Abstract
Background
Abnormal serum sodium levels have been associated with higher mortality among patients with acute coronary syndromes and heart failure. We sought to describe the association between sodium levels and mortality among unselected cardiac intensive care unit (CICU) patients.
Methods and Results
We retrospectively reviewed consecutive adult patients admitted to our cardiac intensive care unit from 2007 to 2015. Hyponatremia and hypernatremia were defined as admission serum sodium <135 and >145 mEq/L, respectively. In‐hospital mortality was assessed by multivariable regression, and postdischarge mortality was evaluated by Cox proportional‐hazards analysis. We included 9676 patients with a mean age of 68±15 years (37.5% females). Hyponatremia occurred in 1706 (17.6%) patients, and hypernatremia occurred in 322 (3.3%) patients; these groups had higher illness severity and a greater number of comorbidities. Risk of hospital mortality was higher with hyponatremia (15.5% versus 7.5%; unadjusted odds ratio, 2.41; 95% CI, 2.06–2.82; P<0.001) or hypernatremia (17.7% versus 8.6%; unadjusted odds ratio, 2.82; 95% CI, 2.09–3.80; P<0.001), with a J‐shaped relationship between admission sodium and mortality. After multivariate adjustment, only hyponatremia was significantly associated with in‐hospital mortality (adjusted odds ratio, 1.42; 95% CI, 1.14–1.76; P=0.002). Among hospital survivors, risk of postdischarge mortality was higher in patients with hyponatremia (adjusted hazard ratio, 1.28; 95% CI, 1.17–1.41; P<0.001) or hypernatremia (adjusted hazard ratio, 1.36; 95% CI, 1.12–1.64; P=0.002).
Conclusions
Hyponatremia and hypernatremia on admission to the cardiac intensive care unit are associated with increased unadjusted short‐ and long‐term mortality. Further studies are needed to determine whether correcting abnormal sodium levels can improve outcomes in cardiac intensive care unit patients.
Keywords: cardiac intensive care unit, coronary care unit, hyponatremia, mortality, sodium
Subject Categories: Nephrology and Kidney, Heart Failure, Mortality/Survival, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Clinical Perspective
What Is New?
Abnormal sodium levels have been associated with adverse outcomes in patients with acute cardiovascular disease or critical illness, but this is the first study to examine the relationship between serum sodium and mortality among unselected patients in the cardiac intensive care unit.
There was a U‐shaped relationship between sodium level and unadjusted hospital mortality; after adjustment, hyponatremia remained associated with higher in‐hospital mortality, whereas both hyponatremia and hypernatremia were associated with higher postdischarge mortality.
What Are the Clinical Implications?
Admission sodium levels and hyponatremia can predict short‐ and long‐term mortality in cardiac intensive care unit patients, and further studies are need to determine whether spontaneous or drug‐induced changes in sodium levels are associated with adverse outcomes, and whether therapeutic manipulation of sodium levels can improve outcomes.
Sodium is the most abundant electrolyte in plasma, and abnormal sodium levels can result from a wide array of disease states and in many different situations.1, 2 Abnormal admission sodium levels have been associated with higher mortality among hospitalized patients with a variety of medical conditions, particularly patients with acute cardiac disease.3, 4, 5, 6, 7 Among patients with acute and chronic heart failure (HF), hyponatremia has been consistently associated with increased mortality.5, 8, 9, 10 In patients with acute coronary syndromes (ACS), hyponatremia has also been associated with an increase in 30‐day reinfarction.6, 11, 12 Dysnatremias, including both hyponatremia and hypernatremia, have been further correlated with adverse outcomes among general intensive care unit patients, resulting in the inclusion of admission sodium in the Acute Physiology and Chronic Health Evaluation score to help identify critically ill patients at risk for in‐hospital mortality.3, 13, 14
Although most earlier studies have focused on patients with specific underlying diagnoses, the available evidence suggests that abnormal sodium levels are associated with adverse outcomes across diverse patient populations. The cardiac intensive care unit (CICU) population includes critically‐ill patients with acute and chronic cardiac disease in addition to multiple underlying noncardiac comorbidities.15 The changing characteristics of the CICU population highlight the challenges in classifying CICU patients into a single diagnosis group, reducing the generalizability of data gathered in noncritically ill patients with a specific underlying diagnosis. There is a growing need to re‐evaluate the applicability of previously determined markers for illness severity for patients in the CICU. To our knowledge, the relationship between admission serum sodium and mortality in patients admitted to a contemporary CICU has not yet been examined. This study sought to clarify this relationship by analyzing the association between admission sodium, including hypernatremia and hyponatremia, with both short‐ and long‐term mortality among unselected CICU patients. We hypothesized that abnormal sodium levels, especially hyponatremia, would be associated with higher in‐hospital mortality among CICU patients.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Participants
This study has been approved by the Mayo Clinic Institutional Review Board and was exempt from informed consent because of the minimal risk posed to enrolled patients. This is a historical cohort analysis using an institutional database of adult patients (aged ≥18 years) admitted to the CICU at Mayo Clinic Hospital between January 1, 2007 and December 31, 2015, as previously described.16, 17, 18, 19 The CICU at Mayo Clinic serves critically ill medical patients with acute and chronic cardiovascular disease and does not admit patients after cardiac surgery. Patients were identified from archived electronic health records.20 Patients who were readmitted to the CICU had only their first admission included in the analysis. We excluded patients who remained hospitalized on December 31, 2015 and those without admission sodium values. In compliance with Minnesota state law statute 144.295, patients who did not provide Minnesota Research Authorization were also excluded from the study.
Definitions
Admission sodium level was defined as the sodium value closest to CICU admission. The highest and lowest sodium values during the CICU stay were also recorded. Serum sodium levels were the default, but plasma or whole‐blood sodium values were substituted if serum sodium was not available. For the primary analysis, patients were grouped based on admission sodium level as follows: normal sodium (admission sodium, 135–144 mEq/L); hyponatremia (admission sodium, <135 mEq/L); and hypernatremia (admission sodium, ≥145 mEq/L). For the secondary analysis, patients were classified based on the highest and lowest sodium levels during the CICU stay as follows: hyponatremia (lowest sodium, <135 mEq/L); hypernatremia (highest sodium, ≥145 mEq/L); and normal sodium (lowest sodium, ≥135 mEq/L and highest sodium, <145 mEq/L) groups. Discharge diagnoses were determined using the review of hospital International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes. Among patients who had not previously undergone dialysis, acute kidney injury (AKI) in the CICU was defined using Kidney Disease: Improving Global Outcomes stages based on serum creatinine, as an increase in serum creatinine ≥0.3 mg/dL or by 50% in the CICU from either the baseline creatinine or hospital admission creatinine (whichever was lower).21 Severe AKI was defined as Kidney Disease: Improving Global Outcomes stage 2 or 3 AKI (ie, doubling of serum creatinine or increase in serum creatinine to ≥4.0 mg/dL or new dialysis initiation in the CICU); AKI that did not meet criteria for severe AKI was considered mild AKI.21 Baseline creatinine was considered to be the latest creatinine within 1 year preceding the index hospital admission.16, 19
Collected Data
Laboratory results, patient demographics, and discharge diagnoses were collected. Sequential Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation III scores along with Acute Physiology and Chronic Health Evaluation IV predicted mortality were automatically generated using electronic medical record data during the first 24 hours of CICU admission; missing variables were imputed as normal as the default.16, 17, 18, 22, 23, 24 Mean and maximum values of all daily Sequential Organ Failure Assessment scores during the first week in the CICU were calculated. Baseline comorbid conditions and the Charlson comorbidity index were electronically derived.25 Length of stay in CICU and hospital, hospital discharge disposition, and all‐cause postdischarge mortality were identified by review of electronic medical records or notification of patient mortality.16, 17, 18, 19, 26 Follow‐up was performed by electronic chart review on February 1, 2018.
Statistical Analysis
The primary study end point was all‐cause in‐hospital mortality, and the secondary study end points were all‐cause CICU mortality and all‐cause 5‐year postdischarge mortality. Groups were compared using ANOVA for continuous variables and chi‐square tests for categorical variables. Logistic regression was used to determine the odds ratio (OR) and 95% CI values and to determine the area under the receiver operator characteristic curve value for prediction of hospital mortality. Multivariable analysis was performed using logistic regression with nonadaptive elastic net penalization, with candidate variables including demographics, comorbidities, illness severity, and CICU therapies and complications.27 Optimal tuning parameters for the elastic net were selected by square root grid search in conjunction with 10‐fold cross‐validation to maximize the area under the receiver operator characteristic curve. Postdischarge survival between groups was assessed using Kaplan–Meier analysis, with groups compared using the log‐rank test. Predictors of 5‐year mortality were determined using step‐wise backward regression (P<0.25 to enter the model, P>0.1 to leave the model), then these variables were included in a Cox proportional‐hazards model to determine predictors of postdischarge survival among hospital survivors. P<0.01 was considered statistically significant. Analyses were performed using JMP Pro software (version 14.1.0; SAS Institute Inc., Cary NC).
Results
Study Population
We screened 12 904 CICU admissions and excluded 2900 patients who were excluded because of meeting predefined exclusion criteria for the study cohort, including 1877 readmissions, 755 patients with no Minnesota Research Authorization, and 268 patients admitted outside of the study period. Of the remaining 10 004 potentially eligible patients, we excluded an additional 328 (3.2%) who did not have an available admission sodium‐level measurement, yielding a final study population of 9676 patients (Figure S1). Mean age of included patients was 68±15 years, with 3627 (37.5%) females. Baseline characteristics of the study population grouped by admission serum sodium level are listed in Table 1. Mean admission sodium value was 137.8±4.4 mEq/L. Hyponatremia was present on admission in 1706 (17.6%) patients, and hypernatremia was present on admission in 322 (3.3%) patients. Patients with hyponatremia or hypernatremia on admission differed from patients with normal admission sodium values in terms of baseline characteristics, comorbidities, illness severity, discharge diagnoses, admission vital signs, admission laboratory values, and procedures and therapies (Table 1). Prevalence of AKI (including severe AKI) was higher in patients with either hyponatremia or hypernatremia, as were CICU and hospital length of stay (all P<0.001).
Table 1.
Baseline Characteristics of Patients With Normal Admission Sodium and Patients With Either Hyponatremia or Hypernatremia on Admission
| Hyponatremia (n=1706) | Normal Sodium (n=7648) | Hypernatremia (n=322) | P Value | |
|---|---|---|---|---|
| Baseline demographics | ||||
| Age, y | 66.9±15.4 | 67.5±15.1 | 70.7±14.3 | <0.001 |
| Female sex | 698 (40.9%) | 2804 (36.7%) | 125 (38.8%) | 0.004 |
| White race | 1551 (90.9%) | 7095 (92.8%) | 290 (90.1%) | 0.010 |
| BMI | 29.7±7.5 | 29.5±6.9 | 29.0±7.5 | 0.23 |
| Comorbidities | ||||
| Charlson comorbidity index | 2.8±2.7 | 2.3±2.6 | 2.8±2.7 | <0.001 |
| Previous MI | 347 (20.4%) | 1522 (20.0%) | 58 (18.1%) | 0.63 |
| Previous HF | 450 (26.4%) | 1385 (18.2%) | 73 (22.7%) | <0.001 |
| Previous DM | 596 (35.0%) | 2073 (27.2%) | 93 (29.0%) | <0.001 |
| Previous stroke | 203 (11.9%) | 935 (12.2%) | 51 (15.9%) | 0.13 |
| Previous liver disease | 70 (4.1%) | 115 (1.5%) | 5 (1.6%) | <0.001 |
| Previous cancer | 419 (24.6%) | 1557 (20.4%) | 87 (27.1%) | <0.001 |
| Previous lung disease | 369 (21.7%) | 1437 (18.8%) | 75 (23.4%) | <0.001 |
| Previous CKD | 419 (24.6%) | 1472 (19.3%) | 90 (28.0%) | <0.001 |
| Previous dialysis | 163 (9.6%) | 383 (5.0%) | 17 (5.3%) | <0.001 |
| Severity of illness | ||||
| APACHE‐III | 69.6±26.0 | 59.5±24.2 | 72.8±32.8 | <0.001 |
| APACHE‐IV | 0.228±0.224 | 0.156±0.190 | 0.260±0.264 | <0.001 |
| Day 1 SOFA | 4.7±3.6 | 3.3±3.0 | 5.2±4.0 | <0.001 |
| Maximum week 1 SOFA | 5.2±3.8 | 3.6±3.1 | 5.4±4.0 | <0.001 |
| Mean week 1 SOFA | 3.9±3.1 | 2.8±2.5 | 4.4±3.6 | <0.001 |
| Procedures and therapies | ||||
| Invasive ventilator | 350 (20.5%) | 1138 (14.9%) | 101 (31.4%) | <0.001 |
| Noninvasive ventilator | 315 (18.5%) | 1086 (14.2%) | 76 (23.6%) | <0.001 |
| Vasoactive drugs | 675 (39.6%) | 1655 (21.6%) | 96 (29.8%) | <0.001 |
| Vasopressors | 576 (33.8%) | 1389 (18.2%) | 86 (26.7%) | <0.001 |
| Inotropes | 287 (16.8%) | 600 (7.8%) | 28 (8.7%) | <0.001 |
| Dialysis | 174 (10.2%) | 286 (3.7%) | 18 (5.6%) | <0.001 |
| CRRT | 83 (4.9%) | 83 (1.1%) | 1 (0.3%) | <0.001 |
| IABP | 201 (11.8%) | 622 (8.1%) | 28 (8.7%) | <0.001 |
| PAC | 208 (12.2%) | 490 (6.4%) | 22 (6.8%) | <0.001 |
| RBC transfusion | 294 (17.2%) | 824 (10.8%) | 52 (16.2%) | <0.001 |
| Coronary angiography | 832 (48.8%) | 4130 (54.0%) | 131 (40.7%) | <0.001 |
| PCI | 459 (26.9%) | 2824 (36.9%) | 58 (18.0%) | <0.001 |
| Admission vital signs | ||||
| Systolic BP | 117.3±27.4 | 124.2±25.7 | 123.5±28.9 | <0.001 |
| Diastolic BP | 66.7±17.7 | 70.0±16.7 | 68.9±18.6 | <0.001 |
| Mean BP | 80.0±19.3 | 84.2±17.6 | 83.8±19.4 | <0.001 |
| Heart rate | 86.2±22.7 | 81.1±23.4 | 84.7±24.8 | <0.001 |
| Shock index | 0.77±0.28 | 0.68±0.26 | 0.73±0.29 | <0.001 |
| Respiratory rate | 19.4±5.7 | 18.2±5.7 | 19.3±6.9 | <0.001 |
| Oxygen saturation | 95.2±6.7 | 95.9±5.6 | 94.0±9.4 | <0.001 |
| Admission laboratory data | ||||
| Serum sodium | 130.9±3.8 | 139.0±2.4 | 146.8±2.6 | <0.001 |
| Serum potassium | 4.4±0.8 | 4.2±0.6 | 4.1±0.7 | <0.001 |
| Serum bicarbonate | 23.2±4.7 | 24.0±4.2 | 23.5±6.0 | <0.001 |
| Serum chloride | 96.8±5.8 | 103.9±4.3 | 109.0±5.2 | <0.001 |
| Serum anion gap | 12.3±4.0 | 11.5±3.4 | 13.3±4.7 | <0.001 |
| Serum magnesium | 2.1±0.4 | 2.0±0.3 | 2.1±0.05 | 0.001 |
| BUN | 34.4±25.6 | 24.7±16.2 | 32.1±21.2 | <0.001 |
| Serum creatinine | 1.7±1.5 | 1.3±1.0 | 1.4±1.0 | <0.001 |
| Serum glucose | 168.0±93.6 | 146.6±65.7 | 154.4±71.3 | <0.001 |
| Discharge ICD‐9 diagnoses | ||||
| Shock | 302 (17.7%) | 685 (9.0%) | 58 (18.0%) | <0.001 |
| Cardiomyopathy | 342 (20.1%) | 956 (12.5%) | 47 (14.6%) | <0.001 |
| HF | 914 (53.6%) | 2721 (35.6%) | 156 (48.4%) | <0.001 |
| AF | 665 (39.0%) | 2289 (30.0%) | 139 (43.2%) | <0.001 |
| Cardiac arrest | 164 (9.6%) | 575 (7.5%) | 46 (14.3%) | <0.001 |
| ACS | 622 (36.5%) | 3417 (44.7%) | 107 (33.2%) | <0.001 |
| Sepsis | 182 (10.7%) | 435 (5.7%) | 32 (9.9%) | <0.001 |
| Respiratory failure | 445 (26.1%) | 16.8%) | 117 (36.3%) | <0.001 |
| Outcomes | ||||
| CICU LOS | 3.4±6.4 | 2.4±4.2 | 2.5±2.8 | <0.001 |
| Hospital LOS | 11.4±15.8 | 7.3±12.6 | 8.9±13.8 | <0.001 |
| CICU mortality | 154 (9.0%) | 345 (4.5%) | 41 (12.7%) | <0.001 |
| Hospital mortality | 265 (15.5%) | 542 (7.1%) | 57 (17.7%) | <0.001 |
Data reported as frequency (percent) or mean±SD. Reported P values are for chi‐squared test (categorical variables) or ANOVA (continuous variables) across groups. ACS indicates acute coronary syndrome; AF, atrial fibrillation; APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CICU, cardiac intensive care unit; CKD, chronic kidney disease; CRRT, continuous renal‐replacement; DM, diabetes mellitus; HF, heart failure; IABP, intra‐aortic balloon pump; LOS, length of stay; MI, myocardial infarction; PAC, pulmonary artery catheterization; PCI, percutaneous intervention; RBC, red blood cell; SOFA, Sequential Organ Failure Assessment.
Admission Sodium and In‐Hospital Mortality
In‐hospital mortality occurred in 864 (8.9%) patients, including 540 (5.6%) patients who died in the CICU. Across the entire population, decreasing admission sodium levels were associated with higher in‐hospital mortality risk (unadjusted OR, 0.95; 95% CI, 0.94–0.97; P<0.001). Compared with patients with a normal sodium, an increased risk of in‐hospital mortality was observed in patients with either hyponatremia (15.5% versus 7.5%; unadjusted OR, 2.41; 95% CI, 2.06–2.82; P<0.001) or hypernatremia (17.7% versus 8.6%; unadjusted OR, 2.82; 95% CI, 2.09–3.80; P <0.001). A J‐shaped relationship was observed between admission sodium level and unadjusted CICU and in‐hospital mortality (Figure 1). Similar J‐shaped relationships were observed between admission sodium level and in‐hospital mortality in patients with or without ACS (Figure 2A), HF (Figure 2B), or previous chronic kidney disease (Figure 2C). A total of 3473 (39.0%) patients developed AKI in the CICU, including 1125 (12.6%) patients with severe AKI. When patients were stratified by the presence and severity of AKI in the CICU, similar J‐shaped relationships were observed between admission sodium value and in‐hospital mortality in patients with either mild or no AKI, but this was not significant for patients with severe AKI (Figure 2D).
Figure 1.
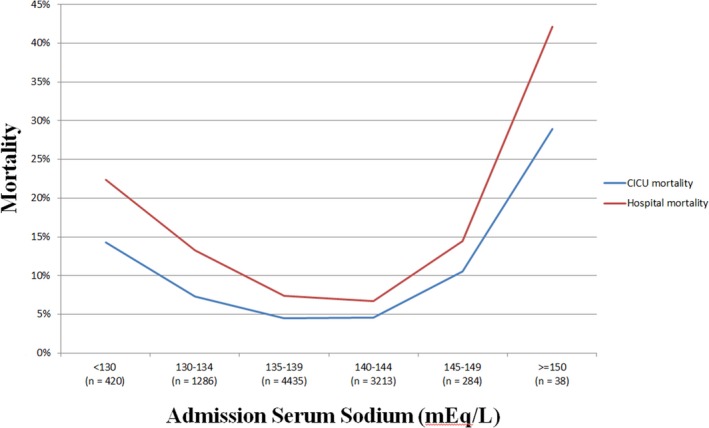
Cardiac intensive care unit (CICU) and in‐hospital mortality as a function of admission sodium in the overall study population (n=9676). P<0.001 for all mortality comparisons between sodium groups.
Figure 2.
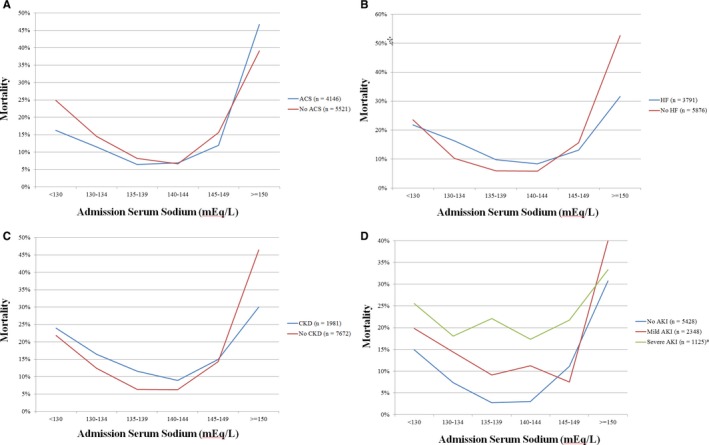
In‐hospital mortality as a function of admission sodium in patients with (A) acute coronary syndrome (ACS); (B) heart failure (HF); (C) chronic kidney disease (CKD), and (D) acute kidney injury (AKI). P<0.001 for all mortality comparisons between sodium groups by chi‐squared test, except in patients with severe AKI (*P=0.30).
After multivariable adjustment for baseline demographics, comorbidities, severity of illness, admission vital signs, admission laboratory values, discharge diagnoses, and procedures and therapies (Table 2), admission sodium level was inversely associated with in‐hospital mortality (adjusted OR, 0.97; 95% CI, 0.95–0.99; P=0.002). Whereas hypernatremia appeared more strongly associated with mortality on unadjusted analysis, after multivariable adjustment, patients with hyponatremia were at increased risk of in‐hospital mortality (adjusted OR, 1.42; 95% CI, 1.14–1.76; P=0.002), whereas patients with hypernatremia were not (adjusted OR, 0.75; 95% CI, 0.47–1.21; P=0.24).
Table 2.
Significant Predictors of Hospital Mortality on Multivariate Logistic Regression With Nonadaptive Elastic Net Penalization
| Adjusted OR | OR 95% CI | P Value | |
|---|---|---|---|
| Age | 1.024 | 1.016 to 1.033 | <0.001 |
| APACHE‐III score | 1.011 | 1.006 to 1.017 | <0.001 |
| Maximum week 1 SOFA | 1.252 | 1.190 to 1.318 | <0.001 |
| Admission sodium level | 0.970 | 0.951 to 0.989 | 0.002 |
| Admission BUN level | 1.010 | 1.004 to 1.016 | <0.001 |
| No. of vasoactive drugs | 1.194 | 1.070 to 1.333 | 0.002 |
| Discharge diagnosis of AF | 0.729 | 0.587 to 0.906 | 0.004 |
| Discharge diagnosis of cardiac arrest | 3.840 | 2.835 to 5.201 | <0.001 |
| Discharge diagnosis of CAD | 0.606 | 0.470 to 0.781 | <0.001 |
| Discharge diagnosis of respiratory failure | 1.727 | 1.300 to 2.296 | <0.001 |
Only predictors with P<0.01 are shown. Predictors with borderline significance (P=0.01–0.1) included in the model were: cardiomyopathy, Charlson comorbidity index, heart rate, HF, hospital days preceding CICU admission, invasive ventilator use, oxygen saturation, PCI, respiratory rate, and systolic BP. Additional predictors included in the model with P≥0.1 were white race, year of CICU admission, noninvasive ventilator use, potassium, bicarbonate, creatinine, previous MI, previous stroke, previous CKD, previous diabetes mellitus, previous lung disease, CRRT, diastolic BP, previous dialysis, new dialysis start, PAC, coronary angiogram, ACS, ESRD, and sepsis. The validation AUC was 0.908 for the final model. ACS indicates acute coronary syndrome; AF, atrial fibrillation; APACHE, Acute Physiology and Chronic Health Evaluation; AUC, area under the curve; BP, blood pressure; BUN, blood urea nitrogen; CAD, coronary artery disease; CICU, cardiac intensive care unit; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; ESRD, end‐stage renal disease; HF, heart failure; MI, myocardial infarction; OR, odds ratio; PAC, pulmonary artery catheterization; PCI, percutaneous intervention; SOFA, Sequential Organ Failure Assessment.
Peak and Nadir Sodium and In‐Hospital Mortality
A total of 9494 (98.1%) patients had a sodium value checked during the CICU stay. All measured sodium values in the CICU were normal in 6263 (66.0%) patients, hyponatremia during the CICU stay was present in 2411 (25.4%) patients, and hypernatremia during the CICU stay was present in 944 (9.9%) patients; 124 (1.3%) patients met criteria for both hyponatremia and hypernatremia during the CICU stay. A step‐wise increase in unadjusted CICU and in‐hospital mortality was observed in patients with hyponatremia, hypernatremia, or both during the CICU stay (Figure 3). Compared with patients with normal sodium during the CICU stay, sequentially higher mortality percentages were noted among those with hyponatremia only (unadjusted OR, 3.42; 95% CI, 2.90–4.05; P<0.001), hypernatremia only (unadjusted OR, 6.19; 95% CI, 5.06–7.58; P<0.001), and both hyponatremia and hypernatremia at different times during the CICU stay (unadjusted OR, 7.8; 95% CI, 5.21–11.88; P<0.001). A reverse J‐shaped relationship was observed between the nadir sodium value during the CICU stay and in‐hospital mortality (Figure 4A). A J‐shaped relationship was observed between the peak sodium value during the CICU stay and in‐hospital mortality (Figure 4B).
Figure 3.
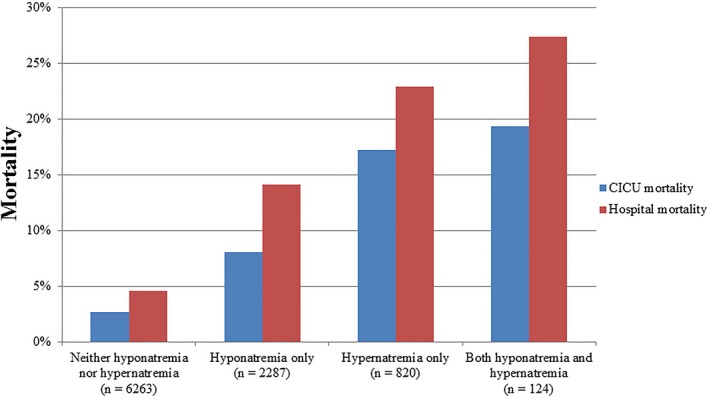
Cardiac intensive care unit (CICU) and in‐hospital mortality as a function of the presence of hyponatremia (minimum sodium <135) or hypernatremia (maximum sodium ≥145) during the CICU stay in the overall study population (n=9494). P<0.001 for all mortality comparisons between sodium groups by chi‐squared test.
Figure 4.
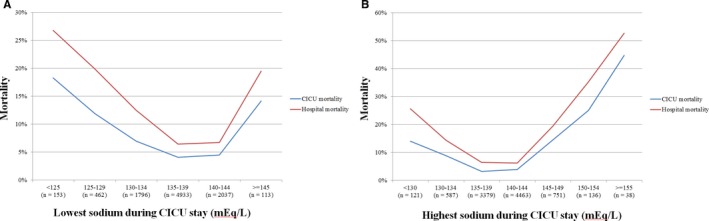
Cardiac intensive care unit (CICU) and in‐hospital mortality as a function of (A) lowest minimum sodium and (B) highest maximum sodium during the CICU stay in the overall study population (n=9494). P<0.001 for all mortality comparisons between sodium groups by chi‐squared test.
Postdischarge Mortality
A total of 2916 (33.1%) of 8812 hospital survivors died during up to 5 years of follow‐up; 1235 (14.0%) patients were lost to follow‐up within 1 year after hospital discharge. On Kaplan–Meier analysis (Figure 5A), hospital survivors with either hyponatremia (n=1441) or hypernatremia (n=265) at the time of CICU admission had lower postdischarge survival (P<0.001 by log‐rank); postdischarge survival was not different for patients with hyponatremia compared with hypernatremia (P=0.30). On Cox proportional‐hazards analysis adjusting for predictors of 5‐year survival (Table 3), risk of postdischarge mortality was higher in patients with either hyponatremia (adjusted hazard ratio, 1.28; 95% CI, 1.17–1.41; P<0.001) or hypernatremia (adjusted hazard ratio, 1.36; 95% CI, 1.12–1.64; P=0.002) at the time of CICU admission, with no difference between these groups (P=0.58). On Kaplan–Meier analysis (Figure 5B), hospital survivors with hyponatremia and/or hypernatremia during the CICU stay had lower postdischarge survival than patients with normal sodium values during the CICU stay (P<0.001 by log‐rank); postdischarge survival was not different between these other groups (all P>0.1).
Figure 5.
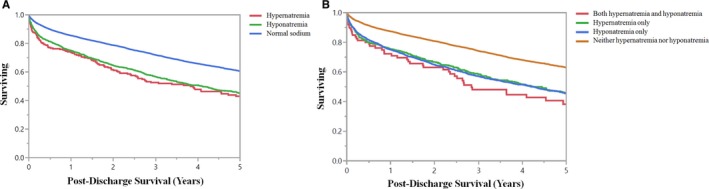
Kaplan–Meier survival curves demonstrating postdischarge survival among hospital survivors (n=8662) as a function of the presence of hyponatremia (minimum sodium <135) or hypernatremia (maximum sodium ≥145) on (A) cardiac intensive care unit (CICU) admission or (B) during the CICU stay. P<0.001 by log‐rank for all groups compared with patients with normal sodium and P>0.1 for other groups compared with each other.
Table 3.
Predictors of 5‐Year Mortality on Cox Proportional‐Hazards Analysis
| Adjusted HR | HR 95% CI | P Value | |
|---|---|---|---|
| Demographics and comorbidities | |||
| Age | 1.029 | 1.025 to 1.032 | <0.001 |
| BMI | 0.982 | 0.977 to 0.988 | <0.001 |
| Charlson comorbidity index | 1.147 | 1.128 to 1.166 | <0.001 |
| Previous cancer diagnosis | 0.834 | 0.755 to 0.921 | <0.001 |
| Previous lung disease diagnosis | 1.143 | 1.046 to 1.248 | 0.003 |
| Illness severity | |||
| APACHE‐III score | 1.006 | 1.003 to 1.009 | <0.001 |
| Maximum week 1 SOFA | 1.051 | 1.030 to 1.073 | <0.001 |
| Admission vital signs | |||
| Admission shock index | 1.393 | 1.213 to 1.601 | <0.001 |
| Respiratory rate | 1.010 | 1.003 to 1.017 | 0.004 |
| CICU therapies and procedures | |||
| Invasive ventilator | 0.815 | 0.703 to 0.945 | 0.007 |
| New dialysis start | 1.629 | 1.195 to 2.219 | 0.002 |
| IABP | 0.727 | 0.624 to 0.846 | <0.001 |
| Coronary angiogram | 0.851 | 0.781 to 0.927 | <0.001 |
| Discharge diagnoses | |||
| HF | 1.406 | 1.291 to 1.530 | <0.001 |
| Sepsis | 1.322 | 1.150 to 1.519 | <0.001 |
| Admission laboratory values | |||
| Serum bicarbonate | 1.031 | 1.022 to 1.040 | <0.001 |
| BUN | 1.007 | 1.005 to 1.010 | <0.001 |
| Admission sodium group | |||
| Hyponatremia vs normal | 1.281 | 1.168 to 1.406 | <0.001 |
| Hypernatremia vs normal | 1.357 | 1.122 to 1.642 | 0.002 |
| Hyponatremia vs hypernatremia | 0.944 | 0.772 to 1.155 | 0.58 |
Only predictors with P<0.01 are shown. Additional predictors included in the model with P≥0.01 were noninvasive ventilator use, oxygen saturation, creatinine, CRRT, dialysis, cardiac arrest, admission MAP, PCI, respiratory failure. APACHE indicates Acute Physiology and Chronic Health Evaluation; ACS, acute coronary syndrome; BMI, body mass index; BUN, blood urea nitrogen; CICU, cardiac intensive care unit; CRRT, continuous renal replacement therapy; HF, heart failure; HR, hazard ratio; IABP, intra‐aortic balloon pump; MAP, mean arterial pressure; PCI, percutaneous intervention; SOFA, Sequential Organ Failure Assessment.
Discussion
We observed a J‐shaped relationship between admission sodium and unadjusted CICU and in‐hospital mortality, including patients with or without a diagnosis of ACS, HF, mild or no AKI, or a history of chronic kidney disease. This is the first study to evaluate the association between mortality and serum sodium in a large cohort of unselected CICU patients. Patients with hyponatremia or hypernatremia had more‐extensive comorbidities, higher illness severity, and required greater use of CICU therapies. After multivariate adjustment for these factors, hyponatremia on admission remained independently associated with in‐hospital mortality, whereas the lower number of patients with hypernatremia did not appear to have an increased risk of in‐hospital mortality. Both the lowest and highest sodium levels during the CICU stay were associated with in‐hospital mortality, particularly among the subgroup of patients exhibiting both hyponatremia and hypernatremia. Hyponatremia or hypernatremia among hospital survivors, both on admission or at some point during the CICU stay, were correlated with an increased risk of postdischarge mortality with no significant difference in survival between dysnatremic groups. Importantly, whereas severe hyponatremia and hypernatremia were uncommon, even mild to moderately abnormal sodium levels appeared to be associated with higher mortality.
Earlier studies have observed a correlation between abnormal sodium levels and mortality in a variety of cardiac populations.5, 6, 7, 8, 9, 10, 11, 12 Hyponatremia is a well‐established risk factor for in‐hospital and postdischarge mortality among hospitalized patients with congestive HF, whereas impact of hypernatremia is less understood.5, 8, 9, 10 A recent retrospective review of 50 932 patients with newly diagnosed diastolic HF by Imran et al demonstrated that both hyponatremia and hypernatremia were significant risk factors for adjusted short‐ (30 day) and long‐term (>30 days) mortality, consistent with our findings in unselected CICU patients.28
Abnormal admission sodium levels have further been linked to adverse outcomes among patients with ACS, with a recent meta‐analysis of 20 studies by Ma et al reporting increased short‐ (30 days) and long‐term (>30 days) mortality associated with hyponatremia in this population.29 After multivariate adjustment, our study demonstrated that only hyponatremia was correlated with in‐hospital mortality, whereas both hyponatremia and hypernatremia were associated with decreased postdischarge survival. The finding that hyponatremia, but not hypernatremia, was associated with in‐hospital mortality after adjustment for other markers of illness severity might suggest an independent pathophysiological contribution of hyponatremia to adverse outcomes. Alternatively, hyponatremia may identify patients with a higher‐risk phenotype or underlying disease process. These data emphasize both the short‐ and long‐term prognostic importance of admission serum sodium levels among patients admitted to the CICU.
Abnormal serum sodium levels are very common among patients admitted to contemporary intensive care units, with an estimated prevalence of 24.6% in 1 recent study.3 The Acute Physiology and Chronic Health Evaluation illness severity score incorporates admission sodium values to identify critically ill patients at risk for in‐hospital mortality.14 Funk et al demonstrated increased in‐hospital mortality among intensive care unit patients with any severity of dysnatremia, including those with only mild hyponatremia (130–135 mEq/L) or mild hypernatremia (145–150 mEq/L).3 Interestingly, a recent observational cohort study noted a survival advantage for intensive care unit patients whose dysnatremias were corrected by day 3 of hospitalization, suggesting that aggressive correction of abnormal sodium levels may be beneficial for critically ill patients.30 We were unable to assess the timing of changes in sodium levels in our study.
Hypernatremia can be secondary to numerous etiologies, including impaired fluid intake, excessive fluid loss, and neurological deficits, among others.1 In our study, patients with admission hypernatremia were sicker, with higher illness severities and a greater number of comorbidities. After multivariate adjustment for these factors, hypernatremia was no longer associated with in‐hospital mortality, which suggests that hypernatremia may identify higher‐risk patients with greater underlying illness severity rather than directly contributing to adverse outcomes. However, hypernatremia was associated with increased adjusted mortality after discharge. There are several possible mechanisms for impaired long‐term survival in this group, given that hypernatremia has numerous physiological effects that are detrimental to cardiac patients. In particular, elevated serum sodium has been associated with decreased left ventricular contractility, increased peripheral insulin resistance, and neuromuscular disturbances.1, 31, 32, 33 Alternatively, patients who are prone to hypernatremia during hospitalization may have an underlying disease process that could contribute to worse long‐term survival. These factors likely contribute to the decreased long‐term survival among patients with hypernatremia and emphasize the need for close outpatient monitoring after discharge.
Hyponatremia can also be secondary to numerous etiologies, including diuretics, excessive gastrointestinal losses, syndrome of inappropriate antidiuretic hormone secretion, endocrinopathies, and intravascular volume depletion.2, 28 It can further be caused by end‐organ dysfunction, such as kidney and liver failure and/or HF, typically producing an inappropriate secretion of antidiuretic hormone secretion with resultant volume overload.34 Among patients with a reduced circulating blood volume, such as those with underlying HF or potentially ACS, hyponatremia develops because of a combination of several mechanisms, including activation of the renin‐angiotensin‐aldosterone system, upregulation of the sympathetic nervous system, and excessive release of antidiuretic hormone. These mechanisms all contribute to increased water retention and overall blood volume.28 This would presumably be problematic for patients with a limited cardiac reserve, and the resultant fluid imbalance may account for the elevated short‐ and long‐term mortality that we witnessed in our patients after adjustment for relevant covariates.
This study has some limitations common to all retrospective cohort studies, including the potential for unmeasured residual confounding factors and inability to establish a causal relationship between abnormal admission sodium levels and mortality despite careful multivariable analysis. The Mayo Clinic CICU population may differ significantly from other populations, potentially limiting external applicability. To facilitate early risk stratification, we focused on admission sodium levels by including laboratory values from either before or after CICU admission and including serum, plasma, and whole‐blood values. We were unable to determine which patients had sodium levels measured by each assay or the reasons that a different assay may have been used in each patient, although the differences between the different assay values and reference ranges are unlikely to impact the main findings. The secondary analysis evaluating maximum and minimum sodium levels during the CICU stay could potentially be confounded by these same analytical issues, as well as lack of data regarding timing of peak and nadir sodium levels. Given the small sample size of in‐hospital mortality among patients with hypernatremia, our finding that it was not an independent risk factor for in‐hospital mortality could represent a type II error. Our final multivariable regression model did not take into account potential statistical interactions between variables or nonlinear effects, which could have influenced our results. Apart from measures of comorbidity and illness severity, we were not able to elucidate the underlying contributing etiologies to dysnatremias in our population. We were not able to obtain data on overall fluid balance or medications administered, which are known to influence sodium levels, such as diuretic therapy. Earlier studies have identified hypermagnesemia as an independent risk factor for mortality among CICU patients.35 We found hypermagnesemia to be more common in patients with hyponatremia or hypernatremia, but did not include serum magnesium levels in our multivariable analysis because of the higher prevalence of missing data compared with other electrolyte levels. The ICD‐9 discharge diagnoses we obtained reflect all diagnoses from the hospitalization, including both acute and chronic conditions, and cannot distinguish the primary admission diagnoses.
Conclusions
Our study has demonstrated a J‐shaped relationship between admission sodium levels and unadjusted CICU and in‐hospital mortality, including patients with or without a diagnosis of ACS, HF, or chronic kidney disease. After multivariate adjustment, hyponatremia was associated with an elevated risk of in‐hospital mortality, but hypernatremia was not, whereas both hyponatremia and hypernatremia were associated with decreased survival after discharge. These data highlight the importance of admission sodium as a prognostic factor that can help to risk stratify contemporary CICU patients. Future studies are needed to explore the underlying etiologies of dysnatremias and effects of aggressive correction of dysnatremias on patient‐centered outcomes in the CICU setting.
Disclosures
None.
Supporting information
Figure S1. Flow diagram demonstrating inclusion/exclusion criteria for the final study population.
Acknowledgments
The authors thank Dr Terry M. Therneau, PhD, for his expert statistical consultation.
(J Am Heart Assoc. 2020;9:e014140 DOI: 10.1161/JAHA.119.014140.)
References
- 1. Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–1499. [DOI] [PubMed] [Google Scholar]
- 2. Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. [DOI] [PubMed] [Google Scholar]
- 3. Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, Metnitz PG. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36:304–311. [DOI] [PubMed] [Google Scholar]
- 4. Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124:197–203. [DOI] [PubMed] [Google Scholar]
- 5. Klein L, O'Connor CM, Leimberger JD, Gattis‐Stough W, Pina IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M; OPTIME‐CHF Investigators . Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) study. Circulation. 2015;111:2454–2460. [DOI] [PubMed] [Google Scholar]
- 6. Singla I, Zahid M, Good CB, Macioce A, Sonel AF. Effect of hyponatremia (<135 mEq/L) on outcome in patients with non‐ST‐elevation acute coronary syndrome. Am J Cardiol. 2007;100:406–408. [DOI] [PubMed] [Google Scholar]
- 7. Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar‐Zadeh K. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O'Connor CM. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007;167:1998–2005. [DOI] [PubMed] [Google Scholar]
- 9. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC; OPTIMIZE‐HF Investigators and Coordinators . Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J. 2007;28:980–988. [DOI] [PubMed] [Google Scholar]
- 10. De Luca L, Klein L, Udelson JE, Orlandi C, Sardella G, Fedele F, Gheorghiade M. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96:19L–23L. [DOI] [PubMed] [Google Scholar]
- 11. Klopotowski M, Kruk M, Przyluski J, Kalinczuk L, Pregowski J, Bekta P, Malek LA, Kepka C, Ciszewski A, Chmielak Z, Demkow M, Karcz M, Witkowski A, Ruzyllo W. Sodium level on admission and in‐hospital outcomes of STEMI patients treated with primary angioplasty: the ANIN Myocardial Infarction Registry. Med Sci Monit. 2009;15:CR477–CR483. [PubMed] [Google Scholar]
- 12. Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D. Prognostic importance of hyponatremia in acute ST‐elevation myocardial infarction. Am J Med. 2004;117:242–248. [DOI] [PubMed] [Google Scholar]
- 13. Lindner G, Funk GC, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, Kramer L, Druml W. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50:952–957. [DOI] [PubMed] [Google Scholar]
- 14. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 15. Goldfarb M, van Diepen S, Liszkowski M, Jentzer JC, Pedraza I, Cercek B. Noncardiovascular disease and critical care delivery in a contemporary cardiac and medical intensive care unit. J Intensive Care Med. 2019;537–543. [DOI] [PubMed] [Google Scholar]
- 16. Jentzer JC, Murphree DH, Wiley B, Bennett C, Goldfarb M, Keegan MT, Murphy JG, Wright RS, Barsness GW. Comparison of mortality risk prediction among patients >/=70 versus <70 years of age in a cardiac intensive care unit. Am J Cardiol. 2018;122:1773–1778. [DOI] [PubMed] [Google Scholar]
- 17. Jentzer JC, Bennett C, Wiley BM, Murphree DH, Keegan MT, Gajic O, Wright RS, Barsness GW. Predictive value of the sequential organ failure assessment score for mortality in a contemporary cardiac intensive care unit population. J Am Heart Assoc. 2018;7:e008169 DOI: 10.1161/JAHA.117.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett CE, Wright RS, Jentzer J, Gajic O, Murphree DH, Murphy JG, Mankad SV, Wiley BM, Bell MR, Barsness GW. Severity of illness assessment with application of the APACHE IV predicted mortality and outcome trends analysis in an academic cardiac intensive care unit. J Crit Care. 2019;50:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brueske B, Sidhu MS, Schulman‐Marcus J, Kashani KB, Barsness GW, Jentzer JC. Hyperkalemia is associated with increased mortality among unselected cardiac intensive care unit patients. J Am Heart Assoc. 2019;8:e011814 DOI: 10.1161/JAHA.118.011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 22. Aakre C, Franco PM, Ferreyra M, Kitson J, Li M, Herasevich V. Prospective validation of a near real‐time EHR‐integrated automated SOFA score calculator. Int J Med Informatics. 2017;103:1–6. [DOI] [PubMed] [Google Scholar]
- 23. Chandra S, Kashyap R, Trillo‐Alvarez CA, Tsapenko M, Yilmaz M, Hanson AC, Pickering BW, Gajic O, Herasevich V. Mapping physicians’ admission diagnoses to structured concepts towards fully automatic calculation of acute physiology and chronic health evaluation score. BMJ Open. 2011;1:e000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keegan MT, Gajic O, Afessa B. Comparison of APACHE III, APACHE IV, SAPS 3, and MPM0III and influence of resuscitation status on model performance. Chest. 2012;142:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, Gajic O, Li G. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ. History of the rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou H, Hastie T. Regularization and variable selection via the elastic net. J Roy Stat Soc B. 2005;67:301–320. [Google Scholar]
- 28. Imran TFKK, Patel YR, Orkaby AR, McLean RR, Ho YL, Cho K, Gaziano JM, Djousse L, Gagnon DR, Joseph J. Serial sodium values and adverse outcomes in heart failure with preserved ejection fraction. Int J Cardiol. 2019;290:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma QQ, Fan XD, Li T, Hao YY, Ma F. Short‐ and long‐term prognostic value of hyponatremia in patients with acute coronary syndrome: a systematic review and meta‐analysis. PLoS One. 13:e0193857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darmon M, Pichon M, Schwebel C, Ruckly S, Adrie C, Haouache H, Azoulay E, Bouadma L, Clec'h C, Garrouste‐Orgeas M, Souweine B, Goldgran‐Toledano D, Khallel H, Argaud L, Dumenil AS, Jamali S, Allaouchiche B, Zeni F, Timsit JF. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 41:394–399. [DOI] [PubMed] [Google Scholar]
- 31. Lenz K, Gossinger H, Laggner A, Druml W, Grimm G, Schneeweiss B. Influence of hypernatremic‐hyperosmolar state on hemodynamics of patients with normal and depressed myocardial function. Crit Care Med. 14:913–914. [DOI] [PubMed] [Google Scholar]
- 32. Bratusch‐Marrain PR, DeFronzo RA. Impairment of insulin‐mediated glucose metabolism by hyperosmolality in man. Diabetes. 32:1028–1034. [DOI] [PubMed] [Google Scholar]
- 33. Kozeny GA, Murdock DK, Euler DE, Hano JE, Scanlon PJ, Bansal VK, Vertuno LL. In vivo effects of acute changes in osmolality and sodium concentration on myocardial contractility. Am Heart J. 1985;109:290–296. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez M, Hernandez M, Cheungpasitporn W, Kashani KB, Riaz I, Rangaswami J, Herzog E, Guglin M, Krittanawong C. Hyponatremia in heart failure: pathogenesis and management. Curr Cardiol Rev. 2019;15:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naksuk N, Hu T, Krittanawong C, Thongprayoon C, Sharma S, Park JY, Rosenbaum AN, Gaba P, Killu AM, Sugrue AM, Peeraphatdit T, Herasevich V, Bell MR, Brady PA, Kapa S, Asirvatham SJ. Association of serum magnesium on mortality in patients admitted to the intensive cardiac care unit. Am J Med. 2017; 130:229.e5–229.e13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram demonstrating inclusion/exclusion criteria for the final study population.


