Abstract
Background
Catheter ablation is an effective treatment for atrial fibrillation (AF), but high levels of post‐procedure inflammation predict adverse clinical events. Ascorbic acid (AA) has shown promise in reducing inflammation but is untested in this population. We sought to test the feasibility, safety, and preliminary effects on inflammatory biomarkers in the CITRIS‐AF (Vitamin C Intravenous Treatment In the Setting of Atrial Fibrillation Ablation) pilot study.
Methods and Results
Patients scheduled to undergo AF ablation (N=20) were randomized 1:1 to double‐blinded treatment with AA (200 mg/kg divided over 24 hours) or placebo. C‐reactive protein and interleukin‐6 levels were obtained before the first infusion and repeated at 24 hours and 30 days. Pain levels within 24 hours and early recurrence of AF within 90 days were recorded. Median and interquartile range were aged 63 (56–70) years, 13 (65%) men, and 18 (90%) white. Baseline data were similar between the 2 groups except ejection fraction. Baseline C‐reactive protein levels were 2.56 (1.47–5.87) mg/L and similar between groups (P=0.48). Change in C‐reactive protein from baseline to 24 hours was +10.79 (+6.56–23.19) mg/L in the placebo group and +3.01 (+0.40–5.43) mg/L in the AA group (P=0.02). Conversely, change in interleukin‐6 was numerically higher in the AA group, though not statistically significant (P=0.32). One patient in each arm developed pericarditis; no adverse events related to the infusions were seen. There were no significant differences between aggregated post‐procedure pain levels within 24 hours or early recurrence of AF (both P>0.05).
Conclusions
High‐dose AA is safe and well tolerated at the time of AF ablation and may be associated with a blunted rise in C‐reactive protein, although consistent findings were not seen in interleukin‐6 levels. Further studies are needed to validate these findings and explore the potential benefit in improving clinically relevant outcomes.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT03148236.
Keywords: ascorbic acid, atrial fibrillation, catheter ablation, C‐reactive protein, inflammation
Subject Categories: Atrial Fibrillation, Inflammation, Clinical Studies, Catheter Ablation and Implantable Cardioverter-Defibrillator
Atrial fibrillation (AF) is highly prevalent, representing the most common arrhythmia worldwide. Transvenous catheter‐based ablation, using either radiofrequency or cryoballoon to perform pulmonary vein isolation, is an important option for treatment of AF.1 However, ≈35% of patients who undergo AF ablation have recurrence within 90 days, a phenomenon which may be caused by the intensity of the inflammatory response following catheter ablation.2 Early recurrence within 90 days predicts long‐term recurrence of AF.3
Intravenous ascorbic acid (AA) has shown promise as an adjunct treatment strategy in other disease states with acute inflammatory spikes, including sepsis,4 percutaneous coronary intervention,5, 6 and cardiac surgery.7, 8 The effects of AA during AF ablation are untested. We therefore designed a phase I pilot study to test the safety and feasibility of AA treatment at the time of AF ablation, with post‐ablation inflammatory biomarkers as a key end point.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. From September 2017 to March 2018, consecutive patients scheduled for a first AF ablation at the Virginia Commonwealth University Health System were screened for eligibility. Inclusion criteria included age ≥21 years and willingness to provide written consent. Exclusion criteria were prior ablation for AF, active renal calculus, malignancy within 5 years, autoimmune or autoinflammatory disease, and recent or active use of immunosuppressive medications. Patients with diabetes mellitus requiring the use of insulin or with glycosylated hemoglobin ≥8% were also excluded in this pilot study because of the potential for high doses of intravenous AA to interfere with point of care glucose testing.
The study was approved by the Institutional Review Board at Virginia Commonwealth University and overseen by an independent Data Safety Monitoring Board. Patients were approached over the phone in the weeks leading up to their scheduled ablation procedure and provided written informed consent on the morning of the ablation. Baseline basic metabolic profile, C‐reactive protein (CRP, measured with a high‐sensitivity assay), interleukin‐6 (IL‐6), von Willebrand Factor, and plasma ascorbate levels were obtained the day of the ablation as the patients were being prepared for the procedure. Following this, subjects were randomized 1:1 in a double‐blinded fashion to treatment with intravenous AA (50 mg/kg administered every 6 hours for a total of 4 doses, with the first dose completed before the ablation procedure) or matched placebo. Repeat laboratory results were obtained at 24 hours and 30 days following ablation. Pain levels were assessed within the first 18 hours following ablation, and recurrence of AF was recorded for all patients to 90 days before unblinding. Patients were screened for any adverse events at all study points (24 hours, 30 days, and 90 days, as well as 6 and 12 months by phone calls), with particular attention to any events which could be attributed to AA infusions such as allergic reactions, renal calculus, or interference with point‐of‐care glucose testing. All adverse events were assessed by the investigators for severity and potential relationship with the study infusions. The randomization order was created by the investigational pharmacy using a computer‐generated sequence in blocks of 10, which was kept blinded from the study investigators until all biomarker data were collected and 90‐day follow up was completed. To maintain blindness, the pharmacy staff prepared all infusions with a shroud to prevent investigators from discerning any pigmentation of the solution which would identify the AA infusions. Additionally, the plasma ascorbate levels were kept blinded from the investigators until the randomization sequence was unlocked.
Descriptive summaries of continuous measurements are reported as median and interquartile ranges; descriptive summaries of categorical measurements are reported as frequencies and proportions. Continuous and categorical variables were assessed between allocation groups using the Mann‐Whitney U test and Fisher exact test, respectively. Wilcoxon Signed Rank test was used to analyze within‐group changes from baseline. Spearman rank order test was used to evaluate correlations between continuous variables. Unadjusted P values are reported throughout, with statistical significance set at the 2‐tailed 0.05 level. The Statistical Package for Social Studies software 25.0 (IBM, Armonk, NY) was used for all analyses.
Results
Twenty patients provided written informed consent and were enrolled into the study. Baseline demographics in each group are shown in Table. Subjects were predominantly in their early 60s and white, with relatively equal split in sex. There was a slightly higher left ventricular ejection fraction in the ascorbic acid group and higher use of cryoballoon ablation in the placebo group, but otherwise baseline data were similar between the 2 groups, including AA and CRP levels. One subject in the AA group had insufficient blood volume obtained to measure 24‐hour IL‐6 levels and was unable to return for the 30‐day biomarker draw because of logistical issues; otherwise there was no loss of follow‐up, no crossover between infusion allocations, and no early termination of the infusions.
Table 1.
Baseline Characteristics
| Placebo (n=10) | Ascorbic Acid (n=10) | P Value | |
|---|---|---|---|
| Epidemiological background | |||
| Age, y | 65 (54–70) | 60.5 (56–69) | 0.796 |
| Men | 7 (70%) | 6 (60%) | 0.639 |
| BMI, kg/m2 | 31 (25–35) | 31 (27–34) | 0.631 |
| Smoking | 2 (20%) | 0 (0%) | 0.136 |
| Hypertension | 4 (40%) | 8 (80%) | 0.068 |
| Diabetes mellitus | 0 (0%) | 0 (0%) | 1.000 |
| Coronary artery disease | 1 (10%) | 0 (0%) | 0.305 |
| Heart failure | 2 (20%) | 1 (10%) | 0.531 |
| Valvular heart disease | 1 (10%) | 0 (0%) | 0.305 |
| Background medications | |||
| Beta blocker | 7 (70%) | 6 (60%) | 0.639 |
| ACE‐inhibitor/ARB | 1 (10%) | 4 (40%) | 0.121 |
| Calcium channel blocker | 2 (20%) | 4 (40%) | 0.329 |
| Statin | 4 (40%) | 4 (40%) | 1.000 |
| Oral vitamin C supplement | 1 (10%) | 1 (10%) | 1.000 |
| Echocardiographic and laboratory parameters | |||
| LV ejection fraction (%) | 55 (45–55) | 60 (56–60) | 0.035 |
| LA diameter, mm | 3.8 (3.5–4.4) | 4.0 (4.0–4.9) | 0.432 |
| LA area, cm2 | 26 (17–27) | 22 (16–27) | 0.556 |
| Ascorbic acid level, μmol/L | 48.3 (35.5–70.4) | 50.5 (35.2–58.5) | 1.000 |
| CRP, mg/L | 3.16 (1.95–8.20) | 2.59 (1.47–5.19) | 0.481 |
| IL‐6, pg/mL | 1.5 (1.2–2.5) | 1.6 (0.8–3.1) | 0.905 |
| vWF, μg/mL | 16.3 (13.7–20.4) | 13.0 (11.3–15.6) | 0.278 |
| Procedure‐related parameters | |||
| Procedure time, min | 152 (124–164) | 151 (114–190) | 1.000 |
| Ablation time, s | 1756 (1387–2658) | 2464 (1620–2778) | 0.481 |
| Mode of ablation | 0.028 | ||
| RFA | 5 (50%) | 9 (90%) | |
| Cryoballoon | 5 (50%) | 0 (0%) | |
| Both | 0 (0%) | 1 (10%) | |
| Acute PV isolation success | 10 (100%) | 9 (90%) | 0.305 |
| Non‐inducibility of AF after ablation | 10 (100%) | 10 (100%) | 1.000 |
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CRP, high sensitivity C‐reactive protein; IL‐6, interleukin‐6; LA, left atrium; LV, left ventricle; PV, pulmonary vein; RFA, radiofrequency ablation; vWF, von Willebrand factor.
Patients treated with AA had a significant rise in AA levels at 24 hours to 321 (156–629) μmol/L, returning to baseline levels by 30 days. There was no significant change at any time point in those receiving placebo.
Subjects allocated to placebo experienced a rise in CRP, from 3.16 (1.95–8.20) mg/L at baseline to 16.0 (9.40–29.29) mg/L at 24 hours; those treated with AA were observed to have a blunted spike in CRP, from 2.59 (1.47–5.19) to 5.31 (4.48–7.92) mg/L, representing changes from baseline of +10.79 (+6.56 to +23.19) and +3.01 (+0.40 to +5.43) mg/L in the placebo and AA groups, respectively (P=0.02, Figure). Both groups experienced increases in interleukin‐6 (IL‐6) levels, from 1.51 (1.17–2.48) and 1.65 (0.84–3.10) pg/mL at baseline to 8.45 (5.10–13.33) and 16.43 (10.06–32.86) at 24 hours for the placebo and AA groups, respectively; though numerically higher in the AA group, the magnitude of IL‐6 change from baseline was not significantly different between groups (P=0.32). There were no significant within‐group or between‐group changes at any time point for von Willebrand factor levels.
Figure 1.
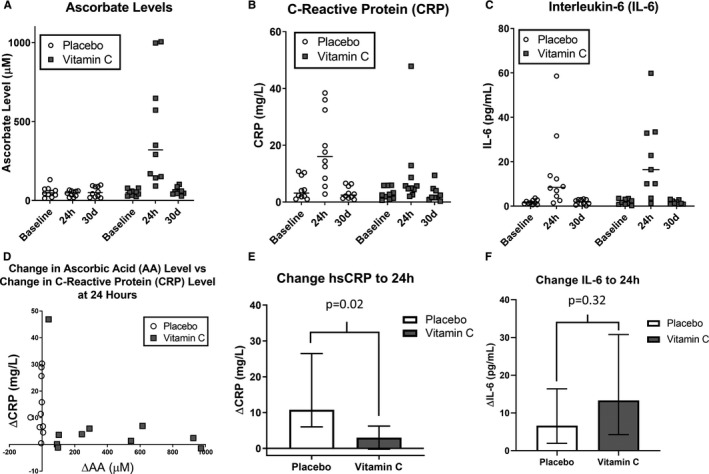
Effects on biomarkers. Trends in ascorbate levels (A), C‐reactive protein (B), and interleukin‐6 (C) based on allocation group at baseline as well as 24 hours and 30 days following ablation. Relationship between changes in ascorbate levels and C‐reactive protein from baseline to 24 hours (D). Changes in C‐reactive protein (E) and interleukin‐6 (F) from baseline to 24 hours based on allocation group. AA indicates ascorbic acid; CRP, C‐reactive protein; hsCRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6. Data are represented as medians and interquartile ranges.
One patient in each group (10%) experienced post‐procedural pericarditis. Sum pain scores within 18 hours of ablation and early recurrence of AF within 90 days (3 in the placebo group, 5 in the AA group, P=0.65) were not significantly different between both groups. There were no allergic reactions, episodes of renal calculus, issues with blood glucose monitoring, or adverse events related to the study infusions.
Discussion
The data obtained from this pilot study establish safety and feasibility of high‐dose intravenous ascorbic acid at the time of AF ablation, while suggesting its potential to modulate the inflammatory response that occurs after the index procedure. Previous investigations have demonstrated that AA administration can reduce markers of oxidative stress, improve microvascular perfusion in patients undergoing percutaneous coronary intervention,5, 6 as well as reduce postoperative rates of AF following cardiac surgery.7 To our knowledge, this is the first evaluation of high‐dose AA in patients undergoing AF ablation.
Blunting the sterile inflammatory response that follows AF ablation could have beneficial effects, including potentially reducing the chances of early or late recurrence of AF. Indeed, the degree of inflammatory biomarker elevation following AF ablation predicts early recurrence of AF,2 which itself predicts long‐term recurrence of AF.3 Prior studies using colchicine have shown promise in reducing inflammatory biomarkers after AF ablation, which has been associated with improved rates of early recurrence in the first study with 161 patients9 and mid‐term recurrence in the follow‐up study with 223 patients.10 While this is an encouraging strategy to use as an adjunct to ablation, the side effect profile, drug‐drug interaction potentials, and real‐world pricing issues involved with colchicine should prompt investigations into alternative anti‐inflammatory agents to improve on these factors.
It is notable that not all prior studies with anti‐inflammatory treatment strategies have shown reductions in recurrent AF. For example, in a randomized clinical trial of 125 patients undergoing AF ablation, Suleiman et al demonstrated that patients who received atorvastatin 80 mg daily starting on postoperative day 1 experienced a decrease in CRP levels at 30 days compared with those randomized to placebo, without significant differences in recurrences in AF.11 Additionally, intravenous AA has previously been studied alone or in combination with other antioxidant supplements; whether the present agent and dosing strategy is most efficacious in achieving the safe anti‐inflammatory effect remains to be determined. Nonetheless, in the present study the AA infusions were initiated before ablation (and the blunted CRP rise noted by postoperative day 1), which may optimally position the treatment strategy to more rapidly resolve early sterile inflammation related to the procedure. Whether this results in clinically relevant improvements (ie, reduced rates of AF recurrence) is unknown.
Limitations to this study include small sample size (limiting power to detect clinically relevant outcomes) and conduct at a single center. Biomarkers were only collected at 24 hours and 30 days following the ablation; it is thus uncertain whether high‐dose AA may have had an effect on IL‐6 levels at day 4, which Deftereos et al found predictive of future recurrence.9 In addition, while there was a significant difference in change in CRP between groups in favor of AA, there was a somewhat discordant finding (albeit not statistically significant) in the IL‐6 values; the significance of this is unclear, and could be elucidated with larger sample sizes as well as additional blood sample time points to more comprehensively describe the rise and fall of the inflammatory biomarkers—and whether those responses may be modified by high‐dose AA. Finally, there was a significantly higher proportion of patients in the placebo group who underwent cryoablation as opposed to radiofrequency ablation, although this might have actually resulted in lower inflammatory biomarker levels in the placebo group because of the expected lower degree of inflammatory response and thus diluted the potential anti‐inflammatory effect of AA. Nonetheless, cryoablation remains an important modality of pulmonary vein isolation for clinical use, and larger sample sizes in future studies that are similarly randomized (perhaps additionally stratified based on anticipated ablation modality) would be better positioned to avoid such differences between treatment groups and remove this confounder. Larger studies, possibly comparing high‐dose intravenous AA with other anti‐inflammatory strategies such as colchicine, are warranted to expand on these findings and explore effects on both short‐ and long‐term clinical outcomes.
Conclusions
High dose ascorbic acid is safe and feasible at the time of catheter ablation for atrial fibrillation and may blunt the rise in CRP 24 hours following the procedure. Future studies are warranted to better characterize the effects of this treatment strategy on the inflammatory response and to explore its effects on clinical outcomes.
Sources of Funding
The study was funded by a grant from the Virginia Commonwealth University Johnson Center for Critical Care and Pulmonary Research. McGuff Pharmaceuticals, Inc. (Santa Ana, CA) provided the study medication (L‐ascorbic acid) at no cost but had no role in the study design, conduct, analysis, or reporting.
Disclosures
None.
Acknowledgments
The authors thank S. Patrick Whalen, MD (Wake Forest University, Winston‐Salem, NC), Antonio Abbate, MD, PhD, and Benjamin W. Van Tassell, PharmD (Virginia Commonwealth University, Richmond, VA) for serving as members of the independent Data Safety Monitoring Board and providing additional safety oversight and review.
(J Am Heart Assoc. 2020;9:e014213 DOI: 10.1161/JAHA.119.014213.)
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Cosedis Nielsen J, Curtis AB, Davies DW, Day JD, D'Avila A, Natasja De Groot NMS, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, MacLe L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim HS, Schultz C, Dang J, Alasady M, Lau DH, Brooks AG, Wong CX, Roberts‐Thomson KC, Young GD, Worthley MI, Sanders P, Willoughby SR. Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:83–89. [DOI] [PubMed] [Google Scholar]
- 3. Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, Deyell MW, Mondesert B, Thibault B, Talajic M, Roy D, Macle L. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:106–116. [DOI] [PubMed] [Google Scholar]
- 4. Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pignatelli P, Tanzilli G, Carnevale R, Di Santo S, Loffredo L, Celestini A, Proietti M, Tovaglia P, Mangieri E, Basili S, Violi F. Ascorbic acid infusion blunts CD40L upregulation in patients undergoing coronary stent. Cardiovasc Ther. 2011;29:385–394. [DOI] [PubMed] [Google Scholar]
- 6. Wang ZJ, Hu WK, Liu YY, Shi DM, Cheng WJ, Guo YH, Yang Q, Zhao YX, Zhou YJ. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol. 2014;30:96–101. [DOI] [PubMed] [Google Scholar]
- 7. Shi R, Li ZH, Chen D, Wu QC, Zhou XL, Tie HT. Sole and combined vitamin C supplementation can prevent postoperative atrial fibrillation after cardiac surgery: a systematic review and meta‐analysis of randomized controlled trials. Clin Cardiol. 2018;41:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill A, Wendt S, Benstoem C, Neubauer C, Meybohm P, Langlois P, Adhikari NKJ, Heyland DK, Stoppe C. Vitamin C to improve organ dysfunction in cardiac surgery patients—review and pragmatic approach. Nutrients. 2018;10:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, Raisakis K, Bouras G, Angelidis C, Theodorakis A, Driva M, Doudoumis K, Pyrgakis V, Stefanadis C. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. 2012;60:1790–1796. [DOI] [PubMed] [Google Scholar]
- 10. Deftereos S, Giannopoulos G, Efremidis M, Kossyvakis C, Katsivas A, Panagopoulou V, Papadimitriou C, Karageorgiou S, Doudoumis K, Raisakis K, Kaoukis A, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid‐term efficacy and effect on quality of life. Heart Rhythm. 2014;11:620–628. [DOI] [PubMed] [Google Scholar]
- 11. Suleiman M, Koestler C, Lerman A, Lopez‐Jimenez F, Herges R, Hodge D, Bradley D, Cha YM, Brady PA, Munger TM, Asirvatham SJ, Packer DL, Friedman PA. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double‐blind, placebo‐controlled, randomized trial. Heart Rhythm. 2012;9:172–178. [DOI] [PubMed] [Google Scholar]


