Abstract
Background
Inflammation is an independent causal risk factor for atherosclerotic cardiovascular diseases (ASCVDs). However, whether hsCRP (high‐sensitivity C‐reactive protein) is prognostic across various levels of atherogenic lipid measures such as low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, apolipoprotein B and total cholesterol/high‐density lipoprotein cholesterol in primary prevention is unknown.
Methods and Results
We studied 9748 ARIC (Atherosclerosis Risk in Communities) study participants who were free of ASCVD at baseline (visit 4, 1996–1998) and had measurements of lipids, apolipoprotein B, and hsCRP. We used multivariable adjusted Cox models to estimate the risk of incident ASCVD events associated with hsCRP levels (less than/greater than or equal to median) in individuals where triple lipid measures combined (low‐density lipoprotein cholesterol + non–high‐density lipoprotein cholesterol + apolipoprotein B) or quadruple measures combined [triple + total cholesterol/high‐density lipoprotein cholesterol] were less than versus greater than or equal to median cut points. Mean age of participants was 62.6±5.6 years; 59% women, 22% black. There were 1574 ASCVD events over median (interquartile range) follow‐up of 18.4 (12.8–19.5) years, and discordance between hsCRP and lipid measures was prevalent in 50% of the population. hsCRP greater than or equal to median (2.4 mg/L), compared with less than median, was associated with an increased risk of ASCVD in individuals with less than median levels of the triple (adjusted hazard ratio, 1.33; 95% CI, 1.09–1.60) and quadruple (adjusted hazard ratio,1.47; 95% CI, 1.18–1.85) lipid measures. Such increased risk was consistent among individuals with low (<7.5%) or high (≥7.5%) estimated risk by the pooled cohort equation. There were no interactions by sex, diabetes mellitus, or statin use.
Conclusions
Our findings suggest that inflammation is independently associated with ASCVD regardless of atherogenic lipid levels and pooled cohort equation risk score in individuals without known ASCVD.
Keywords: apolipoprotein, C‐reactive protein, low density lipoprotein cholesterol, primary prevention
Subject Categories: Primary Prevention, Risk Factors, Cardiovascular Disease
Clinical Perspective
What Is New?
In a primary prevention US cohort, discordance between hsCRP (high‐sensitivity C‐reactive protein) levels, a marker of subclinical inflammation, and atherogenic lipids was common using different discordance definitions (≈50% using the median cut points definition), and elevated hsCRP consistently enhanced atherosclerotic cardiovascular disease risk in individuals regardless of their baseline absolute risk determined by various atherogenic lipid measures and the pooled cohort equation score.
Individuals with favorable atherogenic lipid levels (low‐density lipoprotein cholesterol, non–high‐density lipoprotein cholesterol, apolipoprotein B, and total cholesterol/high‐density lipoprotein cholesterol all relatively low), but a discordantly elevated hsCRP level, had a 30% to 60% greater relative risk of developing atherosclerotic cardiovascular disease events over a long period of time (median follow‐up ≈18 years) compared with those with lower hsCRP; individuals with unfavorable atherogenic lipid levels (all relatively high) and an elevated hsCRP level had a similarly higher atherosclerotic cardiovascular disease risk.
Individuals with higher levels of hsCRP also had an independently enhanced risk of incident heart failure and all‐cause death compared with those with lower hsCRP levels.
What Are the Clinical Implications?
hsCRP levels should be regularly considered along with atherogenic lipid measures in a holistic and personalized risk‐based approach to atherosclerotic cardiovascular disease risk assessment and primary prevention.
Elevated hsCRP levels can serve as a risk enhancer irrespective of baseline absolute risk determined by various atherogenic lipid measures and the pooled cohort equation risk score.
Individuals with elevated hsCRP may additionally benefit from an intensive anti‐inflammatory lifestyle and also possibly from anti‐inflammatory medications such as high‐intensity statin therapy or colchicine; however, this requires further prospective validation in clinical trials.
Introduction
Accumulating evidence from the past 2 decades suggests that inflammation plays a causal role, independent of lipoprotein levels, in the development of atherosclerotic cardiovascular disease (ASCVD; “inflammation hypothesis”).1 Early observations2, 3 led to the landmark JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial, which showed that rosuvastatin therapy reduced ASCVD events in a primary prevention population with low‐density lipoprotein cholesterol (LDL‐C) <130 mg/dL but an elevated hsCRP (high‐sensitivity C‐reactive protein) ≥2 mg/L, a marker of subclinical inflammation.4 However, it remained unclear whether the observed benefits in JUPITER were directly related to reduction of hsCRP, LDL‐C, or both, or other atherogenic lipoproteins. Further, post hoc analyses from multiple lipid‐lowering trials have shown that individuals reaching dual low LDL‐C and hsCRP targets had superior outcomes compared with those who only reached an LDL‐C target.5, 6 Validation for the role of inflammation in ASCVD was strengthened even more recently after the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) showed reduction in recurrent cardiovascular events with canakinumab treatment,7 especially among those who achieved hsCRP <2 mg/L at 3 months on therapy.8
ASCVD events are known to occur more frequently among individuals with relatively low LDL‐C levels who have discordantly elevated levels of atherogenic lipid parameters such as non–high‐density lipoprotein cholesterol (non–HDL‐C), apolipoprotein B,9 and the total cholesterol (TC) to HDL‐C ratio (TC/HDL‐C).10, 11 Given that discordance between these various atherogenic lipid measures is common and clinically relevant, one way to isolate the independent contribution of heightened inflammation to atherosclerosis is to study whether hsCRP levels can provide additional information in situations when these atherogenic lipid and lipoprotein levels are all concordantly favorable or unfavorable. In a large, predominantly biracial population of US adults without known ASCVD, we aimed to evaluate whether hsCRP can provide additional long‐term prognostic information regarding risk of ASCVD independent of lipid and lipoprotein levels.
Methods
The data, analytic methods, and study materials can be made available to other researchers upon request, for purposes of reproducing the results, in accordance with ARIC (Atherosclerosis Risk in Communities) study policies.
Study Population
The ARIC study is a multicenter, prospective cohort of 15 792 middle‐aged men and women, established in 1987 from the following 4 communities in the United States: suburban Minneapolis, Minnesota; Forsyth County, North Carolina; Washington County, Maryland; and Jackson, Mississippi. The ARIC study design has been previously reported.12 Individuals aged 45 to 64 years were enrolled between 1987 and 1989 as part of the baseline (visit 1) clinic examination. For the present study, we used visit 4 (1996–1998) as baseline given that lipid panel, apolipoprotein B and hsCRP were all measured at that visit. Participants also completed questionnaires, underwent a physical exam, and had blood samples collected and stored. Additionally, participants or their proxies were contacted by phone annually to obtain information on occurrence of ASCVD events and their vital status.
For the present analysis, we had the following exclusion criteria: (1) those with prevalent ASCVD at baseline (n=983); (2) those who were neither black nor white, or blacks from Minnesota and Maryland centers (n=69), given that small numbers did not allow for adjustment by race/center groups; and (3) those missing values for lipid or hsCRP variables (n=856). Our final sample included 9748 participants.
Institutional review boards at all participating institutions approved the ARIC study. All participants provided written informed consent at each study visit.
Lipid‐ and hsCRP‐Based Categories
Fasting blood lipid levels were measured according to standard procedures.12 Plasma TC and triglycerides were determined by enzymatic methods, and HDL‐C was measured after dextran‐magnesium precipitation (https://www2.cscc.unc.edu/aric/cohort-manuals). Apolipoprotein B was measured for all participants using World Health Organization/International Federation of Clinical Chemistry standardized reference materials.
For this study, we estimated LDL‐C using our novel Hopkins‐Martin estimation method, which uses 1 of 180 different factors for the ratio of triglycerides to very‐low‐density lipoprotein cholesterol (VLDL‐C) according to non–HDL‐C and TG levels13 and has been externally validated by groups inside and outside the United States.14, 15, 16 In addition to LDL‐C, we calculated the TC/HDL‐C ratio and non–HDL‐C (TC minus HDL‐C).
We performed 3 analyses based on 3 sets of cut points (a, b and c) for our atherogenic lipid measures: (a) median cut points; (b) JUPITER cut points: LDL‐C (130 mg/dL) and non–HDL‐C (160 mg/dL), whereas apolipoprotein B (102 mg/dL) and TC/HDL‐C (4.4) cut points were determined from the ARIC percentile‐equivalent values (57th percentile) to LDL‐C 130 mg/dL; (c) high‐risk cut points: LDL‐C (100 mg/dL) and non–HDL‐C (130 mg/dL), high‐risk targets recommended by international guidelines,17, 18 whereas apolipoprotein B (80 mg/dL) and TC/HDL‐C (3.1) cut points were determined from ARIC percentile‐equivalent values (21st percentile). HDL‐C cut points used were median (40 mg/dL) for (a) and 50 mg/dL for (b) and (c). In addition, we created 2 hsCRP categories defined by less than and greater than or equal to median (2.4 mg/L) for the analysis in (a), and less than and ≥2 mg/L for the analyses in (b) and (c).
Using each set of lipid group cut points (a, b, and c), we compared individuals with hsCRP less than cutpoint to those greater than or equal to cut point in the following groups:
Triple lipid measures combined: [LDL‐C AND non–HDL‐C AND apolipoprotein B] less than cut point versus [LDL‐C AND non–HDL‐C AND apolipoprotein B] greater than or equal to cut point
Quadruple lipid measures combined: [LDL‐C AND non–HDL‐C AND apolipoprotein B AND TC/HDL‐C] less than cut point versus [LDL‐C AND non–HDL‐C AND apolipoprotein B AND TC/HDL‐C] greater than or equal to cut point
For example, we stratified participants with triple lipid measures (LDL‐C AND non–HDL‐C AND apolipoprotein B) less than median levels into those with hsCRP less than median (concordant) versus greater than or equal to median (discordant) and did the same in individuals with triple levels greater than or equal to median [(hsCRP less than median (discordant) and hsCRP greater than or equal to median (concordant)].
We also performed more extensive discordance analyses of hsCRP less than/greater than or equal to cut points across cut points of LDL‐C only followed by the gradual addition of other lipid and lipoprotein cut points in the following order: non–HDL‐C, apolipoprotein B, TC/HDL‐C, and HDL‐C.
Other Covariates
Demographics (age, sex, race/center, education, etc) and cardiovascular risk factors (smoking status, physical activity assessed using a modified interviewer‐administered Baecke Questionnaire, diabetes mellitus, hypertension) were obtained from history, physical examination, and laboratory data at baseline (visit 4). Smoking status was categorized as never/former and current smoker. Body mass index was calculated from measured height and weight. Diabetes mellitus was defined as a fasting (≥8 hours) serum glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self‐reported physician diagnosis of diabetes mellitus, or reported use of hypoglycemic agents. Blood pressure was measured 3 times, and the mean of the second and third measurements was used. Use of lipid‐lowering and antihypertensive medications were self‐reported.
Outcomes
The primary outcome was incident ASCVD determined from hospital discharge codes or death certificates. Incident ASCVD was defined as definite or probable myocardial infarction, definite coronary death, and definite or probable stroke (sudden or rapid onset of neurological symptoms that lasted for 24 hours or led to death in the absence of another cause).19, 20, 21 Additionally, ARIC investigators conducted continuous surveillance for all cardiovascular disease–related hospitalizations and deaths. All ASCVD events were adjudicated by the ARIC study investigators. Study participants contributed follow‐up time from the date of the participant's baseline visit until the date of incident ASCVD event, death, loss to follow‐up, or the end of follow‐up (December 31, 2016), whichever came first.
Secondary outcomes included incident heart failure (HF) and total mortality. Incident HF, defined as the first hospitalization or death related to HF occurring after visit 4 (baseline) until end of follow‐up. Hospitalized HF was determined from hospital discharge codes and HF deaths from the underlying cause assigned on the death certificate. Total mortality was obtained subsequent to visit 4 until the end of follow‐up.
Statistical Analyses
Baseline characteristics of the study population by lipids and hsCRP concordance/discordance categories were described using medians (25th–75th percentiles) for continuous and proportions for categorical variables. Comparisons were performed using Kruskal–Wallis test and chi‐square, respectively, between hsCRP categories within lipid less than cut point and greater than or equal to cut point groups, separately.
For our prospective analysis we constructed 4 Cox proportional hazard models. Model 1 was adjusted by age, sex, and race/center groups. Model 2 was additionally adjusted by physical activity, smoking status, body mass index, systolic blood pressure, treatment for hypertension, diabetes mellitus, and lipid‐lowering medication use. Model 3 was additionally adjusted by log‐transformed triglycerides. Model 4 was additionally adjusted by log–HDL‐C for lipid‐based groups 1 to 3 only. Further, we constructed unadjusted Kaplan–Meier curves for all lipids and hsCRP concordance/discordance groups.
Additionally, all primary analyses were stratified by risk categories defined a priori, estimated by the pooled cohort equations (PCE) (low: <7.5%, high: ≥7.5%).22 Finally, we evaluated effect modification (interaction) by sex, PCE risk categories, statin use, and diabetes mellitus, according to sample sizes shown in Tables 1 and 2.
Table 1.
Baseline Characteristics in Concordant and Discordant Groups by Medians: The ARIC Study
| Overall Population | Four Lipids Less Than Median | Four Lipids Greater Than or Equal to Median | |||||
|---|---|---|---|---|---|---|---|
| hsCRP < Median | hsCRP ≥ Median | P Value | hsCRP < Median | hsCRP ≥ Median | P Value | ||
| Age, y | 62 (58–67) | 62 (57–67) | 62 (58–67) | 0.72 | 62.5 (58–67) | 62 (58–67) | 0.73 |
| Female, n (%) | 5765 (59.1) | 932 (56.1) | 1138 (78.0) | <0.0001 | 611 (41.9) | 1021 (63.1) | <0.0001 |
| Race/center, n (%) | <0.0001 | <0.0001 | |||||
|
Minneapolis, MN, Whites |
2713 (28.0) | 479 (29.1) | 335 (23.1) | 455 (31.4) | 410 (25.5) | ||
| Washington County, MD, Whites | 2658 (27.5) | 406 (24.7) | 313 (21.6) | 449 (31.0) | 512 (31.8) | ||
| Forsyth County, NC, Whites | 2180 (22.5) | 408 (24.8) | 324 (22.4) | 339 (23.4) | 335 (20.8) | ||
| Forsyth County, NC, Blacks | 217 (2.2) | 39 (2.4) | 55 (3.8) | 15 (1.0) | 30 (1.9) | ||
| Jackson, MS, Blacks | 1916 (19.8) | 315 (19.1) | 420 (29.0) | 189 (13.1) | 324 (20.1) | ||
| Body mass index, kg/m2 | 28.0 (24.9–31.6) | 25.5 (22.8–28.4) | 28.5 (24.8–33.4) | <0.001 | 27.6 (25.1–30.4) | 30.2 (27.1–34.2) | <0.0001 |
| LDL‐C, mg/dL | 123.6 (103.3–145.4) | 97.8 (84.0–109.0) | 97.4 (82.8–108.4) | 0.29 | 152.6 (139.1–168.4) | 151.9 (139.4–169.5) | 0.81 |
| Non–HDL‐C, mg/dL | 149 (126–173) | 116 (102–128) | 118 (103–130) | 0.06 | 182 (167–200) | 184 (169–204) | 0.035 |
| TC/HDL‐C | 4.2 (3.3–5.2) | 3.0 (2.5–3.5) | 3.1 (2.6–3.6) | 0.06 | 5.4 (4.8–6.1) | 5.6 (4.9–6.4) | 0.0001 |
| Apolipoprotein B, mg/dL | 98.1 (82.8–113.7) | 78.2 (69.8–85.8) | 79.6 (70.6–87.6) | 0.05 | 117.3 (108.6–129.9) | 119.6 (109.8–132.5) | 0.001 |
| HDL‐C, mg/dL | 48 (39–60) | 58 (48–70) | 57 (47–69) | 0.12 | 42 (36–47) | 41 (35–46) | 0.001 |
| hsCRP, mg/L | 2.4 (1.1–5.4) | 0.9 (0.5–1.5) | 5.7 (3.7–8.9) | <0.0001 | 1.2 (0.8–1.7) | 5.3 (3.7–8.4) | <0.0001 |
| Lipid‐lowering medication use, n (%) | 1104 (11.4) | 138 (8.3) | 90 (6.2) | 0.022 | 133 (9.1) | 125 (7.8) | 0.171 |
| Current smoker, n (%) | 1403 (14.5) | 233 (14.1) | 218 (15.0) | 0.471 | 161 (11.1) | 311 (19.2) | <0.0001 |
| Systolic blood pressure, mm Hg | 125 (114–138) | 122 (111–135) | 126 (114–140) | <0.0001 | 124 (113–137) | 128 (117–141) | <0.0001 |
| Antihypertensive medications, n (%) | 3866 (39.7) | 598 (36.2) | 744 (51.2) | <0.0001 | 589 (40.5) | 838 (51.9) | <0.0001 |
| Diabetes mellitus, n (%) | 1495 (15.4) | 151 (9.2) | 211 (14.6) | <0.0001 | 186 (12.8) | 375 (23.2) | <0.0001 |
| 10‐y ASCVD risk | 8.0 (3.3–14.9) | 6.0 (2.3–11.9) | 5.8 (2.3–11.6) | 0.8966 | 10.5 (5.7–17.5) | 9.7 (4.1–18.5) | 0.0742 |
Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Continuous variables are reported as median (25th–75th percentile). Medians and means were compared using Kruskal‐Wallis and chi‐squared test, respectively. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiple by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol.
Table 2.
Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians: The ARIC Study
| Lipid Target Groups | hsCRP | n ASCVD Events/n Individuals | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|
| Triple lipids < median | < median | 237/2060 | REF | REF | REF | REF |
| (n=3938) | ≥ median | 290/1878 | 1.57 (1.31–1.88) | 1.46 (1.19–1.78) | 1.42 (1.16–1.74) | 1.42 (1.16–1.73) |
| Triple lipids ≥ median | < median | 315/1954 | REF | REF | REF | REF |
| (n=4064) | ≥ median | 442/2110 | 1.56 (1.34–1.81) | 1.33 (1.13–1.57) | 1.32 (1.12–1.56) | 1.31 (1.11–1.55) |
| Quadruple lipids < median | < median | 171/1660 | REF | REF | REF | N/A |
| (n=3120) | ≥ median | 207/1460 | 1.62 (1.31–2.01) | 1.60 (1.26–2.02) | 1.56 (1.23–1.98) | N/A |
| Quadruple lipids ≥ median | < median | 257/1460 | REF | REF | REF | N/A |
| (n=3079) | ≥ median | 362/1619 | 1.52 (1.29–1.79) | 1.30 (1.08–1.56) | 1.29 (1.08–1.55) | N/A |
hsCRP less than median groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non–HDL‐C + apolipoprotein B + TC/HDL‐C. Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Model 1: adjusted for age, sex, race/center. Model 2: Model 1 + physical activity + smoking status + body mass index + systolic blood pressure + treatment for hypertension + diabetes mellitus + lipid‐lowering medication use.
Model 3: Model 2 + log‐triglycerides. Model 4: Model 3 + log‐HDL‐C. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Results
Among the 9748 included participants, the mean age at baseline was 62.5±5.6 years; 59% were women, and 22% were black. At the baseline visit, median levels were LDL‐C, 123 mg/dL; non–HDL‐C, 149 mg/dL; apolipoprotein B: 98 mg/dL; TC/HDL, 4.2; HDL‐C, 40 mg/dL; hsCRP, 2.4 mg/L. The proportion of hsCRP discordance with lipid measures was ≈50% within all lipid groups (Figures S1 and S2). For example, among those with a more favorable lipid profile (quadruple lipid measures less than median), 47% had a discordantly elevated hsCRP greater than or equal to median.
Baseline Characteristics
Table 1 shows the baseline characteristics of study population by concordant/discordant groups when combining the quadruple atherogenic lipid measures versus hsCRP. Among those with a favorable lipid profile (quadruple lipid measures combined less than median), individuals with hsCRP greater than or equal to median were predominantly women, had a higher body mass index, and had a greater proportion of diabetes mellitus and hypertension (P<0.001) but similar 10‐year PCE ASCVD risk, compared with those with hsCRP <median. A comparable pattern was found among those with an unfavorable lipid profile (quadruple lipid measures combined greater than or equal to median).
Discordance Using Median Cutpoints
Over a median follow‐up of 18.4 years (interquartile range, 12.8–19.5), there were 1574 incident ASCVD events (713 had definite/probable myocardial infarction, 304 had coronary death, 557 had definite/probable stroke). Figures 1 and 2 show Kaplan–Meier curves for hsCRP greater than or equal to cut points versus hsCRP less than cut points across various lipid groups. Table 2 shows the prospective analysis for hazard of incident ASCVD by hsCRP level (less than/greater than or equal to median) across triple and quadruple lipid measures stratified by less than/greater than or equal to median cut points. We observed a significant independent ≈30% to 60% increase in ASCVD risk in individuals with hsCRP greater than or equal to median versus less than median regardless of levels of triple or quadruple lipid measures even after adjusting for multiple factors known to be associated with ASCVD and log‐triglyceride levels (Table 2). Of note, this increased risk of ASCVD was similar in our supplementary analysis across all lipid groups starting from discordance with LDL‐C only to the gradual addition of other lipid measures (Table S1).
Figure 1.
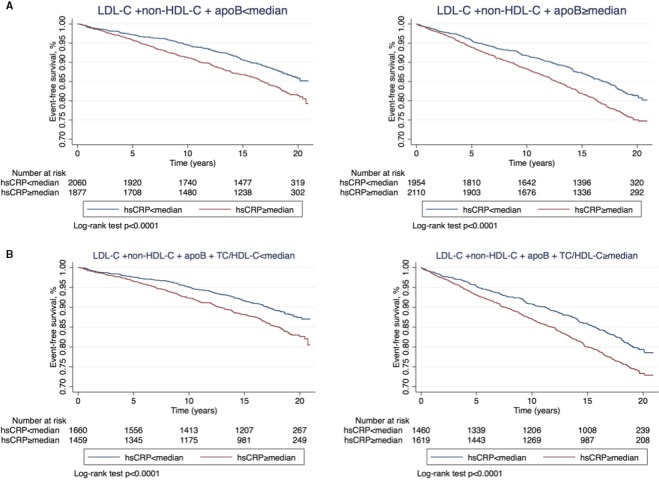
Kaplan–Meier curves for event‐free survival of atherosclerotic cardiovascular events by hsCRP less than and greater than or equal to median among individuals with triple (A), quadruple (B) lipid measures less than median (left) and greater than or equal to median (right). HDL‐C indicates high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol.
Figure 2.
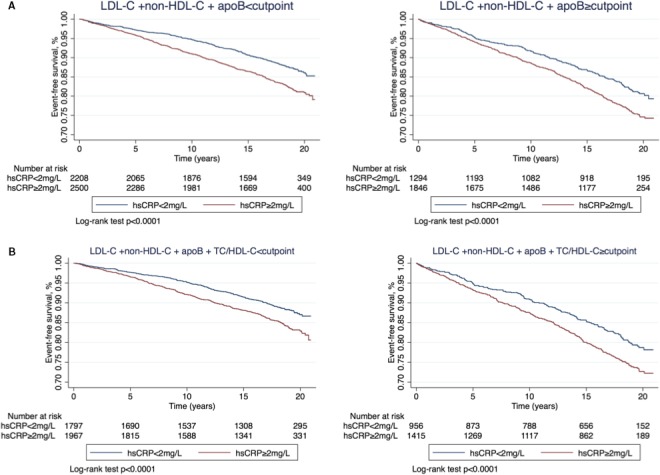
Kaplan–Meier curves for event‐free survival of atherosclerotic cardiovascular events by hsCRP less than and ≥2 mg/L among individuals with triple (A), quadruple (B) measures less than JUPITER cut points (left) and greater than or equal to JUPITER cut points (right). HDL‐C indicates high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; JUPITER, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol.
Further, we found that the increased ASCVD risk associated with elevated hsCRP regardless of atherogenic lipid levels was consistent among individuals with low (<7.5%) or high (≥7.5%) 10‐year estimated risk by the PCE (Table 3 and Table S2).
Table 3.
Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians, Grouped Using the Pooled Cohort Equation Risk Score: The ARIC Study
| Lipid Target Groups | hsCRP | Low Risk (<7.5%) | High Risk (≥7.5%) | ||
|---|---|---|---|---|---|
| ASCVD Events, n/Individuals, n | HR (95% CI) | ASCVD Events, n/Individuals, n | HR (95% CI) | ||
| Triple lipids < median | < median | 80/1091 | REF | 154/948 | REF |
| (n=3938) | ≥ median | 125/1010 | 1.79 (1.35–2.37) | 162/851 | 1.27 (1.02–1.59) |
| Triple lipids ≥ median | < median | 75/779 | REF | 238/1162 | REF |
| (n=4064) | ≥ median | 134/931 | 1.57 (1.18–2.08) | 304/1169 | 1.42 (1.20–1.69) |
| Quadruple lipids < median | < median | 65/955 | REF | 104/688 | REF |
| (n=3120) | ≥ median | 96/857 | 1.73 (1.26–2.37) | 110/591 | 1.35 (1.03–1.77) |
| Quadruple lipids ≥ median | < median | 56/507 | REF | 199/942 | REF |
| (n=3079) | ≥ median | 101/651 | 1.47 (1.06–2.04) | 258/962 | 1.42 (1.18–1.71) |
hsCRP <median groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non–HDL‐C + apolipoprotein B + TC/HDL‐C. Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Models adjusted for lipid‐lowering medication use. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Additionally, we observed consistent results when using incident heart failure (Figure S3, Table 4), total mortality (Figure S4, Table 5) and coronary death as outcomes (Table 6, Table S3).
Table 4.
Hazard Ratios (95%CI) for Incident Heart Failure Across Concordant/Discordant Groups by Medians: The ARIC Study
| Lipid Target Groups | hsCRP | Incident HF, n/Individuals, n | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|
| Triple lipids < median | < median | 268/2044 | REF | REF | REF | REF |
| (n=3888) | ≥ median | 360/1844 | 1.74 (1.48–2.05) | 1.28 (1.07–1.53) | 1.26 (1.05–1.51) | 1.26 (1.05–1.52) |
| Triple lipids ≥ median | < median | 243/1940 | REF | REF | REF | REF |
| (n=4024) | ≥ median | 424/2084 | 1.89 (1.61–2.22) | 1.36 (1.14–1.63) | 1.36 (1.14–1.63) | 1.37 (1.14–1.63) |
| Quadruple lipids < median | < median | 204/1647 | REF | REF | REF | N/A |
| (n=3086) | ≥ median | 258/1439 | 1.70 (1.40–2.07) | 1.35 (1.09–1.66) | 1.33 (1.07–1.64) | N/A |
| Quadruple lipids ≥ median | < median | 182/1449 | REF | REF | REF | N/A |
| (n=3048) | ≥ median | 336/1599 | 1.95 (1.62–2.35) | 1.38 (1.13–1.69) | 1.38 (1.13–1.69) | N/A |
Individuals with prevalent heart failure at visit 4 were excluded from analysis (n=271). hsCRP less than median groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non–HDL‐C + apolipoprotein B + TC/HDL‐C. Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Model 1: adjusted for age, sex, race/center. Model 2: Model 1 + physical activity + smoking status + body mass index+ systolic blood pressure + treatment for hypertension + diabetes mellitus + lipid‐lowering medication use. Model 3: Model 2 + log‐triglycerides. Model 4: Model 3 + log‐HDL‐C. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Table 5.
Hazard Ratios (95% CI) for All‐Cause Mortality Across Concordant/Discordant Groups by Medians: The ARIC Study
| Lipid Target Groups | hsCRP | Deaths, n/Individuals, n | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|
| Triple lipids < median | < median | 699/2060 | REF | REF | REF | REF |
| (n=3938) | ≥ median | 743/1878 | 1.41 (1.26–1.57) | 1.29 (1.14–1.45) | 1.28 (1.14–1.44) | 1.28 (1.14–1.45) |
| Triple lipids ≥ median | < median | 605/1954 | REF | REF | REF | REF |
| (n=4064) | ≥ median | 815/2110 | 1.47 (1.32–1.64) | 1.25 (1.11–1.41) | 1.24 (1.10–1.40) | 1.24 (1.10–1.40) |
| Quadruple lipids < median | < median | 539/1660 | REF | REF | REF | N/A |
| (n=3120) | ≥ median | 574/1460 | 1.49 (1.32–1.69) | 1.42 (1.24–1.63) | 1.41 (1.23–1.62) | N/A |
| Quadruple lipids ≥ median | < median | 479/1460 | REF | REF | REF | N/A |
| (n=3079) | ≥ median | 656/1619 | 1.45 (1.28–1.63) | 1.22 (1.07–1.40) | 1.22 (1.07–1.40) | N/A |
hsCRP less than median groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non–HDL‐C + apolipoprotein B + TC/HDL‐C. Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Model 1: adjusted for age, sex, race/center. Model 2: Model 1 + physical activity + smoking status + body mass index + systolic blood pressure + treatment for hypertension + diabetes mellitus + lipid‐lowering medication use. Model 3: Model 2 + log‐triglycerides. Model 4: Model 3 + log‐HDL‐C. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Table 6.
Hazard Ratios (95%CI) for Coronary Death Across Concordant/Discordant Groups By Medians: The Atherosclerosis Risk in Communities Study
| Lipid Target Groups | hsCRP | Coronary deaths, n/Individuals, n | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|
| Triple lipids < median | < median | 41/2060 | REF | REF | REF | REF |
| (n=3938) | ≥ median | 75/1878 | 2.55 (1.72–3.77) | 1.98 (1.29–3.05) | 1.98 (1.29–3.05) | 1.96 (1.27–3.02) |
| Triple lipids ≥ median | < median | 48/1954 | REF | REF | REF | REF |
| (n=4064) | ≥ median | 92/2110 | 2.25 (1.57–3.22) | 1.78 (1.20–2.65) | 1.76 (1.17–2.63) | 1.74 (1.16–2.60) |
| Quadruple lipids < median | < median | 27/1660 | REF | REF | REF | N/A |
| (n=3120) | ≥ median | 51/1460 | 2.84 (1.75–4.62) | 2.61 (1.54–4.42) | 2.62 (1.55–4.43) | N/A |
| Quadruple lipids ≥ median | < median | 41/1460 | REF | REF | REF | N/A |
| (n=3079) | ≥ median | 78/1619 | 2.17 (1.47–3.21) | 1.73 (1.13–2.65) | 1.73 (1.13–2.65) | N/A |
hsCRP less than median groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non‐HDL‐C + apolipoprotein B + TC/HDL‐C. Medians: LDL‐C: 123 mg/dL, non–HDL‐C: 149 mg/dL, apolipoprotein B: 98 mg/dL, TC/HDL: 4.2, HDL‐C: 40 mg/dL, hsCRP: 2.4 mg/L. Model 1: adjusted for age, sex, race/center. Model 2: Model 1 + physical activity + smoking status + body mass index+ systolic blood pressure + treatment for hypertension + diabetes mellitus + lipid‐lowering medication use. Model 3: Model 2 + log‐triglycerides. Model 4: Model 3 + log–HDL‐C. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Discordance Using JUPITER Cut Points
Table 7 shows hazards of incident ASCVD by hsCRP level (</≥2 mg/L) across the same lipid groups stratified by JUPITER cut points. We observed an independent increase in ASCVD risk of about 28% to 41% in individuals with hsCRP ≥2 versus <2 mg/L across (less than and greater than or equal to JUPITER cut points) of triple and quadruple lipid groups (Table 6) as well as other lipid groups (Table S4). Furthermore, we reproduced our analysis using several hsCRP categories (<1, 1–2, 2–3, and ≥3 mg/L) and found a stepwise increase in ASCVD risk at higher hsCRP levels (Table S5).
Table 7.
Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by JUPITER Cut Points: The ARIC Study
| Lipid Target Groups | hsCRP | ASCVD events, n/Individuals, n | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|
| Triple lipids < cut point | <2 mg/L | 252/2208 | REF | REF | REF | REF |
| (n=4709) | ≥2 mg/L | 398/2501 | 1.60 (1.36–1.89) | 1.45 (1.21–1.73) | 1.41 (1.18–1.68) | 1.40 (1.17–1.68) |
| Triple lipids ≥ cut point | <2 mg/L | 215/1294 | REF | REF | REF | REF |
| (n=3140) | ≥2 mg/L | 392/1846 | 1.46 (1.24–1.73) | 1.27 (1.06–1.54) | 1.27 (1.05–1.53) | 1.26 (1.04–1.52) |
| Quadruple lipids < cut point | <2 mg/L | 189/1797 | REF | REF | REF | N/A |
| (n=3765) | ≥2 mg/L | 279/1968 | 1.52 (1.25–1.84) | 1.43 (1.15–1.76) | 1.39 (1.12–1.72) | N/A |
| Quadruple lipids ≥ cut point | <2 mg/L | 172/1956 | REF | REF | REF | N/A |
| (n=3371) | ≥2 mg/L | 322/1415 | 1.45 (1.20–1.75) | 1.28 (1.04–1.58) | 1.28 (1.04–1.57) | N/A |
hsCRP <2 mg/L groups are reference. Triple lipids: LDL‐C + non–HDL‐C + apolipoprotein B. Quadruple lipids: LDL‐C + non–HDL‐C + apolipoprotein B + TC/HDL‐C. Cut points: LDL‐C: 130 mg/dL (percentile 57), non–HDL‐C: 160 mg/dL, apolipoprotein B: 102 mg/dL, TC/HDL: 4.4, HDL‐C: 50 mg/dL, hsCRP: 2 mg/L. Model 1: adjusted for age, sex, race/center. Model 2: Model 1 + physical activity + smoking status + body mass index + systolic blood pressure + treatment for hypertension + diabetes mellitus + lipid‐lowering medication use. Model 3: Model 2 + log‐triglycerides. Model 4: Model 3 + log‐HDL‐C. International System of Units conversion factors: To convert total, LDL‐C, and HDL‐C to mmol/L, multiply by 0.0249. To convert triglycerides to mmol/L, multiply by 0.0113. ARIC indicates Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; JUPITER, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin; LDL‐C, low‐density lipoprotein cholesterol; N/A indicates not applicable; TC, total cholesterol.
Discordance Using High‐Risk Cut Points
Finally, when using high‐risk cut points equivalent to LDL‐C 100 mg/dL, we observed a similar effect size of 25% to 40% increased risk in individuals with hsCRP ≥2 versus <2 mg/L, although it did not reach statistical significance (defined as P>0.05) in groups with smaller sample size (Table S6).
Of note, there was no evidence for interaction by sex, statin use, or diabetes mellitus when using medians, JUPITER, or high‐risk targets. Finally, we used a time‐varying analysis across multiple visits, and our results did not change (Table S7).
Discussion
In a large biracial cohort of US adults without known ASCVD at baseline and followed for >18 years, individuals with a higher degree of inflammation, determined by hsCRP levels, had heightened risk of ASCVD events, incident HF, and all‐cause death across various levels of atherogenic lipid measures. Approximately 1 in 2 ASCVD‐free individuals with a more favorable lipid pattern (all atherogenic lipid measures less than median) had an elevated hsCRP (greater than or equal to median), which was associated with a 56% increased risk of ASCVD at ≈18 years of follow‐up. This finding was consistent across categories of estimated global ASCVD risk by the PCE, supporting the notion that hsCRP may serve as a risk enhancer regardless of lipid levels and estimated global ASCVD risk. Future studies should examine the potential value of anti‐inflammatory interventions (through lifestyle or novel pharmaceuticals), beyond lowering atherogenic lipids, in the primary prevention of ASCVD.
Subclinical Inflammation in the Current Era of ASCVD Risk Assessment in Primary Prevention
The current trend in primary prevention is to assess individual short‐term (ie, 10‐year) ASCVD risk using scores that incorporate clinical variables and cholesterol measurements.17, 18, 22, 23 Because of the significant role of inflammation in atherogenesis, efforts were made to assess the utility of markers of subclinical inflammation, such as hsCRP, to improve risk assessment when considered in addition to traditional risk assessment tools.24, 25, 26, 27 The most recent Multi‐Society American Heart Association/American College of Cardiology cholesterol treatment guideline published in 201828 gives a class IIb recommendation for using moderate‐intensity statin therapy in borderline risk (PCE score 5 to <7.5%) individuals with an hsCRP level ≥2 mg/L, considered as an ASCVD risk enhancer. In addition, the latest ESC guideline states that inflammation modifies the Systematic Coronary Risk Evaluation risk but does not provide specific guidance to hsCRP targets or treatment strategies in patients with heightened inflammation.
We performed discordance analyses to approach the prognostic value of inflammation in primary prevention from a different angle. In our study, 1 in 2 individuals from ARIC had discordance between ≥1 atherogenic lipid measures and hsCRP, with associated increased ASCVD risk in fully adjusted models, which strengthens the evidence that hsCRP can provide additional risk‐modifying information in primary prevention beyond all lipid levels. This increased risk attributed to hsCRP remained consistent across categories of estimated risk by the PCE (P‐interaction >0.05). Notably, individuals with hsCRP ≥2 mg/L compared with <2 mg/L had a 68% increased risk of ASCVD among individuals categorized as low risk by PCE (score <7.5%). Our findings add to the evidence that anti‐inflammatory lifestyle and pharmacotherapeutic approaches may be considered in a precision‐risk–based approach in individuals with elevated hsCRP.29 A recent study from MESA (Multi‐Ethnic Study of Atherosclerosis) showed that moderate‐to‐vigorous physical activity was associated with a more favorable profile of inflammatory markers, possible attributable to reduction in central adiposity.30 Similarly, dietary regimens such as the Mediterranean diet have been shown to improve markers of inflammation.31 In individuals with elevated hsCRP, reducing the lifelong burden of inflammation (a term we call inflammation pack‐years) using lifestyle interventions or anti‐inflammatory pharmacotherapies, such as high‐intensity statin therapy, should be a cornerstone of primordial and primary prevention.
Implications for JUPITER‐Like Population
The JUPITER trial increased the eligibility of adults for statin therapy in the United States and is the primary evidence behind the most recent guideline recommendations to use a hsCRP level of >2 mg/L as a risk enhancer in intermediate risk patients.32 However, Mora et al33 showed that residual risk associated with elevated hsCRP in the JUPITER trial could be explained by a higher number of atherogenic particles other than LDL‐C. Given the well‐described discordance between LDL‐C and other, more robust atherogenic lipid measures (ie, non–HDL‐C or apolipoprotein B),9 we further refined the JUPITER population by sequentially adding atherogenic lipid cut points of percentile equivalence to LDL‐C 130 mg/dL in order to fully assess the lipid‐independent risk attributed to hsCRP. We found that after ≈18 years of follow‐up (about 10‐fold longer than median JUPITER follow‐up), baseline hsCRP ≥2 mg/L was associated with a 32% to 37% increased risk of ASCVD as compared with hsCRP<2 mg/L among individuals with LDL‐C <130 mg/dL and simultaneously more favorable levels of non–HDL‐C (<160 mg/dL), apolipoprotein B (<102 mg/dL), TC/HDL‐C ratio (<4.4), and HDL‐C (≥50 mg/dL) combined. This increased independent risk was also independent of their global ASCVD risk assessed by the PCE score. We subsequently advanced these concepts by examining whether hsCRP ≥2 mg/L was associated with an increased risk of ASCVD when atherogenic lipid levels were as low as LDL‐C <100 mg/dL, as recommended by some guidelines for high‐risk patients, and percentile equivalent values of non–HDL‐C, apolipoprotein B, and TC/HDL‐C. The results were all consistent, suggesting that we ought to reemphasize the importance of using high‐intensity statin therapy, as recommended by JUPITER, in individuals with hsCRP ≥2 mg/L regardless of their lipid levels or PCE risk score.
But is heightened inflammation, in primary prevention, also associated with other clinical outcomes over a long period of follow‐up? We were able to show that hsCRP is consistently associated with the risk of incident HF and all‐cause death, which suggests the possibility that inflammation reduction might have a broad favorable impact on several clinical outcomes, as proposed by other studies.25, 34
Strengths and Limitations
The present study has several important strengths. First, this is the longest primary prevention study to date (median follow‐up, 18 years) that has examined the association of hsCRP with ASCVD events, and the first to perform an individual‐level discordance analysis to assess the additional prognostic information provided by hsCRP when discordant with LDL‐C and other lipid measures. Second, we used several sets of cut points going from medians (to make our results easier to interpret), to clinical cut points for lipid measures and hsCRP based on observations from the JUPITER trial and recommended guideline targets for individuals at high risk. Third, because direct measurement of LDL‐C by gold‐standard ultra‐centrifugation was not available in ARIC, we used a novel LDL‐C estimation method that provides a more accurate estimation than the Friedewald equation.13, 35 Finally, we demonstrated consistent results when doing time‐sensitive analyses and when analyzing other outcomes such as HF incidence and all‐cause death.
It is also important to acknowledge our limitations. This was an observational study and residual confounding may explain some of the associations seen. Discordance analyses are categorical by nature and do not allow for studying the predictive power of hsCRP as a continuous variable. However, discordance methods using clinically relevant biomarker cut points allow for personalized individual‐level risk discrimination that is more in line with clinical practice and cholesterol treatment guidelines. Finally, this prospective analysis shows that hsCRP predicts ASCVD risk, incident HF, and all‐cause death independent of all atherogenic lipoprotein levels, but it does not prove that inflammation is a direct cause of such events.
Conclusions
Discordance between hsCRP and atherogenic lipoproteins is prevalent (≥50%). Long‐term elevated hsCRP is associated with increased ASCVD risk, incident HF, and all‐cause death even when all atherogenic lipids and HDL‐C levels are more favorable and regardless of estimated PCE risk score. Future studies are needed to assess the use of hsCRP to identify individuals that might benefit from lifestyle and anti‐inflammatory therapies to mitigate this risk.
Sources of Funding
The Atherosclerosis Risk in Communities study was funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I). The publication of this article was funded by the Weill Cornell Medicine ‐ Qatar Distributed eLibrary.
Disclosures
Drs Martin and Jones are listed as coinventors on a pending patent filed by Johns Hopkins University for LDLn‐C estimation. Dr Jones has served as an advisor to Sano/Regeneron. Dr Martin has served as a consultant to Quest Diagnostics, Sano/Regeneron, Amgen, and the Pew Research Center. Dr Puri has received speakers’ fees from Sanofi‐Aventis and Amgen and research honorarium from Cerenis (unrelated to the present work). Unrelated to this work, Dr. Michos received an honorarium from Siemens Healthcare for being a blinded adjudicator of events in a clinical trial. The remaining authors have no disclosures to report.
Supporting information
Table S1. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Table S2. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Risk Categories in Medians: The ARIC Study (1996–2016)
Table S3. Hazard Ratios (95% CI) for Coronary Death (Competing Risk) Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Table S4. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by JUPITER Cut Points: The ARIC Study (1996–2016)
Table S5. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by JUPITER Cut Points and hsCRP Categories: The ARIC Study (1996–2016)
Table S6. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by High‐Risk Cut Points: The ARIC Study (1996–2016)
Table S7. Time‐Varying Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Figure S1. Proportions of hsCRP greater than and less than or equal to median in single, dual, triple, quadruple, and quintuple lipid group less than and greater than or equal to median.
Figure S2. Proportions of hsCRP greater than and ≤2 mg/L in single, dual, triple, quadruple, and quintuple lipid groups less than and greater than or equal to JUPITER cut points.
Figure S3. Kaplan–Meier curves for event‐free survival of incident heart failure by hsCRP less than and greater than or equal to median among individuals with triple (A), quadruple (B) lipid measures less than median (left) and greater than or equal to median (right).
Figure S4. Kaplan–Meier curves for event‐free survival of total mortality by hsCRP less than and greater than or equal to median among individuals with triple (A), quadruple (B) lipid measures less than median (left) and greater than or equal to median (right).
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2020;9:e013600 DOI: 10.1161/JAHA.119.013600.)
References
- 1. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr; Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators . Measurement of C‐reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 5. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low‐density lipoprotein cholesterol and high‐sensitivity C‐reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in improve‐it. Circulation. 2015;132:1224–1233. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low‐density lipoprotein cholesterol <70 mg/dl and C‐reactive protein <2 mg/L: an analysis of the prove‐IT TIMI‐22 trial. J Am Coll Cardiol. 2005;45:1644–1648. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group . Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the cantos randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 9. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elshazly MB, Nicholls SJ, Nissen SE, St John J, Martin SS, Jones SR, Quispe R, Stegman B, Kapadia SR, Tuzcu EM, Puri R. Implications of total to high‐density lipoprotein cholesterol ratio discordance with alternative lipid parameters for coronary atheroma progression and cardiovascular events. Am J Cardiol. 2016;118:647–655. [DOI] [PubMed] [Google Scholar]
- 11. Quispe R, Elshazly MB, Zhao D, Toth PP, Puri R, Virani SS, Blumenthal RS, Martin SS, Jones SR, Michos ED. Total cholesterol/HDL‐cholesterol ratio discordance with LDL‐cholesterol and non‐HDL‐cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. Eur J Prev Cardiol. 2019. DOI: 10.1177/2047487319862401. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low‐density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meeusen JW, Lueke AJ, Jaffe AS, Saenger AK. Validation of a proposed novel equation for estimating LDL cholesterol. Clin Chem. 2014;60:1519–1523. [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Jang S, Son H. Validation of the Martin method for estimating low‐density lipoprotein cholesterol levels in Korean adults: findings from the Korea national health and nutrition examination survey, 2009–2011. PLoS One. 2016;11:e0148147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin SS, Giugliano RP, Murphy SA, Wasserman SM, Stein EA, Ceska R, Lopez‐Miranda J, Georgiev B, Lorenzatti AJ, Tikkanen MJ, Sever PS, Keech AC, Pedersen TR, Sabatine MS. Comparison of low‐density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the Fourier trial. JAMA Cardiol. 2018;3:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 18. Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National Lipid Association recommendations for patient‐centered management of dyslipidemia: Part 1—executive summary. J Clin Lipidol. 2014;8:473–488. [DOI] [PubMed] [Google Scholar]
- 19. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 20. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 21. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 22. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 23. Qureshi WT, Rana JS, Yeboah J, Bin Nasir U, Al‐Mallah MH. Risk stratification for primary prevention of coronary artery disease: roles of C‐reactive protein and coronary artery calcium. Curr Cardiol Rep. 2015;17:110. [DOI] [PubMed] [Google Scholar]
- 24. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC Jr, Psaty BM, Greenland P, Herrington DM. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cainzos‐Achirica M, Miedema MD, McEvoy JW, Cushman M, Dardari Z, Greenland P, Nasir K, Budoff MJ, Al‐Mallah MH, Yeboah J, Blumenthal RS, Comin‐Colet J, Blaha MJ. The prognostic value of high sensitivity C‐reactive protein in a multi‐ethnic population after >10 years of follow‐up: the Multi‐Ethnic Study of Atherosclerosis (mesa). Int J Cardiol. 2018;264:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 30. Vella CA, Allison MA, Cushman M, Jenny NS, Miles MP, Larsen B, Lakoski SG, Michos ED, Blaha MJ. Physical activity and adiposity‐related inflammation: the MESA. Med Sci Sports Exerc. 2017;49:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michos ED, Blumenthal RS. Prevalence of low low‐density lipoprotein cholesterol with elevated high sensitivity C‐reactive protein in the U.S.: implications of the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) study. J Am Coll Cardiol. 2009;53:931–935. [DOI] [PubMed] [Google Scholar]
- 33. Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high‐intensity statin or placebo: the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circulation. 2015;132:2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin‐1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 35. Quispe R, Hendrani A, Elshazly MB, Michos ED, McEvoy JW, Blaha MJ, Banach M, Kulkarni KR, Toth PP, Coresh J, Blumenthal RS, Jones SR, Martin SS. Accuracy of low‐density lipoprotein cholesterol estimation at very low levels. BMC Med. 2017;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Table S2. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Risk Categories in Medians: The ARIC Study (1996–2016)
Table S3. Hazard Ratios (95% CI) for Coronary Death (Competing Risk) Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Table S4. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by JUPITER Cut Points: The ARIC Study (1996–2016)
Table S5. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by JUPITER Cut Points and hsCRP Categories: The ARIC Study (1996–2016)
Table S6. Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by High‐Risk Cut Points: The ARIC Study (1996–2016)
Table S7. Time‐Varying Hazard Ratios (95% CI) for ASCVD Events Across Concordant/Discordant Groups by Medians: The ARIC Study (1996–2016)
Figure S1. Proportions of hsCRP greater than and less than or equal to median in single, dual, triple, quadruple, and quintuple lipid group less than and greater than or equal to median.
Figure S2. Proportions of hsCRP greater than and ≤2 mg/L in single, dual, triple, quadruple, and quintuple lipid groups less than and greater than or equal to JUPITER cut points.
Figure S3. Kaplan–Meier curves for event‐free survival of incident heart failure by hsCRP less than and greater than or equal to median among individuals with triple (A), quadruple (B) lipid measures less than median (left) and greater than or equal to median (right).
Figure S4. Kaplan–Meier curves for event‐free survival of total mortality by hsCRP less than and greater than or equal to median among individuals with triple (A), quadruple (B) lipid measures less than median (left) and greater than or equal to median (right).


