Traditional cytotoxic chemotherapy and novel cancer therapies have various cardiotoxicities, ranging from heart failure to arrhythmias. One of the most exciting developments in cancer treatment is immunotherapy, which uses the immune system to attack malignancies. Among the immunotherapy armamentarium are immune checkpoint inhibitors (ICIs), which have shown promising results.1 ICIs are monoclonal antibodies that target the host immune negative regulation receptors, such as CTLA‐4 (cytotoxic T‐lymphocyte–associated protein 4), programmed cell death receptor 1 (PD‐1), and programmed cell death ligand 1 (PD‐L1). There are currently 7 US Food and Drug Administration–approved ICIs, which include ipilimumab (anti‐CTLA4), nivolumab, pembrolizumab, cemiplimab (anti–PD‐1), avelumab, atezolizumab, and durvalumab (anti–PD‐L1) (Table). The indications for their use in cancer treatment continue to expand for an increasing number of malignancies, and in some as first‐line therapy. Parallel with the increased use, recognition of immune‐related adverse events (IRAEs) has also improved. The most common fatal IRAE is colitis, but the associated mortality is low at 2% to 5%.2 At the other end of the spectrum is ICI‐related myocarditis, which is an uncommon IRAE, but is associated with a high reported mortality.3, 4, 5, 6, 7 There is a need for increased awareness to suspect, diagnose, and treat ICI‐related myocarditis.
Table 1.
FDA‐Approved ICIs
| ICI | Target | FDA Approval Year | Types of Cancers With FDA Approval for Treatment |
|---|---|---|---|
| Ipilimumab | CTLA‐4 | 2011 | Melanoma, renal cell carcinoma, colorectal cancer |
| Nivolumab | PD‐1 | 2014 | Melanoma, non–small‐cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck squamous cell cancer, urothelial carcinoma, colorectal cancer, hepatocellular carcinoma |
| Pembrolizumab | PD‐1 | 2014 | Melanoma, non–small‐cell lung cancer, small‐cell lung cancer, head and neck squamous cell cancer, Hodgkin lymphoma, large B‐cell lymphoma, urothelial carcinoma, colorectal cancer, gastric cancer, esophageal cancer, cervical cancer, hepatocellular carcinoma, Merkel cell carcinoma, renal cell carcinoma |
| Cemiplimab | PD‐1 | 2018 | Cutaneous squamous cell carcinoma |
| Avelumab | PD‐L1 | 2017 | Merkel cell carcinoma |
| Atezolizumab | PD‐L1 | 2016 | Urothelial carcinoma, non–small‐cell lung cancer |
| Durvalumab | PD‐L1 | 2017 | Urothelial carcinoma |
CTLA‐4 indicates cytotoxic T‐lymphocyte–associated antigen 4; FDA, US Food and Drug Administration; ICI, immune checkpoint inhibitor; PD‐1, programmed cell death receptor 1; PD‐L1, programmed cell death ligand 1.
There are few large case series describing cardiotoxicities of ICIs, with the largest coming from adverse event reporting databases. Although these databases provide larger patient numbers and allow comparisons to other cancer therapeutics, they lack granular data on how the myocarditis was diagnosed or treated. Clinical trial data provide fewer patient numbers but allow for a more standardized reporting system of toxicity in the common terminology criteria for adverse events. Although there is a specified adverse event “myocarditis” in the category of “cardiac disorders” in the common terminology criteria for adverse events version 5, this does not provide a standard for how myocarditis is diagnosed or treated. The articles describing use of cardiac imaging studies or endomyocardial biopsy for diagnosis are limited to small case series and case reports, which have a wide variability in the use of either tool for diagnosis. Furthermore, there are even fewer reports of effective treatment using immunomodulators, which have variable dosing and choice of immunomodulation. ICI‐related myocarditis is a new entity that requires further research, and the following review will discuss the current literature available for aiding physicians to better diagnose and treat patients with this infrequent but fatal toxicity.
Epidemiological Characteristics
ICI‐related myocarditis has a reported incidence of 0.04% to 1.14%, but when compared with other IRAEs, it has a significantly higher associated mortality of 25% to 50%.3, 4, 5, 6, 7 In addition, the use of combination ICI therapy has almost twice the incidence of and mortality from myocarditis, although it is still an uncommon adverse event compared with other IRAEs.2, 3, 5, 6 Salem et al described 122 cases reported from 2008 to 2018 in VigiBase, which is the World Health Organization's global database of individual case safety reports.6 The study showed increased reporting of myocarditis over time, with only 15 reported cases between 2013 and 2016 compared with 107 reported cases in 2017 and 2018. Myocarditis was reported for patients on ICI disproportionally compared with the full reporting database, with an increased reporting odds ratio of 11.2.6 The correspondence by Al‐Kindi and Oliveira7 described 250 ICI‐related myocarditis cases from the US Food and Drug Administration Adverse Event Reporting System, showing the same trend of increased reporting over time, with only 18 reported cases between 2012 and 2015, 70 in 2016, and 162 in 2017. Disproportionality analysis also showed increased reporting odds ratio of 5.94 for myocarditis while on ICI when compared with other cancer therapeutics.7 Other cardiovascular toxicities have also been reported, including pericardial disease, myocardial infarction, and vasculitis.6, 7, 8, 9 In a meta‐analysis that included 22 clinical trials of PD‐1 and PD‐L1 inhibitors for lung cancer, the incidence of myocarditis was 0.5%, but the incidence of other cardiovascular toxicities, including pericardial tamponade, myocardial infarction, stroke, cardiac failure, and cardiorespiratory arrest, ranged from 0.7% to 2.0%.9
There is wide variability in the reported time to onset of symptoms after starting ICI. The 35 patients with ICI‐associated myocarditis, described by Mahmood et al, had a median time of onset of 34 days (interquartile range, 21–75 days).4 In this cohort, 81% of patients presented within 3 months of initiating ICI,4 but late presentations of up to 454 days have been reported in the literature.10 The cohort described by Escudier et al had a range of 2 to 454 days and a median of 65 days to a diagnosis of cardiotoxicity after initiation of ICI.10 There was an average of 3 infusions administered before cardiotoxicity diagnosis.10 From a report of 101 patients with ICI‐associated myocarditis, reported in VigiBase, 33 patients had a report of timing from initiation of ICI and 76% of these patients presented in the first 6 weeks of treatment, with a median onset of 27 days.5 Almost two thirds of the patients had received only 1 or 2 doses of therapy before the onset of myocarditis. Taken together, these data suggest that most cases of myocarditis will present within the first 1 to 2 months after initiation of ICI, although clinicians should still consider the diagnosis if patients have been on long‐term ICI therapy. Also, the diagnosis can be suspected even after 1 to 2 doses of immunotherapy.
Mechanisms of Myocardial Toxicity
Immune checkpoints are T‐cell regulatory pathways that inhibit antitumor T‐cell activation. Several receptors and ligands have been identified as targets for immune checkpoint therapy to remove the inhibition of antitumor T‐cell responses. First, this section will discuss the mechanism of immune checkpoint targets discussed above (CTLA‐4, PD‐1, and PD‐L1), followed by proposed mechanisms of cardiotoxicity.
The tumor microenvironment includes not only cancer cells but several immune cells. T cells can recognize tumor neoantigens and become activated and proliferate to attack the tumor. The activation of T cells requires the recognition of tumor neoantigen–major histocompatibility complexes by the T‐cell receptor.11 In addition to neoantigen presentation, T‐cell activation requires costimulation of cluster of differentiation (CD) 28 receptors on the T‐cell surface by B7 molecules on antigen‐presenting cells.12 It has been found that as T cells become activated, they upregulate CTLA‐4 receptor presentation, and CTLA‐4 has much higher affinity for the B7 molecules on antigen‐presenting cells than the costimulatory receptor CD28.13 Although CTLA‐4 was initially thought to be another costimulatory receptor, it was later found to actually downregulate the T‐cell response.14 Thus CTLA‐4 receptors are key to inhibitory pathways for blocking antitumor T‐cell activation. The first US Food and Drug Administration–approved ICI was ipilimumab, an antibody for CTLA‐4, thus removing the inhibitory pathway of T‐cell activation. Since then, other immune checkpoint receptors have been identified, including PD‐1. PD‐1 works through a different mechanism than CTLA‐4 and does not inhibit T‐cell costimulation but rather affects the T‐cell antigen receptor–mediated signaling. In 2014, PD‐1 inhibitors nivolumab and pembrolizumab became US Food and Drug Administration approved; and because they work through distinct mechanisms as CTLA‐4, they began to be studied as combination therapy for various malignancies. PD‐1 also has 2 ligands, including PD‐L1 and PD‐L2, of which PD‐L1 has subsequently been used as a target for immune checkpoint inhibition. Several cell types, including tumor cells, T cells, endothelial cells, and epithelial cells, express PD‐L1. Increased expression in these cell types occurs after activated T cells release interferon‐γ, thus leading to the suspicion that this pathway is involved in protecting cells from T‐cell attack.15
The exact mechanism of ICI‐related myocarditis is unclear. Suggested mechanisms include a shared antigen between the tumor and myocardium, T‐cell receptor targeting a different but homologous muscle antigen as the tumor antigen, or certain T‐cell receptors targeting dissimilar antigens.3 The first 2 possible mechanisms are analogous to proposed mechanisms of viral‐mediated myocarditis in which the heart is targeted by a process of molecular mimicry.16 As in viral myocarditis, ICI‐related myocarditis has been described to have T‐cell infiltration of the myocardium.3 Johnson et al identified T‐cell clonal expansion in both tumor and muscle cells (both striated and cardiac).3 In addition, several studies have identified the expression of PD‐L1 in the myocardium of patients with ICI‐related myocarditis.3 As mentioned above, PD‐L1 expression is upregulated by interferon‐γ, and this is suspected to be a protective response also observed in mice studies.17 PD‐1 knockout mice have been described to develop autoimmune dilated cardiomyopathy and increased mortality.18 The hearts of PD‐1 knockout mice were described to have decreased left ventricular systolic function and wall thinning with dilated right ventricles.18 There was diffuse immunoglobulin G antibody deposition on cardiomyocytes, and the autoantibodies were specific to cardiac structures.18 This suggests the critical role of PD‐1 in regulating autoimmune responses specifically in regard to the heart.18 Another animal study evaluated cynomolgus monkeys that received ipilimumab and nivolumab combination therapy.19 The monkeys had CD4+ and CD8+ T‐cell infiltration of the heart, with fewer numbers of macrophages and B cells described.19 The T‐cell infiltration is similar to that described in humans, and immunohistochemical staining was positive for PD‐1 and PD‐L1.19 These 2 animal studies help to identify the immune cell infiltration of the heart and possible mechanisms tied to PD‐1 and PD‐L1 inhibition. Further research is needed to establish the exact mechanisms of ICI‐related myocarditis.
Clinical Presentation of ICI‐Associated Myocarditis
The clinical presentation of myocarditis likely has a spectrum of mild to severe disease, with a classification system suggested by the American Society of Clinical Oncology (ASCO) clinical practice guidelines for the management of IRAE (Figure 1).20, 21, 22, 23 Thus, the presentation can vary from asymptomatic elevations in cardiac biomarkers to severe decompensation with end‐organ damage. The most concerning is the severe end of the spectrum manifesting as a “fulminant” or life‐threatening presentation, which is also the most reported in the literature. These patients present with a clinical syndrome of cardiogenic shock, which may be accompanied by serious arrhythmias, such as advanced atrioventricular block or ventricular tachycardia.3, 4, 24 The low incidence and high mortality observed are in part explained by almost exclusive reporting of severe cases. The moderate clinical spectrum of disease can manifest in 3 different clinical syndromes also observed in viral myocarditis: acute coronary syndrome like,25 new‐onset heart failure,26 and chronic heart failure.27 These presentations encompass a myriad of symptoms, including chest pain, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, palpitations, and fatigue.
Figure 1.
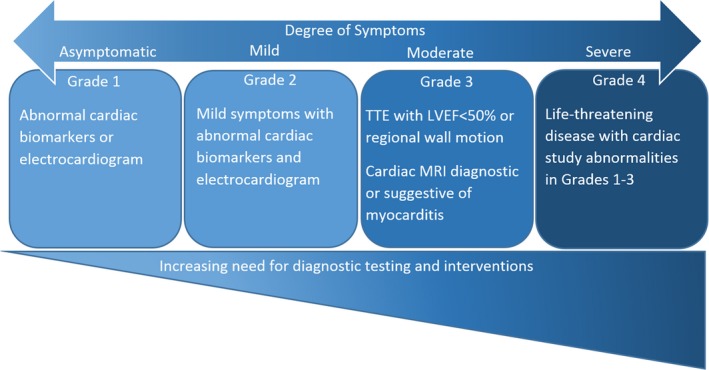
Spectrum of disease in immune checkpoint inhibitor–related myocarditis. The diagnosis of myocarditis is made after ruling out all other causes, such as ischemia or supply/demand mismatch. The grades of severity listed are from the American Society of Clinical Oncology clinical practice guidelines for the management of immune‐related adverse events (Brahmer et al23). LVEF indicates left ventricular ejection fraction; MRI, magnetic resonance imaging; TTE, transthoracic echocardiogram.
In addition to the clinical syndromes mentioned above, patients can also present with concomitant pericardial effusion with or without pericarditis.28 ICIs have been described to cause recurrent pericardial and pleural effusions.8, 29 The 2013 European Society of Cardiology guidelines for myocarditis include pericardial effusion as a supporting diagnostic criterion.27 It is unclear if some of the cases of ICI‐related pericardial effusion may have an underlying myopericarditis. In addition, many malignancies are associated with pericardial effusions, specifically lung cancer, which is one of the more common malignancies treated with ICIs. Therefore, the presence of a new or enlarging pericardial effusion should raise the suspicion of ICI‐related myocarditis but is not diagnostic in isolation from other findings.
An important distinction, which is made in viral myocarditis, is that the syndromes of myocarditis present in the absence of obstructive coronary artery disease (CAD) or other causes of heart failure.27 There are several shared risk factors between malignancy and CAD, and it is not uncommon for patients with malignancy to have underlying CAD. Therefore, in patients with cancer, it is more difficult to differentiate whether their underlying cardiovascular disease is contributing to their symptoms versus a concomitant cardiotoxicity. The clinical presentation alone may be unable to distinguish myocarditis from more common acute cardiac disorders, such as acute coronary syndrome or heart failure due to chronic ischemic heart disease. Additional diagnostic testing plays a pivotal role in the appropriate diagnosis and treatment of patients with suspected ICI cardiotoxicity.
Last, if patients with cardiac symptoms also present with other IRAEs, then the possibility of ICI‐associated myocarditis becomes higher.22 Furthermore, myocarditis has been reported more commonly in association with ICI‐related myasthenia gravis and ICI‐related myositis compared with other IRAEs.2 This is likely secondary to the proposed mechanism of shared antigens given that cardiac muscle shares more antigens with skeletal muscles compared with other organ systems. However, symptoms, such as dyspnea, may also be shared between different IRAEs, and the diagnosis of myocarditis will be dependent on further testing.
Diagnostic Testing
Diagnostic testing should aim not only to confirm the diagnosis of myocarditis, but also to rule out other more common cardiac causes of the clinical manifestations described above, such as acute coronary syndrome, chronic ischemic heart disease with or without heart failure, or other causes of nonischemic heart failure.
Laboratory
The 2 most common laboratory factors that may initially suggest the possibility of myocarditis are elevated serum troponin and natriuretic peptide levels. Troponin I, rather than troponin T, assays are preferentially recommended in the setting of suspected ICI myocarditis.30 Similar to creatine kinase and creatine kinase muscle/brain, troponin T can be also elevated when there is concomitant myositis.30 As noted above, myositis is one of the more common IRAEs known to overlap with ICI‐associated myocarditis and symptoms of dyspnea and fatigue tend to overlap between myositis and cardiac dysfunction. One of the largest clinical case series of 35 patients with ICI‐related myocarditis from multiple institutions evaluated troponin levels with fourth‐generation troponin T assays in most of the patients.4 Troponin elevation was present in 94% of cases of clinically diagnosed myocarditis. They found patients who developed major adverse cardiovascular events (MACEs) had significantly higher troponin levels at admission, peak, and before discharge. MACE was defined as cardiovascular death, cardiogenic shock, cardiac arrest, or complete heart block. The discharge or final troponin T value of ≥1.5 ng/mL was associated with significantly worse prognosis and a 4‐fold increased risk of MACEs.4 Thus, troponin levels aid not only in diagnosis but also in assessing prognosis, similar to their value in several other cardiac and even noncardiac conditions.
The use of troponin has been proposed in screening and surveillance for higher‐risk patients who are treated with combination ICI therapy.4, 23 Lee Chuy et al used this strategy in 76 consecutive patients on ipilimumab and nivolumab but did not identify any patients with overt myocarditis or subclinical myocarditis.31 However, given that myocarditis is uncommon, the evaluation of surveillance strategies in small numbers of patients is limited.31 Better predictors of risk for incident myocarditis are needed to identify those who will benefit most from routine surveillance.
Natriuretic peptides (B‐type natriuretic peptide and NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]) are established biomarkers that aid in the diagnosis of heart failure, and are often elevated in the setting of ICI‐related myocarditis. Mahmood et al4 reported that 66% of the patients in their series with ICI‐related myocarditis had elevated B‐type natriuretic peptide or NT‐proBNP levels. In contrast to troponin T, the elevation in natriuretic peptides did not predict MACEs. Similar to troponin, this class of biomarkers is also nonspecific for ICI‐related myocarditis, and the clinical picture along with imaging modalities should be used to confirm the diagnosis.
ECG/Telemetry
ECGs can help to identify myocarditis, although the findings are not specific. For patients receiving ICI, one should consider the possibility of myocarditis if any of the following are noted: new prolongation of the PR interval, atrioventricular block, ventricular arrhythmias, frequent premature ventricular complexes, ST depression, or diffuse T‐wave inversions (Figure 2). Of course, other causes of these ECG abnormalities, such as acute coronary syndrome, must be ruled out. For patients admitted with suspected myocarditis, the use of telemetry monitoring early in the admission is helpful to identify intermittent arrhythmias, such as ventricular tachycardia or nonsustained ventricular tachycardia, and increasing frequency of premature ventricular complexes. Atrial arrhythmias are also possible, with up to 30% of patients presenting with atrial fibrillation, as reported by Escudier et al.10 Although baseline ECG abnormalities are not predictive of the risk of developing myocarditis, the baseline ECG is useful as a comparison to subsequent studies.4
Figure 2.
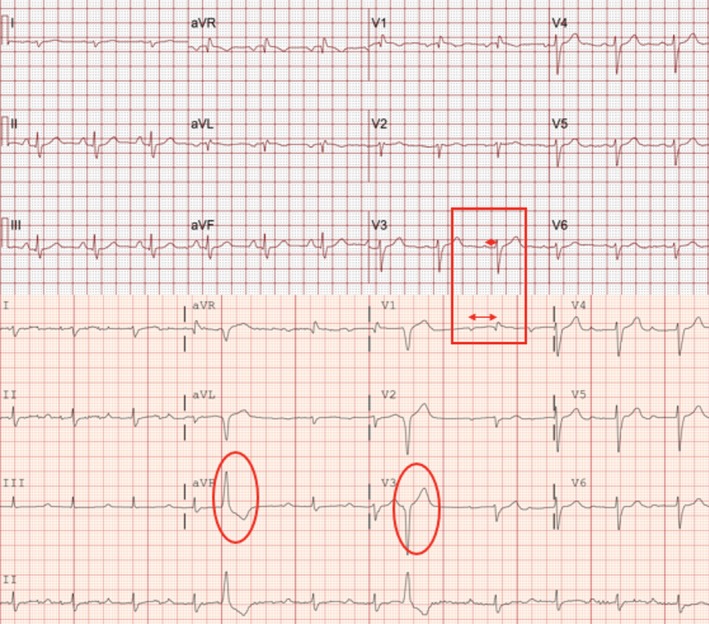
ECG abnormalities: Presented is an example of ECG abnormalities encountered during immune checkpoint inhibitor myocarditis. The severe prolongation of the PR interval on presentation can be a harbinger of potentially serious rhythm abnormalities, such as complete heart block, which occurred in this patient. Top, Baseline ECG before initiating immune checkpoint inhibitors. Normal PR interval (red box with short double arrow) and no premature ventricular complexes. Bottom, After 4 doses of nivolumab, patient presented with dyspnea and decompensated heart failure. ECG reveals new prolongation in PR interval (red box with long double arrow) and frequent premature ventricular complexes (red ovals). The patient had a temporary pacemaker placed and progressed to advanced atrioventricular block, which later recovered, and PR interval returned to normal after initiation of steroids, plasmapheresis, and infliximab.
As with troponin assays, ECGs have been proposed for use in screening and surveillance.4, 23 Lee Chuy et al used ECG in combination with troponin for 76 patients on combination ipilimumab and nivolumab but did not identify any with myocarditis.31 As mentioned above, better predictors are needed to identify those who will benefit most from surveillance.
Echocardiography
Echocardiography is an important tool to aid in the diagnosis of myocarditis, and to monitor the response to treatment. Patients with a severe life‐threatening syndrome of myocarditis may have depressed left ventricular ejection fraction (LVEF) at presentation.4 Patients may also present with regional wall motion abnormalities or even abnormalities of diastolic parameters with a normal LVEF.27 Of note, in the cohort of 35 patients with ICI‐associated myocarditis described by Mahmood et al, 51% had normal LVEF and 38% of those who developed MACEs had normal LVEF.4 Supporting evidence of myocarditis is the presence of a new pericardial effusion, which is easily assessed by echocardiography. The current ASCO clinical practice guidelines for prevention and monitoring of cardiac dysfunction in survivors of adult cancers recommend obtaining a baseline echocardiogram before initiating any potentially cardiotoxic therapy,32 but the specific ASCO guidelines for the management of IRAEs in patients with ICI therapy do not recommend for or against the routine use echocardiography before initiating ICI.23 A baseline echocardiogram could be useful to evaluate for changes with therapy, such as changes in LVEF, diastolic function, new wall motion abnormalities, or pericardial effusion, which could suggest the diagnosis of ICI‐related myocarditis.
Cardiac Magnetic Resonance Imaging
Of all the cardiovascular imaging modalities currently available, the one with the most robust validation to diagnose any myocarditis noninvasively at present is cardiovascular magnetic resonance (CMR).33 This makes CMR an important test to obtain when ICI‐related myocarditis is clinically suspected. There are different imaging techniques within CMR that have been validated to assess for myocarditis, such as T2‐weighted imaging,34, 35, 36 late gadolinium enhancement (LGE),36 extracellular volume fraction,37 T1 mapping,37, 38 and T2 mapping.37, 39 These techniques can provide evidence of myocardial inflammation by demonstrating concomitant myocardial edema and scar/injury (Figure 3). The Lake Louise Criteria, for the CMR diagnosis of myocarditis, have been developed and validated, and have been revised in 2018. They include the following33:
-
Main criteria (2 of 2): If both myocardial edema and nonischemic myocardial injury are identified, then CMR is highly suggestive of myocarditis with greater specificity. Having only 1 main criterion may still support the diagnosis of myocarditis in the correct clinical setting.
-
Myocardial edema:
Abnormal findings in T2 mapping or T2‐weighted images.
-
Nonischemic myocardial injury:
Abnormal findings on T1 mapping, LGE, or extracellular volume fraction.
-
-
Supportive criteria (helpful, suggestive, not definitive): Used alone are not diagnostic of myocarditis but may help support a diagnosis in the correct clinical setting that lacks 2 of 2 main criteria.
-
Pericarditis:
Evidence of pericardial effusion or abnormal LGE/T2 or T1 findings in pericardium.
-
Left ventricular systolic dysfunction:
Regional or global wall motion abnormalities.
-
Figure 3.

Cardiac magnetic resonance imaging of myocarditis. This case meets updated Lake‐Louise Criteria for fibrosis and edema, which is the current definition of myocarditis by cardiac magnetic resonance imaging. Left, Late gadolinium enhancement (LGE) image of replacement fibrosis at the interventricular septum with midmyocardial distribution not following a coronary vascular distribution. Right, Precontrast T2‐weighted image of the same slice location as the image in the left panel. There is an increase in signal in the septum at the same area were the LGE was present.
However, data are limited for findings on CMR specific for ICI‐associated myocarditis. In the case series of systematic assessment of ICI myocarditis, Mahmood et al reported that more than half the affected patients had preserved LVEF (51%).4 Of the patients with CMR diagnosis of ICI‐associated myocarditis, those who had midmyocardial LGE tended to have more major cardiovascular events, although this finding did not reach statistical significance (P=0.06). However, in the same series, 26% of patients who were labeled as having ICI myocarditis did not have evidence of LGE (8/31).4 In another study consisting of a pooled analysis, only 3 of 13 patients who were diagnosed with ICI myocarditis and underwent CMR had presence of LGE.10 This phenomenon of negative LGE myocarditis could be caused by either imaging early in the disease process or an incorrect clinical diagnosis of myocarditis. A repeated CMR in 2 to 3 days could be considered if an endomyocardial biopsy is not possible in cases with an uncertain diagnosis. Further studies will continue to define the role of CMR and specific findings, if any, in this recently described disease process.
Invasive Diagnostics
Endomyocardial biopsy is considered the gold standard diagnostic test for myocarditis. The myocardial tissue is evaluated using the Dallas criteria, which are histologic criteria requiring 2 main components: inflammatory infiltrate and myocardial necrosis.40 The inflammatory infiltrate can be either global or focal with patchy disease. The appearance of the inflammatory infiltrate in myocarditis has been described as similar in appearance to that of rejection seen in transplanted hearts.3 In addition, immunohistochemical staining has typically shown predominantly CD8+ T cells interspersed with CD4+ T cells and macrophages (Figure 4).3 Johnson et al identified T‐cell clonality in the 2 patients reported with similar T‐cell clones in the tumor, skeletal muscle, and cardiac muscle.3 In addition, several articles have described the upregulation and positive staining of PD‐L1 in myocardial tissue that is positive for ICI‐related myocarditis3 (Figure 4).
Figure 4.
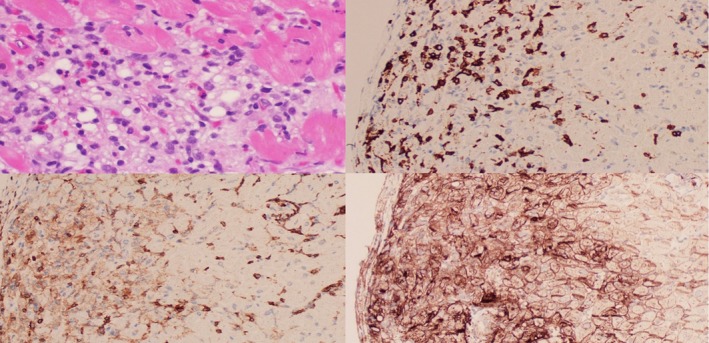
Pathological characteristics of immune checkpoint inhibitor–associated myocarditis. Top left, Hematoxylin and eosin stain of lymphocytic infiltration of myocarditis. Top right, Cluster of differentiation 8+ (CD8+) T‐cell immunohistochemical staining. Bottom left, CD4+ T‐cell immunohistochemical staining. Bottom right, Programmed death‐ligand 1+ immunohistochemical staining.
Endomyocardial biopsy is a fundamental tool for diagnosing ICI‐related myocarditis that also brings insight into mechanisms and pathophysiological characteristics of the disease. Endomyocardial biopsy has its own technical limitations, especially in cases of patchy or focal ICI‐related myocarditis. Traditional endomyocardial biopsy with focus on sampling the right ventricular septal wall may miss the affected myocardium, despite the minimum 4 to 6 samples obtained during the procedure to improve the diagnostic yield.40 Endomyocardial biopsy is an invasive diagnostic procedure with risk of a rare major complication of perforation reported at <1% in experienced centers. Challenges in interpreting the endomyocardial biopsy add another layer of complexity to the diagnosis. Biopsy specimens should be analyzed by a pathologist with experience in myocarditis or heart transplant.
Often, coronary angiography is performed with the endomyocardial biopsy to rule out significant CAD. Coronary angiography does not have a role in diagnosing myocarditis other than to rule out CAD as a cause of the clinical presentation, biomarker increase, or imaging modality abnormalities that may be seen in both disease processes.
Establishing the Diagnosis
The initial guidelines for the management of IRAE, developed by ASCO, separated myocarditis into 4 grades of severity (grade 1–grade 4; Figure 1), with grade 1 being the mildest and grade 4 being the most severe by way of a life‐threatening clinical presentation.23 The guidelines recommended that any grade of severity required further workup, which entailed cardiac biomarkers, ECG, chest x‐ray film, echocardiogram, and cardiology consultation for consideration of CMR and invasive testing, such as coronary angiography and endomyocardial biopsy.23 These recommendations were based on anecdotal data because the reported literature and recognition of myocarditis attributable to ICI therapy at the time were limited. The problem many physicians face is deciding at what point myocarditis becomes a diagnosis. For example, an increase in troponin without symptoms was classified per these guidelines as grade 1 severity myocarditis. However, troponin is a nonspecific test of myocardial injury or loss and many other factors can contribute to its elevation in addition to myocarditis.41 Subsequently, a white paper on myocarditis in the setting of cancer therapeutics attempts to guide physicians to develop more standardized definitions and categorize suspected cases into 3 groups, including definite myocarditis, probable myocarditis, and possible myocarditis, as detailed below.30
-
Definite myocarditis: presence of at least one of the following:
Pathology consistent with myocarditis.
Diagnostic CMR, clinical syndrome of myocarditis, and positive biomarker or ECG.
Echocardiography with wall motion abnormality, clinical syndrome of myocarditis, positive biomarker, positive ECG, and negative angiography for CAD.
-
Probable myocarditis:
Diagnostic CMR without clinical syndrome of myocarditis, positive ECG, or positive biomarker, OR
-
Suggestive CMR with one of the following:
Clinical syndrome of myocarditis.
Positive ECG.
Positive biomarker, OR
Echocardiography with wall motion abnormality and clinical syndrome of myocarditis with either positive ECG or biomarker, OR
Clinical syndrome of myocarditis with positron emission tomography scan evidence and no alternative diagnosis.
-
Possible myocarditis:
Suggestive CMR without clinical syndrome of myocarditis, positive ECG, or positive biomarker, OR
Echocardiography with wall motion abnormality and clinical syndrome of myocarditis or positive ECG, OR
Elevated biomarker with clinical syndrome of myocarditis or positive ECG and no alternative diagnosis.
Some have suggested that CMR has adequate sensitivity and specificity for myocarditis, thus obviating the need for endomyocardial biopsy.41 We suggest that it is ideal for patients who present with a suspicion for myocarditis to have all or most of the diagnostic studies listed above until more definitive data become available (Figure 5). However, we recognize that there may be factors preventing some of this testing, such as patient instability, inability to lie flat, severe thrombocytopenia, and/or nonavailability of some tests. The physician's clinical decision must be centered on patient safety and balance all the available clinical and diagnostic studies to determine if myocarditis is present. The clinical presentation and level of suspicion for myocarditis will then guide the treatment.
Figure 5.
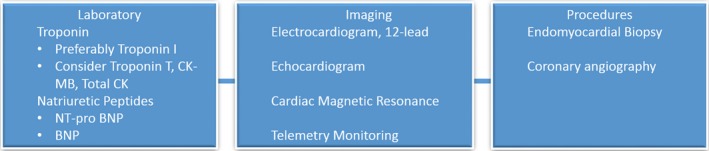
Diagnostic workup of suspected myocarditis. BNP indicates B‐type natriuretic peptide; CK, creatine kinase; CK‐MB, CK muscle/brain; NT‐proBNP, N‐terminal pro‐BNP.
Treatment of ICI‐Associated Myocarditis
The treatment of ICI‐associated myocarditis has largely been based on the use of glucocorticoids, including both oral prednisone and intravenous methylprednisolone.4, 23 Mahmood et al described 86% of their patients receiving glucocorticoids, with lower peak and discharge troponins when high‐dose glucocorticoids were used.4 In addition, the MACEs were lower in patients receiving high‐dose compared with those receiving low‐dose glucocorticoids. Although it is not entirely clear what determined use of high‐dose versus low‐dose steroids in this retrospective series, the authors recommend pulse dose steroids at 1000 mg daily, followed by 1 mg/kg daily of either oral or intravenous steroids.4 The ASCO clinical practice guidelines for IRAE suggest initiation at 1 mg/kg daily of either intravenous or oral steroids.23 There is no consensus on how long the steroids must be continued or how to taper the steroids. The ASCO clinical practice guidelines for IRAE recommend a taper of at least 4 to 6 weeks, which is a shorter taper in comparison to trials of steroid taper for viral myocarditis.23 Trials of viral myocarditis have examined steroid durations of at least 3 months and up to a year.27 For ICI‐related myocarditis, most case reports and case series track the response to steroids by monitoring troponin levels. If the troponin level begins to increase again, steroid dosing is increased and tapered over a longer period, although criteria for the increase are not yet standardized. Using these limited data, we recommend the use of high‐dose steroids at initial presentation, followed by a taper, pending clinical response and troponin monitoring. If there is a lack of adequate clinical or biomarker response to steroids, consideration may be given to other immune modulators.
There have been case reports or small case series of successfully treated ICI‐related myocarditis with intravenous immunoglobulin,20, 42 mycophenolate,42 infliximab,43 anti–thymocyte globulin,44 plasmapheresis,43 alemtuzumab,45 and abatacept.46 The effectiveness of these agents in ICI‐related myocarditis is unclear, and they are generally reserved for those patients who have an inadequate response to glucocorticoids. Even in viral myocarditis, there are limited studies for the use of these agents, with mixed results of their efficacy and with varying end points from overall survival to change in LVEF. We recommend the use of ≥1 of these agents in addition to steroids for those patients who do not respond clinically or continue to have elevated troponin levels despite high‐dose glucocorticoids (Figure 6). With infliximab, caution is required when given to patients with acute decompensated heart failure because of the risk of worsening heart failure. There are limited data suggesting that the lower dose of 5 mg/kg of infliximab may be safer in heart failure as opposed to the 10 mg/kg dose.47 Alemtuzumab is a CD52 monoclonal antibody that has been used in cardiac allograft rejection. A case report described its effectiveness for treating pembrolizumab‐related myocarditis in a patient who did not respond to high‐dose steroids, plasmapheresis, and rituximab.45 Abatacept is a CTLA‐4 agonist, and recent case reports suggest that this may be of benefit in ICI‐related myocarditis, even in patients receiving PD‐1 or PD‐L1 inhibitors because of the upstream effects of CTLA‐4 on PD‐1 and PD‐L1 function.46 However, only one case each has been reported for alemtuzumab and abatacept, and further studies are therefore needed.
Figure 6.
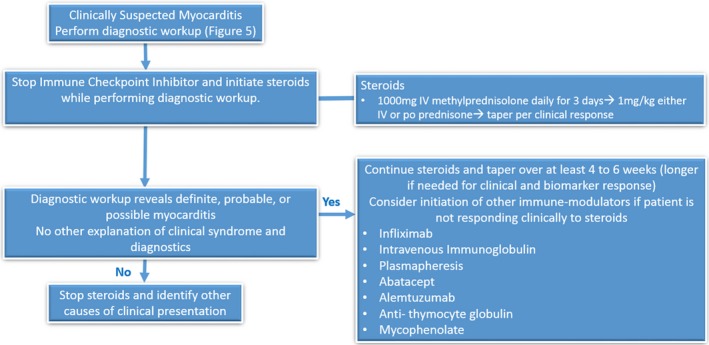
Treatment algorithm for suspected immune checkpoint inhibitor myocarditis.
If there is any suspicion of ICI‐related myocarditis, current consensus recommends holding the ICI, even for mild toxicity. The ASCO guidelines for the management of IRAE grade the severity of toxicity into 4 categories (grade 1–grade 4), with grade 4 being the most severe.23 For most other organ system IRAE, it is not recommended to hold the ICI for grade 1 toxicity, but in the case of ICI‐related myocarditis, it is recommended to hold the ICI even for this mild grade of toxicity.23 This is because of the concern for high mortality related to this IRAE. It is also not known whether ICI therapy can be safely restarted after an episode of successfully treated ICI‐related myocarditis. There is one case report in which a patient developed myocarditis on nivolumab, followed by reinitiation of therapy with a different ICI, pembrolizumab. Within 2 weeks of the first dose, the patient developed worsening heart failure requiring hospitalization, followed by permanent discontinuation of the ICI.48 Therefore, we do not recommend a repeated trial of ICI in patients with previous ICI‐related myocarditis.
In addition to immunosuppression, patients should also be treated with conventional cardiac therapy. For acute decompensated heart failure, intravenous diuretics, inotropes, and mechanical circulatory support are recommended, as per the American College of Cardiology/American Heart Association heart failure guidelines.49, 50 Arrhythmia management for ventricular tachycardia is also guided by the appropriate guidelines.51 Bradyarrhythmias, specifically advanced atrioventricular block, require temporary pacemaker insertion with the expectation that atrioventricular block may improve with treatment of myocarditis and discontinuation of the ICI.52 There are instances when permanent pacemaker insertion is needed, but the timing of the transition is unpredictable and is currently dependent on clinical judgement of the treating cardiologist. After the short‐term stage, guideline‐directed therapy for chronic left ventricular dysfunction and/or heart failure may be needed.49, 50
Conclusions
ICI‐related myocarditis is a complex disease that has similarities in presentation to many other acute cardiac syndromes. This overlap, along with the relative novelty of the entity with only a preliminary understanding about the pathophysiological characteristics, makes this a difficult condition to diagnose and treat. In addition, ICI‐related myocarditis is uncommon, making it difficult to study in a randomized manner, with most guidance being available from case reports and small case series. It is necessary for both the oncologist and cardiologist to have a high suspicion for this ICI‐related cardiac toxicity. Studies evaluating multimodality imaging and invasive testing with endomyocardial biopsy will guide development of better diagnostic algorithms for this condition. Current treatment is largely based on glucocorticoids with a possible role for more targeted immune modulators, depending on the clinical course of individual patients. As the indications and use of ICI continue to expand, more cases of ICI‐related myocarditis will likely be identified. Further research is therefore needed to establish mechanisms and diagnostic strategies, and to guide treatment of this disease entity.
Sources of Funding
Dr Deswal is supported in part by the Ting Tsung and Wei Fong Chao Distinguished Chair.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e013757 DOI: 10.1161/JAHA.119.013757.)
References
- 1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang DY, Salem J‐E, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun‐Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta‐analysis. JAMA Oncol. 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig‐Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moslehi JJ, Salem JE, Sosman JA, Lebrun‐Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet. 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salem JE, Manouchehri A, Moey M, Lebrun‐Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor‐associated myocarditis. Lancet. 2018;392:382–383. [DOI] [PubMed] [Google Scholar]
- 8. Palaskas N, Morgan J, Daigle T, Banchs J, Durand J‐B, Hong D, Naing A, Le H, Hassan SA, Karimzad K, Mouhayar E, Kim P, Lopez‐Mattei J, Thompson K, Yusuf SW, Iliescu C. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123:1351–1357. [DOI] [PubMed] [Google Scholar]
- 9. Hu YB, Zhang Q, Li HJ, Michot JM, Liu HB, Zhan P, Lv TF, Song Y. Evaluation of rare but severe immune related adverse effects in PD‐1 and PD‐L1 inhibitors in non‐small cell lung cancer: a meta‐analysis. Transl Lung Cancer Res. 2017;6:S8–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S, Thuny F. Clinical features, management, and outcomes of immune checkpoint inhibitor‐related cardiotoxicity. Circulation. 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. [DOI] [PubMed] [Google Scholar]
- 12. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. [DOI] [PubMed] [Google Scholar]
- 13. Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA‐4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krummel MF, Allison JP. CD28 and CTLA‐4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. [DOI] [PubMed] [Google Scholar]
- 16. Liu Peter P, Mason Jay W. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. [DOI] [PubMed] [Google Scholar]
- 17. Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death‐1 ligand 1 (PD‐L1) regulates CD8+ T‐cell mediated injury in the heart. Circulation. 2007;116:2062–2071. [DOI] [PubMed] [Google Scholar]
- 18. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD‐1 receptor‐deficient mice. Science. 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 19. Ji C, Roy MD, Golas J, Vitsky A, Ram S, Kumpf SW, Martin M, Barletta F, Meier WA, Hooper AT, Sapra P, Khan NK, Finkelstein M, Guffroy M, Buetow BS. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res. 2019;25:4735–4748. [DOI] [PubMed] [Google Scholar]
- 20. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, Gertler AS, Moslehi JJ, Conry RM. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal myocarditis following treatment with the PD‐1 inhibitor nivolumab. J Forensic Sci. 2018;63:954–957. [DOI] [PubMed] [Google Scholar]
- 22. Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A, Mertz KD. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors‐an autopsy study. J Immunother Cancer. 2016;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez‐Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berg DD, Vaduganathan M, Nohria A, Davids MS, Alyea EP, Torre M, Padera RF Jr. Immune‐related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. Eur J Heart Fail. 2017;19:682–685. [DOI] [PubMed] [Google Scholar]
- 25. Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, Mohri M. Acute lymphocytic myocarditis with anti‐PD‐1 antibody nivolumab. Circ Heart Fail. 2016;9:e003514. [DOI] [PubMed] [Google Scholar]
- 26. Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 28. Amiri‐Kordestani L, Moslehi J, Cheng J, Tang S, Schroeder R, Sridhara R, Karg K, Connolly J, Beaver JA, Blumenthal GM, Pazdur R. Cardiovascular adverse events in immune checkpoint inhibitor clinical trials: a U.S. Food and Drug Administration pooled analysis. J Clin Oncol. 2018;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy—a report of two cases. J Immunother Cancer. 2016;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonaca Marc P, Olenchock Benjamin A, Salem J‐E, Wiviott Stephen D, Ederhy S, Cohen A, Stewart Garrick C, Choueiri Toni K, Di Carli M, Allenbach Y, Kumbhani Dharam J, Heinzerling L, Amiri‐Kordestani L, Lyon Alexander R, Thavendiranathan P, Padera R, Lichtman A, Liu Peter P, Johnson Douglas B, Moslehi J. Myocarditis in the setting of cancer therapeutics. Circulation. 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, Neilan TG, Fradley MG, Ramanathan LV, Wolchok JD, Steingart RM, Gupta D. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center Experience. Oncologist. 2019;24:e196–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 33. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 34. Fernández‐Jiménez R, Sánchez‐González J, Aguero J, del Trigo M, Galán‐Arriola C, Fuster V, Ibáñez B. Fast T2 gradient‐spin‐echo (T2‐GraSE) mapping for myocardial edema quantification: first in vivo validation in a porcine model of ischemia/reperfusion. J Cardiovasc Magn Reson. 2015;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Knobelsdorff‐Brenkenhoff F, Schüler J, Dogangüzel S, Dieringer Matthias A, Rudolph A, Greiser A, Kellman P, Schulz‐Menger J. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2017;10:e005242. [DOI] [PubMed] [Google Scholar]
- 36. Abdel‐Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz‐Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. [DOI] [PubMed] [Google Scholar]
- 37. Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel K‐P, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer‐Trial. J Am Coll Cardiol. 2016;67:1800–1811. [DOI] [PubMed] [Google Scholar]
- 38. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. Native T1‐mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spieker M, Haberkorn S, Gastl M, Behm P, Katsianos S, Horn P, Jacoby C, Schnackenburg B, Reinecke P, Kelm M, Westenfeld R, Bönner F. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. 2017;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 41. Spallarossa P, Tini G, Sarocchi M, Arboscello E, Grossi F, Queirolo P, Zoppoli G, Ameri P. Identification and management of immune checkpoint inhibitor‐related myocarditis: use troponin wisely. J Clin Oncol. 2019;37:2201–2205. DOI: 10.1200/JCO.18.02464. [DOI] [PubMed] [Google Scholar]
- 42. Arangalage D, Delyon J, Lermuzeaux M, Ekpe K, Ederhy S, Pages C, Lebbe C. Survival after fulminant myocarditis induced by immune‐checkpoint inhibitors. Ann Intern Med. 2017;167:683–684. [DOI] [PubMed] [Google Scholar]
- 43. Frigeri M, Meyer P, Banfi C, Giraud R, Hachulla AL, Spoerl D, Friedlaender A, Pugliesi‐Rinaldi A, Dietrich PY. Immune checkpoint inhibitor‐associated myocarditis: a new challenge for cardiologists. Can J Cardiol. 2018;34:92.e1–92.e3. [DOI] [PubMed] [Google Scholar]
- 44. Tay RY, Blackley E, McLean C, Moore M, Bergin P, Gill S, Haydon A. Successful use of equine anti‐thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017;117:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH Jr. Alemtuzumab for immune‐related myocarditis due to PD‐1 therapy. N Engl J Med. 2019;380:2375–2376. [DOI] [PubMed] [Google Scholar]
- 46. Salem J‐E, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, Kerneis M. Abatacept for severe immune checkpoint inhibitor‐associated myocarditis. N Engl J Med. 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 47. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double‐blind, placebo‐controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor‐alpha, in patients with moderate‐to‐severe heart failure: results of the anti‐TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 48. Tajmir‐Riahi A, Bergmann T, Schmid M, Agaimy A, Schuler G, Heinzerling L. Life‐threatening autoimmune cardiomyopathy reproducibly induced in a patient by checkpoint inhibitor therapy. J Immunother. 2018;41:35–38. [DOI] [PubMed] [Google Scholar]
- 49. Yancy Clyde W, Jessup M, Bozkurt B, Butler J, Casey Donald E, Drazner Mark H, Fonarow Gregg C, Geraci Stephen A, Horwich T, Januzzi James L, Johnson Maryl R, Kasper Edward K, Levy Wayne C, Masoudi Frederick A, McBride Patrick E, McMurray John JV, Mitchell Judith E, Peterson Pamela N, Riegel B, Sam F, Stevenson Lynne W, Tang WHW, Tsai Emily J, Wilkoff Bruce L. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 50. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 51. Al‐Khatib Sana M, Stevenson William G, Ackerman Michael J, Bryant William J, Callans David J, Curtis Anne B, Deal Barbara J, Dickfeld T, Field Michael E, Fonarow Gregg C, Gillis Anne M, Granger Christopher B, Hammill Stephen C, Hlatky Mark A, Joglar José A, Kay GN, Matlock Daniel D, Myerburg Robert J, Page Richard L. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 52. Behling J, Kaes J, Munzel T, Grabbe S, Loquai C. New‐onset third‐degree atrioventricular block because of autoimmune‐induced myositis under treatment with anti‐programmed cell death‐1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017;27:155–158. [DOI] [PubMed] [Google Scholar]


