Abstract
Background
This study evaluated the impact of hepatitis C–positive (HCV+) donors on outcomes of heart transplantation in the United States.
Methods and Results
Adults undergoing isolated heart transplantation in the United States between January 1, 2016, and December 31, 2018, were included. The primary outcome was 1‐year post‐transplant survival. Multivariable Cox regression and 2:1 propensity matching were used to compare outcomes between transplants with HCV+ and hepatitis C–negative (HCV−) donors. A subanalysis was performed to evaluate the impact of nucleic acid amplification test positivity on outcomes. Of 7889 isolated heart transplants performed during the study period, 343 (4.4%) used HCV+ donors. Overall unadjusted 1‐year posttransplant survival was not statistically different between HCV− versus HCV+ donors (91.1% versus 90.2%; P=0.86), a finding that persisted after risk adjustment (hazard ratio, 1.05; 95% CI, 0.70–1.58; P=0.80). Propensity matching resulted in 675 well‐balanced patients (437 HCV− and 238 HCV+). Overall 1‐year posttransplant survival was not statistically different in propensity‐matched analysis (89.8% HCV− versus 89.2% HCV+; P=0.88). Rates of 1‐year drug‐treated rejection (21.1% versus 22.1%; P=0.84), postoperative dialysis (11.4% versus 14.7%; P=0.22), and stroke (4.6% versus 2.1%; P=0.10) were also not statistically different between HCV− and HCV+ groups, respectively. Outcomes were not statistically different between nucleic acid amplification test–negative and nucleic acid amplification test–positive HCV+ donors.
Conclusions
Adult heart transplants using HCV+ donors, including those that are nucleic acid amplification test positive, can be performed without an adverse impact on 1‐year survival. Wider implementation of protocols for using HCV+ donors and an assessment of longer‐term outcomes including seroconversion rates will be important in maximizing the effect of HCV+ donors on national donor shortages.
Keywords: heart failure, heart transplantation, hepatitis C, rejection
Subject Categories: Cardiovascular Surgery, Transplantation, Mortality/Survival, Quality and Outcomes, Complications
Clinical Perspective
What Is New?
This is a large, multicenter study evaluating modern outcomes of heart transplantation using hepatitis C–positive donors.
Posttransplant survival, rejection rates, and complications were similar between hepatitis C–positive and –negative donors.
What Are the Clinical Implications?
Adult heart transplantation can be performed using hepatitis C–positive donors without an adverse impact on outcomes.
Further research is needed on specific protocols as well as longer‐term outcomes and seroconversion rates to maximize the potential effect and use of these donors.
Introduction
Although adult heart transplantation can be lifesaving for many patients with end‐stage heart failure with excellent short‐ and long‐term survival, its use is limited by a persistent donor shortage.1 Using hepatitis C–positive (HCV+) donors may be one strategy to help combat the organ shortage for heart transplantation. An earlier report of the UNOS (United Network for Organ Sharing) registry demonstrated inferior survival with the use of HCV+ donors in heart transplantation, with a greater likelihood of death attributable to liver disease and coronary allograft vasculopathy.2 This was likely related to the use of interferon‐based therapy for hepatitis C, and enthusiasm for using HCV+ donors dissipated as a result. Recently, there has been a resurgence of interest in cardiac transplantation using HCV+ donors with the advent of direct‐acting antiviral drugs, with low reported rates of seroconversion and acceptable posttransplant survival in small series.3, 4 The aim of this study was to evaluate national outcomes of adult heart transplantation using HCV+ donors.
Methods
Study Population
The authors declare that all supporting data are available within the article and its online supplementary files. Adult patients aged 18 years or older undergoing isolated heart transplantation in the United States between January 1, 2016, and December 31, 2018, were included in the study. Pediatric patients and those undergoing multiorgan transplantation were excluded. The UNOS registry was used to identify all eligible heart transplants performed during the study period. The institutional review board approved this study. The requirement for informed consent for this study for each individual subject was waived.
Data Analysis
The primary stratification was heart transplants performed using HCV+ versus hepatitis C–negative (HCV−) donors. HCV+ was defined as having a positive antibody to HCV, with further substratification based on nucleic acid amplification test (NAT) positivity. Baseline characteristics, including donor, recipient, recipient‐donor matching, and transplant‐related variables, were compared between HCV+ and HCV− donor transplants.
The primary outcome was overall 1‐year survival following heart transplantation. All causes of mortality were included in the survival analysis. Kaplan–Meier survival curves were generated and compared using the log‐rank test. A multivariable Cox regression analysis was also performed to evaluate the independent effect of HCV+ donor use on posttransplant survival. The multivariable model was constructed using variables that were supported with previously published literature or were associated with posttransplant survival in univariate Cox regression analysis with an exploratory P value of <0.05. Eligible variables were incorporated into the model in a forward and backward stepwise fashion using the likelihood ratio test and Akaike's information criteria in a nested model approach to maximize the explanatory power of our model. Variables with >15% missing data were excluded from the model, as the model was constructed using casewise deletion. The proportional hazards assumption was tested using Schoenfeld residuals and complementary log‐log plots for each covariate.
Secondary outcomes included drug‐treated rejection within 1 year of transplantation. All forms of rejection, including antibody‐mediated rejection, were included in this outcome. Rates of new‐onset postoperative dialysis and postoperative stroke were also compared. For the latter 2 secondary outcomes, postoperative was defined as occurring during the index hospitalization following transplantation. Length of hospitalization following heart transplantation was an additional secondary outcome.
Propensity matching was performed to account for baseline differences. This was done using a greedy matching algorithm with 2:1 nearest neighbor matching without replacement and a caliper of 0.01 of the standard deviation of the propensity score. A subanalysis was performed, limiting the patients to those transplanted only at centers that used HCV+ organs. This was done with both the unmatched and propensity‐matched populations to compare outcomes at these centers using HCV+ versus HCV− donors. Another subanalysis evaluated outcomes of heart transplants using NAT+ versus NAT− HCV+ donors.
Categorical data are presented as number and percentage and compared using the chi‐square test. Normally distributed continuous data are presented as mean with standard deviation and compared with the Student t test. Nonparametric continuous data are presented as median with interquartile range and compared with the Wilcoxon rank‐sum test. All statistical analyses were performed with version 14 STATA software (StataCorp, College Station, TX).
Results
Baseline Characteristics of the Study Population
There were 7889 isolated heart transplants performed in adults in the United States during the study period at 128 centers. Of these, 343 (4.4%) were performed using HCV+ donors at 36 centers. By year of transplant, the percentage of centers performing heart transplants that used HCV+ donors increased from 8.5% in 2016, to 13.2% in 2017, to 29.4% in 2018. Only 15 (4.4%) of the recipients receiving HCV+ donors had a history of treated HCV, with the remaining 328 (95.6%) being HCV−. At baseline, there were significant differences between HCV+ versus HCV− donors (Table S1). HCV+ donors were older and were more likely to be white and blood type O, with drug overdose as the mechanism of death. HCV+ donors also had a lower proportion with inotrope use and a higher terminal serum creatinine.
There were also baseline recipient differences (Table S2). More recipients of HCV+ donors were blood type O. In addition, recipients of HCV+ donors had higher serum creatinine, and more were bridged with the HeartMate 3 (Abbott, Inc, Plymouth, MN) ventricular assist device. Transplants performed using HCV+ donors had a higher percentage of race matching between the donor and recipient (Table S3). Recipients of HCV+ donors had shorter wait list time, with a greater distance between the donor hospital and transplant center along with longer cold ischemic time (Table S3).
Outcomes Before Propensity Matching
The overall 1‐year posttransplant recipient survival was similar between HCV− (91.1%) and HCV+ (90.2%) donors (P=0.86) (Figure S1). In multivariable analysis, the use of an HCV+ donor was not associated with posttransplant survival (hazard ratio, 1.05; 95% CI, 0.70–1.58; P=0.80) (Table 1). The rate of 1‐year drug‐treated rejection was similar (HCV−, 19.6% versus HCV+, 21.6%; P=0.60). The rate of new‐onset postoperative dialysis was comparable (HCV−, 11.2% versus HCV+, 13.7%; P=0.16). The rate of postoperative stroke was also comparable (HCV−, 2.9% versus HCV+, 2.0%; P=0.35). Length of hospitalization was similar in HCV− (median, 16 days; interquartile range, 11–23 days) and HCV+ cases (median, 16 days; interquartile range, 12–24 days) (P=0.36). Similar results were obtained when excluding recipients with a history of treated HCV. The most common causes of death among HCV+ donor recipients were multiorgan failure (n=5), cardiogenic shock (n=3), and sepsis (n=2).
Table 1.
Multivariable Cox Regression Analysis for Posttransplant Mortality in the 675 Propensity‐Matched Patients
| Covariate | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Hepatitis C–positive donor | 1.05 | 0.70–1.58 | 0.80 |
| Donor age (increasing, per year) | 1.01 | 1.01–1.02 | <0.001 |
| Recipient age (increasing, per year) | 1.02 | 1.01–1.02 | <0.001 |
| Etiology of heart failure | |||
| Nonischemic dilated cardiomyopathy | Reference | Reference | Ref. |
| Ischemic cardiomyopathy | 1.12 | 0.95–1.32 | 0.17 |
| Congenital heart disease | 2.36 | 1.59–3.51 | <0.001 |
| Restrictive cardiomyopathy | 1.60 | 1.15–2.24 | 0.006 |
| Valvular heart disease | 0.90 | 0.44–1.86 | 0.78 |
| Failed primary heart transplant | 1.93 | 1.26–2.97 | 0.003 |
| Hypertrophic cardiomyopathy | 0.89 | 0.53–1.49 | 0.65 |
| Other etiology | 0.83 | 0.47–1.48 | 0.53 |
| Diabetes mellitus | 1.22 | 1.05–1.43 | 0.01 |
| Serum creatinine (increasing, per 1 mg/dL) | 1.05 | 1.00–1.11 | 0.07 |
| Serum bilirubin (increasing, per 1 g/dL) | 1.08 | 1.06–1.10 | <0.001 |
| Mechanical ventilation | 1.83 | 1.05–3.20 | 0.03 |
| Bridge with ECMO | 2.74 | 1.59–4.72 | <0.001 |
| Bridge with ventricular assist device | |||
| None | Reference | Reference | Ref. |
| Left ventricular assist device | 1.21 | 1.04–1.42 | 0.01 |
| Right ventricular assist device | 5.53 | 2.25–13.6 | <0.001 |
| Biventricular assist device | 3.62 | 2.27–5.76 | <0.001 |
ECMO indicates extracorporeal membrane oxygenation.
Propensity‐Matched Analysis
Propensity matching yielded 437 HCV− and 238 HCV+ donors. The baseline donor characteristics were not statistically different except for a higher proportion with drug overdose as a mechanism of death in the HCV+ cohort (Table 2). Recipient and donor‐recipient matching characteristics were not statistically different in the propensity‐matched analysis (Tables 3 and 4). Although distance between donor hospital and transplant center was longer in the HCV+ matched cohort, the cold ischemic time was not statistically different between groups after matching (Table 4). In total, propensity matching resulted in well‐balanced groups that had <10% standardized mean difference across all covariates, including mechanism of death (Figure S2).
Table 2.
Comparison of Baseline Donor Characteristics Between Hepatitis C–Negative and Hepatitis C–Positive Donors After Propensity Matching
| Hepatitis C Negative (n=437) | Hepatitis C Positive (n=238) | P Value | |
|---|---|---|---|
| Age, y (IQR) | 32 (24–42) | 33 (27–38) | 0.42 |
| Female, n (%) | 136 (31.1) | 73 (30.7) | 0.90 |
| Race, n (%) | 0.13 | ||
| White | 352 (80.6) | 197 (82.8) | |
| Black | 50 (11.4) | 21 (8.8) | |
| Hispanic | 31 (7.1) | 16 (6.7) | |
| Asian | 3 (0.7) | 0 (0) | |
| Other | 1 (0.2) | 4 (1.7) | |
| Body mass index, kg/m2 (IQR) | 26 (23–31) | 26 (24–31) | 0.98 |
| Blood type, n (%) | 0.45 | ||
| A | 146 (33.4) | 78 (32.8) | |
| AB | 26 (6.0) | 11 (4.6) | |
| B | 64 (14.7) | 27 (11.3) | |
| O | 201 (46.0) | 122 (51.3) | |
| Cytomegalovirus positive | 231 (53.0) | 123 (51.9) | 0.79 |
| Mechanism of donor death, n (%) | <0.001 | ||
| Trauma | 106 (24.3) | 44 (18.5) | |
| Cerebrovascular | 66 (15.1) | 13 (5.5) | |
| Drug overdose | 91 (20.8) | 154 (64.7) | |
| Other | 174 (39.8) | 27 (11.3) | |
| Diabetes mellitus | 19 (4.4) | 8 (3.4) | 0.54 |
| Inotrope use | 157 (35.9) | 81 (34.0) | 0.62 |
| Terminal serum creatinine, mg/dL (IQR) | 1.00 (0.73–1.72) | 1.10 (0.80–1.77) | 0.13 |
| Left ventricular ejection fraction, % (IQR) | 60 (57–65) | 60 (56–65) | 0.43 |
IQR indicates interquartile range.
Table 3.
Comparison of Baseline Recipient Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors After Propensity Matching
| Hepatitis C Negative (n=437) | Hepatitis C Positive (n=238) | P Value | |
|---|---|---|---|
| Age, y (IQR) | 58 (48–64) | 57 (48–64) | 0.91 |
| Female, n (%) | 118 (27.0%) | 62 (26.1%) | 0.79 |
| Race, n (%) | 0.65 | ||
| White | 291 (66.6) | 162 (68.1) | |
| Black | 101 (23.1) | 49 (20.6) | |
| Hispanic | 33 (7.6) | 20 (8.4) | |
| Asian | 12 (2.8) | 6 (2.5) | |
| Other | 0 (0) | 1 (0.4) | |
| Body mass index, kg/m2 (IQR) | 26 (23–31) | 26 (24–31) | 0.98 |
| Blood type, n (%) | 0.45 | ||
| A | 146 (33.4) | 78 (32.8) | |
| AB | 26 (6.0) | 11 (4.6) | |
| B | 64 (14.7) | 27 (11.3) | |
| O | 201 (46.0) | 122 (51.3) | |
| Cytomegalovirus positive, n (%) | 236 (54.0) | 121 (50.8) | 0.43 |
| Etiology of heart failure, n (%) | 0.72 | ||
| Nonischemic dilated cardiomyopathy | 245 (56.1) | 128 (53.8) | |
| Ischemic cardiomyopathy | 132 (30.2) | 70 (29.4) | |
| Congenital heart disease | 12 (2.8) | 8 (3.4) | |
| Restrictive cardiomyopathy | 15 (3.4) | 14 (5.9) | |
| Valvular heart disease | 5 (1.1) | 3 (1.3) | |
| Failed primary heart transplant | 10 (2.3) | 6 (2.5) | |
| Hypertrophic cardiomyopathy | 9 (2.1) | 7 (2.9) | |
| Other etiology | 9 (2.1) | 2 (0.8) | |
| Diabetes mellitus, n (%) | 124 (28.4) | 60 (25.2) | 0.38 |
| Serum creatinine, mg/dL (IQR) | 1.19 (0.97–1.40) | 1.21 (1.00–1.49) | 0.17 |
| Total bilirubin, mg/dL (IQR) | 0.60 (0.40–0.90) | 0.70 (0.50–1.00) | 0.24 |
| Mechanical ventilation, n (%) | 0 (0) | 1 (0.4) | 0.18 |
| Intra‐aortic balloon pump, n (%) | 47 (10.8) | 23 (9.7) | 0.66 |
| ECMO, n (%) | 0 (0) | 0 (0) | 0.99 |
| Bridge with ventricular assist device, n (%) | 0.73 | ||
| None | 237 (54.2) | 130 (54.6) | |
| Left ventricular assist device | 188 (43.0) | 105 (44.1) | |
| Right ventricular assist device | 2 (0.5) | 0 (0) | |
| Biventricular assist device | 4 (0.9) | 1 (0.4) | |
| Total artificial heart | 6 (1.4) | 2 (0.8) | |
| Type of left ventricular assist device, n (%) | |||
| HeartMate 2 | 80 (18.3 | 31 (13.0) | 0.08 |
| HeartWare | 81 (18.5%) | 45 (18.9) | 0.91 |
| HeartMate 3 | 1 (0.2) | 3 (1.3) | 0.10 |
| Other durable device | 32 (7.3) | 26 (10.9) | 0.11 |
| Temporary device | 3 (0.7) | 1 (0.4) | 0.66 |
| Most recent panel reactive antibody, % (IQR) | 0 (0–8) | 0 (0–10) | 0.95 |
ECMO indicates extracorporeal membrane oxygenation; IQR, interquartile range.
Table 4.
Comparison of Baseline Recipient‐Donor Matching and Transplant‐Related Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors After Propensity Matching
| Hepatitis C Negative (n=437) | Hepatitis C Positive (n=238) | P Value | |
|---|---|---|---|
| Sex matched, n (%) | 335 (76.7) | 187 (78.6) | 0.57 |
| Race matched, n (%) | 256 (58.6) | 143 (60.1) | 0.70 |
| HLA matched (≥3 antigens), n (%) | 34 (7.8) | 20 (8.4) | 0.84 |
| Blood type matched, n (%) | 389 (89.0) | 205 (86.1) | 0.27 |
| Cytomegalovirus status matched, n (%) | 213 (48.9) | 132 (55.7) | 0.09 |
| Days on wait list, n (IQR) | 98 (28–281) | 89 (21–298) | 0.36 |
| Donor hospital to transplant center distance, miles (IQR) | 154 (35–331) | 261 (98–436) | <0.001 |
| Cold ischemic time, h (IQR) | 3.5 (2.8–3.9) | 3.5 (3.0–4.0) | 0.18 |
HLA indicates human leukocyte antigen; IQR, interquartile range.
There were no statistical differences in overall 1‐year survival in the propensity‐matched analysis between HCV− (89.8%) and HCV+ (89.2%) donor transplants (P=0.88) (Figure). One‐year drug‐treated rejection rates were statistically comparable (HCV−, 21.1% versus HCV+, 22.1%; P=0.84). Rates of new‐onset postoperative dialysis (HCV−, 11.4% versus HCV+, 14.7%; P=0.22) as well as postoperative stroke (HCV−, 4.6% versus HCV+, 2.1%; P=0.10) were not statistically different, nor was length of hospital stay between HCV− (median, 16 days; interquartile range, 12–25 days) and HCV+ transplants (median, 15.5 days; interquartile range, 11.5–24 days) (P=0.94).
Figure 1.
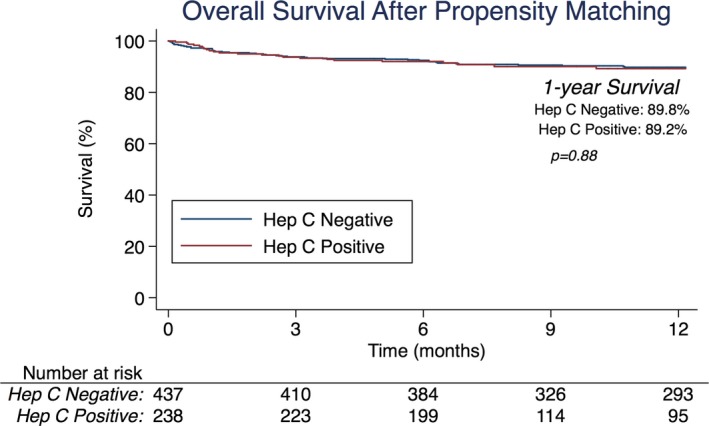
Overall 1‐year survival in the propensity‐matched heart transplants performed using hepatitis C–negative vs. hepatitis C–positive donors.
Subanalysis Limited to Centers Performing Heart Transplants With HCV+ Donors
Of the 128 centers performing heart transplants during the study period, 36 (28.1%) used HCV+ donors. When limiting the analysis to centers performing HCV+ donor transplants, there were 3466 heart transplants performed with HCV− donors from these centers during the same study period. There were no statistical differences in 1‐year survival between HCV− and HCV+ donors at these centers (91.0% versus 90.2%, respectively; P=0.85). There were 223 HCV− and 238 HCV+ donor transplants performed in the propensity‐matched analysis when the analysis was limited to these 36 centers. Again, the 1‐year survival was not statistically different (HCV−, 89.7% versus HCV+, 89.2%, P=0.87).
Subanalysis Evaluating the Impact of NAT+ Donor Use
NAT status was known in 331 (96.5%) HCV+ donor cases. In all, 194 (58.6%) were NAT+ HCV+ donors. There were no statistical differences in 1‐year survival when comparing NAT− HCV+ and NAT+ HCV+ donors (86.9% versus 91.9%, respectively; P=0.16). There were also no differences in 1‐year drug‐treated rejection (NAT−, 18.8% versus NAT+, 25.4%; P=0.41), new‐onset postoperative dialysis (NAT−, 17.5% versus NAT+, 11.9%; P=0.15), or postoperative stroke (NAT−, 2.2% versus NAT+, 2.1%; P=0.94). When directly comparing NAT+ HCV+ donors to HCV− donors, 1‐year survival was again statistically comparable (91.9% versus 91.1%, respectively; P=0.37), as were rates of each of the secondary outcomes of rejection, dialysis, and stroke (each P>0.05).
Discussion
An earlier report that analyzed the UNOS registry for heart transplants performed between 1994 and 2003 in the United States demonstrated a 1‐year mortality rate for transplants using HCV+ donors that was over double that observed in HCV− donors.2 This led many groups to abandon using HCV+ donors for heart transplantation. The recent resurgence in interest in using HCV+ donors largely stems from the development of highly effective direct‐acting antiviral agents that have revolutionized the treatment of hepatitis C.5
A single‐center study reported outcomes of heart transplantation in 8 recipients of HCV+ donors and demonstrated that immediate 4‐week treatment with a direct‐acting antiviral agent resulted in an undetectable viral load in all patients with 100% survival at 6 months with no treatment‐related serious adverse events.3 Another single‐center analysis evaluated heart transplant outcomes in 13 recipients of HCV+ donors, of which 12 were HCV− and 1 had a history of treated HCV.4 In the 12 HCV− recipients, 9 (69%) developed HCV viremia after transplantation and received direct‐acting antiviral agent therapy, with 8 demonstrating cure and 1 patient dying during treatment from a pulmonary embolism. All cases of recipient seroconversion were in transplants using NAT+ HCV+ donors. Another single institution experience of 10 HCV− recipients undergoing heart transplantation with HCV+ donors demonstrated that 9 patients achieved a sustained virologic response at 12 weeks, with the last patient having a positive crossmatch and dying from rejection and multiorgan failure.5
Our current analysis provides a nationwide, larger cohort analysis with heart transplantation using HCV+ donors in the modern era and demonstrates that it can be performed safely with no adverse impact on 1‐year survival. In this current snapshot, it appears that 28% of centers are using HCV+ donors. Although this certainly represents a steep increase from earlier years, one could argue that there is room for broader implementation and use of HCV+ donors.
The potential pool of HCV+ donors is substantial and largely reflective of the rising opioid epidemic in the United States.6 An analysis of heart transplants performed between 2010 and 2017 in the United States showed that 11% were from donors that overdosed on drugs, and these donors were more likely to be HCV+.7 Discarded donor organs from drug overdose were ≈6‐fold more likely to be HCV+.7 Another study of 64 HCV+ donor heart transplants demonstrated that only 5% of donor HCV+ hearts were accepted for transplantation in the United States despite similar posttransplant survival.8
Although our study is supportive of using HCV+ donors for heart transplantation, there are additional factors that should be studied further. Protocols for following and treating seroconversion in HCV− recipients should be evaluated and refined. Granularity regarding seroconversion rates, anti‐HCV therapy initiation and timing, and adverse side effects from therapy are not provided in the UNOS registry but are essential to understanding the impact of HCV+ donor use in heart transplantation. In particular, it is unclear if treatment of all HCV− recipients transplanted with an HCV+ donor should be initiated in advance, particularly with NAT+ donors, or if treatment should be initiated with the first NAT positivity in the recipient.
Another important aspect that should be further studied is longer‐term survival beyond 1‐year. Donor HCV positivity has been associated with the development of allograft coronary artery disease.9, 10 One study demonstrated a 3‐fold greater odds of developing any vasculopathy and over 9‐fold greater risk of developing advanced vasculopathy with the use of HCV+ donors.9 An analysis of heart transplants using HCV+ donors in the more recent era demonstrated that 10% and 25% of recipients developed grade 1 coronary allograft vasculopathy by 6 months and 1 year, respectively, although no patients required percutaneous or surgical revascularization.11
Interestingly, in our analysis, the rates of drug‐treated rejection within 1 year were comparable between HCV− and HCV+ donors both in the unmatched and propensity‐matched analysis. A prior UNOS registry analysis demonstrated that the 4 factors that significantly predicted the risk of drug‐treated rejection in 1 year following heart transplantation included younger recipient age, non‐Asian recipient race, female recipient, and <3 human leukocyte antigens matched between the recipient and donor.12 All of these variables were comparable between HCV− and HCV+ cohorts in our study. Reported risk factors for antibody‐mediated rejection include elevated panel reactive antibody, bridge with ventricular assist device, and redo heart transplant, factors that were also comparable between the cohorts in the current analysis.13, 14, 15
Limitations
This is a retrospective analysis and therefore has inherent limitations related to the study design. Granular aspects of HCV‐related variables such as HCV genotype, anti‐HCV therapy initiation and protocols, and rates of seroconversion were not available in the UNOS registry. Because of the recent resurgence of HCV+ donors in heart transplantation, longer‐term follow‐up was not available for this study period. Details regarding surveillance of donor organs after transplantation including the development of cardiac allograft vasculopathy were not available in the database. There may be data not contained within the UNOS registry that are predictive of outcomes that were not adjusted for in this analysis. Finally, there was a limited number of patients, particularly in the propensity‐matched analysis, which subjects the analyses to type II statistical error. Further validation in larger cohorts is therefore warranted.
Conclusions
This study evaluated outcomes of adult heart transplantation using HCV+ donors in 343 patients in the United States in the modern era. The major finding was that 1‐year posttransplant survival was comparable to HCV− donors. This suggests that heart transplants using HCV+ donors, including those that are NAT+, are safe and portend excellent survival to patients with end‐stage heart failure. Currently, 28% of centers are performing cardiac transplants using HCV+ donors. Refining management protocols related to HCV+ donor heart transplantation, along with education and expansion of these protocols to centers currently not using this potentially large pool of donors, appears warranted.
Disclosures
Dr Kilic is on the Medical Advisory Board for Medtronic, Inc. Dr Gleason is on the Medical Advisory Board for Abbott, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Comparison of Baseline Donor Characteristics Between Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Table S2. Comparison of Baseline Recipient Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Table S3. Comparison of Baseline Recipient‐Donor Matching and Transplant‐Related Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Figure S1. Overall 1‐year survival following heart transplants performed using hepatitis C–negative (HCV−) vs. hepatitis C–positive (HCV+) donors, before propensity matching.
Figure S2. Standardized mean differences across covariates before and after propensity matching.
Acknowledgments
The data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the Organ Procurement and Transplantation Network or the US government.
(J Am Heart Assoc. 2020;9:e014495 DOI: 10.1161/JAHA.119.014495.)
References
- 1. Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gasink LB, Blumberg EA, Localio AR, Desai SS, Israni AK, Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843–1850. [DOI] [PubMed] [Google Scholar]
- 3. Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, Kusztos AE, Johnson ME, Chen K, Haddad EA, Fanikos J, Harrington DP, Camp PC, Baden LR; DONATE HCV Trial Team . Heart and lung transplants from HCV‐infected donors to uninfected recipients. N Engl J Med. 2019;380:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schlendorf KH, Zalawadiya S, Shah AS, Wigger M, Chung CY, Smith S, Danter M, Choi CW, Keebler ME, Brinkley DM, Sacks SB, Ooi H, Perri R, Awad JA, Lewis S, Hayes R, O'Dell H, Darragh C, Carver A, Edmonds C, Ruzevich‐Scholl S, Lindenfeld J. Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct‐acting anti‐viral therapies. J Heart Lung Transplant. 2018;37:763–769. [DOI] [PubMed] [Google Scholar]
- 5. McLean RC, Reese PP, Acker M, Atluri P, Bermudez C, Goldberg LR, Abt PL, Blumberg EA, Van Deerlin VM, Reddy KR, Bloom RD, Hasz R, Suplee L, Sicilia A, Woodards A, Zahid MN, Bar KJ, Porrett P, Levine MH, Hornsby N, Gentile C, Smith J, Goldberg DS. Transplanting hepatitis C virus–infected hearts into uninfected recipients: a single‐arm trial. Am J Transplant. 2019;19:2533–2542. [DOI] [PubMed] [Google Scholar]
- 6. Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J, Friedman J, Goldberg D, Hall S, Ison M, Kaiser T, Klassen D, Klintmalm G, Kobashigawa J, Liapakis A, O'Conner K, Reese P, Stewart D, Terrault N, Theodoropoulos N, Trotter J, Verna E, Volk M. The American Society of Transplantation Consensus Conference on the use of hepatitis C viremic donors in solid organ transplantation. Am J Transplant. 2017;17:2790–2802. [DOI] [PubMed] [Google Scholar]
- 7. Phillips KG, Ranganath NK, Malas J, Lonze BE, Gidea CG, Smith DE, Kon ZN, Reyentovich A, Moazami N. Impact of the opioid epidemic on heart transplantation: donor characteristics and organ discard. Ann Thorac Surg. 2019;108:1133–1139. [DOI] [PubMed] [Google Scholar]
- 8. Moayedi Y, Fan CPS, Gulamhusein AF, Manlhiot C, Ross HJ, Teuteberg JJ, Khush KK. Current use of hearts from hepatitis C viremic donors. Circ Heart Fail. 2018;11:e005276. [DOI] [PubMed] [Google Scholar]
- 9. Haji SA, Starling RC, Avery RK, Mawhorter S, Tuzcu EM, Schoenhagen P, Cook DJ, Ratliff NB, McCarthy PM, Young JB, Yamani MH. Donor hepatitis‐C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23:277–283. [DOI] [PubMed] [Google Scholar]
- 10. Ishizaka N, Ishizaka Y, Takahashi E, Tooda Ei, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid‐artery plaque, and intima‐media thickening. Lancet. 2002;359:133–135. [DOI] [PubMed] [Google Scholar]
- 11. Zalawadiya S, Lindenfeld J, Haddad E, Shah A, Wigger M, Negrotto S, Danter M, Brinkley D, Menachem J, Punnoose L, Brown Sacks S, Ooi H, Balsara K, Perri R, Awad J, Smith S, Fowler R, O'Dell H, Darragh C, Ruzevich‐Scholl S, Schlendorf K. Intracoronary intimal thickness in transplant recipients of hepatitic C–positive donor hearts. J Heart Lung Transplant. 2019;38:S281. [Google Scholar]
- 12. Kilic A, Weiss ES, Allen JG, Conte JV, Shah AS, Baumgartner WA, Yuh DD. Simple score to assess the risk of rejection after orthotopic heart transplantation. Circulation. 2012;125:3013–3021. [DOI] [PubMed] [Google Scholar]
- 13. Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu‐Foca N, Zeevi A, Fishbein MC; International Society for Heart and Lung Transplantation . Acute antibody‐mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–159. [DOI] [PubMed] [Google Scholar]
- 14. Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, Reed EF, Fishbein MC. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. [DOI] [PubMed] [Google Scholar]
- 15. Hammond EH, Wittwer CT, Greenwood J, Knape WA, Yowell RL, Menlove RL, Craven C, Renlund DG, Bristow MR, DeWitt CW. Relationship of OKT3 sensitization and vascular rejection in cardiac transplant patients receiving OKT3 rejection prophylaxis. Transplantation. 1990;50:776–782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Baseline Donor Characteristics Between Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Table S2. Comparison of Baseline Recipient Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Table S3. Comparison of Baseline Recipient‐Donor Matching and Transplant‐Related Characteristics Between Heart Transplants Using Hepatitis C–Negative and Hepatitis C–Positive Donors Before Propensity Matching
Figure S1. Overall 1‐year survival following heart transplants performed using hepatitis C–negative (HCV−) vs. hepatitis C–positive (HCV+) donors, before propensity matching.
Figure S2. Standardized mean differences across covariates before and after propensity matching.


