |
| Monthly income | ||||
| Below poverty line, %b | 46.5 | 50.5 | 47.9 | 0.522 |
| Active tobacco use | ||||
| Smoking cigarettes or inhaling snuff, % | 9.7 | 7.4 | 8.9 | 0.512 |
| Any alcohol use, % | 9.7 | 13.7 | 11.1 | 0.318 |
| Comorbidities (not HIV) | ||||
| ≥1, % | 43.8 | 62.1 | 50.0 | 0.004 |
| Diabetes mellitus, % | 16.2 | 31.6 | 21.4 | 0.003 |
| HIV status | ||||
| Positive, % | 14.1 | 13.7 | 13.9 | 0.534 |
| CVD risk scorec | ||||
| >10%, % | 12.4 | 29.5 | 18.2 | 0.005 |
| CVD pill burden, mean (SD)d | 4.1 (3.5) | 5.5 (4.0) | 4.6 (3.8) | 0.002 |
| Medications named, mean, % | 40.5 | 27.1 | 35.9 | 0.021 |
| Health literacy test, mean, % | 60.0 | 56.6 | 58.8 | 0.282 |
| Suboptimal medication reconciliation, %e | 18.4 | 31.6 | 22.9 | 0.013 |
| No. of clinic visits | ||||
| Time period: 1 y, mean (SD) | 10.6 (5.3) | 10.0 (4.9) | 10.4 (5.2) | 0.389 |
CVD indicates cardiovascular disease.
A total of 220 patients had height measurements to calculate body mass index. This included 73 patients (76.8%) with ≥1 medication error and 147 patients (78.5%) without medication errors.
Below poverty line: <$2/day, <600 pula/month.
World Health Organization Cardiovascular Disease Risk Score.
The number of pills prescribed daily to treat hypertension and diabetes mellitus and prevent CVD.
Patients unable to name their home medications and did not bring their home medications to the study interview.
Medication Reconciliation
The most recent prescribed medication list was available for all patients, and 201 (71.8%) brought their medications to the study visit for review. A total of 64 patients (22.9%) were neither able to name all of their home medications nor brought their home medications and were categorized as having a suboptimal medication reconciliation. All patients underwent medication reconciliation, and 95 (34.0%) had ≥1 discrepancy between the prescribed medication list and the medications patients brought or reported taking. When compared with patients with no medication discrepancies, patients with ≥1 medication discrepancy were able to name fewer of their home medications (average percentage, 40.5% versus 27.1%; P=0.021), had a higher mean CVD pill burden (5.5 versus 4.1; P=0.002), and were more likely to have ≥1 comorbidity (62.1% versus 43.8%; P=0.004), particularly diabetes mellitus (31.6% versus 16.2%; P=0.003) (Table 1). The most commonly prescribed medications were hydrochlorothiazide in 168 patients (60.0%), angiotensin‐converting enzyme inhibitors in 111 patients (40.0%), and calcium channel blockers in 139 patients (49.6%). Patients with medication errors were more often prescribed angiotensin‐converting enzyme inhibitors (51.6% versus 33.5%; P=0.003) and less frequently prescribed hydrochlorothiazide (49.5% versus 68.6%; P=0.002) (Table 2).
Table 2.
Commonly Prescribed Medications by Presence of ≥1 Error
| Commonly Prescribed Medications | No Errors (n=185) | Errors (n=95) | Total (n=280) | P Value |
|---|---|---|---|---|
| Enalapril | 32.4 (60) | 47.4 (45) | 37.5 (105) | 0.015 |
| Captopril | 1.1 (2) | 4.2 (4) | 2.1 (6) | 0.087 |
| ACE inhibitors | 33.5 (62) | 51.6 (49) | 39.6 (111) | 0.003 |
| Hydrochlorothiazide | 68.6 (127) | 49.5 (41) | 60 (168) | 0.002 |
| Nifedipine Extended Release (XL) | 38.4 (71) | 43.2 (41) | 40 (112) | 0.440 |
| Nifedipine Sustained Release (SR) | 5.9 (11) | 9.5 (9) | 7.1 (20) | 0.278 |
| Amlodipine | 1.1 (2) | 5.3 (5) | 2.5 (7) | 0.034 |
| Calcium channel blockers | 45.4 (84) | 57.9 (55) | 49.6 (139) | 0.157 |
| Atenolol | 17.3 (32) | 18.9 (18) | 17.9 (50) | 0.733 |
| Propranolol | 1.6 (3) | 3.2 (3) | 2.1 (6) | 0.401 |
| Carvedilol | 4.3 (8) | 6.3 (6) | 5.0 (14) | 0.469 |
| Bisoprolol | 0.0 | 1.1 (1) | 0.4 (1) | 0.162 |
| β Blockers | 23.2 (43) | 29.5 (28) | 25.4 (71) | 0.257 |
| Hydralazine | 0.5 (1) | 6.3 (6) | 2.5 (7) | 0.003 |
| Furosemide | 3.8 (7) | 16.8 (16) | 8.2 (23) | <0.001 |
| Spironolactone | 3.2 (6) | 6.3 (6) | 4.3 (12) | 0.229 |
| Telmisartan | 1.1 (2) | 6.3 (6) | 2.9 (8) | 0.013 |
| Hydrochlorothiazide+telmisartan (co‐Micardis) | 5.4 (10) | 5.3 (5) | 5.4 (15) | 0.960 |
Data are given as percentage (number). ACE indicates angiotensin‐converting enzyme.
Medication Errors
Among the 95 patients who had medication discrepancies, a total of 118 medication errors were identified. These included 57 of 280 (20.4%) with patient errors, 27 of 280 (9.6%) with dispenser errors, and 34 of 280 (12.1%) with prescriber errors. A total of 19 of 280 (6.8%) of patients with discrepancies had ≥2 types of errors. The most common errors were caused by 31 or 280 (11.1%) of patients taking a medication at a different frequency or dose than prescribed, 22 of 280 (7.9%) of patients omitting a prescribed medication, 21 of 280 (7.5%) of cases of unfilled prescriptions caused by pharmacy stock outs with no medication class substitution, and 16 of 280 (5.7%) of cases of medications prescribed at a dose or frequency inconsistent with guidelines or manufacturer's recommendations (Table 3). A total of 48 of 128 (37.5%), 30 of 128 (31.5%), and 19 of 63 (30.2%) of patients had ≥1 medication error at hospital clinics, community clinics, and health posts, respectively. There was no significant difference in frequency of medication errors among patients enrolled at different types of clinics (P=0.504).
Table 3.
Frequency and Type of Errors Causing Medication Discrepancies Among All Patients
| Type of Error | Patient Errors | Dispenser Errors | Prescriber Errors | All Errors |
|---|---|---|---|---|
| I | 22 (7.9) | 6 (2.1) | 16 (5.7) | … |
| II | 4 (1.4) | 21 (7.5) | 4 (1.4) | … |
| III | 31 (11.1) | … | 8 (2.9) | … |
| IV | … | … | 6 (2.1) | … |
| Total errors | 57 (20.4) | 27 (9.6) | 34 (12.1) | 118 |
Data are given as number (percentage). Patient errors: type I: omission of a prescribed medication (did not refill prescription or missing medication not explained by medication out of stock error); type II: taking a medication not prescribed; type III: taking medication at a different frequency or dose from that prescribed. Dispenser errors: type I: discrepancy between prescribed medication or instruction and dispensed medication or instruction; type II: patient not taking prescribed medication because of medication stock out and no referral back to prescriber for alternative medication option. Prescriber errors: type I: dose or frequency inconsistent with guidelines or manufacturer's recommendations; type II: prescription of ≥2 forms of the same medication or medication class; type III: changes in antihypertensive medication prescription without adequate indication or explanation; type IV: incomplete prescription (omission of dose, frequency, or duration).
Blood Pressure Control
A total of 154 patients (55.2%) had uncontrolled hypertension per an average of the 2 most recent blood pressure measurements performed at study enrollment and study visit. A total of 28 patients (10%) had ≥1 episode of hypotension, and 7 of the 28 (2.5%) had ≥2 episodes of hypotension over the course of 1 year.
When compared with patients with no medication discrepancies, patients with ≥1 medication discrepancy had a higher prevalence of uncontrolled hypertension (73.7% versus 45.4%; P<0.001). The difference in average systolic and diastolic blood pressures between these groups was 11.8 mm Hg (95% CI, 6.6–16.9 mm Hg) and 5.5 mm Hg (95% CI, 2.5–8.4 mm Hg), respectively. The average systolic (P=0.48) and diastolic (P=0.36) blood pressure readings over the course of the preceding 12 months did not significantly vary from the averages of the 2 most recent blood pressure readings or the current blood pressure (Table 4). Overall, patients with ≥1 medication discrepancy had 3.26 higher odds of uncontrolled hypertension. In univariate analyses, patient errors (OR, 3.13; 95% CI, 1.59–6.15; P=0.001) and dispenser errors (OR, 3.13; 95% CI, 1.22–8.02; P=0.017) were significantly associated with uncontrolled blood hypertension, whereas no association was observed between prescriber errors and uncontrolled blood hypertension (OR, 1.55; 95% CI, 0.71–3.36; P=0.271). Frequency of follow‐up was high, with an average of 10.4 recorded blood pressure measurements over the preceding 12 months, of which 60.6% were above blood pressure control targets. Among patients in whom >50.0% of blood pressure readings were above blood pressure control targets, in only 53.7% were any medication adjustments made during the course of the year. In an adjusted multivariable logistic analysis, factors associated with uncontrolled blood pressure included the presence of ≥1 medication discrepancy (aOR, 3.26; 95% CI, 1.75–6.06; P<0.001), male sex (aOR, 2.5; 95% CI, 1.10–5.73; P=0.029), higher CVD pill burden (aOR, 1.23; 95% CI, 1.11–1.37; P<0.001), and receiving care at a community clinic when enrolled in the study (aOR, 3.02; 95% CI, 1.35–6.73; P=0.007) (Table 5).
Table 4.
Varying Definitions of Uncontrolled BP, Stratified by Medication Errors
| Variable | No Medication Discrepancies (n=185) | ≥1 Medication Discrepancy (n=95) | Total Participants (n=280) | P Value |
|---|---|---|---|---|
| Current BP, mean (SD), mm Hg | ||||
| Systolic | 137 (21) | 151 (29) | 141 (25) | <0.001 |
| Diastolic | 82 (13) | 87 (15) | 84 (14) | 0.014 |
| 2‐Reading average BP, mean (SD), mm Hga | ||||
| Systolic | 138 (19) | 149 (24) | 142 (21) | <0.001 |
| Diastolic | 82 (11) | 87 (13) | 84 (12) | <0.001 |
| Yearly average BP, mean (SD), mm Hgb | ||||
| Systolic | 139 (17) | 149 (20) | 142 (19) | <0.001 |
| Diastolic | 82 (11) | 86 (11) | 83 (11) | 0.004 |
| Population % with uncontrolled hypertension per 12‐mo average BPc | 46.5 | 70.8 | 54.8 | <0.001 |
| Population % with uncontrolled hypertensionb | 45.7 | 73.7 | 55.2 | <0.001 |
| Mean frequency of uncontrolled BPc over 12 mo, %d | 54.6 | 72.5 | 60.6 | <0.001 |
BP indicates blood pressure.
Mean of an average of 2 BP readings taken over the course of a month for each participant.
Mean of an average of all BP readings taken over the course of a year for each participant.
Uncontrolled hypertension/BP: systolic or diastolic BP ≥140 and ≥90 mm Hg, respectively, in nondiabetic patients and ≥130 and ≥80 mm Hg, respectively, in patients with diabetes mellitus.
Population mean of the proportion of all individual BP measurements taken over the course of a year that were uncontrolled for each individual.
Table 5.
Multivariate Logistic Regression: Factors Associated With Uncontrolled Blood Pressure
| Variable | Adjusted Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age | 0.98 | 0.95–1.00 | 0.046 |
| Male sex | 2.5 | 1.09–5.73 | 0.029 |
| Clinic typea | |||
| Community clinic | 3.02 | 1.35–6.73 | 0.007 |
| Hospital clinic | 0.90 | 0.42–1.95 | 0.791 |
| Household incomeb | 1.09 | 0.78–1.51 | 0.50 |
| Education (total No. of years) | 0.99 | 0.91–1.07 | 0.778 |
| Active tobacco user (cigarettes or snuff) | 1.94 | 0.69–5.46 | 0.207 |
| No. of comorbidities | 0.96 | 0.61–1.50 | 0.842 |
| Suboptimal medication reconciliationb | 1.97 | 0.96–4.04 | 0.064 |
| Medication errors | 3.26 | 1.75–6.06 | <0.001 |
| CVD pill burdenc | 1.23 | 1.11–1.37 | <0.001 |
| Self‐reported adherence | 1.85 | 0.94–3.65 | 0.075 |
| No. of clinic visits | 0.95 | 0.90–1.01 | 0.081 |
CVD indicates cardiovascular disease. Uncontrolled hypertension/blood pressure: systolic or diastolic blood pressure ≥140 and ≥90 mm Hg, respectively, in nondiabetic patients and ≥130 and ≥80 mm Hg, respectively, in patients with diabetes mellitus, using an average of 2 blood pressure readings taken over the course of a month.
Clinic type at enrollment and study interview: reference: health post.
Unable to name home medications and did not bring home medications to study interview.
The number of pills prescribed daily to treat hypertension and diabetes mellitus and prevent CVD.
Discussion
To our knowledge, this is the first study to date to document an association between medication errors and hypertension management outcomes in LMICs where hypertension is rapidly becoming a major public health challenge. Over half of the patients in this study had uncontrolled blood pressure, despite taking antihypertensive medications and frequent follow‐up, a rate of hypertension control consistent with studies from other LMICs.9, 10, 11, 12 We found a remarkably high prevalence of discrepancies between prescribed and patient‐reported medications. Patients with any medication error were much more likely to have uncontrolled blood pressure compared with patients with no medication errors, and the difference in average systolic and diastolic blood pressures between these groups was clinically significant. Compared with findings in a high‐income context (United States) by Persell et al,18 using the same cutoffs for uncontrolled blood pressure (<140/90 mm Hg and <130/80 mm Hg in diabetic patients), a higher percentage of the population with medication errors (57.7% versus 73.7%) and without medication errors (35.9% versus 45.7%) had uncontrolled hypertension in Botswana. Overall prevalence of uncontrolled hypertension in the 2 studies was comparable (52.7% versus 55.2%).18
The presence of medication discrepancies was the strongest predictor of uncontrolled hypertension among multiple factors included in adjusted analysis. The main clinical implication of our findings is that medication errors may be a major modifiable risk factor for uncontrolled hypertension. Interventions targeting the root causes of the most common errors may thus have the potential to significantly improve blood pressure control among patients already identified and treated for hypertension.
There may be several explanations for the strong association between the presence of medication errors and poor blood pressure control. Patients in our study reported a high rate of medication adherence; and we did not identify an association between self‐reported adherence and blood pressure control, although there was a trend toward significance. The study did not formally evaluate for adherence beyond patient self‐report (eg, pill count); hence, reliability of this measure may be limited in our study. Data from high‐income countries suggest actual adherence may be lower than that reported by patients32 and that patients with better adherence are likely to have better blood pressure control. They may also be more familiar with their medications and better able to avoid medication errors at the patient level as well as prevent errors from being made by prescribers and dispensers.
Medication errors may also reflect the quality of care delivered by prescribers and medication dispensers. Low‐quality care at the level of the prescriber may consist of failure to review a patient's current medication list before updating or changing prescriptions; failure to identify uncontrolled hypertension and make required adjustments; failure to consider potential medication interactions; failure to monitor, identify, and address medication adverse effects; failure to provide adequate patient education; and failure to maintain adequate documentation.12, 13 Poor service quality by medication dispensers may include failure to identify prescriber errors, failure to adequately educate patients and document medication instructions, and failure to communicate with prescribers when problems are identified or medications are out of stock. As demonstrated in this study by the significant percentage of patients with >1 type of error, such quality of care lapses may often coexist. Stock outs of medications were a another prominent cause of medication discrepancies in our study and likely reflect procurement, stock management, and other system quality issues at the facility, district, and national level. Interestingly, we found that patients prescribed angiotensin‐converting enzyme inhibitors were more likely to have medication errors, a finding that may be related to medication stock management and formulation issues. Enalapril is available in 3 different dosage forms (5‐, 10‐, and 20‐mg tablets). The association between angiotensin‐converting enzyme inhibitors and medication errors in our study is likely explained by variable availability of specific dosage forms, which requires dispensers and patients to adjust pill numbers on the basis of the specific dosage forms available at the time of each medication refill. At the district health facility level, reduction in medication errors among our patient population will likely require multicomponent interventions targeting errors generated by patients, prescribers, and medication dispensers. These may include clinician and nurse training on guideline‐driven hypertension management, introduction of routine medication reconciliation on each follow‐up visit, standardization of documentation and dispensing practices, improved communication between dispensers and prescribers, and improvement of patient education practices. As overwork has been previously cited as a cause of self‐reported medication errors among prescribers and dispensers in LMICs,33 the amount of time prescribers and dispensers are able to allocate to each patient encounter is a critical factor in their ability to provide adequate quality of care. Many of the interventions suggested above, such as routine medication reconciliation and improved patient education, may be time‐consuming. Further research is required to assess whether such time investment results in improved outcomes.
Comprehensive interventions will also require involvement of local and national policy makers to engage quality improvement initiatives. Suggested systemic initiatives include maintaining adequate medication supplies, decreasing pill burden by making combination antihypertensive formulations available in public pharmacies, and equipping the healthcare facilities with electronic medical records. The latter has been demonstrated to decrease medication errors in high‐income countries through improved documentation and reduction in prescription and dispensing errors.34
Our study identified multiple errors leading to medication discrepancies but was limited in its ability to identify some of the potential root causes for medication discrepancies and errors.
The study's design limited our ability to draw conclusions on the association between blood pressure control and level of healthcare facility. A strong association was found between study enrollment at community clinics and uncontrolled blood pressure. A random effects regression model could be useful in accounting for correlation between patients at the clinic location and clinic type level. We chose not to use this model as over half of patients in this population reported seeking care at >2 different‐level health facilities over the course of a year. We were unable to assess whether rates of medication errors and uncontrolled hypertension varied between patients seen by a physician, family nurse practitioner, or general nurse at different sites as poor documentation practices made it difficult to consistently identify prescriber level. We do not have sufficient data to determine if the strong correlation we see between clinic type and uncontrolled blood pressure is caused by the clinic environment or individual providers. Nonetheless, there was no difference in the rate of medication errors occurring among the 3 clinic types.
Patients in Botswana's public health system are not typically followed by a single provider, and patients are often seen by a different provider on each follow‐up visit. Prior research shows frequent medication discrepancies with inpatient to outpatient transitions of care.21, 22 The same may be occurring with frequent changes in ambulatory care providers and sites, but we were unable to assess this.
An additional limitation was using a purposive sampling method rather than a randomized sampling method to choose outpatient facilities in the district, which may have introduced selection bias. Given the limited number of facilities in the district, we chose a maximum variation strategy, choosing to balance participants recruited at hospital‐based facilities with those recruited at community primary care centers and health posts of varying volume and distance from the district hospital.
Other limitations of this study include potential for classification errors resulting from imprecise blood pressure measurements (eg, caused by lack of blood pressure machine calibration or inconsistent measurement technique), challenges having working equipment in all clinics to calculate height and weight, and suboptimal medication reconciliation (eg, caused by incomplete medical records or brought‐in or recalled medications). Nevertheless, we believe the reliance on 2 study blood pressure measurements and controlling for patients with suboptimal medication reconciliation are strengths of this study and increase the likelihood that our results are accurate.
Overtreatment and/or patient errors (eg, ingestion beyond the prescribed dose), leading to hypotension, were not classified as medication errors in this study. Hypotension is an important adverse effect to consider in this population of patients being treated for hypertension with medications. The incidence of hypotension was low in this population compared with publications from high‐income countries.35 We were limited in ability to detect episodes of hypotension as our protocol did not include 24‐hour ambulatory blood pressure monitoring.
In summary, our study provides an important addition to the literature by demonstrating a strong association between medication discrepancies and blood pressure control in a resource‐limited middle‐income country. It points to medication reconciliation as an effective method of identifying medication errors and a potential low‐cost intervention for reducing medication discrepancies and improving blood pressure control among patients with hypertension in the outpatient setting. Beyond hypertension, our findings may have implications for other disease conditions that rely on long‐term pharmacologic treatment for their control. Further study is needed to assess whether interventions aimed at improving medication reconciliation and addressing medication errors can improve the control of hypertension and other chronic disease conditions in the ambulatory setting.
Sources of Funding
This work was funded by the Section of Hospital Medicine at Beth Israel Deaconess Medical Center.
Disclosures
None.
Acknowledgments
The authors thank the Beth Israel Deaconess Medical Center Global Health Fellowship Program and the Department of Medicine, including Dr Joseph Li, Dr Rebecca Zash, Dr Jonathan Crocker, and Dr Mitch Ross, for their valuable contributions. We thank Dr Joseph Makhema and the Botswana Harvard Partnership and the Kweneng East District Health Management Team and Study Staff, including Keolebogile Dintwe, Molebatsi Gaotime, Dorothy Legakwa, Phemelo Molthankana, Oliver Basumikili, and Midah Mogonono, for their valuable support and participation in this study.
(J Am Heart Assoc. 2020;9:e013766 DOI: 10.1161/JAHA.119.013766.)
References
- 1. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis‐Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá‐López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas‐Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 2. Fisher NDL, Curfman G. Hypertension‐a public health challenge of global proportions. JAMA. 2018;320:1757–1759. [DOI] [PubMed] [Google Scholar]
- 3. Lackland DT, Weber MA. Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol. 2015;31:569–571. [DOI] [PubMed] [Google Scholar]
- 4. Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol. 2014;30:527–533. [DOI] [PubMed] [Google Scholar]
- 5. Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease, part 2: short‐term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet. 1990;335:827–838. [DOI] [PubMed] [Google Scholar]
- 6. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 7. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 8. Mosepele M, Bennett K, Gaolathe T, Makhema J, Mmalane MO, Holme MP, Lebelonyane R, Powis KM, Leidner J, Jarvis JN, Tapela N, Masupe T, Mokgatlhe L, Wirth K, Lockman S. High prevalence of hypertension in HIV‐infected and HIV‐uninfected adults in Botswana. CROI (Conference of Retroviruses and Opportunistic Infections), March 4‐7, 2018, Boston, MA.
- 9. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez‐Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S; PURE (Prospective Urban Rural Epidemiology) Study investigators . Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959–968. [DOI] [PubMed] [Google Scholar]
- 11. Pilleron S, Aboyans V, Mbelesso P, Ndamba‐Bandzouzi B, Desormais I, Lacroix P, Preux PM, Guerchet M; EPIDEMCA group . Prevalence, awareness, treatment, and control of hypertension in older people in Central Africa: the EPIDEMCA study. J Am Soc Hypertens. 2017;11:449–460. [DOI] [PubMed] [Google Scholar]
- 12. Berry KM, Parker WA, Mchiza ZJ, Sewpaul R, Labadarios D, Rosen S, Stokes A. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017;2:e000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tapela NM, Tshisimogo G, Shatera BP, Letsatsi V, Gaborone M, Madidimalo T, Ovberedjo M, Jibril HB, Tsima B, Nkomazana O, Dryden‐Peterson S, Lockman S, Masupe T, Hirschhorn LR, El Halabi S. Integrating noncommunicable disease services into primary health care, Botswana. Bull World Health Organ. 2019;97:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Country factsheets: Botswana 2018. UNAIDS Available at: <https://www.unaids.org/en/regionscountries/countries/botswana>. Accessed March 15, 2019.
- 15. Adler A, Prabhakaran D, Bovet P, Kazi DS, Mancia G, Mungal‐Singh V, Poulter N. Reducing cardiovascular mortality through prevention and management of raised blood pressure: a world health federation roadmap. Global Heart. 2015;10:111–122. [DOI] [PubMed] [Google Scholar]
- 16. Khatib R, Schwalm JD, Yusuf S, Haynes RB, McKee M, Khan M, Nieuwlaat R. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: a systematic review and meta‐analysis of qualitative and quantitative studies. PLoS One. 2014;9:e84238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarz D, Hirschhorn LR, Kim J, Ratcliffe HL, Bitton A. Continuity in primary care: a critical but neglected component for achieving high‐quality universal health coverage. BMJ Global Health. 2019;4:e001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Persell SD, Bailey SC, Tang J, Davis TC, Wolf MS. Medication reconciliation and hypertension control. Am J Med. 2010;123:182.e9–182.e15. [DOI] [PubMed] [Google Scholar]
- 19. Ernst ME, Brown GL, Klepser TB, Kelly MW. Medication discrepancies in an outpatient electronic medical record. Am J Health Syst Pharm. 2001;58:2072–2075. [DOI] [PubMed] [Google Scholar]
- 20. Manley HJ, Drayer DK, McClaran M, Bender W, Muther RS. Drug record discrepancies in an outpatient electronic medical record: frequency, type, and potential impact on patient care at a hemodialysis center. Pharmacotherapy. 2003;23:231–239. [DOI] [PubMed] [Google Scholar]
- 21. Bedell SE, Jabbour S, Goldberg R, Glaser H, Gobble S, Young‐Xu Y, Graboys TB, Ravid S. Discrepancies in the use of medications. Arch Intern Med. 2000;160:2129. [DOI] [PubMed] [Google Scholar]
- 22. Lehnbom EC, Stewart MJ, Manias E, Westbrook JI. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother. 2014;48:1298–1312. [DOI] [PubMed] [Google Scholar]
- 23. Rose AJ, Fischer SH, Paasche‐Orlow MK. Beyond medication reconciliation: the correct medication list. JAMA. 2017;317:2057–2058. [DOI] [PubMed] [Google Scholar]
- 24. Varkey P, Cunningham J, Bisping DS. Improving medication reconciliation in the outpatient setting. Jt Comm J Qual Patient Saf. 2007;33:286–292. [DOI] [PubMed] [Google Scholar]
- 25. Mekonnen AB, McLachlan AJ, Brien JE, Mekonnen D, Abay Z. Evaluation of the impact of pharmacist‐led medication reconciliation intervention: a single centre pre‐post study from Ethiopia. Int J Clin Pharm. 2018;40:1209–1216. [DOI] [PubMed] [Google Scholar]
- 26. Naicker P, Schellack N, Godman B, Bronkhorst E. Creating and evaluating an opportunity for medication reconciliation in the adult population of South Africa to improve patient care. Hosp Pract (1995). 2018;46:110–120. [DOI] [PubMed] [Google Scholar]
- 27. Demography of Botswana 2015. Statistics Botswana. Available at: <http://botswana.opendataforafrica.org/DEOB2015/demography-ofbotswana-2015>. Accessed August 28, 2018.
- 28. Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. El‐Halabi S, Jibril HB. Botswana Primary Care Guidelines. Gaborone, Botswana: Botswana Ministry of Health; 2016. [Google Scholar]
- 30. Pool LR, Ning H, Wilkins J, Lloyd‐Jones DM, Allen NB. Use of long‐term cumulative blood pressure in cardiovascular risk prediction models. JAMA Cardiol. 2018;3:1096–1100.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. StataCorp . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 32. Lenahan JL, McCarthy DM, Davis TC, Curtis LM, Serper M, Wolf MS. A drug by any other name: patients’ ability to identify medication regimens and its association with adherence and health outcomes. J Health Commun. 2013;18(suppl 1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogunleye OO, Oreagba IA, Falade C, Isah A, Enwere O, Olayemi S, Ogundele SO, Obiako R, Odesanya R, Bassi P, Obodo J, Kilani J, Ekoja M. Medication errors among health professionals in Nigeria: a national survey. Int J Risk Saf Med. 2016;28:77–91. [DOI] [PubMed] [Google Scholar]
- 34. Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scuteri A, Modestino A, Frattari A, Di Daniele N, Tesauro M. Occurrence of hypotension in older participants: which 24‐hour ABPM parameter better correlate with? J Gerontol A Biol Sci Med Sci. 2012;67:804–881. [DOI] [PubMed] [Google Scholar]


