Abstract
Background
Poor engraftment of intramyocardial stem cells limits their therapeutic efficiency against myocardial infarction (MI)‐induced cardiac injury. Transglutaminase cross‐linked Gelatin (Col‐Tgel) is a tailorable collagen‐based hydrogel that is becoming an excellent biomaterial scaffold for cellular delivery in vivo. Here, we tested the hypothesis that Col‐Tgel increases retention of intramyocardially‐injected stem cells, and thereby reduces post‐MI cardiac injury.
Methods and Results
Adipose‐derived mesenchymal stem cells (ADSCs) were co‐cultured with Col‐Tgel in a 3‐dimensional system in vitro, and Col‐Tgel encapsulated ADSCs were observed using scanning electron microscopy and confocal microscopy. Vitality, proliferation, and migration of co‐cultured ADSCs were evaluated. In addition, mice were subjected to MI and were intramyocardially injected with ADSCs, Col‐Tgel, or a combination thereof. ADSCs engraftment, survival, cardiac function, and fibrosis were assessed. In vitro MTT and Cell Counting Kit‐8 assays demonstrated that ADSCs survive and proliferate up to 4 weeks in the Col‐Tgel. In addition, MTT and transwell assays showed that ADSCs migrate outside the edge of the Col‐Tgel sphere. Furthermore, when compared with ADSCs alone, Col‐Tgel‐encapsulated ADSCs significantly enhanced the long‐term retention and cardioprotective effect of ADSCs against MI‐induced cardiac injury.
Conclusions
In the current study, we successfully established a 3‐dimensional co‐culture system using ADSCs and Col‐Tgel. The Col‐Tgel creates a suitable microenvironment for long‐term retention of ADSCs in an ischemic area, and thereby enhances their cardioprotective effects. Taken together, this study may provide an alternative biomaterial for stem cell‐based therapy to treat ischemic heart diseases.
Keywords: 3D culture, mesenchymal stem cells, myocardial infarction, transglutaminase cross‐linked gelatin
Subject Categories: Myocardial Infarction, Cell Therapy, Stem Cells
Clinical Perspective
What Is New?
This study is the first to introduce transglutaminase cross‐linked gelatin in stem cell‐based therapy for ischemic heart disease.
Transglutaminase cross‐linked gelatin encapsulation improves the retention and cardioprotection of intramyocardial transplanted adipose‐derived mesenchymal stem cells for the treatment of acute myocardial infarction.
What Are the Clinical Implications?
Transglutaminase cross‐linked gelatin supports the proliferation and migration of adipose‐derived mesenchymal stem cells in the ischemic microenvironment.
Combination therapy of transglutaminase cross‐linked gelatin with stem cells may be a novel strategy that protects against cardiac injury after myocardial infarction.
Myocardial infarction (MI) remains a major health concern worldwide because of inevitable heart failure that occurs post MI.1 In the clinic, thrombolytic therapy and primary percutaneous coronary intervention are the most effective MI treatments, however, irreversible heart failure cannot be prevented because of limited regenerative capacity of the adult mammalian heart.2 Mesenchymal stem cell (MSC)‐based therapy has been proven a potential strategy for MI treatment.3, 4, 5 However, because of the poor retention of transplanted MSCs in the ischemic environment, favorable results observed in animal studies have not been fully translated to MI patients.6, 7
Unlike organs, such as liver and lung, heart tissue holds less MSCs after cell therapy in mice with or without MI, probably because of its high cell density and mechanical characteristics.8, 9 The strategy to co‐transplant stem cells with synthetic or natural biomaterials that are designed to mimic the in vivo microenvironments not only provides a scaffold for cellular attachment, but also serves a supportive niche for cell engraftment.10, 11 Accumulating studies have demonstrated that natural material hydrogels, including collagen, gelatin, hyaluronic acid, laminin, chitosan, and sodium alginate can increase the retention of transplanted MSCs in the ischemic zone, and thereby promote the therapeutic efficacy of MSCs.12, 13, 14, 15, 16, 17 However, the mechanical strength of the hydrogel, which is required for supporting cell accommodation, needs to be optimized.18
The biomacromolecule gelatin has increasingly been used as an application in biomedicine.19 The appealing advantages of gelatin include its cell‐adhesive characteristics, low costs, off‐the‐shelf availability, high biocompatibility, biodegradability, and low immunogenicity, among others, and make gelatin a desirable candidate for the development of biomaterials for tissue engineering and drug delivery purposes.20 In a recent study, it was shown that MSCs with a gelatin coating improved retention of the heart after MI.21 However, natural gelatin is highly hydrated and possesses poor mechanical stability and durability, which prohibits widespread application in the clinic. Transglutaminase has been used for the preparation of gelatin‐based hydrogels, and is named transglutaminase cross‐linked gelatin (Col‐Tgel). Previous studies have indicated that Col‐Tgel is tailorable, non‐cytotoxic, non‐inflammatory, easy to inject, and stable after gelation.19 In addition, the resorption rates of Col‐Tgel match those reported for neo‐tissue formation. Because of its suitable mechanical strength, and tunable physicochemical properties, including matrix stiffness, Col‐Tgel has been a valuable tool for 3‐dimensional (3D) cell culture.19 Col‐Tgel has shown to be suitable for the proliferation, migration, and cellular delivery of ADSCs.22, 23 However, whether Col‐Tgel increases the retention of ADSCs in contractile heart tissue, as well as their cardioprotection for the treatment of acute MI has not yet been reported.
In the present study, we used Col‐Tgel for 3D culture of ADSCs in vitro, and performed in vivo studies to test whether Col‐Tgel encapsulated ADSCs improved retention and cardioprotection of intramyocardial transplanted cells for the treatment of acute MI.
Materials and Methods
Data are available on request from the authors.
Gel Preparation and Characterization
Col‐Tgel is an enzymatic crosslinking hydrogel, which has been previously reported.24, 25 Briefly, 12% of gelatin Type A 300 Bloom was dissolved in PBS. In addition, transglutaminase from Streptomyces mobaraensis was added to the gelatin solution at 20:1 to accelerate collagen fiber‐generating cross‐linked polymers. Different concentrations of gelatin can create different rigidity and porosity of the substrate. The matrix stiffness of Col‐Tgel was measured using the unconfined compression test, and the porosity was measured using the liquid displacement method as previously described.26 Col‐Tgels with stiffnesses of 1.58, 15.42, and 60.54 kPa were commercially available from Weihui Biotechnology. Because a matrix with stiffnesses of 8 to 17 kPa mimics the characteristics of muscle,27 we selected Col‐Tgels with a stiffness of 15.42±3.11 kPa and porosity of 47% as the proper scaffold for in vivo intramyocardial injection.
Col‐Tgels consist of 2 components: component A (gelatin solution) and crosslinker (transglutaminase). These 2 components were stored at 4°C and −80°C, respectively. Before the generation of 3D transglutaminase‐gels, component A was first thawed at 55°C, and when liquid gelatin was cooled to room temperature, gelatin was mixed with transglutaminase at a ratio of 20:1 (v/v). The mixture would spontaneously solidify after incubation at 37°C for 45 minutes.
Animals
Animal experiments were approved by the Animal Care and Use Committee of the Fourth Military Medical University, and strictly followed the National Institutes of Health Guidelines for the Use of Laboratory Animals (National Institutes of Health publication No. 85‐23, revised 2011). Adult (8–12 weeks) male C57BL/6J mice and male Sprague‐Dawley rats (4–6 weeks) were purchased from the Laboratory Animal Center of the Fourth Military Medical University.
Isolation, Culture, and Identification of ADSCs
ADSCs were isolated from Sprague‐Dawley rats.28 In brief, inguinal subcutaneous adipose tissue was excised, and minced on ice in PBS. Next, the minced tissue was digested for 1.5 hours at 37°C in PBS, containing 1 mg/mL Collagenase I. The digested tissue was filtered through a 70‐μm Cell Strainer and centrifuged at 600 g for 10 minutes at room temperature. After red blood cell lysis with 1×lysis buffer, cells were cultured in complete medium containing a 1:1 mixture of DMEM and F12 medium, 10% fetal bovine serum, and penicillin‐streptomycin. To remove non‐adherent cells, the medium was changed 6 hours after the cells were plated. Adherent cells were cultured in complete medium and split to expand the cells. Cells from passage 3 were used in experiments.
Preparation of Col‐Tgel Encapsulated ADSCs for In Vitro and In Vivo Studies
To prepare the correct number of Col‐Tgel encapsulated ADSCs for in vitro studies, component A was liquefied at 55°C for 5 minutes, then cooled to room temperature for subsequent use according to the manufacturer's guidelines. Passage 3 ADSCs were detached from plates by trypsinization and washed twice with PBS. Then, cells were suspended in the gelatin solution at a cell density of 2×106 cells/mL of gel. Then, 100‐μL cell suspension was mixed with 5 μL of purified transglutaminase crosslinker to create 105‐μL cell‐seeded hydrogels containing 2×105 cells. Cell‐seeded hydrogels were placed as single droplets (per 20 μL containing ≈4×104 cells) on the surface of a 48‐well suspension cell culture plate following incubation at 37°C for 45 minutes. When Col‐Tgel co‐cultures had solidified through enzymatic cross‐linking, 400 μL of complete medium was added to each well.
To collect the exact number of Col‐Tgel encapsulated ADSCs for intramyocardial injection in vivo, we first used 1 mL of component A to resuspend 4×106 ADSCs. Secondly, 100 μL of cell suspension was mixed with 5 μL of purified transglutaminase crosslinker to create 105 μL cell‐seeded hydrogels (containing 4×105 cells). Third, 25 μL of cell‐seeded hydrogels (containing ≈1×105 cells) were intramyocardially injected per mouse.
Detection of Cell Viability and Morphology in the 3D Culture System
The viability and proliferative capacity of PBS resuspended ADSCs (PBS‐ADSC, two‐dimensional [2D]‐culture) or Col‐Tgel encapsulated ADSCs (Tgel‐ADSC, 3D‐culture) were quantified using propidium iodide (PI) staining, 3‐(4, 5‐Dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) staining, and the Cell Counting Kit‐8 during a 28‐day culture period. PI staining was performed to determine cell viability. Briefly, passage 3 ADSCs were seeded in a 6‐well plate for 2D or 3D culture (n=3 independent experiments). After 3 days of incubation, 1‐μg/mL PI solution and 5 μg/mL Hoechst dye were added to cover the cells and were incubated at 37°C for 15 minutes. Then, the staining solution was removed, and the cells were washed 3 times with PBS. Images were obtained using a fluorescence microscope. For the CCK‐8 assay at 5 time points (n=3 independent experiments per time point), co‐culture scaffolds were washed twice with PBS, and incubated in 300 μL of CCK‐8 working solution (CCK‐8 stock solution in complete medium, 1:40) per well for 3 hours at 37°C. Subsequently, 100 μL of supernatant was transferred to 96‐well plates, and the absorbance was measured in a multiplate reader at 450 nm. For the MTT assay at 5 time points (n=3 independent experiments per time point), the reduction of an MTT tetrazolium component into an insoluble dark purple formazan by the mitochondria of the viable cells was evaluated. The 3D culture system was washed twice with PBS, and subsequently incubated with 300 μL of MTT working solution (filtered MTT stock solution [5 mg/mL] in complete medium, 1:10) per well for 3 hours at 37°C. The images were directly taken by light microscopy. This method can not only verify the survival of cells, but also allow observation of the morphology and migration of ADSCs in a 3D Col‐Tgel construct.
Migration of ADSCs in Col‐Tgel
To investigate cell migration, 20 μL of the cell/gel mix was deposited into the middle of the bottom of each well of the 48‐well plate, and the surface tension properties of the gel created a half dome. The gel was set to solidify at 37°C for 45 minutes and then supplemented with 300 μL complete medium. The medium was changed every 3 days. The gel was monitored daily to observe the migration of cells from the gel to the surrounding plate. The migration distance was measured in millimeters at 4 time points (n=3 independent experiments per time point) as the distance from the edge of the original gel to the cellular front. The mean distance was calculated as the average of 4 measurements at each quadrant of the dome. To visualize cell viability and migration, the cells in/out the Col‐Tgel were stained with MTT dye. For the transwell assay, modified 2‐chamber plates, pore size 8 μm, were used. A total of 2×104 ADSCs mixed in 30 μL PBS, Matrigel, or Col‐Tgel were seeded or deposited in the upper chamber for 45 minutes at 37°C. After co‐culture system solidification, serum‐free DMEM‐F12 medium was added in the upper chamber. To stimulate migration, complete medium was added to the bottom chamber. After incubation at 37°C for 4 time points (n=3 independent experiments per time point), cells in the upper chamber were carefully removed with a cotton swab, and cells that had migrated through the membrane were stained with 0.1% Crystal Violet and counted using a microscope.
Animal Study Protocol
MI surgery was performed in C57BL/6J mice by ligating the left anterior descending coronary artery. Immediately after MI, 1×105 rat ADSCs suspended in 25 μL Col‐Tgel or PBS (containing 0.2 mmol/L EDTA, pH=7.3) were injected intramyocardially at 3 different sites into the infarct border zone. MI control animals received Col‐Tgel (25 μL) or PBS (25 μL) following the same injection protocol. Sham control animals were subjected to all surgical procedures, except for ligation of the coronary artery. All mice received an intraperitoneal injection of cyclosporine A (10 mg/kg per day, Signa‐Aldrich) 2 days before transplantation, then daily until the end of the study as previously described.13
The ADSC retention study was performed to evaluate the dynamic retention of transplanted ADSCs. A group of 70 adult male C57BL/6J mice were randomized into the following 2 groups: (1) MI+PBS‐ADSC (1×105 cells [passage 3] were directly injected into the peri‐infarct area immediately post MI, n=35) or (2) MI+Tgel‐ADSC (n=35). Mice were anesthetized by intraperitoneal injection of pentobarbitol sodium (0.04 mg/g of body weight) and euthanized at 3, 7, 14, 28, and 42 days after surgery. All mice that survived the surgery underwent echocardiographic studies at 1 day after MI. MI mice with left ventricular ejection fraction >40% on 1 day were excluded from the study because of a limited infarct size. The numbers of included mice in each group were as follows: MI+ADSC‐PBS (n=26, in which 11 mice were followed‐up ≥28 days and were included in the survival curves), MI+ADSC‐Tgel (n=33, in which 18 mice were followed‐up ≥28 days and were included in the survival curves).
For long‐term observation (including echocardiography, Masson trichrome staining, and survival studies), another 80 mice were randomized into the following 4 groups: MI+PBS (n=20), MI+Tgel (n=20), MI+ADSC‐PBS (n=20), MI+ADSC‐Tgel (n=20). Mice that survived the surgery underwent echocardiographic studies at 1 day after MI. After exclusion of MI mice with a left ventricular ejection fraction >40% on 1 day, the numbers of included mice in each group were as follows: (1) MI+PBS (n=16); (2) MI+Tgel (n=13); (3) MI+PBS‐ADSC (n=11); (4) MI+Tgel‐ADSC (n=19).
Echocardiography
M‐mode images of mice subjected to 1% to 2% isoflurane anesthesia were obtained on 1 day, 2 weeks, and 4 weeks after MI using a Vevo2100 ultrasound system as previously described.29 Hearts were observed in the short‐axis between the 2 papillary muscles. Each measurement was obtained in M‐mode by averaging results from 3 consecutive heartbeats. The left ventricular (LV) end‐systolic diameter and LV end‐diastolic diameter, were obtained. LV ejection fraction was automatically calculated by the echocardiography software using the following equation: ejection fraction (%)=100×[(LV end‐diastolic diameter3− LV end‐systolic diameter)3/ LV end‐diastolic diameter3].
Masson Trichrome Staining
Masson trichrome staining was used to evaluate cardiac interstitial fibrosis and structural changes. Per heart, 5 sections (5‐μm thick) were prepared for Masson trichrome staining as per the manufacturer's instructions. Fibrosis was measured via Olympus cellSens Microscope Imaging Software, and determined by fibrosis area/LV area.
Immunohistochemistry
For histological analysis of the engraftment rate of ADSCs, hearts were perfused with cold PBS and the LV was fixed with 4% paraformaldehyde at 3, 7, 14, 28, and 42 days post MI. Paraffin blocks were prepared, cut into 5‐μm thick sections, and mounted on glass slides. Next, slides were deparaffinized, and subjected to antigen retrieval in hot citric acid buffer. After cooling, slides were permeabilized with 0.2% Triton‐100 at room temperature for 15 minutes and sections were blocked with 1% BSA in PBS for 2 hours, and incubated overnight at 4°C with a TNNT2 Polyclonal Antibody primary antibody (4°C). Primary antibodies were visualized with donkey anti‐rabbit immunoglobulin G (H+L) secondary antibody conjugated with Alexa Fluor 488. Nuclei in both embedded tissues were stained with DAPI. Micrographs of all staining procedures were acquired via Olympus BX51 Fluorescence Microscope and Olympus DP72 camera. Engraftment of ADSCs was quantified as the ratio of Chloromethylbenzamido 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate (CM‐DiI) positive area to total area.
Quantitative Polymerase Chain Reaction
RNA was extracted from heart tissue via RNeasy Mini Kit, per manufacturer's instructions. cDNA was prepared from 1 μg total RNA using SuperScript III First‐Strand Synthesis System, per the manufacturer's guidelines. Polymerase chain reaction was performed via 7900HT Fast Real‐Time PCR System. Samples were analyzed in triplicate 10 μL reactions, per SYBR Green PCR Master Mix protocol. Primers were purchased from Integrated DNA Technologies listed in Table S1). GAPDH and β‐actin served as housekeeping targets.
Statistical Analysis
Data are presented as the mean±SEM and were analyzed using GraphPad Prism 7 software. Survival curves were created and analyzed by the Gehan–Breslow–Wilcoxon test. For analysis of the 2 groups, an unpaired Student t‐test was conducted. One‐way ANOVA with post hoc analysis was performed when >2 groups were compared. For multiple groups over time, 2‐way ANOVA with or without repeated measures was performed, followed by a Bonferroni post hoc test. P<0.05 was considered statistically significant.
Results
Establishment of 3D Col‐Tgel Biological Environment for ADSCs
Col‐Tgel has a network structure of transglutaminase and gelatin (Figure 1A). The network was created by the bond of glutamine residue and lysine residue (Figure 1B). Scanning electron microscopy showed a highly interconnected pore structure and a well‐developed network (Figure 1C). Passage 3 ADSCs were evaluated for surface marker expression and differentiation potential and were used in these experiments.3, 30 We established a 3D culture system and observed that the ADSCs can attach and survive in the Col‐Tgel as observed by scanning electron microscopy (Figure 1D and 1E). In the synthesized 3D images, we clearly demonstrated that the ADSCs almost filled all areas inside the hydrogels after 3 days of culture (Figure 1F).
Figure 1.
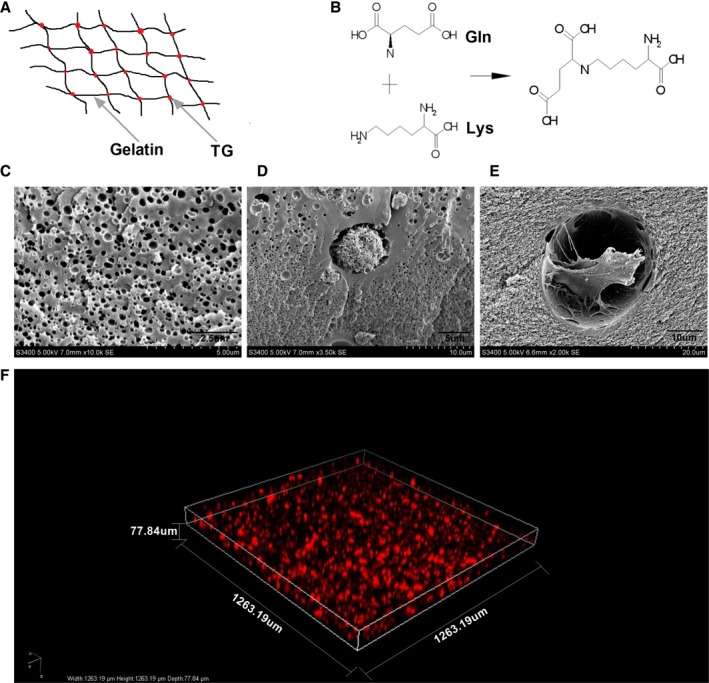
Establishment of a 3D Col‐Tgel biological environment for adipose‐derived mesenchymal stem cells. A, Structure schematic diagrams of Col‐Tgel. B, Glutamine/Lysine enzymatic crosslinking reaction during gelling. C, Scanning electron microscope image of Col‐Tgel. D and E, Scanning electron microscope images of a Col‐Tgel encapsuled adipose‐derived mesenchymal stem cells (ADSC) 3 days after encapsulation. F, 3D view of Col‐Tgel encapsuled ADSCs. Col‐Tgel indicates transglutaminase cross‐linked gelatin; Gln, glutamine; Lys, lysine; PI, propidium iodide; SEM, scanning electron microscope.
Vitality and Proliferation of ADSCs in Col‐Tgels
To validate that Col‐Tgel is non‐toxic for ADSCs, PI staining of ADSCs was performed 3 days after Col‐Tgel encapsulation. As shown in Figure 2A and 2B, PI‐positive ADSCs did not show a significant difference between 2D culture and 3D Col‐Tgel co‐culture. We next tested whether ADSCs could proliferate in the 3D Col‐Tgel by performing MTT and CCK‐8 assays at 1, 3, 7, 14, and 28 days after embedding. The MTT assay demonstrated that ADSCs survived and proliferated in the 3D Col‐Tgel system from 1 to 28 days (Figure 2C). Consistently, CCK‐8 assay revealed that the number of co‐cultured ADSCs increased over time (Figure 2D). Interestingly, 2D‐ and 3D‐cultured ADSCs proliferated at a similar speed within 7 days after seeding. However, when compared with a 2D‐culture, 3D‐cultures showed a significantly increased number of ADSCs on 14 and 28 days after seeding (Figure 2D). We reasoned that a Col‐Tgel‐based 3D culture provided more room for ADSCs expansion. Taken together, Col‐Tgels provided a proper micro‐environment, and more room for long‐term survival and growth of ADSCs.
Figure 2.
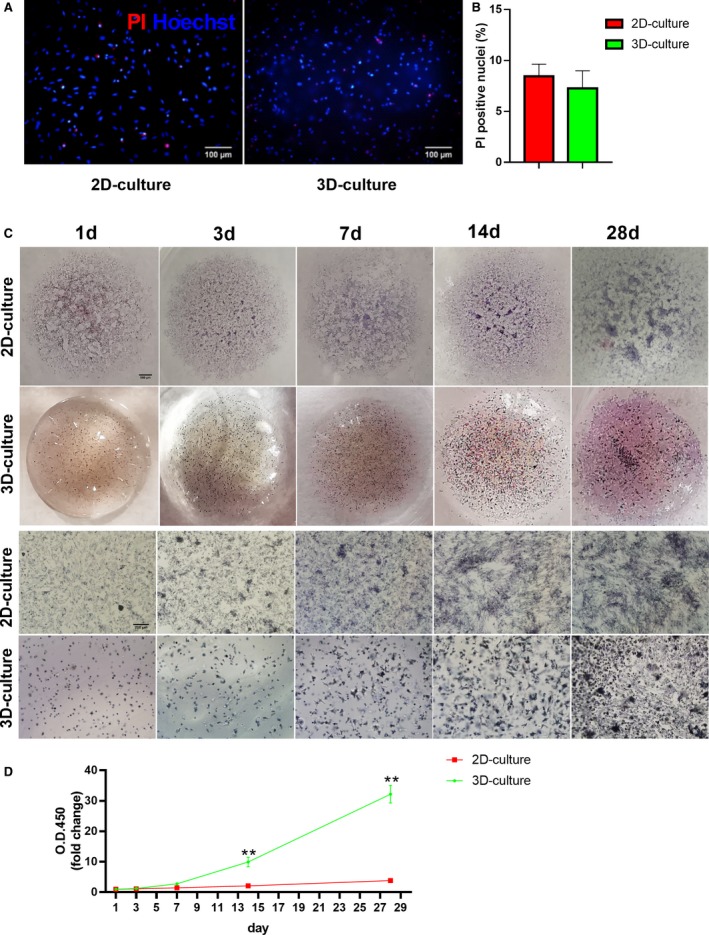
Vitality and proliferation of 2D/3D‐cultured adipose‐derived mesenchymal stem cells. A, Representative images of PI (red) and Hoechst (blue) staining of 2D/3D‐cultured adipose‐derived mesenchymal stem cells (ADSCs) 3 days after Col‐Tgel encapsulation. B, The percentage of PI positive nuclei (n=3 independent experiments). Data were analyzed by unpaired Student t test. C, Representative general and microscopic images of 2D‐ PBS‐resuspended ADSCs and 3D‐Col‐Tgel encapsulated ADSCs at different time points with MTT staining. D, Proliferation curves of 2D‐ PBS‐resuspended ADSCs and 3D‐ Col‐Tgel encapsulated ADSCs determined by CCK‐8 assay (n=3 same samples per group for all 5 time points). Data tested by 2‐way ANOVA with repeated measures, followed by a Bonferroni post hoc test. **P<0.01 vs 2D‐culture. Col‐Tgel indicates transglutaminase cross‐linked gelatin; OD, optical density; PI indicates propidium iodide.
Migration of ADSCs in Col‐Tgel Scaffolds
The migratory capability of stem cells facilitates their navigation to sites of injury, which is pivotal for their cardioprotective effects. However, rapid migration of transplanted stem cells is not good for their cardioprotective effects because the harsh microenvironment of an ischemic myocardium may kill cells. Therefore, we next sought to determine whether Col‐Tgel can slowly release the embedded cells. MTT staining and transwell assay were performed to determine whether ADSCs could migrate from the Col‐Tgel. MTT staining showed that ADSCs migrated outside from the edge of Col‐Tgel sphere initially 3 days after embedding. From 3 to 28 days after embedding, we observed an increased number of migrating ADSCs (Figure 3A). In addition, the migratory distance increased over time (Figure 3B). The migration speed of 3D‐cultured ADSCs was significantly lower than 2D‐cultured ADSCs on 3, 7, and 14 days. However, no significant differences were observed between 2D‐ and 3D‐cultured ADSCs on day 28 after culture. The transwell assay also revealed that Col‐Tgel encapsulated ADSCs could pass through the Col‐Tgel. However, when compared with Matrigel, Col‐Tgel significantly reduced the migration of ADSCs as evaluated at 3, 7, 14, 21 days after seeding (Figure 3C and 3D). Collectively, these results demonstrated that Col‐Tgel encapsulated ADSCs migrated out of the Col‐Tgel, although the migratory capability of ADSCs was limited.
Figure 3.
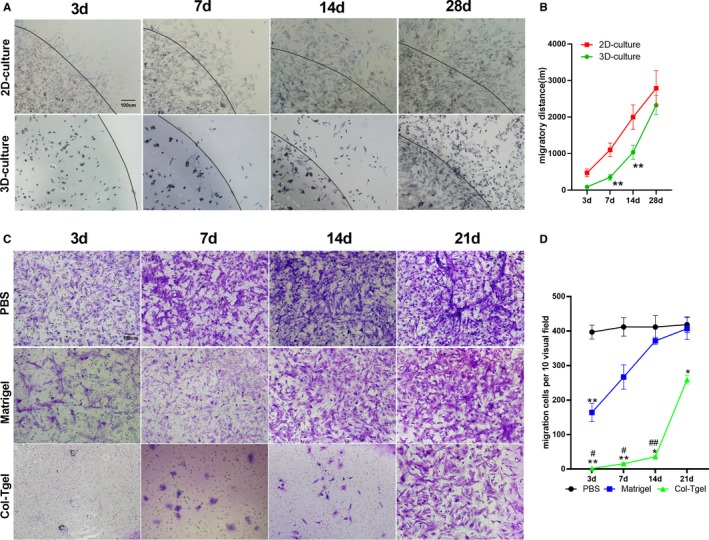
Migration of adipose‐derived mesenchymal stem cells (ADSCs) in the transglutaminase cross‐linked gelatin (Col‐Tgel) scaffold. A, MTT staining of migrated adipose‐derived mesenchymal stem cells from gel spheres edge over time. B, The migratory distance of 2D‐PBS‐resuspended ADSCs and 3D‐Col‐Tgel encapsulated ADSCs (n=3 different samples per group at each time point). **P<0.01 vs 2D‐culture at corresponding time points. C, Transwell assay was performed at indicated time points after mixing ADSCs with PBS (upper), Matrigel (middle), and Col‐Tgel (Lower) compartment (n=3). ADSCs migrating through PBS, Matrigel, and Col‐Tgel were stained with crystal violet. D, The number of ADSCs migrated out of PBS, Matrigel, and the Col‐Tgel (n=3 different samples per group at each time point). Data were tested by 2‐way ANOVA without repeated measures, followed by a Bonferroni post hoc test. *P<0.05, **P<0.01 vs PBS, # P<0.05, ## P<0.01 vs Matrigel at corresponding time points.
Col‐Tgel Encapsulation Significantly Increased Retention of Implanted ADSCs Post MI
To test the retention of Col‐Tgel encapsulated ADSCs (Tgel‐ADSC) and PBS‐resuspended ADSCs (PBS‐ADSC), we firstly performed intramyocardial injection of CM‐DiI‐labeled Tgel‐ADSC and PBS‐ADSC, both of which could be tracked by CM‐DiI immunofluorescence (Figure S1). Next, to investigate whether Col‐Tgel encapsulation increased the engraftment rate of ADSCs, the retention of CM‐DiI‐labeled ADSCs was determined at 3, 7, 14, 28, and 42 days after intramyocardial injection. No significant difference was observed between the number of Tgel‐ADSCs and PBS‐ADSCs in ischemic heart tissue at 3 days after injection (Figure 4). However, when compared with the injection of PBS‐ADSCs, Tgel‐ADSCs resulted in remarkably increased CM‐DiI‐labeled ADSCs from 7 to 42 days after injection (Figure 4). Interestingly, Tgel‐ADSCs were still present, at least 42 days after injection. However, although few PBS‐ADSCs were ingested by macrophages (Figure S2), PBS‐ADSCs were not detected at 28 days after injection. It is important to note that Tgel‐ADSC clusters were scattered from 28 days after injection, while PBS‐ADSC clusters were scattered at 3 days after injection (Figure 4). Taken together, these results demonstrated that Col‐Tgel encapsulation helped ADSCs survive in the harsh ischemic microenvironment.
Figure 4.
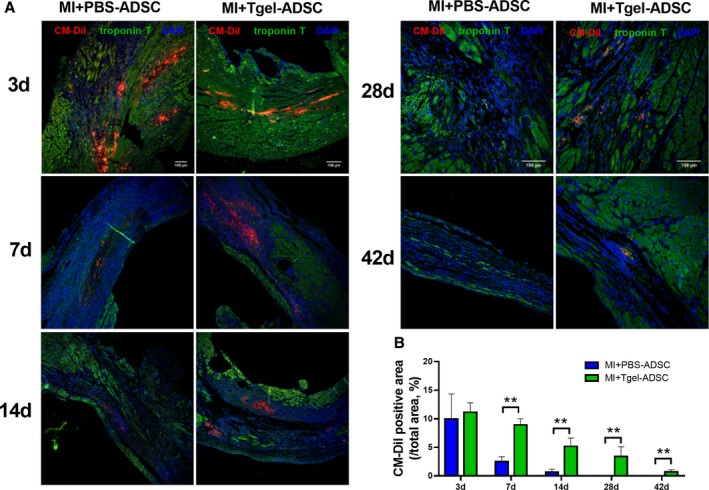
Transglutaminase cross‐linked gelatin significantly increased retention of implanted adipose‐derived mesenchymal stem cells post MI. A, Representative images of CM‐DiI labeled adipose‐derived mesenchymal stem cells (ADSCs) in hearts 3, 7, 14, 28, and 42 days after myocardial infarction (MI). Heart tissue was immunostained for CM‐DiI (red), troponin T (green), and DAPI (blue). B, Quantification of ADSCs in the peri‐infarct area was performed by calculating the percentage of the red‐colored area in the infarcted area (Trop T negative area) (n=20 from 4 mice). CM‐DiI indicates Chloromethylbenzamido 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate; Col‐Tgel, Transglutaminase cross‐linked Gelatin. Data were analyzed by unpaired Student t‐test. **P<0.01.
Col‐Tgel Encapsulation Increased the Cardioprotection of ADSCs Against Post‐MI Cardiac Injury
Given that Col‐Tgel encapsulation increased the retention of ADSCs in ischemic heart tissue, we next aimed to determine whether Col‐Tgel encapsulation increased cardioprotection. Of the 93 mice that were divided into the following 5 groups: sham, MI+PBS, MI+Tgel, MI+PBS‐ADSC, or MI+Tgel‐ADSC, 12 mice died at various time points during follow‐up (Figure 5A). Administration of PBS‐ADSC, Col‐Tgel, and Tgel‐ADSC did not significantly increased the survival rate (all P>0.05 versus MI+PBS). Echocardiography was determined on 1 day, 2 weeks, and 4 weeks after transplantation to evaluate cardiac function post MI (Figure 5B through 5E). Initial cardiac dysfunction was similar in all groups 1 day after MI (Figure 5C through 5E). At 4 weeks after MI, intramyocardial delivery of PBS‐ADSC alone or Col‐Tgel alone did not significantly increase left ventricular ejection fraction, decrease left ventricular end systolic diameter, or decrease left ventricular end diastolic diameter compared with the MI+PBS group. However, Tgel‐ADSC significantly increased left ventricular ejection fraction at 4 weeks after MI (P<0.01 versus MI+PBS group, Figure 5B and 5C). Tgel‐ADSC also significantly decreased left ventricular end systolic diameter (P<0.05, Figure 5B and 5D) but did not significantly decrease the LV end‐diastolic diameter at 4 weeks after MI when compared with PBS treatment (P>0.05, Figure 5B and 5E). Our data showed that either PBS‐ADSC alone or Col‐Tgel alone failed to significantly improve cardiac function post MI or reduce internal diameters (all P>0.05 versus MI+PBS group, Figure 5B through 5E).
Figure 5.
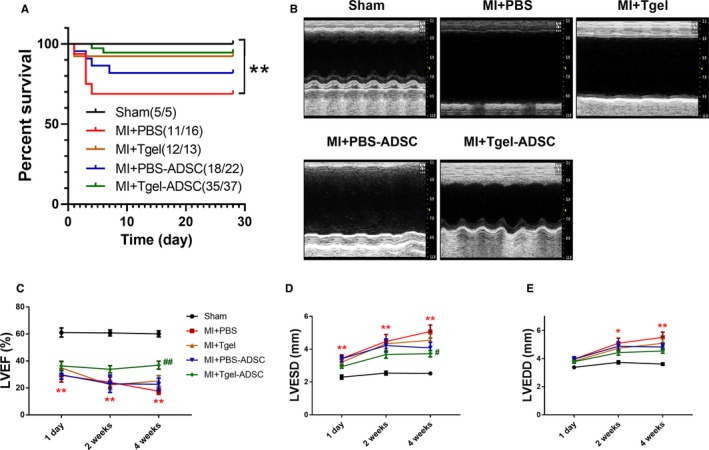
Tgel‐adipose‐derived mesenchymal stem cells improved post‐myocardial infarction (MI) cardiac function. A, Survival curves (n=5–37 mice per group) were analyzed by the Gehan–Breslow–Wilcoxon test. B, Representative echocardiographic images 4 weeks after myocardial infarction. C through E, Left ventricular ejection fraction, (C), left ventricular end systolic diameter, (D), and left ventricular end diastolic diameter, (E) at 1 day, 2 weeks, and 4 weeks after myocardial infarction (n=5–18 mice per group). Unless otherwise indicated, data were analyzed by 1‐way ANOVA, followed by a Bonferroni post hoc test. ADSC indicates adipose‐derived mesenchymal stem cells; Tgel, Transglutaminase cross‐linked Gelatin; LVEDD, left ventricular ejection diastolic diameter; LVESD, left ventricular ejection systolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction. *P<0.05, **P<0.01 vs Sham. # P<0.05, ## P<0.01 vs myocardial infarction+PBS.
Masson trichrome staining of the transverse plane revealed that the myocardial fibrotic area was significantly reduced by Tgel‐ADSC transplantation when compared with the MI+PBS group (P<0.01 versus MI+PBS group, Figure 6A and 6B). However, either PBS‐ADSC alone or Col‐Tgel alone failed to significantly reduce the fibrotic area. Moreover, cardiac remodeling was assessed at the molecular level. Quantitative polymerase chain reaction showed that Tgel‐ADSC transplantation significantly downregulated the mRNA level of atrial natriuretic peptide and brain natriuretic peptide (both P<0.01 versus MI+PBS, Figure 6C and 6D). Taken together, these results clearly showed that Col‐Tgel encapsulation increased the cardioprotection of ADSCs against MI‐induced cardiac injury.
Figure 6.
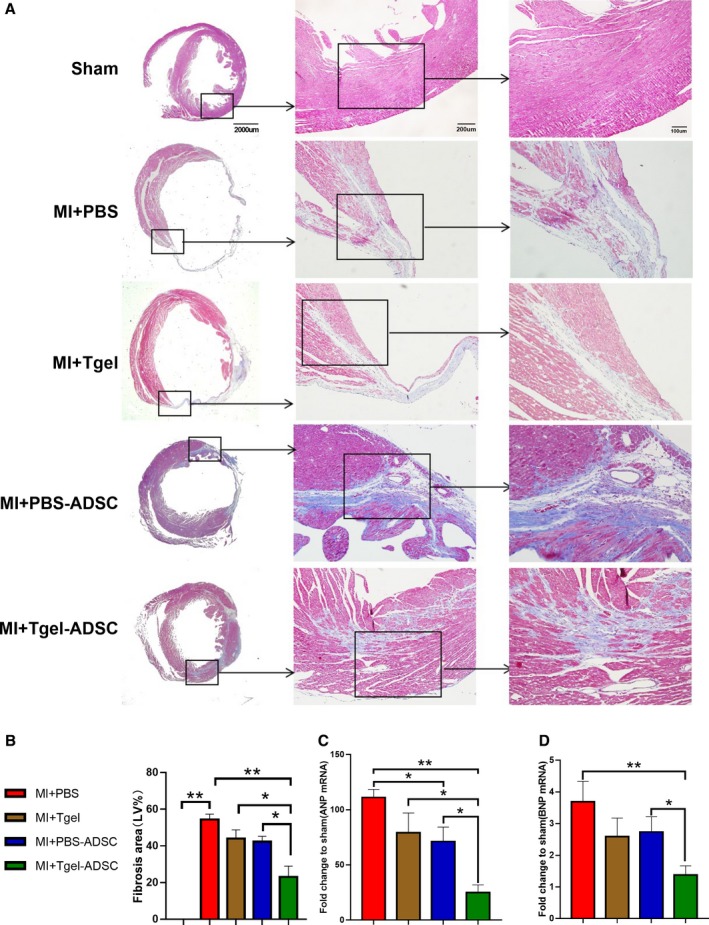
Tgel‐adipose‐derived mesenchymal stem cells improved post‐myocardial infarction (MI) cardiac remodeling. A, Representative images of Masson trichrome staining of the transverse planes of heart sections. B, Quantification of the fibrotic area (n=5–8 mice per group). C and D, Quantitative real time polymerase chain reaction analysis of atrial natriuretic peptide and brain natriuretic peptide mRNA expression in the left ventricle (n=4–6 mice per group per group). Data were analyzed by 1‐way ANOVA, followed by a Bonferroni post hoc test. ADSC indicates adipose‐derived mesenchymal stem cells; Tgel, Transglutaminase cross‐linked Gelatin; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; LV, left ventricular; MI, myocardial infarction. *P<0.05, **P<0.01.
Discussion
MI leads to thinning of the heart wall, myocyte slippage, and ventricular dilation, resulting in progressive damage to the heart‐wall muscle. Based on currently available clinical and basic research findings, cell transplantation is one of the most promising treatment for damaged myocardial tissue.31 Among numerous cell types that can be used for cardiac repair, mesenchymal stem cells (MSCs) have been shown to be the optimal cell type for clinical applications because of their safety and availability.32, 33, 34, 35, 36 Although evidence that MSCs could regenerate new cardiac muscle to replace injured cardiomyocytes is limited, an increased number of preclinical studies have demonstrated that transplantation of MSCs confers cardioprotection by limiting apoptotic cell death and fibrosis, thereby promoting neovascularization in the ischemic heart.37 However, poor survival and engraftment of donated stem cells remains a significant challenge in stem cell therapy for MI.38 As such, significant attempts have been made to enhance cellular and therapeutic efficacy of MSCs by preconditioning, using genetic modification, improving ischemic myocardial microenvironment, and the use of biomaterials such as injectable hydrogels and surgical patches as scaffolds.39, 40, 41, 42
MSCs were first isolated from bone marrow and were also found in many other tissues, including but not limited to: umbilical cord, heart tissue, and adipose tissue.3, 43, 44 Adipose tissue‐derived MSCs (ADSCs) are a cell population with characteristics that are similar, albeit not identical, to those of bone marrow derived MSCs.45 ADSCs offer significant advantages over bone marrow derived MSCs for potential clinical use. Firstly, ADSCs can be easily acquired from subcutaneous adipose tissues through a simple surgical procedure that is less expensive and minimally invasive. Secondly, there is a greater yield (over 5‐fold) of ADSCs from adipose tissue when compared with that from bone marrow.46 Thirdly, ADSCs possess a higher stem cell proliferation rate than bone marrow derived MSCs. Previous studies from our group and others have demonstrated that ADSCs can efficiently incorporate into peri‐infarcted heart tissue when locally transfused by direct intramyocardial injection.3, 47 However, the cardioprotective effects of ADSCs alone are not sufficient and thus need to be improved.
In the present study, we introduced a novel injectable Col‐Tgel hydrogel to improve the retention and cardioprotection of ADSCs against MI. Hydrogels are hydrophilic polymer networks with high water content and diffusivity. Among the diverse types of biomaterials, hydrogels that are primarily prepared from either natural or synthetic hydrophilic, biocompatible polymers, can mimic or include the naturally occurring extracellular matrix.11, 48 To achieve better cell survival and cardiac muscle support, hydrogels have been used in multiple clinical applications, including as vehicles for drug delivery and as scaffolds for cell grafts.21, 49 These injectable hydrogels can be easily administered through minimally invasive procedures, which provide patient convenience as well as site‐specific drug release.10 Previous studies have reported that the gelation of cells‐biomaterial mixture in heart tissue can effectively reduce the loss of transplanted cells caused by myocardial contraction.11 Col‐Tgel is a stiffness‐tunable transglutaminase cross‐linked type of gelatin that has better stability and durability compared with natural gelatin. Col‐Tgel exhibits excellent performance in cellular adhesion and proliferation, and is able to release entrapped cells.22 However, it is still unknown whether Col‐Tgel can be a vehicle for cells for in vivo cell implantation for heart tissue repair.
Here, we established a 3D Col‐Tgel biological environment for ADSCs survival, growth, and migration up to as far as 4 weeks. We investigated the attachment and survival of ADSCs in the Col‐Tgel at 3 days after encapsulation using scanning electron microscopy. Col‐Tgel coating resulted in restricted migration of ADSCs without affecting their survival. We observed no significant evidence of in vitro degradation of Col‐Tgel as far as 4 weeks. The in vitro data not only reported reproducibility of the findings presented in a previous study22 but also demonstrated a longer time of observation on the impact of Col‐Tgel on ADSCs. In the in vivo study, Col‐Tgel encapsulated ADSCs were injected intramyocardially immediately after MI operation. The retention of transplanted cells was monitored by CM‐DiI immunofluorescence of heart sections at 3, 7, 14, 28, and 42 days after injection. Tgel‐ADSC did not show better cardiac retention than PBS‐ADSC 3 days after injection. However, we demonstrated that Col‐Tgel hydrogels significantly prevented the loss of injected cells from 1 to 6 weeks post MI. Interestingly, different from PBS‐ADSC clusters, which were scattered at 3 days after injection, Tgel‐ADSC clusters were scattered from 28 days after injection (Figure 4). These findings might be attributed to the degradation of Col‐Tgel.
Accumulating studies have demonstrated that the benefit of stem cells in cardiac applications is proportional to the number of transplanted cells.5, 50, 51 However, studies using collagen matrix showed improved cardiac recovery even in the absence of stem cell incorporation.52 Our data showed that Col‐Tgel alone increased the mouse survival rate, cardiac function, and decreased fibrosis, although the differences were not significant. Similarly, intramyocardial injection of PBS‐ADSC also slightly increased the survival rate, and improved MI‐induced cardiac remodeling and function cardiac function, which was consistent with our previous findings.3 More importantly, Col‐Tgel encapsulated ADSCs further increased the survival rate, cardiac function, and reduced fibrosis as compared with PBS, Col‐Tgel, and PBS‐ADSC. Our results confirmed the increased number of ADSC survival in the peri‐infarct region exerted better cardioprotective effects against MI injury.
Conclusions
We demonstrated successful encapsulation of ADSCs using Col‐Tgel. Col‐Tgel provides a suitable microenvironment for maintaining survival, proliferation, and migration of ADSCs in ischemic heart tissue, which are pivotal for the cardioprotection of ADSCs. Encapsulated ADSCs achieved a higher number and longer survival in the ischemic myocardium after transplantation, and thereby exert better cardioprotection against post‐MI cardiac remodeling and dysfunction. This methodology is capable of clinical translation and can improve the success and reproducibility of cell therapy studies and cardiac regeneration studies in general.
Sources of Funding
This work was supported by National Key R&D Program of China Grant 2018YFA0107400 and by Program for National Science Funds of China Grants 81970212, 81600310, 81800229, and 81670229. Xijing Hospital Supporting Program Grant XJZT18MJ40.
Disclosures
None.
Supporting information
Table S1. Primer List
Figure S1. Tracking of both Tgel‐adipose‐derived mesenchymal stem cells (Tgel‐ADSC) or PBS‐adipose‐derived mesenchymal stem cells (PBS‐ADSC) in heart section prepared immediately after injection.
Figure S2. Co‐localization of F4/80 and CM‐DiI in the injected PBS‐ADSCs 2 weeks after myocardial infarction and cell injection.
Acknowledgments
The work presented here was performed in collaboration between all authors. Dr Youhu Chen, Congye Li, and Prof Chengxiang Li designed methods and experiments, performed the laboratory experiments, and wrote the paper. Dr Jiangwei Chen, Yan Li, Huaning Xie, Dr Chen Lin, Dr Miaomiao Fan, Yongzhen Guo, and Prof Erhe Gao helped to perform in vivo experiments. Dr Wenjun Yan and Prof Ling Tao designed and supervised the study, and revised the manuscript critically.
(J Am Heart Assoc. 2020;9:e013784 DOI: 10.1161/JAHA.119.013784.)
Contributor Information
Wenjun Yan, Email: yanyan517032@163.com.
Ling Tao, Email: lingtao@fmmu.edu.cn.
References
- 1. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 2. Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan W, Guo Y, Tao L, Lau WB, Gan L, Yan Z, Guo R, Gao E, Wong GW, Koch WL, Wang Y, Ma X. C1q/tumor necrosis factor‐related protein‐9 regulates the fate of implanted mesenchymal stem cells and mobilizes their protective effects against ischemic heart injury via multiple novel signaling pathways. Circulation. 2017;136:2162–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell‐based therapy, and engineered heart tissue. Physiol Rev. 2016;96:1127–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta‐analysis of preclinical studies and clinical trials. Circ Res. 2017;120:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bobi J, Solanes N, Fernandez‐Jimenez R, Galan‐Arriola C, Dantas AP, Fernandez‐Friera L, Galvez‐Monton C, Rigol‐Monzo E, Aguero J, Ramirez J, Roque M, Bayes‐Genis A, Sanchez‐Gonzalez J, Garcia‐Alvarez A, Sabate M, Roura S, Ibanez B, Rigol M. Intracoronary administration of allogeneic adipose tissue‐derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction. J Am Heart Assoc. 2017;6:e005771 DOI: 10.1161/JAHA.117.005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. [DOI] [PubMed] [Google Scholar]
- 8. Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously delivered mesenchymal stem cells: systemic anti‐inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120:1598–1613. [DOI] [PubMed] [Google Scholar]
- 9. Hwang Y, Kim JC, Tae G. Significantly enhanced recovery of acute liver failure by liver targeted delivery of stem cells via heparin functionalization. Biomaterials. 2019;209:67–78. [DOI] [PubMed] [Google Scholar]
- 10. Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv. 2014;32:449–461. [DOI] [PubMed] [Google Scholar]
- 11. Yao X, Liu Y, Gao J, Yang L, Mao D, Stefanitsch C, Li Y, Zhang J, Ou L, Kong D, Zhao Q, Li Z. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials. 2015;60:130–140. [DOI] [PubMed] [Google Scholar]
- 12. Xu B, Li Y, Deng B, Liu X, Wang L, Zhu QL. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp Ther Med. 2017;13:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Zhan Y, Wang Y, Han D, Tao B, Luo Z, Ma S, Wang Q, Li X, Fan L, Li C, Deng H, Cao F. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post‐myocardial infarction in rats. Acta Biomater. 2018;80:154–168. [DOI] [PubMed] [Google Scholar]
- 14. Sonnenberg SB, Rane AA, Liu CJ, Rao N, Agmon G, Suarez S, Wang R, Munoz A, Bajaj V, Zhang S, Braden R, Schup‐Magoffin PJ, Kwan OL, DeMaria AN, Cochran JR, Christman KL. Delivery of an engineered HGF fragment in an extracellular matrix‐derived hydrogel prevents negative LV remodeling post‐myocardial infarction. Biomaterials. 2015;45:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blocki A, Beyer S, Dewavrin JY, Goralczyk A, Wang Y, Peh P, Ng M, Moonshi SS, Vuddagiri S, Raghunath M, Martinez EC, Bhakoo KK. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long‐term retention of MSCs in infarcted myocardium. Biomaterials. 2015;53:12–24. [DOI] [PubMed] [Google Scholar]
- 16. Muscari C, Bonafe F, Martin‐Suarez S, Valgimigli S, Valente S, Fiumana E, Fiorelli F, Rubini G, Guarnieri C, Caldarera CM, Capitani O, Arpesella G, Pasquinelli G. Restored perfusion and reduced inflammation in the infarcted heart after grafting stem cells with a hyaluronan‐based scaffold. J Cell Mol Med. 2013;17:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sougawa N, Miyagawa S, Fukushima S, Yokoyama J, Kitahara M, Harada A, Mochizuki‐Oda N, Sato‐Nishiuchi R, Sekiguchi K, Sawa Y. Laminin‐511 supplementation enhances stem cell localization with suppression in the decline of cardiac function in acute infarct rats. Transplantation. 2019;103:e119–e127. [DOI] [PubMed] [Google Scholar]
- 18. Ji ST, Kim H, Yun J, Chung JS, Kwon S. Promising therapeutic strategies for mesenchymal stem cell‐based cardiovascular regeneration: from cell priming to tissue engineering. Stem Cells Int. 2017;2017:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He X, Wu R, Xu X, Wang J, Yin Y, Chen F. Macrophage involvement affects matrix stiffness‐related influences on cell osteogenesis under three‐dimensional culture conditions. Acta Biomater. 2018;71:132–147. [DOI] [PubMed] [Google Scholar]
- 20. Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015;37:2139–2145. [DOI] [PubMed] [Google Scholar]
- 21. Gottipati A, Chelvarajan L, Peng H, Kong R, Cahall CF, Li C, Tripathi H, Al‐Darraji A, Ye S, Elsawalhy E, Abdel‐Latif A, Berron BJ. Gelatin based polymer cell coating improves bone marrow‐derived cell retention in the heart after myocardial infarction. Stem Cell Rev. 2019;15:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Xiao Z, Ren X, Long H, Qian H, Ma K, Guo Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue‐derived stromal cells. PeerJ. 2016;4:e2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alarake NZ, Frohberg P, Groth T, Pietzsch M. Mechanical properties and biocompatibility of in situ enzymatically cross‐linked gelatin hydrogels. Int J Artif Organs. 2017;40:159–168. [DOI] [PubMed] [Google Scholar]
- 24. Kuwahara K, Yang Z, Slack GC, Nimni ME, Han B. Cell delivery using an injectable and adhesive transglutaminase‐gelatin gel. Tissue Eng Part C Methods. 2010;16:609–618. [DOI] [PubMed] [Google Scholar]
- 25. Fang JY, Tan S, Wu Y, Yang Z, Hoang BX, Han B. From competency to dormancy: a 3D model to study cancer cells and drug responsiveness. J Transl Med. 2016;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan S, Fang JY, Yang Z, Nimni ME, Han B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials. 2014;35:5294–5306. [DOI] [PubMed] [Google Scholar]
- 27. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi M, Suzuki E, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Adipose tissue‐derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Physiol Heart Circ Physiol. 2010;298:H415–H423. [DOI] [PubMed] [Google Scholar]
- 29. Xia Y, Zhang F, Zhao S, Li Y, Chen X, Gao E, Xu X, Xiong Z, Zhang X, Zhang J, Zhao H, Wang W, Wang H, Guo Y, Liu Y, Li C, Wang S, Zhang L, Yan W, Tao L. Adiponectin determines farnesoid X receptor agonism‐mediated cardioprotection against post‐infarction remodelling and dysfunction. Cardiovasc Res. 2018;114:1335–1349. [DOI] [PubMed] [Google Scholar]
- 30. He Y, Guo Y, Xia Y, Guo Y, Wang R, Zhang F, Guo L, Liu Y, Yin T, Gao C, Gao E, Li C, Wang S, Zhang L, Yan W, Tao L. Resistin promotes cardiac homing of mesenchymal stem cells and functional recovery after myocardial ischemia‐reperfusion via the ERK1/2‐MMP‐9 pathway. Am J Physiol Heart Circ Physiol. 2019;316:H233–H244. [DOI] [PubMed] [Google Scholar]
- 31. Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017;121:135–154. [DOI] [PubMed] [Google Scholar]
- 32. Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES‐cell‐derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. [DOI] [PubMed] [Google Scholar]
- 34. Hare JM, Fishman JE, Gerstenblith G, DiFede VD, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis‐Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong PFC, Ruiz P, Amador A, Da SJ, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, Sougawa N, Kawamura T, Daimon T, Shimizu T, Okano T, Toda K, Sawa Y. Enhanced survival of transplanted human induced pluripotent stem cell‐derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013;128:S87–S94. [DOI] [PubMed] [Google Scholar]
- 36. Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ. Essential role of ICAM‐1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. [DOI] [PubMed] [Google Scholar]
- 37. Chen Z, Chen L, Zeng C, Wang WE. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018;2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohsin S, Troupes CD, Starosta T, Sharp TE, Agra EJ, Smith S, Duran JM, Zalavadia N, Zhou Y, Kubo H, Berretta RM, Houser SR. Unique features of cortical bone stem cells associated with repair of the injured heart. Circ Res. 2015;117:1024–1033. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka Y, Shirasawa B, Takeuchi Y, Kawamura D, Nakamura T, Samura M, Nishimoto A, Ueno K, Morikage N, Hosoyama T, Hamano K. Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am J Transl Res. 2016;8:2222–2233. [PMC free article] [PubMed] [Google Scholar]
- 40. Bortolotti F, Ruozi G, Falcione A, Doimo S, Dal Ferro M, Lesizza P, Zentilin L, Banks L, Zacchigna S, Giacca M. In vivo functional selection identifies cardiotrophin‐1 as a cardiac engraftment factor for mesenchymal stromal cells. Circulation. 2017;136:1509–1524. [DOI] [PubMed] [Google Scholar]
- 41. Baldari S, Di Rocco G, Trivisonno A, Samengo D, Pani G, Toietta G. Promotion of survival and engraftment of transplanted adipose tissue‐derived stromal and vascular cells by overexpression of manganese superoxide dismutase. Int J Mol Sci. 2016;17:E1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ichihara Y, Kaneko M, Yamahara K, Koulouroudias M, Sato N, Uppal R, Yamazaki K, Saito S, Suzuki K. Self‐assembling peptide hydrogel enables instant epicardial coating of the heart with mesenchymal stromal cells for the treatment of heart failure. Biomaterials. 2018;154:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naftali‐Shani N, Levin‐Kotler LP, Palevski D, Amit U, Kain D, Landa N, Hochhauser E, Leor J. Left ventricular dysfunction switches mesenchymal stromal cells toward an inflammatory phenotype and impairs their reparative properties via Toll‐like receptor‐4. Circulation. 2017;135:2271–2287. [DOI] [PubMed] [Google Scholar]
- 44. Lim M, Wang W, Liang L, Han ZB, Li Z, Geng J, Zhao M, Jia H, Feng J, Wei Z, Song B, Zhang J, Li J, Liu T, Wang F, Li T, Li J, Fang Y, Gao J, Han Z. Intravenous injection of allogeneic umbilical cord‐derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther. 2018;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization‐associated molecules on multi‐lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. [DOI] [PubMed] [Google Scholar]
- 46. Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose‐derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. [DOI] [PubMed] [Google Scholar]
- 47. Wang WE, Yang D, Li L, Wang W, Peng Y, Chen C, Chen P, Xia X, Wang H, Jiang J, Liao Q, Li Y, Xie G, Huang H, Guo Y, Ye L, Duan DD, Chen X, Houser SR, Zeng C. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose‐derived stem cells in infarcted myocardium. Circ Res. 2013;113:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sepantafar M, Maheronnaghsh R, Mohammadi H, Rajabi‐Zeleti S, Annabi N, Aghdami N, Baharvand H. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol Adv. 2016;34:362–379. [DOI] [PubMed] [Google Scholar]
- 49. Lin T, Lu Y, Zhang X, Gong L, Wei C. Treatment of dry eye by intracanalicular injection of a thermosensitive chitosan‐based hydrogel: evaluation of biosafety and availability. Biomater Sci. 2018;6:3160–3169. [DOI] [PubMed] [Google Scholar]
- 50. Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel‐Latif A, Zuba‐Surma EK, Dawn B. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ Res. 2015;117:558–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. [DOI] [PubMed] [Google Scholar]
- 52. Blackburn NJ, Sofrenovic T, Kuraitis D, Ahmadi A, McNeill B, Deng C, Rayner KJ, Zhong Z, Ruel M, Suuronen EJ. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials. 2015;39:182–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer List
Figure S1. Tracking of both Tgel‐adipose‐derived mesenchymal stem cells (Tgel‐ADSC) or PBS‐adipose‐derived mesenchymal stem cells (PBS‐ADSC) in heart section prepared immediately after injection.
Figure S2. Co‐localization of F4/80 and CM‐DiI in the injected PBS‐ADSCs 2 weeks after myocardial infarction and cell injection.


