Abstract
Background
Lamin A/C cardiomyopathy is a malignant and highly penetrant inheritable cardiomyopathy. Competitive sports have been associated with adverse events in these patients, but data on recreational exercise are lacking. We aimed to explore associations between exercise exposure and disease severity in patients with lamin A/C genotype.
Methods and Results
Lamin A/C genotype positive patients answered a questionnaire on exercise habits from age 7 years until genetic diagnosis. We recorded exercise hours >3 metabolic equivalents and calculated cumulative lifetime exercise. Patients were grouped in active or sedate based on lifetime exercise hours above or below median. We performed echocardiography, 12‐lead ECG, Holter monitoring, and biomarkers including NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide). We defined left ventricular ejection fraction <45% as a clinically significant impairment of left ventricular function. We included 69 patients (age 42±14 years, 41% probands, 46% women) with median lifetime exercise 4160 (interquartile range 1041–6924) hours. Active patients were more frequently probands (53% versus 29%, P=0.04), had lower left ventricular ejection fraction (43±13% versus 51±11%, P=0.006), and higher NT‐proBNP (78 [interquartile range 32–219] pmol/L versus 30 [interquartile range 13–64] pmol/L, P=0.03) compared with sedate, while age did not differ (45±13 years versus 40±16 years, P=0.16). The decrease in left ventricular ejection fraction per tertile increment in lifetime exercise was 4% (95% CI −7% to −0.4%, P=0.03), adjusted for age and sex and accounting for dependence within families. Left ventricular ejection fraction <45% was observed at a younger age in active patients (log rank P=0.007).
Conclusions
Active lamin A/C patients had worse systolic function compared with sedate which occurred at younger age. Our findings may improve exercise recommendations in patients with lamin A/C.
Keywords: arrhythmias, dilated cardiomyopathy, exercise, genetics, lamin A/C
Subject Categories: Cardiomyopathy, Exercise, Genetics
Clinical Perspective
What Is New?
Lamin A/C genotype positive patients with greater exercise exposure had earlier penetrance of dilated cardiomyopathy than those with less.
A dose‐response relationship was illustrated by a linear relationship between increasing tertile of exercise duration and dilated cardiomyopathy.
Recreational exercise may have an adverse impact on cardiac function in lamin A/C positive patients, and family members identified by genetic screening demonstrated earlier penetrance and lower left ventricular ejection fraction with increasing exercise exposure.
What Are the Clinical Implications?
It may be reasonable to advise patients with lamin A/C mutations to avoid competitive and/or vigorous exercise.
This advice may also apply to genotype positive family members with incomplete disease penetrance at the time of genetic diagnosis.
Exercise has known health benefits and should not be unnecessarily restricted, however, safe exercise thresholds in patients with lamin A/C mutations are still unknown.
Introduction
Dilated cardiomyopathy (DCM) is often familial, and a genetic substrate is found in up to half of the cases.1 Mutations in the lamin A/C gene account for 5% to 8% of familial DCM,2, 3 and up to 33% in those with conduction defects.4 Lamin A/C cardiomyopathy is characterized by early onset atrioventricular block, supraventricular arrhythmias, ventricular arrhythmias, and progressive DCM.5 The natural history is highly malignant, ventricular arrhythmias and sudden cardiac death may occur before clinical manifestation of DCM.6, 7, 8, 9 Lamin A/C cardiomyopathy has an autosomal dominant pattern of inheritance with a lifetime penetrance of nearly 100%, and patients develop heart disease in early adulthood.10 The cardiomyopathy can be penetrant either separately or in combination with skeletal muscle disease.4
Regular physical activity is a cornerstone of a healthy lifestyle, and is inversely related to lifestyle diseases dominating in the western world.11 On the other hand, vigorous exercise aggravates and accelerates cardiac disease in arrhythmogenic cardiomyopathy.12, 13, 14, 15, 16 The impact of exercise on lamin A/C disease is not as thoroughly described, although a study by Pasotti et al10 indicated competitive sports as a marker for worse outcome.10 A recent experimental study on lamin A/C genotype positive mice has indicated earlier development of DCM in exercising mice compared with sedate mice.17 In lamin A/C positive humans, the effects of non‐competitive exercise, and association of exercise with skeletal muscle disease are unknown.
We aimed to explore the effects of exercise habits on left ventricular (LV) dimensions and function, on electrical disease, including atrioventricular block and arrhythmias in lamin A/C cardiomyopathy. We hypothesized that more exercise is associated with worse cardiac phenotypes in lamin A/C patients.
Methods
Data Availability
The authors do not have the authority to share the data used in the analyses described in this article. The approval of the Regional Committee for Medical Research Ethics limits sharing data with researchers outside of Norway for purposes of reproducing the results or replicating the procedures. The data can only be made available to any additional researchers if a formal request is filled with the Regional Committee for Medical Research Ethics and explicit consent is given from every study subject.
Study Population
We conducted a single‐center cross sectional study including consecutively recruited lamin A/C genotype positive probands, and genotype positive family members from the Unit for Genetic Cardiac Diseases, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Oslo, Norway.2 Probands were defined as the first affected individual in a family who sought medical attention for lamin A/C related disease and with a pathogenic lamin A/C genotype. Genotype positive family members were identified by cascade genetic screening.
The study complied with the Declaration of Helsinki, and the research protocol was approved by the Regional Committee for Medical Research Ethics. All study patients gave written informed consent.
Exercise Questionnaire
In March 2017 we performed an exercise survey among our lamin A/C genotype positive patients using a structured phone interview or a questionnaire by mail, as previously described.18 Exercise data were obtained retrospectively from age 7 until March 2017, and were reported as type of activity/sport, each graded at intensity light, moderate, or vigorous.
The intensity of the reported activities were quantified as metabolic equivalents,19 and rated according to Compendium of Physical Activities with updated online tools.20 We summarized all exercise hours above 3 metabolic equivalents21 from age 7 until the study echocardiogram, which coincided in time with the diagnosis of lamin A/C genotype, and defined this quantitative measure as cumulative lifetime exercise (Figure 1).
Figure 1.
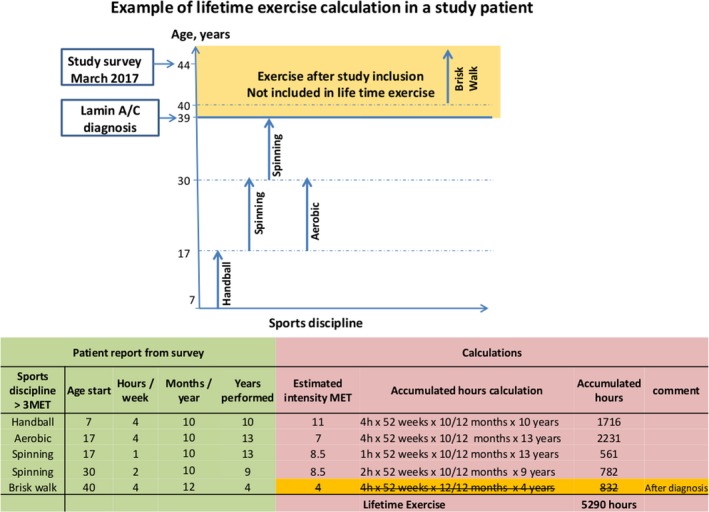
Diagram showing exercise discipline >3 METs and duration performed at different ages. Orange field indicates exercise after lamin A/C diagnosis, not included in lifetime exercise. Table showing exercise reported by the patient (green) and calculations of lifetime exercise (red). Estimated intensity (metabolic equivalents) from compendium of physical activities20 was used for defining vigorous exercise (>6 metabolic equivalents). MET indicates metabolic equivalents.
Patients with cumulative lifetime exercise > median were defined as active, and those with cumulative lifetime exercise ≤ median were defined as sedate. To address potential age confounding, we calculated the exercise habits by standardizing by the number of years over which the lifetime exercise hours accumulated in each patient, defined as standardized lifetime exercise (lifetime exercise/[52 weeks×age at diagniosis−7]). Patients exercising above median standardized lifetime exercise were defined as standardized active patients as opposed to standardized sedate patients.
We analyzed exercise irrespective of duration by averaging the intensity of each patient's main activities in the presumably healthy decade between 15 and 25 years of age. Patients with average intensities >6 metabolic equivalents were defined as vigorous exercisers.11 Patients with average intensities ≤6 metabolic equivalents were defined as non‐vigorous exercisers, similar to a previous definition.10
Echocardiography
We analyzed the echocardiographic study with good image quality closest to the time of diagnosis of lamin A/C genotype. Transthoracic echocardiography was performed using Vivid 7 or Vivid E9 (GE Healthcare, Horten, Norway) and data were analyzed off‐line (EchoPac; GE Healthcare).
LV end‐diastolic diameter were measured by M‐mode or 2‐dimensional imaging. LV end‐diastolic volume, end‐systolic volume, and LV ejection fraction (LVEF) were calculated by modified Simpson biplane method.22 Clinically significant impaired LV systolic function was defined as LVEF <45% in line with previous studies on lamin A/C patients.5 We defined DCM as LVEF <45%, or end‐diastolic diameter ≥60 mm (men) or 54 mm (women).2, 5 We performed LV strain analyses by 2‐dimensional speckle tracking, traced from the 3 apical views at a frame rate of >50 frames/s. Peak negative longitudinal strain was assessed in 16 LV segments and averaged to LV global longitudinal strain (GLS).23
Cardiac Magnetic Resonance
In a subset of patients without cardiac implantable electronic devices, we performed cardiac magnetic resonance (CMR) with late gadolinium enhancement, using 1.5 T clinical scanner (Magnetom Sonata or Magnetom Avanto Siemens, Erlangen, Germany) as previously described.24 We analyzed LV end‐diastolic and end‐systolic volumes and LVEF. Cardiac fibrosis was assessed by late gadolinium enhancement and analyzed and dichotomized as present or non‐present by an expert radiologist.
Conduction Disease and Arrhythmias
Twelve‐lead ECG was recorded at the time of study echocardiogram. We noted rhythm, PR‐interval, QRS duration, and presence of atrioventricular block. All patients underwent Holter monitoring. Arrhythmias were recorded from 12‐lead resting ECG, exercise ECG, Holter monitoring, and interrogation of implantable cardiac electronic devices (implantable cardioverter defibrillator or cardiac resynchronization therapy‐defibrillator).
Sustained ventricular arrhythmia was defined as aborted cardiac arrest or ventricular tachycardia with a frequency ≥120/min lasting >30 seconds or appropriate implantable cardioverter defibrillator therapy. Non‐sustained ventricular tachycardia was defined as ≥3 consecutive ventricular beats with a rate ≥120/min lasting <30 seconds.5
Skeletal Muscle Function
All patients were screened for skeletal muscle function, and patients with suspected skeletal muscle dysfunction were referred for neurological exam. Skeletal muscle dysfunction was noted as present in patients with symptoms of muscular weakness, with either positive findings on neurological exam, or pathological creatinine kinase.
Genetic and Laboratory Analyses
Genetic analyses were performed as part of the clinical evaluation as previously described.24 We grouped all patients by family, based on the pedigree of each proband. We measured creatinine kinase and NT‐proBNP (N‐terminal pro b‐type natriuretic peptide) at time of echocardiogram.
Statistical Analysis
Normally distributed data were presented as mean±SD, and categorical data as frequencies with percentages. Comparisons were performed using unpaired Student t test or by χ2, χ2 for trend or Fisher exact test, as appropriate. Data on exercise were not normally distributed and were presented as median (interquartile range) and compared by Mann–Whitney U test. Not normally distributed biomarkers were log‐transformed and presented as geometric mean (interquartile range). We used Spearman rho to explore the correlation between lifetime exercise and LV systolic function. Parameters which differed significantly (P<0.05) between active and sedate patients were adjusted for age at diagnosis and sex by multivariate logistic regression. Exercise was divided in tertiles to explore if a dose response relationship existed between lifetime exercise/standardized lifetime exercise and LV systolic function. Kaplan–Meier curves showed age‐related survival free from LVEF <45% (SPSS version 25, SPSS Inc Chicago, IL, USA). Subgroups of probands and family members were analyzed separately. Because of the dependence of the measurements of cardiac function within a family we performed mixed‐models linear regression with random family clustering (STATA version 15.0, StataCorp LLC 4905 Lakeway Drive College Station, TX 77845, USA). Two‐sided P<0.05 were considered significant.
Results
Study Patients
We conducted the exercise survey in March 2017 and approached 83 live lamin A/C genotype positive patients. Of these, 69 (83%) responded to the exercise survey and were included in the study (age 42±14 years, 46% women, 41% probands, and 27 families). Lamin A/C genotypes are summarized in Table S1.
At the time of study echocardiogram, 33 (48%) patients had overt DCM (Table 1) and 25 (36%) patients had LVEF <45%. The majority (67%) of the patients were treated with cardiac medication (Table S2). In all, 33 (48%) patients had an implantable cardiac electronic device, 15 (22%) had implantable cardioverter defibrillator and 18 (26%) had cardiac resynchronization therapy‐defibrillator. Paroxysmal or permanent atrial fibrillation (AF) was present in 36 (52%), non‐sustained ventricular tachycardia had occurred in 31 (45%), and 7 (10%) had experienced sustained ventricular arrhythmia. Fifteen (22%) of the patients had skeletal muscle dysfunction (Table 1).
Table 1.
Characteristics of 69 Lamin A/C Mutation Positive Patients Related to Lifetime Exercise
| Characteristics | Total (n=69) | Sedate Patients (n=35) | Active Patients (n=34) | P Value | OR | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Adjusted for age and sex | |||||||
| Clinical characteristics | |||||||
| Proband, n (%) | 28 (41) | 10 (29) | 18 (53) | 0.04 | 2.4 | 0.85–6.89 | 0.10 |
| Women, n (%) | 32 (46) | 17 (49) | 15 (44) | 0.71 | |||
| Proband | 12 (43) | 5 (50) | 7 (39) | 0.70 | |||
| Family member | 20 (48) | 12 (48) | 8 (50) | 0.90 | |||
| Age, y | 42±14 | 40±16 | 45±13 | 0.16 | |||
| Proband | 48±12 | 43±16 | 50±9 | 0.12 | |||
| Family member | 39±15 | 39±16 | 38±14 | 0.97 | |||
| Body mass index, kg/m2 | 25±5 | 25±6 | 26±4 | 0.30 | |||
| Proband | 26±4 | 26±6 | 26±4 | 0.82 | |||
| Family member | 25.0±4.7 | 24.2±5.1 | 26.1±3.9 | 0.20 | |||
| DCM, n (%) | 33 (48) | 11 (31) | 22 (65) | <0.01 | 3.7 | 1.3–10.4 | 0.01 |
| Proband | 19 (68) | 6 (60) | 13 (72) | 0.68 | |||
| Family member | 14 (34) | 5 (20) | 9 (56) | 0.02 | 7.1 | 1.5–34.6 | 0.02 |
| Skeletal muscle dysfunction, n (%) | 15 (22) | 7 (20) | 8 (24) | 0.70 | |||
| Proband | 10 (36) | 4 (40) | 6 (33) | 1.00 | |||
| Family member | 5 (12) | 3 (12) | 2 (13) | 1.00 | |||
| Electrophysiologic characteristics | |||||||
| PR interval*, ms | 222±76 | 201±76 | 245±72 | 0.06 | |||
| Proband | 271±94 | 140±13 | 300±76 | 0.02 | |||
| Family member | 205±63 | 207±77 | 201±19 | 0.79 | |||
| QRS width†, ms | 99±21 | 95±20 | 104±22 | 0.11 | |||
| Proband | 112±26 | 115±37 | 111±23 | 0.78 | |||
| Family member | 93±15 | 90±11 | 98±20 | 0.15 | |||
| Atrioventricular ‐block, n (%) | 43 (62) | 18 (51) | 25 (74) | 0.06 | |||
| Proband | 25 (89) | 8 (80) | 17 (94) | 0.53 | |||
| Family member | 18 (44) | 10 (40) | 8 (50) | 0.53 | |||
| Atrial fibrillation, n (%) | 36 (52) | 13 (37) | 23 (68) | 0.01 | 3.8 | 1.1–13.0 | 0.04 |
| Proband | 20 (71) | 7 (70) | 13 (72) | 1.00 | |||
| Family member | 16 (39) | 6 (24) | 10 (63) | 0.01 | 15.7 | 2.1–119.2 | <0.01 |
| ICD, n (%) | 15 (22) | 7 (20) | 8 (24) | 0.72 | |||
| Proband | 9 (32) | 3 (30) | 6 (33) | 1.00 | |||
| Family member | 6 (15) | 4 (16) | 2 (13) | 1.00 | |||
| CRT‐D, n (%) | 18 (26) | 4 (11) | 14 (41) | <0.01 | 5.1 | 1.3–20.0 | 0.02 |
| Proband | 11 (39) | 2 (20) | 9 (50) | 0.23 | |||
| Family member | 7 (17) | 2 (8) | 5 (31) | 0.09 | |||
| Sustained ventricular arrhythmia, n (%) | 7 (10) | 2 (6) | 5 (15) | 0.30 | |||
| Proband | 6 (21) | 2 (20) | 4 (22) | 1.00 | |||
| Family member | 1 (2.4) | 0 (0) | 1 (6.3) | 0.39 | |||
| NSVT, n (%) | 31 (45) | 12 (34) | 19 (56) | 0.07 | |||
| Proband | 17 (61) | 5 (50) | 12 (67) | 0.44 | |||
| Family member | 14 (34) | 7 (28) | 7 (44) | 0.30 | |||
| Laboratory characteristics | |||||||
| CK, microkatal/L | 2.1 (1.3–3.4) | 2.3 (1.5–3.8) | 1.9 (1.0–3.1) | 0.40 | |||
| Proband | 1.9 (1.0–4.3) | 2.2 (0.7–5.8) | 1.8 (1.0–3.7) | 0.67 | |||
| Family member | 2.2 (1.7–2.9) | 2.4 (1.6–3.1) | 2.0 (1.7–2.4) | 0.42 | |||
| NT‐proBNP, pmol/L | 51 (18–164) | 30 (13–64) | 78 (32–219) | 0.03 | 1.06‡ | 0.95–1.18 | 0.31 |
| Proband | 108 (44–281) | 71 (27–251) | 134 (47–403) | 0.32 | |||
| Family member | 26 (8–64) | 18 (8–52) | 38 (8–121) | 0.17 | |||
| Echocardiographic findings | |||||||
| Heart rate, beats/min | 66±14 | 69±16 | 62±11 | 0.07 | |||
| Proband | 62±16 | 68±25 | 59±9 | 0.21 | |||
| Family member | 68±13 | 69±14 | 66±12 | 0.43 | |||
| EDV, mL | 122±40 | 111±25 | 133±49 | 0.02 | 1.02 | 0.99–1.04 | 0.06 |
| Proband | 133±52 | 115±27 | 142±60 | 0.20 | |||
| Family member | 115±28 | 110±25 | 123±31 | 0.13 | |||
| ESV, mL | 67±34 | 55±22 | 79±39 | <0.01 | 1.03 | 1.01–1.1 | 0.02 |
| Proband | 81±40 | 67±28 | 88±43 | 0.19 | |||
| Family member | 57±25 | 50±17 | 68±31 | 0.03 | 1.1 | 1.01–1.13 | 0.03 |
| EDD, mm | 54±7 | 52±7 | 57±6 | 0.07 | 1.1 | 1.01–1.2 | 0.03 |
| Proband | 56±7 | 52±6 | 59±7 | 0.01 | 1.22 | 1.002–1.5 | 0.04 |
| Family member | 53±6 | 52±7 | 54±5 | 0.4 | |||
| LVEF, % | 47±13 | 51±11 | 43±13 | <0.01 | 0.95 | 0.90–0.99 | 0.02 |
| Proband | 40±13 | 41±14 | 39±12 | 0.69 | |||
| Family member | 52±11 | 55±7 | 47±13 | 0.01 | 0.88 | 0.79–0.92 | 0.02 |
| GLS, % | −16.5±6.7 | −18.2±5.8 | −15.0±7.2 | 0.04 | 1.1 | 0.98–1.2 | 0.11 |
| Proband | −14.4±7.0 | −13.4±9.2 | −15.0±6.0 | 0.61 | |||
| Family member | −18.0±6.1 | −20.0±2.5 | −15.0±9.0 | 0.01 | 1.5 | 1.06–2.1 | 0.02 |
Values are mean±SD, frequency (%), or geometric mean (interquartile range). P values are calculated by Student t‐test, Chi square test, or Fisher exact test as appropriate. BMI indicates body mass index; CK, creatinine kinase; CRT‐D, cardiac resynchronization therapy‐defibrillator; DCM, dilated cardiomyopathy; EDD, end diastolic diameter; EDV, end diastolic volume; ESV, end systolic volume; GLS, global longitudinal strain; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NSVT, non‐sustained ventricular tachycardia; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide.
*n=43, †n=51, ‡odds for 50 increments increase.
Relationship Between Exercise, Clinical Characteristics, and Cardiac Function
Median lifetime exercise was 4160 (interquartile range 1041–6924) hours, and median standardized lifetime exercise was 2.2 hours of exercise per week. In total, 34 patients were by definition active with accumulated lifetime exercise equivalent to 4.5 hours of exercise per week, and 35 patients were sedate with equivalents of 0.8 exercise hours per week. None of our patients performed professional sports and only 2 patients exercised >10 hours per week. There were no differences in age, sex or skeletal muscle function between the active and sedate patients (Table 1).
Active patients were more likely to be probands, had lower LVEF and more frequently DCM compared with sedate patients (Table 1). Exercise duration was associated with lower LVEF and DCM, also when adjusted for age and sex (both P<0.05) (Table 1). Impaired LV systolic function in active patients was further supported by worse GLS and higher NT‐proBNP levels compared with the sedate patients (Table 1). Similarly, using standardized lifetime exercise supported the finding with worse cardiac function in standardized active versus sedate patients (LVEF 42±14% versus 52±14%, P=0.003, and GLS −15±8% versus −18±5%, P=0.04). LVEF decreased with 4% per tertile increment in lifetime exercise (adjusted LVEF −4% 95% CI [−7% to −0.4%, P=0.02]), when adjusted for age at diagnosis, sex, and accounting for dependence within families. Similarly, LVEF decreased by 4% 95% CI (−7% to −1%, P=0.01) using tertiles of standardized lifetime exercise (Figure 2A and 2C).
Figure 2.
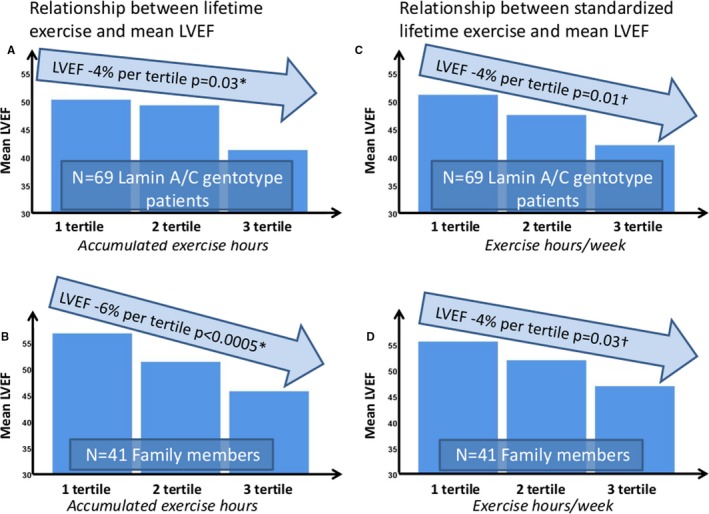
Values in light blue arrows are regression coefficients with P values calculated by linear mixed model regression with random family clustering effects. Left panels: Bar charts displaying mean LVEF related to increasing tertiles of lifetime exercise in 69 lamin A/C genotype positive patients (A), and in 41 lamin A/C genotype positive family members (B). Right panels: Bar charts displaying mean LVEF related to increasing tertiles of standardized lifetime exercise in 69 lamin A/C genotype positive patients (C), and in 41 lamin A/C genotype positive family members (D). *Adjusted age at diagnosis and sex. †Adjusted for sex. LVEF indicates left ventricular ejection fraction.
Active patients were younger when diagnosed with LVEF <45% (Log rank P=0.007) (Figure 3A) compared with sedate. There was a significant linear trend between increase in lifetime exercise/standardized lifetime exercise in tertiles and LVEF <45% (Figure S1A and S1C). Furthermore, patients with LVEF <45% had accumulated more hours of exercise (Table 2).
Figure 3.
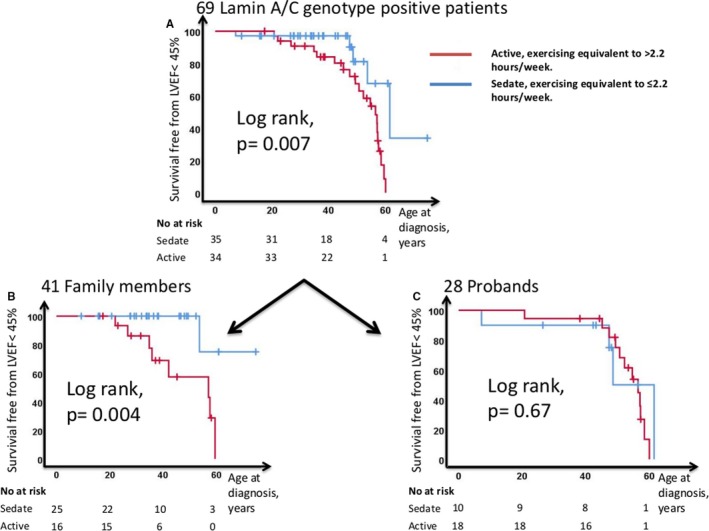
Kaplan–Meier plots displaying age‐related survival free from LVEF <45% in sedate (blue line) and active (red line) lamin A/C patients (n=69. A). B, The subgroup of 41 family members (n=41). C, The subgroup of 28 probands (n=28). LVEF indicates left ventricular ejection fraction.
Table 2.
Distribution of Exercise Parameters for Patients With LVEF Above and Below 45%
| Total (n=69) | LVEF ≥45% (n=44) | LVEF <45% (n=25) | P Value | |
|---|---|---|---|---|
| Age, y | 42±14 | 40±14 | 47±14 | 0.06 |
| Exercise data | ||||
| Lifetime exercise, h | 4160 (1041–6924) | 1845 (756–5225) | 6630 (4826–9851) | <0.01 |
| Probands lifetime exercise, h | 5276 (733–10 184) | 3660 (670–7500) | 6431 (1792–10 888) | 0.19 |
| Family members lifetime exercise, h | 2122 (1132–6619) | 1732 (860–4700) | 6630 (4945–7911) | <0.01 |
Values are mean±SD, median (interquartile range). P values are calculated by Student t‐test, or Mann–Whitney U test. LVEF indicates left ventricular ejection fraction.
By using continuous variables for lifetime exercise and cardiac function, we observed a moderate correlation between lifetime exercise and cardiac function (LVEF: ρ=−0.28, P=0.02 and GLS: ρ=0.30, P=0.01).
Because of a high frequency of implantable electronic cardiac devices, CMR was performed in only a subset of patients (n=21), 0.6 (interquartile range 1.5–4.5) months after the study echocardiogram. LVEF by CMR was reduced in the active compared with the sedate patients (Table 3).
Table 3.
CMR Characteristics of 21 Lamin A/C Genotype Positive Patients
| CMR Characteristics, (n) | Total (n=21) | Sedate (n=10) | Active (n=11) | P Value |
|---|---|---|---|---|
| Proband, n (%) | 4 (19) | 0 (0) | 4 (36) | |
| Family Member, n (%) | 17 (80) | 10 (100) | 7 (64) | |
| Age at CMR, y | 38±11 | 37±12 | 39±11 | 0.70 |
| Family member | 36±11 | 37±13 | 36±8 | 0.90 |
| Women, n (%) | 12 (57) | 5 (50) | 7 (64) | 0.67 |
| Family member | 10 (59) | 5 (50) | 5 (71) | 0.62 |
| EDV, mL | 176±41 | 163±30 | 188±47 | 0.16 |
| Family member | 165±30 | 163±30 | 169±26 | 0.67 |
| ESV, mL | 81±28 | 68±16 | 92±31 | 0.03 |
| Family member | 73±19 | 68±16 | 80±22 | 0.17 |
| LVEF, % | 55±7 | 59±4 | 52±7 | 0.01 |
| Family member | 56±6 | 59±4 | 53±7 | 0.04 |
| LGE*, n (%) | 12 (63) | 5 (56) | 7 (70) | 0.65 |
| Family member | 9 (56) | 5 (56) | 4 (57) | 1.00 |
Values are mean±SD, frequency (%). P values are calculated by Student t‐test, Chi square test, or Fisher exact test as appropriate. CMR indicates cardiac magnetic resonance; EDV, end diastolic volume; ESV, end systolic volume; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
n=19.
Relationship Between Exercise, Conduction Abnormalities, and Arrhythmias
Because of high frequencies of AF and total atrioventricular block, PR‐interval was available only in 43 (62%), and QRS duration in 51 (74%) patients. We observed no statistically significant differences in PR‐interval, QRS duration, or occurrence of atrioventricular block between the active and sedate patients in this limited cohort (Table 1), nor when using standardized exercise categories (data not shown). The active patients had more frequently cardiac resynchronization therapy‐defibrillator and AF independently of age and sex (Table 1). History of sustained ventricular arrhythmia or non‐sustained ventricular tachycardia did not differ between active and sedate patients.
Exercise and Cardiac Phenotype in Subgroup Analyses of Family Members
The probands were older than family members at study inclusion and therefore, as expected, had more severe disease (Table S3). Furthermore, there were more probands in the active group. To avoid that age‐related disease progression confounded our results, we analyzed the 41 family members separately (16 active and 25 sedate). Importantly, age did not differ between active and sedate family members (Table 1). Active family members had reduced LV systolic function by LVEF (echocardiography and CMR), and worse GLS (Tables 1 and 3). This was also evident when using standardized lifetime exercise (LVEF 47±12% versus 55±7%, P=0.02, CMR LVEF 52±7% versus 58±4%, P=0.04, GLS −16±8% versus −20±2%, P=0.02). Importantly, LVEF decreased by 6% per tertile increment in lifetime exercise (adjusted LVEF −6% 95% CI [−10% to −3%, P<0.0005]) when adjusted for age at diagnosis and sex and accounting for relatedness within families. Similarly, with tertiles of standardized lifetime exercise (adjusted LVEF −4% 95% CI [−7% to −0.3%, P=0.03]) (Figure 2B and 2D).
Active family members were younger when diagnosed with LVEF <45% (log rank P=0.004) (Figure 3B). Lifetime exercise showed a dose‐response relationship with LV systolic function. None of the family members in the lowest exercise tertile had LVEF <45%, while almost half of family members in the highest exercise tertile had LVEF <45% (Figure S1B).
We observed moderate and significant correlations between lifetime exercise and cardiac function (LVEF ρ=−0.35, P=0.03, GLS ρ=0.44, P=0.004).
Active family members had more frequently AF compared with the sedate, also when adjusted for age and sex (Table 1). Family members with a history of vigorous exercise had lower LVEF (echocardiographic LVEF 46% versus 54%, P=0.03, CMR LVEF 52±8% versus 58±4%, P=0.04) compared with family members with non‐vigorous exercise.
In the subgroup of probands, differences between active and sedate were less clear (Tables 1 and 3; Figure 3C).
Discussion
This study showed that exercise was associated with worse LV systolic function and higher prevalence of AF in lamin A/C genotype positive patients. Active patients had worse cardiac function independent of age and sex, indicating a harmful effect of exercise not explained by age‐related disease progression. Furthermore, our results indicated that recreational exercise may be sufficient to negatively affect disease progression.
Atrioventricular block and ventricular arrhythmias were not clearly associated with lifetime exercise, but these results must be interpreted with care because of a limited number of observations.
Exercise and Cardiac Function
LV systolic function was impaired in active patients, independently of age and sex. LV systolic function was assessed by multi‐modality imaging (echocardiography and CMR) and results were supported by biomarkers showing higher NT‐proBNP in active patients.
We used 3 different exercise parameters including lifetime exercise, standardized exercise by age and vigorous exercise. By all parameters, we demonstrated a relationship between impaired cardiac function and exercise. Our study included non‐competitive sports indicating that also recreational exercise may have an impact on cardiac function. In summary, our results support previous reports by Pasotti et al10 and indicate a dose‐response relationship of exercise on LV systolic function in lamin A/C disease that has not been shown previously.
The differences between active and sedate were most prominent in the younger family members representing individuals identified by family genetic screening and not necessarily symptomatic. The active family members were younger at DCM diagnosis and showed a clear dose response relationship between LV systolic function and exercise (Figure 2B and 2D). The greater exercise effect observed in family members compared with probands is possibly explained by their younger age and a wider spectrum of cardiac function. In all, our findings indicated that exercise aggravates cardiac function and leads to earlier DCM penetrance in lamin A/C disease.
Active patients were more frequently probands. Probands were older than the family members which made them likely to have more advanced disease, and more likely to have accumulated more hours of exercise. In probands, LVEF was low in both the active and sedate and the majority presented an end stage phenotype. The high prevalence of severely depressed LVEF in probands limited the interpretation of exercise effect on their LV systolic function.
Exercise has beneficial and anti‐remodeling effects and can be performed safely in the majority of heart failure patients.25, 26 In specific cardiomyopathies however, including lamin A/C disease, the impact of exercise may be more complex.10, 12, 27, 28 The role of lamin A/C mutations in initiating cardiomyopathy is unknown. We can speculate that lamin A/C mutations may result in maladaptive gene transcription in response to mechanical strain on the cell29 which may provide the link to exercise induced maladaptive cardiac remodeling in lamin A/C.
Active patients had larger cardiac dimensions compared with sedate patients and in probands cardiac dimensions exceeded those associated with athlete's heart.30, 31 Our patients mostly performed recreational sports and did not perform exercise at a level associated with cardiac morphological changes.32, 33, 34, 35, 36 We therefore believe that enlarged cardiac dimensions in our active patients were results of pathological DCM development, possibly aggravated by exercise, and not a physiologic consequence of athletic activity.
Exercise, Conduction Disease, and Arrhythmias
The active patients had more AF, independently of age both in the total group and in the subgroup of family members, while there were no differences in the subgroup of probands. The high prevalence of age‐related AF in probands probably limited the interpretation of the exercise effect on AF in this subgroup.
We found no significant association between exercise and atrioventricular block or ventricular arrhythmia. The study by Pasotti et al10 showed an association between former competitive athletes and ventricular arrhythmias in the lamin A/C population. Our data should be interpreted with care because of the relatively low sample size, and possible differences in age and medical treatment of the patients compared with the patients studied by Pasotti et al. Furthermore, higher exercise intensities or durations may be required to increase the risk of ventricular arrhythmias than those investigated in our study.
Exercise and Skeletal Muscle Affection
We found no clear association between exercise and skeletal muscle disease or creatinine kinase‐levels. One might speculate that skeletal muscle function may be less affected by exercise, in line with experimental studies.17 Importantly, our findings indicated that skeletal muscle disease did not limit exercise in our patients with about 80% of patients with normal skeletal function; these numbers are in line with previous studies including patients from cardiology departments.5
Clinical Implications
Our findings indicated that patients with lamin A/C genotype should avoid engaging in long‐term and/or vigorous exercise. Our data did not identify potential tolerable exercise thresholds but indicated a dose response relationship and that even recreational, non‐competitive sports may have an impact on cardiac function and cardiac disease progression. Because of the known health benefits of an active lifestyle, it may be reasonable to recommend avoidance of vigorous sports until more data are available. Further studies are needed to indicate safe exercise levels in patients with lamin A/C.
Limitations
Our study was a cross sectional single‐center study with assessment of exercise retrospectively which may limit external validity. We cannot exclude potential residual confounding. Only exercise habits before diagnosis of lamin A/C were investigated in this study. Therefore, we cannot conclude about exercise restriction following genetic diagnosis.
Quantification of self‐reported activity may be subject to recall bias. Possible patient knowledge about the adverse effect of competitive sports before study entry may have influenced their self‐reporting of lifetime exercise. However, it is difficult to conclude whether this information lead to under‐ or over‐reporting. More objective metrics for activity, ie accelerometry, would increase the validity of our findings and should be addressed in prospective studies.
Lifetime exercise hours are age‐dependent which could influence our data. To overcome this limitation, we standardized exercise data for age, and we used statistical age adjustments. Ideally, a study should randomize patients to exercise, which is not feasible for ethical and practical reasons. In addition, separate analyses of the younger family members limited the confounding effect of age‐related penetrance. Furthermore, we used the age independent exercise variable assessing history of vigorous exercise in the decade between age 15 to 25 years, which supported our results and was in accordance with the previous study of Pasotti et al.10
Members within families are likely to be correlated with each other. Dependence in data attributable to patient relatedness was handled by mixed effects regression models allowing family clustering.
Conclusions
Longer lifetime exercise duration was a marker of impaired LV systolic function by echocardiography, CMR, and biomarkers in patients with lamin A/C genotype, independent of age and sex. DCM occurred at younger age in active patients and exercise hours correlated with impaired LV systolic function. These results were most evident in the younger subgroup of genotype positive family members presenting with non‐complete penetrance. Exercise was associated with AF, while the associations with atrioventricular block and ventricular arrhythmias were marginal. Our findings may improve exercise recommendations in patients with lamin A/C.
Sources of Funding
This work was supported by the Norwegian Research Council (203489/030).
Disclosures
None.
Supporting information
Table S1. Lamin A/C Genotypes in Sedate and Active Patients
Table S2. Medication in 69 Lamin A/C Genotype Positive Patients
Table S3. Baseline Characteristics in 41 Lamin A/C Family Members and 28 Lamin A/C Probands
Figure S1. Bar charts showing distribution of LVEF below 45% (red bars) in increasing tertiles of exercise.
(J Am Heart Assoc. 2020;9:e012937 DOI: 10.1161/JAHA.119.012937.)
References
- 1. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze‐Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace. 2011;13:1077–1109. [DOI] [PubMed] [Google Scholar]
- 2. Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, Edvardsen T, Haugaa KH. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacoby D, McKenna WJ. Genetics of inherited cardiomyopathy. Eur Heart J. 2012;33:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arbustini E, Pilotto A, Repetto A, Grasso M, Negri A, Diegoli M, Campana C, Scelsi L, Baldini E, Gavazzi A, Tavazzi L. Autosomal dominant dilated cardiomyopathy with atrioventricular block: a lamin A/C defect‐related disease. J Am Coll Cardiol. 2002;39:981–990. [DOI] [PubMed] [Google Scholar]
- 5. van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans‐van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP, Pilotto A, Pasotti M, Jenkins S, Rowland C, Aslam U, Wilde AA, Perrot A, Pankuweit S, Zwinderman AH, Charron P, Pinto YM. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59:493–500. [DOI] [PubMed] [Google Scholar]
- 6. Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med. 2006;354:209–210. [DOI] [PubMed] [Google Scholar]
- 7. Sen‐Chowdhry S, McKenna WJ. Sudden death from genetic and acquired cardiomyopathies. Circulation. 2012;125:1563–1576. [DOI] [PubMed] [Google Scholar]
- 8. van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, Yaou RB, Duboc D, Rossenbacker T, Heidbuchel H, de Visser M, Crijns HJ, Pinto YM. Meta‐analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med (Berl). 2005;83:79–83. [DOI] [PubMed] [Google Scholar]
- 9. Hookana E, Junttila MJ, Sarkioja T, Sormunen R, Niemela M, Raatikainen MJ, Uusimaa P, Lizotte E, Peuhkurinen K, Brugada R, Huikuri HV. Cardiac arrest and left ventricular fibrosis in a Finnish family with the lamin A/C mutation. J Cardiovasc Electrophysiol. 2008;19:743–747. [DOI] [PubMed] [Google Scholar]
- 10. Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, Mannarino S, Gambarin F, Favalli V, Grasso M, Agozzino M, Campana C, Gavazzi A, Febo O, Marini M, Landolina M, Mortara A, Piccolo G, Vigano M, Tavazzi L, Arbustini E. Long‐term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–1260. [DOI] [PubMed] [Google Scholar]
- 11. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. [DOI] [PubMed] [Google Scholar]
- 12. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail. 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. [DOI] [PubMed] [Google Scholar]
- 14. Ruwald AC, Marcus F, Estes NA III, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier MC, Mustafic H, Toussaint JF, Desnos M, Rieu M, Benameur N, Le Heuzey JY, Empana JP, Jouven X. Sports‐related sudden death in the general population. Circulation. 2011;124:672–681. [DOI] [PubMed] [Google Scholar]
- 16. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. [DOI] [PubMed] [Google Scholar]
- 17. Cattin ME, Ferry A, Vignaud A, Mougenot N, Jacquet A, Wahbi K, Bertrand AT, Bonne G. Mutation in lamin A/C sensitizes the myocardium to exercise‐induced mechanical stress but has no effect on skeletal muscles in mouse. Neuromuscul Disord. 2016;26:490–499. [DOI] [PubMed] [Google Scholar]
- 18. Dejgaard LA, Haland TF, Lie OH, Ribe M, Bjune T, Leren IS, Berge KE, Edvardsen T, Haugaa KH. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2018;250:157–163. [DOI] [PubMed] [Google Scholar]
- 19. Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol (1985). 2005;99:1112–1119. [DOI] [PubMed] [Google Scholar]
- 20. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 21. Landry CH, Allan KS, Connelly KA, Cunningham K, Morrison LJ, Dorian P. Sudden cardiac arrest during participation in competitive sports. N Engl J Med. 2017;377:1943–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 23. Edvardsen T, Haugaa KH. Imaging assessment of ventricular mechanics. Heart. 2011;97:1349–1356. [DOI] [PubMed] [Google Scholar]
- 24. Hasselberg NE, Edvardsen T, Petri H, Berge KE, Leren TP, Bundgaard H, Haugaa KH. Risk prediction of ventricular arrhythmias and myocardial function in lamin A/C mutation positive subjects. Europace. 2014;16:563–571. [DOI] [PubMed] [Google Scholar]
- 25. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise‐based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lie OH, Dejgaard LA, Saberniak J, Rootwelt C, Stokke MK, Edvardsen T, Haugaa KH. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–753. [DOI] [PubMed] [Google Scholar]
- 28. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age‐related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog Biophys Mol Biol. 2014;115:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. [DOI] [PubMed] [Google Scholar]
- 31. Caselli S, Montesanti D, Autore C, Di Paolo FM, Pisicchio C, Squeo MR, Musumeci B, Spataro A, Pandian NG, Pelliccia A. Patterns of left ventricular longitudinal strain and strain rate in Olympic athletes. J Am Soc Echocardiogr. 2015;28:245–253. [DOI] [PubMed] [Google Scholar]
- 32. Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130:23–31. [DOI] [PubMed] [Google Scholar]
- 33. Abergel E, Chatellier G, Hagege AA, Oblak A, Linhart A, Ducardonnet A, Menard J. Serial left ventricular adaptations in world‐class professional cyclists: implications for disease screening and follow‐up. J Am Coll Cardiol. 2004;44:144–149. [DOI] [PubMed] [Google Scholar]
- 34. Caselli S, Di Paolo FM, Pisicchio C, Di Pietro R, Quattrini FM, Di Giacinto B, Culasso F, Pelliccia A. Three‐dimensional echocardiographic characterization of left ventricular remodeling in Olympic athletes. Am J Cardiol. 2011;108:141–147. [DOI] [PubMed] [Google Scholar]
- 35. Spirito P, Pelliccia A, Proschan MA, Granata M, Spataro A, Bellone P, Caselli G, Biffi A, Vecchio C, Maron BJ. Morphology of the “athlete's heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol. 1994;74:802–806. [DOI] [PubMed] [Google Scholar]
- 36. Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol. 1986;7:190–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Lamin A/C Genotypes in Sedate and Active Patients
Table S2. Medication in 69 Lamin A/C Genotype Positive Patients
Table S3. Baseline Characteristics in 41 Lamin A/C Family Members and 28 Lamin A/C Probands
Figure S1. Bar charts showing distribution of LVEF below 45% (red bars) in increasing tertiles of exercise.
Data Availability Statement
The authors do not have the authority to share the data used in the analyses described in this article. The approval of the Regional Committee for Medical Research Ethics limits sharing data with researchers outside of Norway for purposes of reproducing the results or replicating the procedures. The data can only be made available to any additional researchers if a formal request is filled with the Regional Committee for Medical Research Ethics and explicit consent is given from every study subject.


