Abstract
Background
Although changes in left ventricular end‐systolic volume (LVESV), left ventricular end‐diastolic volume, and global circumferential strain occur during cancer treatment, the relationship of these changes to the 2‐year post–cancer‐treatment measures of left ventricular ejection fraction (LVEF) are unknown.
Methods and Results
In a prospective, continuously recruited cohort of 95 patients scheduled to receive potentially cardiotoxic chemotherapy for breast cancer, lymphoma, or soft tissue sarcoma, measures of left ventricular end‐diastolic volume, LVESV, global circumferential strain, and LVEF were acquired via cardiac magnetic resonance imaging before and then 3 and 24 months after initiating treatment by individuals blinded to all patient identifiers. Participants had an average age of 54±15 years; 68% were women, and 82% were of white race. LVEF declined from 62±7% to 58±9% over the 24 months (P<0.0001), with 42% of participants experiencing a >5% decline in LVEF at 24 months. Predictors of a 24‐month >5% decline in LVEF included the following factors from baseline to 3 months into treatment: (1) >3‐mL increases in LVESV (P=0.033), (2) >3‐mL increases in LVESV or 10‐mL declines in left ventricular end‐diastolic volume with little change in LVESV (P=0.001), or (3) ≥10% deteriorations in global circumferential strain with little change in LVESV (P=0.036).
Conclusion
During receipt of potentially cardiotoxic chemotherapy, increases in LVESV, the absence of its deterioration during decreases of left ventricular end‐diastolic volume, or the deterioration of global circumferential strain without a marked decrease in LVESV help identify those who will develop more permanent 2‐year declines in LVEF.
Keywords: cardiotoxicity, chemotherapy, global circumferential strain, left ventricular ejection fraction
Subject Categories: Heart Failure, ,
Clinical Perspective
What Is New?
Up to 42% of adults receiving cardiotoxic anthracycline chemotherapy for treatment of breast cancer, lymphoma, leukemia, or soft tissue sarcoma experience a >5% reduction in left ventricular ejection fraction (LVEF) 2 years after initiating treatment.
Three months into receiving these cardiotoxic treatments, patients sustaining an increase in left ventricular end‐systolic volume or those who experience little change in their left ventricular end‐systolic volume with a drop in left ventricular end‐diastolic volume are at high risk of developing a >5% decline in LVEF 2 years after initiating therapy.
During this same early month period, those hospital patients experiencing LVEF declines due to simultaneous large declines in left ventricular end‐diastolic and end‐systolic volumes do not experience an increased risk of 2 year decline in LVEF.
What Are the Clinical Implications?
Evaluating cancer therapy–associated changes in left ventricular volume helps identify patients who will develop declines in LVEF 2 years after cancer treatment.
Particular attention should be paid to evaluating changes in both left ventricular end‐diastolic and end‐systolic volumes when measuring LVEF to identify cardiac dysfunction after receipt of potentially cardiotoxic chemotherapy.
During the early phases of receiving potentially cardiotoxic chemotherapy, patients may develop increases in left ventricular (LV) end‐systolic volume (LVESV) that are associated with declines in LV ejection fraction (LVEF) or deteriorations in global circumferential strain (GCS).1, 2, 3, 4 However, LVEF or GCS also may deteriorate because of decreases in LV end‐diastolic volume (LVEDV) that may be related to reduced LV preload or hypovolemia (due to decreased oral intake or hyperemesis) as opposed to myocardial injury resulting from chemotherapy treatment.3, 4 It is currently unknown how changes in LV volume (LVEDV or LVESV) that occur early during receipt of cardiotoxic chemotherapy forecast long‐term post–cancer‐treatment assessments of LVEF.
Accordingly, we performed this study to determine whether changes in LVESV or LVEDV before to 3 months after initiation of chemotherapy—particularly as they relate to GCS—forecast declines in LVEF 24 months after initiation of chemotherapy. Our goal was to examine this question in patients who survived their cancer and its treatment who were healthy 24 months after initiating cancer treatment. To accomplish this evaluation, we measured LVEDV, LVESV, GCS, and LVEF in a cohort of patients before and then surviving to 3 and 24 months after initiating potentially cardiotoxic chemotherapy for breast cancer, lymphoma, or soft tissue sarcoma.
Methods
Study Population and Design
The data that support the findings of this study are available from the corresponding author on reasonable request. This prospective cohort study was approved by the institutional review board of the Wake Forest School of Medicine, and all participants provided written informed consent. The study was funded by the National Institutes of Health (NIH) grant R01CA167821. We consecutively enrolled 95 patients from the hematology/oncology outpatient clinics at the Comprehensive Cancer Center of the Wake Forest School of Medicine. These patients were scheduled to receive cardiotoxic chemotherapy without a contraindication to cardiovascular magnetic resonance (CMR) and agreed to participate for serial examinations before and then 3 and 24 months after initiation of their cancer therapy.
At the time of enrollment, demographic data including age, race, and sex were collected, and height and weight were used to calculate body mass index. Baseline data on cardiovascular disease risk factors including the presence of coronary artery disease, hypertension, diabetes mellitus, hyperlipidemia, and smoking status (ever in lifetime) also were recorded. A cardiovascular disease score was calculated by counting the number of cardiovascular disease risk factors (hypertension, diabetes mellitus, known coronary artery disease, smoking, or elevated cholesterol; thus, in a range of 0–5). Prior cancer treatment (yes/no) and current cancer treatment, including type of treatment and cumulative dose, were assessed. Medication data, including the use of cardioprotective medications throughout the course of the study, and the type of cancer were recorded.
Determination of LV Volumes, GCS, and LVEF
All participants underwent CMR imaging on a 1.5‐T Avanto scanner (Siemens). CMR imaging was chosen to assess LVEDV, LVESV, GCS, and LVEF because of its accuracy and prior use in NIH‐funded initiatives such as MESA (Multi‐Ethnic Study of Atherosclerosis)5 and the Jackson Heart Study.6 LV volumes and GCS measures were obtained by QMASS (v6.1.5; Medis Medical Imaging Systems) through a mid‐SAX view with previously published methods. Cine white blood steady‐state free precession techniques were used with a 256×128 matrix, a 40‐cm field of view, 10‐ms repetition time, 4‐ms echo time, a 20° flip angle, an 8‐mm thick slice with a 2‐mm gap, and 40‐ms temporal resolution.5, 7 All images were analyzed by readers blinded to all identifiers and conducted unpaired reads. Heart rate (beats/min) and systolic and diastolic blood pressure (mm Hg) were also measured during CMR imaging.
Baseline to 24‐month change in LVEF was categorized as a significant drop if this change resulted in a decline of >5% or a drop below 50% at the 24‐month visit.8 In addition, we performed analyses to identify those who transitioned to stage B or C heart failure. A transition to stage B heart failure by the 24‐month visit was defined as a decline in LVEF to a value below 50% or an LVEF decline to below 53% caused by an absolute drop in LVEF of at least 10%.9, 10, 11 The Minnesota Living with Heart Failure Questionnaire was used to distinguish stage C heart failure.12 A subset of 5 items included (1) leg swelling, (2) fatigue, (3) low energy, (4) dyspnea on exertion, and (5) shortness of breath. The participants rated each item on a scale from 0 to 5, with a higher number indicating a more negative impact on quality of life. The items were summed to obtain a score that ranged from 0 to 25. Participants were classified as having stage C heart failure if they met the criteria for stage B heart failure and had a score for baseline to 24‐month change in heart failure that was >1 SD above the mean.9, 10, 11, 12
Changes in LVEDV, LVESV, and GCS from baseline to 3 months were calculated and dichotomized at various threshold intervals as described in the next section.
Statistical Analysis
Counts and percentages were reported for all categorical variables, and mean±SD was reported for all continuous variables. Paired Student t tests were conducted to determine whether changes between baseline and follow‐up visits were different from zero. Two sample t tests were conducted to test for differences in means between participants with or without a 24‐month drop of >5% in LVEF. Chi‐square tests were performed to test for differences in percentages between categorical variables. All tests used a 5% level of significance, and thus P≤0.05 was considered significant.
The thresholds for dichotomizing baseline to 3‐month changes in LVEDV and LVESV were selected from clinical measures appreciable as clinically detectable from CMR.8, 13 After selection, these measures were verified using logistic regression modeling. For LVEDV and LVESV, we dichotomized at 5‐ and 3‐mL intervals, respectively. The best predictors of a baseline to 24=month drop of >5% in LVEF were verified by selecting the model with the lowest Bayesian information criterion statistic for each 3‐month predictor (ie, 1 threshold was chosen for each of LVEDV and LVESV). Logistic regression was used to compute odds ratios and 95% CIs for each predictor of 24‐month drop of >5% in LVEF and composites of these predictors. Each model was computed both unadjusted and adjusted for sex, age, anthracycline use as of the 3‐month visit, and cardiovascular disease score. Models were also adjusted separately for any cardioprotective medication use over the 2 years of the study and cancer type. Analyses were performed using SAS v9.4 (SAS Institute).
Results
Participant disposition status is described in Figure 1. Seventy‐one participants had complete CMR data at all 3 visits and were included in the analysis. To examine whether the participants who did not complete the study differed from those who did, we compared sex, age, race, ethnicity, cardiovascular risk factors, chemotherapy treatment regimen, and the baseline mean LVEDV, LVESV, LVEF, blood pressure, and body mass index and found no differences. However, we found that cancer type was associated with likelihood of completing the study (P=0.0004), with breast cancer patients (88%) being more likely to complete the study and acute myeloid leukemia (0%) and sarcoma (42%) patients being less likely to complete the study.
Figure 1.
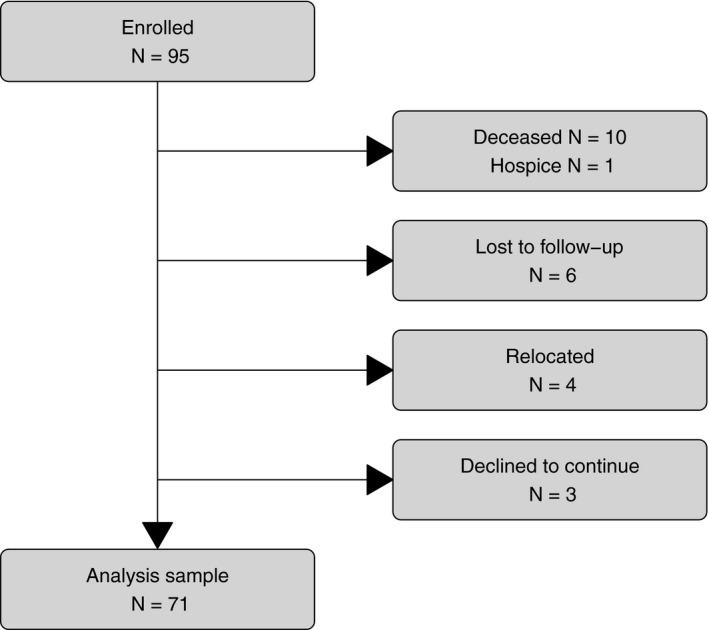
Participant disposition at 24 months. Withdrawal from the study was recorded at either the 3‐month visit or the 24‐month visit. Lost to follow‐up includes 4 participants who would not return calls and 2 participants who did not come to their scheduled visit.
Demographic data for the 71 participants are shown in Table 1. The participants were 68% female, 82% white, and averaged 54±15 years in age. Data regarding participants’ heart rates, systolic and diastolic blood pressures, LVEDVs, LVESVs, stroke volumes, LVEFs, LV mass, and GCSs are reported across all 3 visits (Table 2. Twelve participants (16.9%) had an LVEF below 50%. Ten participants (14.1%) met criteria for stage B, and 5 (7.0%) met criteria for stage C heart failure at the 24‐month visit. Moreover, 42% of patients experienced a >5% decline in LVEF 24 months after initiating potentially cardiotoxic chemotherapy. Eight participants experienced a >5% decline to below 50%, 22 participants experienced a >5% to at least 50%, and 2 participants experienced a ≤5% decline to below 50%.
Table 1.
Study Population (n=71)
| Characteristic | Result |
|---|---|
| Age | 53.7±14.5 |
| Height, m | 1.7±0.10 |
| Weight, kg | 84.3±18.7 |
| Sex | |
| Male | 23 (32.4) |
| Female | 48 (67.6) |
| Race | |
| Black | 13 (18.3) |
| White | 58 (81.7) |
| Coronary artery disease | 3 (4.2) |
| Hypertension | 36 (50.7) |
| Diabetes mellitus | 12 (16.9) |
| Hyperlipidemia | 8 (11.4) |
| Smoker at any time | 8 (11.6) |
| Body mass index ≥30 | 30 (42.3) |
| Cancer | |
| Breast | 29 (40.8) |
| Lymphoma | 37 (52.1) |
| Sarcoma | 5 (7.0) |
| Chemotherapy before study | 13 (18.3) |
| Chemotherapy treatment regimen | |
| Anthracycline | 48 (67.6) |
| Trastuzumab | 2 (2.8) |
| Taxane | 28 (39.4) |
| Cyclophosphamide | 49 (69.0) |
| Other chemotherapy | 45 (63.4) |
| Immunotherapy | 23 (32.4) |
| Cardioprotective medication | 39 (54.9) |
Values are n (%) or mean±SE. Cardioprotective medications include use of angiotensin‐converting enzyme inhibitors (22.5%), β‐blockers (18.3%), diuretics (26.8%), and statins (28.2%) at any point during the study.
Table 2.
Hemodynamic and Cardiac Measurements (n=71)
| Mean±SD | P Value | ||||
|---|---|---|---|---|---|
| Before Initiating Chemotherapy | 3 mo After Initiating Chemotherapy | 24 mo After Initiating Chemotherapy | 3‐mo Change | 24‐mo Change | |
| Heart rate, beats/min | 73±13 | 81±12 | 71±11 | <0.0001 | 0.21 |
| Systolic blood pressure, mm Hg | 117±16 | 109±14 | 115±17 | <0.0001 | 0.20 |
| Diastolic blood pressure, mm Hg | 69±12 | 65±9 | 68±10 | 0.005 | 0.25 |
| LVEDV, mL | 126±36 | 120±37 | 123±41 | 0.02 | 0.44 |
| LVEDV index, mL/m2 | 65±14 | 62±16 | 63±17 | 0.08 | 0.30 |
| LVESV, mL | 48±20 | 49±20 | 53±24 | 0.53 | 0.03 |
| LVESV index, mL/m2 | 25±9 | 25±9 | 27±11 | 0.28 | 0.07 |
| LV stroke volume, mL | 77±19 | 71±20 | 71±23 | 0.0003 | 0.0006 |
| LV stroke volume index, mL/m2 | 40±8 | 37±9 | 36±10 | 0.001 | 0.0003 |
| LVEF, % | 62±7 | 60±7 | 58±9 | 0.0007 | <0.0001 |
| LV mass, g | 105±28 | 103±25 | 103±28 | 0.39 | 0.28 |
| LV mass index, g/m2 | 54±10 | 54±9 | 52±11 | 0.90 | 0.20 |
| LV circumferential strain, ν | −20±4 | −18±4 | −17±4 | <0.0001 | <0.0001 |
Values are mean±SE. P≤0.05 were considered significant. LV indicates left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume.
Bayesian information criterion fit statistics for various cutoffs for 3‐month LVEDV, LVESV, and LVEF change were evaluated. Three‐month LVESV increases of >3 mL and 3‐month LVEDV drops of >10% were the best predictors of 24‐month significant or >5% LVEF drop.
Figure 2 displays baseline to 3‐month changes in LVEDV and LVESV by 24‐month LVEF drop status among participants without a baseline to 3‐month LVESV increase >3 mL. Alone, the magnitude of LVEDV decline at 3 months did not forecast 24‐month 5% declines in LVEF (P=0.716). However, among participants without a baseline to 3‐month LVESV increase of >3 mL, a small 3‐month drop in LVESV was associated with an LVEF decline >5% or to below 50% at 24 months (P=0.005).
Figure 2.
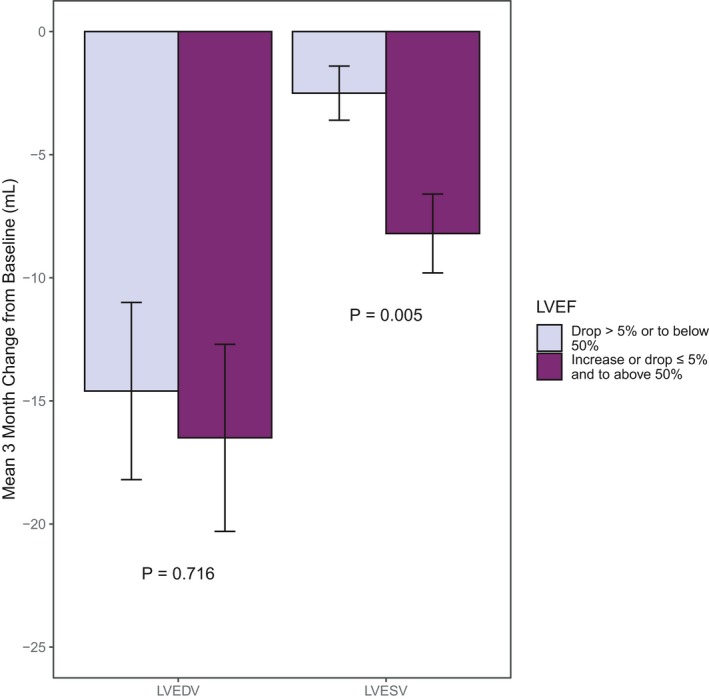
Mean 3‐month change in left ventricular end‐diastolic volume (LVEDV) and left ventricular end‐systolic volume (LVESV) by 24‐month left ventricular ejection fraction (LVEF) status among patients without a 3‐month >3‐mL increase in LVESV. There is a significant difference in short‐term LVESV drop but not short‐term LVEDV drop by long‐term LVEF drop status among patients without a short‐term >3‐mL increase in LVESV.
Figure 3 illustrates the percentages of participants with or without a >5% LVEF drop from baseline to 24 months by 3‐month LVESV status. Sixty percent of participants who experienced a 3‐month LVESV increase of >3 mL had experienced a >5% drop in LVEF at 24 months (P=0.031).
Figure 3.
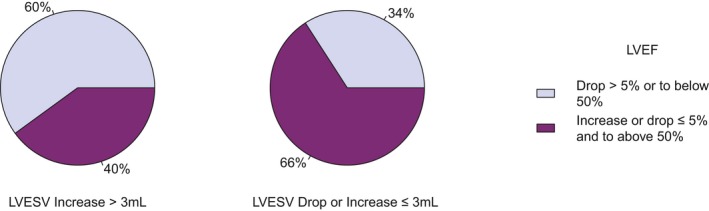
Twenty‐four–month left ventricular ejection fraction (LVEF) status by 3‐month change in left ventricular end‐systolic volume (LVESV). A higher percentage of participants experienced LVEF drops of >5% or to below 50% at 24 months among those with early increases in LVESV.
Figure 4 presents odds ratios and corresponding 95% CIs for various measures of 3‐month change in LV systolic function that predicted >5% drops in LVEF 24 months after receipt of potentially cardiotoxic chemotherapy. All significant models remained significant after controlling for cancer type (P=0.001 to P=0.037). Additional analyses related to the utility of these models for predicting large 24‐month declines in LVEF are provided in Data S1.
Figure 4.
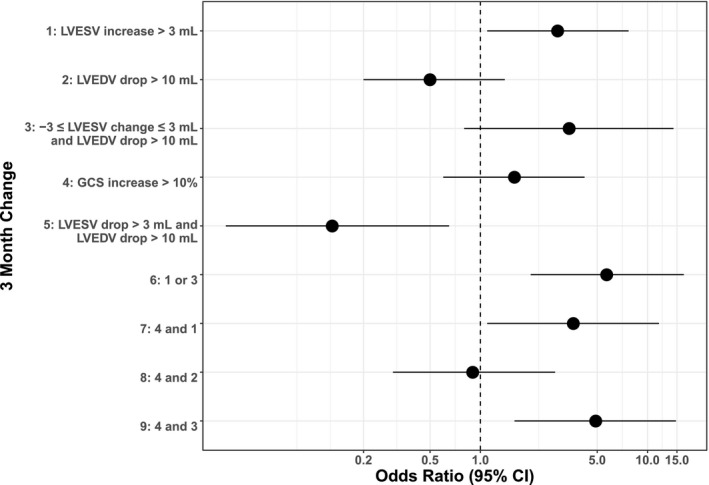
Predictors of left ventricular ejection fraction (LVEF) decline of >5% or to below 50% at 24 months. Forest plots graph the odds ratios for various factors, such as left ventricular end‐systolic volume (LVESV), left ventricular end‐diastolic volume (LVEDV), and global circumferential strain (GCS), that possibly predict LVEF decline at 24 months. All predictors measure changes from baseline to 3 months. Error bars represent 95% CIs.
Discussion
This prospective cohort study of patients receiving potentially cardiotoxic chemotherapy for breast cancer, lymphoma, or soft tissue sarcoma has several important results. First, 42% of these patients experience a >5% decline in their LVEF 2 years after initiating their cancer treatment. Second, measurements of LV volumes, strain, and their relationship with one another obtained within 3 months of initiating cancer treatment for these forms of cancer predict patients who will develop 24‐month declines of >5% in LVEF. The following predictors exist before therapy to 3 months after initiating therapy: (1) LVESV increases of >3 mL, (2) LVESV increases of >3 mL or a small (<3‐mL) change in LVESV when LVEDV declines by >10 mL, (3) GCS increases of >10% accompanied by an LVESV increase of >3 mL, and (4) GCS relative increases of >10% accompanied by a small (<3 mL) change in LVESV when the LVEDV declines by >10 mL. Importantly, early (3 months into therapy) decrements in LVEF caused by simultaneous large declines in LVEDV and LVESV did not forecast subsequent declines in LVEF of >5% at 24 months after initiation of cancer treatment. Third, the optimal sensitivity and specificity for identifying a 24‐month decline in LVEF of >5% includes the combination of either of 2 measures of LV volumes at 3 months: a >3‐mL increase in LVESV at 3 months or a small change in LVESV in combination with a >10‐mL decrease in LVEDV. Finally, the predictive value of these baseline to 3‐month changes in LVEDV, LVESV, or GCS for identifying patients with >5% declines in LVEF at 24 months after initiating potentially cardiotoxic chemotherapy remains present after accounting for cardiovascular risk factors; the types of chemotherapy received; demographic variables including age, sex, and body mass index; receipt of potentially cardioactive medications; and cancer type.
The results of this study identified that 42% of the individuals in this cohort of breast cancer, lymphoma, and sarcoma patients experienced a >5% decline in LVEF over a 2‐year period. This finding occurred independent of the presence of cardiovascular disease risk factors. Prior reports of LVEF change after receipt of potentially cardiotoxic chemotherapy have focused on relatively large declines in LVEF of >10%.3, 9, 10, 14, 15, 16 The majority of these prior reports implemented echocardiographic or radio‐isotope techniques to measure LVEF, and few reported on change in LV volumes. In the current study, we followed a prospective, consecutively recruited cohort with serial CMR studies that provided precise measurements of LV volumes, GCS, and LVEF. The results of this study highlight the fact that the presence of breast cancer, lymphoma, or sarcoma and their respective treatment promote a subclinical decline in LVEF in 42% of patients. Although current guideline documents may not classify this observation as a conversion from stage A to B heart failure, further studies are necessary to determine the long‐term effects of this subclinical decline in LVEF. Although our study was underpowered to be able to detect an effect on a lower prevalence outcome such as stage B or C heart failure, enrolling an additional 36 to 57 patients who would be projected to sustain 23 to 27 events could address this issue.
Using CMR in this study, changes in measurements of LVEDV and LVESV were evaluated as they related to LVEF and GCS measures and, importantly, how changes in GCS, LVEDV, and LVESV before to 3 months after treatment initiation forecasted measurements of LVEF 2 years after initiation of cancer treatment. As shown Figure 3, >3‐mL increases in LVESV before to 3 months into treatment forecasted 2‐year declines in LVEF of >5% in 60% of patients. These findings more likely result from the impact of potentially cardiotoxic chemotherapy on processes that raise LVESV by interfering with LV contraction or raising LV afterload. In addition, only those deteriorations in GCS that were associated with at least 3‐mL increases in LVESV and occurred before to 3 months after cancer treatment initiation were associated with 2‐year more permanent declines in LVEF (Figure 4). This latter finding suggests that global strain measures obtained during receipt of potentially cardiotoxic chemotherapy should be interpreted in the context of changes in LV volumes, as shown previously.17
A noteworthy finding related to changes in LVEF, due to declines in LVEDV, before to 3 months after initiation of cancer treatment. In a previous study, up to 20% of large deteriorations in LVEF (>10% to values <50%) or strain (>10% to 15%) during cancer treatment were related to isolated declines in LVEDV that occurred presumably because of a decline in LV preload from decreased oral intake or extraneous loss (eg, diarrhea or vomiting).3 One would expect those experiencing declines in LVEF related to declines in LVEDV not to experience long‐term LVEF declines. In the current study, 3 months into cancer treatment, those patients who experienced >10 mL declines in LVEDV combined with concomitant >3 mL declines in LVESV were less likely to experience declines in LVEF of >5% at 2 years after cancer treatment initiation. Importantly, however, those patients who experienced in these same 3 months into cancer treatment >10 mL declines in LVEDV with virtually no change (<3‐mL decline or <3‐mL increase) in LVESV did experience a decline in LVEF of >5% at 2 years after initiation of cancer treatment. These latter data suggest that a relatively large decline in LVEDV can mask a rise in LVESV 3 months into cancer treatment that would otherwise be associated with a future 2‐year decline in LVEF. Thus, it can be determined that those experiencing LVEDV declines related to decrements in LV preload may also experience myocardial dysfunction related to cancer or its treatment.
We performed analyses to determine the combination of changes in LV volumes, GCS, and LVEF at 3 months into treatment; these analyses possessed the highest sensitivity and specificity for determining a 2‐year posttreatment deterioration in LVEF of >5%. As shown in Figure 4, a 3‐month increase in LVESV >3 mL or a mild change in LVESV (increase or decrease of <3 mL) accompanied by a large drop in LVEDV (>10 mL) was the strongest predictor of a 24‐month drop in LVEF. These results further support the assessment and clinical reporting of LVEF volumes along with interpretation of GCS and LVEF measures when using noninvasive assessments of LV function to forecast future declines in LVEF after receipt of potentially cardiotoxic chemotherapy for treatment of breast cancer, lymphoma, or sarcoma.
Our study exhibits the following limitations. First, several participants did not complete the 24‐month follow‐up visit (Figure 1). Of several demographic and health measures examined, only cancer type was associated with study attrition. Thus, although we are uncertain whether LV dysfunction at 24 months could have been present to a higher or lower degree in these 24 individuals who did not complete the study, we have little baseline or planned treatment information to suggest differences for these patients relative to those who completed the study. Second, although the precision of CMR measures of LV function reduced the variability of our outcome measures, this same small sample size reduced the biological variability of our patient population. Third, we did not have power to predict larger drops in LVEF that lead to heart failure, given our small sample size. Further studies in larger numbers of patients should investigate larger drops in LVEF and may identify additional combinations of variables noted during cancer treatment that forecast declines in LV performance in cancer survivors. Fourth, given the small sample size, the thresholds for dichotomizing baseline to 3‐month changes in LVEDV and LVESV were selected using the same data that were used to construct prediction models. These cutoffs may differ in studies of other patient populations.
In conclusion, 42% of patients treated with potentially cardiotoxic chemotherapy for breast cancer, lymphoma, or sarcoma experienced 2‐year posttreatment >5% decrements of LVEF. During the receipt of potentially cardiotoxic chemotherapy, measurements of LV volumes in concert with GCS predict more permanent 2‐year declines in LVEF. In particular, increases in LVESV or little change in LVESV with a sharp drop in LVEDV are predictive of 2‐year post–cancer‐treatment declines in LVEF.
Sources of Funding
This research was supported in part by National Institutes of Health grants R01CA167821 and R33CA12196 and Susan G. Komen Foundation grant BCTR07007769.
Disclosures
None.
Supporting information
Data S1. Statistical Modeling.
(J Am Heart Assoc. 2020;9:e015400 DOI: 10.1161/JAHA.119.015400.)
References
- 1. Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: an overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen KL, Hu P, Ennis DB, Shao J, Pham KA, Chen JJ. Cardiac MRI: a translational imaging tool for characterizing anthracycline‐induced myocardial remodeling. Curr Oncol Rep. 2016;18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meléndez GC, Sukpraphrute B, D'Agostino RB, Jordan JH, Klepin HD, Ellis L, Lamar Z, Vasu S, Lesser G, Burke GL, Weaver KE, Ntim WO, Hundley WG. Frequency of left ventricular end‐diastolic volume–mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol. 2017;119:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging. 2018;11:1150–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch‐Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spahillari A, Talegawkar S, Correa A, Carr JJ, Terry JG, Lima J, Freedman JE, Das S, Kociol R, de Ferranti S, Mohebali D, Mwasongwe S, Tucker KL, Murthy VL, Shah RV. Ideal cardiovascular health, cardiovascular remodeling, and heart failure in blacks: the Jackson Heart Study. Circ Heart Fail. 2017;10:e003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jolly MP, Jordan JH, Meléndez GC, McNeal GR, D'Agostino RB, Hundley WG. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio‐toxic chemotherapy. J Cardiovasc Magn Reson. 2017;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drafts BC, Twomley KM, D'Agostino R, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline‐based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for modality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 10. Jones DN, Jordan JH, Meléndez GC. Frequency of transition from Stage A to Stage B heart failure after initiating potentially cardiotoxic chemotherapy. JACC Heart Fail. 2018;6:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunt SA, Abraham MH, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 12. Rector TS, Cohn KN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025. [DOI] [PubMed] [Google Scholar]
- 13. Jordan JH, Castellino SM, Melendez GC, Klepin HD, Ellis LR, Lamar Z, Vasu S, Kitzman DW, Ntim WO, Brubaker PH, Reichek N, D'Agostino RB, Hundley GH. Left ventricular mass change after anthracycline chemotherapy. Circ Heart Fail. 2018;11:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic Z, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. [DOI] [PubMed] [Google Scholar]
- 15. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Indepdendent and incremental value of deformation indices for prediction of trastuzumab‐induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. [DOI] [PubMed] [Google Scholar]
- 17. Jordan JH, Sukpraphrute B, Meléndez GC, Jolly MP, D'Agostino RB Jr, Hundley WG. Early myocardial strain changes during potentially cardiotoxic chemotherapy may occur as a result of reductions in left ventricular end‐diastolic volume: the need to interpret left ventricular strain with volumes. Circulation. 2017;135:2575–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Statistical Modeling.


