Abstract
Background
In nonvalvular atrial fibrillation (AF), oral anticoagulants prevent ischemic strokes and transient ischemic attacks (TIAs), but nonpersistence with vitamin K antagonist (VKA) oral anticoagulant therapy (20–50% at 1 year) is problematic. The precise risk of stroke/TIA after VKA cessation and its time course during extended follow‐up is unknown.
Methods and Results
The study cohort of incident AF in patients receiving initial VKA between 2001 and 2013 was identified from the UK Clinical Practice Research Datalink (linked hospitalizations and causes of death). Using a nested case‐control analysis, patients with incident stroke/TIA were matched to patients without stroke/TIA (controls). Relative risk with time since VKA cessation compared with current VKA use was approximated from conditional logistic regression. We studied 16 696 patients with incident AF and initial VKA treatment. There were 489 stroke/TIA cases matched to 2137 controls (mean CHA 2 DS 2‐VASc score 4.3). Compared with current VKA use, the excess incidence rate of stroke/TIA following VKA cessation in the first year after AF diagnosis was 2.29 (95% CI, 0.98–3.90) per 100 person‐years of VKA cessation or 1 additional stroke/TIA per 43 patients per year discontinuing VKA, compared with 1.43 (95% CI, 0.97–1.88) per 100 person‐years corresponding to 1 additional stroke/TIA per 70 patients per year, when VKA was discontinued more than 1 year after AF diagnosis.
Conclusions
VKA cessation is associated with a continuous excess thromboembolic stroke/TIA risk. Increasing oral anticoagulant persistence, especially in the year after AF diagnosis, should be a therapeutic target to reduce stroke/TIA in AF.
Keywords: anticoagulants, atrial fibrillation, stroke, transient ischemic attack, treatment cessation
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Transient Ischemic Attack (TIA), Anticoagulants, Compliance/Adherence
Clinical Perspective
What Is New?
In patients with newly diagnosed atrial fibrillation (AF), stroke and transient ischemic attack risk after cessation of oral anticoagulant treatment is highest when therapy is stopped in the first year after AF diagnosis.
Risk of stroke and transient ischemic attack after cessation of oral anticoagulant therapy in patients with AF remains elevated at twice the risk of patients who continue this therapy, for at least 3 years after anticoagulant therapy has been stopped.
What Are the Clinical Implications?
Nonpersistence with oral anticoagulant thromboprophylaxis for stroke in patients with newly diagnosed AF is a significant and highly preventable cause of stroke in patients with AF.
Therapeutic interventions are needed to target nonpersistence in patients who are commenced on oral anticoagulants, in particular in the first year after AF diagnosis.
Introduction
Vitamin K antagonist (VKA) oral anticoagulants (OACs), principally warfarin, decrease the risk of cardioembolic cerebral ischemic events in atrial fibrillation (AF), with a 64% reduction in stroke and 26% reduction in death.1 The non‐VKA OACs (NOACs) have some advantages in safety and efficacy.2 Therefore, OACs are recommended for the prevention of stroke and systemic thromboembolism in patients with additional CHA2DS2‐VASc stroke risk factors in all AF guidelines.3, 4
Real‐world and registry studies show that only 50% to 80% of patients with AF are actually taking OACs.5, 6 Optimal stroke prevention requires not only OAC prescription but also treatment persistence (treatment continued long term). Fear of bleeding or actual bleeds, difficulty in achieving good international normalized ratio control,2 requirement for regular laboratory monitoring, and multiple drug/food interactions have led to VKA discontinuation rates between 20% and >50% at 1 year.7, 8, 9, 10 OAC therapy nonpersistence is therefore potentially as great an issue for effective stroke prevention as nonprescription. This could have implications for OAC choice with significantly greater early persistence shown for NOACs than for VKA in patients with AF.11, 12, 13
It is conventional wisdom that patients who discontinue OAC will carry their baseline risk of thromboembolic complications. What is less clear is the actual level, duration, and time course of risk after OAC cessation. To better define the penalty of VKA nonpersistence, we studied patients with newly diagnosed AF commenced on VKA soon after diagnosis and examined the association between VKA discontinuation and the risk of ischemic strokes and transient ischemic attacks (TIAs) during extended follow‐up.
Methods
Design, Study Setting, and Study Population
We performed a cohort study with a nested case‐control analysis using data from the subset of individuals in the UK Clinical Practice Research Datalink (CPRD) who were linked to the Hospital Episode Statistics (HES) and Office for National Statistics (ONS) mortality data.14 CPRD collates routinely collected anonymized electronic health record data from general practices. Because of CPRD's licensing restrictions, the data presented herein will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Data include demographics, lifestyle information, medical diagnoses, symptoms, signs, laboratory tests, and prescriptions issued by the general practitioner. HES include dates of hospital admission and discharge, primary and other main reasons for treatment recorded, and surgical operations and procedures performed during the hospital stay. The study cohort of incident OAC‐naive nonvalvular AF was formed using a previously described and validated algorithm.15 The cohort consisted of all patients in CPRD‐HES aged 45 to 89 years between 2001 and 2013 with a hospital or general practitioner–based AF diagnosis and VKA treatment initiation within 60 days. Cohort entry was defined as 2 days after the hospital discharge for AF or 1 day after general practitioner diagnosis of AF. Patients with active cancer or stroke/TIA between AF diagnosis and cohort entry were excluded, as were patients with a history of dementia or palliative care, as these could be reasons for discontinuation of VKA. End of follow‐up was the earliest of the following events: stroke/TIA, active cancer (defined as 90 days before a recording for cancer or chemotherapy), dementia diagnosis, palliative care initiation, reinitiation of anticoagulant treatment after previous discontinuation, switching to NOAC or parenteral anticoagulant therapy, death, transfer out of the general practitioner practice, end of data collection of the general practitioner practice, or end of study period.
Case Definition
The composite outcome consisted of community‐acquired ischemic stroke or TIA (stroke/TIA) recorded in general practice, hospitalization discharge, or death certificate (primary or secondary cause of death). Patient summaries of all potential cases of stroke/TIA were manually reviewed by one author (C.M.). Hemorrhagic strokes, intracerebral/subarachnoid hemorrhage, and strokes not resulting in hospitalization were excluded. The date of stroke/TIA was defined as the index day.
Selection of Controls
Controls were sampled from the cohort with VKA‐treated incident AF as follows: for each stroke/TIA (case) up to 5 controls without stroke/TIA were randomly selected and matched on sex, date of AF±365 days, time from cohort entry until the index day, age at index day±1 year, and CHA2DS2‐VASc score (excluding age/sex). The control's cohort entry date plus time from cohort entry until the stroke/TIA of the corresponding case became the index day of the control. Controls had to be free of stroke/TIA between start of follow‐up and index day.
Exposures
For all cases and their matched controls, we estimated VKA use from all VKA prescriptions, international normalized ratio tests, and medical codes indicating discontinuation of oral anticoagulants recorded before the index date. We considered individuals to be exposed for the length to VKA use plus a 30‐day grace period to account for any remaining medication, lack of patient compliance, or residual effects of the VKA medication. We considered cases and controls to be current users if their VKA exposure (prescription length+30 days) extended to or beyond the index date. Past use was use that (including the 30‐day grace period) ended at least 1 day before the index date.
Covariates
Covariates were assessed on the index day and included CHA2DS2‐VASc score, defined from individual CHA2DS2‐VASc components3 assessed from general practitioner records and hospital discharge diagnoses, body mass index, smoking habits, and mode of presentation of AF, ie, ambulatory, primary, and nonprimary hospital discharge AF diagnosis.
Data Analysis
Relative risks (RRs) of the association between VKA use and stroke/TIA were approximated from crude and adjusted odds ratios derived from conditional logistic regression for matched case‐control data.16 The main analysis included time since VKA discontinuation, ie, ≤365 or >365 days as of the index date, compared with current VKA treatment, and CHA2DS2‐VASc score at day of first AF (ie, any score and score ≥2). RRs were adjusted for residual imbalance of all specified covariates among cases and controls:
Several sensitivity analyses on estimation of RRs were performed by: (1) restricting the study outcome to ischemic strokes only, (2) assuming a 60‐day grace period for duration of VKA use instead of 30 days, (3) excluding patients with a history of stroke/TIA, (4) excluding stroke/TIA in the first 60 days after AF, and (5) restricting the study period to incident AF after January 2008.
To obtain an absolute measure of the excess incidence rate, we transformed the adjusted RRs to a rate difference by using the incidence rate of stroke/TIA after cohort entry, the exposure prevalence in the entire AF cohort, and respective RR estimates stratified by time since AF diagnosis.17
Quadratic splines were used to illustrate the RR of stroke/TIA as a function of duration of VKA discontinuation, using current VKA users as reference.
Statistical procedures were performed using Stata MP version 14.2 (StataCorp LLC). This study was approved by the Independent Scientific Advisory Committee for CPRD research (Protocol 16_132), and no informed consent was required.
Results
We identified 19 912 patients with any recording of AF and VKA use within 60 days following incident AF. Of those, 3216 were excluded, eg, because of an uncertain date of AF, resulting in a cohort of 16 696 patients (Figure 1). The mean age was 72.7±9.4 years and 57.5% were men. Of all patients with newly diagnosed AF, 9256 (55.4%) presented in the ambulatory setting, 4141 (24.8%) in the hospital setting with a primary AF discharge diagnosis, and 3299 (19.8%) with a nonprimary AF discharge diagnosis.
Figure 1.
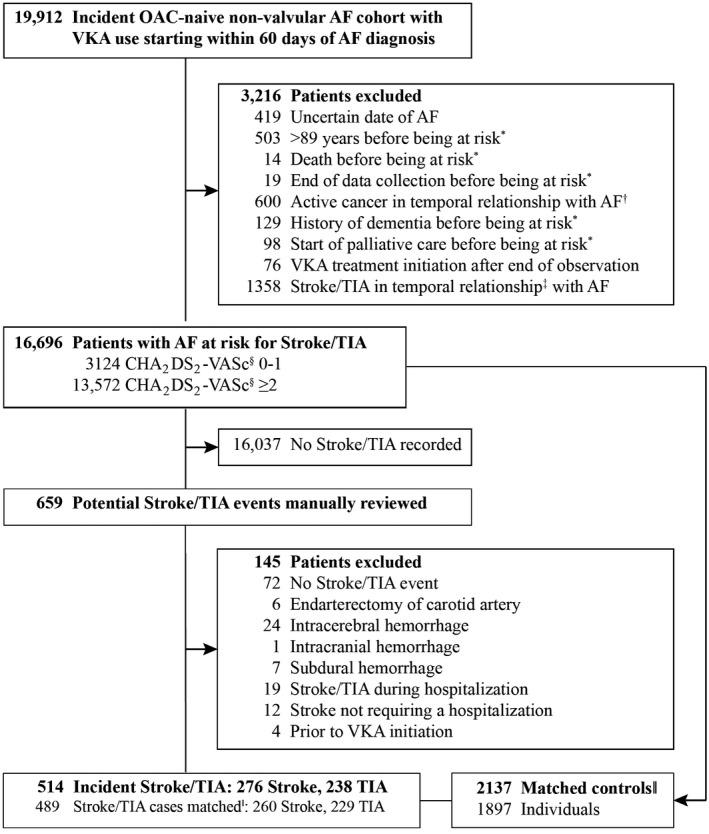
Ascertainment of cases and controls for outcome stroke/transient ischemic attacks (TIA) after atrial fibrillation (AF). OAC indicates oral anticoagulant. *Patient is at risk 2 days after hospital discharge or 1 day after general practitioner AF diagnosis. †Active cancer defined as cancer recording between 90 days before first AF diagnosis and 90 days after being at risk. ‡Stroke/TIA diagnosis or discharge day of stroke/TIA hospitalization between 14 days before AF and start of being at risk, or before the first vitamin K antagonist (VKA) prescription. §At day of AF. ‖Matching on age at index day ±1 year, sex, calendar day of AF ±1 year, CHA2DS2‐VASc score at index day (without points for age and sex), and index day.
A total of 514 incident strokes/TIAs (45 [8.8%] fatal or death within 28 days) were observed during 31 990 person‐years of observation. Of 514 patients with stroke/TIA, 489 were matched to 2137 controls with AF (Figure 1). As a result of matching, sex and age distribution were similar (Table 1). The proportion of current smokers was higher among cases. Mean CHADS2 score was 2.4±1.3 and CHA2DS2‐VASc score was 4.3±1.7 among cases and virtually identical to controls. The prevalence of hypertension, diabetes mellitus, and vascular disease was lower in cases, whereas stroke/TIA or thromboembolism history was more frequent among cases (Table 1).
Table 1.
Characteristics of Cases and Controls at Index Day
| Stroke/TIA | Matched Controlsa | |
|---|---|---|
| Total, No. | 489 | 2137 |
| Male | 252 (51.5) | 1096 (51.5) |
| Age, yb | 77.4±7.9 | 77.4±7.9 |
| BMI, kg/m2 c | 27.3±4.9 | 27.9±5.5e |
| Current smokerc | 41 (8.5) | 142 (6.4) |
| Source of AF diagnosis | ||
| General practitioner | 275 (56.2) | 1196 (55.6) |
| Primary hospital discharge diagnosis | 115 (23.5) | 495 (23.1) |
| Nonprimary hospital discharge diagnosis | 99 (20.2) | 446 (21.5) |
| CHADS2 scoreb | 2.4±1.3 | 2.3±1.3e |
| 0 to 1 | 131 (26.8) | 622 (28.8) |
| ≥2 | 358 (73.2) | 1515 (71.2) |
| CHA2DS2‐VASc scoreb | 4.3±1.7 | 4.3±1.7 |
| 0 to 1 | 19 (3.9) | 76 (4.1) |
| ≥2 | 470 (96.1) | 2061 (95.9) |
| Non‐age and nonsex components of CHA2DS2‐VASc scoreb | ||
| CHF/LVD | 130 (26.6) | 580 (28.4) |
| Hypertension | 337 (68.9) | 1558 (73.0) |
| Diabetes mellitus | 76 (15.5) | 472 (23.9)e |
| Stroke/TIA/thromboembolism | 158 (32.3) | 445 (23.7)e |
| Vascular disease | 194 (39.7) | 891 (42.5) |
| Charlson indexb , d | 2.0±1.7 | 2.1±1.8 |
Values are expressed mean±SD or number (percentage). BMI indicates body mass index; CHF, congestive heart failure; LVD, left ventricular dysfunction; TIA, transient ischemic attack.
Matched on age at index day ±1 year, sex, calendar day of atrial fibrillation (AF) ±1 year, CHA2DS2‐VASc score at index day (without points for age and sex).
On index day.
Latest information available before index day.
Not including age.
P<0.05 from ANOVA for comparison of means or Fisher exact text for comparison of proportions.
Of 489 matched patients with stroke/TIA, 61.8% were currently taking VKA and 38.2% had discontinued VKA treatment before the index stroke/TIA without subsequent use of other anticoagulants compared with 79.3% and 20.7% among controls, respectively. This revealed an adjusted RR for VKA discontinuation and stroke/TIA of 2.57 (95% CI, 2.01–3.28). Stratification by time since incident AF yielded comparable RRs for VKA discontinuation. Of the 489 strokes/TIAs, 223 (45.6%) occurred in the year following incident AF and 266 (54.4%) occurred >1 year after initial AF diagnosis. The RR for stroke/TIA after VKA discontinuation was significantly increased regardless of the number of years since incident AF and in all strata of time since VKA discontinuation, with estimates ranging from 2.27 to 3.28. While overall excess incidence rate of stroke/TIA was significantly increased in all strata, this amounted to 2.29 additional strokes/TIAs per 100 person‐years for VKA discontinuation in the first year after AF diagnosis compared with 1.43 for VKA discontinuation >1 year after AF diagnosis. Similarly, the excess stroke/TIA incidence rate in patients with CHA2DS2‐VASc ≥2 was higher (2.66 versus 1.85 per 100 person‐years, respectively) when VKA was discontinued in the first year following AF diagnosis versus >1 year after diagnosis (Table 2).
Table 2.
Discontinuation of VKA Treatment and the Risk of Stroke/TIA, by Time Since Incident AF
| Cases, No. (%) | Controls, No. (%) | Crude RR (95% CI) | Adjusted RRa (95% CI) | Adjusted excess IRa/100 PY (95% CI) | |
|---|---|---|---|---|---|
| Complete AF cohort | |||||
| Any time after incident AF | |||||
| Total | 489 | 2137 | |||
| Current VKA use | 302 (61.76) | 1694 (79.27) | 1 | 1 | 0 |
| VKA discontinued | 187 (38.24) | 443 (20.73) | 2.52 (1.99–3.20) | 2.57 (2.01–3.28) | 1.75 (1.13–2.37) |
| VKA discontinued ≤365 d | 83 (16.97) | 184 (8.61) | 2.60 (1.91–3.55) | 2.64 (1.91–3.63) | 1.91 (1.01–2.81) |
| VKA discontinued ≤120 d | 43 (8.79) | 98 (4.59) | 2.40 (1.61–3.59) | 2.52 (1.67–3.80) | 1.94 (0.42–3.47) |
| VKA discontinued >120 to ≤365 d | 40 (8.18) | 86 (4.02) | 2.87 (1.86–4.45) | 2.79 (1.78–4.36) | 2.32 (0.45–4.19) |
| VKA discontinued >365 d | 104 (21.27) | 259 (12.12) | 2.45 (1.78–3.37) | 2.50 (1.80–3.47) | 1.34 (0.77–1.90) |
| ≤1 y after incident AF | |||||
| Total | 223 | 1051 | |||
| Current VKA use | 183 (82.06) | 959 (91.25) | 1 | 1 | 0 |
| VKA discontinued ≤365 d | 40 (17.94) | 92 (8.75) | 2.34 (1.51–3.62) | 2.45 (1.56–3.86) | 2.29 (0.98–3.90) |
| VKA discontinued ≤120 d | 29 (13.00) | 70 (6.66) | 2.17 (1.34–3.53) | 2.27 (1.38–3.75) | 1.96 (0.61–3.80) |
| VKA discontinued >120 to ≤365 d | 11 (4.93) | 22 (2.09) | 3.06 (1.29–7.24) | 3.28 (1.32–8.14) | 3.53 (0.55–9.21) |
| >1 y after incident AF | |||||
| Total | 266 | 1086 | |||
| Current VKA use | 119 (44.74) | 735 (67.68) | 1 | 1 | 0 |
| VKA discontinued | 147 (55.26) | 351 (32.32) | 2.61 (1.96–3.46) | 2.61 (1.94–3.50) | 1.43 (0.97–1.88) |
| VKA discontinued ≤365 d | 43 (16.17) | 92 (8.47) | 2.91 (1.87–4.55) | 2.89 (1.83–4.55) | 1.68 (0.74–3.02) |
| VKA discontinued ≤120 d | 14 (5.26) | 28 (2.58) | 3.02 (1.48–6.14) | 3.17 (1.52–6.62) | 1.93 (0.48–4.82) |
| VKA discontinued >120 to ≤365 d | 29 (10.90) | 64 (5.89) | 2.87 (1.72–4.78) | 2.78 (1.65–4.67) | 1.59 (0.60–3.13) |
| VKA discontinued >365 d | 104 (39.10) | 259 (23.85) | 2.48 (1.80–3.43) | 2.50 (1.79–3.48) | 1.34 (0.79–1.91) |
| CHA2DS2‐VASc ≥2 | |||||
| Any time after incident AF | |||||
| Total | 440 | 1886 | |||
| Current VKA use | 280 (63.64) | 1538 (81.55) | 1 | 1 | 0 |
| VKA discontinued | 160 (36.36) | 348 (18.45) | 2.61 (2.03–3.36) | 2.67 (2.06–3.46) | 2.17 (1.36–2.97) |
| VKA discontinued ≤365 d | 72 (16.36) | 157 (8.32) | 2.51 (1.81–3.49) | 2.55 (1.82–3.58) | 2.17 (1.06–3.29) |
| VKA discontinued ≤120 d | 39 (8.86) | 90 (4.77) | 2.26 (1.49–3.44) | 2.39 (1.55–3.67) | 1.99 (0.29–3.69) |
| VKA discontinued >120 to ≤365 d | 33 (7.50) | 67 (3.55) | 2.90 (1.80–4.66) | 2.79 (1.72–4.54) | 2.53 (0.13–4.92) |
| VKA discontinued >365 d | 88 (20.00) | 191 (10.13) | 2.75 (1.94–3.88) | 2.81 (1.97–4.02) | 1.84 (1.04–2.64) |
| ≤1 y after incident AF | |||||
| Total | 210 | 969 | |||
| Current VKA use | 174 (82.86) | 886 (91.43) | 1 | 1 | 0 |
| VKA discontinued ≤365 d | 36 (17.14) | 83 (8.57) | 2.23 (1.42–3.51) | 2.41 (1.51–3.86) | 2.66 (1.03–4.72) |
| VKA discontinued ≤120 d | 28 (13.33) | 65 (6.71) | 2.16 (1.31–3.55) | 2.32 (1.39–3.88) | 2.47 (0.75–4.88) |
| VKA discontinued >120 to ≤365 d | 8 (3.81) | 18 (1.86) | 2.57 (1.01–6.57) | 2.84 (1.05–7.64) | 3.44 (0.11–10.77) |
| >1 y after incident AF | |||||
| Total | 230 | 917 | |||
| Current VKA use | 106 (46.09) | 652 (71.10) | 1 | 1 | 0 |
| VKA discontinued | 124 (53.91) | 265 (28.90) | 2.81 (2.07–3.82) | 2.80 (2.04–3.85) | 1.85 (1.25–2.47) |
| VKA discontinued ≤365 d | 36 (15.65) | 74 (8.07) | 2.87 (1.78–4.64) | 2.81 (1.72–4.59) | 1.86 (0.76–3.50) |
| VKA discontinued ≤120 d | 11 (4.78) | 25 (2.73) | 2.53 (1.16–5.51) | 2.64 (1.17–5.94) | 1.68 (0.16–4.92) |
| VKA discontinued >120 to ≤365 d | 25 (10.87) | 49 (5.34) | 3.04 (1.75–5.29) | 2.89 (1.64–5.07) | 1.94 (0.66–3.98) |
| VKA discontinued >365 d | 88 (38.26) | 191 (20.83) | 2.77 (1.95–3.94) | 2.79 (1.93–4.02) | 1.84 (1.06–2.66) |
IR indicates incidence rate; PY, person‐years; RR, relative risk; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Adjusted for smoking, body mass index, presentation of atrial fibrillation (AF) (ie, ambulatory, primary hospital discharge, and nonprimary hospital discharge diagnosis), and all CHA2DS2‐VASc score components except for age and sex.
The incidence rate of stroke/TIA after AF diagnosis in patients continuing VKA treatment was highest in the first month, falling gradually over the first year to a stable level up to 3 years (Figure 2). The RR of stroke/TIA as a function of time since discontinuation of VKA use increased for 4 months, then declined gradually to a plateau and remained significantly elevated for at least 3 years (Figure 3).
Figure 2.
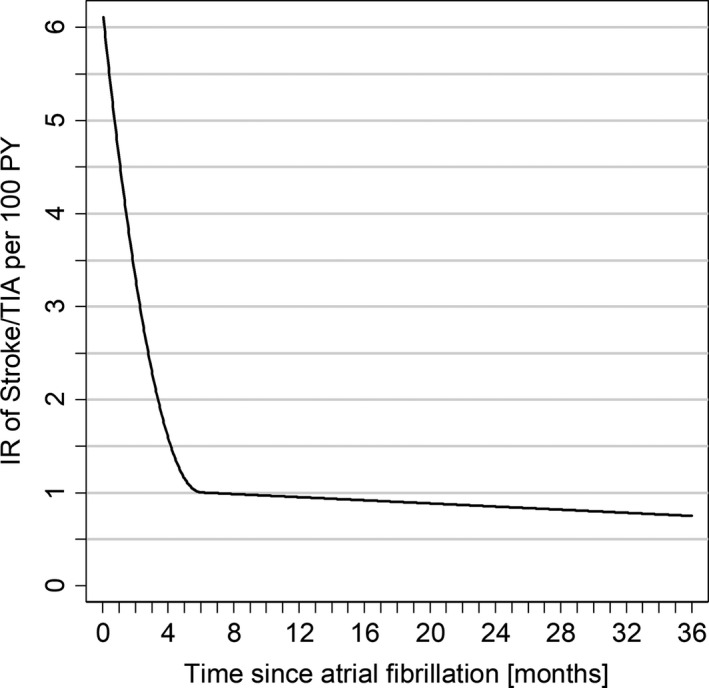
Incidence rate of stroke/transient ischemic attack (TIA) during initial vitamin K antagonist (VKA) treatment by time since atrial fibrillation (AF) diagnosis. PY indicates person‐years; Incidence rate (IR) of stroke/TIA during VKA treatment were calculated per month since AF diagnosis. IR function by time since AF was fitted using quadratic splines and weighting for variances of the estimated IRs.
Figure 3.
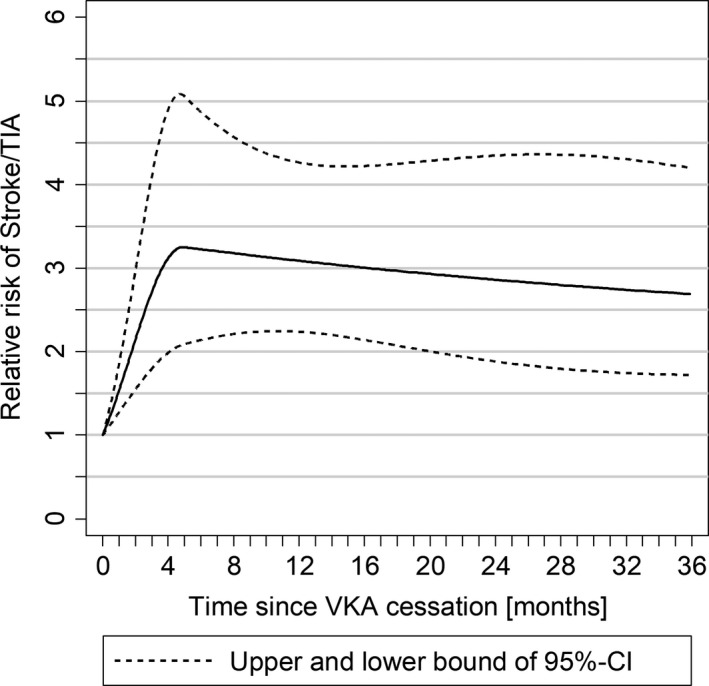
Risk of stroke/transient ischemic attack (TIA) by time since vitamin K antagonist (VKA) discontinuation in patients with CHA 2 DS 2‐VASc ≥2. Relative risks of stroke/TIA using quadratic splines and adjusted for smoking, body mass index, presentation of atrial fibrillation (ie, ambulatory, primary hospital discharge, and nonprimary hospital discharge diagnosis), and all CHA 2 DS 2‐VASc score components except for age and sex.
Sensitivity analyses using ischemic strokes alone as the outcome, a grace period of 60 days for concatenation of VKA recordings, excluding patients with a stroke/TIA history, for the subset with incident AF after January 2008, and excluding patients with a stroke/TIA event within 60 days after AF diagnosis revealed consistent findings (Table S1).
Discussion
For the first time, we have been able to quantify the magnitude of the effect and temporal relationship between VKA discontinuation and stroke/TIA risk and time after AF diagnosis in patients with incident AF. We found a significant increase in stroke/TIA risk in the first year after VKA cessation and also in the years following, with RRs between 2.3 and 3.3 compared with current VKA use, and an excess stroke/TIA incidence rate between 1.3 and 3.5 per 100 patient‐years, higher in the first year after AF diagnosis. Modeling of the risk over time showed an increase in the first 4 months after VKA cessation, which plateaued by 3 years after anticoagulant cessation, with RRs approaching 2.5 at that time. Restriction to patients with CHA2DS2‐VASc ≥2 did not materially affect the RR of VKA discontinuation, although absolute risk was higher in patients with higher CHA2DS2‐VASc scores. The absolute excess incidence rate of stroke/TIA peaked at 2.7 stroke/TIA per 100 patient‐years in patients with CHA2DS2‐VASc ≥2 in the first year after AF diagnosis and remained stable thereafter. Our findings indicate a high penalty of VKA cessation, particularly in the first year after AF diagnosis, and continuing excess stroke/TIA risk even 3 years after discontinuation.
Others have also noted the higher risk of stroke in the first year after AF diagnosis.18 While the pathophysiological mechanism is unclear, a number of potential mechanisms have been suggested as contributing to this risk, including left atrial stasis, endothelial dysfunction, hyperaggregability and hypercoagulability, endothelial dysfunction, and inflammation.18
The issue of risk of VKA cessation has received relatively little attention. Prior studies on this topic showed a significant increase in stroke after VKA cessation19, 20, 21, 22, 23 but none investigated the risk of ischemic strokes and TIAs by time from AF diagnosis until discontinuation and only 1 by time since VKA discontinuation (only for 1 year), which would result in an underestimation of the stroke risk. We showed the risk not only depended on time after discontinuation, but, crucially, on time after AF diagnosis, with a high excess rate of stroke following VKA discontinuation within the first year after AF diagnosis. This high early stroke risk after AF diagnosis has recently been noted by others.18 Failure to investigate the timing and duration of VKA use and VKA discontinuation would result in underestimation of the association between VKA cessation and stroke if overall risk estimates are based on a high proportion of patients with a small risk, a phenomenon known as depletion of susceptibles.24 The peak we observed in the first 4 months may also be attributable to rebound hypercoagulability as we have previously demonstrated after discontinuation of therapy for venous thromboembolism.25
The high risk of stroke/TIA after VKA cessation in the first year after AF diagnosis is of particular concern given the poor early persistence with VKA therapy we and others have shown in AF.10, 11, 26 One can extrapolate from the stroke/TIA risk that for every 100 patients starting on VKA and allowing for the discontinuation rate of 36.4% at 1 year (34.7% in those with CHA2DS2‐VASc ≥ 2) shown in our previous study,11 there would be a predicted excess of 0.9 stroke/TIA by the end of the first year just caused by discontinuation. An increase in persistence with NOACs to 83%11 would translate to prevention of 1 stroke/TIA in the first year after AF diagnosis per 212 patients with CHA2DS2‐VASc ≥2 starting on NOACs instead of VKAs.
We showed a long‐lasting increase of the stroke/TIA risk at least 3 years after VKA discontinuation, which was highest about 4 months after VKA discontinuation. This translates to an absolute increase of 1.6 strokes/TIAs per 100 patients in the first year and 3.5 strokes/TIAs per 100 patients in 3 years as a result of VKA discontinuation after newly diagnosed AF. While patient education and counseling may improve adherence to anticoagulant therapy,27, 28 future research should target maximizing OAC persistence to prevent avoidable stroke in patients with AF.
Strengths and Limitations
This was a relatively large, homogeneous cohort with recent‐onset AF started on VKA, and over 450 strokes/TIAs, compared in a nested case‐control design with a control cohort closely matched on known stroke risk factors in AF. Unusually for AF studies, over 50% of cases had the initial AF diagnosis made in general practice. Most other cohorts include only hospital discharge AF diagnoses, increasing the generalizability of our results.
As in all observational studies, despite the strong matching we used, including days from AF diagnosis to index stroke/TIA, unmeasured confounding or hidden bias might exist. The main limitation is information bias resulting from missing data on inpatient VKA use and medications dispensed at hospital discharge and in anticoagulation clinics. Patients with a recording of VKA use in the 60 days following the AF and thereafter seen exclusively in anticoagulation clinics, would appear to have discontinued VKA use. This would underestimate the association between VKA cessation and stroke risk.
The date of VKA discontinuation was calculated mainly from VKA prescriptions and international normalized ratio measurements, which included a grace period of 30 days. Patients who had discontinued VKA use within these 30 days would appear to be current VKA users, which would decrease the contrast between VKA discontinuation and current use resulting in an underestimation of the effect of VKA discontinuation.
Because of a lack of information about international normalized ratio control, the group of current VKA users in our study included an unknown proportion of patients with poor anticoagulation control. Thus, the difference between patients who discontinued VKAs and patients optimally treated with VKAs may be underestimated in our study.
The analysis relies on the accuracy and completeness of coding of symptoms and diagnoses as well as discharge summaries, and the effect of incomplete medical records, especially for patients referred to specialists or switching practices, is unknown. ONS data rely on death certificates and these greatly underestimate the frequency of stroke‐related deaths because of the difficulty in diagnosis.
Conclusions
VKA cessation is associated with continuing excess risk of stroke/TIA, greatest in the year after initial AF diagnosis. Increasing persistence with anticoagulant therapy especially in the year after AF diagnosis should be a therapeutic target to reduce stroke/TIA.
Disclosures
C.M., C.W., and S.R. report grants to the institution from Bayer, CSL Behring, Merz Pharma and Bristol‐Myers Squibb (significant), outside the submitted work. B.F. reports grants to the institution, personal fees and nonfinancial support from Bayer, grants to the institution (significant), personal fees and nonfinancial support from Bristol‐Myers Squibb–Pfizer (significant), personal fees and nonfinancial support from Daiichi Sankyo (modest), and nonfinancial support from Alivecor (modest), outside the submitted work.
Supporting information
Table S1. Sensitivity Analyses on Discontinuation of VKA Treatment and the Risk of Stroke/TIA, by Time Since Incident AF—(A) Using a 60‐Day Grace Period Instead of a 30‐Day Grace Period, (B) Excluding Patients With AF With Previous Stroke/TIA, (C) Restricting Analysis to a Subset With AF Onset After January 2008, (D) Excluding Patients With AF With Stroke/TIA Within 60 Days After AF and (E) a Subset With Ischemic Strokes Only
Acknowledgments
C.M. is the guarantor of this article and takes full responsibility for the conduct of the study and controlled the decision to publish. B.F. and C.M. conceived the study. C.M. and B.F. drafted the article. C.W., S.R., and C.M conducted the data analysis. All authors participated in the study design, data acquisition, data analysis, and interpretation. All authors contributed to the critical revision and approved the final article.
(J Am Heart Assoc. 2020;9:e014376 DOI: 10.1161/JAHA.119.014376.)
References
- 1. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 5. Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR pinnacle registry. JAMA Cardiol. 2016;1:55–62. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Rushton‐Smith SK, Goldhaber SZ, Fitzmaurice DA, Mantovani LG, Goto S, Haas S, Bassand JP, Camm AJ, Ambrosio G, Jansky P, Al Mahmeed W, Oh S, van Eickels M, Raatikainen P, Steffel J, Oto A, Kayani G, Accetta G, Kakkar AK; GARFIELD‐AF Investigators . Does sex affect anticoagulant use for stroke prevention in nonvalvular atrial fibrillation? The prospective global anticoagulant registry in the field‐atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:S12–S20. [DOI] [PubMed] [Google Scholar]
- 7. Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, Brensinger CM, Newcomb CW, Samaha FF, Gross R. The influence of patient adherence on anticoagulation control with warfarin: results from the international normalized ratio adherence and genetics (in‐range) study. Arch Intern Med. 2007;167:229–235. [DOI] [PubMed] [Google Scholar]
- 8. Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. [DOI] [PubMed] [Google Scholar]
- 9. Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. [DOI] [PubMed] [Google Scholar]
- 10. Simons LA, Ortiz M, Freedman SB, Waterhouse BJ, Colquhoun D, Thomas G. Improved persistence with non‐vitamin‐K oral anticoagulants compared with warfarin in patients with atrial fibrillation: recent australian experience. Curr Med Res Opin. 2016;32:1857–1861. [DOI] [PubMed] [Google Scholar]
- 11. Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non‐valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost. 2016;115:31–39. [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali‐Serdoz L, Petrescu L, Darabantiu D, Crijns HJ, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP, Boriani G. Prognosis and treatment of atrial fibrillation patients by european cardiologists: one year follow‐up of the EURObservational research programme‐atrial fibrillation general registry pilot phase (EORP‐AF pilot registry). Eur Heart J. 2014;35:3365–3376. [DOI] [PubMed] [Google Scholar]
- 13. Forslund T, Wettermark B, Hjemdahl P. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72:329–338. [DOI] [PubMed] [Google Scholar]
- 14. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez C, Katholing A, Wallenhorst C, Granziera S, Cohen AT, Freedman SB. Increasing incidence of non‐valvular atrial fibrillation in the uk from 2001 to 2013. Heart. 2015;101:1748–1754. [DOI] [PubMed] [Google Scholar]
- 16. Peacock PB. The non‐comparability of relative risks from different studies. Biometrics. 1971;27:903–907. [Google Scholar]
- 17. Suissa S. The quasi‐cohort approach in pharmacoepidemiology: upgrading the nested case‐control. Epidemiology. 2015;26:242–246. [DOI] [PubMed] [Google Scholar]
- 18. Procter NE, Stewart S, Horowitz JD. New‐onset atrial fibrillation and thromboembolic risk: cardiovascular syzygy? Heart Rhythm. 2016;13:1355–1361. [DOI] [PubMed] [Google Scholar]
- 19. Spivey CA, Liu X, Qiao Y, Mardekian J, Parker RB, Phatak H, Masseria C, Kachroo S, Abdulsattar Y, Wang J. Stroke associated with discontinuation of warfarin therapy for atrial fibrillation. Curr Med Res Opin. 2015;31:2021–2029. [DOI] [PubMed] [Google Scholar]
- 20. Deitelzweig SB, Buysman E, Pinsky B, Lacey M, Jing Y, Wiederkehr D, Graham J. Warfarin use and stroke risk among patients with nonvalvular atrial fibrillation in a large managed care population. Clin Ther. 2013;35:1201–1210. [DOI] [PubMed] [Google Scholar]
- 21. Gallego P, Roldan V, Marin F, Romera M, Valdes M, Vicente V, Lip GY. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb Haemost. 2013;110:1189–1198. [DOI] [PubMed] [Google Scholar]
- 22. Qureshi AI, Jahangir N, Malik AA, Afzal MR, Orfi F, Suri MF. Risk of ischemic stroke in high risk atrial fibrillation patients during periods of warfarin discontinuation for surgical procedures. Cerebrovas Dis. 2016;42:346–351. [DOI] [PubMed] [Google Scholar]
- 23. Rivera‐Caravaca JM, Roldan V, Esteve‐Pastor MA, Valdes M, Vicente V, Lip GYH, Marin F. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb Haemost. 2017;117:1448–1454. [DOI] [PubMed] [Google Scholar]
- 24. Miettinen OS, Caro JJ. Principles of nonexperimental assessment of excess risk, with special reference to adverse drug reactions. J Clin Epidemiol. 1989;42:325–331. [DOI] [PubMed] [Google Scholar]
- 25. Martinez C, Katholing A, Folkerts K, Cohen AT. Risk of recurrent venous thromboembolism after discontinuation of vitamin K antagonist treatment: a nested case‐control study. J Thromb Haemost. 2016;14:1374–1383. [DOI] [PubMed] [Google Scholar]
- 26. Beyer‐Westendorf J, Ebertz F, Forster K, Gelbricht V, Michalski F, Kohler C, Werth S, Endig H, Pannach S, Tittl L, Sahin K, Daschkow K, Weiss N. Effectiveness and safety of dabigatran therapy in daily‐care patients with atrial fibrillation. Results from the dresden NOAC registry. Thromb Haemost. 2015;113:1247–1257. [DOI] [PubMed] [Google Scholar]
- 27. Clarkesmith DE, Pattison HM, Lip GY, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the treat randomised trial. PLoS ONE. 2013;8:e74037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al‐Khalidi HR, He W, Xian Y, Ciobanu AO, Kamath DY, Fox KA, Rao MP, Pokorney SD, Berwanger O, Tajer C, de Barros ESPGM, Roettig ML, Huo Y, Granger CB. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT‐AF): an international, cluster‐randomised trial. Lancet. 2017;390:1737–1746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity Analyses on Discontinuation of VKA Treatment and the Risk of Stroke/TIA, by Time Since Incident AF—(A) Using a 60‐Day Grace Period Instead of a 30‐Day Grace Period, (B) Excluding Patients With AF With Previous Stroke/TIA, (C) Restricting Analysis to a Subset With AF Onset After January 2008, (D) Excluding Patients With AF With Stroke/TIA Within 60 Days After AF and (E) a Subset With Ischemic Strokes Only


