Abstract
Background
We examined the longitudinal associations between changes in cardiovascular biomarkers and cancer therapy–related cardiac dysfunction (CTRCD) in patients with breast cancer treated with cardotoxic cancer therapy.
Methods and Results
Repeated measures of high‐sensitivity cardiac troponin T (hs‐cTnT), NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), myeloperoxidase, placental growth factor, and growth differentiation factor 15 were assessed longitudinally in a prospective cohort of 323 patients treated with anthracyclines and/or trastuzumab followed over a maximum of 3.7 years with serial echocardiograms. CTRCD was defined as a ≥10% decline in left ventricular ejection fraction to a value <50%. Associations between changes in biomarkers and left ventricular ejection fraction were evaluated in repeated‐measures linear regression models. Cox regression models assessed the associations between biomarkers and CTRCD. Early increases in all biomarkers occurred with anthracycline‐based regimens. hs‐cTnT levels >14 ng/L at anthracycline completion were associated with a 2‐fold increased CTRCD risk (hazard ratio, 2.01; 95% CI, 1.00–4.06). There was a modest association between changes in NT‐proBNP and left ventricular ejection fraction in the overall cohort; this was most pronounced with sequential anthracycline and trastuzumab (1.1% left ventricular ejection fraction decline [95% CI, −1.8 to –0.4] with each NT‐proBNP doubling). Increases in NT‐proBNP were also associated with CTRCD (hazard ratio per doubling, 1.56; 95% CI, 1.32–1.84). Increases in myeloperoxidase were associated with CTRCD in patients who received sequential anthracycline and trastuzumab (hazard ratio per doubling, 1.28; 95% CI, 1.04–1.58).
Conclusions
Cardiovascular biomarkers may play an important role in CTRCD risk prediction in patients with breast cancer who receive cardiotoxic cancer therapy, particularly in those treated with sequential anthracycline and trastuzumab therapy.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01173341.
Keywords: biomarker, cardiomyopathy, cardio‐oncology, cardiotoxicity
Subject Categories: Infectious Endocarditis, Inflammatory Heart Disease, Heart Failure
Clinical Perspective
What Is New?
This is the largest prospective cohort study of patients with breast cancer treated with anthracyclines and/or trastuzumab with detailed cardiovascular phenotyping, including biomarkers, echocardiography, and clinical data, over an extended follow‐up.
High‐sensitivity cardiac troponin T elevation is common following anthracycline therapy, and its assessment specifically at the completion of anthracyclines may be informative in cancer therapy–related cardiac dysfunction risk prediction.
Changes in N‐terminal pro‐B‐type natriuretic peptide show temporal associations with changes in imaging measures of cardiac dysfunction and predict the risk of cancer therapy–related cardiac dysfunction, particularly in patients treated with sequential anthracycline and trastuzumab therapy, and myeloperoxidase shows promise as a candidate novel biomarker of cardiotoxicity with anthracycline‐based cancer therapy regimens.
What Are the Clinical Implications?
Biomarkers can play an important role in cancer therapy–related cardiac dysfunction risk prediction in patients with breast cancer who receive cardiotoxic cancer therapy.
The findings of the study motivate randomized controlled trials aimed at investigating whether biomarker‐guided strategies are of utility in mitigating cardiovascular risk.
Breast cancer is the most common cancer in women. Although marked improvements in the detection and treatment of breast cancer have led to substantial gains in disease‐specific survival, cardiovascular morbidity and mortality associated with commonly used cardiotoxic cancer therapies remain a significant concern. Declines in cardiac function with agents such as anthracyclines and trastuzumab can result in treatment interruptions and delays in the short term. In the long term, the risk of cardiovascular mortality may exceed that of recurrent cancer, particularly in older women.1
Within cardio‐oncology, there has been a focus on the early detection of cancer therapy–related cardiac dysfunction (CTRCD) to avoid treatment delays and interruptions and decrease long‐term cardiovascular risk.2, 3 Current guidelines recommend routine cardiac surveillance in patients with breast cancer treated with anthracyclines and/or trastuzumab.4, 5, 6 Because of widespread availability, echocardiographic assessment of left ventricular ejection fraction (LVEF) is a recommended monitoring strategy. However, LVEF assessment is limited in the sensitive detection of early, subclinical changes in cardiac function.
Cardiac biomarkers are promising tools for the early detection and prediction of CTRCD.7, 8, 9 Some prior studies have suggested that elevations in cardiac troponins (cTns) are common in patients treated with anthracyclines, with or without trastuzumab, and that elevations predict the development of cardiac dysfunction.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Our own published data have supported an association between troponin concentrations at the completion of anthracyclines and CTRCD but no association with serial troponin assessment at postanthracycline time points.16, 17 However, the reproducibility of these findings and a clear characterization of the utility of troponin assessment for the prediction of CTRCD are lacking. In addition, unanswered questions pertaining to the potential predictive value of natriuretic peptides and several newer biomarkers including myeloperoxidase, placental growth factor (PIGF), and growth differentiation factor 15 (GDF‐15) also exist.16, 17, 18, 19, 20, 21
To better characterize the role of biomarkers in patients with breast cancer who receive potentially cardiotoxic therapies, we comprehensively examined the longitudinal associations between changes in multiple cardiovascular biomarkers, including high‐sensitivity cTn T (hs‐cTnT), NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), myeloperoxidase, PIGF, and GDF‐15, and changes in LVEF and the risk of CTRCD in 323 patients with breast cancer treated with anthracyclines and/or trastuzumab, and determined differences according to cancer therapy regimen. The specific study objectives were to: (1) characterize the changes in multiple cardiovascular biomarkers in patients with breast cancer treated with anthracyclines and/or trastuzumab; (2) evaluate the longitudinal associations between changes in individual biomarkers and LVEF changes; (3) evaluate the associations between changes in biomarkers and the risk of CTRCD; (4) explore whether these associations differ according to cancer therapy regimen; and (5) describe the potential predictive value of these biomarkers.
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The CCT (Cardiotoxicity of Cancer Therapy) study is a prospective cohort of patients with breast cancer from the Rena Rowan Breast Center of the Abramson Cancer Center at the University of Pennsylvania (Philadelphia, Pennsylvania). Patients aged 18 years and older with breast cancer who were treated with anthracycline‐ and/or trastuzumab‐based regimens were included. Exclusion criteria included pregnancy or an inability or unwillingness to provide informed consent. Treatment regimen was determined by the treating oncologist and consisted of either doxorubicin (240 mg/m2 divided into 4 cycles of 60 mg/m2 each) and cyclophosphamide followed by paclitaxel (doxorubicin group), doxorubicin (240 mg/m2 divided into 4 cycles of 60 mg/m2 each) and cyclophosphamide followed by paclitaxel and trastuzumab (doxorubicin+trastuzumab group), or trastuzumab with docetaxel and either cyclophosphamide or carboplatin (trastuzumab group). The study was approved by the institutional review board of the University of Pennsylvania. All participants provided written informed consent. B.D. and B.K. had full access to all of the data in the study and take responsibility for their integrity and the data analysis.
Study Procedures
Detailed clinical data were collected using standardized questionnaires at baseline (ie, before treatment initiation) and during prespecified follow‐up visits, and were further verified by reviewing medical records. Blood samples were drawn at standardized time intervals according to cancer therapy regimen (Figure 1).22 In the doxorubicin group, blood samples were collected at baseline, during doxorubicin treatment (≈1 month), at the completion of doxorubicin (≈2 months), at the completion of paclitaxel (≈4 months), and then annually. In the doxorubicin+trastuzumab group, blood samples were collected at baseline, during doxorubicin treatment (≈1 month), at the completion of doxorubicin (≈2 months), every 6 weeks during trastuzumab therapy, and then annually. The median (interquartile range) interval between the completion of doxorubicin and initiation of trastuzumab therapy was 14 (14–15) days. In the trastuzumab group, blood samples were collected at baseline, every 6 weeks during trastuzumab therapy, and then annually. Blood samples were processed and stored at −80°C until assay. In addition, in the doxorubicin group, transthoracic echocardiograms were performed at baseline, at the completion of paclitaxel (≈4 months), and then annually. In the doxorubicin+trastuzumab group, echocardiograms were performed at baseline, at the completion of doxorubicin (≈2 months), every 3 months during trastuzumab therapy, and then annually. In the trastuzumab group, echocardiograms were performed at baseline, every 3 months during trastuzumab therapy, and then annually. Two‐dimensional images were acquired using Vivid 7, E9, or E95 machines (GE Healthcare) and digitally archived for post hoc analyses.
Figure 1.
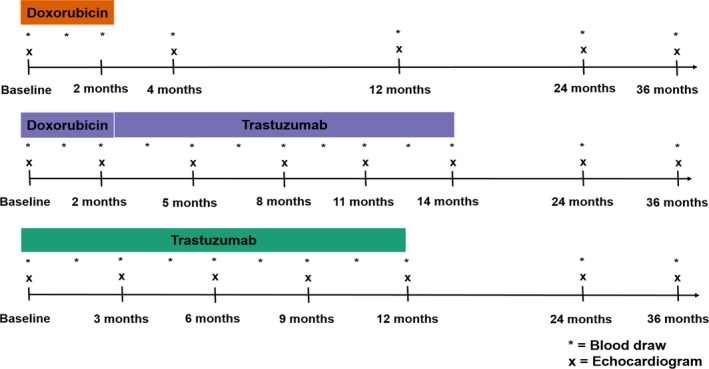
Study protocol. Timeline of blood draws and echocardiography assessment according to cancer therapy regimen.
Biomarker Measurements
Four biomarkers including hs‐cTnT, NT‐proBNP, PIGF, and GDF‐15 were assessed in all treatment groups. hs‐cTnT was measured using the fourth‐generation Elecsys TnT‐hs assay on the Cobas platform (Roche Diagnostics). NT‐proBNP was measured using the Elecsys NT‐proBNP assay on the Cobas platform. PIGF was measured using the Elecsys PIGF immunoassay. GDF‐15 was measured with the Elecsys GDF‐15 immunoassay. Myeloperoxidase was measured only in the doxorubicin and doxorubicin+trastuzumab groups, given its purported biologic relevance to anthracyclines specifically, using an ELISA assay (Cleveland HeartLab Inc). Detailed assay information is provided in Data S1.
Echocardiography Quantitation and CTRCD Definition
Quantitative echocardiography was performed by a single blinded observer at the University of Pennsylvania Center for Quantitative Echocardiography (Philadelphia, PA) using the TomTec Imaging Systems platform. Left ventricular end‐diastolic and end‐systolic volumes were calculated using the Simpson's method of discs and were utilized to derive LVEF. In addition, longitudinal and circumferential strain were analyzed. Intraobserver coefficients of variation were 4.9%, 10.9%, and 9.4% for LVEF, longitudinal strain, and circumferential strain, respectively.23 CTRCD was defined as a ≥10% absolute decline in LVEF to a value <50%.23, 24
Statistical Analysis
Baseline characteristics were summarized using proportions for categorical variables, and median (interquartile range) were presented for continuous variables. Given the skewed distributions, all biomarkers were log2 transformed. This also facilitated the comparison of the associations across all biomarkers. On the log2 scale, each 1‐unit increase is equivalent to a doubling in biomarker levels.
To characterize the changes in biomarker levels according to individual treatment groups, mean estimated changes from baseline were plotted over time. Mean changes were determined using repeated‐measures linear regression estimated via generalized estimating equations. Each model was adjusted for the baseline values of the biomarker under consideration and time since treatment initiation modeled nonparametrically using a cubic spline.
Contemporaneous associations between changes in biomarkers from baseline and changes in LVEF were determined using repeated‐measures linear regression estimated via generalized estimating equations. Here, we used the independence working correlation structure. All models were adjusted for cancer therapy regimen, baseline LVEF, baseline biomarker value, time from treatment initiation (modeled nonparametrically using a cubic spline, whereby its effect was allowed to vary across treatment groups by including an interaction term), age, hypertension, smoking, and body mass index. Associations between changes from baseline in biomarker levels over time and subsequent changes in LVEF and longitudinal and circumferential strain were assessed similarly. Differences in the associations between changes in biomarkers and LVEF across the different treatment groups were evaluated by including biomarker‐treatment interaction terms. Wald test was used to test statistical significance.
Associations between baseline biomarker values and time to CTRCD were assessed using Cox proportional hazards models. Longitudinal associations between repeated assessments of changes in biomarkers from baseline and time to CTRCD were determined using partly conditional survival models.25 This approach provides a flexible, computationally efficient framework for longitudinal risk prediction and is well suited for analyses of relatively small sample sizes.26 All models were adjusted for cancer therapy regimen, baseline LVEF, baseline biomarker value, age, hypertension, smoking, and body mass index. The proportional hazards assumption was evaluated for both the baseline biomarker and time‐varying biomarker change variables by plotting Schoenfeld residuals over time. This assumption was not violated for any of the biomarker variables with the exception of baseline GDF‐15 and change in NT‐proBNP. Here, there was a notable decrease in the estimated residuals at late follow‐up time points (Figures S1 and S2). We therefore conducted the analyses for baseline GDF‐15 and change in NT‐proBNP with follow‐up time limited to 2 years, given the proportional hazards assumption was valid over this shorter follow‐up period (Figure S3). To determine the incremental predictive value of biomarker changes to baseline clinical variables, the change in the concordance index was quantified.
To gain further insight into the predictive value of biomarkers, we performed additional analyses. We explored the association between elevated hs‐cTnT (ie, >14 ng/L based on the sex‐specific 99th percentile cutoff limit for the cTn assay used) at the completion of anthracyclines and subsequent CTRCD risk, given prior studies indicating the importance of troponin assessment at this time point in patients treated with anthracycline‐based regimens.14, 16, 18, 19 We then quantified time‐dependent sensitivity and specificity, and calculated the positive predictive value (PPV) and negative predictive value (NPV) at this threshold. In an exploratory analysis, we further examined these parameters at an hs‐cTnT threshold of 5 ng/L (ie, limit of detection of the assay) as we postulated that this threshold could identify a subgroup with a low risk of CTRCD at the completion of anthracyclines. Furthermore, we explored test characteristics and the associated PPV and NPV at NT‐proBNP thresholds of 125 ng/L, 150 ng/L, and 300 ng/L, based on previously published studies.27, 28, 29
Two‐sided α levels <0.05 were considered statistically significant. All analyses were performed using R 3.4.0 (R Foundation for Statistical Computing).
Results
Study Population
In total, 323 patients had at least 1 biomarker measurement at baseline and 1 follow‐up visit. The number of available measurements per biomarker is provided in Table S1. Baseline characteristics and biomarker values are summarized in Table 1. At baseline, the trastuzumab group tended to have higher median NT‐proBNP levels, in particular, and patients in this group tended to be older, with higher systolic blood pressure, and a greater prevalence of hypertension compared with the other treatment groups.
Table 1.
Baseline Characteristics of the Overall Analysis Cohort and Stratified According to Cancer Therapy Regimen
| Variable | Overalla (N=323) | Doxorubicin (n=199) | Trastuzumab (n=71) | Doxorubicin+ Trastuzumab (n=53) |
|---|---|---|---|---|
| Age, y | 48 [41–57] | 49 [41–56] | 51 [44–58] | 43 [38–54] |
| Race | ||||
| Black | 81 (25.1) | 58 (29.2) | 7 (9.9) | 16 (30.2) |
| White | 224 (69.3) | 130 (65.3) | 60 (84.5) | 34 (64.1) |
| Other/unknown | 18 (5.6) | 11 (5.5) | 4 (5.6) | 3 (5.7) |
| Breast cancer side | ||||
| Left | 147 (45.6) | 91 (45.8) | 32 (45.7) | 24 (45.3) |
| Right | 157 (48.8) | 97 (48.7) | 37 (52.9) | 23 (43.4) |
| Bilateral | 18 (5.6) | 11 (5.5) | 1 (1.4) | 6 (11.3) |
| Metastases or recurrence | 4 (1.2) | 1 (0.5) | 3 (4.2) | 0 (0) |
| Breast cancer stage | ||||
| 1 | 69 (21.3) | 27 (13.6) | 32 (45.1) | 10 (18.9) |
| 2 | 178 (55.1) | 123 (61.8) | 28 (39.4) | 27 (50.9) |
| 3 | 69 (21.4) | 47 (23.6) | 6 (8.5) | 16 (30.2) |
| 4 | 7 (2.2) | 2 (1.0) | 5 (7.0) | 0 (0) |
| Radiation therapy | ||||
| None | 119 (37.0) | 72 (36.4) | 30 (42.2) | 17 (32.1) |
| Left‐sided | 97 (30.1) | 59 (29.8) | 19 (26.8) | 19 (35.8) |
| Right‐sided | 95 (29.5) | 61 (30.8) | 21 (29.6) | 13 (24.5) |
| Bilateral | 11 (3.4) | 6 (3.0) | 1 (1.4) | 4 (7.6) |
| LVEF, % | 53 [51–56] | 53 [50–56] | 53 [52–56] | 54 [53–57] |
| Body mass index, kg/m2 | ||||
| <25 | 128 (39.7) | 77 (38.7) | 30 (42.2) | 21 (39.6) |
| 25 to 30 | 97 (30.0) | 60 (30.1) | 20 (28.2) | 17 (32.1) |
| ≥30 | 98 (30.3) | 62 (31.2) | 21 (29.6) | 15 (28.3) |
| Systolic blood pressure, mm Hg | 126 [116–135] | 125 [115–135] | 128 [117–140] | 125 [116–134] |
| Diastolic blood pressure, mm Hg | 74 [69–81] | 75 [68–82] | 73 [68–81] | 74 [70–79] |
| Heart rate, beats per min | 79 [72–89] | 79 [72–89] | 80 [72–90] | 78 [74–87] |
| Current or prior smoking | 128 (40.0) | 83 (42.3) | 25 (35.2) | 20 (37.7) |
| History of diabetes mellitus | 27 (8.4) | 19 (9.5) | 6 (8.5) | 2 (3.8) |
| History of hypertension | 99 (30.7) | 60 (30.3) | 28 (39.4) | 11 (20.8) |
| History of hyperlipidemia or statin use | 70 (21.7) | 45 (22.6) | 14 (20.0) | 11 (20.8) |
| ACEI/ARB or β‐blocker use | 61 (18.9) | 43 (21.6) | 13 (18.3) | 5 (9.4) |
| ACEI use | 28 (8.7) | 21 (10.6) | 5 (7.0) | 2 (3.8) |
| ARB use | 22 (6.8) | 13 (6.5) | 8 (11.3) | 1 (1.9) |
| β‐Blocker use | 22 (6.8) | 17 (8.5) | 3 (4.2) | 2 (3.8) |
| hs‐cTnT, ng/L | 3 [3–4] | 3 [3–4] | 3 [3–5] | 3 [3–4] |
| NT‐proBNP, ng/L | 68 [37–124] | 58 [35–103] | 140 [79–236] | 60 [30–112] |
| GDF‐15, ng/L | 629 [509–892] | 698 [538–897] | 587 [425–945] | 580 [519–707] |
| PIGF, ng/L | 13 [10–16] | 13 [10–15] | 15 [11–17] | 13 [10–16] |
| Myeloperoxidase, pmol/L | 307 [219–457] | 303 [213–476] | 325 [241–436] | |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; GDF‐15, growth differentiation factor 15; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PIGF, placental growth factor.
Patients with at least 1 available biomarker measurement at baseline and during at least 1 follow‐up visit were included. Values are expressed as count (percentage) for categorical variables and median [interquartile range] for continuous variables. Myeloperoxidase was not measured in the trastuzumab group.
Biomarker Trajectories According to Cancer Therapy Regimen
Changes in biomarkers over time according to the 3 cancer therapy regimens are presented in Figure 2A through 2E. In addition, changes in the combined doxorubicin groups as compared with the trastuzumab group are presented in Figure S4. In the doxorubicin and doxorubicin+trastuzumab groups, hs‐cTnT increased by ≈4‐fold in the first 6 months and then declined to baseline levels. hs‐cTnT levels remained low throughout follow‐up in the trastuzumab group. On average, NT‐proBNP demonstrated an early, modest elevation in the doxorubicin group but no substantial change with doxorubicin+trastuzumab. In the trastuzumab group, NT‐proBNP significantly declined in the first 6 months; possibly in part related to the higher baseline levels in this treatment group.
Figure 2.
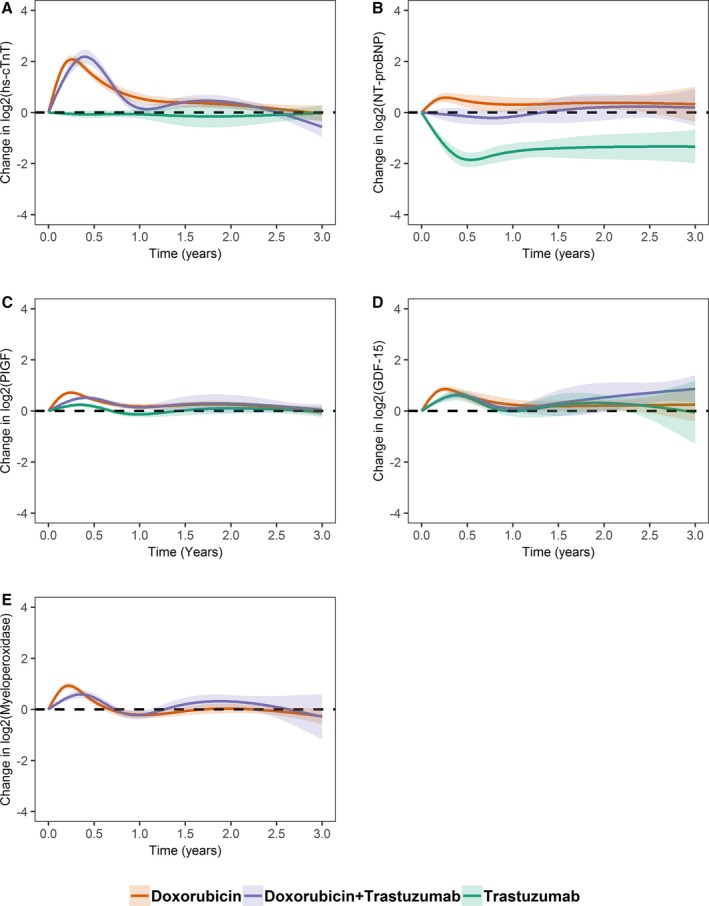
Mean estimated changes in biomarkers over time according to cancer therapy regimen. The solid line represents mean estimated changes over time and the width of the surrounding band represents the corresponding 95% CI. Biomarker levels were log2 transformed (a unit increment from baseline should be interpreted as doubling); (A) high‐sensitivity cardiac troponin T (hs‐cTnT), (B) NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), (C) placental growth factor (PIGF), (D) growth differentiation factor 15 (GDF‐15), and (E) myeloperoxidase.
For PIGF, early increases were observed in the doxorubicin, doxorubicin+trastuzumab, and to a lesser extent, in the trastuzumab groups, with a return to baseline levels over time. Early increases were followed by a decline to baseline in all treatment groups for GDF‐15. Early increases were also observed for myeloperoxidase in the doxorubicin and doxorubicin+trastuzumab groups.
Associations Between Baseline and Changes in Biomarkers With LVEF Changes
This analysis was performed in 254 patients with quantitated echocardiograms at baseline and at least 1 follow‐up visit. Baseline characteristics and biomarker values are summarized according to treatment group in this subset of patients in Table S2.
First, we determined the association between baseline biomarker levels and changes in LVEF. No significant associations were observed (Table S3). Next, we evaluated the contemporaneous associations between changes in biomarkers and changes in LVEF (ie, changes in biomarker levels and changes in LVEF over the same time interval). Changes in hs‐cTnT and NT‐proBNP from baseline had modest associations with LVEF changes at the same visit; LVEF declined by ≈0.6% per doubling of each of these biomarkers (P<0.05). No statistically significant associations were seen for the other biomarkers (Table 2).
Table 2.
Associations Between Changes in Biomarker Levels and Changes in LVEF
| Biomarker | Contemporaneous | Subsequent Visit | ||
|---|---|---|---|---|
| Beta (95% CI) | P Value | Beta (95% CI) | P Value | |
| hs‐cTnT | −0.6 (−1.1 to −0.1) | 0.019 | −0.3 (−0.8 to 0.2) | 0.313 |
| NT‐proBNP | −0.6 (−1.1 to −0.2) | 0.003 | −0.7 (−1.2 to −0.2) | 0.004 |
| PIGF | 0.6 (−0.1 to 1.4) | 0.100 | 1.1 (0–2.2) | 0.056 |
| GDF‐15 | −0.2 (−0.9 to 0.5) | 0.608 | −0.3 (−1.4 to 0.7) | 0.544 |
| Myeloperoxidase | 0.4 (−0.1 to 0.8) | 0.105 | 0.4 (−0.2 to 0.9) | 0.225 |
Biomarker levels were log2 transformed. Beta estimates represent the absolute change in the left ventricular ejection fraction (LVEF) for each doubling of biomarker levels from baseline to the same (contemporaneous) visit or the subsequent change in LVEF for each doubling in biomarker levels from baseline to the prior visit. Associations were adjusted for baseline variables including cancer therapy regimen, baseline LVEF, baseline biomarker levels, age, hypertension, smoking, body mass index, and time since treatment initiation (modeled nonparametrically using a cubic spline, its effect allowed to vary across treatment groups by including an interaction term). GDF‐15 indicates growth differentiation factor 15; hs‐cTnT, high‐sensitivity cardiac troponin T; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PIGF, placental growth factor.
Next, to gain insight into the ability of biomarkers to predict subsequent LVEF changes, we examined the association between preceding changes in biomarkers and LVEF changes at the subsequent visit. On average, there was a 0.7% (95% CI, −1.2 to −0.2) decline in LVEF at each subsequent visit per doubling of NT‐proBNP (P=0.004), whereby the median time between biomarker and LVEF assessments was 2.1 months. No statistically significant associations were observed with the other biomarkers (Table 2). Associations between biomarker changes and circumferential strain were consistent with the LVEF findings; however, there were no significant associations between biomarker changes and longitudinal strain with the exception of myeloperoxidase (Table S4).
We also explored the associations between biomarker and LVEF changes according to treatment regimen, at both the contemporaneous and subsequent visits. There was a significant interaction between NT‐proBNP and treatment regimen on the associations between biomarker and LVEF changes at the same (P=0.030) and subsequent visits (P=0.017). The most pronounced effect was observed in the doxorubicin+trastuzumab group; on average, LVEF declined by 1.1% to 1.3% per doubling of NT‐proBNP from baseline for these associations (Figure 3 and Table S5).
Figure 3.
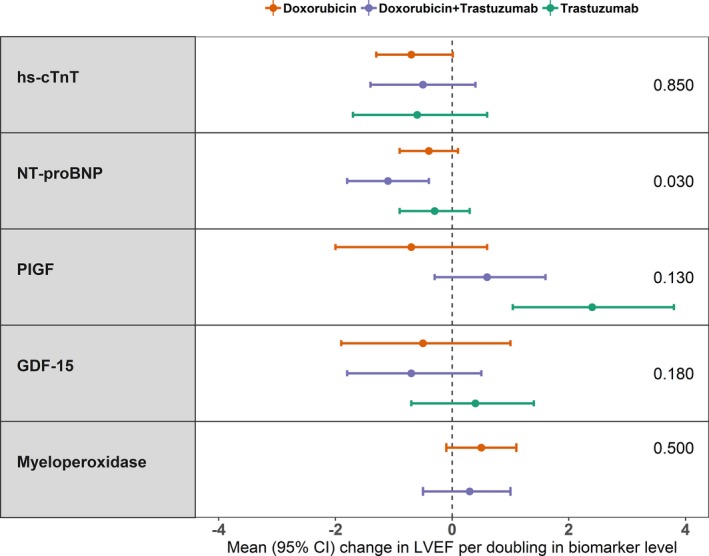
Associations between changes in biomarkers and changes in left ventricular ejection fraction (LVEF) according to cancer therapy regimen. Each point corresponds to mean absolute change in LVEF from baseline for each doubling of a biomarker from baseline to the same visit. The last column on the right side presents P values for interaction. GDF‐15 indicates growth differentiation factor 15; hs‐cTnT, high‐sensitivity cardiac troponin T; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PIGF, placental growth factor.
Associations Between Baseline and Changes in Biomarker and CTRCD
Among the 254 patients with quantitated echocardiograms at baseline and during at least 1 follow‐up visit, CTRCD occurred in 22 (14.2%), 9 (17.0%), and 18 (39.1%) patients in the doxorubicin, trastuzumab, and doxorubicin+trastuzumab groups, respectively, over a maximum period of 3.7 years of follow‐up, consistent with previously published rates.2, 30, 31 The majority had New York Heart Association class I heart failure (75.5%), while 24.5% developed New York Heart Association class II/III heart failure. The median (interquartile range) time to the development of CTRCD was 12 (5–22), 8 (5–9), and 8 (5–11) months from initiation of therapy in the doxorubicin, trastuzumab, and doxorubicin+trastuzumab groups, respectively.
First, we determined the associations between baseline biomarkers and CTRCD risk. A significant association was observed for myeloperoxidase. The hazard of CTRCD increased by 30% (hazard ratio, 1.30; 95% CI, 1.01–1.68) per doubling in baseline myeloperoxidase value (P=0.041). No other statistically significant associations were observed (Table 3).
Table 3.
Associations Between Baseline and Changes in Biomarker Levels and CTRCD (Defined as ≥10% Decline in LVEF to <50%)
| Biomarker | Baseline | Change From Baseline | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| hs‐cTnT | 0.94 (0.55–1.59) | 0.82 | 1.06 (0.87–1.31) | 0.528 |
| NT‐proBNPa | 1.00 (0.78–1.28) | >0.99 | 1.56 (1.32–1.84) | <0.001 |
| PIGF | 0.95 (0.53–1.70) | 0.87 | 0.93 (0.87–1.31) | 0.786 |
| GDF‐15a | 1.12 (0.56–2.20) | 0.75 | 0.96 (0.64–1.44) | 0.834 |
| Myeloperoxidase | 1.30 (1.01–1.68) | 0.041 | 1.10 (0.92–1.31) | 0.300 |
Biomarker levels were log2 transformed. Hazard ratios (HRs) are for each doubling of biomarker level. Associations were adjusted for baseline biomarker levels and baseline variables including cancer therapy regimen, left ventricular ejection fraction (LVEF), age, hypertension, smoking, and body mass index. Associations between baseline biomarker levels and cancer therapy–related cardiac dysfunction (CTRCD) were modeled using Cox proportional hazards models, and associations between repeated assessments of change in biomarkers from baseline and CTRCD was modeled using partly conditional Cox models. hs‐cTnT indicates high‐sensitivity cardiac troponin T; PIGF, placental growth factor.
For baseline growth differentiation factor 15 (GDF‐15) and change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) from baseline, the analyses were limited to the first 2 years of follow‐up to address the violation of the proportional hazards assumption at late follow‐up times (Figures S1 through S3).
We next evaluated the associations between changes in biomarker levels from baseline and CTRCD risk in the overall cohort. We observed a significant association between changes in NT‐proBNP and CTRCD. Each doubling in NT‐proBNP from baseline was associated with a 56% increased risk (hazard ratio, 1.56; 95% CI, 1.32–1.84, P<0.001) over a maximum follow‐up of 2 years (Table 3). Because of the limited number of CTRCD events within the different treatment groups, particularly in patients treated with trastuzumab alone, cancer therapy regimen–based stratified analysis was not performed. However, we specifically evaluated the association between changes in myeloperoxidase and CTRCD in the doxorubicin+trastuzumab group to validate previous findings from our group that demonstrated predictive value of myeloperoxidase with this cancer therapy regimen.16, 17 Here, increases in myeloperoxidase were associated with increased CTRCD risk (hazard ratio per doubling 1.28; 95% CI, 1.04–1.57, P=0.019).
We then explored the incremental predictive value of biomarker changes to a baseline clinical model comprising cancer therapy regimen, LVEF, age, hypertension, body mass index, and smoking (Table S6). The addition of NT‐proBNP change to this model improved the concordance index from 0.694 to 0.724. The incremental value of the other biomarkers was limited.
Predictive Utility of hs‐cTnT and NT‐proBNP at the Completion of Anthracycline Therapy
Based on a priori hypothesis, we investigated the predictive value of hs‐cTnT elevation at the time of completion of anthracycline chemotherapy among patients in the doxorubicin or doxorubicin+trastuzumab groups. Among 170 patients with available hs‐cTnT at this time point, 71 (41.8%) had elevated hs‐cTnT (ie, >14 ng/L). In multivariable analysis, elevated hs‐cTnT at this time point was associated with a doubling of CTRCD risk (hazard ratio, 2.01; 95% CI, 1.00–4.06, P=0.052), consistent with previously published findings from our group and others.13, 16, 18, 19 At this threshold, hs‐cTnT had a sensitivity and specificity of 60.3% and 62.5% for the prediction of CTRCD within 1 year after completion of anthracycline therapy; accompanying PPV and NPV were 26.5% and 87.5%, respectively (Table 4). Interestingly, an hs‐cTnT level <5 ng/L at the completion of anthracycline therapy had 100% sensitivity and NPV for CTRCD at 1 year. However, <10% of the patients had hs‐cTnT levels <5 ng/L at this time point (Table S7).
Table 4.
Time‐Dependent Sensitivity, Specificity, PPV, and NPV Estimates for hs‐cTnT and NT‐proBNP at the Completion of Anthracycline Therapy in the Doxorubicin or Doxorubicin+Trastuzumab Groups
| Biomarker | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|
| hs‐cTnT thresholds | ||||
| 5 ng/L | 100 | 10.2 | 20.0 | 100 |
| 14 ng/L | 60.3 | 62.5 | 26.5 | 87.5 |
| NT‐proBNP thresholds | ||||
| 125 ng/L | 42.3 | 70.1 | 24.3 | 84.3 |
| 150 ng/L | 37.7 | 85.1 | 36.4 | 85.8 |
| 300 ng/L | 22.0 | 94.2 | 46.4 | 84.2 |
hs‐cTnT indicates high‐sensitivity cardiac troponin T; NPV, negative predictive value; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PPV, positive predictive value.
In terms of NT‐proBNP elevations, 56 (33.1%) had levels >125 ng/L at completion of anthracyclines (Table S7). Elevations above this threshold at this time point had a sensitivity of 42.3% and NPV of 84.3% (Table 4). NT‐proBNP concentrations at thresholds of 150 and 300 ng/L had higher specificity (ie, >85%) and PPV, but worse sensitivity and NPV (Table 4). The estimates of the PPV were 36.4% and 46.4% at 150 ng/L and 300 ng/L, respectively.
Discussion
To our knowledge, this is the largest prospective cohort study of patients with breast cancer treated with anthracyclines and/or trastuzumab with detailed cardiovascular phenotyping, including biomarkers, echocardiography, and clinical data, over an extended follow‐up period of 3.7 years. This study comprehensively evaluated the potential predictive utility of both traditional and novel cardiovascular biomarkers at multiple time points during standard breast cancer systemic therapy. We demonstrate 4 key findings that advance our understanding of the role of biomarkers in cardio‐oncology: (1) hs‐cTnT elevations are common after anthracycline therapy; (2) elevated hs‐cTnT specifically at the time of completion of anthracycline therapy may be predictive of subsequent CTRCD risk; (3) changes in NT‐proBNP are significantly associated with changes in LVEF and CTRCD risk, and these associations are most pronounced in patients who receive sequential anthracycline and trastuzumab therapy; and (4) higher baseline levels of myeloperoxidase are associated with an increased risk of CTRCD, and changes in myeloperoxidase may have potential utility in predicting the risk of CTRCD, particularly in patients treated with sequential anthracycline and trastuzumab therapy.
cTns are the most widely studied biomarkers in cardio‐oncology. In early published work, cTnI elevation was reported in up to a third of patients treated with high‐dose chemotherapy, and the pattern of release predicted the risk for cardiac events.10, 11 Recent studies utilizing high‐sensitivity assays indicate that cTn elevation is even more common in patients treated with anthracycline‐based regimens. However, the association between serial changes in cTn concentrations and the development of CTRCD have been less consistent.10, 11, 12, 13, 14, 15, 16, 17, 32
Our findings indicate that levels of hs‐cTnT, on average, increase by nearly 4‐fold early after the initiation of therapy, thereby suggesting that anthracyclines inflict cardiac injury broadly. However, changes in hs‐cTnT levels were only modestly associated with changes in LVEF and circumferential strain. In addition, neither baseline values nor repeated assessment of changes in hs‐cTnT were consistently associated with the development of CTRCD. Therefore, overall, our findings do not support the routine serial evaluation of hs‐cTnT in patients with breast cancer treated with anthracyclines with or without trastuzumab for the prediction of systolic dysfunction. However, our data do suggest 2 important potential applications for hs‐cTnT in this population, specifically at the time point of anthracycline completion. Here, hs‐cTnT levels >14 ng/L were associated with a >2‐fold increased risk of CTRCD, and this threshold had a PPV of 26.5% for the prediction of CTRCD at 1 year following the completion of therapy (which is 8% greater than the incidence of CTRCD within the subgroup of patients who received anthracyclines). This suggests that hs‐cTnT elevation at the time of completion of anthracycline therapy might be considered as a possible enrichment strategy for defining a high‐risk patient subpopulation. Another important observation was that none of the patients with hs‐cTnT levels <5 ng/L after the completion of anthracycline therapy developed CTRCD over the subsequent year of follow‐up (ie, 100% NPV). Although further validation is needed, this finding would suggest that hs‐cTnT levels below the assay limit of detection at this time point might be considered as a possible strategy for identifying a low‐risk subpopulation of patients. It is worth noting that our results do not exclude a role for hs‐cTnT as a surrogate measure of subclinical injury with anthracycline‐based regimens in the clinical trial setting.32, 33, 34, 35, 36, 37 Finally, our study further clarifies the role of hs‐cTnT testing in patients treated with trastuzumab only. Our findings provide definitive evidence that, on average, hs‐cTnT elevations are uncommon with trastuzumab therapy alone.38
Natriuretic peptides are also widely studied in patients treated with cardiotoxic cancer therapy. However, the predictive value of these biomarkers has not been consistent.16, 17, 18, 19, 20, 21 Prior studies mainly focused on the changes in natriuretic peptides during a relatively short follow‐up time, mostly restricted to the time period during cancer therapy. Our study systematically examined the temporal associations between repeated assessments of changes in NT‐proBNP and changes in imaging measures of cardiac function and CTRCD over an extended follow‐up period. We observed consistent associations between increases in NT‐proBNP and LVEF declines, which were particularly notable in the sequential anthracycline and trastuzumab group. Preceding increases in NT‐proBNP were also associated with subsequent LVEF decline and worsening in circumferential strain, suggestive of a temporal association with these 2 measures of cardiac function. Importantly, the addition of NT‐proBNP provided incremental value to baseline clinical risk factors for the prediction of CTRCD. Based on these results, we propose that routine serial assessment of NT‐proBNP has the greatest potential utility in the cardiac surveillance of patients with breast cancer, particularly in those treated with sequential anthracycline and trastuzumab therapy. In exploratory analysis, we also observed that NT‐proBNP concentrations at thresholds of 150 ng/L or 300 ng/L can identify subpopulations with increased subsequent CTRCD risk with high specificity and PPV that is 2‐ to 3‐fold the incidence of CTRCD specifically in patients treated with the anthracycline‐based cancer therapy regimen. Future studies are needed to validate these thresholds and further define their clinical utility in the setting of cardio‐oncology.39
Among the newer biomarkers, myeloperoxidase has demonstrated promise as a biomarker of cardiac dysfunction in anthracycline‐based treatment groups. As oxidative stress is a central mechanism in anthracycline‐induced cardiotoxicity, myeloperoxidase is a mechanistically plausible marker of cardiotoxicity in patients treated with anthracyclines.22, 40, 41 Prior small studies from our group showed that increased levels of myeloperoxidase during the course of therapy are associated with increased risk of cardiotoxicity with sequential anthracycline and trastuzumab therapy.16, 17 In line with these findings, our study showed that increases in myeloperoxidase, as well as baseline values, are associated with an increased risk of CTRCD with this cancer therapy regimen. These results would indicate that myeloperoxidase may be a candidate biomarker for the prediction of cardiotoxicity, particularly in patients treated with sequential anthracycline and trastuzumab therapy. However, further validation in larger studies is needed before it can be considered for clinical use as a marker of cardiotoxicity.
Modest early elevations in PIGF and GDF‐15 were also observed with both anthracycline‐based regimens and trastuzumab alone, suggesting that these agents might result in other pathophysiologic changes related to inflammation and angiogenesis. However, neither PIGF nor GDF‐15 showed significant associations with changes in LVEF and strain or CTRCD. Our findings do not support the role of PIGF and GDF‐15 as predictive biomarkers of anthracycline and/or trastuzumab cardiotoxicity. This may appear to be in contrast with our previously published results;17 however, we note that in the prior study, we studied the temporal changes in 8 biomarkers measured at 3‐month intervals during doxorubicin and trastuzumab therapy, for a maximum follow‐up time of 15 months. We sought to define both the concurrent and associations with subsequent cardiotoxicity and evaluated biomarkers over the entire course of follow‐up and allowed for recurrent events (in contrast to the first event). Moreover, in prior studies, cardiotoxicity was defined by the Cardiac Review and Evaluation Committee criteria.42 Thus, our exposures and outcomes were both defined and analyzed differently.
Study Strengths
Our study expands on prior work in this field and provides important findings that further clarify the role of biomarkers in the prediction of cardiac dysfunction in patients with breast cancer exposed to cardiotoxic cancer therapy. The current study has several strengths, particularly related to study design and methodology, which make it unique from previous studies. First, the prospective, longitudinal cohort study design coupled with the relatively large sample size and longer follow‐up duration extending well beyond the time of completion of cancer therapy is an important strength as compared with previous studies. Moreover, biomarkers were evaluated at multiple prespecified time points, including both during and after completion of cancer therapy. Previous studies have mainly focused on changes in biomarker levels during the course of cancer therapy. Third, our study was composed of a nonclinical trial population, which enhances the generalizability of our findings. Fourth, the current study is the first to systematically characterize differences in the pattern of biomarker changes and their associations with changes in measures of cardiac dysfunction across different cancer therapy regimens. Fifth, the utilization of more robust statistical tools for the analysis of longitudinal data including generalized estimating equations and partly conditional survival models is another novel aspect of the study.
Our results motivate additional research. An important next step should be establishing validated biomarker thresholds that can be used as benchmarks to facilitate the detection of cardiotoxicity and prediction of subsequent risk, particularly for hs‐cTnT and NT‐proBNP. This can enhance the clinical applicability of biomarker information in daily clinical practice. Moreover, dynamic risk prediction models combining clinical data with serial measurements of multiple biomarkers could be developed in larger data sets and externally validated. More importantly, randomized controlled trials are needed to determine whether biomarker‐guided cardioprotective treatment strategies are of utility in mitigating cardiovascular risk. Lastly, the value of serial assessment of biomarker changes appears to be primarily in patients treated with sequential anthracycline and trastuzumab therapy. This highlights a significant need for biomarker discovery research to identify novel mechanistic biomarkers of cardiotoxicity with anthracyclines or trastuzumab alone.22
Study Limitations
Our study has limitations that warrant consideration. The hs‐cTn assay used is relatively less sensitive than some even newer methods. It thus remains possible that our results might differ if other, more highly sensitive methods were used for cTn assessment. As a result of the relatively small size of the treatment groups and number of CTRCD events, we could not reliably evaluate biomarker‐treatment regimen interactions for the CTRCD outcome, and these analyses were limited to LVEF change alone as the outcome measure. We also did not adjust for multiple comparisons. As such, the association models should be interpreted in that context. Another limitation is that myeloperoxidase was not measured in the trastuzumab group. Importantly, our findings may not be generalizable to other cancer populations receiving additional cardiotoxic therapies and with different cardiovascular risk profiles. As it relates to NT‐proBNP, we did observe decreases during trastuzumab therapy, particularly in the trastuzumab group. The reasoning behind this is not clear at this stage, although this is unlikely to have impacted any associations with CTRCD. We explored whether this may in part be attributable to confounding by variables such as age and hypertension, as the trastuzumab group tended to be older and more hypertensive with higher baseline NT‐proBNP levels. However, adjustment for potential confounders did not significantly alter the pattern of change over time observed for NT‐proBNP with this treatment regimen (Figure S5). We cannot exclude the possibility of an assay interaction between trastuzumab and NT‐proBNP, but further work is needed to clarify this and should be a topic of future basic studies.
Conclusions
Our study demonstrates that hs‐cTnT elevations are common in patients with breast cancer treated with anthracycline‐based cancer therapy regimens, and assessment at the completion of anthracycline therapy may have clinical utility in cardiotoxicity risk prediction. We determined a consistent temporal association between changes in NT‐proBNP and cardiotoxicity, which was particularly notable in the sequential anthracycline and trastuzumab group. The study also provides incremental data to support the potential role for myeloperoxidase as a candidate novel biomarker of cardiotoxicity in patients treated with sequential anthracycline and trastuzumab therapy. Altogether, our findings inform clinically relevant strategies to mitigate cardiovascular risk in patients with breast cancer who receive cardiotoxic therapies.
Sources of Funding
This work is supported by NHLBI R01‐HL118018 (B.K.), McCabe Fellow Award (Philadelphia, PA; B.K.), American Cancer Society Institutional Research Grant ‐78‐002‐30 (Atlanta, GA; B.K.), NHLBI K23‐HL095661 (B.K.). Assay support was provided by Roche Diagnostics, Inc. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the manuscript for publication.
Disclosures
Dr Hubbard reports grants from the National Institute of Health. Dr Zhang reports grants from the University of Ottawa Heart Institute. Dr Januzzi reports grants from Roche and has served as a consultant for Roche, Siemens, Prevencio, and Abbott. Dr Tang reports grants from the National Institute of Health. Dr Liu reports grants from Roche. Dr Ky reports serving as a consultant for Roche and an investigator initiated research award from Roche, and grants from the National Institute of Health. All other authors report no conflict of interest relevant to the content of this study.
Supporting information
Data S1. Supplemental Methods.
Table S1. Number of Available Biomarker Measurements in the Analysis Patient Population
Table S2. Baseline Characteristics Stratified According to Cancer Therapy Regimen in the Subset of Patients With Quantitated Echocardiograms at Baseline and During at Least 1 Follow‐Up Visit (N=254)
Table S3. Associations Between Baseline Biomarker Levels and Changes in LVEF
Table S4. Associations Between Changes in Biomarker Levels and Changes in Circumferential and Longitudinal Strain
Table S5. Associations Between Changes in Biomarker Levels From Baseline to a Visit and Change in LVEF at the Subsequent Visit: Interactions With Cancer Therapy Regimen
Table S6. Incremental Predictive Value of Biomarker Change Variables When Added to a Baseline Clinical Model
Table S7. Proportion of Patients With Elevated hs‐cTnT and NT‐proBNP at the Completion of Anthracycline Therapy
Figure S1. Plots of Schoenfeld residuals against time for the associations between baseline biomarkers and time to cancer therapy–related cardiac dysfunction.
Figure S2. Plots of Schoenfeld residuals against time for the associations between changes in biomarkers and time to cancer therapy–related cardiac dysfunction.
Figure S3. Plots of Schoenfeld residuals against time for the associations between baseline growth differentiation factor 15 (GDF‐15) and change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and time to cancer therapy–related cardiac dysfunction limiting maximum follow‐up time to 2 years.
Figure S4. Mean estimated changes in biomarkers over time according to cancer therapy regimen. The doxorubicin group includes both patients treated with doxorubicin alone and sequential doxorubicin and trastuzumab therapy. The solid line represents mean estimated changes over time and the width of the surrounding band represents the corresponding 95% CI. Biomarker levels were log2 transformed (a unit increment from baseline should be interpreted as doubling); (A) high‐sensitivity cardiac troponin T (hs‐cTnT), (B) NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), (C) placental growth factor (PIGF), (D) growth differentiation factor 15 (GDF‐15), (E) myeloperoxidase.
Figure S5. Covariate‐adjusted marginal mean estimates of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) change at 3, 6, 12, 24, and 36 months after initiation of cancer therapy according to cancer therapy regimen. Covariates include baseline NT‐proBNP and baseline variables including age, hypertension, body mass index, and smoking. The effect of time was allowed to differ according to cancer therapy regimen by including a time×treatment interaction term.
(J Am Heart Assoc. 2020;9:e014708 DOI: 10.1161/JAHA.119.014708.)
References
- 1. Abdel‐Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population‐based study of cardiovascular mortality following early‐stage breast cancer. JAMA Cardiol. 2017;2:88–93. [DOI] [PubMed] [Google Scholar]
- 2. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation. 2006;114:2474–2481. [DOI] [PubMed] [Google Scholar]
- 3. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland A, Storas TH, Hagve TA, Rosjo H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 5. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 6. Hamo CE, Bloom MW, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, Butler J. Cancer therapy‐related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9:e002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu AF, Ky B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart. 2016;102:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ky B, Carver JR. Biomarker approach to the detection and cardioprotective strategies during anthracycline chemotherapy. Heart Fail Clin. 2011;7:323–331. [DOI] [PubMed] [Google Scholar]
- 9. Shah KS, Yang EH, Maisel AS, Fonarow GC. The role of biomarkers in detection of cardio‐toxicity. Curr Oncol Rep. 2017;19:42. [DOI] [PubMed] [Google Scholar]
- 10. Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high‐dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. [DOI] [PubMed] [Google Scholar]
- 11. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high‐dose chemotherapy. Circulation. 2004;109:2749–2754. [DOI] [PubMed] [Google Scholar]
- 12. Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. [DOI] [PubMed] [Google Scholar]
- 13. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy‐treated patients. Am J Cardiol. 2011;107:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C‐reactive protein are commonly detected in patients with breast cancer treated with dose‐dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. [DOI] [PubMed] [Google Scholar]
- 16. Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer‐Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, Al‐Sakaff N, Piccart‐Gebhart MJ, de Azambuja E. Role of troponins I and T and N‐terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early‐stage human epidermal growth factor receptor 2‐positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35:878–884. [DOI] [PubMed] [Google Scholar]
- 19. de Vries Schultink AH, Boekhout AH, Gietema JA, Burylo AM, Dorlo TPC, van Hasselt JG, Schellens JH, Huitema AD. Pharmacodynamic modeling of cardiac biomarkers in breast cancer patients treated with anthracycline and trastuzumab regimens. J Pharmacokinet Pharmacodyn. 2018;45:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romano S, Fratini S, Ricevuto E, Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C, Penco M. Serial measurements of NT‐proBNP are predictive of not‐high‐dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy‐related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9:e96736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finkelman BS, Putt M, Wang T, Wang L, Narayan H, Domchek S, DeMichele A, Fox K, Matro J, Shah P, Clark A, Bradbury A, Narayan V, Carver JR, Tang WHW, Ky B. Arginine‐nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J Am Coll Cardiol. 2017;70:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Narayan HK, French B, Khan AM, Plappert T, Hyman D, Bajulaiye A, Domchek S, DeMichele A, Clark A, Matro J, Bradbury A, Fox K, Carver JR, Ky B. Noninvasive measures of ventricular‐arterial coupling and circumferential strain predict cancer therapeutics‐related cardiac dysfunction. JACC Cardiovasc Imaging. 2016;9:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin adjuvant (HERA) Trial Study Team . Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med. 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 25. Zheng Y, Heagerty PJ. Partly conditional survival models for longitudinal data. Biometrics. 2005;61:379–391. [DOI] [PubMed] [Google Scholar]
- 26. Maziarz M, Heagerty P, Cai T, Zheng Y. On longitudinal prediction with time‐to‐event outcome: comparison of modeling options. Biometrics. 2017;73:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gustafsson F, Steensgaard‐Hansen F, Badskjaer J, Poulsen AH, Corell P, Hildebrandt P. Diagnostic and prognostic performance of N‐terminal ProBNP in primary care patients with suspected heart failure. J Card Fail. 2005;11:S15–S20. [DOI] [PubMed] [Google Scholar]
- 28. Januzzi JL Jr, Chen‐Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, Peacock WF, Rivers EJ, Walters EL, Gaggin HK; ICON‐RELOADED Investigators . N‐terminal pro‐B‐type natriuretic peptide in the emergency department: the ICON‐RELOADED study. J Am Coll Cardiol. 2018;71:1191–1200. [DOI] [PubMed] [Google Scholar]
- 29. Taylor CJ, Roalfe AK, Iles R, Hobbs FD. The potential role of NT‐proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4:e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jordan JH, D'Agostino RB Jr, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Hundley WG. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1‐weighted and T2‐weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014;7:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary approach to novel therapies in cardio‐oncology research (MANTICORE 101‐Breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol. 2017;35:870–877. [DOI] [PubMed] [Google Scholar]
- 32. Gulati G, Heck SL, Rosjo H, Ree AH, Hoffmann P, Hagve TA, Norseth J, Gravdehaug B, Steine K, Geisler J, Omland T. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (prevention of cardiac dysfunction during adjuvant breast cancer therapy) study. J Am Heart Assoc. 2017;6:s006513 DOI: 10.1161/JAHA.117.006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM; ICOS‐ONE Study Investigators . Anthracycline‐induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society‐one trial. Eur J Cancer. 2018;94:126–137. [DOI] [PubMed] [Google Scholar]
- 34. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Goncalves Brandao SM, Rigaud VO, Higuchi‐Dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for prevention of chemotherapy‐related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. [DOI] [PubMed] [Google Scholar]
- 35. Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE, Packard CJ, Mills NL. High‐sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;68:2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jhund PS, Claggett BL, Voors AA, Zile MR, Packer M, Pieske BM, Kraigher‐Krainer E, Shah AM, Prescott MF, Shi V, Lefkowitz M, McMurray JJ, Solomon SD; PARAMOUNT Investigators . Elevation in high‐sensitivity troponin T in heart failure and preserved ejection fraction and influence of treatment with the angiotensin receptor neprilysin inhibitor LCZ696. Circ Heart Fail. 2014;7:953–959. [DOI] [PubMed] [Google Scholar]
- 37. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR; RELAX‐AHF Investigators . Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX‐AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. [DOI] [PubMed] [Google Scholar]
- 38. Ponde N, Bradbury I, Lambertini M, Ewer M, Campbell C, Ameels H, Zardavas D, Di Cosimo S, Baselga J, Huober J, Izquierdo M, Fumagalli D, Bozovic‐Spasojevic I, Maetens M, Harbeck N, Pusztai L, Berghorn M, Im YH, Borrego MR, Chen DR, Rodeheffer R, Piccart M, Suter T, de Azambuja E. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib‐induced cardiotoxicity in patients with HER2‐positive early‐stage breast cancer: a NeoALTTO sub‐study (BIG 1‐06). Breast Cancer Res Treat. 2018;168:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 40. Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–359. [DOI] [PubMed] [Google Scholar]
- 41. Schindhelm RK, van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–1470. [DOI] [PubMed] [Google Scholar]
- 42. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Number of Available Biomarker Measurements in the Analysis Patient Population
Table S2. Baseline Characteristics Stratified According to Cancer Therapy Regimen in the Subset of Patients With Quantitated Echocardiograms at Baseline and During at Least 1 Follow‐Up Visit (N=254)
Table S3. Associations Between Baseline Biomarker Levels and Changes in LVEF
Table S4. Associations Between Changes in Biomarker Levels and Changes in Circumferential and Longitudinal Strain
Table S5. Associations Between Changes in Biomarker Levels From Baseline to a Visit and Change in LVEF at the Subsequent Visit: Interactions With Cancer Therapy Regimen
Table S6. Incremental Predictive Value of Biomarker Change Variables When Added to a Baseline Clinical Model
Table S7. Proportion of Patients With Elevated hs‐cTnT and NT‐proBNP at the Completion of Anthracycline Therapy
Figure S1. Plots of Schoenfeld residuals against time for the associations between baseline biomarkers and time to cancer therapy–related cardiac dysfunction.
Figure S2. Plots of Schoenfeld residuals against time for the associations between changes in biomarkers and time to cancer therapy–related cardiac dysfunction.
Figure S3. Plots of Schoenfeld residuals against time for the associations between baseline growth differentiation factor 15 (GDF‐15) and change in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and time to cancer therapy–related cardiac dysfunction limiting maximum follow‐up time to 2 years.
Figure S4. Mean estimated changes in biomarkers over time according to cancer therapy regimen. The doxorubicin group includes both patients treated with doxorubicin alone and sequential doxorubicin and trastuzumab therapy. The solid line represents mean estimated changes over time and the width of the surrounding band represents the corresponding 95% CI. Biomarker levels were log2 transformed (a unit increment from baseline should be interpreted as doubling); (A) high‐sensitivity cardiac troponin T (hs‐cTnT), (B) NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), (C) placental growth factor (PIGF), (D) growth differentiation factor 15 (GDF‐15), (E) myeloperoxidase.
Figure S5. Covariate‐adjusted marginal mean estimates of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) change at 3, 6, 12, 24, and 36 months after initiation of cancer therapy according to cancer therapy regimen. Covariates include baseline NT‐proBNP and baseline variables including age, hypertension, body mass index, and smoking. The effect of time was allowed to differ according to cancer therapy regimen by including a time×treatment interaction term.


