Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is characterized by high arrhythmic burden and progressive heart failure, which can prompt referral for heart transplantation. Cardiopulmonary exercise testing (CPET) has an established role in risk stratification for advanced heart failure therapies, but has not been described in ARVC/D. This study sought to determine the safety and prognostic utility of CPET in patients with ARVC/D.
Methods and Results
Using the Johns Hopkins ARVC/D Registry, we examined patients with ARVC/D undergoing CPET. Baseline characteristics and transplant‐free survival were compared on the basis of peak oxygen consumption (pVO2) (≤14 or >14 mL/kg per minute) and ventilatory efficiency (Ve/VCO 2 slope ≤34 or >34). Thirty‐eight patients underwent 50 CPETs. There were no sustained arrhythmic events. Twenty‐nine patients achieved a maximal test. Patients with pVO2 ≤14 mL/kg per minute were more often men (P=0.042) compared with patients with pVO2 >14 mL/kg per minute. Patients with Ve/VCO 2 slope >34 tended to have more moderate/severe right ventricular dilation (7/9 [78%] versus 10/26 [38%]; P=0.060) and clinical heart failure (8/9 [89%] versus 13/26 [50%]; P=0.056) compared with patients with Ve/VCO 2 slope ≤34. Patients who underwent heart transplantation were more likely to have clinical heart failure (10/10 [100%] versus 13/28 [46%]; P=0.003). Patients with Ve/VCO 2 slope >34 had worse transplant‐free survival compared with patients with Ve/VCO 2 slope ≤34 (n=35; hazard ratio, 6.57 [95% CI, 1.28–33.72]; log‐rank P=0.010), whereas transplant‐free survival was similar on the basis of pVO2 groups (n=29; hazard ratio, 3.38 [95% CI, 0.75–15.19]; log‐rank P=0.092).
Conclusions
CPET is safe to perform in patients with ARVC/D. Ve/VCO 2 slope may be used for risk stratification and guide referral for heart transplantation in ARVC/D.
Keywords: arrhythmias, cardiomyopathy, exercise testing, genetics, heart failure, transplantation
Subject Categories: Arrhythmias, Heart Failure, Cardiomyopathy, Transplantation
Clinical Perspective
What Is New?
This is the first study to demonstrate the safety of cardiopulmonary exercise testing in patients with arrhythmogenic right ventricular (RV) cardiomyopathy/dysplasia, a population of patients in whom many clinicians may be hesitant to recommend exercise testing.
In addition, it shows how traditional parameters from cardiopulmonary exercise testing, such as peak oxygen consumption, may be less beneficial in this unique population. Instead, ventilatory efficiency may have utility in risk stratifying patients with arrhythmogenic RV cardiomyopathy/dysplasia.
What Are the Clinical Implications?
Arrhythmogenic RV cardiomyopathy/dysplasia is a rare disorder, but with increasing recognition, cascade screening, and implantable cardioverter‐defibrillator implantation, patients with arrhythmogenic RV cardiomyopathy/dysplasia are surviving longer and progressing toward heart failure.
Given the unique nature of this predominantly RV cardiomyopathy, the presentation and management of these patients differs from patients with traditional heart failure.
Therefore, risk stratification and timely therapeutic interventions can be challenging. Cardiopulmonary exercise testing provides an objective tool in the clinician's arsenal to potentially identify higher‐risk patients appropriate for heart transplant referral.
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a genetic cardiomyopathy marked by fibrofatty replacement of cardiomyocytes, resulting in right ventricular (RV) dysfunction and life‐threatening ventricular arrhythmias.1, 2, 3 Sudden cardiac death was a frequent initial presentation of disease but with increasingly prompt recognition and intervention (such as implantation of cardiac defibrillators), patients are living longer to develop other progressive manifestations, primarily right‐ and left‐sided heart failure (HF).2, 4, 5, 6
Exercise intolerance is a cardinal manifestation of HF. Cardiopulmonary exercise testing (CPET) allows assessment of maximal exercise capacity by measuring peak oxygen consumption (pVO2), as well as ventilatory patterns during submaximal exercise.7 CPET can be used to inform prognosis and patient selection for advanced HF therapies, such as cardiac transplantation and ventricular assist devices.8, 9 pVO2 is the best‐studied variable in HF, but more recently ventilatory efficiency (Ve/VCO2 slope) has been shown to have prognostic implications, particularly in RV cardiomyopathies.10, 11, 12, 13
The role of CPET has not been described in patients with ARVC/D. This may be because of the rarity of the disease, hesitancy to refer because of perceived arrhythmic risk during exercise, and limited understanding of application of CPET in RV predominant disease states. The aim of this study was to demonstrate the safety and prognostic ability of CPET in a large US ARVC/D cohort. Specifically, we hypothesized that CPET is safe to perform and that Ve/VCO2 slope may serve as a prognostic marker in patients with ARVC/D.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
The Johns Hopkins ARVC/D Program Registry was established in 1999 and prospectively enrolls patients referred for possible ARVC/D and their family members. We queried the registry and included patients who (1) met the 2010 Revised Task Force Criteria for ARVC/D by last follow‐up, (2) had undergone CPET at any point in their disease course, and (3) were aged ≥18 years at the time of CPET. The Johns Hopkins Institutional Review Board approved the study protocol. Written, informed consent was obtained from each patient.
Clinical Data Collection
Baseline demographic and clinical data, including ARVC/D presentation, comorbidities, symptoms, medications, implantable cardioverter‐defibrillator (ICD), imaging studies, and clinical events, were obtained from the ARVC/D registry accessed on August 15, 2018. The data set includes medical records and patient questionnaires about major clinical events, which are updated prospectively at yearly intervals after patient enrollment. For the subset of patients who underwent a CPET, additional chart review and medical record collection were performed up to data query date of August 15, 2018. Almost all the patients had multiple transthoracic echocardiograms during the study period. Data from the transthoracic echocardiogram closest in absolute time, either before or after, to CPET were used to assess ventricular function, as follows: (1) RV dilation and dysfunction were qualitatively assessed and categorized as normal/mild or moderate/severe on the basis of transthoracic echocardiogram; and (2) left ventricular (LV) dysfunction was defined as LV systolic ejection fraction <45%, and LV diastolic dimension was measured in the parasternal long axis on transthoracic echocardiogram. The presence of HF at the time of CPET was determined using patient symptoms and physical examination findings for HF during clinical encounters, as we have previously described.4 HF signs and symptoms included shortness of breath, dyspnea on exertion, fatigue, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, abdominal swelling/ascites, S3 summation gallop, jugular venous distention, and rales. Life‐threatening ventricular arrhythmias were defined as a composite of spontaneous sustained ventricular tachycardia (VT), appropriate ICD intervention, sudden cardiac arrest, or sudden cardiac death. The primary outcome was transplant‐free survival.
CPET Data
Cardiopulmonary exercise test result reports were abstracted and included if VO2 testing and respiratory exchange ratio (RER) data were available. Patient height and weight, pulmonary function parameters, absolute and normalized pVO2, percentage of predicted pVO2, VO2 at anaerobic threshold, Ve/VCO2 slope, RER, exercise protocol used, peak heart rate, and reason for stopping were recorded for each CPET. Adverse events were adjudicated from CPET visit documentation, which routinely includes patient symptoms and procedural complications, CPET ECG, and chart review of subsequent clinical encounters. If a patient underwent multiple CPETs, all CPETs were used for safety analysis; however, only the patient's last CPET was used for the remainder of analyses. A test was considered submaximal if RER <1.05 and thus excluded from peak VO2 analyses. On the basis of established prognostic CPET variable cutoffs, subjects were divided into groups by pVO2 (≤14 or >14 mL/kg per minute)10 and Ve/VCO2 slope (≤34 or >34).8, 13 Sensitivity analyses were also performed using Ve/VCO2 slope cutoff of 36 and percentage of predicted pVO2 (<70% versus ≥70%), given the utility of this latter variable over pVO2 in a younger cohort.
Statistical Analysis
All continuous variables are presented as median and interquartile range (IQR) and categorical variables as numbers (percentages). We performed bivariate analyses to compare baseline variables according to pVO2 and Ve/VCO2 slope categories. In addition, subjects were compared on the basis of whether they met the end point of death or transplant. Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test. Overall and transplant‐free survival rates were estimated using the Kaplan‐Meier method. Differences in survival between groups were evaluated using the log‐rank test. Two‐sided P values were used for all tests, and P<0.050 was considered statistically significant. Statistical analyses were performed using Stata, version 14.2.
Results
Study Population
A total of 50 CPETs were performed in 38 patients meeting the ARVC/D 2010 Revised Task Force Criteria. Eight patients had multiple CPETs over the study period, with the interval between testing ranging from 7 months to 8 years; 2 patients had 3 CPETs, and 1 patient underwent 4 CPETs. Baseline characteristics are described in Table 1. Most patients had an identified pathogenic mutation associated with ARVC/D (n=27 [71%]). In regard to arrhythmic risk, most patients had a history of life‐threatening ventricular arrhythmia before CPET (n=32 [84%]) and were on a β blocker at the time of CPET (n=32 [84%]). Clinical HF was present in 23 patients (61%). LV systolic dysfunction was present in 9 patients (24%). Medical comorbidities were uncommon.
Table 1.
Baseline Characteristics of Patients With ARVC/D Undergoing CPET
| Variable | No Transplant (n=28) | Transplant (n=10) | P Valuea | All Patients (n=38) |
|---|---|---|---|---|
| Men | 13 (46) | 8 (80) | 0.136 | 21 (55) |
| White | 27 (96) | 10 (100) | 1.000 | 37 (97) |
| Age at CPET, y | 38.4 (24.3) | 42.0 (13.3) | 0.765 | 38.8 (17.4) |
| Proband | 25 (89) | 9 (90) | 1.000 | 34 (89) |
| Pathogenic mutation, any | 22 (79) | 5 (50) | 0.116 | 27 (71) |
| Type of mutation | 0.564 | |||
| PKP2 | 10 (36) | 5 (50) | 15 (39) | |
| DSP | 5 (18) | 0 (0) | 5 (13) | |
| DSG2 | 1 (4) | 0 (0) | 1 (3) | |
| DSC2 | 1 (4) | 0 (0) | 1 (3) | |
| JUP | 1 (4) | 0 (0) | 1 (3) | |
| TMEM43 | 1 (4) | 0 (0) | 1 (3) | |
| PLN | 1 (4) | 0 (0) | 1 (3) | |
| CH/HO/DG | 2 (7) | 0 (0) | 2 (5) | |
| Age at presentation, y | 23.0 (22.8) | 29.9 (16.8) | 0.507 | 26.0 (22.0) |
| Age at meeting task force criteria, y | 31.1 (23.3) | 33.1 (18.7) | 0.974 | 31.1 (22.0) |
| No. of major criteria met | 3.0 (2.0) | 3.0 (1.0) | 0.657 | 3.0 (2.0) |
| No. of minor criteria met | 3.0 (2.5) | 3.0 (3.0) | 0.695 | 3.0 (3.0) |
| Total No. of criteria met | 6.0 (2.5) | 7.0 (4.0) | 0.471 | 6.0 (3.0) |
| Type of presentation | 0.362 | |||
| Sudden cardiac arrest | 2 (7) | 1 (10) | 3 (8) | |
| Symptomatic | 20 (71) | 9 (90) | 29 (76) | |
| Asymptomatic | 6 (21) | 0 (0) | 6 (16) | |
| Hypertension | 1 (4) | 1 (10) | 0.462 | 2 (5) |
| Coronary artery disease | 0 (0) | 0 (0) | 1.000 | 0 (0) |
| Cerebrovascular accident | 1 (4) | 0 (0) | 1.000 | 1 (3) |
| Diabetes mellitus | 0 (0) | 0 (0) | 1.000 | 0 (0) |
| Hyperlipidemia | 2 (7) | 2 (20) | 0.279 | 4 (11) |
| β Blocker | 25 (89) | 7 (70) | 0.310 | 32 (84) |
| ACEi/ARB | 14 (54) | 6 (60) | 1.000 | 21 (55) |
| Aldosterone receptor blocker | 6 (21) | 3 (30) | 0.673 | 9 (24) |
| Antiarrhythmic | 13 (46) | 7 (70) | 0.278 | 20 (53) |
| ICD | 23 (82) | 9 (90) | 1.000 | 32 (84) |
| Clinical heart failure | 13 (46) | 10 (100) | 0.003 | 23 (61) |
| LVEF, % | 55 (13) | 55 (15) | 0.310 | 55 (15) |
| LVEF <45% | 6 (21) | 3 (30) | 0.673 | 9 (24) |
| Left ventricular end diastolic diameter, cm | 5.0 (0.7) | 4.9 (0.4) | 0.416 | 4.9 (0.7) |
| Moderate/severe RV dysfunction | 13 (46) | 6 (60) | 0.714 | 19 (50) |
| Moderate/severe RV dilation | 11 (39) | 8 (80) | 0.062 | 19 (50) |
Categorical variables are expressed as number (percentage). Continuous variables are expressed as median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; CPET, cardiopulmonary exercise testing; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; RV, right ventricle.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
CPET Safety
CPET protocols used were as follows: 28 staged cycle, 6 Bruce treadmill, 4 Naughton treadmill, 3 unspecified treadmill, and 9 unknown. Most CPETs were performed with patients having an ICD (n=43/50 [86%]). There were no deaths, sustained arrhythmias, ICD interventions, or other life‐threatening events during or immediately after any CPET. CPET was terminated in 3 (6%) because of heart rate approaching ICD threshold for ventricular arrhythmia therapies. Arrhythmias were nonsustained, infrequent, and asymptomatic, as follows: premature ventricular contractions (n=7 [14%]), bigeminy (n=2 [4%]), and nonsustained VT (n=2 [4%]).
CPET Parameters and Clinical Characteristics
CPET performance characteristics are shown in Table 2. Median normalized pVO2 was 21.1 (IQR, 9.8) mL/kg per minute and median Ve/VCO2 slope was 30.0 (IQR, 8.3). Twenty‐nine patients achieved an RER ≥1.05, and 35 patients had Ve/VCO2 slope reported. The 9 submaximal tests (RER <1.05) were limited because of fatigue (n=2), heart rate approaching ICD therapy threshold (n=2), dyspnea/leg tingling, dizziness, patient fear of arrhythmia, per patient request, and unknown.
Table 2.
CPET Characteristics in Patients With ARVC/D
| Characteristic | No Transplant (n=28) | Transplant (n=10) | P Valuea | Total (n=38) |
|---|---|---|---|---|
| Absolute pVO2, mL/min | 1697 (766) | 1232 (1056) | 0.037 | 1659 (785) |
| Normalized pVO2, mL/kg per min | 22.6 (10.9) | 15.8 (10.3) | 0.006 | 21.1 (9.8) |
| Predicted VO2, % | 73.0 (34.6) | 55.0 (31.3) | 0.084 | 71.5 (36.1) |
| Ve/VCO2 slope (n=35) | 29.0 (5.6) | 37.2 (12.3) | <0.001 | 30.0 (8.3) |
| VO2 at anaerobic threshold (n=32), mL/kg per min | 14.7 (6.2) | 11.5 (5.6) | 0.068 | 13.0 (5.3) |
| RER | 1.14 (0.14) | 1.08 (0.11) | 0.353 | 1.10 (0.13) |
| Peak HR, beats per min | 155 (51) | 121 (31) | 0.111 | 140 (51) |
| Predicted HR, % | 82 (22) | 68 (17) | 0.101 | 81 (24) |
| Submaximal test (RER <1.05) | 6 (21) | 3 (30) | 0.673 | 9 (24) |
All variables are expressed as median (interquartile range) and n=38, unless noted. ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; CPET, cardiopulmonary exercise testing; HR, heart rate; pVO2, peak VO2; RER, respiratory exchange ratio; Ve/VCO2 slope, ventilatory efficiency; VO2, oxygen consumption.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
When compared with patients with pVO2 >14 mL/kg per minute, those with pVO2 ≤14 mL/kg per minute were more often men (10/25 [40%] versus 4/4 [100%]; P=0.042) but there were no observed differences in age, mutation presence, comorbidities, or presence of clinical HF (Table 3). Patients with pVO2 ≤14 mL/kg per minute had higher Ve/VCO2 slope compared with patients with pVO2 >14 mL/kg per minute (51.0 [IQR, 26.4] versus 29.6 [IQR, 6.1]; P=0.009). In the sensitivity analysis using percentage of predicted pVO2 (<70% versus ≥70%), baseline differences were notable for higher proportion of men (8/11 [73%] versus 6/18 [33%]; P=0.042) and larger LV diastolic dimension (5.1 [IQR, 1.0] versus 4.8 [IQR, 0.8] cm; P=0.016) in the group with pVO2 <70% (Table 4).
Table 3.
Comparison of Patient Characteristics Based on Normalized pVO2 Category
| Characteristic | pVO2 ≤14 mL/kg per min (n=4) | pVO2 >14 mL/kg per min (n=25) | P Valuea |
|---|---|---|---|
| Men | 4 (100) | 10 (40) | 0.042 |
| White | 4 (100) | 24 (96) | 0.862 |
| Age at CPET, y | 42.1 (14.4) | 38.2 (23.8) | 0.343 |
| Proband | 3 (75) | 23 (92) | 0.371 |
| Pathogenic mutation, any | 1 (25) | 17 (68) | 0.139 |
| Age at presentation, y | 41.0 (22.0) | 25.8 (21.2) | 0.312 |
| Age at meeting task force criteria, y | 41.0 (22.0) | 30.8 (20.1) | 0.487 |
| No. of major criteria met | 3.5 (2.0) | 3.0 (2.0) | 0.897 |
| No. of minor criteria met | 3.0 (1.5) | 3.0 (3.0) | 0.846 |
| Total No. of criteria met | 6.0 (3.5) | 6.0 (3.0) | 0.749 |
| Type of presentation | 1.000 | ||
| Sudden cardiac arrest | 0 (0) | 1 (4) | |
| Symptomatic | 4 (100) | 19 (76) | |
| Asymptomatic | 0 (0) | 5 (20) | |
| β Blocker | 2 (50) | 22 (88) | 0.127 |
| ACEi/ARB | 3 (75) | 11 (44) | 0.330 |
| Aldosterone receptor blocker | 0 (0) | 6 (24) | 0.553 |
| Antiarrhythmic | 1 (25) | 13 (52) | 0.598 |
| ICD | 3 (75) | 20 (80) | 1.000 |
| Clinical heart failure | 4 (100) | 13 (52) | 0.121 |
| LVEF, % | 47.5 (22.5) | 55.0 (5.0) | 0.255 |
| LVEF <45% | 2 (50) | 4 (16) | 0.180 |
| Left ventricular end diastolic diameter, cm | 4.9 (0.1) | 4.94 (0.7) | 0.824 |
| Moderate/severe RV dysfunction | 3 (75) | 11 (44) | 0.330 |
| Moderate/severe RV dilation | 4 (100) | 11 (44) | 0.100 |
| Absolute pVO2, mL/min | 877 (301) | 1911 (594) | |
| Normalized pVO2, mL/kg per min | 11.7 (0.7) | 22.8 (8.4) | |
| Predicted VO2 (n=27), % | 30.5 (17) | 83.2 (38) | |
| Ve/VCO2 slope (n=26) | 51.0 (26.4) | 29.6 (6.1) | 0.009 |
| VO2 at AT (n=26), mL/kg per min | 8.4 (1.6) | 15.0 (6.2) | 0.006 |
| Respiratory exchange ratio | 1.17 (0.16) | 1.16 (0.10) | 0.727 |
| Peak heart rate, beats per min | 130 (24) | 150 (37) | 0.311 |
| Predicted heart rate, % | 75.5 (18.6) | 82 (19) | 0.569 |
Categorical variables are expressed as number (percentage). Continuous variables are expressed as median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AT, anabolic threshold; CPET, cardiopulmonary exercise testing; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; pVO2, peak VO2; RV, right ventricle; Ve/VCO2 slope, ventilatory efficiency; VO2, oxygen consumption.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
Table 4.
Comparison of Patient Characteristics Based on Percentage Predicted pVO2
| Characteristic | pVO2 >70% (n=18) | pVO2 ≤70% (n=11) | P Valuea |
|---|---|---|---|
| Men | 6 (33) | 8 (73) | 0.042 |
| White | 17 (94) | 11 (100) | 0.621 |
| Age at CPET, y | 39.6 (28.7) | 38.3 (14.6) | 0.857 |
| Proband | 18 (100) | 8 (73) | 0.371 |
| Pathogenic mutation, any | 11 (61) | 7 (64) | 0.139 |
| Age at presentation, y | 29.1 (22.5) | 25.8 (24.0) | 0.857 |
| Age at meeting task force criteria, y | 35.2 (22.7) | 30.8 (22.0) | 0.686 |
| No. of major criteria met | 3.0 (2.0) | 3.0 (2.0) | 0.729 |
| No. of minor criteria met | 3.0 (3.0) | 4.0 (3.0) | 0.872 |
| Total No. of criteria met | 6.0 (2.0) | 7.0 (3.0) | 0.480 |
| Type of presentation | 0.598 | ||
| Sudden cardiac arrest | 1 (6) | 0 (0) | |
| Symptomatic | 15 (83) | 8 (73) | |
| Asymptomatic | 2 (11) | 3 (27) | |
| β Blocker | 15 (83) | 9 (82) | 0.127 |
| ACEi/ARB | 8 (44) | 6 (55) | 0.330 |
| Aldosterone receptor blocker | 3 (17) | 3 (27) | 0.553 |
| Antiarrhythmic | 9 (50) | 5 (45) | 0.598 |
| ICD | 13 (72) | 10 (91) | 1.000 |
| Clinical heart failure | 8 (44) | 9 (82) | 0.121 |
| LVEF, % | 58 (5) | 55 (23) | 0.071 |
| LVEF <45% | 2 (11) | 4 (36) | 0.180 |
| Left ventricle end diastolic diameter, cm | 4.8 (0.8) | 5.1 (1.0) | 0.016 |
| Moderate/severe RV dysfunction | 7 (39) | 7 (64) | 0.330 |
| Moderate/severe RV dilation | 8 (44) | 7 (64) | 0.100 |
| Absolute pVO2, mL/min | 1843 (892) | 1554 (676) | 0.150 |
| Normalized pVO2, mL/kg per min | 25.6 (14.6) | 19.0 (9.2) | 0.002 |
| Ve/VCO2 slope | 30.0 (4.2) | 29.6 (13.5) | 0.916 |
| VO2 at AT (n=26), mL/kg per min | 15.9 (7.1) | 11.8 (5.6) | 0.016 |
| Respiratory exchange ratio | 1.16 (0.09) | 1.15 (0.20) | 0.822 |
| Peak heart rate, beats per min | 160 (25) | 127 (23) | 0.025 |
| Predicted heart rate, % | 85 (10) | 70 (18) | 0.021 |
Categorical variables are expressed as number (percentage). Continuous variables are expressed as median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AT, anabolic threshold; CPET, cardiopulmonary exercise testing; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; pVO2, peak VO2; RV, right ventricle; Ve/VCO2 slope, ventilatory efficiency; VO2, oxygen consumption.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
When compared by Ve/VCO2 slope, patients with Ve/VCO2 slope ≤34 (n=26) had no observed differences from patients with Ve/VCO2 slope >34 (n=9) in regard to age, sex, race, or mutation presence (Table 5). There was a trend toward patients with Ve/VCO2 slope >34 more frequently having clinical HF (8/9 [89%] versus 13/26 [50%]; P=0.056) and more moderate/severe RV dilation (7/9 [78%] versus 10/26 [38%]; P=0.060). Those with Ve/VCO2 slope >34 also had a lower pVO2 than those with Ve/VCO2 ≤34 (15.9 [IQR, 10.6] versus 21.8 [IQR, 12.0] mL/kg per minute; P=0.025). Sensitivity analysis using higher Ve/VCO2 cutoff (≤36 versus >36) is described in Table 6 and showed similar results as well as a statistically significant higher degree of RV dilation in the Ve/VCO2 slope >36 group. Invasive hemodynamic data were available in 17 patients. A statistically significant inverse correlation between cardiac output and Ve/VCO2 slope was noted, with r 2=0.5 and P=0.002.
Table 5.
Comparison of Patient Characteristics Based on Ve/VCO2 Slope Category
| Characteristic | Ve/VCO2 Slope ≤34 (n=26) | Ve/VCO2 Slope >34 (n=9) | P Valuea |
|---|---|---|---|
| Men | 13 (50) | 6 (67) | 0.460 |
| White | 25 (96) | 9 (100) | 0.740 |
| Age at CPET, y | 38.8 (24.3) | 42.9 (14.0) | 0.706 |
| Proband | 23 (88) | 8 (89) | 1.000 |
| Pathogenic mutation, any | 19 (73) | 6 (67) | 0.694 |
| Age at presentation, y | 26.1 (21.1) | 26.2 (18.8) | 0.678 |
| Age at meeting task force criteria, y | 33.3 (23.4) | 26.2 (20.4) | 0.850 |
| No. of major criteria met | 3.0 (2.0) | 3.0 (1.0) | 0.110 |
| No. of minor criteria met | 3.0 (3.0) | 3.0 (1.0) | 0.427 |
| Total No. of criteria met | 6.0 (3.0) | 6.0 (4.0) | 0.717 |
| Type of presentation | 0.410 | ||
| Sudden cardiac arrest | 2 (8) | 1 (11) | |
| Symptomatic | 18 (69) | 8 (89) | |
| Asymptomatic | 6 (23) | 0 (0) | |
| β Blocker | 23 (88) | 7 (78) | 0.586 |
| ACEi/ARB | 16 (62) | 4 (44) | 0.451 |
| Aldosterone receptor blocker | 6 (23) | 2 922) | 1.000 |
| Antiarrhythmic | 12 (46) | 6 (67) | 0.443 |
| ICD | 22 (85) | 9 (100) | 0.553 |
| Clinical heart failure | 13 (50) | 8 (89) | 0.056 |
| LVEF, % | 55 (15) | 55 (10) | 0.847 |
| LVEF <45% | 6 (23) | 2 (22) | 1.000 |
| Left ventricle end diastolic diameter, cm | 5.1 (0.8) | 4.8 (0.6) | 0.059 |
| Moderate/severe RV dysfunction | 13 (50) | 4 (44) | 1.000 |
| Moderate/severe RV dilation | 10 (38) | 7 (78) | 0.060 |
| Absolute pVO2, mL/min | 1743 (740) | 1255 (344) | 0.006 |
| Normalized pVO2, mL/kg per min | 21.8 (12.0) | 15.9 (10.6) | 0.025 |
| Predicted peak VO2, % | 73 (35) | 60 (36) | 0.071 |
| VO2 at AT (n=32), mL/kg per min | 13.5 (5.4) | 10.2 (6.1) | 0.041 |
| Respiratory exchange ratio | 1.10 (0.16) | 1.11 (0.11) | 0.692 |
| Peak heart rate, beats per min | 152 (52) | 121 (20) | 0.395 |
| Predicted heart rate, % | 82 (25) | 68 (17) | 0.282 |
Categorical variables are expressed as number (percentage). Continuous variables are expressed as median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AT, anabolic threshold; CPET, cardiopulmonary exercise testing; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; pVO2, peak VO2; RV, right ventricle; Ve/VCO2 slope, ventilatory efficiency; VO2, oxygen consumption.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
Table 6.
Comparison of Patient Characteristics Based on Ve/VCO2 Slope Category Using 36 as the Cutoff
| Characteristic | Ve/VCO2 Slope ≤36 (n=29) | Ve/VCO2 Slope >36 (n=6) | P Valuea |
|---|---|---|---|
| Men | 14 (48) | 5 (83) | 0.460 |
| White | 28 (97) | 6 (100) | 1.000 |
| Age at CPET, y | 38.6 (21.0) | 43.1 (25.5) | 0.358 |
| Proband | 26 (90) | 5 (83) | 1.000 |
| Pathogenic mutation, any | 21 (72) | 4 (67) | 0.694 |
| Age at presentation, y | 23.9 (21.2) | 33.4 (20.4) | 0.484 |
| Age at meeting task force criteria, y | 31.5 (22.7) | 33.4 (20.4) | 0.965 |
| No. of major criteria met | 3.0 (2.0) | 4.0 (2.0) | 0.039 |
| No. of minor criteria met | 3.0 (3.0) | 3.0 (3.0) | 0.964 |
| Total No. of criteria met | 6.0 (3.0) | 8.0 (4.0) | 0.215 |
| Type of presentation | 0.586 | ||
| Sudden cardiac arrest | 3 (10) | 0 (0) | |
| Symptomatic | 20 (69) | 6 (100) | |
| Asymptomatic | 6 (21) | 0 (0) | |
| β Blocker | 25 (86) | 5 (83) | 0.586 |
| ACEi/ARB | 17 (59) | 3 (50) | 0.451 |
| Aldosterone receptor blocker | 7 (24) | 1 (17) | 1.000 |
| Antiarrhythmic | 14 (48) | 4 (67) | 0.443 |
| ICD | 25 (86) | 6 (100) | 0.553 |
| Clinical heart failure | 16 (55) | 5 (83) | 0.056 |
| LVEF, % | 55 (15) | 55 (10) | 0.755 |
| LVEF <45% | 7 (24) | 1 (17) | 1.000 |
| Left ventricle end diastolic diameter, cm | 5.0 (0.6) | 4.7 (0.7) | 0.048 |
| Moderate/severe RV dysfunction | 13 (45) | 4 (67) | 1.000 |
| Moderate/severe RV dilation | 11 (38) | 6 (100) | 0.060 |
| Absolute pVO2, mL/min | 1682 (695) | 1236 (344) | 0.049 |
| Normalized pVO2, mL/kg per min | 22.5 (9.3) | 14.0 (5.1) | 0.022 |
| Predicted VO2, % | 73 (32) | 49 (41) | 0.025 |
| VO2 at AT (n=32), mL/kg per min | 13.7 (4.7) | 9.43 (2.6) | 0.032 |
| Respiratory exchange ratio | 1.10 (0.16) | 1.10 (0.07) | 0.948 |
| Peak heart rate, beats per min | 144 (52) | 121 (19) | 0.220 |
| Predicted heart rate, % | 82 (24) | 68 (17) | 0.237 |
Categorical variables are expressed as number (percentage). Continuous variables are expressed as median (interquartile range). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AT, anabolic threshold; CPET, cardiopulmonary exercise testing; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; pVO2, peak VO2; RV, right ventricle; Ve/VCO2 slope, ventilatory efficiency; VO2, oxygen consumption.
Continuous variables were analyzed using Wilcoxon rank‐sum test (Mann‐Whitney U test), and categorical data were analyzed using Fisher exact test.
Long‐Term Outcomes
Median follow‐up time from the last CPET was 252 (IQR, 555) days. Ten patients (26%) met the end point of heart transplantation; there were 2 deaths, both occurring after heart transplantation. No significant differences in baseline demographics (Table 1) or ability to exercise during CPET (as assessed by heart rate, exercise duration, and RER) (Table 2) were seen between those who did and did not undergo heart transplantation. All 5 patients with pathogenic mutation who also underwent heart transplantation had a PKP2 gene mutation; this, however, was not significantly different than the proportion of patients with a PKP2 mutation who did not receive a transplant. Patients who underwent heart transplantation were more likely to have had clinical HF (10/10 [100%] versus 13/28 [46%]; P=0.003). Eight patients were transplanted for advanced HF (New York Heart Association functional class IV symptoms), one for incessant VT leading to HF, and one for a combination of VT and significant symptomatic RV failure. Of the 10 patients who underwent transplant, 7 were transplanted within 1 year of their last CPET, 2 within 18 months, and 1 not until 4 years later. There was no difference between groups in number of submaximal tests (3/10 [30%] versus 6/28 [21%]; P=0.673).
Kaplan‐Meier curves for transplant‐free survival based on pVO2 and Ve/VCO2 slope are shown in Figure 1. Patients with pVO2 ≤14 mL/kg per minute had similar transplant‐free survival as patients with pVO2 >14 mL/kg per minute (n=29; hazard ratio, 3.38 [95% CI, 0.75–15.19]; log‐rank P=0.092; Figure 1A). Patients with Ve/VCO2 slope >34 had worse transplant‐free survival compared with patients with Ve/VCO2 slope ≤34 (n=35; hazard ratio, 6.57 [95% CI, 1.28–33.72]; log‐rank P=0.010; Figure 1B). There was no difference based on percentage predicted pVO2 ≤70% versus >70% (n=29; hazard ratio, 3.27 [95% CI, 0.60–18.00]; log‐rank P=0.148; Figure 2A). Transplant‐free survival remained significantly different with sensitivity analysis using Ve/VCO2 slope cutoff >36 (n=35; hazard ratio, 4.25 [95% CI, 1.06–17.09]; log‐rank P=0.026; Figure 2B).
Figure 1.
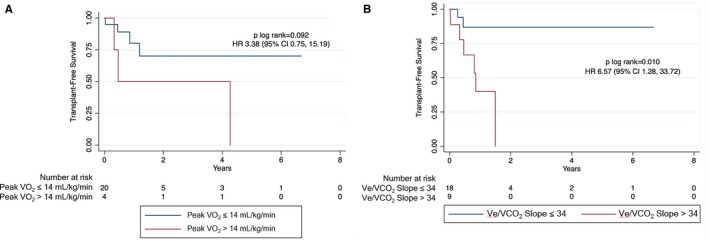
Transplant‐free survival based on peak oxygen consumption (VO 2) (A) and ventilatory efficiency (Ve/VCO 2 slope) (B). Comparison of survival curves for patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia undergoing cardiopulmonary exercise testing based on peak VO 2 (≤14 vs >14 mL/kg per minute) and Ve/VCO 2 slope (≤34 vs >34). HR indicates hazard ratio.
Figure 2.
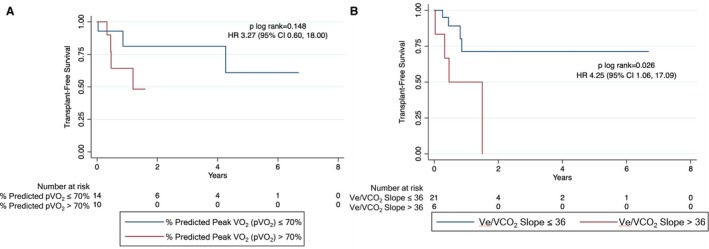
Transplant‐free survival based on percentage predicted peak oxygen consumption (pVO 2) (A) and ventilatory efficiency (Ve/VCO 2 slope) (B). Comparison of survival curves for patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia undergoing cardiopulmonary exercise testing based on percentage predicted pVO 2 (<70% vs ≥70%) and Ve/VCO 2 slope (≤36 vs >36). HR indicates hazard ratio.
Discussion
This study is the first, to our knowledge, to examine CPET in patients with ARVC/D. We describe CPET safety, performance characteristics, and outcomes in this special patient population. We found that CPET is safe to perform even in patients with high arrhythmic burden and history of life‐threatening ventricular arrhythmias. We also demonstrated that Ve/VCO2 slope, rather than pVO2, is associated with clinical HF and transplant‐free survival. This study provides important prognostic insight for patients with ARVC/D who are increasingly presenting with progressive HF as arrhythmic mortality decreases.
Safety of Exercise Testing in ARVC/D
CPET was safe without any sustained arrhythmias in this ARVC/D cohort. Exercise testing is generally considered a safe procedure in appropriately selected patients, with surveys suggesting 0 to 6 deaths or cardiac arrests and 2 to 10 myocardial infarctions per 10 000 tests.7 The ARVC/D population generally lacks coronary artery risk factors, as confirmed in our current cohort, so myocardial infarction is less likely when compared with the broader population referred for exercise testing.
ARVC/D is a disease of the cardiac desmosome, which are specialized adhesion junctions providing the mechanical connection between cardiac myocytes. In this study, two thirds of patients carried a desmosomal mutation and just over half of those were in the PKP2 gene. Alterations in desmosomal structure as well as increased RV wall stress during exercise have been implicated as triggers for arrhythmia in ARVC/D.1, 14 It is well established that non‐ARVC/D patients with severe LV systolic dysfunction also have an increased risk of ventricular arrhythmia.15, 16 Despite this inherent risk, in the HF‐ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training Study), only 2 of 4411 (0.04%) CPETs performed in patients with LV ejection fraction <35% were complicated by ventricular arrhythmia, and only 27 (0.6%) were stopped for nonsustained VT.17 However, compared with our ARVC/D cohort, in which two thirds had a prior life‐threatening arrhythmia, in HF‐ACTION only 23% of those with an ICD had a history of ICD firing before CPET.17 Despite this, there were no sustained ventricular arrhythmias observed during or immediately after CPET in our cohort. Premature ventricular contractions (PVCs) may be seen more commonly, for example, at a rate of 55% in a large study of non‐HF patients referred for exercise testing primarily for ischemia.18 In our study, 14% had PVCs noted during testing. Overall, the rate of arrhythmic events in our cohort was similar to or lower than in previous studies in other populations.
Exercise alone can worsen arrhythmic outcomes in ARVC/D, and work from our group has demonstrated the relationship between longitudinal exercise exposure and disease progression, which has resulted in guidelines for exercise limitation in ARVC/D.14 These guidelines can create hesitation for both clinician referral and patient participation (perhaps contributing to several submaximal tests in our cohort) for CPET risk stratification. Therefore, this is an appropriate setting to rely more so on submaximal parameters, such as Ve/VCO2 slope. Also, the ill effects of exercise on ARVC/D are related to longer‐term exercise exposure (in units of hours per year) and aerobic intensity, whereas the exercise required for CPET is of short duration (average exercise time of 10.5 minutes in present study). Of note, in our study, 76% (29 patients) were able to perform a maximal test (RER ≥1.05).
Ve/VCO2 Slope and RV Cardiomyopathy
Early work on CPET in HF mostly focused on pVO2 in LV systolic dysfunction, although correlation of pVO2 with survival has also been seen in disease models with pure RV systolic dysfunction.19 However, interpretation of pVO2 is limited by need for maximal exercise effort, which was not achieved in 24% of our ARVC/D cohort. In ARVC/D in particular, as discussed above, achievement of an adequate RER can be limited for several reasons, including patient counseling to avoid maximal exercise, concern for arrhythmia or ICD intervention at higher heart rates, and heavy β blockade (84% of patients on β blocker in this study) and antiarrhythmic use. Although percentage predicted pVO2 may be a better measure in a younger patient population (such as ARVC/D) than absolute normalized pVO2, our sensitivity analysis using percentage predicted pVO2 did not demonstrate predictive ability and this measurement also relies on maximum exercise. Therefore, a submaximal CPET parameter may be more suited to use in the ARVC/D population (namely, Ve/VCO2 slope).
Although there is an established relationship between RV function and Ve/VCO2 slope, to date, most analyses have been limited to patients with concomitant left‐sided HF and/or those with pulmonary hypertension and RV pressure overload.13, 20, 21 In one such study, Lewis et al studied 30 patients with left‐sided heart disease with simultaneous CPET and invasive hemodynamic monitoring and showed an inverse correlation between Ve/VCO2 slope and RV ejection fraction, as measured using radionuclide ventriculography.13 It is less clear, however, if Ve/VCO2 slope increases as a result of pulmonary vascular disease, RV dysfunction, or both. Our study presented the unique opportunity to study this relationship in a patient population enriched with intrinsic RV pathological features, not caused by RV pressure overload from left‐sided heart disease or pulmonary vascular disease. Interestingly, we found an inverse relationship between pulmonary artery systolic pressure and Ve/VCO2 slope (r 2=0.27; P=0.031). However, the clinical significance of this finding may be limited, as none of our patients had truly elevated pulmonary artery pressure (pulmonary artery systolic pressure range, 11–32 mm Hg; mean pulmonary artery pressure, all <20 mm Hg) and we only had a subset of patients with invasive hemodynamic data.
Although our data did not demonstrate a clear relationship between RV function and Ve/VCO2 slope, we were limited by relying on clinical echocardiography and resting state measurements of RV function. Indeed, there is increasing evidence that RV reserve may be a more robust way to assess RV function, correlating better with both symptoms and outcomes.22, 23, 24, 25, 26 Using multibeat pressure‐volume relations, the gold standard to assess ventricular function, Hsu and colleagues found an inverse relationship between Ve/VCO2 slope and both RV–pulmonary artery coupling and RV reserve.21 Guazzi et al studied 97 patients with HF and grouped them first on the basis of whether they had normal RV function using tricuspid annular plane systolic excursion and then on the basis of whether those with abnormal tricuspid annular plane systolic excursion improved with exercise.20 They demonstrated not only increasing Ve/VCO2 slope with impaired RV reserve but also a higher percentage of symptomatic HF (based on New York Heart Association class) in the reduced RV reserve group. One population that can demonstrate similar physiological features to ARVC/D is adult congenital heart disease with RV involvement, with or without pulmonary vascular changes. DeFaria Yeh et al studied a heterogeneic group of 147 patients with adult congenital heart disease and found that RV reserve, measured using exercise CPET and radionuclide ventriculography, was a powerful predictor of event‐free survival.27
These studies, combined with our present data, suggest that impaired RV reserve may be contributing to HF symptoms in ARVC/D. Pathologically, contractile cardiomyocytes are replaced by noncontractile fibrous and fatty tissue in ARVC/D, thus decreasing the ability of the heart to accommodate increased demands of exercise, with possible resultant symptoms. As we demonstrated, worse Ve/VCO2 slope tended to correlate with clinical HF, emphasizing the importance of recognizing symptomatic HF in patients with ARVC/D because this may be a marker for future need for advanced therapies.4 In addition, longer‐term management can be aided by Ve/VCO2 slope by supplementing the subjective symptoms with objective parameters of cardiac function.
Limitations
The main limitations of this study stem from its observational and retrospective design, reliance on registry data, and small sample size. Given the referral nature of our ARVC/D program and the rarity of the disease, we elected to include CPETs done at other institutions. This limits our ability to propose any conclusions based on exercise time or protocol, which can impact pVO2 measurements.28 After exclusion of submaximal tests for outcome analyses, we were left with a small number in the group with pVO2 ≤14 mL/kg per minute, likely limiting our ability to detect significant differences between groups if they exist. Last, our assessment of RV function was based on echocardiogram at rest rather than during exercise. Given the potential role of impaired RV reserve in ARVC/D, future studies should incorporate dynamic assessment of RV function during CPET under a standardized protocol for exercise and RV assessment. Despite these limitations, this is the first study to report safety, clinical characteristics, and outcomes of patients with ARVC/D undergoing CPET.
Conclusions
CPET is safe to perform in patients with arrhythmogenic RV cardiomyopathy/dysplasia. In addition, Ve/VCO2 slope is associated with transplant‐free survival and allows submaximal testing in a patient cohort who may hesitate to perform maximal exercise. With increasing incidence of HF in ARVC/D, this study encourages providers to incorporate CPET into risk stratification of these patients. Future prospective multicenter studies are needed to further elucidate the prognostic value of serial CPET in ARVC/D.
Sources of Funding
The Johns Hopkins ARVD/C Program is supported by the Leonie‐Wild Foundation (Heidelberg, Germany), the Dr Francis P. Chiaramonte Private Foundation (Alexandria, VA), the Leyla Erkan Family Fund for ARVD Research, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins (Baltimore, MD), the Bogle Foundation, the Healing Hearts Foundation, the Campanella Family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments. The authors also wish to acknowledge a grant from the Fondation Leducq’ (Dr Calkins) (Paris, France).
Disclosures
None of the authors has conflicts to disclose relevant to the present work. Dr Scheel, Dr Florido, Dr Hsu, B. Murray, J. Agafonova, Dr Russell, and Dr Gilotra report no nonrelevant disclosures. Dr Tandri is part of the speaker bureau for Abbott. Dr Judge reports payments from Alnylam, Pfizer, GSK, and Blade Therapeutics as a scientific advisor and clinical trial support from Array Biopharma and Eidos Therapeutics. Dr Tedford is on the research advisory board for Abiomed. Dr Calkins is a consultant for Medtronic Inc and St Jude Medical/Abbott. Dr Calkins receives research support from Boston Scientific Corp. C. Tichnell and Dr James receive salary support from this grant. Dr James has received a lecture fee from Abbott.
Acknowledgments
We are grateful to the patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and families who have made this work possible.
(J Am Heart Assoc. 2020;9:e013695 DOI: 10.1161/JAHA.119.013695.)
References
- 1. Calkins H. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ J. 2015;79:901–913. [DOI] [PubMed] [Google Scholar]
- 2. Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. [DOI] [PubMed] [Google Scholar]
- 3. Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Am Coll Cardiol. 2018;72:784–804. [DOI] [PubMed] [Google Scholar]
- 4. Gilotra NA, Bhonsale A, James CA, te Riele ASJ, Murray B, Tichnell C, Swant A, Ong CS, Judge DPJ, Russell SD, Calkins H, Tedford RJ. Heart failure is common and under‐recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail. 2017;10:1–9. [DOI] [PubMed] [Google Scholar]
- 5. Lemola K, Brunckhorst C, Helfenstein U, Oechslin E, Jenni R, Duru F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: long term experience of a tertiary care centre. Heart. 2005;91:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruiz‐Salas A, Cabrera‐Bueno F, García‐Pinilla JM, Barrera‐Cordero A, Pena‐Hernandez J, Fernandez‐Pastor J, Medina‐Palomo C, Alzueta‐Rodriguez J. Long‐term prognosis of patients with arrhythmogenic right ventricular cardiomyopathy and implantable defibrillator. Int J Cardiol. 2014;174:794–796. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 8. Malhotra R, Bakken K, D'Elia E, Lewis G. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4:607–616. [DOI] [PubMed] [Google Scholar]
- 9. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckerman A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant. 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 10. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 11. Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Pina IL, Leifer ES, Fleg JL, Blackbum G, Fonarow GC, Chase PJ, Piner L, Vest M, O'Connor CM, Ehrman JK, Walsh MN, Ewald G, Bensimhon D, Russel SD. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. 2016;67:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJS. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2 . Eur Heart J. 2000;21:154–161. [DOI] [PubMed] [Google Scholar]
- 13. Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age‐related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy–associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadish A, Dyer A, Daubert JP, Quigg R, Estes M, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylatic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 16. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylatic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 17. Keteyian SJ, Isaac D, Thadani U, Roy BA, Bensimhon DR, McKelvie R, Russell SD, Hellkamp AS, Kraus WE. Safety of symptom‐limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J. 2009;158:S72–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dewey FE, Kapoor JR, Williams RS, Lipinski MJ, Ashley EA, Hadley D, Myers J, Froelicher VF. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med. 2008;168:225–234. [DOI] [PubMed] [Google Scholar]
- 19. Müller J, Hager A, Diller GP, Derrick G, Buys R, Dubowy KO, Takken T, Orwal S, Inuzuka R, Vanhees L, Gatzoulis M, Giardini A. Peak oxygen uptake, ventilatory efficiency and QRS‐duration predict event free survival in patients later after surgical repair of tetralogy of Fallot. Int J Cardiol. 2015;196:158–164. [DOI] [PubMed] [Google Scholar]
- 20. Guazzi M, Villani S, Generati G, Ferraro OE, Pellegrino M, Alfonzetti E, Labate V, Gaeta M, Sugimoto T, Bandera F. Right ventricular contractile reserve and pulmonary circulation uncoupling during exercise challenge in heart failure: pathophysiology and clinical phenotypes. JACC Heart Fail. 2016;4:625. [DOI] [PubMed] [Google Scholar]
- 21. Hsu S, Houston B, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, Damico RL, Kolb TM, Hummers LK, Shah AA, McMahan Z, Corona‐Villalobos CP, Zimmerman SL, Wigley FM, Hassoun PM, Kass DA, Tedford RJ. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133:2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grunig E, Tiede H, Enyimayew EO, Ehlken N, Seyfarth HJ, Bossone E, D'Andrea A, Jaeije R, Olschewski H, Ulrich S, Nagel C, Halank M, Fischer C. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–2015. [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto K, Tanaka H, Onishi A, Motoji Y, Tatsumi K, Sawa T, Miyoshi T, Imanishi J, Mochizuki Y, Hirata K. Bi‐ventricular contractile reserve offers an incremental prognostic value for patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:1213–1223. [DOI] [PubMed] [Google Scholar]
- 24. Borlaug BA, Kane GC, Melenovksy V, Olson TP. Abnormal right ventricular‐pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3294–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma T, Lau EMT, Choudhary P, Torzillo PJ, Munoz PA, Simmons LR, Naeije R, Celermajer DS. Dobutamine stress for evaluation of right ventricular reserce in pulmonary arterial hypertension. Eur Respir J. 2015;45:700–708. [DOI] [PubMed] [Google Scholar]
- 26. Hsu S, Kokkonen‐Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, Mukherjee M, Shah AA, Wigley FM, Margulies KB, Hassoun PM, Halushka MK, Tedford RJ, Kass DA. Right ventricular myofilament functional differences in humans with systemic sclerosis‐associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137:2360–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeFaria Yeh D, Stefanescu Schmidt AC, Eisman AS, Serfas JD, Naqvi M, Youniss MA, Ryfa AD, Khan AA, Safi L, Tabtabai SR, Bhatt AB, Lewis GB. Impaired right ventricular reserve predicts adverse cardiac outcomes in adults with congenital right heart disease. Heart. 2018;0:1–7. [DOI] [PubMed] [Google Scholar]
- 28. Lockwood PA, Yoder JE, Deuster PA. Comparison and cross‐validation of cycle ergometry estimates of VO2max . Med Sci Sports Exerc. 1997;29:1513–1520. [DOI] [PubMed] [Google Scholar]


