Recently, the field of oncology has witnessed a surge of new therapeutic agents that have collectively led to improved rates of progression‐free and overall survival for many cancers.1 However, these advances have also led to increased morbidity attributable to treatment‐related side effects, in particular, cardiac toxicities. Cancer therapeutics may negatively affect the heart in a host of ways, whether it is through direct tissue damage or indirectly by exacerbating cardiovascular risk factors.2, 3, 4, 5, 6 Given that cardiovascular disease is the most common cause of death among cancer survivors, increased recognition and understanding of these cardiovascular toxicities is paramount to successful management of this ever increasing survivorship population.1, 5, 7, 8 Cardiac imaging has been essential to elucidating mechanisms for a growing number of cardiotoxicities, and its role in toxicity surveillance and management continues to evolve.9, 10, 11, 12
Approaching this review from the perspective of treatment‐related cardiovascular toxicities, we examine evolving applications of imaging in contemporary cardio‐oncology with specific focus on the more novel and emerging anticancer therapies. In doing so, we will examine broad categories of cancer therapeutics, highlight specific agents that have been implicated in cardiotoxicity, and discuss optimal cardiac imaging modalities for each class.3 We will also consider amyloidosis, in light of recent interest. Guideline‐driven recommendations and position paper statements pertaining to cardiac imaging will be highlighted wherever possible.12 We recognize that cardiac imaging position statements are largely unavailable for certain drug categories, particularly for more novel agents. In these instances, primary literature and specific clinical cases pertaining to cardiac imaging applications for each cardiotoxicity will be reviewed.
Prominent cardiotoxicities as related to cancer therapies are detailed below:
Systolic dysfunction and heart failure
Coronary artery disease and myocardial ischemia
Myocarditis
Amyloidosis
Pericardial disease
Peripheral vascular disease and vascular dysfunction
Cancer Treatment–Related Systolic Dysfunction and Heart Failure
Multiple cancer therapeutic agents are associated with myocardial dysfunction and heart failure including anthracyclines, biologic agents, proteasome inhibitors, radiation therapy, and certain tyrosine kinase inhibitors. Anthracyclines remain among the most commonly used traditional chemotherapies and have been well implicated as a cause of heart failure.13 This toxicity is thought to be mediated by topoisomerase‐Iiβ, which in turn activates oxidative phosphorylation and other apoptotic pathways.14 Heart failure symptoms typically manifest within the first year of exposure and follow a dose‐response relationship. Toxicity often presents when the dose exceeds 350 mg/m2.15 Additionally, patients with traditional cardiovascular risk factors, older age, prior systolic dysfunction, prior high‐dose radiotherapy (≥30 Gy), or exposure to prior cardiotoxic therapies are at a higher risk of toxicity.16 The mortality associated with anthracycline cardiotoxicity ranges between 30% and 70%.16 Multiple imaging modalities have been employed to study anthracycline cardiac toxicity, and this continues to evolve (Figure 1A and 1B).
Figure 1.
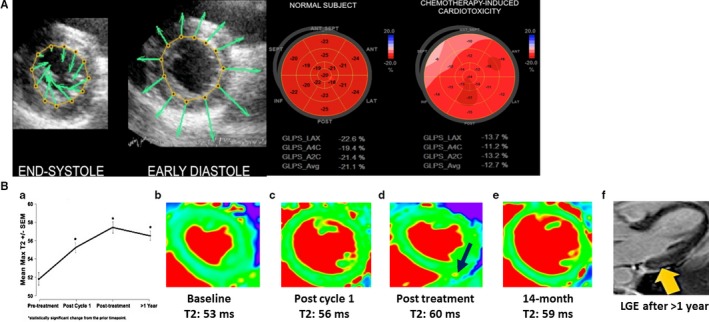
A, (Left) Velocity vector imaging tracking endomyocardial border throughout the cardiac cycle. (Right) Bull's‐eye plot of average peak systolic global longitudinal strain in a normal subject compared with that of a patient with chemotherapy‐induced cardiotoxicity. Adapted from Mele et al17 under the Creative Commons Attribution Non‐Commercial‐NoDerivs (CC‐BY‐NC‐ND) license. B, Objective increase in myocardial T2 mapping values (a marker of inflammation/edema) after cardiotoxic anthracycline therapy exposure (A through E), even before cardiac fibrosis (F; yellow arrow, LGE) and clinical heart failure (with left ventricular ejection fraction decline) development among breast cancer patients via use of T2 mapping. Note: maximum T2 plateaus and actually declines after cancer therapy cessation (A, D, and E; black arrow in D, high T2). Adapted with permission from Lustberg et al18 Copyright ©2019, Wolters Kluwer Health, Inc. LGE indicates late gadolinium enhancement.
Biologic agents such as trastuzumab and pertuzumab are monoclonal antibodies that target HER2/neu receptors and are primarily used for the treatment of breast cancer. The cytotoxicity produced by these agents is attributable to activation of the PI3K (phosphatidyl 3‐kinase)/AkT (protein kinase B)/mTOR (mammalian target of rapamycin)/Ras/MAPK (mitogen‐activated protein kinase) pathways.19 Cardiac side effect is primarily limited to left ventricular (LV) dysfunction and is thought to be reversible upon drug discontinuation.20 LV dysfunction is not dose dependent but is related to traditional cardiovascular risk factors and prior anthracycline use.20, 21
Proteasome inhibitors such as bortezomib and carfilzomib cause disruption of normal protein homeostasis and have revolutionized the treatment of multiple myeloma. While beneficial in their pro‐apoptotic effects on cancer cells, proteasome inhibitors are also associated with various, albeit largely reversible, cardiotoxic effects. Four percent to 7% of patients treated with bortezomib and carfilzomib will develop heart failure with more recent studies reporting an incidence of grade 3 or 4 heart failure upwards of 20%. Case series data demonstrate that patients with proteasome cardiotoxicity can have moderate‐to‐severe global LV hypokinesis with ejection fraction ranging between 20% and 50%.22 These agents are also linked to hypertension, pulmonary hypertension, and diastolic dysfunction.23, 24
Targeted therapies comprise a broad range of agents, including small‐molecule tyrosine kinase inhibitors (TKIs), which modulate signal transduction, gene expression, angiogenesis, and apoptosis pathways in a variety of malignancies. TKIs each have their own mechanism of action, and their associated cardiac side effect profile is heterogeneous. For example, sunitinib is linked to hypertension and heart failure, the latter on the order of 10%.25, 26 Ibrutinib has been associated with rare cases of heart failure, atrial and ventricular arrhythmias, and sudden cardiac death.27, 28 Sorafenib, dasatinib, and pazopanib are other members of the TKI class that have prominent cardiotoxic profiles and are discussed elsewhere.
Radiotherapy‐associated cardiovascular disease is a major cause of morbidity and mortality in cancer survivors. The mechanism of toxicity predominantly originates from endothelial damage with a subsequent inflammatory response driving neointimal proliferation, fibrosis, and atherosclerosis.29, 30 Radiotherapy‐associated diastolic dysfunction and cardiac remodeling results in subsequent fibrosis and heart failure with preserved ejection fraction. This has been well demonstrated in the Hodgkin lymphoma cohort31, 32 as well as in the breast cancer population, with associated reduced functional capacity and survival.33, 34 Associated risk factors for radiotherapy‐induced cardiac dysfunction include radiation dose over 30 Gy or any radiation exposure with concomitant anthracycline treatment, younger age of exposure, location of radiation, and preexisting cardiovascular disease.
Echocardiography and Treatment‐Related Myocardial Dysfunction
Because of its wide accessibility, low cost, and negligible side effect profile, echocardiography is the cornerstone of myocardial dysfunction assessment. Echo is the most common modality used to monitor patients who are on traditional chemotherapeutic regimens such as anthracyclines, given the strong causal relationship between these agents and cardiomyopathy. Guidelines recommend using echocardiography in clinical scenarios of heart failure presentation, surveillance during therapy, and follow‐up until 1 year after treatment completion.9 Specific echo guidelines regarding monitoring anthracyclines have been proposed by multiple organizations and are detailed in Table 1. The 2014 expert consensus statement by the American Society of Echocardiography35 recognized echocardiographic strain as a valuable modality when following patients receiving cardiotoxic therapies since reduction in myocardial strain, particularly global longitudinal strain (GLS), has been shown to be predictive of future myocardial dysfunction36 (Figure 1A). Hence, there has been introduction of strain imaging in certain monitoring guidelines.10
Table 1.
Existing Cardiac Imaging Recommendations Based on Prior Data
| Anti‐Cancer Agent | Monitoring Guidelines | Organization/Society Recommending |
|---|---|---|
| Anthracyclines | Echo | |
| Recommended for those with symptoms of heart failure (E,B,1)a | ASCO9 | |
| Recommended for surveillance of those undergoing treatment, frequency based on clinical discretion (E,B,2) | ASCO9 | |
| Recommended to perform in asymptomatic patients 6 to 12 mo after completion of therapy in those felt to be at a higher risk for CTRCD (E,B,2) | ASCO9 | |
| LVEF measurement at baseline and duringb treatment (2D/3D) and GLS with treatment or risk factor modification at LVEF ≥60%, 50%–59%, 40%–49%, and <40% | Liu et al37 | |
| LVEF at baseline and at end of treatment. Regular LVEF monitoring if cumulative dose exceeds 240 mg/m2. Recommendation based on use of 2D echocardiogram and GLS | SEOM,10 ESC12 | |
| Measurement of LVEF at baseline, every 3 mo during chemotherapy, at the end of treatment (within 1 mo), every 3 mo during the first year after chemotherapy, every 6 mo during the following 4 y, and yearly afterward | Cardinale et al15 | |
| CMR | ||
| Recommended instead of echo only if echo unavailable or not technically feasible (E,B,2) | ASCO9 | |
| Recommendation to perform in asymptomatic patients 6 to 12 mo after completion of therapy in those felts to be at a higher risk for CTRCD and not a good candidate for echocardiogram (E,B,2) | ASCO9 | |
| Utility of CMR over LVEF monitoring in terms of myocardial fibrosis and inflammation quantification | Jordan et al38 | |
| Multigated angiocardiography (MUGA) | ||
| Recommended instead of echo only if echo unavailable or not technically feasible and CMR unavailable (E,B,2) | ASCO9 | |
| Recommended to perform in asymptomatic patients 6 to 12 mo after completion of therapy in those felt to be at a higher risk for CTRCD and not a good candidate for echocardiogram and CMR unavailable (E,B,2) | ASCO15 | |
LVEF >50% at baseline
|
ASNC39 | |
LVEF <50% at baseline
|
ASNC39 | |
| Trastuzumab | Echo | |
| Recommended for surveillance of metastatic breast cancer patients receiving trastuzumab indefinitely (E,C,2) | ASCO9 | |
Recommended:
|
SEOM10 ASCO15 |
|
Transthoracic echocardiograms that includes comprehensive 2D, 3D, and strain imaging.
|
SAFE‐HEaRt study42, 43 | |
| Checkpoint inhibitors |
|
ASCO44 |
| Tyrosine kinase inhibitors | Echo | |
|
ASE/EACVI45 | |
| Radiation therapy | Echo | |
Baseline and repeated echo after radiation therapy involving the heart are recommended for the diagnosis and follow‐up of valvular heart disease
|
ASE/EACVI45 | |
| Cardiac MRI | ||
| Recommended in those with suboptimal echocardiography or discrepant results | ESC12 | |
| Coronary CT angiography/calcium artery calcium score | ||
| Reasonable to perform ≥5 y post radiotherapy, and further workup (eg, coronary angiography, functional testing) is indicated for risk stratification if there is concern for severe ischemic heart disease | SCAI46 | |
| SPECT | ASE45 | |
|
||
| Prior exposure (not currently on therapy) | Echo | |
| Recommended for those with symptoms of heart failure (E,B,1) | ASCO9 | |
| CMR | ||
| Recommended instead of echo only if echo unavailable or not technically feasible (E,B,2) | ASCO16 | |
| Potential cardiotoxic therapy | Echo | |
| LVEF measurement at baseline and during$ treatment (2D/3D) and GLS with treatment or risk factor modification at LVEF ≥60%, 50%–59%, 40%–49%, and <40% | Liu et al37 | |
ASCO indicates American Society of Clinical Oncology; ASE, American Society of Echocardiography; ASNC, American Society of Nuclear Cardiology; CAD, coronary artery disease; CMR, cardiac magnetic resonance imaging; CTRCD, cancer therapeutics‐related cardiac dysfunction; EACVI, European Association of Cardiovascular Imaging; ESC, European Society of Cardiology; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; SCAI, Society for Cardiovascular Angiography and Interventions; MRI, magnetic resonance imaging; SEOM, Spanish Society of Medical Oncology; SPECT, single‐photon emission computed tomography; VEGF, vascular endothelial growth factor.
Evidence‐based Y/N=E/N, level of evidence high/intermediate/low=A/B/C, strength of recommendation strong/moderate/weak=1/2/3.
During treatment frequency not defined.
Echo is important for assessing myocardial dysfunction related to biologic agents such as trastuzumab and pertuzumab. The largest study of echocardiographic monitoring of vascular endothelial growth factor inhibitors, including biologic agents, comprised of 40 individuals who were followed via a 2‐dimensional speckle tracking echocardiography protocol with baseline echocardiography and repeat 2‐dimensional speckle tracking echocardiography at 1, 3, and 6 months.47 Trastuzumab has been extensively studied, and monitoring guidelines are summarized well in a recent review by Jerusalem et al48 and detailed in Table 1, including the protocol of ongoing SAFE‐HEaRT (Cardiac Safety Study in Patients With HER2 + Breast Cancer) trial.42, 43
Echo also has a central role in the surveillance of suspected TKI cardiotoxicity as reflected by the US Food and Drug Administration's prescription to discontinue certain agents when a decline in LV ejection fraction is noted.26 Derangement in GLS is known to precede LV dysfunction in patients who had received a variety of TKIs, suggestive of a class effect.47, 49 Professional societies have published recommendations summarized in Table 1 regarding baseline echocardiography and screening intervals in patients receiving TKI therapy.12, 35
Though no established guidelines exist, echocardiography is also important in evaluating suspected proteasome‐induced cardiac toxicity. Gavazzoni et al observed that mean systolic and diastolic pressure increased and echo parameters (namely, E and E`) used to assess diastolic function worsened in patients treated with carfilzomib.50 As with other cardiotoxicities, GLS with speckle tracking echocardiography can be employed to detect subclinical LV dysfunction, particularly with carfilzomib.51 Echo remains the initial imaging modality of choice when assessing radiation‐induced myocardial function and valvular disease. Radiotherapy‐associated diastolic dysfunction is commonly attributable to adverse cardiac remodeling. Echo can assess diastolic dysfunction patterns via tissue Doppler and strain imaging. Imaging surveillance recommendations from professional societies for this patient population are noted in Table 1.
Cardiac Magnetic Resonance Imaging and Treatment‐Related Myocardial Dysfunction
Cardiac magnetic resonance imaging (CMR) is the gold standard for evaluation of chamber quantification and function. In patients in whom echo‐derived LV function is equivocal, in question, or marginally decreased, CMR can be a useful arbiter in the decision to withhold or continue therapy. CMR is an important adjunctive diagnostic tool when the etiology of a new cardiomyopathy is in question or in prognostication.
Increasingly, CMR has been recognized as a valuable tool for following patients on anthracycline therapy given not only its accurate assessment of LV function but also its ability to detect fibrotic (late gadolinium enhancement [LGE] and T1 mapping) and inflammatory patterns (T2 mapping), which may help in early diagnosis and prognostication of anthracycline‐induced cardiomyopathy52, 53 (Figure 1B). Currently, additional prospective studies testing the potential of CMR‐derived indices (ie, T1/T2) to detect meaningful toxicity before clinical presentation are under way. CMR has been used to study the diastolic pattern, fibrosis, and myocardial strain after treatment with trastuzumab therapy.54, 55 CMR usage continues to increase among this population, as reflected by its role in high‐profile trials, like MANTICORE 101‐Breast (Multidisciplinary Approach to Novel Therapies in Cardio‐Oncology Research) and others.56
CMR is a valuable tool for soft tissue characterization and its role in the evaluation of patients with suspected proteasome‐induced myocardial dysfunction continues to evolve. Much of the information regarding CMR findings in this population is based on case report level data.22, 50, 51, 57, 58 Typical CMR findings include mild‐moderate LV dilation, global hypokinesis, and subendocardial to midwall myocardial linear or circumferential stripes of delayed myocardial enhancement consistent with fibrosis. T2 mapping may show hyperintense signals consistent with myocardial edema. These findings are relatively nonspecific and can be seen in other nonischemic cardiomyopathies. Other cases series, however, showed that these CMR findings, in particular the LGE findings, are not universal in patients with suspected proteasome‐induced heart failure.22
CMR is important for assessing radiotherapy‐associated myocardial dysfunction and fibrosis burden, particularly when the etiology of heart failure is unclear. CMR provides accurate volumetric and mass information in addition to tissue characterization via T1 mapping and fibrosis imaging (Figure 2). Emerging CMR technologies also have strain packages that do not necessitate speckle tracking requirements.
Figure 2.
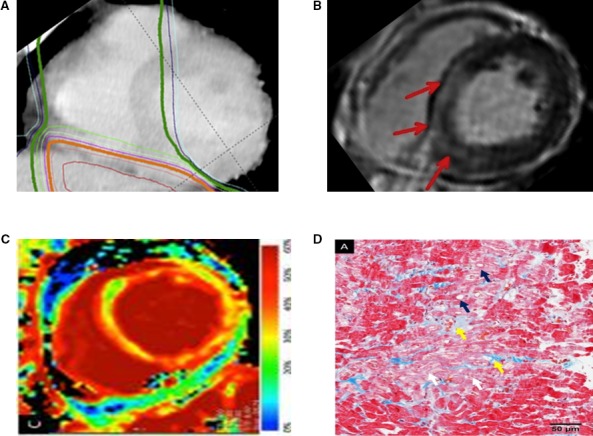
Correlation between radiotherapy (A) and discrete myocardial fibrosis (LGE, red arrows; B). Cardiac magnetic resonance imaging‐detectable diffuse fibrosis (ECV; C) corresponding to biopsy‐proven fibrosis (D), after esophageal cancer radiotherapy. Specifically, histopathologically, interstitial fibrosis (yellow arrow) and myocardial degeneration such as irregular arrangement (white arrow) or vacuolar changes (blue arrow) are seen. Adapted with permission from Mukai‐Yatagai et al.59 Copyright ©2018, Elsevier. ECV indicates extracellular volume; LGE, late gadolinium enhancement.
In patients treated with TKIs who developed LV dysfunction without a clear ischemic etiology, the role of CMR is less clear. Available smaller observational‐level data do not demonstrate characteristic CMR resting perfusion defects or LGE patterns.60, 61 Clinical data regarding the use of parametric mapping for tissue characterization in this subgroup are lacking. This is of particular interest given increasing data describing change in myocardial and vascular character with various TKIs. Further validated studies are needed to elucidate the role of CMR in evaluating suspected TKI‐induced myocardial dysfunction.
Nuclear Imaging and Treatment‐Related Myocardial Dysfunction
Historically, a multigated angiocardiography (MUGA) scan was one of the most common modalities employed for measuring LV ejection fraction.9, 15, 39 However, MUGA has been largely supplanted by more advanced imaging modalities such as 3‐dimensional echo, and CMR. That is mainly because MUGA scan provides limited information,62 is less accurate,63 and has a higher radiation exposure (8 mSv in MUGA versus 0 in CMR/3‐dimensional echo)64, 65 to patients compared with the aforementioned modalities. Specific single‐photon emission computed tomography tracers have been developed for assessing left ventricular function in patients with suspected anthracycline‐induced cardiomyopathy, but clinical use of these agents is not widespread.66
Fludeoxyglucose (FDG) positron emission tomography (PET) has a role in assessing myocardial dysfunction, particularly in those who have received prior radiation treatment. Radiotherapy‐associated cardiomyopathy may manifest not only with fibrosis but also with elevated plasma levels of brain natriuretic peptide.67 These patients with elevated brain natriuretic peptide may demonstrate abnormal FDG accumulation on PET imaging along irradiated regions (Figure 3).67, 68, 69 This clinical utility suggests that cardiac PET may have an adjunctive role in assessing cardiotoxicity in this patient population.
Figure 3.
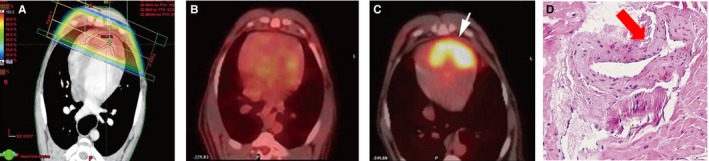
Within the radiation field (A), change in positron emission tomography (PET)‐measured myocardial metabolic activity from before (B) to 3 months after (C, white arrow) radiotherapy treatment, with corresponding fibrosis (D, red arrow) observed on histopathological examination following local heart irradiation in beagles. Echocardiographic parameters remained unchanged. Adapted from Yan et al69 under the Creative Commons Attribution Non‐Commercial (CC‐BY‐NC) License.
Cancer Treatment–Related Atherosclerosis and Myocardial Ischemia
A number of oncologic agents have been associated with myocardial ischemia, thrombosis, and coronary artery disease, including traditional chemotherapy drugs, TKIs, hormonal agents, and radiation therapy. Cisplatin is a traditional chemotherapeutic alkylating agent that is employed in the treatment of a variety of solid‐tumor malignancies and is associated with a 1% to 12% incidence of myocardial infarction. Bleomycin is an antitumor antibiotic that interferes with DNA crosslinking and is associated with a 1% to 3% incidence of coronary vasospasm and myocardial infarction. 5‐Fluorouracil is associated with 1% to 19% incidence of vasospasm, myocardial infarction, and Takotsubo cardiomyopathy.46 A number of TKIs are associated with cardiac ischemia attributable to the modulation of cardiovascular homeostatic pathways. Sorafenib is a multi‐target TKI that is associated with cardiac ischemia (3%) and hypertension (10%–17%).70 Pazopanib is associated with a variety of cardiac toxicities including ischemia, hypertension, LV dysfunction, and QT prolongation.71
Selective estrogen receptor modulators (ie, tamoxifen) and aromatase inhibitors (anastrozole, letrozole, and exemestane) work to counteract growth in certain subtypes of hormonally responsive breast cancers and in turn reduce recurrence and improve disease‐free survival.72, 73 However, these agents have notable cardiac side effects. Tamoxifen is associated with higher rates of stroke and pulmonary embolism when compared with placebo.74 Pooled data analyses from large trials demonstrate a modest increase in myocardial infarction, angina, and heart failure with aromatase inhibitors when compared with tamoxifen.75
Androgen deprivation therapy (ie, leuprolide, goserelin, flutaminde, and abiraterone) is the mainstay of treatment of metastatic prostate cancer. They are collectively associated with hyperinsulinemia, hypertension, peripheral artery disease, and hypercholesterolemia. Data are conflicting about the direct impact of these agents on cardiovascular mortality with more recent meta‐analyses demonstrating a modestly increased risk of myocardial infarction, stroke, and nonfatal cardiovascular disease, predominantly in men who had preexisting cardiovascular disease or cardiac risk factors.76, 77, 78, 79, 80
Radiotherapy leads to endothelial damage with a subsequent inflammatory response driving atherosclerosis and subsequent clinically relevant ischemic events.29, 30 As studied in the Hodgkin lymphoma and breast cancer survivor populations, those who have received radiation treatment have an elevated relative risk of developing coronary artery disease (2.2 and 1.27, respectively) when compared with the general population.33, 81 The risk of coronary artery disease significantly increases with time since radiotherapy. In addition, these patients suffer worse long‐term outcomes after cardiac surgery.82 A major concern in this population is the lack of traditional symptoms on presentation for obstructive coronary disease, which is attributable to radiation nerve damage to the chest wall.
Clonal hematopoiesis of indeterminate significance (CHIP) refers to somatic mutations that occur within hematopoietic stem cells and is associated with increased cardiovascular risk, including myocardial infarction, and decreased overall survival.83, 84 Interestingly, an increased prevalence of CHIP has been noted in patients who have received prior cancer treatment, particularly radiotherapy.85 Though a mechanistic link between CHIP and cardiac mortality has yet to be definitively established, it is plausible that CHIP alleles confer cardiovascular risk via atherosclerosis mechanisms.83, 84 The optimal management strategies require further investigation.
Cardiac Computed Tomography and Treatment‐Related Atherosclerosis and Myocardial Ischemia
Although traditional ischemic evaluations include nuclear or echocardiographic modalities, cardiac computed tomography (CT)—including coronary artery calcium scanning and CT coronary angiography—has become more prominent because of its excellent diagnostic and prognostic information via anatomic evaluation of atherosclerosis. Coronary artery calcium scanning is a non–contrast‐enhanced CT image acquisition of the coronary arteries to assess atherosclerotic plaque burden and in turn has shown to have important prognostic value for future adverse cardiac events in asymptomatic individuals.86 Coronary CT angiography offers a non‐invasive alternative to left heart catheterization with comparable diagnostic accuracy and important prognostication in patients who present with stable chest pain.87 While coronary CT angiography adds further radiation exposure, the typical dose ranges from 2 to 5 mSv, with improved technology resulting in <1 mSv. As traditional therapies such as 5‐fluorouracil, certain TKI agents, and radiotherapy have all been associated with myocardial ischemia, coronary CT angiography is an excellent modality to exclude clinically significant coronary artery disease in such patients who are undergoing an ischemic evaluation (Figure 4). This is a particularly appealing alternative where invasive angiography may be untenable given elevated bleeding risks commonly noted in this population. In the aforementioned CHIP population, Jaiswal et al84 showed that these patients had a 3‐fold higher risk of having high coronary artery calcium score (>615 Agatston units). Given the increased cardiovascular mortality risk noted with these patients, coronary artery calcium scoring may have a role in primary prevention paradigms in this population. This is an exploratory application in this respect and for the cardio‐oncology population as a whole, and represents an exciting area for future study.
Figure 4.
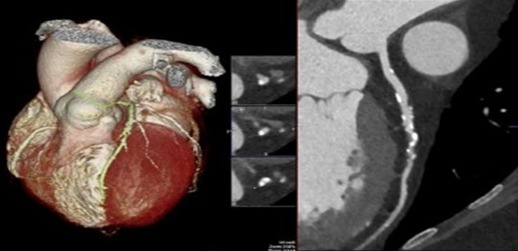
(Left) Cardiac computed tomography (CT) with 3‐dimensional reconstruction rendering allowing for (right) omniplane visualization of coronary artery plaque and calcification. Adapted with permission from Layoun et al.88 Copyright © 2019, Springer Science Business Media, LLC, part of Springer Nature.
Cardiac PET and Treatment‐Related Atherosclerosis and Myocardial Ischemia
Cardiac PET offers not only accurate assessments of myocardial function and ischemia. This is particularly useful in assessing ischemic events in those receiving vasospastic chemotherapy like 5‐fluorouracil.89 However, there are no guideline recommendations regarding FDG‐PET in this population, outside of established methodologies for ischemic evaluations and cardiac viability assessments. Additional applications are demonstrated in animal literature, where PET is being studied as a noninvasive method to detect alterations in cardiac metabolism that potentially predates overt cardiotoxicity.90 In human subjects, similar observations have been made in case‐level data where aberrations in cardiac PET imaging had preceded clinical coronary events in a patient treated with a TKI.91 It remains to be seen how these findings can be applied to routine clinical practice and population‐wide screening schemas in this population.
Other Imaging Modalities and Treatment‐Related Atherosclerosis and Myocardial Ischemia
Stress echocardiography has an established role in ischemic evaluations in the cardio‐oncology population.35 In line with that of the general population, it can be used for the detection and prognostication of stable coronary artery disease or in the evaluation of those with intermediate pretest probability for CAD. This should particularly be considered if the patient is undergoing treatment with regimens associated with ischemia including 5‐fluorouracil, bevacizumab, sorafenib, and sunitinib.35 Stress echocardiography may also be useful in the evaluation of subclinical LV dysfunction and the evaluation of contractile reserve in patients with treatment‐related cardiac dysfunction. Regarding the use of single‐photon emission computed tomography, there are no guideline recommendations outside of established methodologies for ischemic evaluations. CMR can also provide quantitative myocardial perfusion mapping to directly quantify regional myocardial perfusion reserve and detect prior myocardial infarction using LGE when an ischemic insult is suspected92 (Figure 5).
Figure 5.
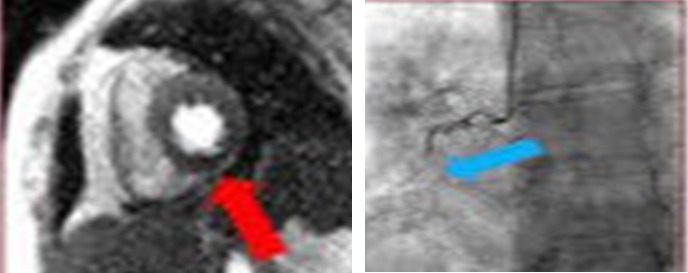
Cardiac magnetic resonance imaging scan of short‐axis plane of left ventricle in a breast cancer survivor showing inferior wall perfusion defect consistent with prior myocardial infarction (left) with corresponding occlusion of the right coronary artery (right). Adapted from Vasu et al93 under the Creative Commons Attribution (CC‐BY) license.
Immunotherapy‐Related Myocarditis
Immunotherapies, such as immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T‐cell transfer therapy, amplify T‐cell–mediated immune responses against cancer cells and represent an exciting evolution in oncologic therapeutics. Many tumors express inhibitory cellular membrane ligands that effectively act as cloaking mechanisms to avoid host immune detection. Blockade of these ligands and/or the corresponding receptors on host cells with monoclonal antibodies activate the immune system to recognize and target cancer cells.94 ICIs have led to progression‐free survival in a variety of metastatic disease states but are associated with various cardiac side effects, most notably myocarditis.95, 96 Initial clinical trial data indicated that the prevalence of severe myocarditis was rare (0.06%), with a higher occurrence (0.27%) reported in patients receiving multiple ICIs.97, 98, 99 Yet more recent data suggest that the prevalence may in fact be higher on the order of 1% to 2%.100 A recent consensus statement by Bonaca et al101 elaborated on the definition of myocarditis in relation with ICI and further evaluation with multiple imaging modalities, which are detailed in Table 2.
Table 2.
Recommended Modality Without Specific Guidelinesa
| Therapeutic Agent | Imaging Modality | Source |
|---|---|---|
| Immune checkpoint inhibitors | Echo | |
|
Consider echo with strain imaging when suspicion of ICI toxicity exists: Definite myocarditis: New wall motion abnormality on echo not explained by another diagnosis (ie, acute coronary syndrome, stress‐induced cardiomyopathy, sepsis) and all of the following: (1) clinical syndrome consistent with myocarditis, (2) elevated biomarker of cardiac myonecrosis, (3) ECG evidence of myopericarditis, (4) negative angiography or other testing to exclude obstructive coronary disease Probable myocarditis: New wall motion abnormality on echo with a clinical syndrome consistent with myocarditis not otherwise explained by another diagnosis and either: (1) elevated biomarker of cardiac myonecrosis or (2) ECG evidence of myopericarditis Possible myocarditis: New wall motion abnormality on echo one of the following: (1) clinical syndrome consistent with myocarditis not explained by alternate diagnosis, (2) ECG evidence of myopericarditis |
Bonaca et al101 Adawalla et al102 Waheed et al103 |
|
| CMR | ||
|
Consider CMR when suspicion for ICI‐induced myocarditis exists: Definite myocarditis: CMR diagnostic of myocarditis, a clinical syndrome not explained by alternate diagnosis, and one of following: (1) elevated biomarker of cardiac myonecrosis or (2) ECG evidence of myopericarditis Probable myocarditis: CMR with findings diagnostic of myocarditis not explained by alternate clinical diagnosis with any of the following: (1) clinical syndrome consistent with myocarditis, (2) elevated biomarker of cardiac myonecrosis, or (3) ECG evidence of myopericarditis Possible myocarditis: Nonspecific CMR findings suggestive of myocarditis with one or more of the following: (1) clinical syndrome consistent with myocarditis not explained by alternate clinical diagnosis, (2) elevated biomarker of cardiac myonecrosis, (3) ECG evidence of myopericarditis |
Mahmood et al100 Bonaca et al101 Salem et al104 |
|
| 18 FDG‐PET | ||
| Scenario meeting criteria for possible myocarditis (see above) with PET showing patchy cardiac FDG uptake without another explanation | Bonaca et al101 | |
| Tyrosine kinase inhibitors | Echo | |
| In context of appropriate symptoms, screening echo test of choice to evaluate pulmonary pressures, right ventricular dysfunction or hypertrophy, septal deviation to the left to provide supporting evidence of pulmonary hypertension (Dasatinib useb) | Moslehi et al105 | |
| CMR | ||
| Consider CMR during evaluation of suspected TKI‐related ischemia (sorafenibb) | Sudasena et al92 | |
| 18 FDG‐PET | ||
| Consider cardiac PET during evaluation of suspected TKI‐related ischemia (sorafenibb) |
Sudasena et al92 Toubert et al91 |
|
| Proteasome inhibitors | Echo | |
| Consider echo with strain imaging when evaluating LV systolic and diastolic parameters for suspected proteasome inhibitor LV dysfunction |
Gavazzoni et al50 Iannaccone et al51 |
|
| Radiation therapy | CMR | |
| Consider T1‐weighted mapping in the evaluation in suspected radiation induced myocardial fibrosis | Mukai‐Yatagai et al59 | |
18‐FDG PET indicates 18‐fluorodeoxyglucose positron emission tomography; CMR, cardiac magnetic resonance imaging; GLS, global longitudinal strain; ICI, immune checkpoint inhibitor; LVEF, left ventricular ejection fraction; TKI, tyrosine kinase inhibitor.
Reflects emerging data that may show efficacy to additional applications of cardiac CT, MRI, and PET in broader applications.
Recommendations apply only to specific agent, not class.
Chimeric antigen receptor T‐cell therapy is a technique wherein a patient's T cell is genetically modified ex vivo with a fusion protein receptor that is specific for a tumor antigen. Once reinfused back into the patient, engagement of this receptor with a tumor antigen results in activation of the T cell against the tumor cell.106 Clinical trials demonstrated an array of toxicities with these agents, including cytokine release syndrome, neurotoxicity, and prolonged cytopenias. Cardiac‐related events such as arrhythmias and cardiomyopathies have ranged from 29% to 30%, including rare reports of cardiac arrest.107, 108, 109, 110, 111, 112 Single‐center data suggest that many of these toxicities are associated with cytokine release syndrome and resolve within 6 months of follow‐up.113 The pathophysiology of these toxicities—whether mediated directly by the chimeric antigen receptor T‐ cell product itself, indirectly by a cytokine‐mediated process, or an alternative mechanism—is not well understood.
Echocardiography and Immunotherapy‐Related Myocarditis
Echo has an important role in the assessment of cardiac function with guidelines recommending a screening echo when the suspicion of ICI cardiotoxicity arises.44 Aside from standard echocardiographic parameters, there has been recent work on the use of GLS in assessing LV function in patients treated with ICIs. Awadalla et al102 assessed GLS in patients treated with ICI who developed myocarditis and showed that GLS is reduced with both preserved and reduced ejection fraction. They also noted that lower GLS is associated with subsequent major adverse cardiac events in those with ICI‐related myocarditis. Waheed et al103 similarly demonstrated a decrease in GLS in patients treated with ICIs who developed myocarditis despite the LV ejection fraction remaining unchanged. These studies support observations noted in other cardiomyopathies that strain, particularly GLS, can be used to detect LV dysfunction despite a normal ejection fraction assessment.
Cardiac Magnetic Resonance Imaging and Immunotherapy‐Related Myocarditis
The end result of many immunotherapies is to elicit an inflammatory response against a target tumor site. Yet off‐target actions may also produce systemic and cardiac inflammatory injury and subsequent fibrosis.44 In this sense, CMR is a powerful tool in the diagnosis of ICI cardiotoxicity, as it allows for assessment of myocardial edema, inflammatory injury, and fibrosis. Although there are no guideline‐driven CMR recommendations specifically pertaining to ICI‐related myocarditis, generally speaking, CMR is the noninvasive test of choice for the assessment of myocarditis.114 Condition‐specific protocols include contrast‐enhanced T1‐weighted, noncontrast T2‐weighted, and more advanced quantitative tissue characterization using parametric (T1 and T2) mapping sequences. Inflammatory processes tend to cause cell injury and permeability of cellular membranes, in turn leading to global and regional edema (Figure 6A). T2 reflects the free‐water content of the tissue, and thus inflammation and edema lead to elevated T2. LGE imaging can reveal high signal intensity, diffuse subepicardial or midwall distribution patterns; however, signal intensity on LGE images can be elevated with either edema or fibrosis. By combining information from T1, T2, and LGE imaging, CMR can identify myocarditis with sensitivity of 76% and specificity of 96% when compared with invasive histologic confirmation.115, 116 These techniques have allowed for a major step forward in the assessment of cardiotoxic injury, by providing a novel avenue to evaluate quantitative, rather than traditional subjective/qualitative, early cardiotoxic change. These observations extend beyond more traditional markers of cardiotoxicity as measured by echocardiography alone, including GLS. The ability of CMR to aid in myocyte‐level tissue characterization offers a valuable tool, which may prove vital in the understanding, diagnosis, and management of ICI‐related myocarditis events. Moreover, this modality may assist in the serial assessment of potential steroid (or even nonsteroid) treatment(s) response over time. With limited numbers, Mahmood et al100 showed there was no difference in cardiac events between those patients with and without LGE noted on their CMR who had been diagnosed with myocarditis. However, additional studies are needed to confirm the efficacy of parametric mapping after ICI or other immune‐based therapy exposure.
Figure 6.
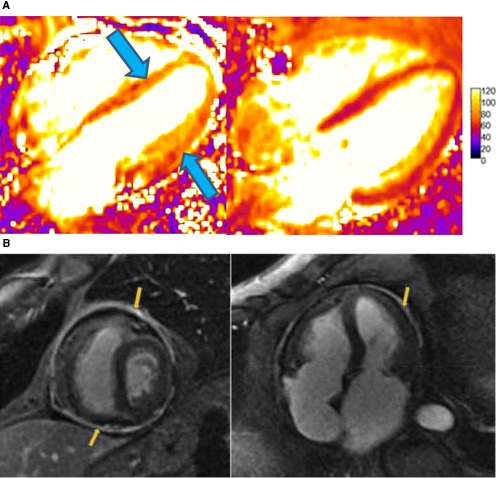
A, (Left) Cardiac MRI T2 maps at diagnosis of myocarditis in a patient treated with pembrolizumab showing global hypokinesis with moderate systolic dysfunction (LVEF, 41%) and diffusely elevated T2 signal (arrows). (Right) T2 maps after withdrawal of pembrolizumab and 1‐month course of prednisone 1 mg/kg with resultant normalized systolic function (LVEF, 59%) and improved T2 signal. B, Cardiac magnetic resonance imaging scan late gadolinium enhancement (LGE) sequences showing thickened and enhanced pericardium in a patient with constrictive pericarditis following radiation therapy. Adapted with permission from Aldweib et al.123 Copyright © 2018, Eureka Science (FZC). LVEF, left ventricular ejection fraction.
Cardiac PET and Immunotherapy‐Related Myocarditis
FDG‐PET is widely used to assess tumor burden and response to therapy, and it has an established role in the diagnosis and follow‐up evaluation of certain inflammatory myopathies.117 However, there are no existing guidelines regarding the use of cardiac PET in evaluation of immunotherapy‐related myocarditis. Nevertheless, the application of cardiac PET in patients receiving such agents is an active field of study. Wang et al118 noted that the degree of tumor burden assessed via PET in patients with non‐Hodgkin lymphoma receiving chimeric antigen receptor T‐cell transfer therapy correlated with the degree of cytokine release syndrome. They showed how PET could be used to assess myocarditis, pericardial effusions, and treatment response in a patient with diffuse large B‐cell lymphoma with cardiac involvement. Others have shown that the development of cytokine release syndrome did not confound PET findings, a particular concern given the inflammatory milieu involved in this common side effect.119 As murine and early human studies have shown, PET has the potential to visualize minuscule concentrations of tracers linked to T‐cell populations and show promise in tracking T‐cell clonal response.120 Whether this can be applied to better assess patients with suspected chimeric antigen receptor T‐cell therapy cardiotoxicity, including myocarditis, remains to be seen.
Pericardial Disease
Radiotherapy is the prototypical agent associated with pericarditis. Radiation can induce acute pericarditis early after treatment, which is usually self‐limited. However, rare presentations of recurrent pericarditis have been noted and can result in constrictive physiology. Cancer survivors receiving chest radiotherapy have ≈13 times higher likelihood of requiring a pericardiectomy as compared with matched cohorts.121
Imaging Modalities and Treatment‐Related Pericardial Disease
Echocardiography is the first‐line modality in evaluation of pericardial disease, as it can assess for pericardial effusion in addition to providing valuable hemodynamic data indicative of tamponade or constrictive physiology. Cardiac CT may provide anatomic evaluation of the pericardium via the presence of a pericardial effusion in the acute phase or calcification in the chronic phase. CMR can be used to evaluate the pericardium via hemodynamic assessment through interventricular dependence and mitral/tricuspid inflow variation,122 in addition to pericardial inflammation through edema and fibrosis imaging123 (Figure 6B).
Cardiac Amyloidosis
Cardiac amyloidosis is heterogeneous group of infiltrative cardiomyopathies typified by the deposition of extracellular amyloid protein resulting progressive heart failure, arrhythmias, and death. Incidence, prognosis, diagnostic evaluation, and treatment all depend on the subtype, chiefly among them being amyloid light chain (AL) and acquired transthyretin amyloidosis. A recent study conducted in the United Kingdom estimates that the incidence of AL amyloidosis is 8.0/100 000 per year, with greater than half of patients demonstrating cardiac involvement.124, 125 The median survival for acquired transthyretin amyloidosis with cardiac involvement is 3 to 5 years, while median survival for AL amyloidosis with cardiac involvement is reportedly <12 months.126, 127 Contemporary treatment regimens for AL and acquired transthyretin have led to improvements in quality of life, decreased hospitalization, and extended overall survival.124, 128 Thus, early identification and risk stratification of patients with cardiac amyloid infiltration is vital to reducing the death toll of this fatal disease.
While endomyocardial biopsy was traditionally used for diagnosis, advances in noninvasive imaging in echocardiography, nuclear medicine, and CMR have provided early and accurate detection in a previously underdiagnosed population. Echo findings include LV hypertrophy in the setting of a low to normal QRS amplitude on electrocardiography, systolic dysfunction, restrictive physiology, and pericardial effusion. Strain imaging may demonstrate a characteristic pattern of global dysfunction with relative apical sparring.129 Technetium‐labeled bone scintigraphy is a highly sensitive technique for the diagnosis acquired transthyretin cardiac amyloidosis, with myocardial tracer uptake showing a >99% sensitivity and 86% specificity (Figure 7).130, 131 CMR is able to accurately diagnose cardiac amyloidosis through characteristic global subendocardial LGE and abnormal myocardial gadolinium kinetics.132 CMR also provides further incremental clinical value via prognostication and treatment response through T1 mapping.133 As AL treatment regimens often include proteasome inhibitor agents such as bortezomib, which in turn are associated with LV dysfunction and heart failure, echo and CMR have an important role in evaluating treatment‐related cardiac toxicity as detailed elsewhere.22, 51, 57, 58
Figure 7.
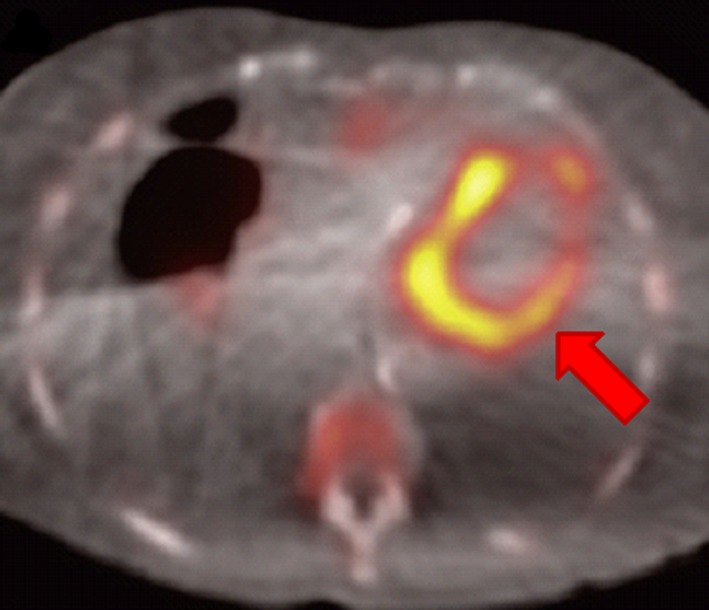
88‐year‐old female with aortic stenosis and amyloid transthyretin cardiac amyloidosis by 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scan; arrow points to regions of increased uptake. Adapted from Scully et al131 under the Creative Commons Attribution (CC‐BY) license.
Peripheral Vascular Disease and Vascular Dysfunction
Vascular disease, namely venous thromboembolism and hypertension, is a pervasive side effect of a number of cancer therapies. Additional vascular complications include pulmonary hypertension, arterial thromboembolism, and vasospasm. Traditional therapies associated with vascular side effects include fluoropyrimidines, taxanes,134 vinca alkaloids, platinum compounds, cyclophosphamide, anthracyclines,135 and bleomycin136 among others.
Therapeutic agents specifically associated with systemic or pulmonary hypertension including androgen deprivation therapies, proteasome inhibitors, biologic agents, and various TKIs, particularly those that modulate the vascular endothelial growth factor pathway. Vascular endothelial growth factor stimulates endothelial cells to release nitric oxide and prostacyclin, causing vasodilation. Thus, inhibiting this pathway is thought to lead to hypertension. Though commonly classified as a biologic agent, bevacizumab is a recombinant humanized monoclonal antibody against vascular endothelial growth factor used in the treatment of a variety of malignancies including colon, lung, and renal cancer. Bevacizumab can lead to hypertension in roughly 35% of patients, which in turn, is thought to be responsible for downstream myocardial dysfunction.47 Sunitinib, sorafenib, pazopanib, ibrutinib, and axitinib are a few prominent TKI agents associated with hypertension, with the former being the most well studied. Up to 80% of patients on sunitinib may experience systolic hypertension (median blood pressure of 160 mm Hg) by the end of the second cycle of treatment.137 Dasatinib is a second‐generation TKI commonly used in the treatment of chronic myelogenous leukemia and has been linked to pulmonary hypertension with a variably low incidence.105 Dasatinib‐induced pulmonary hypertension is thought to be partially reversible and, if confirmed, should be permanently discontinued.105, 138
Finally, radiation therapy causes acute and chronic vascular inflammatory effects resulting in a range of vascular dysfunction including premature atherosclerotic disease, venous stenosis and thrombosis, and dysautonomia, to name a few.139, 140, 141, 142, 143 The mechanisms of each of these side effects are summarized in an American Heart Association Statement by Campia et al.2
Imaging Modalities and Cancer Treatment–Related Vascular Disease
Vascular imaging is governed by the type of the pathology, availability, and local expertise rather than the specific chemotherapy used.2, 12, 144, 145 Peripheral arterial and carotid imaging ranges from inexpensive modalities like duplex ultrasound to highly accurate but expensive modalities like magnetic resonance angiography and invasive angiography. Duplex ultrasound, CT angiography and ventilation‐perfusion scans have been widely used in the evaluation of venous thromboembolic events. Whenever therapy‐induced pulmonary hypertension is suspected, echo is the first imaging test of choice.47 Given that cancer treatment–related hypertension can lead to downstream myocardial dysfunction, an echocardiogram is also an important initial imaging test for this cohort as well.47, 146 Phase contrast magnetic resonance angiography provides important information on vascular morphology and flow quantification.147 A new technology that has been introduced in the space of magnetic resonance angiography is 4‐dimensional flow. Four‐dimensional flow magnetic resonance angiography provides 3‐dimensional vascular morphology as well as dynamic quantitative flow information across the cardiac cycle.147 This technology has added a new dimension in vascular imaging with the ability to derive parameters such as wall shear stress, pressure gradients, and turbulence. There are many potential uses148 of this technique including evaluation of valvular disease in radiation‐induced heart disease and ruling out renal vascular disease as a cause of secondary hypertension in setting of cancer therapy–related hypertension.
Future Perspective and Direction
Cancer survival continues to improve in the era of emerging anticancer therapies.149 Given this trend and the fact that cardiovascular disease is the most common cause of death among cancer survivors, there is pressing need to identify and treat patients with adverse cardiovascular outcomes related to prior and ongoing cancer therapies. There remain a number of unmet needs in the cardio‐oncology realm, including the role of primary prevention in cardiotoxicity, appropriate therapeutic response and surveillance in secondary prevention, and the optimal mode and timing of cardiac imaging in this population as a whole.
Multimodality cardiac imaging will continue to have an essential role in the evaluation of treatment‐related cardiac toxicity. Our review offers a comprehensive summary of guideline‐directed recommendations on this subject, where available. In areas where there is a paucity of data, we highlight primary literature detailing novel and evolving applications of cardiac imaging as related to treatment toxicity. With these data, along with our single‐center experience, we offer our practical approach to multimodality imaging for evaluation of cardiotoxicity, as summarized in Table 3.
Table 3.
Practical Considerations in Multimodality Imaging for Evaluation of Cardiotoxicity
| Volume and Functional Assessment | Tissue/Mass Characterization | Myocarditis/Inflammation | Valve Disease | Pericardial Disease | Coronary Disease/Ischemia | Radiation Exposure | Reproducibility/Accuracy | Cost | Availability | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac MRI | +++ | +++ | +++ | ++ | +++ | ++ | None | +++ | + | + |
| CT Coronary | ++ | + | − | + | ++ | +++ | + | +++ | ++ | + |
| PET/Nuclear | +++ | ++ | +++ | − | + | +++ | +++ | +++ | ++ | ++ |
| Echo | +/++ | ++ | − | +++ | +/++ | −/+ (wall motion) | None | + | +++ | +++ |
Adapted with modifications from Seraphim et al150 under the Creative Commons Attribution (CC‐BY) license. CT indicates computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Echocardiography has served as the cornerstone of baseline and surveillance assessments, displacing MUGA in a whole host of diagnostic algorithms. Applications of strain imaging continue to expand, though more studies are needed to validate its use as a reliable method of detecting subclinical LV dysfunction, particularly with newer agents. CMR is unparalleled in a variety of applications including evaluation of chamber quantification, myocardial tissue characterization, and multidimensional quantitative vascular assessment. Although CMR had previously been confined primarily to academic centers, accessibility continues to improve given the ever decreasing costs of CMR, growing expertise, and expanded use in traditionally limited patient populations including those with pacemaker/defibrillator devices and severe renal dysfunction.151, 152 Given this, we expect CMR to assume a broader role in cardiotoxicity diagnostic and surveillance imaging. More data are needed to drive standardization of CMR protocols in the evaluation of specific cardiac toxicities.
Previously mentioned animal studies and case reports demonstrate novel applications of cardiac PET beyond its traditional role of ischemia and viability assessments. For example, the use of cardiac PET in tracking specific T‐cell clones in those treated with immunotherapy or who develop cardiac events with such agents holds promise. Given that the withdrawal of an anticancer agent may hold grave clinical consequences for patients, such dynamic imaging techniques could provide an additional tool to help clinicians avoid unwarranted discontinuation of therapy.
Coronary artery calcium scanning and coronary CT angiography applications continue to expand, particularly as newer gating techniques have allowed lower radiation exposure and high‐fidelity imaging acquisition. As coronary CT angiography is the only modality mentioned above that allows for an accurate noninvasive anatomic assessment of coronary vasculature, it is uniquely positioned for evaluating patients with suspected ischemic insults from cardiotoxic agents, particularly where invasive angiography is undesirable.
Our review highlights the important and varied roles that cardiac imaging has in the assessment suspected cardiotoxicity in the oncologic population. Multimodality cardiac imaging will continue to be essential to filling unmet needs and knowledge gaps, and in turn, augment best practice recommendations in the management of the cardio‐oncology patient.
Sources of Funding
This work was supported in part by NIH grant P30 CA016058, and by an NIH K12‐CA133250 (Addison) grants.
Disclosures
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
(J Am Heart Assoc. 2020;9:e013755 DOI: 10.1161/JAHA.119.013755.)
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Campia U, Moslehi JJ, Amiri‐Kordestani L, Barac A, Beckman JA, Chism DD, Cohen P, Groarke JD, Herrmann J, Reilly CM, Weintraub NL. Cardio‐oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139:e579–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 4. Beyer AM, Bonini MG, Moslehi J. Cancer therapy‐induced cardiovascular toxicity: old/new problems and old drugs. Am J Physiol Heart Circ Physiol. 2019;317:H164–H167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, Dos‐Santos‐Silva I, Smeeth L, Bhaskaran K. Medium and long‐term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population‐based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonsu J, Charles L, Guha A, Awan F, Woyach J, Yildiz V, Wei L, Jneid H, Addison D. Representation of patients with cardiovascular disease in pivotal cancer clinical trials. Circulation. 2019;139:2594–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379:2438–2450. [DOI] [PubMed] [Google Scholar]
- 8. Guha A, Buck B, Biersmith M, Arora S, Yildiz V, Wei L, Awan F, Woyach J, Lopez‐Mattei J, Plana‐Gomez JC, Oliveira GH, Fradley MG, Addison D. Contemporary impacts of a cancer diagnosis on survival following in‐hospital cardiac arrest. Resuscitation. 2019;142:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 10. Virizuela JA, Garcia AM, de Las Penas R, Santaballa A, Andres R, Beato C, de la Cruz S, Gavila J, Gonzalez‐Santiago S, Fernandez TL. SEOM clinical guidelines on cardiovascular toxicity (2018). Clin Transl Oncol. 2019;21:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. [DOI] [PubMed] [Google Scholar]
- 12. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 13. Armenian SH, Armstrong GT, Aune G, Chow EJ, Ehrhardt MJ, Ky B, Moslehi J, Mulrooney DA, Nathan PC, Ryan TD, van der Pal HJ, van Dalen EC, Kremer LCM. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36:2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med. 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 15. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 16. Armenian S, Bhatia S. Predicting and preventing anthracycline‐related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018;38:3–12. [DOI] [PubMed] [Google Scholar]
- 17. Mele D, Rizzo P, Pollina AV, Fiorencis A, Ferrari R. Cancer therapy‐induced cardiotoxicity: role of ultrasound deformation imaging as an aid to early diagnosis. Ultrasound Med Biol. 2015;41:627–643. [DOI] [PubMed] [Google Scholar]
- 18. Lustberg MB, Reinbolt R, Addison D, Ruppert AS, Moore S, Carothers S, Suresh A, Das H, Berger M, Ramaswamy B, Wesolowski R, Binkley P, Raman SV, Shapiro CL. Early detection of anthracycline‐induced cardiotoxicity in breast cancer survivors with T2 cardiac magnetic resonance. Circ Cardiovasc Imaging. 2019;12:e008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35–46. [DOI] [PubMed] [Google Scholar]
- 20. Rushton M, Johnson C, Dent S. Trastuzumab‐induced cardiotoxicity: testing a clinical risk score in a real‐world cardio‐oncology population. Curr Oncol. 2017;24:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472 DOI: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015;21:138–144. [DOI] [PubMed] [Google Scholar]
- 23. Kyprolis (Carfilzomib). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202714lbl.pdf. Revised in 7/2012. Accessed August 1, 2019.
- 24. Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, Carver JR, Cohen AD, Engelhardt BG, Garfall AL, Goodman SA, Harrell SL, Kassim AA, Jadhav T, Jagasia M, Moslehi J, O'Quinn R, Savona MR, Slosky D, Smith A, Stadtmauer EA, Vogl DT, Waxman A, Lenihan D. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med. 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 26. SUTENT (sunitinib). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/ 2011/021938s13s17s18lbl.pdf. Revised 5/2011. Accessed August 17, 2019.
- 27. Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, Jones JA, Walewska R, Savage KJ, Michaud GF, Moslehi JJ, Brown JR. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guha A, Derbala MH, Zhao Q, Wiczer TE, Woyach JA, Byrd JC, Awan FT, Addison D. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. [DOI] [PubMed] [Google Scholar]
- 30. Addison D, Seidelmann SB, Janjua SA, Emami H, Staziaki PV, Hallett TR, Szilveszter B, Lu MT, Cambria RP, Hoffmann U, Chan AW, Wirth LJ, Neilan TG. Human papillomavirus status and the risk of cerebrovascular events following radiation therapy for head and neck cancer. J Am Heart Assoc. 2017;6:e006453 DOI: 10.1161/JAHA.117.006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. [DOI] [PubMed] [Google Scholar]
- 32. Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE. Cardiovascular status in long‐term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. [DOI] [PubMed] [Google Scholar]
- 33. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) ; Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast‐conserving surgery on 10‐year recurrence and 15‐year breast cancer death: meta‐analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clasen SC, Scherrer‐Crosbie M. Applications of left ventricular strain measurements to patients undergoing chemotherapy. Curr Opin Cardiol. 2018;33:493–497. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Banchs J, Mousavi N, Plana JC, Scherrer‐Crosbie M, Thavendiranathan P, Barac A. Contemporary role of echocardiography for clinical decision making in patients during and after cancer therapy. JACC Cardiovasc Imaging. 2018;11:1122–1131. [DOI] [PubMed] [Google Scholar]
- 38. Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging. 2018;11:1150–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell RR, Alexander J, Jain D, Poornima IG, Srivastava AV, Storozynsky E, Schwartz RG. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J Nucl Cardiol. 2016;23:856–884. [DOI] [PubMed] [Google Scholar]
- 40. Manufacturer Information Herceptin. Available at: https://www.gene.com/download/pdf/herceptinprescribing.pdf. Revised: 11/2018. Accessed August 24, 2019.
- 41. Manufacturer Label Perjeta. Available at: https://www.gene.com/download/pdf/perjetaprescribing.pdf. Revised: 12/2018. Accessed August 24, 2019.
- 42. Lynce F, Barac A, Tan MT, Asch FM, Smith KL, Dang C, Isaacs C, Swain SM. SAFE‐HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2‐positive breast cancer and reduced left ventricular function. Oncologist. 2017;22:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, Gallagher C, Pohlmann PR, Nunes R, Herbolsheimer P, Warren R, Srichai MB, Hofmeyer M, Cunningham A, Timothee P, Asch FM, Shajahan‐Haq A, Tan MT, Isaacs C, Swain SM. Prospective evaluation of the cardiac safety of HER2‐targeted therapies in patients with HER2‐positive breast cancer and compromised heart function: the SAFE‐HEaRt study. Breast Cancer Res Treat. 2019;175:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez‐Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; Network NCC . Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lancellotti P, Nkomo VT, Badano LP, Bergler‐Klein J, Bogaert J, Davin L, Cosyns B, Coucke P, Dulgheru R, Edvardsen T, Gaemperli O, Galderisi M, Griffin B, Heidenreich PA, Nieman K, Plana JC, Port SC, Scherrer‐Crosbie M, Schwartz RG, Sebag IA, Voigt JU, Wann S, Yang PC; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance and American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, Society of Cardiovascular Computed Tomography . Expert consensus for multi‐modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. [DOI] [PubMed] [Google Scholar]
- 46. Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Marmagkiolis K. SCAI expert consensus statement: evaluation, management, and special considerations of cardio‐oncology patients in the cardiac catheterization laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologıa Intervencionista). Catheter Cardiovasc Interv. 2016;87:895–899. [DOI] [PubMed] [Google Scholar]
- 47. Nhola LF, Abdelmoneim SS, Villarraga HR, Kohli M, Grothey A, Bordun KA, Cheung M, Best R, Cheung D, Huang R, Barros‐Gomes S, Pitz M, Singal PK, Jassal DS, Mulvagh SL. Echocardiographic assessment for the detection of cardiotoxicity due to vascular endothelial growth factor inhibitor therapy in metastatic renal cell and colorectal cancers. J Am Soc Echocardiogr. 2019;32:267–276. [DOI] [PubMed] [Google Scholar]
- 48. Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat. 2019;177:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moreo A, Vallerio P, Ricotta R, Stucchi M, Pozzi M, Musca F, Meani P, Maloberti A, Facchetti R, Di Bella S, Giganti MO, Sartore‐Bianchi A, Siena S, Mancia G, Giannattasio C. Effects of cancer therapy targeting vascular endothelial growth factor receptor on central blood pressure and cardiovascular system. Am J Hypertens. 2016;29:158–162. [DOI] [PubMed] [Google Scholar]
- 50. Gavazzoni M, Lombardi CM, Vizzardi E, Gorga E, Sciatti E, Rossi L, Belotti A, Rossi G, Metra M, Raddino R. Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: early in vivo cardiovascular effects. Eur J Pharmacol. 2018;838:85–90. [DOI] [PubMed] [Google Scholar]
- 51. Iannaccone A, Bruno G, Ravera A, Gay F, Salvini M, Bringhen S, Sabia L, Avenatti E, Veglio F, Milan A. Evaluation of cardiovascular toxicity associated with treatments containing proteasome inhibitors in multiple myeloma therapy. High Blood Press Cardiovasc Prev. 2018;25:209–218. [DOI] [PubMed] [Google Scholar]
- 52. Ferreira de Souza T, Quinaglia T, Neilan TG, Coelho‐Filho OR. Assessment of cardiotoxicity of cancer chemotherapy: the value of cardiac MR imaging. Magn Reson Imaging Clin N Am. 2019;27:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jeong D, Gladish G, Chitiboi T, Fradley MG, Gage KL, Schiebler ML. MRI in cardio‐oncology: a review of cardiac complications in oncologic care. J Magn Reson Imaging. 2019;50:1349–1366. [DOI] [PubMed] [Google Scholar]
- 54. Gong IY, Ong G, Brezden‐Masley C, Dhir V, Deva DP, Chan KKW, Graham JJ, Chow CM, Thavendiranathan P, Dai D, Ng MY, Barfett JJ, Connelly KA, Yan AT. Early diastolic strain rate measurements by cardiac MRI in breast cancer patients treated with trastuzumab: a longitudinal study. Int J Cardiovasc Imaging. 2019;35:653–662. [DOI] [PubMed] [Google Scholar]
- 55. Fallah‐Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II‐positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. [DOI] [PubMed] [Google Scholar]
- 56. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary approach to novel therapies in cardio‐oncology research (MANTICORE 101‐breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. [DOI] [PubMed] [Google Scholar]
- 57. Diwadkar S, Patel AA, Fradley MG. Bortezomib‐induced complete heart block and myocardial scar: the potential role of cardiac biomarkers in monitoring cardiotoxicity. Case Rep Cardiol. 2016;2016:3456287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fradley MG, Groarke JD, Laubach J, Alsina M, Lenihan DJ, Cornell RF, Maglio M, Shain KH, Richardson PG, Moslehi J. Recurrent cardiotoxicity potentiated by the interaction of proteasome inhibitor and immunomodulatory therapy for the treatment of multiple myeloma. Br J Haematol. 2018;180:271–275. [DOI] [PubMed] [Google Scholar]
- 59. Mukai‐Yatagai N, Haruki N, Kinugasa Y, Ohta Y, Ishibashi‐Ueda H, Akasaka T, Kato M, Ogawa T, Yamamoto K. Assessment of myocardial fibrosis using T1‐mapping and extracellular volume measurement on cardiac magnetic resonance imaging for the diagnosis of radiation‐induced cardiomyopathy. J Cardiol Cases. 2018;18:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu CF, Chuang WP, Li AH, Hsiao CH. Cardiac magnetic resonance imaging in sunitinib malate‐related cardiomyopathy: no late gadolinium enhancement. J Chin Med Assoc. 2009;72:323–327. [DOI] [PubMed] [Google Scholar]
- 61. Ederhy S, Massard C, Dufaitre G, Balheda R, Meuleman C, Rocca CG, Izzedine H, Cohen A, Soria JC. Frequency and management of troponin I elevation in patients treated with molecular targeted therapies in phase I trials. Invest New Drugs. 2012;30:611–615. [DOI] [PubMed] [Google Scholar]
- 62. Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. [DOI] [PubMed] [Google Scholar]
- 63. Huang H, Nijjar PS, Misialek JR, Blaes A, Derrico NP, Kazmirczak F, Klem I, Farzaneh‐Far A, Shenoy C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, Cerqueira MD, Cullom SJ, DeKemp R, Dickert NW, Dorbala S, Fazel R, Garcia EV, Gibbons RJ, Halliburton SS, Hausleiter J, Heller GV, Jerome S, Lesser JR, Raff GL, Tilkemeier P, Williams KA, Shaw LJ. Patient‐centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 2014;63:1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, Ting HH, Shah ND, Nasir K, Nallamothu BK. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population‐based analysis. J Am Coll Cardiol. 2010;56:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carrió I, Lopez‐Pousa A, Estorch M, Duncker D, Berná L, Torres G, de Andrés L. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium‐111‐antimyosin monoclonal antibody studies. J Nucl Med. 1993;34:1503–1507. [PubMed] [Google Scholar]
- 67. D'Errico MP, Grimaldi L, Petruzzelli MF, Gianicolo EA, Tramacere F, Monetti A, Placella R, Pili G, Andreassi MG, Sicari R, Picano E, Portaluri M. N‐terminal pro‐B‐type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left‐sided breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:e239–e246. [DOI] [PubMed] [Google Scholar]
- 68. Jingu K, Nemoto K, Kaneta T, Oikawa M, Ogawa Y, Ariga H, Takeda K, Sakayauchi T, Fujimoto K, Narazaki K, Takai Y, Nakata E, Fukuda H, Takahashi S, Yamada S. Temporal change in brain natriuretic peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1417–1423. [DOI] [PubMed] [Google Scholar]
- 69. Yan R, Song J, Wu Z, Guo M, Liu J, Li J, Hao X, Li S. Detection of myocardial metabolic abnormalities by 18F‐FDG PET/CT and corresponding pathological changes in beagles with local heart irradiation. Korean J Radiol. 2015;16:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. NEXAVAR (sorafenib). Available at: https://www.accessdata.fda.gov/drugsatfdadocs/label/2010/021923s008s009lbl.pdf. Revised 10/2010. Accessed August 17, 2019.
- 71. Lenihan DJ, Kowey PR. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist. 2013;18:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node‐negative breast cancer who have estrogen‐receptor‐positive tumors. N Engl J Med. 1989;320:479–484. [DOI] [PubMed] [Google Scholar]
- 73. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin‐Boes G, Houghton J, Locker GY, Tobias JS; Group AT . Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. [DOI] [PubMed] [Google Scholar]
- 74. Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan‐Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P‐1 Study. J Natl Cancer Inst. 1998;90:1371–1388. [DOI] [PubMed] [Google Scholar]
- 75. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta‐analysis. J Natl Cancer Inst. 2011;103:1299–1309. [DOI] [PubMed] [Google Scholar]
- 76. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. [DOI] [PubMed] [Google Scholar]
- 77. Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85‐31. J Clin Oncol. 2009;27:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alibhai SM, Duong‐Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, Paszat LF. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nanda A, Chen MH, Braccioforte MH, Moran BJ, D'Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease‐induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. [DOI] [PubMed] [Google Scholar]
- 80. Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta‐analysis. Eur Urol. 2015;68:386–396. [DOI] [PubMed] [Google Scholar]
- 81. Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, Tate DJ, Horning SJ, Hoppe RT, Hancock SL. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–49. [DOI] [PubMed] [Google Scholar]
- 82. Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, Griffin BP, Desai MY. Long‐term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1485. [DOI] [PubMed] [Google Scholar]
- 83. Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landén M, Höglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Grönberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood‐cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, Arcila ME, Ladanyi M, Tallman MS, Levine RL, Berger MF. Therapy‐related clonal hematopoiesis in patients with non‐hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382.e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 87. Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC; Investigators S‐H . Coronary CT angiography and 5‐year risk of myocardial infarction. N Engl J Med. 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 88. Layoun ME, Yang EH, Herrmann J, Iliescu CA, Lopez‐Mattei JC, Marmagkiolis K, Budoff MJ, Ferencik M. Applications of cardiac computed tomography in the cardio‐oncology population. Curr Treat Options Oncol. 2019;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dean AP, Higgs D, Robins P, Stobie P, Craven P, Daly C, Carija S. Use of FDG PET scanning to evaluate 5‐FU myocardial toxicity as a global metabolic effect rather than vascular spasm. J Clin Oncol. 2018;36:792–792. [Google Scholar]
- 90. O'Farrell AC, Evans R, Silvola JM, Miller IS, Conroy E, Hector S, Cary M, Murray DW, Jarzabek MA, Maratha A, Alamanou M, Udupi GM, Shiels L, Pallaud C, Saraste A, Liljenbäck H, Jauhiainen M, Oikonen V, Ducret A, Cutler P, McAuliffe FM, Rousseau JA, Lecomte R, Gascon S, Arany Z, Ky B, Force T, Knuuti J, Gallagher WM, Roivainen A, Byrne AT. A novel positron emission tomography (PET) approach to monitor cardiac metabolic pathway remodeling in response to sunitinib malate. PLoS One. 2017;12:e0169964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Toubert ME, Vercellino L, Faugeron I, Lussato D, Hindie E, Bousquet G. Fatal heart failure after a 26‐month combination of tyrosine kinase inhibitors in a papillary thyroid cancer. Thyroid. 2011;21:451–454. [DOI] [PubMed] [Google Scholar]
- 92. Sudasena D, Balanescu DV, Donisan T, Hassan S, Palaskas N, Kim P, Karimzad K, Lopez‐Mattei J, Arain S, Gould KL, Iliescu C. Fulminant vascular and cardiac toxicity associated with tyrosine kinase inhibitor sorafenib. Cardiovasc Toxicol. 2019;19:382–387. [DOI] [PubMed] [Google Scholar]
- 93. Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: an overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. [DOI] [PubMed] [Google Scholar]
- 95. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD‐1 blockade with pembrolizumab in advanced Merkel‐cell carcinoma. N Engl J Med. 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig‐Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Opdivo (nivolumab) [package insert]. Princeton, NJ: Bristol‐Meyers Squibb; 2019. [Google Scholar]
- 99. Yervoy (ipilimumab) [package insert]. Princeton, NJ: Bristol‐Meyers Squibb; 2019. [Google Scholar]
- 100. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, Stewart GC, Choueiri TK, Di Carli M, Allenbach Y, Kumbhani DJ, Heinzerling L, Amiri‐Kordestani L, Lyon AR, Thavendiranathan P, Padera R, Lichtman A, Liu PP, Johnson DB, Moslehi J. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio‐oncology. Circulation. 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Awadalla MMS, Groarke J, Liu S, Hassan M, Murphy S, Jones‐O'Connor M, Nohria A, Heinzerling L, Sullivan R, Chen C, Gupta D, Ganatra S. Decrease global longitudinal strain with myocarditis from immune checkpoint inhibitors and occurrence of major adverse cardiac events. J Am Coll Cardiol. 2019;73:1532. [Google Scholar]
- 103. Waheed N, Mahmoud A, Shah C, Wu Y, March K, Pepine C, Gong Y. Global longitudinal strain among patients with immune checkpoint inhibitor‐induced cardiotoxicity: a case series. J Am Coll Cardiol. 2019;73:1595. [Google Scholar]
- 104. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, Kerneis M. Abatacept for severe immune checkpoint inhibitor‐associated myocarditis. N Engl J Med. 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 105. Moslehi JJ, Deininger M. Tyrosine kinase inhibitor‐associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br J Haematol. 2015;169:463–478. [DOI] [PubMed] [Google Scholar]
- 107. Stirrups R. CAR T‐cell therapy in refractory large B‐cell lymphoma. Lancet Oncol. 2018;19:e19. [DOI] [PubMed] [Google Scholar]
- 108. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu‐Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric antigen receptor T cells in refractory B‐cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in children and young adults with B‐cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kymriah‐epar‐product‐information. Available at: https://www.ema.europa.eu/documents/productinformation/kymriah-epar-product-informationen.pdf. Revised: 9/2018. Accessed August 2, 2019.
- 111. Yescarta‐epar‐product‐information. Available at: https://www.ema.europa.eu/documents/product-information/yescartaepar-product-information_en.pdf. Revised: 10/19/2018. Accessed August 2, 2019.
- 112. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single‐institution experience. Biol Blood Marrow Transplant. 2018;24:1590–1595. [DOI] [PubMed] [Google Scholar]
- 114. Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta‐analysis. JACC Cardiovasc Imaging. 2018;11:1583–1590. [DOI] [PubMed] [Google Scholar]
- 115. Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23:602–619. [DOI] [PubMed] [Google Scholar]
- 116. Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. [DOI] [PubMed] [Google Scholar]
- 117. Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in ¹⁸F‐fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. [DOI] [PubMed] [Google Scholar]
- 118. Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, Zhang Y, Chen D, Wu Z, Xiao L, Chang AH, Huang H, Zhao K. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non‐Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25:1092–1098. [DOI] [PubMed] [Google Scholar]
- 119. Shah NN, Nagle SJ, Torigian DA, Farwell MD, Hwang WT, Frey N, Nasta SD, Landsburg D, Mato A, June CH, Schuster SJ, Porter DL, Svoboda J. Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor ‐T‐cell therapy in B‐cell non‐Hodgkin lymphomas. Cytotherapy. 2018;20:1415–1418. [DOI] [PubMed] [Google Scholar]
- 120. Minn I, Huss DJ, Ahn HH, Chinn TM, Park A, Jones J, Brummet M, Rowe SP, Sysa‐Shah P, Du Y, Levitsky HI, Pomper MG. Imaging CAR T cell therapy with PSMA‐targeted positron emission tomography. Sci Adv. 2019;5:eaaw5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Galper SL, Yu JB, Mauch PM, Strasser JF, Silver B, Lacasce A, Marcus KJ, Stevenson MA, Chen MH, Ng AK. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. [DOI] [PubMed] [Google Scholar]
- 122. Thavendiranathan P, Verhaert D, Walls MC, Bender JA, Rajagopalan S, Chung YC, Simonetti OP, Raman SV. Simultaneous right and left heart real‐time, free‐breathing CMR flow quantification identifies constrictive physiology. JACC Cardiovasc Imaging. 2012;5:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Aldweib N, Farah V, Biederman RWW. Clinical utility of cardiac magnetic resonance imaging in pericardial diseases. Curr Cardiol Rev. 2018;14:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, Dungu J, Banypersad SM, Wechalekar AD, Whelan CJ, Hawkins PN, Gillmore JD. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. [DOI] [PubMed] [Google Scholar]
- 127. Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, Lachmann HJ, Wechalekar A, Gillmore JD, Whelan CJ, Hawkins PN, Anderson LJ. CMR‐based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–142. [DOI] [PubMed] [Google Scholar]
- 128. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; Investigators A‐AS . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 129. Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two‐dimensional speckle‐tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. [DOI] [PubMed] [Google Scholar]
- 130. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 131. Scully PR, Treibel TA, Fontana M, Lloyd G, Mullen M, Pugliese F, Hartman N, Hawkins PN, Menezes LJ, Moon JC. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71:463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kwong RY, Falk RH. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:122–124. [DOI] [PubMed] [Google Scholar]
- 133. Kotecha T, Martinez‐Naharro A, Treibel TA, Francis R, Nordin S, Abdel‐Gadir A, Knight DS, Zumbo G, Rosmini S, Maestrini V, Bulluck H, Rakhit RD, Wechalekar AD, Gilbertson J, Sheppard MN, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71:2919–2931. [DOI] [PubMed] [Google Scholar]
- 134. Numico G, Garrone O, Dongiovanni V, Silvestris N, Colantonio I, Di Costanzo G, Granetto C, Occelli M, Fea E, Heouaine A, Gasco M, Merlano M. Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer. 2005;103:994–999. [DOI] [PubMed] [Google Scholar]
- 135. Duquaine D, Hirsch GA, Chakrabarti A, Han Z, Kehrer C, Brook R, Joseph J, Schott A, Kalyanaraman B, Vasquez‐Vivar J, Rajagopalan S. Rapid‐onset endothelial dysfunction with adriamycin: evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8:101–107. [DOI] [PubMed] [Google Scholar]
- 136. Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J. 1988;9:552–556. [DOI] [PubMed] [Google Scholar]
- 137. Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Food and Drug Administration . FDA drug safety communication: sprycel (dasatinib) and risk of pulmonary arterial hypertension. 2011. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety communication‐sprycel‐dasatinib‐and‐risk‐pulmonary‐arterial‐hypertension. Accessed August 19, 2019.
- 139. Beckman JA, Thakore A, Kalinowski BH, Harris JR, Creager MA. Radiation therapy impairs endothelium‐dependent vasodilation in humans. J Am Coll Cardiol. 2001;37:761–765. [DOI] [PubMed] [Google Scholar]
- 140. Henson KE, McGale P, Taylor C, Darby SC. Radiation‐related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29:2391–2403. [DOI] [PubMed] [Google Scholar]
- 142. Zhou W, Bush RL, Lin PH, Lumsden AB. Radiation‐associated venous stenosis: endovascular treatment options. J Vasc Surg. 2004;40:179–182. [DOI] [PubMed] [Google Scholar]
- 143. Kamath MV, Halton J, Harvey A, Turner‐Gomes S, McArthur A, Barr RD. Cardiac autonomic dysfunction in survivors of acute lymphoblastic leukemia in childhood. Int J Oncol. 1998;12:635–640. [DOI] [PubMed] [Google Scholar]
- 144. Lancellotti P, Nkomo VT, Badano LP, Bergler‐Klein J, Bogaert J, Davin L, Cosyns B, Coucke P, Dulgheru R, Edvardsen T, Gaemperli O, Galderisi M, Griffin B, Heidenreich PA, Nieman K, Plana JC, Port SC, Scherrer‐Crosbie M, Schwartz RG, Sebag IA, Voigt JU, Wann S, Yang PC; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography, Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, Society of Cardiovascular Computed Tomography . Expert consensus for multi‐modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. [DOI] [PubMed] [Google Scholar]
- 145. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL, Oeffinger KC, Rosenthal DN, Sable CA, Sallan SE, Singh GK, Steinberger J, Cochran TR, Wilkinson JD; American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Basic Cardiovascular Sciences, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiology . Long‐term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. [DOI] [PubMed] [Google Scholar]
- 146. Szmit S, Nurzynski P, Szalus N, Opolski G, Szczylik C. Reversible myocardial dysfunction in a young woman with metastatic renal cell carcinoma treated with sunitinib. Acta Oncol. 2009;48:921–925. [DOI] [PubMed] [Google Scholar]
- 147. Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C‐J, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson. 2015;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sierra‐Galan LM, Francois CJ. Clinical applications of MRA 4D‐flow. Curr Treat Options Cardiovasc Med. 2019;21:58. [DOI] [PubMed] [Google Scholar]
- 149. Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez‐Jaramillo P, Gupta R, Diaz R, Avezum A, Oliveira GBF, Wielgosz A, Parambath SR, Mony P, Alhabib KF, Temizhan A, Ismail N, Chifamba J, Yeates K, Khatib R, Rahman O, Zatonska K, Kazmi K, Wei L, Zhu J, Rosengren A, Vijayakumar K, Kaur M, Mohan V, Yusufali A, Kelishadi R, Teo KK, Joseph P, Yusuf S. Variations in common diseases, hospital admissions, and deaths in middle‐aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2019. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(19)32007-0. [Epub ahead of print]. Accessed January 10, 2020. [DOI] [PubMed] [Google Scholar]
- 150. Seraphim A, Westwood M, Bhuva AN, Crake T, Moon JC, Menezes LJ, Lloyd G, Ghosh AK, Slater S, Oakervee H, Manisty CH. Advanced imaging modalities to monitor for cardiotoxicity. Curr Treat Options Oncol. 2019;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek E, Kwan A, Berger RD, Calkins H, Lardo AC, Kraut MA, Kamel IR, Zimmerman SL, Halperin HR. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377:2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schieda N, Maralani PJ, Hurrell C, Tsampalieros AK, Hiremath S. Updated clinical practice guideline on use of gadolinium‐based contrast agents in kidney disease issued by the Canadian Association of Radiologists. Can Assoc Radiol J. 2019;70:226–232. [DOI] [PubMed] [Google Scholar]


