Abstract
Background
Circulating levels of sFLT‐1 (soluble fms‐like tyrosine kinase 1), the extracellular domain of vascular endothelial growth factor (VEGF) receptor 1, and its ratio to levels of placental growth factor are markers of the occurrence and severity of preeclampsia.
Methods and Results
C57BL/6 pregnant mice on embryonic day 14.5 (E14.5), male, and non‐pregnant female mice were exposed to air or to Br2 at 600 ppm for 30 minutes and were treated with vehicle or with VEGF‐121 (100 μg/kg, subcutaneously) daily, starting 48 hours post‐exposure. Plasma, bronchoalveolar lavage fluid, lungs, fetuses, and placentas were collected 120 hours post‐exposure. In Br2‐exposed pregnant mice, there was a time‐dependent and significant increase in plasma levels of sFLT‐1 which correlated with increases in mouse lung wet/dry weights and bronchoalveolar lavage fluid protein content. Supplementation of exogenous VEGF‐121 improved survival and weight gain, reduced lung wet/dry weights, decreased bronchoalveolar lavage fluid protein levels, enhanced placental development, and improved fetal growth in pregnant mice exposed to Br2. Exogenous VEGF‐121 administration had no effect in non‐pregnant mice.
Conclusions
These results implicate inhibition of VEGF signaling driven by sFLT‐1 overexpression as a mechanism of pregnancy‐specific injury leading to lung edema, maternal mortality, and fetal growth restriction after bromine gas exposure.
Keywords: hemodynamics, lung edema, preeclampsia, pregnancy, vascular biology
Subject Categories: Animal Models of Human Disease, Basic Science Research, Cell Signalling/Signal Transduction, Inflammation, Pulmonary Biology
Clinical Perspective
What Is New?
Our data indicate that inhibition of vascular endothelial growth factor signaling by sFLT‐1 (soluble fms‐like tyrosine kinase 1) plays a role in the development of lung edema in pregnant mice exposed to Br2 and that treatment with vascular endothelial growth factor‐121 reverses lung injury.
What Are the Clinical Implications?
Although inhibition of vascular endothelial growth factor signaling by sFLT‐1 has been well‐established as a mechanism resulting in elevated blood pressure in animal models of preeclampsia, our results indicate that it plays a role in ‘the development of’ lung injury as well.
Introduction
Preeclampsia is a leading cause of maternal and perinatal morbidity and mortality, affecting up to 10% of pregnancies. In addition to the estimated 60,000 worldwide preeclampsia‐related deaths and the additional burden related to acute peripartum morbidity, survivors of preeclampsia suffer from an increased rate of previously underappreciated long‐term cardiovascular health risks.1 Increasing evidence implicates placenta‐derived sFLT‐1 (soluble fms‐like tyrosine kinase 1) in the maternal circulation as a critical mechanistic driver of the disease.2, 3 sFLT‐1, the soluble extracellular domain of FLT‐1 (Vascular Endothelial Growth Factor Receptor 1) is known to act as a decoy receptor capable of binding circulating vascular endothelial growth factor and placental growth factor (VEGF and PlGF, respectively). However, since sFLT‐1 lacks transmembrane and cytoplasmic components, binding of ligand fails to transduce a signal inside the cell. Subsequent diminished VEGF signaling results in endothelial dysfunction and hypertension.4, 5 Circulating sFLT‐1 and ratios of sFLT‐1/PlGF have been shown to be predictive markers for the occurrence and severity of preeclampsia in clinical studies.6
With over 56 million tons of halogens (e.g. chlorine (Cl2) or bromine (Br2)) produced and transported through populated areas worldwide every year, accidental or malicious exposure to these gases poses a significant threat to public health. For example, unintentional releases of transported halogens have occurred in Geneva Switzerland (1984, Br2), Graniteville, SC (2005, Cl2), Chelyabinsk Russia (2011, Br2), and Dimona Israel (2016, Br2) and resulted in significant short‐ and long‐term morbidity and mortality.7, 8, 9 In addition to these major industrial/transportation accidents, halogen inhalation injuries often occur in residential areas because of swimming pool sanitation accidents.10
We have shown that exposure of pregnant mice to the toxic gas bromine (Br2) induces a pregnancy‐specific pathology that is distinct from the course of injury in non‐pregnant mice.11 More precisely, pregnant mice, exposed to Br2 at E14.5 develop systemic and pulmonary hypertension, fetal growth restriction and a lung injury that is characterized by increased wet/dry weight ratios and elevated bronchoalveolar lavage fluid (BALF) protein levels. Accompanying these symptoms, we detected elevated circulating levels of tumor necrosis factor‐a, interleukin‐6, C‐X‐C Motif Chemokine Ligand 1 (CXCL 1), and sFLT‐1 in the systemic circulation and elevated levels of sFLT‐1 and tumor necrosis factor‐a mRNA in the placentas of Br2 exposed pregnant mice. None of these symptoms or mediators were present in non‐pregnant mice exposed to the same level of Br2. The majority of pregnancy‐specific symptoms and mediators that we detected in Br2 exposed pregnant mice correspond to symptoms and mediators observed in human preeclampsia. We hypothesized that the mechanism by which Br2 induces a preeclampsia‐like phenotype in pregnant mice is via reduced functional VEGF attributable to increased circulating levels of placental‐derived sFLT‐1 in maternal plasma acting as a competitive inhibitor for the Vascular Endothelial Growth Factor Receptor 1 and 2. We further hypothesized that treatment with exogenous VEGF‐121, a non‐heparin spliced variant of VEGF, can overcome the increase in sFLT‐1 by out‐competing sFLT‐1 binding to VEGF receptors and restoring VEGF signaling. In a series of experiments we found that exposure of pregnant mice at gestational day 14.5 (E14.5) to Br2 in concentrations likely to be encountered in industrial accidents (600 parts per million (ppm) for 30 minutes) caused significant and steady increases in circulating sFLT‐1 not seen in identically exposed non‐pregnant mice.12 These increases were accompanied by similar increases in markers of lung injury, blood pressure, and fetal growth restriction. Furthermore, maternal mortality and morbidity, in addition to fetal growth restriction, were improved by maternal administration of VEGF‐121 post Br2 exposure. We chose to administer the VEGF variant VEGF‐121 because it is the most abundant VEGF isoform and has been found to lower blood pressure in animal models of placental ischemia‐induced hypertension.13, 14
Methods
Animals
Specific pathogen‐free and sexually mature, male (20–25 g) and non‐pregnant female (20–25 g) C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the University of Alabama at Birmingham Animal Facility under standard conditions with food and water access ad libitum. Timed pregnant females were either ordered from Charles River Laboratories or were generated in house by mating mature males and females in estrus overnight.
Study Approval
All studies involving mice were pre‐approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (#20 343). Procedures followed were in accordance with institutional guidelines.
Exposure of Mice to Br2
Mice were exposed 2 at a time in a cylindrical glass container to Br2 (Airgas, Birmingham, AL) for 30 minutes at 600 ppm Br2 (balance air) with a flow rate of 5 L/min. All exposures were performed between 6:00 AM and 12:00 PM. Cylinder concentrations were certified to within 2% by both chemical analysis and periodic Br2 detection (Interscan Corporation, Sima Valley, CA). Br2 tanks were replaced after pressures decreased <500 psi. After exposure, the mice were returned to room air with ad libitum access to both food and water. Control mice were exposed to air for 30 minutes. Mice were monitored hourly for the first 12 hours following exposure and every 24 hours thereafter. Body weight was measured pre‐exposure and every 24 hours thereafter. Weights are reported as percent of baseline pre‐exposure body weight.
Measurement of sFLT‐1
Circulating sFLT‐1 in murine plasma was measured by ELISA (R&D Systems #DY471, Minneapolis, MN).
VEGF‐121 Administration
The mouse VEGF splice variant VEGF‐121 (ProSpec, East Brunswick, NJ, CYT‐574), was reconstituted with sterile saline to achieve dosage of 100 μg/kg body weight and volume of 50 μL. For administration, mice were anesthetized with 2% isoflurane mixed with compressed air. VEGF‐121 or saline vehicle was administered via subcutaneous injection at the site of loose skin over the shoulders 48 hours‐post‐exposure and every 24 hours thereafter.
Controls
For survival studies, all mice were injected with vehicle or VEGF‐121. Age‐matched non‐pregnant male and female mice were used in all studies. Separate groups of mice were used for BAL studies and measurements of lung weights. Otherwise, multiple measurements were performed in the same mice.
Blood Collection
General anesthesia was induced with 5% isoflurane and maintained with 2% isoflurane. After appropriate depth of anesthesia was ensured a midline incision was made through the skin, subcutaneous tissue, and peritoneum. Two incisions were made laterally perpendicular to the primary midline incision to allow exposure of all abdominal organs. Bowels were then gently displaced laterally to allow visualization of the inferior vena cava and abdominal aorta. A 25 G needle attached to a 1 mL syringe loaded with 25 μL of heparin was inserted into the abdominal aorta. After insertion, blood was slowly drawn into the syringe until a volume of >500 μL was retrieved.
Bronchoalveolar Lavage
For bronchoalveolar lavage (BAL) fluid collection, a 5 mm incision was made on the anatomical right at the mid‐clavicular line where the diaphragm attaches to the thoracic cavity, as described previously.15, 16, 17 After the initial incision, the diaphragm was cut away from the thoracic cavity and 2 incisions were made on either side of the thorax lateral to each internal thoracic artery. The ventral thoracic cavity was removed with a perpendicular incision across the chest wall exposing the heart and lungs. The skin and subcutaneous tissue of the neck were removed along with the thyroid gland. The trachea was isolated via dissection and visualized from the cricoid cartilage to the tracheal bifurcation as it entered the thoracic cavity. Using spring scissors, a 3 mm perpendicular incision was made in between the cartilaginous rings of the most superior portion of the trachea. A 1 mL syringe filled with 1 mL of chilled 1x PBS was attached to a 3 mm endotracheal cannula (Exel 18G x 1” catheter cut at a 45° angle). The catheter was inserted into the opening in between the tracheal rings and a suture was tightened around the trachea and endotracheal catheter to maintain an adequate seal. Then 1 mL of cold PBS was slowly injected which expanded the lungs. The fluid was pulled back fully and pushed back into the lungs again 2 times. On the final retrieval there was 0.70 to 0.90 mL of bronchoalveolar lavage (BAL) fluid recovered in all groups. Recovered lavage fluid was kept on ice and centrifuged (4000 rpm, 5 minutes, 4°C) to separate the cell pellet from the supernatant cells. Supernatant was isolated and stored for further analysis at −80°C. BAL fluid protein concentration was determined via BCA Protein Assay Kit (Fisher Scientific, Waltham, MA, 23 225) as described previously.17
Fetal and Placental Collection
The fetuses and placentas in the adult pregnant females were excised following maternal abdominal incision and weighed. Placentas were divided, flash frozen in liquid nitrogen, and stored at −80°C or placed in 70% ethanolic formalin for histology (Fisher Scientific, Waltham, MA, 89 900). Paraffin‐embedded tissues were cut into 4‐μm sections, deparaffinized, and rehydrated using CitriSolv (d‐limonene‐based solvent, Fisher Scientific, Waltham, MA, 04‐355‐12.) and isopropanol, respectively. The histological sections were stained with hematoxylin and eosin.
Lung Collection
Lungs collected for analysis (except wet:dry weight ratio analysis) were flushed with 10 mL of 0.9% saline through the left and right ventricles of the heart to ensure that there was no contamination from the procedure.
Lung Wet/Dry Weight Ratio
Whole lungs were removed, isolated, blotted, weighed (for determination of wet weight), placed in an oven at 70°C for 7 days, and then weighed again for determination of the dry weight.
Placental Injury
Placental injury was evaluated based on hematoxylin and eosin staining of placental sections and scored by quantification of the junctional zone size. For the assessment of junctional zone area we used the following criteria: on the decidual side we considered the area of the giant cells to be the boundary and traced along them. On the labyrinth side we defined the boundary based on the clear morphological difference between trophoblasts and cells in the labyrinth.
Statistical Analysis
Survival between treatment groups under different experimental conditions and at different time points was evaluated using the Pearson Chi‐squared test, and Fisher exact test. The overall survival rates were estimated according to the Kaplan‐Meier method and compared using the log‐rank test. No outliers were identified via the ROUT method (robust regression followed by outlier identification).18 Unpaired Student t test was used to compare 2 groups of continuous variables. One‐way ANOVA was used to compare >2 groups of continuous variables with Tukey post hoc analysis to determine statistical significance. A P<0.05 was considered statistically significant in the 2‐tailed statistical tests that were used. Tests of normality were performed in GraphPad Prism and if normal distribution could not be established non‐parametric tests were used. To analyze body weight percentage over time a multiple unpaired t test adjusted for the number of time points was used correcting for multiple comparisons using the Holm‐Sidak method to test for statistical significance (P<0.05). Each row was analyzed individually without assuming a consistent standard deviation. All data are represented as mean±SEM. Analysis was conducted using GraphPad Prism ver. 7 software (La Jolla, CA). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Br2. Exposure Causes Time Dependent Increases in sFLT‐1 in Pregnant Mice
We exposed non‐pregnant and pregnant mice to Br2 (600 ppm for 30 minutes) and returned them to room air as described previously.11 Pregnant Br2‐exposed mice demonstrated a linear increase in plasma sFLT‐1 over time (R 2=0.8151, P<0.0001) not observed in Br2‐exposed non‐pregnant animals (R 2=0.0323, P=0.4010). The pregnant Br2‐exposed mice had increased levels of circulating sFLT‐1 in the plasma (2060.0±281.1 pg/mL; mean±1 SEM) at E16.5 (48 hours post‐exposure), 5993.0±1107.0) at E17.5 and (8445.0±635.4 pg/mL) at E18.5; the corresponding levels in pregnant air‐exposed mice at the same time points were 329.4±84.8, 87.7±51.2, and 608.3±394.8 pg/mL (Figure 1A). Notably, male and non‐pregnant female mice exposed to Br2 at the same level showed no changes in circulating sFLT‐1 at any of the time points post‐exposure (Figure 1B).
Figure 1.
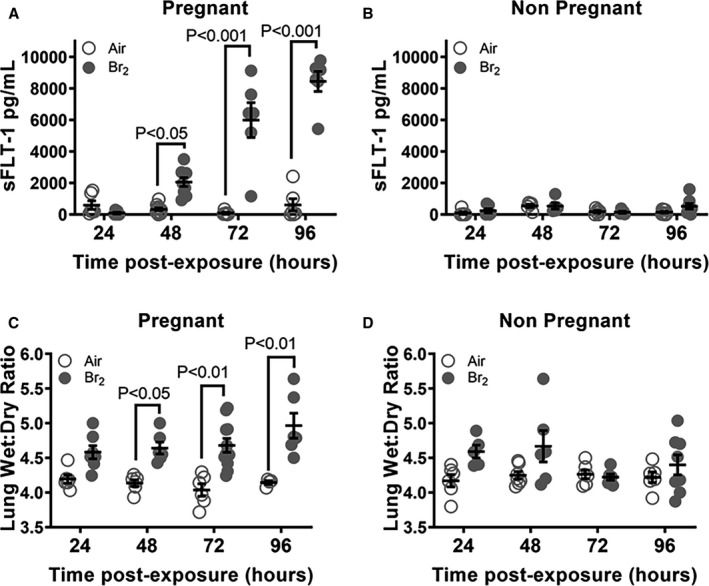
Br2‐exposed pregnant mice exhibit progressively increased circulating sFLT‐1 (soluble fms‐like tyrosine kinase 1) with a concomitant increase of lung wet/dry weight. Non‐pregnant and pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. sFLT‐1 levels in the plasma of (A) pregnant Br2‐exposed mice increased 48‐hour post‐exposure vs air controls and continued to increase until euthanasia at 96 hours. This increase was linear (R 2=0.8151, P<0.0001). Similarly exposed (B) non‐pregnant mice exhibited no increase at any time point; n=6 to 8; ANOVA. Lung wet:dry weight ratios of (C) pregnant Br2‐exposed mice increased 72‐hour post‐exposure compared with air controls and continued to increase until euthanasia at 96 hours. Similarly exposed (D) non‐pregnant mice exhibited no increase at any time point; n=6 to 8; ANOVA. All data are individual values and means±SEM. sFLT‐1 indicates soluble fms‐like tyrosine kinase 1.
Male and non‐pregnant female mice exposed to Br2 at 600 ppm for 30 minutes had no significant increase of the lung wet:dry weight as compared with their air control counterparts within 4 days post‐exposure (Figure 1D). However, pregnant females exposed to a similar Br2 regimen at E14.5 had significantly increased lung wet:dry weights at 48, 72 and 96 hours (4.68±0.09 and 4.965±0.18) vs air‐exposed pregnant mice at similar time points (4.04±0.09 and 4.15±0.03) (Figure 1C) indicating the presence of pulmonary edema.
Administration of VEGF‐121 Decreases Post‐Br2 Exposure‐Induced Mortality and Lung Injury in Pregnant Mice
Pregnant mice were exposed to 600 ppm Br2 for 30 minutes at E14.5. Survivors at 48 hours were administered 100 μg/kg VEGF‐121 or vehicle subcutaneously each day until 120 hours post‐exposure. At 120 hours post‐exposure, pregnant mice in the VEGF‐121 group exhibited significantly increased survival (76%) (Figure 2A) as compared with the vehicle control group (48% survival). Non‐pregnant mice exhibited no difference in survival between the groups treated with VEGF‐121 or vehicle (Figure 2B). Additionally, pregnant mice receiving VEGF‐121 gained weight at 96 hours (91.4%±3.3%) (P=0.050) and 120 hours (93.2%±3.9%) (P=0.046) post‐exposure as opposed to the vehicle control group that showed no improvement in weight gain (79.3%±2.8% and 78.2%±3.8%) (Figure 2C), although their body weights lagged behind of those non‐exposed to Br2. Non‐pregnant mouse body weight was unaffected by administration of VEGF‐121 (Figure 2D).
Figure 2.
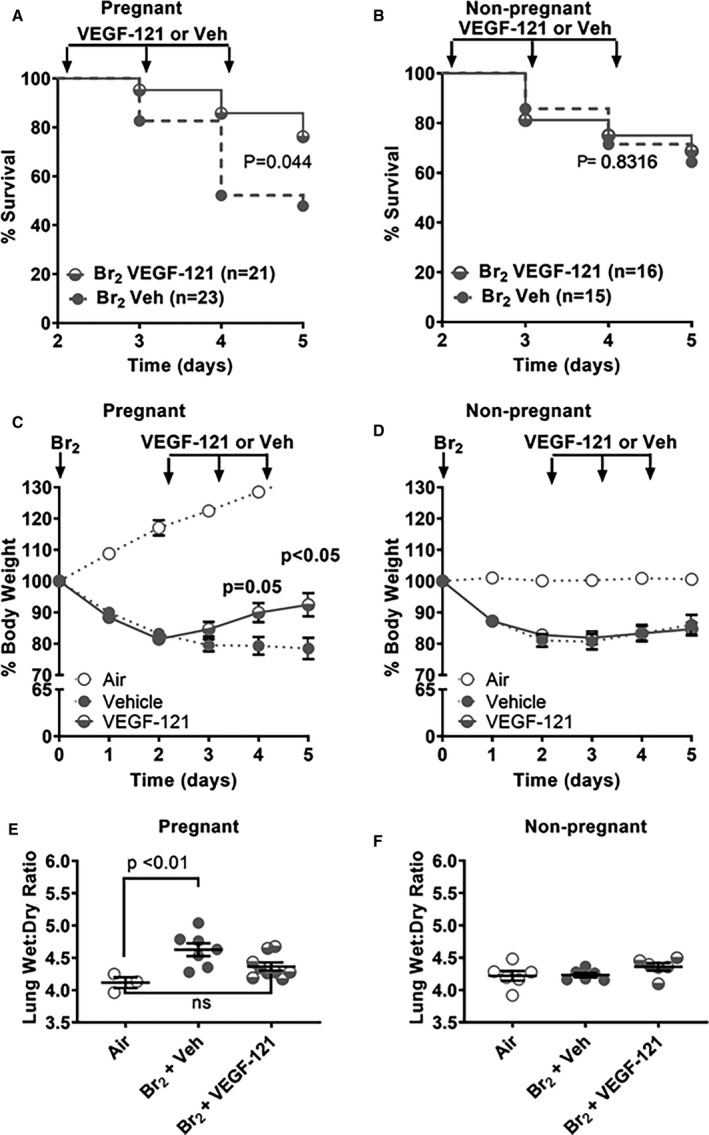
Treatment of pregnant mice exposed to Br2 with vascular endothelial growth factor (VEGF)‐121 decreases mortality, weight loss, and lung wet/dry weights. Non‐pregnant and pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. At 48‐hour post‐exposure and every 24 hours thereafter, survivors were administered VEGF‐121 (100 μg/kg subcutaneously) or vehicle. A, Pregnant Br2‐exposed mice treated with VEGF‐121 demonstrated significantly improved survival at E19.5 compared with vehicle controls; n=21 to 23; Log Rank Mantel‐Cox. B, Survival curves of non‐pregnant mice exposed to Br2 and receiving VEGF‐121 at 48 hours post‐exposure were not significantly different than similarly Br2 exposed non‐pregnant mice receiving vehicle only; n=15 to 16; Log Rank Mantel‐Cox. C, The same mice were monitored for body weight loss with pregnant Br2‐exposed mice treated with VEGF‐121 at 48 hours post Br2 exposure demonstrating significantly reduced percent body weight loss compared with similarly exposed vehicle controls at E18.5 and E19.5; n=21 to 23; multiple unpaired t test with Holm‐Sidak method. D, Body weight loss following exposure of non‐pregnant mice to Br2 is not affected by VEGF‐121 therapy at any measured time point in non‐pregnant mice; n=9 to 11; Student t test. E, Lung wet:dry weight ratios at 120‐hour post‐exposure indicate that exposure of pregnant mice to Br2 results in increased lung wet/dry weights at 120 hours post‐exposure and that lung wet/dry weights of Br2 exposed pregnant mice treated with VEGF‐121 were not different from air‐exposed control mice at the same time point. F, Lung wet/dry weights were unaltered in Br2‐exposed non‐pregnant mice with or without VEGF‐121 treatment as compared with air‐exposed non‐pregnant mice. ANOVA. VEGF indicates vascular endothelial growth factor. All data are means±SEM.
At 120 hours post‐Br2–exposure, pregnant mice with vehicle treatment had lung wet:dry weight ratios with a mean of 4.65±0.13 (Figure 2E), significantly higher than lung wet:dry weights of air‐exposed mice (4.15±0.03). Br2‐exposed pregnant mice treated with VEGF‐121, however, showed a reduction in lung wet:dry weight ratio (4.35±0.07) not significantly different from lung wet:dry ratios of air‐exposed mice. Non‐pregnant mice subjected to the same regimen of Br2 exposure followed by treatment with VEGF‐121 or vehicle control demonstrated no significant differences between treatment groups (4.36±0.06 vs 4.23±0.03, respectively) and were not different from lung wet:dry ratios observed in air‐exposed non‐pregnant mice (4.22±0.07) (Figure 2F). BALF was collected at 120 hours post‐exposure in surviving animals. Pregnant females exposed to Br2 and treated at 48 hours with either vehicle or VEGF‐121 had higher levels of BALF protein than air‐exposed pregnant mice (Figure 3A) (mean difference 896 μg/mL, 95% CI 562–1230 μg/mL, P<0.0001 and mean difference 377 μg/mL, 95% CI 43–710 μg/mL, P<0.0252, respectively) Following VEGF‐121 treatment we observed a significant decrease in BALF protein level compared with vehicle‐treated control mice (Figure 3A) (mean difference 520 μg/mL, 95% CI 186–854 μg/mL, P=0.0024). Total BALF cell count differed between air‐exposed pregnant mice and Br2‐exposed pregnant mice treated with vehicle (P<0.0001) and VEGF‐121 animals (P=0.0072) (Figure 3B). VEGF‐121 treated pregnant mice demonstrated a lower total cell count than vehicle treated controls (P=0.0406) (Figure 3B). BALF neutrophil count was elevated in Br2 exposed pregnant mice compared with air‐exposed controls in both vehicles treated (P=<0.0001) and VEGF‐121 treated cohorts (P<0.0001) (Figure 3C). Pregnant mice exposed to Br2 and treated with VEGF‐121 demonstrated a significantly lower BALF neutrophil count compared with vehicle‐treated control mice (P=0.0274) (Figure 3C). Macrophage cell count within the BALF was increased in vehicle treated Br2‐exposed pregnant mice compared with air‐exposed controls (P=0.0031) (Figure 3D). In pregnant mice exposed to Br2 and treated with VEGF‐121 there was no significant difference in macrophage cell count compared with air‐exposed controls or when compared with vehicle treated Br2‐exposed mice (Figure 3D).
Figure 3.
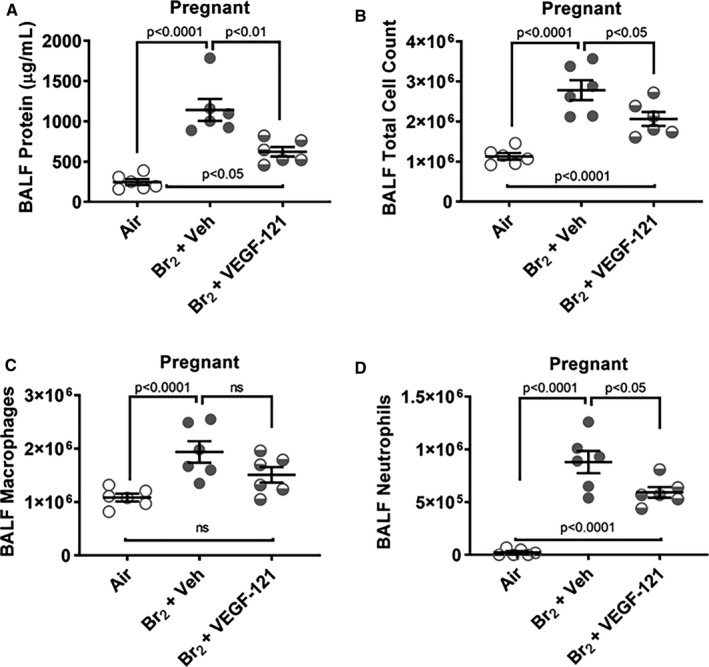
Br2‐exposed pregnant mice have higher protein content and total cell counts in bronchoalveolar lavage fluid. Non‐pregnant and pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. Survivors at 48 hours were administered vascular endothelial growth factor (VEGF)‐121 or vehicle subcutaneously with subsequent doses every 24 hours thereafter until 120 hours. Bronchoalveolar lavage fluid was collected at 120‐hours post‐Br2 exposure. A, Br2‐exposed pregnant mice treated with vehicle and those treated with VEGF‐121 both demonstrated higher levels of bronchoalveolar lavage fluid protein compared with air‐exposed pregnant mice. Br2‐exposed mice treated with VEGF‐121 demonstrated a significantly lower bronchoalveolar lavage fluid protein level than those treated with vehicle. B, Total cell count was higher in Br2‐exposed pregnant mice treated with vehicle and VEGF‐121. The VEGF‐121 treated pregnant mice demonstrated a lower total cell count than vehicle treated controls. C, Neutrophil count was elevated in Br2‐exposed pregnant mice compared with air‐exposed controls in both vehicle treated and VEGF‐121 treated groups. The group treated with VEGF‐121 had significantly lower neutrophil counts compared with vehicle treated mice. D, Macrophage cell count was increased in vehicle treated Br2‐exposed pregnant mice compared with air‐exposed controls. Mice treated with VEGF‐121 did not demonstrate a significant difference in macrophage cell count compared with air controls or Br2‐exposed vehicle treated mice. n=6; ANOVA. BALF indicates bronchoalveolar lavage fluid; VEGF, vascular endothelial growth factor. ns indicates non‐significant. All data are individual values and means±SEM.
Treatment With VEGF‐121 Partially Rescues Severe Fetal Growth Restriction Induced by Maternal Br2 Exposure
Pregnant mice were exposed to Br2 at 600 ppm or air for 30 minutes at E14.5 and then returned to room air. Br2‐exposed mice surviving at 48 hours post‐exposure were administered 100 μg/kg VEGF‐121 or vehicle subcutaneously each day until 120 hours post‐exposure. Fetal weights of pregnant mice exposed to air with no treatment were 1.38±0.02 g at 120 hours post‐exposure (E 19.5) (Figure 4B). Fetuses of Br2‐exposed, vehicle‐treated mice exhibited severe growth restriction both by visual inspection and by quantitative assessment of body weight (0.58±0.02 g, P<0.001 vs air) at the same time point (Figure 4A and 4B). Fetuses of Br2‐exposed, VEGF‐121 treated mice were visibly larger (Figure 4A) and demonstrated significantly increased body weights (0.88±0.03 g) compared with fetal weights of Br2‐exposed vehicle treated mice (P<0.001), although still remained significantly smaller than fetuses of air‐exposed mice (P<0.001).
Figure 4.
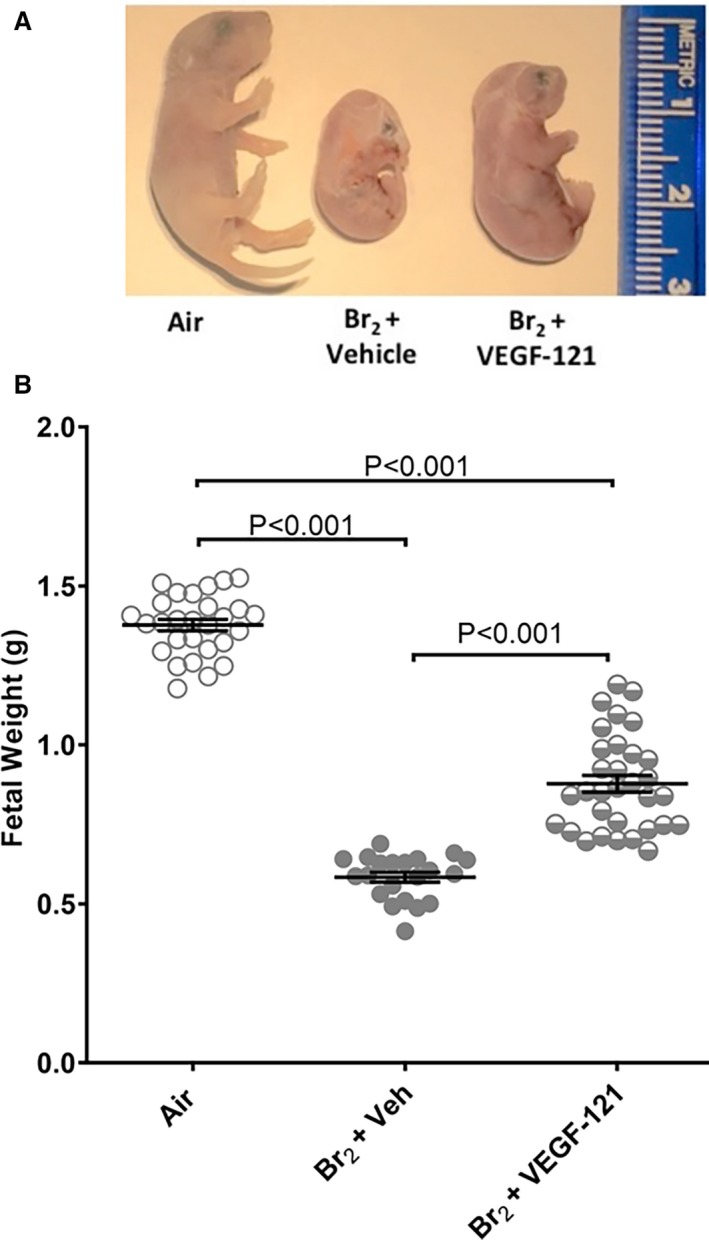
Fetal growth restriction induced by maternal exposure to Br2 is mitigated by vascular endothelial growth factor‐121 treatment. Pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. At 48 hours post‐exposure and every 24 hours thereafter, survivors were administered vascular endothelial growth factor‐121 (100 μg/kg subcutaneously) or vehicle. A, Representative photograph of paraformaldehyde‐fixed fetuses at E19.5 for the indicated conditions. Fetuses of Br2‐exposed pregnant mice exhibit severe fetal growth restriction. Treatment with vascular endothelial growth factor‐121 improves fetal growth. B, Fetal weights were recorded after extraction of fetuses at E19.5. Fetuses from Br2‐exposed pregnant mice weighed considerably less than air controls. Fetal weight was partially rescued by maternal vascular endothelial growth factor‐121 administration; n=pups (21–33); ANOVA; All data are means±SEM. VEGF indicates vascular endothelial growth factor.
Treatment With VEGF‐121 Alleviates Br2 Exposure‐Induced Compromise of Placenta Development
Pregnant mice were exposed to Br2 at 600 ppm or air for 30 minutes at E14.5 and then returned to room air. Br2‐exposed mice surviving at 48 hours post‐exposure were administered 100 μg/kg VEGF‐121 or vehicle subcutaneously each day until 120 hours post‐exposure. Placenta development was assessed by quantifying the area of junctional zone on hematoxylin and eosin stained histological cross‐sections of the placentas prepared at the centerline of 2 to 3 placentas from each pregnant mouse in each treatment group as described above. Junctional zone boundaries are indicated on Figure 5 where on the high magnification inserts the blue arrows point to the giant cells and yellow arrows point to the boundary between trophoblasts (identifiable by size and stain color) and cells of the labyrinth. The area of junctional zone was visibly decreased in placentas of Br2‐exposed vehicle‐treated mice as compared with air‐exposed mice (Figure 5B vs A and Figure 5D middle column vs left column). Treatment of Br2‐exposed mice with VEGF‐121 resulted in increased area of junctional zone as compared with Br2‐exposed vehicle‐treated mice (Figure 5C vs 5B and Figure 5D middle column vs right column). Glycogen‐containing cells, a feature of a normally developed junctional zone, were abundant in air‐exposed placentas (magenta arrows, Figure 5A, enlarged panel on the right). In Br2‐exposed vehicle‐treated mice, glycogen‐containing cells were almost completely absent (Figure 5B, enlarged panel on the right). In Br2‐exposed VEGF‐121 treated mice, glycogen containing cells were readily identifiable (magenta arrows, Figure 5C, enlarged panel on the right).
Figure 5.
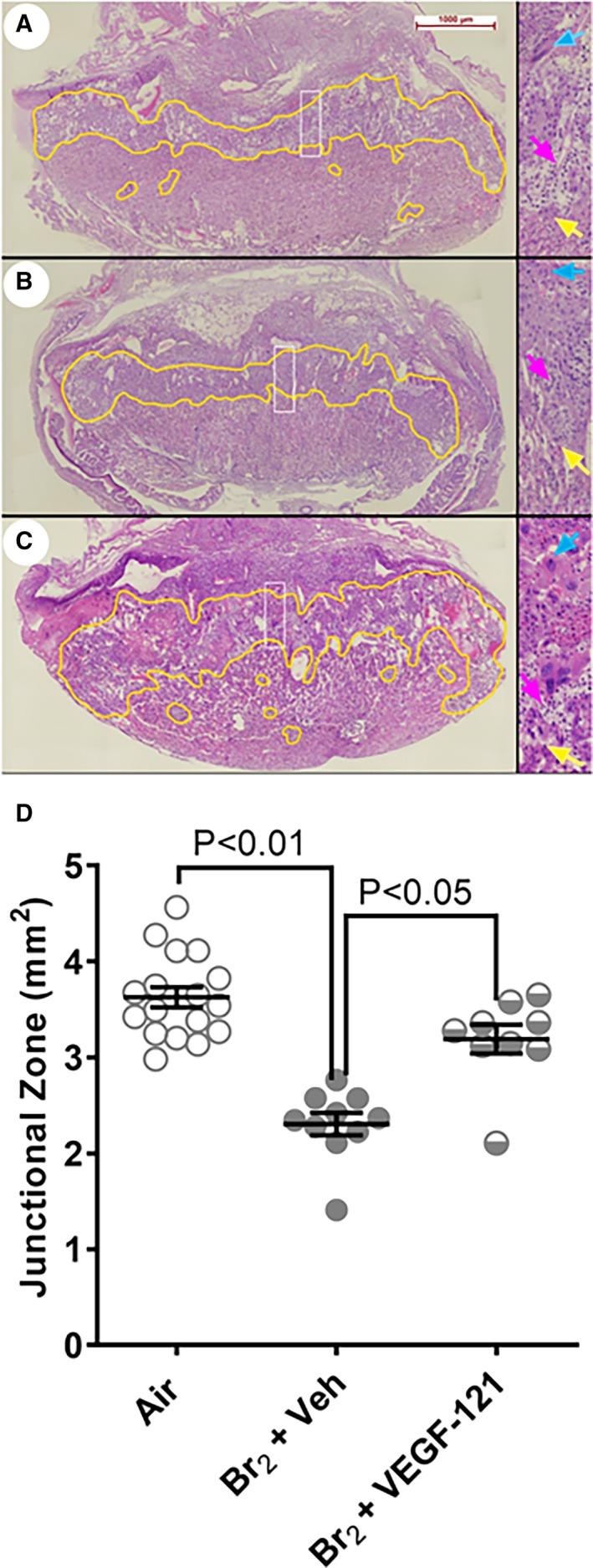
Reduction of placental junctional zone induced by maternal exposure to Br2 is mitigated by treatment with vascular endothelial growth factor (VEGF)‐121. Pregnant (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 minutes and returned to room air. At 48 hours post‐exposure and every 24 hours thereafter, survivors were administered 100 μ/kg b.w. VEGF‐121 or vehicle subcutaneously. A through C, depict representative images of hematoxylin and eosin stained histological sections from air‐exposed (A), Br2‐exposed vehicle‐treated (B), and Br2‐exposed VEGF 121‐treated (C) pregnant mice. Increased (×4) magnification of the area within the white rectangle is depicted to the right. Blue arrows demarcate giant cells and yellow arrows point to the boundary between trophoblasts (identifiable by size and stain color) and cells of the labyrinth. Magenta arrows point to glycogen‐containing cells. D, Quantification of junctional zones in the groups indicated in (A through C). There is a decrease in the area of junctional zone in Br2‐exposed vehicle‐treated animals (B, D; middle column. Treatment with VEGF‐121 results in significantly increased areas of junctional zone. Statistical test ANOVA; P values indicated on graph. VEGF indicates vascular endothelial growth factor.
Discussion
Exposure to Br2 has the potential to cause significant injury and death in human populations. Worldwide, Br2 production exceeds 550 000 tons per year to meet the manufacturing needs for medicinal compounds, flame‐retardants, agricultural chemicals, gasoline additives, dyes, photographic chemicals, bleaching agents, and water disinfectants. Main producers include the United States, China, and Israel. Following production in central facilities, Br2 is transported to surrounding industrial sites via rail or road. Major accidents have occurred during these routine passages of Br2. Chelyabinsk, Russia (pop. 1.1 million) recently experiencing an accidental release of railway Br2. There is a paucity of data available on Br2 gas toxicity, and only 1 study includes pregnant animals despite 4% of women in the United States being pregnant at any given time (US Census Bureau).
To our knowledge, we demonstrate for the first time the mechanistic role reduced VEGF signaling plays in generating the lung edema, impaired placental development, impaired fetal development, and overall maternal mortality seen in pregnant mice following exposure to the oxidant and electrophile gas Br2 in concentrations likely to be encountered by a victim of an accidental industrial exposure. These findings were substantiated by (1) the levels of circulating sFLT‐1, which antagonizes VEGF signaling, rose as a function of time from 48 hours post‐exposure to 120 hours post‐exposure in Br2‐exposed pregnant mice correlating with increasing lung wet/dry weight ratios and increases in BALF protein content. (2) sFLT‐1 levels, lung wet/dry ratios, and BALF protein levels were not different in Br2‐exposed non‐pregnant mice as compared with air‐exposed non‐pregnant mice. (3) Treatment with VEGF‐121 (100 μg//kg, SC, q.d.) reduced lung wet/dry weights, reduced BALF protein content, improved survival, weight gain, placental development, and fetal growth in Br2 exposed pregnant mice as compared with air‐exposed pregnant mice.
Previously, we have shown that exposure of pregnant mice to Br2 resulted in diminished placental development and reduced fetal growth, accompanied by increased plasma levels of inflammatory cytokines and sFLT‐1, elevated maternal blood pressure, and pulmonary edema: symptoms reminiscent of those seen in pregnant women with preeclampsia.11 Increased sFLT‐1 and inflammatory cytokine plasma levels, lung edema, and increased blood pressure were absent from non‐pregnant mice exposed to identical levels of Br2. The present findings mechanistically link circulating sFLT‐1 and inhibition of VEGF signaling with the pregnancy‐specific effects of Br2 exposure.
We reason that the increase in sFLT‐1 beginning at 48 hours post‐Br2 exposure and continuing to rise until euthanasia of the animal at 96 hours accounts for the pregnancy‐specific phenotype of increased lung wet/dry weights, increased BALF protein, high mortality, and fetal growth restriction. sFLT‐1 is considered to play a role in the development of preeclampsia in pregnant women. It is produced by the placenta and due to its anti‐angiogenic effects sFLT‐1 overexpression is increasingly viewed as a key component of the systemic endothelial dysfunction observed in preeclampsia.4, 19 Released into maternal circulation, sFLT‐1 acts as a decoy receptor for VEGF, thus reducing VEGF signaling and downstream effects such as angiogenesis and vasodilation. In animal models, overexpression of exogenous sFLT‐1 in pregnant mice by adenoviral vectors resulted in endothelial dysfunction and increased blood pressure. This constellation is similar to what is observed in the reduced uterine perfusion pressure model of preeclampsia in mice and rats, a model whose preeclampsia phenotype is also accompanied by elevated sFLT‐1 levels.20, 21 In the aforementioned animal models, administration of VEGF alleviated hypertension and endothelial dysfunction.14 Intriguingly, the 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A (HMG CoA) reductase inhibitor pravastatin is currently in human clinical trials to treat preeclampsia (NCT01717586). One pleiotropic effect of pravastatin is a reduction in sFLT‐1 levels and an increase in VEGF levels.22, 23
Maternal exposure to Br2 may lead to placental sFLT‐1 overexpression in pregnant mice because of acutely reduced placental blood flow and/or impairment in tissue oxygenation (tissue hypoxia insult) triggering sFLT‐1 overexpression that then potentiates this phenotype after the acute Br2 exposure. Br2 exposure results in a sustained respiratory acidosis in pregnant mice consistent with conditions that may impair placental perfusion.24 This hypothesis is supported by the improvement in fetal growth observed with VEGF‐121 treatment. Exogenous VEGF administration may rescue fetal growth restriction by 2 complementary mechanisms; (1) increased endothelial nitric oxide synthase (eNOS) activity leading to vasodilation and improved placental blood flow and (2) improved maternal pulmonary endothelial function resulting in better maternal and consequently better fetal oxygenation. Furthermore, in previous studies we have demonstrated that halogen gasses (chlorine or bromine) interact with lung plasmalogens leading to formation of halogenated aldehyde which are either reduced to alcohol or oxidized to halogenated fatty acids detectable in the plasma even at 24 hours post‐exposure.25, 26 These brominated aldehydes contribute to cardiac injury in rats exposed to Br2. 24 It is possible that systemically circulating brominated aldehydes are similarly causing placental derangements in pregnant mice.
Our findings indicating increased pulmonary endothelial dysfunction are particularly interesting in light of the well‐established role VEGF signaling plays in increasing endothelial permeability via stimulation of vascular endothelial (VE)‐cadherin endocytosis.27 With increases in sFLT‐1 a sequestration of VEGF is anticipated and one might expect to observe decreased endothelial permeability. In both preeclampsia and high‐altitude pulmonary edema increased permeability is observed despite increased plasma ratios of sFLT‐1/VEGF.28, 29 An important distinction is that in human preeclampsia the concentration of serum VEGF remained low or in some cases (between 37 and 41 weeks gestation) was lower in women with preeclampsia than in healthy pregnancies. Conversely, in subjects with high‐altitude pulmonary edema the plasma VEGF level was increased compared with control subjects without high‐altitude pulmonary edema while sFLT‐1 levels were increased to a much greater extent, thus establishing the noted increase in sFLT‐1/VEGF ratio. It should be noted that this recent work runs counter to previous research published 13 years prior where lower sFLT‐1 was noted in subjects who developed high‐altitude pulmonary edema under different experimental conditions and with different sampling time points.30 The commonality between these 2 entities and our Br2 exposure model may be hypoxia and further work to understand the mechanism linking sFLT‐1 overexpression and pulmonary edema is needed.
The timing of injury observed differs between pregnant and non‐pregnant mice. Both groups demonstrate evidence of acute injury after initial exposure. In pregnant mice, however, surviving mice demonstrate progressive worsening of lung wet:dry ratios overtime until euthanasia or death not seen in non‐pregnant mice (Figure 1C). This progressive worsening is accompanied by the increased serum sFLT‐1 levels noted in Figure 1A providing evidence correlating the pregnant state with increased susceptibility to ongoing and progressing lung injury.
Treatment with VEGF‐121 requires caution as high levels of VEGF have been shown to result in abnormal endothelial proliferation and decreased endothelial barrier function.31 At the dose administered, we did not observe any adverse effects of VEGF‐121 on either pregnant or non‐pregnant animals. Notably, a recent study found that treatment of cultured endothelial cells with VEGF reduced endothelial barrier function, unless the cells were first pre‐treated with PlGF. PlGF is not exclusively expressed during pregnancy, however, as its name implies PlGF is avidly produced by the placenta resulting in a gradual increase in plasma levels until the 32nd week of pregnancy.32 The abundance of PlGF in pregnancy may explain the selectively beneficial effect of exogenous VEGF administration during pregnancy.
We chose to administer the VEGF variant VEGF‐121 because it is the most abundant VEGF isoform and was previously shown to lower blood pressure in animal models of placental ischemia‐induced hypertension.13, 14 Our findings indicate that administration of VEGF‐121 does reduce pregnancy‐specific effects of Br2 exposure. The protective effects may be attributable to a variety of mechanisms. First, VEGF binding to endothelial cell receptors is known to trigger a vasodilatory response via the Protein kinase B/ endothelial nitric oxide synthase signal pathway.33 Such a mechanism may alleviate the systemic endothelial dysfunction that we documented in Br2‐exposed pregnant mice in our previous publication. VEGF has also been shown to protect alveolar epithelium and has a role in the repair of the airway epithelium following lung injury.34 Normally VEGF is highly compartmentalized in the alveolus with up to 500x the levels seen in plasma.35 The increased BALF protein is indicative of alveolar endothelial disruption and increased permeability consistent with the endothelial dysfunction observed in human preeclampsia and linked to sFLT‐1 overexpression. In addition to serving as a marker for increased endothelial and epithelial permeability the increased protein content within the lung may contribute to surfactant inactivation, further exacerbating pulmonary dysfunction.36, 37, 38 Lastly, sFLT‐1 binds to placental growth factor (PlGF) in addition to VEGF. PlGF, like VEGF, is primarily associated with angiogenesis, however PlGF plays a greater role in placental trophoblast cells responsible for invasion of maternal arteries and proper placental development.39 VEGF‐121 binding to sFLT‐1 could potentially allow for increased PlGF/full‐length receptor binding and subsequent placental development.
Conclusions
Diminished VEGF signaling in pregnant C57BL/6 mice exposed to Br2 at E14.5 plays a role in halogen‐inhalation induced pregnancy‐specific outcomes including increased lung wet/dry weights, BALF protein content, fetal growth restriction, and maternal mortality. Unlike similarly exposed non‐pregnant mice, pregnant mice show increased circulating sFLT‐1 beginning 48 hours post‐exposure, along with lung edema appearing at 48 hours post‐exposure. VEGF‐121 administered 48 hours post‐Br2 exposure increases survival exclusively in pregnant mice, reduces maternal lung edema and partially rescues fetal growth restriction. To our knowledge, this is the first demonstration that increased morbidity and mortality following brief exposure of pregnant mice to Br2 is mechanistically driven by antagonism of VEGF signaling by sFLT‐1.
In summary, we show that inhalational exposure to Br2 causes lung edema, increased mortality, and fetal growth restriction in pregnant mice due to reduced VEGF signaling. We demonstrate that sFLT‐1 in maternal plasma, maternal blood pressures, and lung injury increase in tandem following Br2 gas exposures in pregnant mice. Administering the VEGF isoform VEGF‐121 restores body weights, increases survival, and reduces signs of lung injury seen in pregnant mice exposed to Br2.
Sources of Funding
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, the National Institute of Environmental Health Sciences, Grant Numbers U01ES026458 (Matalon), U01 ES027697 (Matalon and Jilling). Dylan Addis is supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award under award number T32HL129948. James Lambert was supported in part by the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant, T32HL007918.
Disclosures
None.
Acknowledgments
Author contributions: Dr Addis and Dr Lambert designed, performed, and analyzed experiments and wrote drafts of this paper; Dr Ren bred the mice, Mr Doran exposed mice to bromine; Dr Jilling designed performed and analyzed experiments and edited all versions of this manuscript. He also looked at all the original data. Dr Matalon designed experiments, edited all versions of this manuscript and examined all data. Dr Jilling and Dr Matalon are responsible for the contents of this study.
(J Am Heart Assoc. 2020;9:e013238 DOI: 10.1161/JAHA.119.013238.)
References
- 1. American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. [DOI] [PubMed] [Google Scholar]
- 3. Radulescu C, Bacarea A, Hutanu A, Gabor R, Dobreanu M. Placental growth factor, soluble FMS‐like tyrosine kinase 1, soluble endoglin, IL‐6, and IL‐16 as biomarkers in preeclampsia. Mediators Inflamm. 2016;2016:3027363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu F, Longo M, Tamayo E, Maner W, Al‐Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over‐expression of SFLT‐1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396.e391–397.e391; discussion 396.e397. [DOI] [PubMed] [Google Scholar]
- 5. Amraoui F, Spijkers L, Hassani Lahsinoui H, Vogt L, van der Post J, Peters S, Afink G, Ris‐Stalpers C, van den Born BJ. SFLT‐1 elevates blood pressure by augmenting endothelin‐1‐mediated vasoconstriction in mice. PLoS ONE. 2014;9:e91897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, Denk B, Stepan H. The SFLT‐1/PLGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e51–58.e58. [DOI] [PubMed] [Google Scholar]
- 7. Mackie E, Svendsen E, Grant S, Michels JE, Richardson WH. Management of chlorine gas‐related injuries from the graniteville, south carolina, train derailment. Disaster Med Public Health Prep. 2014;8:411–416. [DOI] [PubMed] [Google Scholar]
- 8. Morabia A, Selleger C, Landry JC, Conne P, Urban P, Fabre J. Accidental bromine exposure in an urban population: an acute epidemiological assessment. Int J Epidemiol. 1988;17:148–152. [DOI] [PubMed] [Google Scholar]
- 9. Clark KA, Karmaus WJ, Mohr LC, Cai B, Balte P, Gibson JJ, Ownby D, Lawson AB, Vena JE, Svendsen ER. Lung function before and after a large chlorine gas release in Graniteville, South Carolina. Ann Am Thorac Soc. 2016;13:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agabiti N, Ancona C, Forastiere F, Di Napoli A, Lo Presti E, Corbo GM, D'Orsi F, Perucci CA. Short term respiratory effects of acute exposure to chlorine due to a swimming pool accident. Occup Environ Med. 2001;58:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambert JA, Carlisle MA, Lam A, Aggarwal S, Doran S, Ren C, Bradley WE, Dell'Italia L, Ambalavanan N, Ford DA, Patel RP, Jilling T, Matalon S. Mechanisms and treatment of halogen inhalation‐induced pulmonary and systemic injuries in pregnant mice. Hypertension. 2017;70:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res. 2016;26:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia‐induced hypertension. Hypertension. 2010;55:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mateus J, Bytautiene E, Lu F, Tamayo EH, Betancourt A, Hankins GD, Longo M, Saade GR. Endothelial growth factor therapy improves preeclampsia‐like manifestations in a murine model induced by overexpression of SVEGFR‐1. Am J Physiol Heart Circ Physiol. 2011;301:H1781–H1787. [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal S, Jilling T, Doran S, Ahmad I, Eagen JE, Gu S, Gillespie M, Albert CJ, Ford D, Oh JY, Patel RP, Matalon S. Phosgene inhalation causes hemolysis and acute lung injury. Toxicol Lett. 2019;312:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aggarwal S, Ahmad I, Lam A, Carlisle MA, Li C, Wells JM, Raju SV, Athar M, Rowe SM, Dransfield MT, Matalon S. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI insight. 2018;;3:120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, Pittet JF, Aggarwal S, Garantziotis S, Song W, Matalon S. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am J Physiol Lung Cell Mol Physiol. 2018;314:L808–L821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble FMS‐like tyrosine‐1 (SFLT‐1) production response to placental ischemia/hypoxia: role of tumor necrosis factor‐alpha. Am J Physiol Regul Integr Comp Physiol. 2013;304:R130–R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. Enos deficiency acts through endothelin to aggravate SFLT‐1‐induced pre‐eclampsia‐like phenotype. J Am Soc Nephrol. 2012;23:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bytautiene E, Lu F, Tamayo EH, Hankins GD, Longo M, Kublickiene K, Saade GR. Long‐term maternal cardiovascular function in a mouse model of SFLT‐1‐induced preeclampsia. Am J Physiol Heart Circ Physiol. 2010;298:H189–H193. [DOI] [PubMed] [Google Scholar]
- 22. Saad AF, Kechichian T, Yin H, Sbrana E, Longo M, Wen M, Tamayo E, Hankins GD, Saade GR, Costantine MM. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod Sci. 2014;21:138–145. [DOI] [PubMed] [Google Scholar]
- 23. Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia‐induced hypertension. Hypertension. 2013;61:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N, Louch WE, Ford DA, Doran SF, Matalon S, Dell'Italia LJ. Bromine inhalation mimics ischemia‐reperfusion cardiomyocyte injury and calpain activation in rats. Am J Physiol Heart Circ Physiol. 2019;316:H212–H223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post‐chlorine gas exposure. J Lipid Res. 2016;57:1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J Lipid Res. 2018;59:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gavard J, Gutkind JS. VEGF controls endothelial‐cell permeability by promoting the beta‐arrestin‐dependent endocytosis of VE‐cadherin. Nat Cell Biol. 2006;8:1223–1234. [DOI] [PubMed] [Google Scholar]
- 28. Zhang S, Liu J, Jiang D, Wuren T, Ma S, Du Y, Yi X, Wu S. The plasma level changes of VEGF and soluble VEGF receptor‐1 are associated with high‐altitude pulmonary edema. J Med Invest. 2018;65:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 30. Tissot van Patot MC, Leadbetter G, Keyes LE, Bendrick‐Peart J, Beckey VE, Christians U, Hackett P. Greater free plasma VEGF and lower soluble VEGF receptor‐1 in acute mountain sickness. J Appl Physiol (1985). 2005;98:1626–1629. [DOI] [PubMed] [Google Scholar]
- 31. Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF‐mediated src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil A, Maiz N, Garcia‐Mandujano R, Penco JM, Nicolaides KH. Longitudinal changes in maternal serum placental growth factor and soluble FMS‐like tyrosine kinase‐1 in women at increased risk of pre‐eclampsia. Ultrasound Obstet Gynecol. 2016;47:324–331. [DOI] [PubMed] [Google Scholar]
- 33. Feliers D, Chen X, Akis N, Choudhury GG, Madaio M, Kasinath BS. VEGF regulation of endothelial nitric oxide synthase in glomerular endothelial cells. Kidney Int. 2005;68:1648–1659. [DOI] [PubMed] [Google Scholar]
- 34. Ohwada A, Yoshioka Y, Iwabuchi K, Nagaoka I, Dambara T, Fukuchi Y. VEGF regulates the proliferation of acid‐exposed alveolar lining epithelial cells. Thorax. 2003;58:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaner RJ, Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- 36. Günther A, Ruppert C, Schmidt R, Markart P, Grimminger F, Walmrath D, Seeger W. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res. 2001;2:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hallman M, Glumoff V, Ramet M. Surfactant in respiratory distress syndrome and lung injury. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:287–294. [DOI] [PubMed] [Google Scholar]
- 38. Martinez Sarrasague M, Cimato A, Rubin de Celis E, Facorro G. Influence of serum protein and albumin addition on the structure and activity of an exogenous pulmonary surfactant. Respir Physiol Neurobiol. 2011;175:316–321. [DOI] [PubMed] [Google Scholar]
- 39. Knuth A, Liu L, Nielsen H, Merril D, Torry DS, Arroyo JA. Placenta growth factor induces invasion and activates p70 during rapamycin treatment in trophoblast cells. Am J Reprod Immunol. 2015;73:330–340. [DOI] [PubMed] [Google Scholar]


