Abstract
Background
Because of the increasing numbers of congenital patients surviving into adulthood, early diagnosis and prevention of acquired cardiovascular disease is reasonable. The aim of this study was to detect diagnostic subgroups of adults with congenital heart disease (ACHD) that have increased carotid intima‐media thickness (cIMT), a subclinical marker of cardiovascular damage.
Methods and Results
This study enrolled 831 ACHD patients (392 women, aged 38.8±11.7 years) from May 2015 to February 2019 at their regular outpatient visit. Far wall cIMT was measured using a semiautomatic ultrasound system at 4 angles. Age, sex, height, weight, blood pressure, smoking status, and antihypertensive medication were registered and entered in a multiple linear regression model to compare diagnostic subgroups to 191 healthy controls (111 women, aged 36.7±13.5 years). There were no significant differences in cIMT of ACHD (0.538±0.086 mm) compared with healthy controls (0.541±0.083 mm; P=0.649) after adjusting for the aforementioned covariates. Only patients with coarctation of the aorta showed significantly higher cIMT values (0.592±0.075 mm; P<0.001) compared with healthy controls. In addition, ACHD patients who were men (P=0.032), older (P<0.001), and were prescribed antihypertensive medications (P=0.003) were all found to have thicker cIMT values.
Conclusions
Overall, we determined that within the ACHD cohort, only those patients with a history of coarctation have higher cIMT values. To better determine the mechanism of abnormal vasculature, further basic research is needed.
Keywords: adults with congenital heart disease, carotid intima‐media thickness, coarctation of the aorta
Subject Categories: High Blood Pressure, Congenital Heart Disease
Clinical Perspective
What Is New?
Adults with a history of coarctation of the aorta have increased carotid intima‐media thickness values compared with healthy controls.
Higher age, male sex, and prescribed antihypertensive medication are associated with higher carotid intima‐media thickness in patients with congenital heart disease.
Most diagnostic subgroups of patients with congenital heart disease show normal carotid intima‐media thickness values.
What Are the Clinical Implications?
Patients with coarctation of the aorta should be screened in order to early detect vascular disease involving the carotid arteries.
Patients with transposition of the great arteries after arterial switch demonstrated a trend toward increased carotid intimal thickness, which will need to be further studied to determine if screening is indicated.
Introduction
The decreasing mortality rate in children with congenital heart defects (CHDs)1, 2 leads to more attention for the management and care of adults with congenital heart disease (ACHD). While most of these patients are not cured but palliated and face long‐term cardiac sequelae, they need special care to live with their chronic conditions.3 With an aging population, there are new challenges for cardiologists, caregivers, and researchers.4, 5, 6
Late effects of CHD as well as further acquired heart defects are prominent.4 In this context, age‐associated cardiovascular diseases in ACHD patients become an additional problem. Atherosclerosis already starts in childhood and leads to cardiovascular disease in adults.7 Identifying diagnostic subgroups or patients at risk is an important step to prevent premature cardiovascular events in these patients in later life.
The aforementioned subclinical signs of atherosclerosis can be detected by measuring carotid intima‐media thickness (cIMT). This measurement is noninvasive, and it facilitates the early detection of vascular impairments.8 Another advantage of this diagnostic method is the early detection of vascular damage in otherwise healthy‐appearing people.9
In a prior study,10 we outlined that children and adolescents with coarctation of the aorta (CoA) and transposition of the great arteries (TGA) after arterial switch have higher cIMT values than the following groups: healthy controls, patients with a univentricular heart, isolated shunts, tetralogy of Fallot, and pulmonic stenosis. The aim of this study was to check if these conspicuous values are still prevalent in ACHD or whether other diagnostic subgroups show vascular changes in adulthood.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Subjects
The present study prospectively enrolled 831 patients with CHD (392 women) aged 18 to 81 years (38.8±11.7 years). All subjects were recruited during their outpatient visit at the German Heart Centre in Munich between May 2015 and February 2019. Age, sex, height, and weight as well as cIMT and blood pressure were recorded. Afterwards, all patients were classified in diagnostic subgroups: aortic stenosis (n=112), atrial septal defect/ventricular septal defect/atrioventricular septal defect (isolated shunts=138), CoA (n=69), tetralogy of Fallot (ToF, n=154), transposition of the great arteries after arterial switch (n=21), transposition of the great arteries after Rastelli and congenitally corrected TGA (n=37), transposition of the great arteries after Mustard/Senning repair (n=88), pulmonary stenosis or regurgitation (n=40), Ebstein anomaly (n=40), Fontan circulation (n=44), cyanotic (native or palliated; n=33), or others (n=55).
Patients’ data were compared with 191 healthy controls (111 women) of the same age range (36.7±13.5 years). All controls were investigated in a similar setting with the same ultrasound protocol, equipment, and staff.
The study was approved by the local ethical board of the Technical University of Munich (project number: 515/15) and is part of the CARING (Cardiovascular Risk in Grown‐up Congenital Heart Disease) project, which is registered in the “Deutsches Register Klinischer Studien” with the number DRKS00015248. All subjects gave written informed consent for participation.
Study Protocol
Since our overall study population was young, and therefore the likely prevalence of atherosclerotic plaque was low, we chose to measure cIMT as a subclinical marker. A well‐standardized study protocol is very important in scanning cIMT.8 We previously described our standardized protocol for measuring cIMT,10 which follows the guidelines of the Cardiovascular Prevention Working Group of the Association for European Paediatric Cardiology.11 These guidelines for scanning and analyzing cIMT are based on the Mannheim Consensus12 but are more specific and therefore important for future longitudinal studies following children to adulthood. In brief, all measurements were performed using the Cardiohealth Station of Panasonic (Jakohama, Japan), a semiautomated ultrasound system. With B‐Mode, 4 measurements of cIMT at the far wall of the common carotid artery, 1 cm distal to the bulb, were recorded, and a mean value was calculated and used for all following analyses (Figure 1). The central frequency of the linear‐array transducer was 8.9 MHz.
Figure 1.
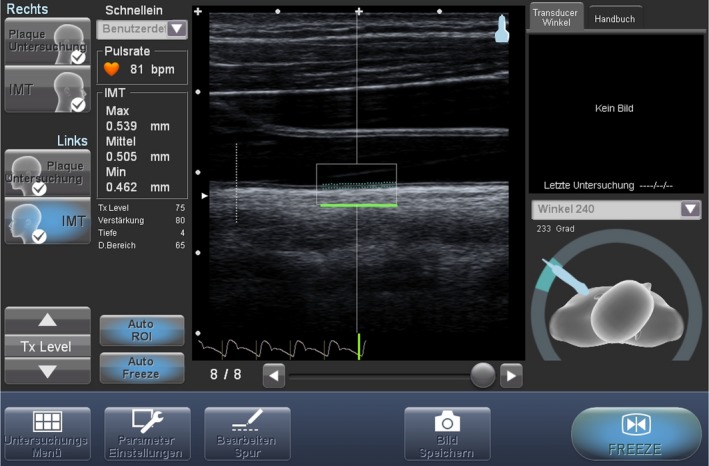
Visualization of the carotid intima‐media thickness measurement.
Age, sex, height, weight, and use of antihypertensive/heart failure medications (diuretics, β‐blocker, angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists) were recorded for all participants. Systolic and diastolic blood pressure were measured after 5 minutes of rest in supine position using the oscillometric Mobil‐O‐Graph (IEM Healthcare, Stolberg, Germany) device. Mean arterial pressure was calculated by the formula: [(2×diastolic)+systolic]/3.
Data Analyses
Descriptive data are reported as mean±SD in case of continuous variables and as counts±percentages in case of categorical variables. Comparison of demographic and clinical variables of ACHD and healthy controls were performed with t tests for unpaired samples and chi‐square tests if appropriate. As seen in our recently published paper on children with CHD, a multiple linear regression model was used to compare cIMT values between ACHD patients and healthy controls, after adjusting for age, sex, height, weight, mean arterial pressure, smoking status, and intake of antihypertensive medication.10 The same model, including the same covariates, was applied when comparing diagnostic subgroups with and without healthy controls. To find parameters associated with increased cIMT in patients with CHD, a multivariate regression model was used in this group.
All results are given as (estimated) mean and SD. Two‐sided P<0.05 were considered as statistically significant. SPSS 23.0 software (IBM Corp., Armonk, NY) was used for all statistical analysis of data.
Results
There were no notable differences in cIMT between the 2 general groups of ACHD and healthy controls (CHD=0.538±0.086 mm versus controls=0.541±0.083 mm; P=0.649) after correction for sex, age, height, weight, mean arterial pressure, smoking, and use of antihypertensive/heart failure agents (Table 1).
Table 1.
Overview of Study Characteristics
| n | Sex | Age (y) | Height (cm) | Weight (kg) | MAP (mm Hg) | Antihypertensive Medication | cIMTa (mm) | |
|---|---|---|---|---|---|---|---|---|
| Female n (%) | (Mean±SD) | (Mean±SD) | (Mean±SD) | (Mean±SD) | n (%) | (Mean±SD) | ||
| Aortic stenosis | 112 | 30 (25) | 35.7±10.4 | 175.4±9.0 | 78.0±15.9 | 89.7±9.9 | 38 (33.9) | 0.540±0.074 |
| Coarctation of the aorta | 69 | 29 (42) | 37.5±10.6 | 173.2±8.5 | 72.5±14.3 | 90.5±10.3 | 42 (60.9 | 0.592±0.075 |
| Isolated shunts (ASD, VSD, AVSD) | 138 | 80 (58) | 44.0±14.0 | 170.1±10.0 | 74.3±16.2 | 93.1±11.4 | 53 (38.4) | 0.535±0.082 |
| Pulmonic stenosis/regurgitation | 40 | 18 (45) | 38.9±12.6 | 172.7±11.3 | 72.0±14.2 | 91.3±9.9 | 11 (27.5) | 0.530±0.076 |
| Tetralogy of Fallot | 154 | 71 (46) | 38.0±10.2 | 171.2±9.1 | 72.3±14.9 | 88.8±10.1 | 39 (25.3) | 0.526±0.074 |
| TGA after arterial switch | 21 | 6 (29) | 27.0±7.8 | 178.7±9.4 | 74.6±14.4 | 86.5±9.1 | 6 (28.6) | 0.584±0.078 |
| TGA after Senning/Mustard | 88 | 32 (36) | 37.7±5.4 | 172.8±8.0 | 75.2±13.8 | 88.1±9.3 | 31 (35.2) | 0.545±0.075 |
| TGA after Rastelli and congenitally corrected TGA | 37 | 24 (65) | 38.9±12.2 | 170.4±8.5 | 67.8±12.8 | 84.7±8.4 | 19 (51.4) | 0.541±0.079 |
| Ebstein anomaly | 40 | 28 (70) | 43.1±15.2 | 171.4±7.3 | 73.3±13.4 | 90.5±9.3 | 16 (40) | 0.524±0.076 |
| Fontan circulation | 44 | 19 (43) | 35.4±8.7 | 170.2±10.6 | 68.8±14.8 | 85.1±9.8 | 35 (79.5) | 0.526±0.080 |
| Cyanotic (native or palliated) | 33 | 21 (64) | 44.5±11.4 | 167.2±9.8 | 66.1±14.1 | 85.8±10.1 | 23 (69.7) | 0.517±0.080 |
| Others | 55 | 34/21 (62) | 38.5±12.7 | 175.3±12.8 | 74.2±16.2 | 88.4±8.8 | 32 (58.2) | 0.519±0.082 |
| Congenital heart defect | 831 | 392 (47) | 38.8±11.7 | 172.1±9.7 | 73.2±15.1 | 89.3±10.3 | 348 (42) | 0.538±0.086 |
| Controls | 191 | 111 (58) | 36.7±13.5 | 173.8±8.8 | 72.2±14.2 | 89.8±9.0 | 9 (4.7) | 0.541±0.083 |
| P valueb | ··· | 0.008c | 0.032c | 0.043c | 0.371 | 0.584 | <0.001c | 0.649 |
ASD/VSD/AVSD indicates atrial septal defect/ventricular septal defect/atrioventricular septal defect; cIMT, carotid intima‐media thickness; MAP, mean arterial pressure; TGA, transposition of the great arteries.
Values are adjusted for sex, age, height, weight, mean arterial pressure, smoking status (yes/no), and antihypertensive medication (diuretics, β‐blocker, angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists).
Comparing CHD vs. controls with a t test and chi‐square test if appropriate.
Statistically siginificant difference (p<0.05).
In a more detailed analysis regarding diagnostic subgroups, only patients with CoA showed thicker cIMT values (0.592±0.075 mm; P<0.001) in comparison with healthy controls and almost all other subgroups (Figure 2 and Table 1).
Figure 2.
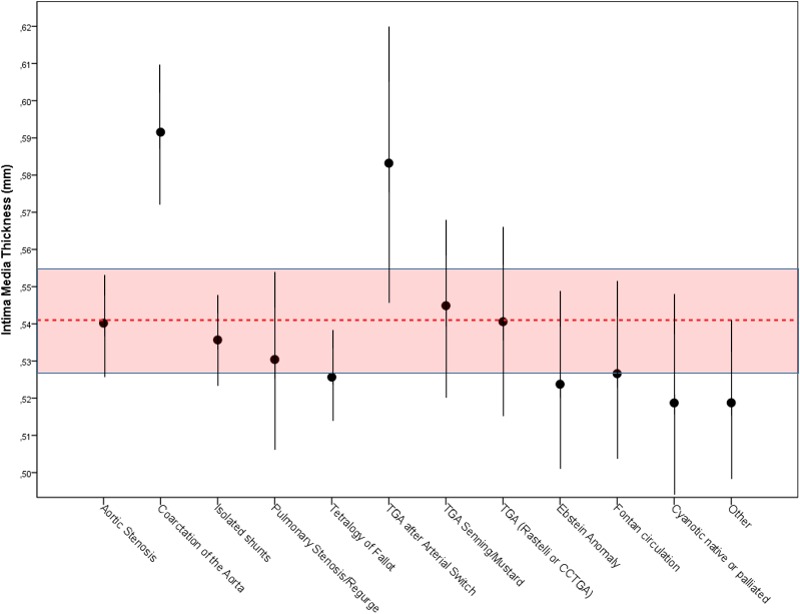
Carotid intima‐media thickness (cIMT) of adults with congenital heart disease in comparison with healthy controls after correction for sex, age, height, weight, mean arterial pressure, smoking status, and hypertensive agents.
In addition, in ACHD higher age (β=0.556, P<0.001), intake of antihypertensive/heart failure medication (β=0.103; P=0.001) and male sex (β=0.078, P=0.031) were associated with thicker cIMT in a multivariate regression model (Table 2).
Table 2.
Parameter Associated With Increased Carotid Intima‐Media Thickness in CHD in a Multivariate Regression Model
| β | P Value | |
|---|---|---|
| Sex (0=male, 1=female) | −0.078 | 0.032a |
| Age, y | 0.559 | <0.001a |
| Height, cm | −0.001 | 0.981 |
| Weight, kg | 0.032 | 0.396 |
| Mean arterial pressure, mm Hg | 0.014 | 0.631 |
| Antihypertensive medication (0=no, 1=yes) | 0.090 | 0.003a |
| Smoking (0=no, 1=yes) | 0.009 | 0.760 |
Statistically siginificant difference (p<0.05).
In addition, 8.9% of patients with CHD and 12.6% of healthy controls reported smoking histories.
Discussion
The findings of the present study confirm abnormal cIMT values for CoA patients in adulthood. In contrast to our prior study, adult patients with TGA after arterial switch do not demonstrate significantly increased cIMT values, compared with the other ACHD patients and healthy controls. One reason for the differing results between our 2 studies, the earlier of which compared cIMT in children,10 may be the small patient sample of adults with TGA after arterial switch in our adult cohort. Nevertheless, this group shows the second‐highest cIMT values and is the only group that does not significantly differ to the abnormal values of CoA patients. Therefore, we continue to recommend early, regular screening and management of acquired cardiovascular risk factors in the coarctation and arterial switch population. Patients with other CHD diagnoses did not show increased cIMT values at all.
Patients with CoA demonstrated highest cIMT values and are therefore at risk for later cardiovascular events. This is in accordance with the results of other studies13, 14 and the fact that estimated survival of CoA patients is still lowered.15, 16 Several studies16, 17, 18 showed that coronary artery disease is the most common cause of death in these patients. Further, their premature deaths are attributed to abnormal vascular characteristics19, 20, 21 and early atherosclerosis.13,15
Aortic arch geometry22 and autonomic cardiovascular dysregulation23 are only some factors that are discussed as influencing parameters of cardiovascular function in later life. Hypertension (at rest or exercise induced), for example, is one serious problem in patients with CoA.14, 18, 24 However, because normotensive children without residual aortic arch obstruction also show vascular abnormalities, Meyer et al14 supported the thesis of additional factors that promote early atherosclerosis. Even the theory that vascular changes might be a congenital problem in patients with CoA is possible, since vascular changes were also seen in patients with early repair and independent of the time of surgery.14
Cyanotic patients have a high morbidity and mortality25 but rarely show signs of atherosclerosis. Low blood pressure and good cholesterol levels might be responsible for good cIMT values26 by positively influencing arterial function and structure.
Because of hypoxemia, upregulated nitric oxide, hyperbilirubinemia, and thrombocytopenia, cyanotic patients are believed to have a reduced risk of atherosclerosis.26, 27 In contrast to this, the authors of a multicenter study28 concluded that there is neither a beneficial plasma lipoprotein profile nor a decreased atherosclerotic burden of this patient group.28 This is in accordance with our findings regarding cIMT values.
Conclusions
Answering our primary aim of this study, we can clearly confirm high cIMT values in adults with CoA. For patients with TGA after arterial switch, a higher number of patients is needed to make a clear assessment of their vascular structure. Both diagnostic subgroups should be screened for early prevention. For closer understanding of the vascular structure of patients with CHD, further basic research is needed to determine whether vascular changes directly influence the premature cardiovascular morbidity in this special cohort.
Limitations
The German Heart Centre Munich is a tertiary center, and all patients were under regular follow‐up. Therefore, a selection bias is possible. Although our total sample size of patients with CHD is huge, the findings for some subgroups (in particular, patients with TGA after arterial switch) could be strengthened by a higher number of cases. Further, the usefulness of cIMT values has been critically discussed in the past decade, as some studies showed that cIMT values are less predictive than plaque detection and coronary artery calcium score. Therefore, the authors do not recommend cIMT as parameter for risk stratification.29, 30 On the other hand, there are studies that recommend the common use of cIMT and plaque for risk stratification.31, 32
Sources of Funding
The study was funded by an unrestricted grant from the “Deutsche Gesellschaft für Pädiatrische Kardiologie (DGPK)” and “Friede‐Springer‐Herz‐Stiftung”. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e013536 DOI: 10.1161/JAHA.119.013536.)
References
- 1. Khairy P, Ionescu‐Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. [DOI] [PubMed] [Google Scholar]
- 2. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumgartner H, Budts W, Chessa M, Deanfield J, Eicken A, Holm J, Iserin L, Meijboom F, Stein J, Szatmari A, Trindade PT, Walker F. Recommendations for organization of care for adults with congenital heart disease and for training in the subspecialty of ‘Grown‐up Congenital Heart Disease’ in Europe: a position paper of the Working Group on Grown‐up Congenital Heart Disease of the European Society of Cardiology. Eur Heart J. 2014;35:686–690. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt Ami B, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S, Davidson William R, Earing Michael G, Ghoshhajra Brian B, Karamlou T, Mital S, Ting J, Tseng Zian H. Congenital heart disease in the older adult. Circulation. 2015;131:1884–1931. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation. 2016;133:2716–2733. [DOI] [PubMed] [Google Scholar]
- 7. Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki‐Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima‐media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. [DOI] [PubMed] [Google Scholar]
- 8. Liao X, Norata GD, Polak JF, Stehouwer CD, Catapano A, Rundek T, Ezhov M, Sander D, Thompson SG, Lorenz MW; PROG‐IMT Study Group , Balakhonova T, Safarova M, Grigore L, Empana J‐P, Lin H‐J, McLachlan S, Bokemark L, Ronkainen K, Schminke U, Lind L, Willeit P, Yanez DN, Steinmetz H, Poppert H, Desvarieux M, Ikram MA, Johnsen SH, Iglseder B, Friera A, Xie W, Plichart M, Su T‐C, Srinivasan SR, Schmidt C, Tuomainen T‐P, Völzke H, Nijpels G, Willeit J, Franco OH, Suarez C, Zhao D, Ducimetiere P, Chien K‐L, Robertson C, Bergström G, Kauhanen J, Dörr M, Dekker JM, Kiechl S, Sitzer M, Bickel H, Sacco RL, Hofman A, Mathiesen EB, Gabriel R, Liu J, Berenson G, Kavousi M, Price JF. Normative values for carotid intima media thickness and its progression: are they transferrable outside of their cohort of origin? Eur J Prev Cardiol. 2016;23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 9. de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JJ. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–III38. [DOI] [PubMed] [Google Scholar]
- 10. Reiner B, Oberhoffer R, Häcker AL, Ewert P, Müller J. Carotid intima‐media thickness in children and adolescents with congenital heart disease. Can J Cardiol. 2018;34:1618–1623. [DOI] [PubMed] [Google Scholar]
- 11. Dalla Pozza R, Ehringer‐Schetitska D, Fritsch P, Jokinen E, Petropoulos A, Oberhoffer R; Association for European Paediatric Cardiology Working Group on Cardiovascular Prevention . Intima media thickness measurement in children: a statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. 2015;238:380–387. [DOI] [PubMed] [Google Scholar]
- 12. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luijendijk P, Lu HL, Heynneman FB, Huijgen R, de Groot EE, Vriend JWJ, Vliegen HW, Groenink M, Bouma BJ, Mulder BJM. Increased carotid intima‐media thickness predicts cardiovascular events in aortic coarctation. Int J Cardiol. 2014;176:776–781. [DOI] [PubMed] [Google Scholar]
- 14. Meyer AA, Joharchi MS, Kundt G, Schuff‐Werner P, Steinhoff G, Kienast W. Predicting the risk of early atherosclerotic disease development in children after repair of aortic coarctation. Eur Heart J. 2005;26:617–622. [DOI] [PubMed] [Google Scholar]
- 15. Vriend JWJ, Mulder BJM. Late complications in patients after repair of aortic coarctation: implications for management. Int J Cardiol. 2005;101:399–406. [DOI] [PubMed] [Google Scholar]
- 16. Toro‐Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long‐term follow‐up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89:541–547. [DOI] [PubMed] [Google Scholar]
- 17. Vriend JW, de Groot E, de Waal TT, Zijta FM, Kastelein JJ, Mulder BJ. Increased carotid and femoral intima‐media thickness in patients after repair of aortic coarctation: influence of early repair. Am Heart J. 2006;151:242–247. [DOI] [PubMed] [Google Scholar]
- 18. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long‐term follow‐up and prediction of outcome after surgical correction. Circulation. 1989;80:840–845. [DOI] [PubMed] [Google Scholar]
- 19. Swan L, Kraidly M, Vonder Muhll I, Collins P, Gatzoulis MA. Surveillance of cardiovascular risk in the normotensive patient with repaired aortic coarctation. Int J Cardiol. 2010;139:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voges I, Kees J, Jerosch‐Herold M, Gottschalk H, Trentmann J, Hart C, Gabbert DD, Pardun E, Pham M, Andrade AC, Wegner P, Kristo I, Jansen O, Kramer HH, Rickers C. Aortic stiffening and its impact on left atrial volumes and function in patients after successful coarctation repair: a multiparametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kühn A, Baumgartner D, Baumgartner C, Horer J, Schreiber C, Hess J, Vogt M. Impaired elastic properties of the ascending aorta persist within the first 3 years after neonatal coarctation repair. Pediatr Cardiol. 2009;30:46–51. [DOI] [PubMed] [Google Scholar]
- 22. Ou P, Celermajer DS, Mousseaux E, Giron A, Aggoun Y, Szezepanski I, Sidi D, Bonnet D. Vascular remodeling after “successful” repair of coarctation: impact of aortic arch geometry. J Am Coll Cardiol. 2007;49:883–890. [DOI] [PubMed] [Google Scholar]
- 23. Polson JW, McCallion N, Waki H, Thorne G, Tooley MA, Paton JF, Wolf AR. Evidence for cardiovascular autonomic dysfunction in neonates with coarctation of the aorta. Circulation. 2006;113:2844–2850. [DOI] [PubMed] [Google Scholar]
- 24. Yogeswaran V, Connolly HM, Al‐Otaibi M, Ammash NM, Warnes CA, Said SM, Egbe AC. Prognostic role of hypertensive response to exercise in patients with repaired coarctation of aorta. Can J Cardiol. 2018;34:676–682. [DOI] [PubMed] [Google Scholar]
- 25. Engelfriet P, Boersma E, Oechslin E, Tijssen J, Gatzoulis MA, Thilen U, Kaemmerer H, Moons P, Meijboom F, Popelova J, Laforest V, Hirsch R, Daliento L, Thaulow E, Mulder B. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow‐up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2005;26:2325–2333. [DOI] [PubMed] [Google Scholar]
- 26. Duffels MG, Mulder KM, Trip MD, de Groot E, Gort J, van Dijk AP, Hoendermis ES, Daliento L, Zwinderman AH, Berger RM, Mulder BJ. Atherosclerosis in patients with cyanotic congenital heart disease. Circ J. 2010;74:1436–1441. [DOI] [PubMed] [Google Scholar]
- 27. Fyfe A, Perloff JK, Niwa K, Child JS, Miner PD. Cyanotic congenital heart disease and coronary artery atherogenesis. Am J Cardiol. 2005;96:283–290. [DOI] [PubMed] [Google Scholar]
- 28. Tarp JB, Sorgaard MH, Christoffersen C, Jensen AS, Sillesen H, Celermajer D, Eriksson P, Estensen ME, Nagy E, Holstein‐Rathlou NH, Engstrom T, Sondergaard L. Subclinical atherosclerosis in patients with cyanotic congenital heart disease. Int J Cardiol. 2019;277:97–103. [DOI] [PubMed] [Google Scholar]
- 29. Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten‐year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J. 2010;31:2041–2048. [DOI] [PubMed] [Google Scholar]
- 30. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M‐R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41:111–188. Available at: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 31. Gardin JM, Bartz TM, Polak JF, O'Leary DH, Wong ND. What do carotid intima‐media thickness and plaque add to the prediction of stroke and cardiovascular disease risk in older adults? The Cardiovascular Health Study. J Am Soc Echocardiogr. 2014;27:998–1005.e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naqvi TZ, Lee MS. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. [DOI] [PubMed] [Google Scholar]


