Viewed dispassionately, the cardiac loop seems an unconventional, even an odd, evolutionary adaptation. The ventricular U‐turn loop, which occurs in all reptiles, mammals, and birds, forces blood to travel back on itself with a 180° turn between the atrioventricular and the semilunar valves. This U‐turn arrangement is prone to fail, because of relatively small anatomic perturbations that cause left ventricular outflow tract (LVOT) obstruction attributable to systolic anterior motion (SAM) and mitral septal contact.1, 2, 3, 4, 5 In the course of caring for patients with obstructive hypertrophic cardiomyopathy (HCM), we have often wondered about the origin of the ventricular U‐turn loop, whose “failure” is the cause of LVOT pressure gradients and severe symptoms. We review (1) the clinical scenarios of LVOT obstruction and their similarities; (2) as clues to the origin of the ventricular U‐turn loop, we review how it emerges in the developing embryo and when the loop appears in vertebrate evolution; (3) although by its nature a conjectural endeavor because of its remote beginnings, we propose that the ventricular U‐turn loop may add to the pumping efficiency of the heart of lung‐breathing vertebrates, and propose that this may explain why the loop has emerged because of evolutionary pressures. A nuanced understanding of how failure of the loop may emerge, and how its failure causes LVOT obstruction, should promote better treatments. These concepts particularly pertain to optimal septal reduction therapy when it is clinically indicated.
“Loop Failure”: Clinical Scenarios of LVOT Obstruction
Ventricular U‐turn looping in susceptible individuals causes a crucial overlap of the inflow and outflow portions of the left ventricle (LV), resulting in SAM, LVOT obstruction, and secondary mitral regurgitation2, 3, 4, 5(Figure 1, 2 through 3). In these patients, circumstances that decrease the size of the LV cavity, such as dehydration, standing, eating, or Valsalva maneuver, increase the overlap and may increase obstruction. The most commonly observed clinical scenario is in obstructive HCM. Interventricular septal thickening, anterior positioning of the mitral apparatus, and leaflet elongation render patients susceptible to the crucial overlap, and thus to SAM.
Figure 1.
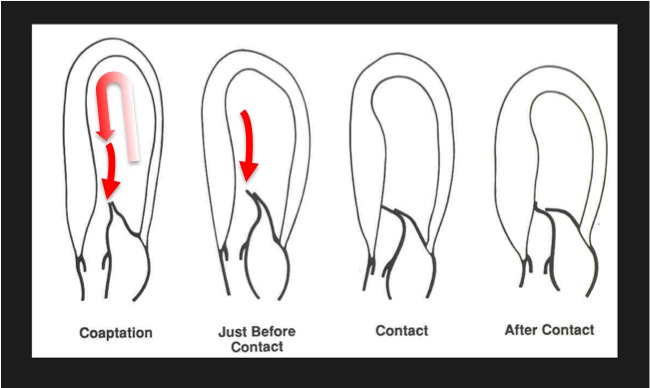
The cardiac loop and systolic anterior motion (SAM). Because of the U‐turn redirection from the cardiac loop, flow turns around mitral valve. Because of the intimate relation of the inflow and outflow portions of the left ventricle, this arrangement is prone to fail, because of relatively small anatomic perturbations that cause SAM. In obstructive hypertrophic cardiomyopathy, the mitral valve is swept into the septum by the pushing force of flow.1, 2, 3, 4, 5
Figure 2.

The cardiac loop. Four‐dimensional magnetic resonance imaging in a normal volunteer showing early diastolic flow (top), late diastolic vortical flow (middle), and early systolic flow (bottom). Mitral valve leaflets are shown in red. Note how the flow turns around the tip of the anterior mitral leaflet. Because of the intimate relation of the inflow and outflow portions of the left ventricle, this U‐turn arrangement is prone to failure. Courtesy of Drs Teodora Chitboi and Leon Axel, NYU Langone Health.
Figure 3.
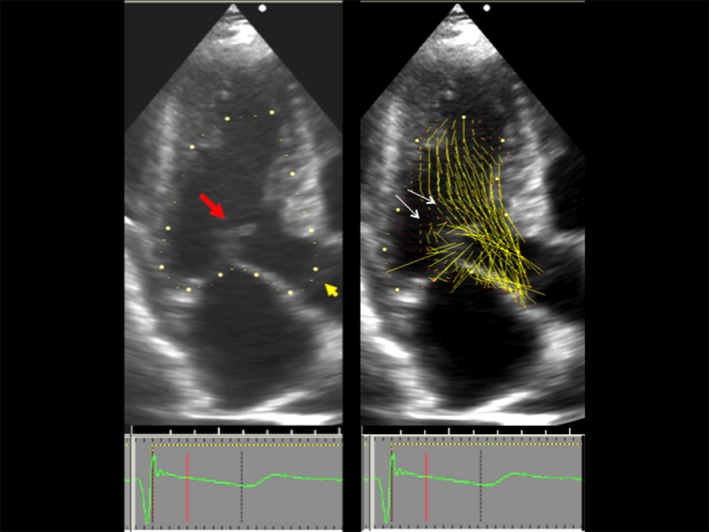
Overlap between left ventricular outflow and the mitral valve. Left, Two‐dimensional echocardiogram, 3‐chamber view, showing the beginning of systolic anterior motion in systole. Red arrow points to mitral valve. Orange arrow points to the still closed aortic valve, also seen closed in the right panel. Right, Vector flow map of the same moment in systole. Local flow velocity is depicted as yellow lines proportional to, and in the direction of, local velocity, indicated by red head of the vector. The overlap between the ejection flow in the outflow tract and the mitral valve is seen. The septal bulge displaces ejection flow so that it comes from a more posterior direction. Note that vector flow impacts the posterior surface of the mitral leaflets with high angle of attack (white arrows).3
Outflow obstruction results in high intraventricular pressures, cardiac work, and supply‐demand myocardial ischemia; these lead to heart failure symptoms, exercise intolerance, ischemic chest pain, and syncope. Dynamic SAM causes mitral insufficiency. Moreover, LVOT obstruction is associated with premature mortality.6 Thus, the clinician caring for patients with obstructive HCM is confronted daily with a “failing” of the ventricular U‐turn loop.7
Current thinking holds that flow drag, the pushing force of flow, is the dominant hydrodynamic force that causes SAM.1, 2, 3, 4, 5, 8, 9 The anatomic perturbations that predispose to SAM are frequently caused by mutations coding for sarcomeric proteins.8 The thickened septum redirects ejection flow so that it comes from relatively posterior and lateral direction in the LV. Frequently, elongated mitral leaflets occur that protrude into the outflow tract, where ejection flow catches them from behind and sweeps them into the septum (Figure 3). The recently developed echocardiographic technique, vector flow mapping, visualizes the interaction of early systolic flow and the mitral valve. Ro et al3 observed color flow and velocity vector flow posterior to the mitral leaflets, impacting them in the early systolic frames of 95% of obstructive HCM, 22% of nonobstructive HCM, and 11% of normal patients. The average overlap was 8.3 mm posterior to coaptation in individuals with obstructive HCM, compared with −0.7 mm in normal subjects.3
Patients who are resistant to pharmacologic therapy are referred for septal reduction. Its history began with the limited myectomy developed in the 1960s, and it was refined by extending it further down into the LV to the level of the papillary muscles and releasing the anterolateral muscle from the anterior wall. This modification explicitly separates the inflow and outflow portions of the LV and allows ejection flow to escape without catching the mitral leaflets.9, 10, 11, 12 The change in surgical technique, which Messmer indicated was promoted by a revised “overlap” understanding of the pathophysiological characteristics of SAM,11 has improved surgical outcomes. Ancillary procedures performed at myectomy release the papillary muscles or transect thickened, fibrotic secondary chordae, allowing the valve to drop posteriorly in the LV. Others directly address the residual leaflet of the anterior mitral leaflet by excision or plication.13 The alternative widely applied procedure, septal thinning with alcohol ablation, also separates the 2 portions of the ventricle.
However, there are other conditions that cause SAM and LVOT obstruction besides obstructive HCM. Certain symptomatic patients may develop LVOT obstruction at rest or after exercise even in the absence of LV thickening; these patients have mitral valve abnormalities that are the substrate for their SAM.14 Mid‐LV obstruction from muscular systolic apposition of the walls is not caused by an overlap of the inflow and outflow portions of the LV and thus not loop failure, as we have characterized it herein.
Other causes of LV thickening can cause SAM and symptomatic LVOT obstruction. Both amyloidosis and Fabry disease can mimic obstructive HCM.15, 16 The salient point is that the morphologic features of loop failure that predispose to SAM are not exclusive to HCM. Any condition that produces overlap of the mitral valve and LV outflow can cause SAM, provided that there is slack in the mitral leaflets.2, 3, 4, 5
After mitral valve repair to relieve severe mitral regurgitation caused by another condition, myxomatous degeneration, patients may develop SAM, a surgical complication. This is attributable to anterior position of the mitral leaflets causing a postoperative overlap between the valve and ejection.17 Therefore, efforts are made to ensure that the mitral coaptation plane is posterior instead of anterior in the LV.
After an emerging technique not yet ready for clinical use, transcatheter mitral valve replacement, the new mitral annular ring may displace the anterior leaflet of the residual native mitral valve into the outflow tract, causing SAM and disastrous LVOT obstruction. Novel strategies have been developed to avoid this complication.
In congenital heart disease, SAM and LVOT obstruction can occur in d‐looped transposition of the great arteries and congenitally corrected transposition of the great arteries, when overlap between the mitral valve and LV outflow occur. The inverted role of ventricles displaces the septum posteriorly. In congenitally corrected transposition of the great arteries, SAM has occurred in the venous LV when a severely dilated systemic right ventricle deviates the septum toward the subpulmonary mitral apparatus.
Ventricular U‐Turn Loop Formation in Higher Vertebrate Embryos: The Phases of Development
The basic design of the ventricular U‐turn loop evolves from the initially straight embryonic heart tube during a process called “cardiac looping” or “ventricular looping.” Subsequent to looping morphogenesis, the basic design of the LV U‐turn loop of the developing hearts of avian and mammalian species is modified in a ventricle‐specific manner by the formation of the interventricular septum and by a process called “wedging.” Wedging brings the developing aortic root into its mature “wedged” position between the tricuspid and mitral valves and, thereby, sets the scene for the development of fibrous continuity between the aortic and mitral valves.18
Manner, elaborating on earlier work of Patten, Taber, and others, described 3 sequential phases of ventricular looping in higher vertebrate embryos: (1) dextral or C‐looping, (2) early S‐looping, and (3) late S‐looping.19, 20, 21, 22, 23, 24, 25, 26, 27, 28 The early embryonic heart has the form of a straight tube harbored within the primitive pericardial cavity and aligned along the craniocaudal body axis. It has a venous pole at its caudal end and an arterial pole at its cranial end. During the phase of dextral C‐looping, the ventricular portion of the initially straight embryonic heart tube becomes deformed into a coiled ventricular loop, the form of which is usually described as being C‐shaped. The convexity of the “C” normally points to the right (dextral) side of the body. The normal loop, therefore, is usually classified as a right‐handed loop or D‐(dextral)‐loop. C‐looping mainly results from 2 biomechanical processes: (1) bending toward the original ventral surface of the ventricular tube and (2) rightward torsion.
The S‐Loop: Ascent of the Atria, Growth‐Induced Buckling, and Intrinsic Bending
During the subsequent phases of S‐looping, the tubular embryonic heart becomes deformed into a looped tube of more complex helical shape, the form of which is usually described in a simplified way as being S‐shaped.19, 29 With respect to the emergence of the ventricular U‐turn loop, 2 processes are of main importance: (1) shortening of the distance between the arterial and venous ends of the heart and (2) shifting of the developing atria from their original position caudal to the ventricles into their final position craniodorsal to the ventricles (“ascent of the atria”). It is the ascent of the atria that establishes the final 180° (U‐turn) ventricular loop found in higher vertebrates. A diagram of the cardiac loop showing the craniodorsal ascent of the atria is shown in Figure 4, and the electron micrographs are shown in Figure 5.21, 30
Figure 4.
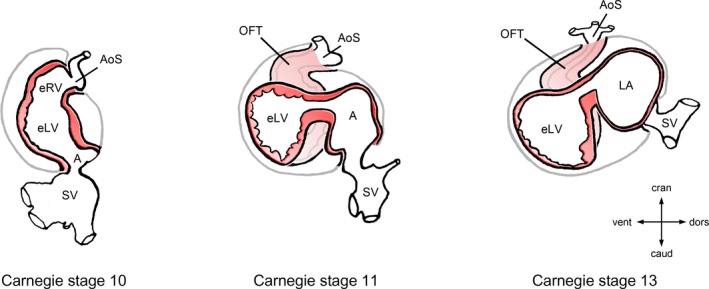
Ascent of the atria during looping morphogenesis of the embryonic heart. This diagram depicts the progressive changes of the topographical relationship between the developing atrial and ventricular chambers of the human embryonic heart during the fourth and fifth week of development (gestational weeks 6 and 7). Hearts are shown in left lateral views. During the initial phase of heart looping (C‐looping; Carnegie stage 10), the embryonic atrium is caudal and dorsal to the embryonic ventricles. During S‐looping (Carnegie stages 11–13), the future atrial chambers are shifted from their original caudal position, first toward a dorsal position (Carnegie stage 11) and, later, toward their definitive position dorsal and cranial to the embryonic ventricles (Carnegie stage 13). The ascent of the atria occurs in all terrestrial vertebrates and sets the scene for establishment of the 180° U turn between the atrioventricular and semilunar valves found in the mature heart of birds and mammals. A indicates embryonic atrium; AoS, aortic sac; caud, caudal; cran, cranial; dors, dorsal; eLV, embryonic left ventricle; eRV, embryonic right ventricle; LA, left half of the common embryonic atrium; OFT, outflow tract; SV, sinus venosus; vent, ventral.
Figure 5.
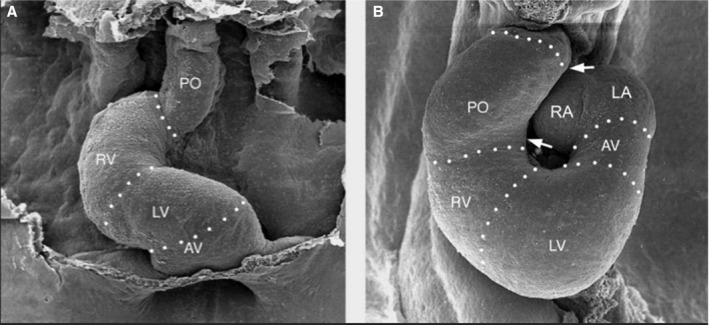
Ascent of the atria. Scanning electron micrographs, viewed from front, show the heart loops of chick embryos at the end of C‐looping (left) and later after early S‐looping (right). Note that at the end of C‐looping, the venous pole of the heart is in a primitive position caudal to the embryonic ventricles. During early S‐looping, the future atrial chambers are shifted from their original caudal position toward their definitive position cranial to the embryonic ventricles. AV indicates atrioventricular canal; LA, left half of the common atrium; LV, embryonic left ventricle; PO, proximal part of outflow tract; RA, right half of the common atrium; RV, embryonic right ventricle. Reprinted from Männer30 with permission. Copyright ©2008, John Wiley and Sons.
The morphogenetic process of cardiac looping does not only occur in higher vertebrate embryos. It also occurs in fish embryos. Compared with higher vertebrates, however, the extent of the atrial ascent during S‐looping is less in fish embryos. S‐looping of the embryonic fish heart places the atrium dorsal to the ventricle and thereby produces only a 90° turn of the ventricular flow path.31
Contemporary mechanistic hypotheses attribute the helical deformations of the tubular embryonic heart to forces inside and outside the heart. This is a modern iteration of an old hypothesis that has been termed the “growth‐induced buckling hypothesis.”19, 29, 32 It is posited that the lengthening embryonic heart becomes too long to be encompassed by the primordial pericardial sack. The length of the fully looped embryonic chick heart is 3.4 times longer than the craniocaudal length of the pericardial sack; the human embryonic heart loop is 2 times longer. The bends permit the enlarging heart to stay intrapericardial. In elastic tube models, elongation of a linear tube within a fixed containment, subjected to axial compressive forces, is deformed in a helical manner, or “buckling.”19, 26, 28, 33, 34 In addition to growth‐induced buckling, bending of the heart tube is also driven by internal biomechanical forces. Intrinsic bending as well as the elongation of the developing heart tube are under the control of various heart‐expressed genes, as indicated by the occurrence of cardiac looping defects in several genetically modified model organisms.31, 35
Besides Compact Packing, Is There Functional Advantage From the Loop?
On a granular level, different mechanisms are proposed for the sequence of bends and twists that are observed, but their evolutionary advantage has not been completely explored. Although the mechanics of the helical structure are yielding their secrets to molecular biology and thoughtful experimentation, the question remains: why bend at all? Romer wrote: “But during development, the anterior part of the heart tube tends to fold back ventrally (and somewhat to the right) in an S‐shaped curve, thus combining length with compact structure,”36 suggesting an advantage of compact structure that would fit in the pericardium. But, the pericardium could have continued to grow in length to accommodate a straight tubular heart. The evolution of the vertebrate heart has been reviewed with an eye toward its successful adaptations37; however, there is limited discussion about what selective advantage atrial ascent and the 180° loop might provide.38 In a recent study, physical models were used to test the physical plausibility of the hypothesis that looping morphogenesis may improve the efficiency of valveless pumping, such as that found in the early embryonic heart tube. In periodically compressed tubular vessels simulating the early embryonic heart tube, a looped tube configuration generated higher maximum pressure heads and higher average flow rates than a straight tube.39 This suggested that the ventricular loop might add to the efficiency of blood transport early in development in the embryonic vertebrate heart, and data from zebrafish embryos have shown that failure of cardiac looping is associated with a decrease in the pumping efficiency of the valveless embryonic heart tube.40, 41
Clues to the selective advantage of the ventricular U‐turn loop in the adult vertebrate heart may be deduced by when it evolved during evolution, and how its particular “plumbing” solution may offer a selective advantage to highly active land‐based, air‐breathing animals. Ascended atria offer advantages to animals subject to the force of gravity.
The Cardiac Loop in Vertebrate Evolution
In lower, water‐living and gill‐breathing, vertebrates, such as sharks and teleost fish, the heart is a 2‐chambered pump with a dorsally‐caudally positioned atrial chamber relative to the ventricular chamber, making a roughly 90° turn from the atrioventricular to the semilunar valves. On the cranial end, the aorta flows ventrally and cranially to the gill loops. In terrestrial vertebrates, the cranial disposition of the aorta is preserved, evolved from the gill loops. But there is now cranial ascent of the atrial chambers.21, 30, 37 It is this atrial ascent, seen in embryonic development during the phase of S‐looping, that causes the 180° U‐turn, the completed cardiac loop (Figure 4, 5 through 6).
Figure 6.
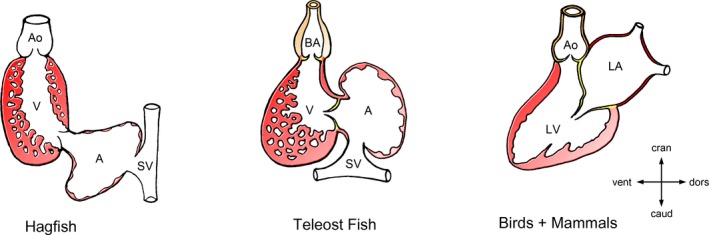
Ascent of the atria during vertebrate evolution. This diagram depicts the progressive modification of the topographical relationship between the atrial and ventricular chambers during vertebrate evolution from primitive fish (hagfish) to higher vertebrates (birds and mammals). Note that there is progressive ascent of the atria from an initially dorsocaudal to a dorsocranial position. The present work posits that evolution has placed the atria cranial and dorsal to the ventricles to harness the force of gravity to promote passive filling of the ventricles. It is the ascent of the atria that establishes U turn of ventricular flow, the cardiac loops. A indicates atrial chamber; Ao, aorta; BA, bulbus arteriosus; caud, caudal; cran, cranial; dors, dorsal; LA, left atrium; LV, left ventricle; SV, sinus venosus; V, ventricular chamber; vent, ventral.
The ventricular U‐turn loop appeared when tetrapods emerged from the sea and became land‐living animals, which developed lungs and a double circuit to provide blood oxygenation, as opposed to gills. Thus, low‐pressure, low‐velocity oxygenated pulmonary venous return occurs at the same point in evolution as the ascent of the atria.
Evolutionary Pressures and the Development of the Ventricular U‐Turn Loop
For both the low‐pressure oxygenated pulmonary venous return and the systemic venous return, we propose that the more craniodorsal atrial position provides a favorable hydrostatic position for animals subject to gravity. This topography may be contrasted with the caudal venous terminus of primitive fish, such as the hagfish, whose structure has remained virtually constant for 300 million years. In the water, where these organisms evolved, the effect of gravity on the blood within the circulatory system is small because the gravitational forces are largely canceled out by the hydrostatic pressure outside of the body. In water, the potential increase in pressure with increasing height of the blood column is counteracted by an increased hydrostatic pressure with increasing depth.42 Land vertebrates have horizontal or upright torsos, depending generally on whether they are quadrupedal or bipedal. Craniodorsal ascent of the atria places these chambers above the ventricles regardless of whether the animal's torso is horizontal or upright (Figure 6). The craniodorsal atrial position satisfies 3 compelling needs of terrestrial vertebrates, summarized in the following paragraphs.
A Low Pressure System
The pulmonary veins and left atrium must function at low pulmonary venous pressure and velocity to protect the pulmonary capillaries; evolution has provided the low‐pressure, low‐velocity system to prevent exudation of fluid into the alveoli. The thin pulmonary capillary membranes facilitate oxygen transfer from the alveoli, and the low pressure prevents leakage of interstitial fluid.37 The frothy bloody secretions of patients with pulmonary edema when pulmonary venous pressure increases bear testimony to evolutionary pressures favoring low pulmonary venous pressure.
Evolution's prevention of this sort of damage can be observed in other species. Snakes provide a living demonstration of how evolutionary adaptations have protected the pulmonary capillary bed from high pressures and gravity, depending on individual habitat. Blood vessels can be thought of as cylindrical tubes filled with fluid. In an upright position, there is a vertical pressure gradient between the top of the tube (low pressure) and the bottom (higher pressure). Thin‐walled veins may become distended, and vessels may pool and leak blood. Snakes vary in the length of their lungs, depending on whether they are aquatic, nonclimbing terrestrial, or tree‐climbing species. In tree snakes that climb upright, evolution has provided short lungs to decrease the distance of the pulmonary venous gravitational column; evolution has provided shorter lungs to decrease the hydrostatic pressure on the pulmonary capillaries.43 Sea snakes, in contrast, function well with longer lungs because gravitational hydrostatic pressure gradients within blood vessels are small in the aquatic environment. However, when sea snakes with long lungs are placed in an upright posture out of the water, they develop pulmonary edema because of the long gravitational column between the central pulmonary veins and the bottom of their lungs. This example underscores why, in terrestrial and upright animals, there is a crucial evolutionary pressure for a low pulmonary venous pressure; however, a low‐pressure, low‐velocity system would not fill the ventricles well if the venous terminus was caudal and dependent.
Harnessing Gravity
Given the obligate requirement of a low pulmonary venous pressure to protect the alveolar capillaries, the benefit of the ascended atria is that they harness gravity to passively fill the ventricles even at low pressure. If the pulmonary venous return was a high‐velocity, high‐pressure system to fill the ventricles, this would not be an issue. Atrial ascended position promotes gravity‐assisted passive filling of the ventricles even at low pressure when the animal is upright during daily activity when evolutionary pressures operate (ie, feeding, fleeing, or fighting). Data examining LV filling in various postures show its dependence on gravity.
Data in the Supine Posture: Gravity Acts Against Pulmonary Venous Acceleration
In the supine dorsal‐down position, gravity operates against filling of the left atrium and LV, but this is not a time when a high metabolic rate, high cardiac output, and related evolutionary adaptations are required. In the supine posture, where blood must flow ventrally to fill the heart, gravity acts against pulmonary vein flow acceleration. Flow velocity from the pulmonary veins into the left atrium is provided by the impetus produced by right ventricular contraction and transmitted through the pulmonary capillary bed.44 Diastolic velocities vary from only 57 to 35 cm/s in 20‐ to 70‐year‐old individuals, respectively.45 In the supine position, acting against gravity, measured diastolic pulmonary venous accelerations range from 3.8 to 2.4 m/s2.45 In contrast, the acceleration of gravity is much higher, 9.8 m/s2. The difference in the magnitude of these accelerations is striking. In the supine position, low pulmonary venous flow velocities render ventricular filling vulnerable to the effects of gravity. A caudal posterior terminus of the pulmonary veins would present an even worse “uphill battle” to fill the heart. Active relaxation and ventricular suction occur, but these early diastolic processes require energy; in contrast, in the upright or prone position, gravity promotes more efficient passive ventricular filling and conservation of energy. This offers a selective advantage because ventricular energy efficiency is crucial because of obligate high cardiac energy consumption.46
Data in the Upright Posture: Gravity Acts in Concert With Flow Velocity
Evolutionary pressures have positioned the atria of the hearts of lung‐breathing vertebrates in a craniodorsal position relative to the ventricles, at the top, rather than at the bottom, of a gravitational well. The craniodorsal position of the left atrium places it above the ventricle, when the torso is horizontal or upright during daily activity when evolutionary pressures are likely to operate, during feeding, fleeing, or fighting. In the upright position, the acceleration of flow from the pulmonary veins and gravity act in concert, not in opposition. The effect of gravity was seen in normal human volunteers in whom transmitral Doppler E‐wave velocities varied with position, from head up, to horizontal supine, to head down because of alterations in preload; E velocity was 80, 73, and 66 cm/s, respectively (P=0.001).47 This variation in preload with tilt shows the dependence of ventricular preload on gravity; fighting gravity would only be aggravated by a caudal location of the atria.
Passive Drainage of the Upper Body
On the systemic venous side, 35% of the systemic venous return comes from the superior vena cava in humans. To the extent that the head is above the heart, this venous blood does not have to fight gravity as it traverses the right atrium and tricuspid valve. Venous return from the legs is pumped back to the heart by the phasic exercise of large muscle contractions that compress the veins and squeeze the blood back to the heart. But this mechanism is not available in the brain; hence, for the brain, there is an advantage of passive drainage, in concert with gravity. With any elevation of the head above the heart, the gravity column extends from the head, through the open tricuspid valve, into the right ventricle, filling it. A caudal position of the atria would be a distinct disadvantage here. Even in a horizontal torso, if the confluence of the vena cavas was in a caudal location, blood returning from the brain and upper torso would have to be pumped or suctioned actively backward from this caudal confluence to the right ventricle. Thus, the advantage of ascent of the right atrium is to avoid circuitous routing of the venous return from the upper body. This circuitous routing would not be an efficient chamber configuration for rapidly ramping up the cardiac output in situations of fight or flight, when selection pressure is greatest.
The vulnerability of the venous return to the effect of gravity is highlighted by the difference in complications between the Glenn and Fontan shunt procedures. Both procedures are used to palliate single‐ventricle physiological characteristics by connecting the superior or both cavas, respectively, directly to the pulmonary artery, bypassing the atrial and ventricular pumps. As such, both shunts are passive conduits. Both shunts are subject to congestion if left atrial or pulmonary pressure increases (eg, because of atrial tachyarrhythmia, LV dysfunction, or increased pulmonary vascular resistance). However, complications are less frequent in the Glenn procedure because gravity promotes passive drainage from the upper body. In contrast, patients undergoing the Fontan procedure are prone to edema, lymphedema, protein‐losing enteropathy, and cirrhosis because venous return must overcome gravity all the way back to the pulmonary artery.48
Microgravity
The importance of this passive drainage mechanism is underscored by the intracranial hypertension observed in astronauts subjected to microgravity.49 Microgravity eliminates the hydrostatic gravitational gradients in the vascular system.50 The puffy face and thinned legs (“puffy face, chicken leg syndrome”) that are observed in astronauts are a direct effect of microgravity caused by a redistribution of fluid from the legs to the upper torso. Astronauts may also experience changes in visual acuity associated with choroidal folds, retinal nerve fiber layer thickening, hyperopic shifts, cotton wool spots, and optic disc edema: the magnetic resonance imaging spectrum of intraorbital and intracranial findings is similar to those in idiopathic intracranial hypertension.49 The causal factor is cephalad fluid shifts, resulting from a loss of hydrostatic gradients on the lower extremity venous system, manifest as facial congestion, and thought to increase intracranial pressure.
In microgravity, despite a decrease in blood volume, there is a marked 40% dilatation of internal jugular vein cross‐sectional area. Loss of hydrostatic pressure from gravity and redistribution of the blood volume result in cephalic and thoracic blood stagnation and even spontaneous internal jugular thrombosis.51, 52, 53 Despite the distinct plethora of the upper body, transmitral Doppler E‐wave velocity decreased during spaceflight, compared with values obtained on the ground before the flight,50 from an average of 76 to 59 cm/s. Microgravity decreases transmitral flow velocities. The converse holds: on terra firma, as described above, with the head up, gravity increases transmitral flow velocities.47 The opposite effects on preload of microgravity versus head‐up position on earth show the vulnerability of ventricular preload to gravity, and hence, by extrapolation, to the cranial versus caudal location of the atria. This contrast supports the hypothesis that ascent of the atria is an adaptation to capture the beneficial effect of gravity for the passive movement into the heart of the systemic and pulmonary venous return. Without gravity, nature's finely crafted system to provide preload falls apart.
Another Hypothesis
Another proposal for the teleology of the ventricular U‐turn loops has been advanced.38 The anatomic restraints of the ventricular loop redirect blood out the LVOT in late diastole and isovolumic systole so that when ejection subsequently begins, blood is already moving toward the aortic valve, thus preserving its momentum. Our hypothesis for the loop's origin does not exclude operation of this explanation; however, its proposed benefit to substantially preserve momentum and energy has been questioned.54
The helical deformation of the embryonic heart tube, which generates the ventricular U‐turn loop, is, furthermore, suspected to act as the base for the development of the helical myocardial band of Torrent‐Guasp that envelops both ventricles in a continuous helical muscle band.55 However, Manner has pointed out that the helical configuration of the S‐shaped embryonic heart loop cannot be related to the spatial configuration of Torrent‐Guasp's helical band. In addition, a study in situs‐inversus hearts showed that the helical orientation of ventricular muscle fibers does not depend on the handedness (solitus or inversus) of the hearts, which is determined during C‐looping.56, 57
Conclusions
The S‐looping of the tubular embryonic heart with ascent of the atria brings them into their approximate final position dorsal and cranial to the primordial ventricles, forming the ventricular U‐turn loop. Although inherently a conjectural endeavor, we propose explanations why evolutionary pressures have determined this location for the atria, all of which are a response to life in a strong gravitational field. First, if the confluence of the vena cavas was in a caudal location, blood returning from the brain and upper torso would have to be pumped or suctioned actively backward from this caudal confluence to the right ventricle. In contrast, in their craniodorsal location, the atria are in a position to harness gravity to fill their respective ventricles. On the left side, the low‐pressure pulmonary venous return, which protects the pulmonary capillaries, is poised in early diastole to flow passively into the LV, as opposed to requiring energy to overcome gravity. On the right side, although the legs have an active pumping mechanism to return blood to the heart, such a mechanism is not present for the upper torso and brain. Evolutionary pressures, resulting in placement of the right atria above the ventricle (and not caudal to it), promote passive emptying of the brain. As an evolutionary adaptation, ascent of the atria, and thus the ventricular U‐turn loop, ensures adequate preload without the expense of energy. This may particularly provide efficiency during periods of high heart rate or threat. The loop's principle liability is its vulnerability to genetic and acquired anatomic perturbations, rendering it vulnerable to LV outflow obstruction because of an overlap between LV ejection flow and the mitral valve. A nuanced understanding of why the loop has developed, how failure of the loop can emerge in patients, and how its failure causes LVOT obstruction should promote better treatments. When septal reduction therapy is clinically necessary, separation of the inflow and outflow portions of the LV results in the best resolution of LVOT obstruction.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e014857 DOI: 10.1161/JAHA.119.014857.)
References
- 1. Jiang L, Levine RA, King ME, Weyman AE. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am Heart J. 1987;113:633–644. [DOI] [PubMed] [Google Scholar]
- 2. Levine RA, Vlahakes GJ, Lefebvre X, Guerrero JL, Cape EG, Yoganathan AP, Weyman AE. Papillary muscle displacement causes systolic anterior motion of the mitral valve: experimental validation and insights into the mechanism of subaortic obstruction. Circulation. 1995;91:1189–1195. [DOI] [PubMed] [Google Scholar]
- 3. Ro R, Halpern D, Sahn DJ, Homel P, Arabadjian M, Lopresto C, Sherrid MV. Vector flow mapping in obstructive hypertrophic cardiomyopathy to assess the relationship of early systolic left ventricular flow and the mitral valve. J Am Coll Cardiol. 2014;64:1984–1995. [DOI] [PubMed] [Google Scholar]
- 4. Schwammenthal E, Levine RA. Dynamic subaortic obstruction: a disease of the mitral valve suitable for surgical repair? J Am Coll Cardiol. 1996;28:203–206. [DOI] [PubMed] [Google Scholar]
- 5. Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;36:1344–1354. [DOI] [PubMed] [Google Scholar]
- 6. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 7. Sherrid MV, Shetty A, Winson G, Kim B, Musat D, Alviar CL, Homel P, Balaram SK, Swistel DG. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first‐line therapy with beta‐blockade or verapamil. Circ Heart Fail. 2013;6:694–702. [DOI] [PubMed] [Google Scholar]
- 8. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2703–2738. [DOI] [PubMed] [Google Scholar]
- 9. Sherrid MV, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy: echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg. 2003;75:620–632. [DOI] [PubMed] [Google Scholar]
- 10. Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy–the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med. 2007;4:503–512. [DOI] [PubMed] [Google Scholar]
- 11. Messmer BJ. Extended myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 1994;58:575–577. [DOI] [PubMed] [Google Scholar]
- 12. Nakatani S, Schwammenthal E, Lever HM, Levine RA, Lytle BW, Thomas JD. New insights into the reduction of mitral valve systolic anterior motion after ventricular septal myectomy in hypertrophic obstructive cardiomyopathy. Am Heart J. 1996;131:294–300. [DOI] [PubMed] [Google Scholar]
- 13. Swistel DG, Sherrid MV. The surgical management of obstructive hypertrophic cardiomyopathy: the RPR procedure‐resection, plication, release. Ann Cardiothorac Surg. 2017;6:423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowin EJ, Maron BJ, Chokshi A, Kannappan M, Arkun K, Wang W, Rastegar H, Maron MS. Clinical spectrum and management implications of left ventricular outflow obstruction with mild ventricular septal thickness in hypertrophic cardiomyopathy. Am J Cardiol. 2018;122:1409–1420. [DOI] [PubMed] [Google Scholar]
- 15. Oh JK, Tajik AJ, Edwards WD, Bresnahan JF, Kyle RA. Dynamic left ventricular outflow tract obstruction in cardiac amyloidosis detected by continuous‐wave Doppler echocardiography. Am J Cardiol. 1987;59:1008–1010. [DOI] [PubMed] [Google Scholar]
- 16. Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM. Prevalence of Anderson‐Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. [DOI] [PubMed] [Google Scholar]
- 17. Loulmet DF, Yaffee DW, Ursomanno PA, Rabinovich AE, Applebaum RM, Galloway AC, Grossi EA. Systolic anterior motion of the mitral valve: a 30‐year perspective. J Thorac Cardiovasc Surg. 2014;148:2787–2793. [DOI] [PubMed] [Google Scholar]
- 18. Kirby ML, Waldo KL. Neural crest and cardiovascular patterning. Circ Res. 1995;77:211–215. [DOI] [PubMed] [Google Scholar]
- 19. Bayraktar M, Manner J. Cardiac looping may be driven by compressive loads resulting from unequal growth of the heart and pericardial cavity: observations on a physical simulation model. Front Physiol. 2014;5:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butler JK, Keith J. An experimental analysis of cardiac loop formation in the chick. 1952. MS Thesis, University of Texas.
- 21. Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec. 2000;259:248–262. [DOI] [PubMed] [Google Scholar]
- 22. Manning A, McLachlan JC. Looping of chick embryo hearts in vitro. J Anat. 1990;168:257–263. [PMC free article] [PubMed] [Google Scholar]
- 23. Patten BM. The formation of the cardiac loop in the chick. Am J Anat. 1922;30:373–397. [Google Scholar]
- 24. Shi Y, Yao J, Xu G, Taber LA. Bending of the looping heart: differential growth revisited. J Biomech Eng. 2014;136:0810021–08100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soufan AT, van den Berg G, Ruijter JM, de Boer PA, van den Hoff MJ, Moorman AF. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res. 2006;99:545–552. [DOI] [PubMed] [Google Scholar]
- 26. Taber LA. Biophysical mechanisms of cardiac looping. Int J Develop Biol. 2006;50:323–332. [DOI] [PubMed] [Google Scholar]
- 27. Taber LA. Morphomechanics: transforming tubes into organs. Curr Opin Genet Dev. 2014;27:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voronov DA, Alford PW, Xu G, Taber LA. The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev Biol. 2004;272:339–350. [DOI] [PubMed] [Google Scholar]
- 29. Manner J. On the form problem of embryonic heart loops, its geometrical solutions, and a new biophysical concept of cardiac looping. Ann Anat. 2013;195:312–323. [DOI] [PubMed] [Google Scholar]
- 30. Männer J. The anatomy of cardiac looping: a step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat. 2009;22:21–35. [DOI] [PubMed] [Google Scholar]
- 31. Lombardo VA, Heise M, Moghtadaei M, Bornhorst D, Männer J, Abdelilah‐Seyfried S. Morphogenetic control of zebrafish cardiac looping by Bmp signaling. Development. 2019;146(22), (pii): dev180091 DOI: 10.1242/dev.180091. [DOI] [PubMed] [Google Scholar]
- 32. Taber LA, Voronov DA, Ramasubramanian A. The role of mechanical forces in the torsional component of cardiac looping. Ann N Y Acad Sci. 2010;1188:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manner J, Seidl W, Steding G. Correlation between the embryonic head flexures and cardiac development: an experimental study in chick embryos. Anat Embryol (Berl). 1993;188:269–285. [DOI] [PubMed] [Google Scholar]
- 34. Ramasubramanian A, Chu‐Lagraff QB, Buma T, Chico KT, Carnes ME, Burnett KR, Bradner SA, Gordon SS. On the role of intrinsic and extrinsic forces in early cardiac S‐looping. Develop Dynamics. 2013;242:801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durst R, Kimberly Sauls K, Peal DS, Slaugenhaupt SA. Mutations in DCHS1 cause mitral valve prolapse. Nature. 2015;525:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romer AS. The Vertebrate Body. 3rd ed. Philadelphia, PA: Saunders; 1962. [Google Scholar]
- 37. Bettex DA, Pretre R, Chassot PG. Is our heart a well‐designed pump? The heart along animal evolution. Eur Heart J. 2014;35:2322–2332. [DOI] [PubMed] [Google Scholar]
- 38. Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000;404:759–761. [DOI] [PubMed] [Google Scholar]
- 39. Hiermeier F, Manner J. Kinking and torsion can significantly improve the efficiency of valveless pumping in periodically compressed tubular conduits: implications for understanding of the form‐function relationship of embryonic heart tubes. J Cardiovasc Develop Dis. 2017;4(4), (pii): E19 DOI: 10.3390/jcdd4040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalogirou S, Malissovas N, Moro E, Argenton F, Stainier DYR, Beis D. Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc Res. 2014;104:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berndt C, Poschmann G, Stühler K, Holmgren A, Bräutigam L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014;2:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandblom E, Axelsson M. The venous circulation: a piscine perspective. Comp Biochem Physiol A. 2007;148:785–801. [DOI] [PubMed] [Google Scholar]
- 43. Lillywhite HB. Snakes, blood circulation and gravity. Sci Am. 1988;259:92–99.3070746 [Google Scholar]
- 44. Firstenberg MS, Greenberg NL, Smedira NG, Prior DL, Scalia GM, Thomas JD, Garcia MJ. Doppler echo evaluation of pulmonary venous‐left atrial pressure gradients: human and numerical model studies. Am J Physiol Heart Circ Physiol. 2000;279:H594–H600. [DOI] [PubMed] [Google Scholar]
- 45. Gentile F, Mantero A, Lippolis A, Ornaghi M, Azzollini M, Barbier P, Beretta L, Casazza F, Corno R, Faletra F, Giagnoni E, Gualtierotti C, Lombroso S, Mattioli R, Morabito A, Pepi M, Todd S, Pezzano A. Pulmonary venous flow velocity patterns in 143 normal subjects aged 20 to 80 years old: an echo 2D colour Doppler cooperative study. Eur Heart J. 1997;18:148–164. [DOI] [PubMed] [Google Scholar]
- 46. Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 47. Voutilainen S. Effects of head‐up and head‐down tilt on the transmitral flow velocities in relation to age: a Doppler echocardiographic study in healthy persons. Clin Physiol. 1994;14:561–567. [DOI] [PubMed] [Google Scholar]
- 48. Day RW, Etheridge SP, Veasy LG, Jenson CB, Hillman ND, Di Russo GB, Thorne JK, Doty DB, McGough EC, Hawkins JA. Single ventricle palliation: greater risk of complications with the Fontan procedure than with the bidirectional Glenn procedure alone. Int J Cardiol. 2006;106:201–210. [DOI] [PubMed] [Google Scholar]
- 49. Zhang LF, Hargens AR. Spaceflight‐induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev. 2018;98:59–87. [DOI] [PubMed] [Google Scholar]
- 50. Hamilton DR, Sargsyan AE, Martin DS, Garcia KM, Melton SL, Feiveson A, Dulchavsky SA. On‐Orbit Prospective Echocardiography on International Space Station Crew. Echocardiography. 2011;28:491‐501. [DOI] [PubMed] [Google Scholar]
- 51. Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short‐ and long‐term head‐down tilt and spaceflights. Eur J Appl Physiol. 2001;86:157–168. [DOI] [PubMed] [Google Scholar]
- 52. Diedrich A, Paranjape SY, Robertson D. Plasma and blood volume in space. Am J Med Sci. 2007;334:80–85. [DOI] [PubMed] [Google Scholar]
- 53. Marshall‐Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón‐Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz‐Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of Jugular Venous Blood Stasis and Thrombosis During Spaceflight. JAMA Netw Open. 2019;2:e1915011 DOI: https://101001/jamnetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watanabe H, Sugiura S, Hisada T. The looped heart does not save energy by maintaining the momentum of blood flowing in the ventricle. Am J Physiol Heart Circ Physiol. 2008;294:H2191–H2196. [DOI] [PubMed] [Google Scholar]
- 55. Torrent‐Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras‐Costa F, Flotats A, Cosin‐Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. [DOI] [PubMed] [Google Scholar]
- 56. Delhaas T, Decaluwe W, Rubbens M, Kerckhoffs R, Arts T. Cardiac fiber orientation and the left‐right asymmetry determining mechanism. Ann N Y Acad Sci. 2004;1015:190–201. [DOI] [PubMed] [Google Scholar]
- 57. Manner J. Ontogenetic development of the helical heart: concepts and facts. Eur J Cardiothorac Surg. 2006;29(suppl 1):S69–S74. [DOI] [PubMed] [Google Scholar]


