Introduction
In almost 4 decades since the start of the HIV epidemic, the world has witnessed an unprecedented evolution of this disease from a debilitating and rapidly fatal syndrome to a chronic condition, effectively managed with combination antiretroviral therapy (cART). Today people living with HIV (PLWH) who receive treatment have nearly the same life expectancy as HIV‐negative individuals.1 With the exception of 2 people cured by human stem‐cell transplantation,2, 3 a widely applicable cure for HIV remains elusive and infection still requires lifelong therapy for PLWH. Although effective cART suppresses viral replication, inflammation and immune activation persist for PLWH and are driven by a combination of HIV‐dependent and HIV‐independent factors.4 These immune factors contribute to an excess of non‐AIDS comorbidities in PLWH, including cardiovascular disease (CVD), frailty, malignancy, neurocognitive disease, osteoporosis, and renal and liver diseases.4 It is increasingly recognized that as the population of PLWH ages, targeting non‐AIDS comorbidities is essential to effectively care for and treat this population.
CVD is the leading cause of death worldwide, accounting for 56.9 million deaths in 2016.5 The relative risk of CVD in PLWH is significantly higher than in HIV‐negative controls, including: higher rates of acute myocardial infarction6 and increased risk for ischemic stroke,7 heart failure,8 and sudden cardiac death.9 In fact, it is estimated that the HIV‐associated risk for CVD may be similar to that of traditional risk factors such as smoking, hyperlipidemia, diabetes mellitus, and hypertension.10 Despite several studies showing the higher risk of cardiovascular events in PLWH, the greatest challenge has been defining the overarching mechanisms by which HIV‐mediated immune activation and chronic inflammation increase the risk for CVD.11 This has made it difficult to identify effective interventions to target and reduce cardiovascular risk in this population despite considerable efforts.
In this review, we examine the effects of HIV‐associated inflammation and immune activation on the cardiovascular system with a focus on atherosclerotic CVD and discuss existing and proposed therapeutic strategies targeting inflammation to reduce CVD risk. The factors contributing to immune activation and CVD in PLWH are summarized in Figure 1 below.
Figure 1.
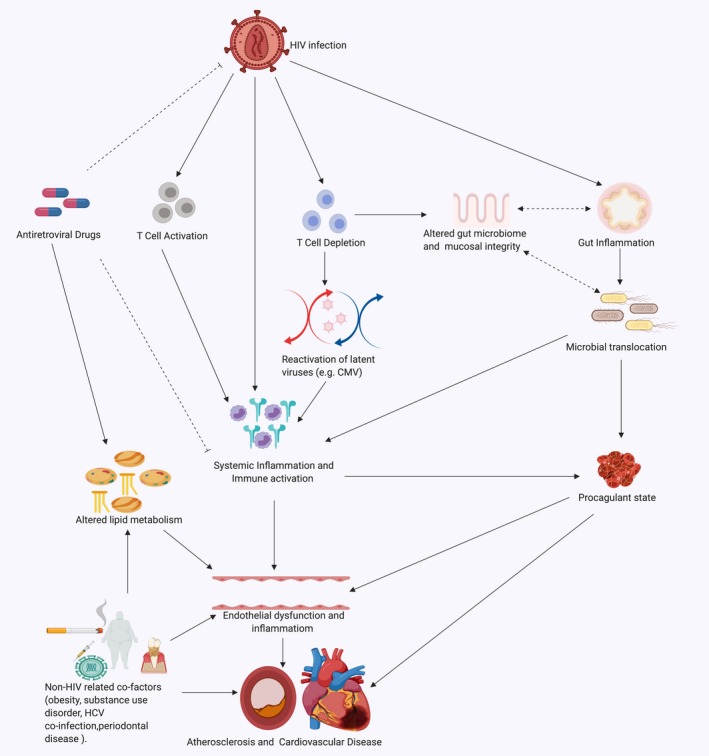
Factors contributing to immune activation and cardiovascular disease in PLWH. Solid line arrows indicate a contributory effect; dotted line arrows represent a potential yet uncertain relationship; dotted terminal line indicates an inhibitory effect. ART indicates antiretroviral therapy; CMV, cytomegalovirus; HCV, hepatitis C virus; PLWH, people living with HIV. This figure was created using http://www.biorender.com software.
Mechanisms of Chronic Inflammation and Immune Activation in HIV Infection
Infection with HIV triggers a generalized activation of the immune system. This immune activation is both specific and nonspecific, involving several mechanisms.
Persistent Viral Production and Replication
During HIV infection, uncontrolled viral replication leads to progressive CD4+ T‐cell decline, but also systemic inflammation and immune activation. In the SMART (Strategies for Management of Antiretroviral Therapy) trial, continuous suppression of HIV replication was associated with decreased risk of CVD when compared with intermittent therapy, suggesting a direct role for uncontrolled viral replication as a risk factor for CVD.12, 13 Subsequent studies have gone on to show an association between uncontrolled HIV replication and vascular endothelial dysfunction,14, 15 further highlighting the importance of cART to reduce cardiovascular risk in PLWH. This is especially relevant in Sub‐Saharan Africa, which harbors 26 million PLWH with an estimated 40% of these individuals not on cART.16 The Ndlovu cohort study, founded in 2017, aims to provide insight into the burden of CVD and contribution of HIV infection in a rural area of Sub‐Saharan Africa with high HIV prevalence.17 This study will include a total of 1000 HIV‐positive and 1000 HIV‐negative participants, with a male‐to‐female ratio of 1:1, and should provide useful information on the burden of CVD in this context as well as the implications of virological suppression with cART on the risk of CVD.17 In a prospective study of 82 treatment‐naïve patients enrolled to initiate cART in the United States, treatment of HIV leading to virological suppression and immune reconstitution resulted in rapid improvement in brachial artery flow‐mediated dilation (FMD), a measure of endothelial dysfunction.14 A smaller study by Baker et al also demonstrated that untreated HIV infection was associated with impaired arterial elasticity, measured by pulse waveform analysis, in both small and large vessels.
In elite controllers who maintain high CD4+ T‐cell counts and suppress HIV viremia in the absence of cART, there is also evidence of innate immune activation and elevated serum markers for inflammation associated with increased risk for clinical events, higher rates of hospitalization, and CVD.18, 19, 20, 21, 22, 23 In a longitudinal study of 40 elite controllers, 98% of these individuals had measurable HIV RNA during a 16‐month median follow‐up period, often at levels higher than observed in patients receiving cART.24 These findings suggest that low‐level viral replication, which may not be consistently detectable in elite controllers, may still be a significant driver of immune activation and inflammation in this group. These persistent T‐cell activation levels in elite controllers may contribute to progressive CD4+ T‐cell decline even in the absence of consistently detectable viremia,25 thus highlighting a potential role for treating elite controllers with cART in order to prevent non‐AIDS‐related comorbidities.19, 20, 26
T‐Cell Activation, Dysfunction, and Depletion
CD4+ T‐cell activation and depletion as well as CD8+ T‐cell activation are central to the pathogenesis of HIV infection. Activated CD4+ T cells (CD38+ HLA‐DR+) are significantly elevated in HIV‐positive individuals compared with negative controls25 and decrease with early initiation of cART.27 Several studies have examined the association between CD4+ T‐cell depletion and CVD and shown an independent association between lower CD4+ T‐cell counts and increased cardiovascular risk.7, 28, 29, 30 In 1 study of 114 HIV‐positive women compared with HIV‐negative controls, women with HIV infection were found to have higher levels of CD38+ HLA‐DR+ CD4+ T cells. This higher level of activated T cells was associated with increased carotid artery stiffness in this group.31 In the HIV‐negative population, activated CD4+ T cells are frequently found in atherosclerotic lesions. It is plausible to speculate that in HIV infection, activated CD4+ T cells generate cytokines which are part of the cascade leading to atherosclerosis. A causal link is yet to be established between T‐cell activation and risk for CVD.20, 32
Cytotoxic CD8+ T cells are important components of the adaptive immune response to HIV infection. Elevated levels of activated CD8+ T cells (CD38+ HLA‐DR+) have been associated with subclinical carotid artery disease in HIV‐positive individuals.31, 33 In the SATURN‐HIV (Stopping Atherosclerosis and Treating Unhealthy bone with Rosuvastatin in HIV) trial, higher levels of CD8+ CD38+ HLA‐DR+ T cells were associated with increased carotid media intima thickness (CIMT).33 The reasons behind persistent CD8+ T‐cell activation, even among people on cART, are likely a combination of a persistent antiviral response to HIV infection as well as stimulation from other viral infections like cytomegalovirus. In a study of 93 HIV positive individuals compared with 37 HIV negative controls, cytomegalovirus‐specific T‐cell responses was shown to be independently associated with CIMT in HIV‐positive individuals compared with negative controls.34
Monocyte/Macrophage Activation
There is considerable evidence to support that activation of monocytes and macrophages also enhances atherosclerosis in chronic HIV infection.35, 36, 37, 38 Activated monocytes and macrophages release soluble CD163 (sCD163) during inflammation, a serum marker associated with aortic inflammation and plaque volume in PLWH.35, 36, 39 Soluble CD14 (sCD14), another serum marker of macrophage activation, has also been shown to correlate with subclinical atherosclerosis in HIV‐positive individuals,37 further implicating the innate immune response in the pathogenesis of CVD in chronic HIV infection. The central role of monocyte/macrophage activation in the pathogenesis of atherosclerosis in the general population is well recognized.40 Circulating monocytes adhere to vascular endothelium and transform into tissue macrophages, where they phagocytize oxidized low‐density lipoprotein (LDL) molecules to become lipid‐laden foam cells.41 Consequently, this triggers release of proinflammatory cytokines and adhesion molecules central to plaque formation and growth.41 The chronic state of inflammation and immune activation associated with HIV infection likely amplifies monocyte/macrophage inflammation and tissue damage in arterial plaque formation.
Effects of HIV‐Mediated Immune Activation and Chronic Inflammation on the Cardiovascular System
Suppressing viral replication with cART significantly reduces immune activation for PLWH, but it does not eliminate it. PLWH have persistently higher levels of systemic inflammation when compared with HIV‐negative controls both before and while receiving cART. This is reflected by higher levels of serum biomarkers (including hs‐CRP [high‐sensitivity C‐reactive protein], IL [interleukin]‐6, sCD163, sCD14, and D‐dimer) observed in PLWH on cART compared with negative controls, driven by mechanisms previously discussed.35, 36, 38, 42, 43, 44 The resulting effects on the cardiovascular system are far reaching and likely explain the increased risk for cardiovascular events noted in this population, even when accounting for traditional cardiovascular risk factors.
Lipid Metabolism and Atherosclerosis
Vascular endothelial dysfunction and inflammation are central to the pathogenesis of atherosclerosis. A detailed discussion of atherosclerosis and lipid metabolism are beyond the scope of the current review. Briefly, in response to proatherogenic stimuli, such as oxidized LDL, the vascular endothelium produces nitric oxide, which alters vascular tone, upregulates production of chemokines and adhesion molecules, and increases endothelial permeability. This leads to increased deposition of low‐density lipids in the vascular wall. Macrophage uptake of oxidized LDL induces transformation to foam cells, which are characterized by the further release of chemokines, proinflammatory cytokines, and growth factors.41 This causes progressive intimal thickening and plaque formation characteristic of atherosclerotic disease. If the plaque ruptures or erodes, this exposes the highly thrombogenic collagenous matrix and lipid core to the circulation. This, in turn, triggers platelet aggregation, fibrin deposition, and ultimately thrombus formation, which, at its worse, may ultimately cause obstruction of the vessel.41
HIV infection alters markers of vascular endothelial dysfunction through mechanisms which remain incompletely understood. As previously described, endothelial dysfunction is an important precursor of atherogenesis. In nonhuman primate studies, focal endothelial proliferation and increased subendothelial inflammatory cells were identified in thoracic aortas of simian immunodeficiency virus–infected macaques compared with negative controls.45 FMD of the brachial artery has been used as a measure of endothelial dysfunction and a predictor of CVD in the general population.46 To date, 1 prospective randomized controlled trial (RCT) has demonstrated a reduction in flow‐mediated dilatation FMD in PLWH treated with cART.14 Furthermore, soluble VCAM‐1 (vascular cell adhesion molecule‐1), a marker for vascular endothelial activation, has also been shown to be elevated in HIV‐positive patients, and levels were reduced by cART.47 In vitro studies provide some evidence for the direct toxicity of viral proteins gp120 and Tat (transactivator of viral replication) to cardiac and vascular cell lines, suggesting a potential direct role of HIV replication in mediating endothelial dysfunction.48, 49 This likely does not fully explain the endothelial dysfunction observed in PLWH, given that markers of endothelial dysfunction remain higher in PLWH on cART with effective viral suppression.50 Finally, cART itself has been implicated in endothelial dysfunction and further confounds the mechanistic drivers of this process in PLWH.51, 52
In addition to effects on the endothelium, lipid metabolism appears to be directly altered by HIV infection.53, 54 Before the rollout of cART, clinical studies of untreated HIV‐positive individuals demonstrated lipid disorders, including increased triglycerides, increased LDL, and low high‐density lipoprotein serum levels, in patients with advanced disease and severe immunosuppression.55, 56, 57 Chronic inflammation and immune activation may play a role in dyslipidemia observed in individuals with untreated HIV infection. In untreated PLWH, high levels of IFN (interferon)‐alpha may decrease clearance of triglycerides, leading to elevated serum levels,55, 56 whereas low levels of serum high‐density lipoprotein have been associated with elevated IL‐6 and D‐dimer levels.57 Treatment with cART generally leads to some improvement in low high‐density lipoprotein cholesterol, but does not normalize the lipid profile in treated PLWH.58, 59 Several antiretroviral drug classes have also been implicated in altering lipid metabolism and increasing oxidative stress in PLWH, potentially independently contributing to increased cardiovascular risk (reviewed in Cunha60).
Hypercoagulability
Several markers of chronic inflammation have been linked with increased cardiovascular risk in the general population and in HIV‐positive individuals.15, 61 IL‐6 and hs‐CRP are associated with increased mortality from CVD in PLWH and hs‐CRP to increased CIMT in this population.8, 43 D‐dimer, a product of the coagulation cascade and inflammation marker, is also increased in HIV infection and has been shown to have similar associations with CVD risk as IL‐6 and hs‐CRP.32 Besides being a marker of inflammation, high levels of D‐dimer are associated with a procoagulable state. It is reasonable to hypothesize that a procoagulable state would increase the risk of cardiovascular events such as myocardial infarction (MI), stroke, and acute thrombosis.54, 61 A causal relationship between D‐dimer elevation and cardiovascular events in PLWH has not yet been demonstrated and may simply be a reflection of the underlying chronic inflammatory state. The coagulation cascade remains, however, an attractive target for interventions aimed at reducing the risk of CVD in HIV infection.
Targeting Inflammation and Immune Activation to Reduce the Risk of CVD in HIV
Given the overwhelming evidence supporting the increased risk of CVD in patients in PLWH, several traditional and novel interventions are being investigated to reduce cardiovascular risk in this patient population (summarized in Table 1 below).
Table 1.
Interventions Targeting Cardiovascular Inflammation in HIV‐1: Outcomes, Benefits, and Limitations
| Intervention | Mode of Action | Effect on Markers of Inflammation and Immune Activation in PLWH | Benefits | Limitations |
|---|---|---|---|---|
| Traditional interventions | ||||
| Aspirin | Inhibition of the COX‐1 pathway | No effect on sCD14, IL‐16, SCD163, d‐dimer and endothelial function after 12‐weeks62 | Primary prevention of cardiovascular events in individuals with high ASCVD risk63 |
Non‐negligible risk of bleeding especially in elderly No effect on HIV‐mediated inflammation and immune activation |
| Dipyramidole | Adenosine reuptake inhibitor |
No effect on sCD14, sCD163and IL‐664 Modest decrease in CD8+ T cells and decrease in activated CD4+ T cells64 |
FDA approved for treatment of peripheral arterial disease and coronary artery disease | Associated bleeding risk |
| Statins | Inhibition of HMA‐CoA enzyme |
Rosuvastatin reduced markers T‐cell activation, vascular inflammation, and immune activation65 Atorvastatin reduced noncalcified plaque volumes66 |
Established benefits and safety in reducing ASCVD risk in HIV‐negative individuals | Unclear benefit in a more‐generalizable population of PLWH Ongoing REPRIEVE trial should provide more information |
| Canakinumab | Monoclonal antibody that binds IL‐1β | Reduced markers of inflammation and monocyte activation as well as aortic inflammation measured by FDG‐PET67 |
Reduces rates of recurrent cardiovascular events in HIV‐negative individuals68 Demonstrated to be safe in PLWH67 |
Expensive intervention Unclear medium‐ and long‐term efficacy and safety in PLWH |
| Novel interventions | ||||
| Bilirubin | Scavenges free radicals | Elevated bilirubin levels correlated with decreased CVD in HIV‐positive and ‐negative individuals | Elevated bilirubin levels can be induced be pharmacologically induced, thus potential for harnessing this effect to target CVD | No large clinical trial of interventions raising bilirubin levels as a strategy for targeting CVD in PLWH |
| Tocilizumab | Monoclonal antibody that binds IL‐6 | Modest effect on markers of inflammation and immune activation in small group of PLWH69 | Effective anti‐inflammatory in rheumatological disease with some improvement of endothelial function in high‐risk populations of HIV‐negative individuals70 |
Elevation of LDL‐ cholesterol and alterations in lipid profiles in both HIV‐positive and ‐negative individuals of unclear significance Expensive intervention |
| Sirolimus | mTOR inhibitor |
Reduced CCR5 expression Reduced markers of cell cycling and immune exhaustion71 |
FDA approved as immunosuppressive therapy in organ transplant recipients |
Associated hypercholesterolemia and ‐triglyceridemia Well‐described drug‐related toxicities and increased risk for infections |
| Valgancyclovir | Competitive inhibitor of deoxyguanosine triphosphate inhibiting viral DNA polymerases | Reduced markers of T‐cell activation72 | FDA approved for treatment of invasive cytomegalovirus disease |
Unclear benefit in reducing the risk for CVD in PLWH Associated renal toxicity |
| Probiotics | Alters gut microbiome |
May cause changes in gut‐associated lymphoid tissues and improve biomarkers of microbial translocation73, 74, 75 No effect on markers of inflammation and immune activation76 |
Potentially a low‐cost intervention with favorable tolerance profile | Unclear impact, if any, on gut microbial translocation and HIV pathogenesis Optimal timing of intervention, duration, and dosing present challenges |
| Methotrexate |
Inhibits dihydrofolate reductase enzyme Inhibits binding of IL‐1B to its surface receptor |
No effect on endothelial function No effect on markers of inflammation and immune activation Significant decrease in CD8+ T cells77 |
Demonstrated safety in select population of PLWH |
Failed to show any benefit as tool for reducing CVD risk Pre‐existing T‐cell abnormalities in people with chronic HIV may overpower immune‐modulatory effects of low‐dose methotrexate Well‐described adverse effects of increased risk for infection |
| Edoxaban | Direct inhibitor of factor Xa |
Reduction in D‐dimer levels No effect on markers of inflammation or immune activation78 |
May impact HIV‐associated hypercoagulability through effect on D‐dimer |
Non‐negligible bleeding risk Unknown impact on markers of endothelial function and atherosclerosis |
| Jak‐inhibitors | Small molecules targeting specific JAKs in the JAK/STAT pathway |
Modest decrease in sCD14 No significant effect on IL‐6 levels noted79 |
Good tolerability and specificity in targeting inflammation Ruxolitinib shown to be safe in PLWH Role in impeding seeding of the HIV reservoir gives them potential for use in functional cure strategies80 |
Effect on endothelial function and atherosclerosis yet to be determined High‐dose tofacitinib and baricitinib associated with increased risk for thrombosis Relatively new molecules and some may be associated with significant associated cost |
ASCVD indicates atherosclerotic cardiovascular disease; CCR5, C‐C chemokine receptor type 5; COX‐1, cyclooxygenase 1; CVD, dardiovascular disease; FDA, US Food and Drug Administration; FDG‐PET, fluorodeoxyglucose positron emission tomography; HMA‐CoA, β‐hydroxy β‐methylglutaryl coenzyme A; IL‐1β, interleukin‐1 beta; IL‐16, interleukin‐16; JAK, Janus kinase; LDL, low‐density lipoprotein; mTOR, mammalian target of rapamycin; PLWH, people living with HIV; REPRIEVE, Randomized Trial to Prevent Vascular Events in HIV; sCD14, soluble CD14; sCD163, soluble CD163; STAT, signal transducer and activator of transcription.
Directly Acting Modulators of Inflammation and Immune Activation
Antiplatelet Agents
Platelets play a key role in thrombus formation and atherogenesis and may also contribute to inflammation. Increased platelet activation is associated with an increased risk of cardiovascular events. As a result, antiplatelet therapy is recommended in those at highest risk for CVD and with low risk of bleeding. Aspirin, an antiplatelet drug that irreversibly inhibits the COX‐1 (cyclooxygenase‐1) pathways, has been the choice drug for this intervention based on clinical trial data.63
The effect of low‐dose aspirin on markers of inflammation and immune activation for PLWH has been assessed in several observational studies and clinical trials. An open‐label, uncontrolled study showed that 1 week of low‐dose aspirin therapy in HIV‐positive patients on cART reduced platelet aggregation and markers of T‐cell/monocyte activation compared with untreated controls.81 This promising observation led to a prospective, blinded RCT with a longer intervention period of 12 weeks, comparing the effects of low‐dose (100 mg) aspirin with a higher (300 mg) dose and a placebo on the markers of immune activation and inflammation in PLWH on cART. The larger trial did not show any effect of 100 or 300 mg of aspirin compared with placebo on levels of sCD14, IL‐16, sCD163, D‐dimer, or other markers of monocyte and T‐cell activation. Endothelial function measured by FMD was also unaffected.62
Anti‐inflammatory effects of aspirin are mediated by its ability to selectively block the COX‐1 pathway, causing a downstream inhibition of prostaglandin and paracrine hormone production and propagating a broad variety of effects. These effects are highly nonspecific, which might explain the inability of aspirin to have a measurable effect on inflammation and immune activation for HIV‐positive individuals, suggesting that more‐targeted anti‐inflammatory interventions may be required in order to observe an effect. Recent evidence has demonstrated the significant risk of major bleeding associated with the use of aspirin for primary prevention of CVD82 and highlighted the need to better define high‐risk individuals with clinical atherosclerosis (such as those with a high coronary artery calcium score) in whom the benefits of this intervention may outweigh the bleeding risk.83 This is a consideration which is highly relevant in HIV‐positive individuals, given that individuals in this group are more likely to have a pre‐existing risk of bleeding features such as thrombocytopenia.
IL‐1ß Antagonists
IL‐1ß is an important inflammatory cytokine that drives the IL‐6 signaling pathway. IL‐1ß plays a significant role in development of atherothrombotic plaque. It promotes coagulation, increases adhesion of leukocytes and monocytes to the vascular endothelium, and upregulates growth of smooth muscle cells.84, 85, 86 The CANTOS (Canakinumab Anti‐Inflammatory Thrombosis Outcomes Study) trial was designed to test the inflammatory hypothesis of atherothrombosis by using canakinumab, a therapeutic monoclonal antibody which specifically targets IL‐1ß, to treat HIV‐negative individuals.68 In patients with a previous MI and baseline elevated hs‐CRP levels, 150 mg of canakinumab led to significantly reduced rates of recurrent cardiovascular events than placebo, independent of lipid lowering.68
The landmark findings from the CANTOS trial encouraged investigation of IL‐1ß antagonists to target inflammation and immune activation in PLWH. More recently, a nested case‐control study and 183 HIV‐negative controls from Denmark explored the IL‐1 inflammation pathway as a predictor of first‐time MI in PLWH. This study found that PLWH had higher levels of the IL‐1 receptor antagonist at all time points leading to first‐time MI, and these higher levels of IL‐1 receptor antagonist were associated with a 1.5‐fold increase in MI risk.87 These findings further support targeting the IL‐1 activation pathway to reduce CVD for PLWH.
A small, open‐label pilot study including 10 participants evaluated the safety and efficacy of canakinumab in targeting inflammation and immune activation for PLWH virologically suppressed with cART.67 This study showed that canakinumab therapy was safe and led to decreased levels of markers of inflammation and monocyte activation and also a reduction in aortic inflammation as measured by fluorodeoxyglucose positron emission tomography.67 Whereas these findings are encouraging, they are limited by the small numbers recruited and the short follow‐up time. A larger and longer RCT (ClinicalTrials.gov Identifier: NCT02272946) is underway, which should help clarify the short‐ and medium‐term efficacy and safety of canakinumab in PLWH. It is worth mentioning that the IL‐1ß antagonist, canakinumab, carries a treatment cost of approximately $200 000 per year for its current US Food and Drug Administration (FDA)‐approved indications and recently failed to gain approval from the regulatory body for use in CVD prevention in HIV‐negative individuals.
Low‐Dose Methotrexate
Methotrexate is a chemotherapeutic and anti‐inflammatory drug used to treat cancer and chronic rheumatic conditions such as rheumatoid arthritis (RA). The mechanism involves a competitive inhibition of the dihydrofolate reductase enzyme and also inhibition of IL‐1ß binding to its cell‐surface receptor. Several large cohort studies have suggested the benefit of low‐dose methotrexate therapy (LD MTX) for reducing atherosclerotic CVD risk.88, 89, 90, 91, 92 This prompted the investigation of LD MTX as a strategy to lower cardiovascular inflammation in a large RCT excluding PLWH. The CIRT (Cardiovascular Inflammation Reduction Trial), NCT01594333, aimed to randomize 7000 patients with previous MI and either type 2 diabetes mellitus or metabolic syndrome to LD MTX or placebo for 3 to 5 years. The trial was halted in early 2018 because of failure to show an effect on markers of inflammation or cardiovascular events compared with placebo.93 Hsue et al, in a smaller observational study, looked at the safety and impact of LD MTX on endothelial function in PLWH on cART.77 Although the intervention was safe, it did not have any demonstrable effects on endothelial function measured by brachial artery FMD or inflammatory biomarkers, but was associated with a significant reduction in CD8+ T cells.77
The failure of this intervention to show an effect again suggests that the pathways of inflammation in HIV infection likely differ significantly from those of inflammatory rheumatologic conditions, such as RA and psoriasis, for which methotrexate is effective treatment. Furthermore, the pre‐existing T‐cell abnormalities, even in virologically suppressed PLWH on cART, as included in this study, may overpower the immunomodulatory effects of LD MTX.77
IL‐6 Antagonists
Elevated levels of IL‐6 have been linked to an increased risk of cardiovascular events70 and predict morbidity and mortality for individuals living with HIV infection.94 The IL‐6 antagonist, tocilizumab, is approved for treatment of RA.95 Given the central role of IL‐6 as a driver of cardiovascular inflammation and atherogenesis, there has been interest in the use of IL‐6 antagonists to reduce cardiovascular inflammation. In safety trials of tocilizumab for treatment of RA, lipid levels increased for some patients receiving the study drug,70, 96, 97, 98 raising a concern as to whether it would be beneficial or harmful as a potential agent to reduce the risk of CVD.
In 1 community‐based, prospective study, tocilizumab was shown to improve endothelial function in a high‐risk population despite its effect of increasing levels of LDL cholesterol.99 At the 2019 CROI (Conference on Retroviruses and Opportunistic Infections), a small RCT looking at the effects of tocilizumab among treated HIV‐positive patients showed that the drug significantly altered lipid profiles for individuals on cART, but also seemed to modestly lower some markers of inflammation and immune activation in this patient population.69 Further studies are warranted to fully examine the impact of tocilizumab‐induced lipid changes on CVD risk for PLWH.
Janus Kinase Inhibitors
The JAK (Janus kinase)/STAT (signal transduction and activator of transcription) pathway has been implicated as an important driver in the pathogenesis of inflammatory, hematological, and autoimmune conditions.100, 101, 102 Binding of a variety of ligands, such as cytokines, interleukins, and interferons, to cell‐surface receptors causes activation of JAKs. Activation of JAKs triggers cross‐phosphorylation of these proteins and further downstream phosphorylation of a family of STAT proteins.100 Activation of the JAK/STAT pathway ultimately culminates in inducing transcription of genes for a range of proinflammatory and immunomodulatory cytokines.
Signaling pathways for important inflammatory cytokines TNF (tumor necrosis factor)‐alpha, IL‐6, and IL‐1 have been successfully targeted by biological agents in treatment of autoimmune diseases and cancer. The success of tocilizumab (a monoclonal antibody targeting IL‐6 for the treatment of RA) validated the potential for JAK1, JAK2, and TYK2 (tyrosine kinase 2) as viable therapeutic targets of inflammation.102, 103 Biological agents are highly successful and have revolutionized the treatment of autoimmune conditions and cancer in recent years, but also present significant limitations. Biologics are large proteins with the potential to be immunogenic, require administration by intravenous infusion or subcutaneous dosing, and treatments are associated with significant financial cost. Their use has also been associated with opportunistic infections, making them less attractive for use in PLWH.
Currently, 3 JAK inhibitors are FDA approved for clinical use—tofacitinib (RA and ulcerative colitis), baricitinib (RA), and ruxolinitib (myeloproliferative neoplasms)104, 105, 106—and at least 12 more drugs are being tested in clinical trials. Tolerability and specificity of JAK inhibitors present a unique advantage over traditional anti‐inflammatory agents like NSAIDs. They are small molecules with oral bioavailability, an added advantage over biological agents. The earlier‐generation JAK inhibitors, such as tofacitinib, which was the first of its class to be FDA approved, have the disadvantage of not being highly selective in its ability to block JAKs. Tofacitinib specifically blocks JAK3, but has a lower affinity for JAK1/JAK2. JAK3 blockade is associated with lower neutrophil and natural killer cell counts, which results in an increased risk for infections associated with its use.102 This observation led to a more‐targeted approach in developing newer JAK inhibitors more specific for blocking JAK1/2. Ruxolitinib and its structural analog, baricitinib, specifically inhibit JAK1/2.107 Baricitinib has been demonstrated to be safe in pediatric populations in treatment of autoinflammatory interferonopathies108 and is under investigation for other indications in this population. Another important advantage is its low dose (2 or 4 mg) and minimal metabolism (poor substrate for cytochrome P450) and hence reduced potential for interaction with other drugs.109
When it comes to JAK inhibitors and their potential role in targeting HIV‐related immune activation/inflammation and the implied CVD risk, these agents present an attractive option worthy of further exploration. Inflammatory cytokines, which are generated through the JAK/STAT pathway (IL‐1, IL‐6, IL‐10, and IFN‐alpha and ‐gamma), remain elevated in treated and untreated HIV‐positive individuals and are associated with increased cardiovascular risk, morbidity, and mortality in this group. In addition, immunomodulatory cytokines, such as IL‐2, IL‐7, and IL‐15, play an important role in triggering the JAK/STAT pathway and in recent reports have been implicated in proliferation of the HIV reservoir.110, 111 This presents a unique dual target for JAK inhibitors, both as potential modulators of chronic inflammation/activation and part of a functional cure strategy, by targeting expansion of the viral reservoir. A recent study by Gavegnano et al demonstrated the role of the JAK/STAT pathway in HIV persistence and showed the ability of ruxolitinib and tofacitinib to impede seeding and maintenance of the HIV reservoir,80 further supporting this theory.
Safety of JAK‐inhibitors in HIV‐positive people is currently being examined in clinical trials. The ACTG (AIDS Clinical Trials Group) 5336 open‐label RCT recently assessed the safety, tolerability, and immunological activity of 5 weeks of ruxolitinib 10 mg in HIV‐positive people on cART.79 This study showed that in a highly selected cohort of HIV‐positive individuals, ruxolitinib was safe and led to a modest decrease in sCD14, though a nonsignificant reduction was noted in IL‐6 levels for individuals receiving ruxolitinib compared with the control group.79 The findings of this trial provide support for future studies aimed at exploring the use of JAK‐inhibitors to target residual inflammation and immune dysregulation in treated HIV infection. Cardiovascular outcomes have been examined in patients receiving tofacitinib for RA and psoriasis, with some indication that among treated individuals, it may lead to lower incidence of major cardiovascular events.105, 112 Of note, postmarketing reports of increased risk of thrombosis associated with high doses of tofacitinib and baracitinib to a lesser degree have been reported by the FDA and will need to be considered with any new clinical indications for this drug class.113, 114 The benefit of JAK inhibitors in specifically targeting cardiovascular inflammation should be examined in the near future.
Indirectly Acting Modulators of Inflammation and Immune Activation
Statins
These potent lipid‐lowering drugs act by inhibiting the β‐hydroxy β‐methylglutaryl coenzyme A reductase enzyme and reduce the risk of CVD. Anti‐inflammatory properties have also been attributed to statins, and landmark clinical trials have demonstrated their effects in reducing cardiovascular risk when used in both primary and secondary prevention for HIV‐negative populations.115, 116 Among patients with HIV infection, statins have also been shown to reduce several markers of immune activation and CVD. Large retrospective studies suggest dramatic benefits of statins in reduction of all‐cause mortality for PLWH.117 In contrast, within a large retrospective study of 3601 participants from the ALLRT (AIDS Clinical Trials Group Longitudinal Linked Randomized Trials) cohort, statins did not reduce time to non‐AIDS‐defining events.118
Statins exhibit antiviral properties against HIV in vitro119 but prospective and retrospective clinical studies on the effects of statins on HIV‐1 RNA levels have yielded mixed results.120, 121, 122 An early small double blind placebo RCT that included 24 PLWH, high dose atorvastatin was found to decrease markers of T‐cell activation, but had no effect on HIV‐1 RNA levels.123 In the INTREPID (HIV‐infected patients and treatment with pitavastatin vs pravastatin for dyslipidemia) study, an RCT comparing pitavastatin to pravastatin for treatment of dyslipidemia in PLWH, pitavastatin was associated with a reduction in markers of monocyte activation (sCD14) and arterial inflammation (oxidozed LDL and lipoprotein‐associated phospholipase 2).124 More recently, a medium‐size study with 303 PLWH on long‐term suppressive cART examined the relationship between markers of immune activation/inflammation and viral persistence. With the exception of increasing IP‐10 (IFN‐γ‐inducible protein 10), statin therapy in this cohort did not significantly lower markers of inflammation (IL‐6, neopterin, sCD14, sCD163, and TNF‐alpha) and did not have an effect on the viral reservoir.125
In the SATURN‐HIV RCT comparing 10 mg of rosuvastatin to placebo for 48 weeks, rosuvastatin showed a reduction in markers of monocyte and T‐cell activation for HIV‐positive participants receiving antiretroviral therapy.65 CIMT progression was also found to be slower in the intervention group compared with placebo, and this effect was greatest in study participants who had the highest levels of inflammatory biomarkers.126, 127 A second RCT by Lo et al showed dramatic reductions in noncalcified plaque volumes measured by computed tomography angiography for patients with treated HIV infection receiving 20 or 40 mg of atorvastatin.66 Whereas some studies suggest that more‐potent statins will be beneficial in reducing the risk of CVD in PLWH otherwise at low risk,127 other studies have failed to demonstrate an effect on immune activation/inflammation,125 raising questions as to the true effect of statins for PLWH.
The ongoing multicenter REPRIEVE trial will assess whether pitavastatin compared with placebo will reduce risk of CVD in a more‐generalizable population of PLWH receiving cART, more representative of a “real world scenario.”128 Participants are being enrolled based on the American College of Cardiology/American Heart Association atherosclerotic CVD risk score and LDL cholesterol level, with the goal of identifying individuals most likely to benefit from this intervention.128, 129 Whether the cardiovascular risk reduction attributed to statins for PLWH is attributable to a reduction in LDL cholesterol level versus a reduction in inflammation and immune activation or a combination of both remains unclear, and subanalyses from this trial are also planned with the aim of addressing this question.129 The REPRIEVE trial, which plans to recruit >7000 participants in 120 sites across 11 countries, should hopefully provide some mechanistic insights into how statins mediate CVD risk reduction among PLWH.
Valganciclovir
HIV infection is also indirectly responsible for stimulating the immune system through reactivation of latent forms of other viruses, including cytomegalovirus and Epstein–Barr virus.130 Depletion of CD4+ T cells during HIV infection may lead to a decreased ability of the immune system to control these persistent herpes viruses, allowing their reactivation and replication.131 Reactivation of cytomegalovirus during HIV infection has been shown to be associated with higher levels of cytomegalovirus‐specific T‐cell responses in coinfected individuals.54 In a WIHS (Women's Interagency HIV Study) cohort study of women living with HIV, higher serum titers of cytomegalovirus immunoglobulin G were associated with having a subclinical lesion in the carotid arteries.130 In the HIV‐negative population, cytomegalovirus has been isolated from atherosclerotic lesions132 and shown to predict mortality in patients with coronary artery disease.133 Cytomegalovirus is a relatively ubiquitous pathogen with the ability to infect endothelial cells and smooth muscle cells.134 As a result, it has been cited as a possible pathogen for atherosclerosis,135 although there is a paucity of evidence to support a direct causal relationship. A small prospective study explored the role of targeting cytomegalovirus replication in PLWH with valganciclovir. In the valganciclovir‐treated arm, reduced T‐cell activation was noted in HIV‐positive people on cART72; however, it remains unclear whether this translates to an overall decrease in CVD risk over time.
Probiotics
Dietary supplementation with probiotics as a strategy to alter the gut microbiome and reduce CVD for HIV‐negative individuals has been extensively investigated in several studies (reviewed in Ettinger et al136). A meta‐analysis of 13 RCTs including 485 participants showed that a probiotic‐rich diet reduces LDL and total cholesterol levels in individuals with high, borderline, and normal cholesterol levels.137 Gut microbial translocation during HIV infection contributes to immune activation and chronic inflammation.37, 138, 139 Targeting this microbial translocation to reduce inflammation for PLWH is an attractive proposition, especially considering the potential collateral effects of reducing non‐AIDS chronic conditions associated with HIV infection. There is some evidence, both in vivo and ex vivo, that probiotics may improve local and systemic inflammation in patients with inflammatory bowel disease.140 In chronically simian immunodeficiency virus–infected macaques, the combination of cART plus a probiotic led to an increase in gut mucosal CD4+ T cells and antigen‐presenting cell frequency and function as well as reduced fibrosis.141
Several studies of probiotics for PLWH have also reported changes in gut‐associated lymphoid tissues and improvement in biomarkers of microbial translocation.73, 74, 75 More recently, an RCT assessed the effect of probiotics on inflammation and immune activation in treated HIV infection.76 In this study, 47 participants were randomized to receive the probiotic, Visbiome ES, for 24 weeks compared with 46 participants in the placebo arm. Addition of probiotics to cART did not alter markers of inflammation and immune activation, had no effect on lipid profiles, and showed a modest effect on gut microbiome populations.76
Use of probiotics as a potential intervention to target inflammation and immune activation for PLWH poses unique challenges. The lack of an effect in the recent RCT may be attributable to the wrong target, the wrong population, or inadequate dosing or choice of probiotics. HIV infection appears to have its most significant impact on the gut in the early stages of infection, and as a result we may be less likely to observe a notable effect for interventions targeting chronically infected patients who have been on cART and are virologically suppressed. Nonetheless, this intervention, if demonstrated to be beneficial, would present a preventative option that is affordable, easy to administer, and with few adverse effects.
There is a need for more studies to clarify a potential role for probiotics in PLWH. It is unclear to what degree the pathogenesis of HIV infection in the gut can truly be altered by the use of these agents. Improving the microbial composition of the gut may not necessarily reverse its leakiness and the immune dysfunction at that interface. Furthermore, the changes in microbial composition of the gut may not be permanent once the probiotic is withdrawn.
Bilirubin
Bilirubin has been shown to have antiatherogenic characteristics attributed, in part, to its antioxidant properties.142 Individuals with Gilbert syndrome, who have a hereditary inability to conjugate bilirubin and elevated levels of unconjugated bilirubin as a result, have lower rates of stroke, coronary artery disease, and other inflammatory conditions.143 This property has been studied as a possible intervention to reduce the risk of CVD for HIV‐negative individuals with diabetes mellitus. Through the use of pharmacological interventions, which increase serum bilirubin levels, several studies have shown a decrease in CVD with elevated bilirubin levels in patients with diabetes mellitus.144, 145, 146 More recently, analyses of 96,381 participants in the Veterans Aging Cohort Study, one‐third of whom were HIV positive, showed that an elevated bilirubin level is inversely correlated with CVD among both HIV‐positive and ‐negative individuals.147 Bilirubin elevations are common during treatment with the protease inhibitor, atazanavir. In a prospective RCT on CIMT, elevated bilirubin in study participants receiving atazanavir appeared to favorably influence CIMT progression rates.148 These findings raise the interesting prospect of harnessing the protective effect of elevated bilirubin to reduce cardiovascular risk in PLWH.
Mammalian Target of Rapamycin Inhibitors
These drugs inhibit the mTOR (mammalian target of rapamycin), which is an important cellular kinase mediating growth, cellular metabolism, and proliferation.149 The mTOR inhibitor, sirolimus, is frequently used for immunosuppression to reduce organ rejection in solid organ transplant patients.150 A retrospective analysis of the use of sirolimus in HIV‐positive renal transplant recipients suggested that this drug may lower levels of proviral HIV DNA in CD4+ T cells.151 Recently, the potential role of sirolimus in modulating immune function and the HIV reservoir was investigated in a small, prospective, open‐label single‐arm study including 32 participants. Sirolimus was associated with a reduction in CCR5 (C‐C chemokine receptor type 5) expression and T‐cell markers of cell cycling and immune exhaustion; however, there was a high discontinuation rate attributed to associated drug toxicities and adverse effects.71 mTOR inhibitors are associated with considerable adverse effects, which may outweigh any potential benefits they have in targeting immune activation to reduce CVD risk or the viral reservoir in PLWH. A study investigating CVD risk in 1812 liver transplant recipients who received sirolimus as part of initial immunosuppression (sirolimus cohort) found that sirolimus was associated with hypertriglyceridemia and –cholesterolemia, but did not increase the risk of MI or other CVD compared with a nonsirolimus control group.152 Considering that the sirolimus cohort has more baseline cardiovascular risk factors and nearly double the 10‐year cardiovascular risk of the control group in this study, the investigators argue that a similar incidence of CVD events in both the sirolimus and nonsirolimus group is suggestive of a cardioprotective effect of sirolimus in liver transplant recipients.152 More studies are needed to further clarify the role of sirolimus in CVD and as adjunctive therapy for modulating immune dysregulation in PLWH.
Factor Xa Antagonists
As discussed earlier, elevated D‐dimer levels have been linked to a higher incidence of non‐AIDS‐related morbidity and death for PLWH.32, 153, 154 It is hypothesized that HIV‐associated coagulopathy may contribute to disease by amplifying inflammatory pathways in addition to direct effects from thrombotic occlusions. Coagulation activity may drive immune activation by protease‐activated receptors with downstream effects on the function of vascular endothelial surfaces.155, 156, 157, 158 Targeting the coagulation cascade as a way of reducing inflammation and immune activation for PLWH is an attractive area for investigation, especially considering potential implications for cardiovascular risk reduction and other non‐AIDS‐related morbidities.
Factor Xa is a key activator of protease‐activated receptors 1 and 2, inhibition of which may reduce inflammation along vascular surfaces. The anti‐inflammatory properties of factor Xa inhibitors pertaining to vascular inflammation in the absence of HIV infection have been reported in several studies.159, 160, 161, 162 In the TACTICAL HIV (Targeted Anticoagulation Therapy to Reduce Inflammation and Cellular Activation in Long‐term HIV Disease) study, a crossover RCT, safety and efficacy of 30 mg of edoxaban (an antagonist of factor Xa) were assessed in participants with treated HIV infection.78 Whereas edoxaban led to significant reduction in D‐dimer levels, it had no effect on markers of inflammation and immune activation.78 Use of edoxaban was also associated with a higher bleeding risk. In preliminary results presented at CROI 2019, the investigators did not specifically assess markers of endothelial function and atherosclerosis. These results provide promising leads, but also highlight the need for more studies to better understand the mechanisms of HIV‐associated coagulopathy. The failure to notice an effect on inflammation and immune activation suggest that some of these mechanisms of coagulation may be distinct from the drivers of inflammation in PLWH. Other coagulation factors, notably factor IIa (thrombin), have been implicated in vascular inflammation, and targeting this factor with its direct inhibitor dabigatran improved endothelial function and reduced atherosclerosis in mice.163 This strategy has not been explored in the setting of HIV infection and presents an interesting avenue for future strategies targeting the coagulation cascade.
Adenosine Reuptake Inhibitors
Targeting adenosine reuptake has recently been explored as a way of targeting HIV‐associated chronic inflammation and cardiovascular risk. Adenosine has potent immunoregulatory effects in response to cellular stress triggered by tissue damage and inflammation.164 Increased production of adenosine in the extracellular environment leads to downstream signaling effects initiated by the binding of adenosine to its cellular receptors.165 This culminates in increased production of cAMP within the cell and a dampening of immune response.166 Dipyramidole inhibits cellular reuptake of adenosine and raises its extracellular levels. It is also approved by the FDA as an antiplatelet agent for treatment of peripheral arterial disease and coronary artery disease.167 Macatangay et al, in a recent RCT, hypothesized that increasing extracellular levels of adenosine through use of dipyramidole could potentially dampen chronic inflammation and reduce cardiovascular risk in HIV‐positive individuals.64
To test this hypothesis, they conducted a placebo‐controlled, 2‐arm, crossover pilot study in which dipyramidole was administered to 17 HIV‐positive individuals on suppressive cART compared with 18 HIV‐positive individuals who received placebo. Markers of endothelial dysfunction and inflammation were assessed after 12 weeks of treatment.64 Following the treatment period, there were no significant changes in sCD14, sCD163, or IL‐6 levels in the intervention and control arms of the study. They did, however, note a modest decrease in activated CD8+ T cells as well as a significant decrease in activated CD4+ T cells in pooled analyses. There was no significant difference in FMD between treatment and control groups at the end of the study period.64
The small decrease in activated T cells following treatment with dipyramidole suggests that alterations in adenosine metabolism may contribute to T‐cell activation in HIV‐positive individuals who are treated and virologically suppressed. However, the lack of an effect on markers of inflammation and endothelial dysfunction indicates that adenosine metabolism may not play a significant role in monocyte/macrophage activation in PLWH and thus only partly explains the degree of residual inflammation and immune activation observed in chronic HIV infection. Similar to the other interventions discussed in this review, the selection of a healthy group of HIV‐positive individuals with immune reconstitution and virological suppression may limit the ability to detect a measurable effect of the intervention.
Future Directions and Conclusions
The role of HIV infection as an independent predictor for CVD is now well established, but the mechanisms by which this occurs remain to be fully defined. There is a growing body of evidence suggesting that chronic inflammation and immune activation, the direct effects of HIV proteins, and treatment with cART may be important in the pathogenesis of CVD among PLWH compared with their HIV‐negative counterparts. The degree to which each of these factors contribute to the pathogenesis and pathways of CVD remain uncertain, hence the challenge in identifying clear therapeutic targets. One plausible theory is that the aforementioned factors create an environment of increased oxidative stress at the endovascular interface, leading to increased endothelial dysfunction and damage, which triggers atherogenesis and its consequent nefarious effects. This theory supports exploring strategies which aim to directly reduce inflammation and immune activation and oxidative stress by targeting reactive oxygen species. Whether these strategies would be sufficient in reversing the risk of CVD in PLWH or need to be combined with traditional interventions targeting other factors remain to be seen (see Figure 2 and Table 2, 168, 169, 170, 171, 172, 173, 174, 175 below).
Figure 2.
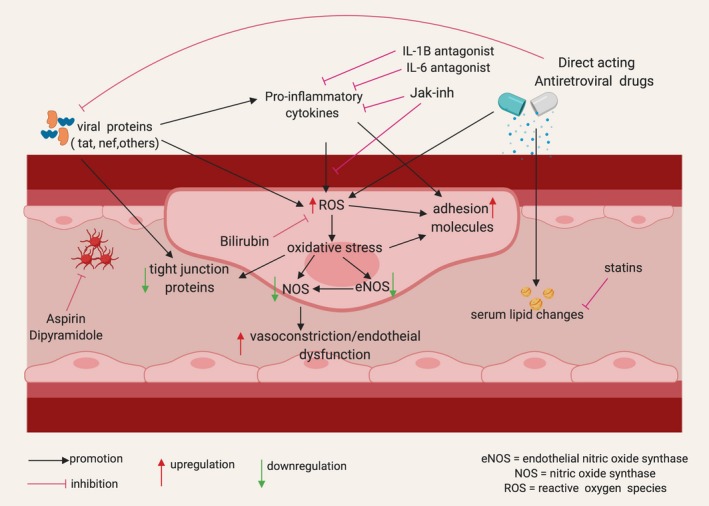
Pathogenesis of endothelial damage attributable to HIV infection and its treatment: Viral proteins, inflammatory cytokines, and cART all lead to increase in reactive oxygen species (ROS). Oxidative stress induces endothelial nitic oxide synthase (eNOS) uncoupling leading to decreased nitric oxide (NO) availability. These events trigger endothelial dysfunction which is an important early step in endovascular damage and atherogenesis. This figure was created using http://www.biorender.com software. cART indicates combination antiretroviral therapy; IL‐1β, interleukin‐1 beta; IL‐16, interleukin‐16; Jak‐inh, Janus kinase inhibitor.
Table 2.
Future Directions: Potential Strategies Which May Directly or Indirectly Target Inflammation to Reduce ASCVD, Not Yet Explored in PLWH
| Target | Potential Agent | Rationale | Challenges/Limitations |
|---|---|---|---|
| IL‐1R | IL‐1R antagonist:
|
Anakinra‐ FDA‐approved IL‐1R antagonist can improve endothelial function in patients with RA168 and decrease inflammation in response to acute MI.169 |
|
| TNF‐alpha | TNF‐alpha inhibitors
|
Significant increase of total and HDL cholesterol in patients treated with TNF‐alpha inhibitors170 Infliximab treatment associated with improved endothelial function All 3 agents are FDA approved for treatment of RA171 |
|
| B cells | Anti‐CD20 antibody
|
B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions.171 Emerging data that rituximab may have a role in targeting the HIV reservoir172 |
|
| Lipid lowering agents with anti‐inflammatory potential | Inhibition of ATP‐citrate lyase and activation of AMP activated protein kinase in the liver
|
Shown to attenuate inflammatory cytokine expression and proinflammatory signaling in full‐length aorta of mice173 Has good oral bioavailability with once‐daily administration and was recently FDA approved for use as lipid‐lowering agent in patient with high 10‐year ASCVD risk and intolerance to statins174 |
|
| Coagulation cascade | Factor IIa inhibitor
|
Good oral bioavailability FDA approved for anticoagulation Shown to improve endothelial function and atherosclerosis in mice163 |
|
ASCVD indicates atherosclerotic cardiovascular disease; FDA, US Food and Drug Administration; HDL, high‐density lipoprotein; IgM, immunoglobulin M; IL‐1R, interleukin‐1 receptor; MI, myocardial infarction; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
So far, robust RCT data that support targeting inflammation to reduce CVD risk in HIV‐positive individuals are limited, with most of the observational data discussed in this review yielding mixed results. These studies have almost exclusively included relatively healthy virologically suppressed HIV‐positive individuals on cART and may explain the limited ability to detect clear benefits of the interventions tested. Furthermore, larger studies encompassing the full spectrum of PLWH are needed to ascertain that therapies inhibiting inflammatory response in individuals with HIV will bring positive effects rather than deleterious ones. The currently available evidence highlights the need for studies aimed at better understanding the mechanisms of CVD in HIV, defining clear intervention targets, identifying clinically relevant markers to measure response, and the subset of patients most likely to benefit from these interventions.
Sources of Funding
This work was supported, in part, by the Emory University Center for AIDS Research (NIH grant 2P30‐AI‐050409) and the NIH (grant R01‐MH‐116695 to Schinazi and R01AI110334 to Marconi).
Disclosures
Marconi has received consulting honoraria and/or research support from Lilly, ViiV, Gilead, and Bayer. The remaining authors have no disclosures to report.
References
- 1. Teeraananchai S, Kerr S, Amin J, Ruxrungtham K, Law M. Life expectancy of HIV‐positive people after starting combination antiretroviral therapy: a meta‐analysis. HIV Med. 2017;18:256–266. [DOI] [PubMed] [Google Scholar]
- 2. Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long‐term control of HIV by CCR5 Delta32/Delta32 stem‐cell transplantation. N Engl J Med. 2009;360:692–698. [DOI] [PubMed] [Google Scholar]
- 3. Gupta RK, Abdul‐Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez‐Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E. HIV‐1 remission following CCR5Delta32/Delta32 haematopoietic stem‐cell transplantation. Nature. 2019;568:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis‐Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila‐Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda‐Orjuela CA, Castillo‐Rivas J, Catalá‐López F, Choi JY, Christensen H, Cirillo M, Cooper L, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi‐Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés‐Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor‐Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta‐analysis. HIV Med. 2012;13:453–468. [DOI] [PubMed] [Google Scholar]
- 7. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez‐Barradas MC, Lim J, Kazis LE, Gottlieb S, Justice AC, Freiberg MS. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsue PY, Waters DD. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation. 2018;138:1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Currier JS, Hsue P. The role of inflammation in HIV‐associated atherosclerosis—one size may not fit all. J Infect Dis. 2019. pii: jiz256. DOI: 10.1093/infdis/jiz256. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Strategies for Management of Antiretroviral Therapy (SMART) Study Group , Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, Girard PM, Grund B, Law M, Losso MH, Palfreeman A, Wood R. Major clinical outcomes in antiretroviral therapy (ART)–naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. [DOI] [PubMed] [Google Scholar]
- 13. Strategies for Management of Antiretroviral Therapy (SMART) Study Group , El‐Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fätkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 14. Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Stein JH; ACTG 5152s Study Team . Endothelial function in human immunodeficiency virus‐infected antiretroviral‐naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) study 5152s. J Am Coll Cardiol. 2008;52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker JV, Duprez D, Rapkin J, Hullsiek KH, Quick H, Grimm R, Neaton JD, Henry K. Untreated HIV infection and large and small artery elasticity. JAIDS. 2009;52:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. UNAIDS . UNAIDS global HIV and AIDS Statistics—2019 Fact Sheet. Available at: https://www.unAIDS.org/en/resources/fact-sheet. Accessed November 16, 2019.
- 17. Vos A, Tempelman H, Deville W, Barth R, Wensing A, Kretzschmar M, Klipstein‐Grobusch K, Hoepelman A, Tesselaar K, Aitken S, Madzivhandila M, Uiterwaal C, Venter F, Coutinho R, Grobbee DE. HIV and risk of cardiovascular disease in Sub‐Saharan Africa: rationale and design of the Ndlovu Cohort Study. Eur J Prev Cardiol. 2017;24:1043–1050. [DOI] [PubMed] [Google Scholar]
- 18. Krishnan S, Wilson EMP, Sheikh V, Rupert A, Mendoza D, Yang J, Lempicki R, Migueles SA, Sereti I. Evidence for innate immune system activation in HIV type 1‐infected elite controllers. J Infect Dis. 2014;209:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, Abbara S, Grinspoon SK. Increased coronary atherosclerosis and immune activation in HIV‐1 elite controllers. AIDS. 2012;26:2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN, Deeks SG. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV‐associated atherosclerosis. AIDS. 2009;23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, Hale B, Crum‐Cianflone N, Delmar J , Barthel V, Quinnan G, Agan BK, Dolan MJ; Infectious Disease Clinical Research Program (IDCRP) HIV Working Group . Clinical outcomes of elite controllers, viremic controllers, and long‐term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200:1714–1723. [DOI] [PubMed] [Google Scholar]
- 22. Leon A, Perez I, Ruiz‐Mateos E, Benito JM, Leal M, Lopez‐Galindez C, Rallon N, Alcami J, Lopez‐Aldeguer J, Viciana P, Rodriguez C, Grau E, Iribarren J, Gatell JM, Garcia F; EC and Immune Pathogenesis Working group of the Spanish AIDS Research Network . Rate and predictors of progression in elite and viremic HIV‐1 controllers. AIDS. 2016;30:1209–1220. [DOI] [PubMed] [Google Scholar]
- 23. Crowell TA, Gebo KA, Blankson JN, Korthuis PT, Yehia BR, Rutstein RM, Moore RD, Sharp V, Nijhawan AE, Mathews WC, Hanau LH, Corales RB, Beil R, Somboonwit C, Edelstein H, Allen SL, Berry SA. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211:1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn‐Williams J, Hunt PW, Hoh R, Stramer SL, Linnen JM, McCune JM, Martin JN, Busch MP, Deeks SG. Evidence for persistent low‐level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page‐Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG. Relationship between t cell activation and cd4+t cell count in HIV‐seropositive individuals with undetectable plasma HIV rna levels in the absence of therapy. J Infect Dis. 2008;197:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowell TA, Hatano H. Clinical outcomes and antiretroviral therapy in ‘elite’ controllers: a review of the literature. J Virus Erad. 2015;1:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee T‐H, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T‐cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, Weber R, D'Arminio Monforte A, Reiss P, Kamara D, El‐Sadr W, Pradier C, Dabis F, Law M, Lundgren J; D:A:D Study Group . Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013;27:2735–2748. [DOI] [PubMed] [Google Scholar]
- 30. Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP, Klein DB. Immunodeficiency and risk of myocardial infarction among HIV‐positive individuals with access to care. JAIDS. 2014;65:160–166. [DOI] [PubMed] [Google Scholar]
- 31. Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, Anastos K, Tien PC, Sharrett AR, Hodis HN. Low CD4+ T‐cell count as a major atherosclerosis risk factor in HIV‐infected women and men. AIDS. 2008;22:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, Natarajan V, Rehm C, Hadigan C, Sereti I. Traditional risk factors and D‐dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA. Markers of inflammation and CD8 T‐cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV‐infected individuals. HIV Med. 2013;14:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, Deeks SG. Increased carotid intima‐media thickness in HIV patients is associated with increased cytomegalovirus‐specific t‐cell responses. AIDS. 2006;20:2275–2283. [DOI] [PubMed] [Google Scholar]
- 35. Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti‐retroviral therapy. J Infect Dis. 2011;204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV‐infected patients. J Infect Dis. 2011;204:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV‐1 infection. J Infect Dis. 2012;206:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr, Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, Tracy RP, Brown TT, Post WS. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA. 2012;308:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Ridker PM, Hansson GK. Leducq transatlantic network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weissberg PL. Atherogenesis: current understanding of the causes of atheroma. Indian Heart J. 2000;52:467–472. [PubMed] [Google Scholar]
- 42. Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, Dube MP, Murphy R, Yang OO, Currier JS, McComsey GA. Changes in inflammation and immune activation with atazanavir‐, raltegravir‐, darunavir‐based initial antiviral therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ. Inflammation, coagulation and cardiovascular disease in HIV‐infected individuals. PLoS One. 2012;7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rudy BJ, Kapogiannis BG, Worrell C, Squires K, Bethel J, Li S, Wilson CM, Agwu A, Emmanuel P, Price G, Hudey S, Goodenow MM, Sleasman JW; Adolescent Trials Network for HIVAIDS Interventions . Immune reconstitution but persistent activation after 48 weeks of antiretroviral therapy in youth with pre‐therapy CD4 >350 in ATN 061. JAIDS. 2015;69:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Panigrahi S, Freeman ML, Funderburg NT, Mudd JC, Younes SA, Sieg SF, Zidar DA, Paiardini M, Villinger F, Calabrese LH, Ransohoff RM, Jain MK, Lederman MM. SIV/SHIV infection triggers vascular inflammation, diminished expression of kruppel‐like factor 2 and endothelial dysfunction. J Infect Dis. 2016;213:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults. Circulation. 2007;115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 47. Ross AC, Rizk N, O'Riordan MA, Dogra V, El‐Bejjani D, Storer N, Harrill D, Tungsiripat M, Adell J, McComsey GA. Relationship between inflammatory markers, endothelial activation markers, and carotid intima‐media thickness in HIV‐infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schecter AD, Berman AB, Yi L, Mosoian A, McManus CM, Berman JW, Klotman ME, Taubman MB. HIV envelope GP120 activates human arterial smooth muscle cells. Proc Natl Acad Sci USA. 2001;98:10142–10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paladugu R, Fu W, Conklin BS, Lin PH, Lumsden AB, Yao Q, Chen C. HIV tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549–555; discussion, 555–546. [DOI] [PubMed] [Google Scholar]
- 50. de Gaetano Donati K, Rabagliati R, Iacoviello L, Cauda R. HIV infection, haart, and endothelial adhesion molecules: current perspectives. Lancet Infect Dis. 2004;4:213–222. [DOI] [PubMed] [Google Scholar]
- 51. Auclair M, Afonso P, Capel E, Caron‐Debarle M, Capeau J. Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir Ther. 2014;19:773–782. [DOI] [PubMed] [Google Scholar]
- 52. Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti‐human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rose H, Hoy J, Woolley I, Tchoua U, Bukrinsky M, Dart A, Sviridov D. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV‐infected adults. J Infect Dis. 2012;205:S375–S382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, Pierson RN Jr, Feingold KR. Circulating interferon‐alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991;90:154–162. [PubMed] [Google Scholar]
- 56. Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. [DOI] [PubMed] [Google Scholar]
- 57. Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, Duprez D, Neaton JD. High‐density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stein JH, Komarow L, Cotter BR, Currier JS, Dube MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Parker RA, Torriani FJ; ACTG 5152s Study Team . Lipoprotein changes in HIV‐infected antiretroviral‐naive individuals after starting antiretroviral therapy: ACTG study A5152s stein: lipoprotein changes on antiretroviral therapy. J Clin Lipidol. 2008;2:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dube MP, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, Roubenoff R, Shafer RW, Wininger DA, Meyer WA III, Snyder SW, Mulligan K. Glucose metabolism, lipid, and body fat changes in antiretroviral‐naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. [DOI] [PubMed] [Google Scholar]
- 60. Cunha JD. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus‐infected patients: old and new drugs. World J Virol. 2015;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Brien MP, Hunt PW, Kitch DW, Klingman K, Stein JH, Funderburg NT, Berger JS, Tebas P, Clagett B, Moisi D, Utay NS, Aweeka F, Aberg JA. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus‐infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis. 2017;4:ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eidelman RS, Hebert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med. 2003;163:2006. [DOI] [PubMed] [Google Scholar]
- 64. Macatangay BJC, Jackson EK, Abebe KZ, Comer D, Cyktor J, Klamar‐Blain C, Borowski L, Gillespie DG, Mellors JW, Rinaldo CR, Riddler SA. A randomized, placebo‐controlled, pilot clinical trial of dipyridamole to decrease human immunodeficiency virus–associated chronic inflammation. J Infect Dis. 2019. pii: jiz344. DOI: 10.1093/infdis/jiz344. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin reduces vascular inflammation and T‐cell and monocyte activation in HIV‐infected subjects on antiretroviral therapy. JAIDS. 2015;68:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high‐risk plaque morphology in HIV‐infected patients with subclinical atherosclerosis: a randomised, double‐blind, placebo‐controlled trial. Lancet HIV. 2015;2:e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, Ganz P, Ridker PM, Deeks SG, Tawakol A. IL‐1beta inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. 2018;72:2809–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ridker PM, Everett BM, Thuren T, Macfadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 69. Emily Bowman MC, Reidl K, Baum J, Freeman LM, Lederman MM, Rodriguez B, Funderburg N. Tocilizumab alters lipids in HIV+ individuals in a randomized double‐blind study. Conferences on Retroviruses and Opportunistic Infections (CORI). 2019, Poster abstract number 647.
- 70. McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: measure, a randomised, placebo‐controlled study. Ann Rheum Dis. 2015;74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Henrich TJ, Bosch R, Godfrey C, Mar H, Nair A, Hawkins E, Fichtenbaum C, Keefer M, Li JZ, Kuritzkes DR, Lederman MM, Hsue P, Deeks SG. Sirolimus reduces t‐cell cycling and immune checkpoint marker expression, ACTG A5337, Conference on Retroviruses and Opportunistic infections (CROI), 2019. Oral abstract number 131. Available at: http://www.croiconference.org/sessions/sirolimus-reduces-t-cell-cycling-and-immune-checkpoint-marker-expression-actg-a5337. Accessed January 14, 2020. [Google Scholar]
- 72. Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. Valganciclovir reduces t cell activation in HIV‐infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. d'Ettorre G, Rossi G, Scagnolari C, Andreotti M, Giustini N, Serafino S, Schietroma I, Scheri GC, Fard SN, Trinchieri V, Mastromarino P, Selvaggi C, Scarpona S, Fanello G, Fiocca F, Ceccarelli G, Antonelli G, Brenchley JM, Vullo V. Probiotic supplementation promotes a reduction in t‐cell activation, an increase in th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in art‐treated HIV‐1‐positive patients. Immun Inflamm Dis. 2017;5:244–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheri GC, Fard SN, Schietroma I, Mastrangelo A, Pinacchio C, Giustini N, Serafino S, De Girolamo G, Cavallari EN, Statzu M, Laghi L, Vullo A, Ceccarelli G, Vullo V, d'Ettorre G. Modulation of tryptophan/serotonin pathway by probiotic supplementation in human immunodeficiency virus‐positive patients: preliminary results of a new study approach. Int J Tryptophan Res. 2017;10:1178646917710668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. D'Ettorre G, Ceccarelli G, Giustini N, Serafino S, Calantone N, De Girolamo G, Bianchi L, Bellelli V, Ascoli‐Bartoli T, Marcellini S, Turriziani O, Brenchley JM, Vullo V. Probiotics reduce inflammation in antiretroviral treated, HIV‐infected individuals: results of the “probio‐HIV” clinical trial. PLoS One. 2015;10:e0137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Overton ET, Yeh E, Presti R, Jacobson J, Williams B, Wilson C, Landay A, Brenchley J, Dube M, Fichtenbaum C, Utay NS, Kitch DW, Andrade A; for the ACTG A5350 Study Team . Assessing the probiotic effect in treated HIV: results of ACTG A5350. Conference on retroviruses and opportunistic infections (CROI), 2019. Oral abstract number 35.
- 77. Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, Daar ES, Havlir D, Yeh E, Tawakol A, Lederman M, Currier JS, Stein JH. Safety and impact of low‐dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS clinical trials group study A5314. Clin Infect Dis. 2018;68:1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baker JV, Wolfson J, Peterson T, Garcia‐Myers K, Klaphake J, Mooberry M, Gissel M, Brummel‐Ziedins K, Sereti I, Key N, Tracy R. Factor X inhibition reduces coagulation but not inflammation in persons with HIV. Conference on retroviruses and opportunistic infections (CROI), 2019, Oral Abstract number 36. [Google Scholar]
- 79. Marconi VC, Moser C, Gavegnano C, Tsibris A, Kantor A, Overton ET, Flexner CW, Hunt PW, Sekaly R‐P, del Rio C, Lederman MM, Tressler R, Deeks SG, Lennox JJ, Schinazi RF. Safety, tolerability and immunologic activity of ruxolitinib added to suppressive ART. Conference on retroviruses and opportunistic infections (CROI), 2019. Oral abstract number 37LB.
- 80. Gavegnano C, Brehm JH, Dupuy FP, Talla A, Ribeiro SP, Kulpa DA, Cameron C, Santos S, Hurwitz SJ, Marconi VC, Routy JP, Sabbagh L, Schinazi RF, Sékaly RP. Novel mechanisms to inhibit HIV reservoir seeding using JAK inhibitors. PLoS Pathog. 2017;13:e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. O'Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Gettenberg G, Cavanagh K, Aberg JA, Bhardwaj N, Berger JS. Aspirin attenuates platelet activation and immune activation in HIV‐1‐infected subjects on antiretroviral therapy: a pilot study. JAIDS. 2013;63:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta‐analysis. JAMA. 2019;321:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dinarello CA. Interleukin‐1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin‐1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin‐1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- 87. Hoel H, Ueland T, Knudsen A, Kjær A, Michelsen AE, Sagen EL, Halvorsen B, Yndestad A, Nielsen SD, Aukrust P, Lebech AM, Trøseid M. Soluble markers of IL‐1 activation as predictors of first‐time myocardial infarction in HIV‐infected individuals. J Infect Dis. 2019. May 11. pii: jiz253. DOI: 10.1093/infdis/jiz253. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 88. Naranjo A, Sokka T, Descalzo MA, Calvo‐Alen J, Horslev‐Petersen K, Luukkainen RK, Combe B, Burmester GR, Devlin J, Ferraccioli G, Morelli A, Hoekstra M, Majdan M, Sadkiewicz S, Belmonte M, Holmqvist AC, Choy E, Tunc R, Dimic A, Bergman M, Toloza S, Pincus T; QUEST‐RA Group . Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST‐RA study. Arthritis Res Ther. 2008;10:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, Schneeweiss S. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–3798. [DOI] [PubMed] [Google Scholar]
- 90. van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease‐modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262–267. [DOI] [PubMed] [Google Scholar]
- 92. Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. [DOI] [PubMed] [Google Scholar]
- 93. Ridker PM. Methotrexate for prevention of cardiovascular events. Reply. N Engl J Med. 2019;380:2277. [DOI] [PubMed] [Google Scholar]
- 94. Borges ÁH, O'Connor JL, Phillips AN, Neaton JD, Grund B, Neuhaus J, Vjecha MJ, Calmy A, Koelsch KK, Lundgren JD; INSIGHT SMART Study and ESPRIT Groups . Interleukin 6 is a stronger predictor of clinical events than high‐sensitivity C‐reactive protein or D‐dimer during HIV infection. J Infect Dis. 2016;214:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gabay C, McInnes IB, Kavanaugh A, Tuckwell K, Klearman M, Pulley J, Sattar N. Comparison of lipid and lipid‐associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez‐Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, Genovese MC. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the ambition study. Ann Rheum Dis. 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, Woodworth T, Alten R; OPTION Investigators . Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet. 2008;371:987–997. [DOI] [PubMed] [Google Scholar]
- 99. Bacchiega BC, Bacchiega AB, Usnayo MJG, Bedirian R, Singh G, Pinheiro GDRC. Interleukin 6 inhibition and coronary artery disease in a high‐risk population: a prospective community‐based clinical study. J Am Heart Assoc. 2017;6:e005038 DOI: 10.1161/JAHA.116.005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK‐STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Levine RL, Wernig G. Role of JAK‐STAT signaling in the pathogenesis of myeloproliferative disorders. Hematology. 2006;2006:233–239. [DOI] [PubMed] [Google Scholar]
- 102. T Virtanen A, Haikarainen T, Raivola J, Silvennoinen O. Selective jakinibs: prospects in inflammatory and autoimmune diseases. BioDrugs. 2019;33:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 104. Bryan JC, Verstovsek S. Overcoming treatment challenges in myelofibrosis and polycythemia vera: the role of ruxolitinib. Cancer Chemother Pharmacolol. 2016;77:1125–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Charles‐Schoeman C, Wicker P, Gonzalez‐Gay MA, Boy M, Zuckerman A, Soma K, Geier J, Kwok K, Riese R. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral janus kinase inhibitor. Semin Arthritis Rheum. 2016;46:261–271. [DOI] [PubMed] [Google Scholar]
- 106. Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, Yakushin S, Ishii T, Emoto K, Beattie S, Arora V, Gaich C, Rooney T, Schlichting D, Macias WL, de Bono S, Tanaka Y. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–662. [DOI] [PubMed] [Google Scholar]
- 107. Choy EH. Clinical significance of janus kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, Schalm S, Murias S, Dare JA, Brown D, Stone DL, Gao L, Klausmeier T, Foell D, De Jesus AA, Chapelle DC, Kim H, Dill S, Colbert RA, Failla L, Kost B, O'Brien M, Reynolds JC, Folio LR, Calvo KR, Paul SM, Weir N, Brofferio A, Soldatos A, Biancotto A, Cowen EW, Digiovanna JJ, Gadina M, Lipton AJ, Hadigan C, Holland SM, Fontana J, Alawad AS, Brown RJ, Rother KI, Heller T, Brooks KM, Kumar P, Brooks SR, Waldman M, Singh HK, Nickeleit V, Silk M, Prakash A, Janes JM, Ozen S, Wakim PG, Brogan PA, Macias WL, Goldbach‐Mansky R. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128:3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shi JG, Chen X, Lee F, Emm T, Scherle PA, Lo Y, Punwani N, Williams WV, Yeleswaram S. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54:1354–1361. [DOI] [PubMed] [Google Scholar]
- 110. Chomont N, El‐Far M, Ancuta P, Trautmann L, Procopio FA, Yassine‐Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sékaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antiviral Chem Chemother. 2009;20:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu JJ, Strober BE, Hansen PR, Ahlehoff O, Egeberg A, Qureshi AA, Robertson D, Valdez H, Tan H, Wolk R. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long‐term extension data in patients with plaque psoriasis. J Am Acad Dermatol. 2016;75:897–905. [DOI] [PubMed] [Google Scholar]
- 113. Verden A, Dimbil M, Kyle R, Overstreet B, Hoffman KB. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41:357–361. [DOI] [PubMed] [Google Scholar]
- 114. Scott IC, Hider SL, Scott DL. Thromboembolism with janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 2018;41:645–653. [DOI] [PubMed] [Google Scholar]
- 115. Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti‐inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 117. Rasmussen LD, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N. Statin therapy and mortality in HIV‐infected individuals; a Danish nationwide population‐based cohort study. PLoS One. 2013;8:e52828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Overton ET, Kitch D, Benson CA, Hunt PW, Stein JH, Smurzynski M, Ribaudo HJ, Tebas P. Effect of statin therapy in reducing the risk of serious non‐AIDS‐defining events and nonaccidental death. Clin Infect Dis. 2013;56:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV‐1 assembly and release. Proc Natl Acad Sci USA. 2001;98:13925–13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sklar PA, Masur H, Grubb JR, Voell J, Witek J, Ono A, Freed EO, Maldarelli F. Pravastatin does not have a consistent antiviral effect in chronically HIV‐infected individuals on antiretroviral therapy. AIDS. 2005;19:1109–1111. [DOI] [PubMed] [Google Scholar]
- 121. Moncunill G, Negredo E, Bosch L, Vilarrasa J, Witvrouw M, Llano A, Clotet B, Este JA. Evaluation of the anti‐HIV activity of statins. AIDS. 2005;19:1697–1700. [DOI] [PubMed] [Google Scholar]
- 122. Waters L, Stebbing J, Jones R, Mandalia S, Bower M, Stefanovic M, Nelson M, Gazzard B. The effect of statins on HIV rebound and blips. JAIDS. 2005;39:637–638. [PubMed] [Google Scholar]
- 123. Ganesan A, Crum‐Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, Brandt C, Vita J, Decker CF, Sklar P, Bavaro M, Tasker S, Follmann D, Maldarelli F. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV‐1 rna levels: results of a double‐blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, Pate MM, Aberg JA, Zanni MV, Grinspoon SK. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in intrepid. AIDS. 2017;31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bedimo RJ, Mar H, Bosch RJ, Drechsler H, Cyktor JC, Macatangay BJC, Lalama C, Rinaldo C, Collier A, Godfrey C, Hogg Evelyn, Hensel C, Eron JJ, McMahon DK, Mellors JW, Tebas P, Gandhi RT. No evidence for an association between statin use and lower biomarkers of HIV persistence or immune activation/inflammation during effective art. JAIDS. 2019;82:e27–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS. 2016;30:2195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Longenecker CT, Eckard AR, McComsey GA. Statins to improve cardiovascular outcomes in treated HIV infection. Curr Opin Infect Dis. 2016;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, Malvestutto C, Lu MT, Currier JS, Sponseller CA, Waclawiw M, Alston‐Smith B, Cooper‐Arnold K, Klingman KL, Desvigne‐Nickens P, Hoffmann U, Ribaudo HJ, Douglas PS; REPRIEVE Investigators . Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J. 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hoffmann U, Lu MT, Olalere D, Adami EC, Osborne MT, Ivanov A, Aluru JS, Lee S, Arifovic N, Overton ET, Fichtenbaum CJ, Aberg JA, Alston‐Smith B, Klingman KL, Waclawiw M, Burdo TH, Williams KC, Zanni MV, Desvigne‐Nickens P, Cooper‐Arnold K, Fitch KV, Ribaudo H, Douglas PS, Grinspoon SK; REPRIEVE Investigators . Rationale and design of the mechanistic substudy of the randomized trial to prevent vascular events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J. 2019;212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dunn HS, Haney DJ, Ghanekar SA, Stepick‐Biek P, Lewis DB, Maecker HT. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J Infect Dis. 2002;186:15–22. [DOI] [PubMed] [Google Scholar]
- 131. Appay V, Sauce D. Immune activation and inflammation in HIV‐1 infection: causes and consequences. J Pathol. 2008;214:231–241. [DOI] [PubMed] [Google Scholar]
- 132. Melnick JL, Adam E, DeBakey ME. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990;263:2204–2207. [PubMed] [Google Scholar]
- 133. Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, Anderson JL. Cytomegalovirus seropositivity and C‐reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation. 2000;102:1917–1923. [DOI] [PubMed] [Google Scholar]
- 134. Jarvis MA, Nelson JA. Human cytomegalovirus tropism for endothelial cells: not all endothelial cells are created equal. J Virol. 2007;81:2095–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Maniar A, Ellis C, Asmuth D, Pollard R, Rutledge J. HIV infection and atherosclerosis: evaluating the drivers of inflammation. Eur J Prev Cardiol. 2013;20:720–728. [DOI] [PubMed] [Google Scholar]
- 136. Ettinger G, Macdonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, Sun ZH, Zhang HP, Chen W. Influence of consumption of probiotics on the plasma lipid profile: a meta‐analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:844–850. [DOI] [PubMed] [Google Scholar]
- 138. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira‐Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 139. Cassol E, Malfeld S, Mahasha P, Van Der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent microbial translocation and immune activation in HIV‐1–infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. [DOI] [PubMed] [Google Scholar]
- 140. Plaza‐Díaz J, Ruiz‐Ojeda F, Vilchez‐Padial L, Gil A. Evidence of the anti‐inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quiñones M, Deming CB, Perkins M, Hazuda DJ, Miller MD, Lederman MM, Segre JA, Lifson JD, Haddad EK, Estes JD, Brenchley JM. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in siv‐infected macaques. J Clin Invest. 2013;123:903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stocker R, Ames BN. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci USA. 1987;84:8130–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vitek L, Jirsa M, Brodanova M, Kalab M, Marecek Z, Danzig V, Novotny L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449–456. [DOI] [PubMed] [Google Scholar]
- 144. Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower‐extremity peripheral arterial disease: National health and nutrition examination survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:166–172. [DOI] [PubMed] [Google Scholar]
- 145. Dekker D, Dorresteijn MJ, Pijnenburg M, Heemskerk S, Rasing‐Hoogveld A, Burger DM, Wagener FA, Smits P. The bilirubin‐increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:458–463. [DOI] [PubMed] [Google Scholar]
- 146. Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca‐Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. [DOI] [PubMed] [Google Scholar]
- 147. Marconi VC, Duncan MS, So‐Armah K, Re VL, Lim JK, Butt AA, Goetz MB, Rodriguez‐Barradas MC, Alcorn CW, Lennox J, Beckman JA, Justice A, Freiberg M. Bilirubin is inversely associated with cardiovascular disease among HIV‐positive and HIV‐negative individuals in VACS (Veterans Aging Cohort Study). J Am Heart Assoc. 2018;7:e007792 DOI: 10.1161/JAHA.117.007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stein JH, Ribaudo HJ, Hodis HN, Brown TT, Tran TT, Yan M, Brodell EL, Kelesidis T, McComsey GA, Dube MP, Murphy RL, Currier JS. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS. 2015;29:1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Geissler EK. The influence of mtor inhibitors on immunity and the relationship to post‐transplant malignancy. Transplant Res. 2013;2:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ventura‐Aguiar P, Campistol JM, Diekmann F. Safety of MTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15:303–319. [DOI] [PubMed] [Google Scholar]
- 151. Stock PG, Barin B, Hatano H, Rogers RL, Roland ME, Lee TH, Busch M, Deeks SG. Reduction of HIV persistence following transplantation in HIV‐infected kidney transplant recipients. Am J Transplant. 2014;14:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McKenna GJ, Trotter JF, Klintmalm E, Ruiz R, Onaca N, Testa G, Saracino G, Levy MF, Goldstein RM, Klintmalm GB. Sirolimus and cardiovascular disease risk in liver transplantation. Transplantation. 2013;95:215–221. [DOI] [PubMed] [Google Scholar]
- 153. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cirino G, Vergnolle N. Proteinase‐activated receptors (PARS): crossroads between innate immunity and coagulation. Curr Opin Pharmacol. 2006;6:428–434. [DOI] [PubMed] [Google Scholar]
- 156. Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. [DOI] [PubMed] [Google Scholar]
- 157. Krupiczojc M, Scotton C, Chambers R. Coagulation signalling following tissue injury: focus on the role of factor Xa. Int J Biochem Cell Biol. 2008;40:1228–1237. [DOI] [PubMed] [Google Scholar]
- 158. Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease‐activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83:1309–1322. [DOI] [PubMed] [Google Scholar]
- 159. Pham PT, Fukuda D, Yagi S, Kusunose K, Yamada H, Soeki T, Shimabukuro M, Sata M. Rivaroxaban, a specific FXA inhibitor, improved endothelium‐dependent relaxation of aortic segments in diabetic mice. Sci Rep. 2019;9:11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Sata M. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in apoe‐deficient mice. Atherosclerosis. 2015;242:639–646. [DOI] [PubMed] [Google Scholar]
- 161. Hara T, Phuong PT, Fukuda D, Yamaguchi K, Murata C, Nishimoto S, Yagi S, Kusunose K, Yamada H, Soeki T, Wakatsuki T, Imoto I, Shimabukuro M, Sata M. Protease‐activated receptor‐2 plays a critical role in vascular inflammation and atherosclerosis in apolipoprotein e‐deficient mice. Circulation. 2018;138:1706–1719. [DOI] [PubMed] [Google Scholar]
- 162. Zhou Q, Bea F, Preusch M, Wang H, Isermann B, Shahzad K, Katus HA, Blessing E. Evaluation of plaque stability of advanced atherosclerotic lesions in apo E‐deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm. 2011;2011:432080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lee IO, Kratz MT, Schirmer SH, Baumhakel M, Bohm M. The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E‐deficient mice. J Pharmacol Exp Ther. 2012;343:253–257. [DOI] [PubMed] [Google Scholar]
- 164. Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. de Lera Ruiz M, Lim YH, Zheng J. Adenosine a2a receptor as a drug discovery target. J Med Chem. 2014;57:3623–3650. [DOI] [PubMed] [Google Scholar]
- 166. Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7–15. [DOI] [PubMed] [Google Scholar]
- 167. Whayne TF. A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol. 2012;21:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ikonomidis I, Tzortzis S, Lekakis J, Paraskevaidis I, Andreadou I, Nikolaou M, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos P, Kremastinos DT. Lowering interleukin‐1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart. 2009;95:1502–1507. [DOI] [PubMed] [Google Scholar]
- 169. Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin‐1 receptor antagonist therapy on markers of inflammation in non‐ST elevation acute coronary syndromes: the MRC‐ILA heart study. Eur Heart J. 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti‐TNF‐alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–419. [DOI] [PubMed] [Google Scholar]
- 171. Cardillo C, Schinzari F, Mores N, Mettimano M, Melina D, Zoli A, Ferraccioli G. Intravascular tumor necrosis factor alpha blockade reverses endothelial dysfunction in rheumatoid arthritis. Clin Pharmacol Ther. 2006;80:275–281. [DOI] [PubMed] [Google Scholar]
- 172. Serra‐Peinado C, Grau‐Exposito J, Luque‐Ballesteros L, Astorga‐Gamaza A, Navarro J, Gallego‐Rodriguez J, Martin M, Curran A, Burgos J, Ribera E, Raventos B, Willekens R, Torrella A, Planas B, Badia R, Garcia F, Castellvi J, Genesca M, Falco V, Buzon MJ. Expression of CD20 after viral reactivation renders HIV‐reservoir cells susceptible to rituximab. Nat Commun. 2019;10:3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Samsoondar JP, Burke AC, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Pinkosky SL, Newton RS, Huff MW. Prevention of diet‐induced metabolic dysregulation, inflammation, and atherosclerosis in LDLR(‐/‐) mice by treatment with the atp‐citrate lyase inhibitor bempedoic acid. Arterioscler Thromb Vasc Biol. 2017;37:647–656. [DOI] [PubMed] [Google Scholar]
- 174. Honigberg MC, Natarajan P. Bempedoic acid for lowering LDL cholesterol. JAMA. 2019;322:1769–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low‐density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the clear wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]


