Abstract
Background
Although several studies have indicated that lipoprotein(a) is a useful prognostic predictor for patients following percutaneous coronary intervention (PCI), previous observations have somewhat been limited by either small sample size or short‐term follow‐up. Hence, this study aimed to evaluate the impact of lipoprotein(a) on long‐term outcomes in a large cohort of stable coronary artery disease patients after PCI.
Methods and Results
In this multicenter and prospective study, we consecutively enrolled 4078 stable coronary artery disease patients undergoing PCI from March 2011 to March 2016. They were categorized according to both the median of lipoprotein(a) levels and lipoprotein(a) values of <15 (low), 15 to 30 (medium), and ≥30 mg/dL (high). All patients were followed up for occurrence of cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and stroke. During an average of 4.9 years of follow‐up, 315 (7.7%) cardiovascular events occurred. The events group had significantly higher lipoprotein(a) levels than the nonevents group. Compared with the low lipoprotein(a) group, Kaplan–Meier analysis showed that the high lipoprotein(a) group had a significantly lower cumulative event‐free survival rate, and multivariate Cox regression analysis further revealed that the high lipoprotein(a) group had significantly increased cardiovascular events risk. Moreover, adding continuous or categorical lipoprotein(a) to the Cox model led to a significant improvement in C‐statistic, net reclassification, and integrated discrimination.
Conclusions
With a large sample size and long‐term follow‐up, our data confirmed that high lipoprotein(a) levels could be associated with a poor prognosis after PCI in stable coronary artery disease patients, suggesting that lipoprotein(a) measurements may be useful for patient risk stratification before selective PCI.
Keywords: cardiovascular events, coronary artery disease, lipoprotein(a), percutaneous coronary intervention
Subject Categories: Clinical Studies, Lipids and Cholesterol, Cardiovascular Disease
Clinical Perspective
What Is New?
Evidence concerning the association between plasma lipoprotein(a) and cardiovascular outcomes in patients following percutaneous coronary intervention is clinically limited.
In this large‐sample‐size and long‐term follow‐up study, data from real‐world practice suggested the prognostic value of lipoprotein(a) in stable coronary artery disease patients after percutaneous coronary intervention.
What Are the Clinical Implications?
This study provides convincing evidence for the role of lipoprotein(a) in predicting cardiovascular events in patients undergoing percutaneous coronary intervention.
Lipoprotein(a) measurements before selective percutaneous coronary intervention may be valuable for risk stratification and clinical decision making in stable coronary artery disease patients.
Introduction
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality worldwide. Percutaneous coronary intervention (PCI) and statin use have significantly reduced adverse cardiovascular events (CVEs) in patients with CAD.1, 2, 3, 4 However, patients following PCI and who are receiving statin therapy still suffer from a high risk of recurrent CVEs,5, 6, 7 which is referred to as residual cardiovascular risk. To improve long‐term prognosis in post‐PCI patients under statin treatment, first and foremost, further enhancement of risk stratification and identification of high‐risk patients are urgent.
In past decades, multiple biomarkers have been identified and used for risk stratification and outcome prediction, among which lipoprotein(a) is one of the most attractive and promising cardiovascular risk factors.8 As is well known, lipoprotein(a) consists of a low‐density lipoprotein (LDL)‐like particle bound to apolipoprotein(a) and apolipoprotein B100.9 Therefore, it contains structural homology to plasminogen and the most proatherogenic subtype, resulting in enhanced thrombo‐ and atherogenic properties.9 A large number of studies have suggested that elevated plasma lipoprotein(a) levels consequently play an important role in promoting cardiovascular disease (CVD) in primary prevention10, 11, 12 and predicting subsequent CVEs in secondary prevention.9, 13, 14, 15, 16 However, the association between lipoprotein(a) and CVEs requires sustained exploration in different populations for the purpose of elucidating its potential role as a marker of cardiovascular risk and a target for treatment, especially in high‐risk patients according to real clinical practice and guidelines. To date, the association between lipoprotein(a) and cardiovascular outcomes in some special populations, including postmenopausal women, premature CAD, acute coronary syndrome (ACS), diabetes mellitus (DM), familial hypercholesterolemia, and so on, has already been evaluated by studies, including ours.17, 18, 19, 20, 21 Nevertheless, there have been only a few small‐sample‐size studies with no more than 1500 subjects exploring the association between lipoprotein(a) and CVEs in patients after PCI, who still suffer from a high risk of CVEs as stated above.5, 14, 22, 23, 24 Notably, they also did not achieve consistent evidence. Therefore, the prognostic value of lipoprotein(a) in stable CAD patients after PCI is not well established.
Recently, we have demonstrated the clinical significance of plasma lipoprotein(a) in cardiovascular risk prediction in patients with impaired glucose metabolism and familial hypercholesterolemia.19, 20, 25 In the present study, we aimed to further evaluate the impact of lipoprotein(a) on long‐term clinical prognosis of patients with stable CAD following PCI in the era of statins, with a large sample size and long‐term follow‐up.
Methods
We will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
This study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (FuWai Hospital & National Center for Cardiovascular Diseases, Beijing, China). Each participant provided written informed consent before enrollment.
As shown in Figure 1, from March 2011 to March 2016, 6979 patients scheduled for coronary angiography because of angina‐like chest pain and/or positive treadmill exercise test and/or significant stenosis as indicated by coronary computed tomography angiography were recruited consecutively from 3 medical centers, including FuWai hospital, XuanWu Hospital, and AnZhen hospital, according to the same protocol. Blood samples used for testing lipoprotein(a) concentrations were sent to FuWai hospital for unified measurement. On admission, 32 patients declined to participate. Next, based on elevated myocardial enzyme levels (cardiac troponin I, creatine kinase, and creatine kinase‐MB), typical ECG changes, positive findings by coronary angiography, and treatment during hospitalization, 2022 patients without indication of PCI, with failed PCI, or who underwent coronary artery bypass grafting and 665 patients who had ACS were excluded. Furthermore, 161 patients were excluded because of missing detailed laboratory data, uncontrolled decompensated heart failure, unstable hemodynamic status, thyroid dysfunction, infectious or systematic inflammatory disease, severe hepatic and/or renal insufficiency, or malignant disease. During the study, 21 patients were lost to follow‐up. Thus, the resulting population consisted of 4078 stable CAD patients receiving PCI treatment. According to the median of lipoprotein(a) concentrations (15.3 mg/dL), the study population was divided into 2 groups. Next, they were divided into 3 groups defined as low (<15 mg/dL; n=2005), medium’(≥15 and <30 mg/dL; n=826), and high (≥30 mg/dL; n=1247) lipoprotein(a).9, 15, 26 All enrolled patients were prescribed aspirin and clopidogrel before PCI and at least 12 months following PCI, unless contraindicated, and then continued to take aspirin without ischemic or bleeding events.
Figure 1.
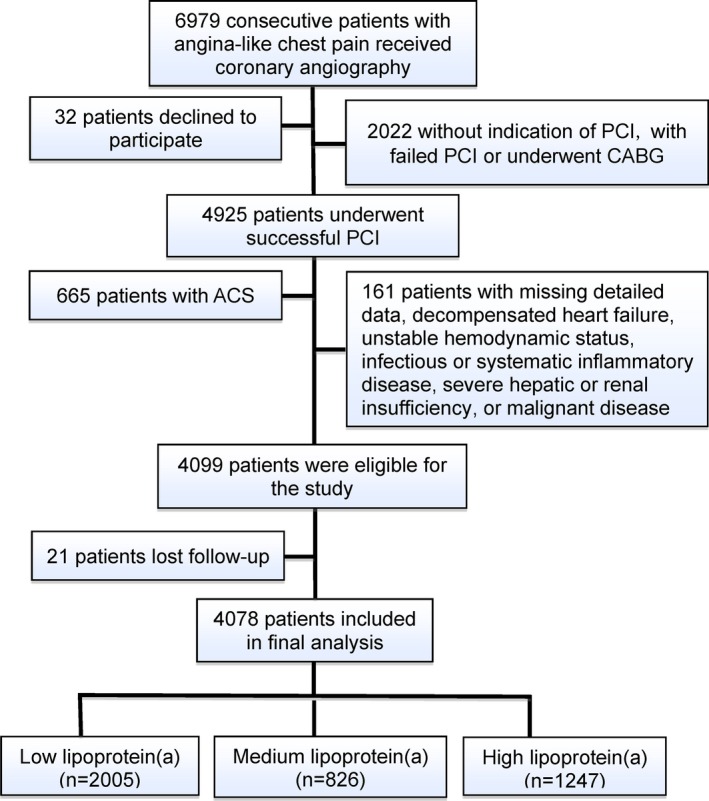
Flowchart illustrating study population. ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Clinical Assessment and Biochemical Analysis
Baseline information on demographic factors, personal health habits, medication use, medical history, and revascularization procedure‐related factors were collected from each patient. Traditional risk factors were defined as follows27: DM was diagnosed by fasting plasma glucose ≥7.0 mmol/L or 2‐hour plasma glucose of the oral glucose tolerance test ≥11.1 mmol/L or currently using hypoglycemic drugs or insulin. Hypertension was defined as a self‐reported hypertension, currently taking antihypertensive drugs, or recorded systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg for ≥3 consecutive times. Smoking was ascertained as subjects who had smoked regularly within the previous 12 months. The definition of baseline medication use was taking drugs continuously for at least 3 months before admission. Medications at follow‐up referred to continuous medication use for at least 3 months before end of follow‐up.
Blood samples for measurement of lipoprotein(a) and other biomarkers were collected from each patient after at least 12 hours of fasting in the morning before PCI (second day after admission). As described in our previous studies,19, 20 lipoprotein(a) was measured by an immunoturbidimetry method (LASAY lipoprotein(a) auto; SHIMA Laboratories Co., Ltd, Tokyo, Japan) with a normal value of <30 mg/dL. A lipoprotein(a) protein‐validated standard was used to calibrate the examination, and intra‐ and interassay coefficients of variation were <10%. LDL cholesterol concentration was analyzed by a selective solubilization method (Low Density Lipid Cholesterol Test Kit; Kyowa Medex, Tokyo, Japan) with a coefficient of variation of <5% and a total imprecision of <10%. The detection limit was 0.026 mmol/L. High‐density lipoprotein cholesterol concentration was determined by a homogeneous method (Determiner L HDL; Kyowa Medex, Tokyo, Japan) with a coefficient of variation of <5% and a total imprecision of <10%. The detection limit was 0.026 mmol/L. Total cholesterol and triglyceride levels were measured by enzymatic assay. Concentrations of glucose were measured by an enzymatic hexokinase method. Glycosylated hemoglobin was measured using the Tosoh Automated Glycohemoglobin Analyser (HLC‐723G8; Tosoh Corporation, Tokyo, Japan). Concentrations of hs‐CRP (high‐sensitivity C‐reactive protein) were determined using immunoturbidimetry (Beckmann Assay 360; Beckman Coulter, Brea, CA), whereas fibrinogen concentration was measured by the Clauss method and a Stagoauto analyzer with an STA Fibrinogen kit (Diagnostica Stago, Taverny, France).
Follow‐up
After enrollment, all patients were actively followed‐up at 6‐month intervals through clinical visits and/or telephone contacts until February 2019 by well‐trained nurses or cardiologists, who were blinded to the aim of this study. Primary end points included cardiovascular death, nonfatal myocardial infarction (MI), and stroke. All available relevant data from any reported possible event were collected. Death of a participant was reported by his or her relatives, the general practitioner, or the specialist who treated the participant. Nonfatal MI was diagnosed as positive cardiac troponins along with typical chest pain or typical ECG serial changes. Stroke was defined as persistent neurological dysfunction with documentation of acute cerebral infarction on computed tomography and/or magnetic resonance imaging. Three experienced cardiologists who were masked to any of the study data classified the events independently.
Statistical Analysis
Continuous variables are expressed as mean±SD or median (interquartile range), as appropriate. The Kolmogorov–Smirnov test was used to test the distribution pattern, and differences between groups were determined using the Student's t test, ANOVA, or nonparametric test, where appropriate. Categorical variables are presented as number (percentage) and analyzed by chi‐squared statistic test or Fisher's exact test. Event‐free survival rates among groups were estimated by the Kaplan–Meier method and compared by the log‐rank test. Uni‐ and multivariate Cox regression analyses were performed to calculate hazard ratios (HRs) and 95% CIs. Additionally, we performed a sensitivity analysis of the association of plasma lipoprotein(a) concentration for prediction of CVEs by 3 methods, that is, separately adjusting for each of the other significant variables in the univariate analysis, excluding subjects with lipoprotein(a) levels in the top or the bottom 5%, and rejecting participants with CVEs developed during the first year. To assess whether adding plasma lipoprotein(a) levels to established cardiovascular risk factors is associated with improvement in prediction of future CVEs, we calculated measures of discrimination for censored time‐to‐event data: Harrell's C‐statistic, the continuous net reclassification improvement, and integrated discrimination improvement.28, 29 Established cardiovascular risk factors included age, sex, current smoking, hypertension, DM, systolic blood pressure, glycosylated hemoglobin, hs‐CRP, triglyceride, LDL cholesterol, number of lesion vessels, and baseline statin use. Two‐tailed P<0.05 was considered statistically significant. Statistical analyses were performed with SPSS software (version 24.0; SPSS Inc., Chicago, IL) and R language (version 3.5.2, Feather Spray; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
In the overall population, lipoprotein(a) levels had a skewed distribution with a tail toward the highest levels, which was consistent with previous studies15, 19 (Figure 2). As presented in Figure 2, according to lipoprotein(a) concentrations, 49.2% of patients were allocated to the low lipoprotein(a) group (<15 mg/dL), 20.2% to the medium lipoprotein(a) group (≥15 and <30 mg/dL), and the remaining 30.6% to the high lipoprotein(a) group (≥30 mg/dL). Baseline and procedural characteristics of study participants are detailed in Table 1. Participants in the high lipoprotein(a) group had lower diastolic blood pressure and fasting plasma glucose levels and a higher percentage of baseline statin use compared with those in the other 2 groups. Of note, the medium lipoprotein(a) group had higher DM incidence and glycosylated hemoglobin levels than the high lipoprotein(a) group. However, the linear trend test did not show a significant relationship between lipoprotein(a) and DM prevalence (P=0.103). Patients with medium lipoprotein(a) levels also had higher hs‐CRP levels compared with those with low lipoprotein(a) concentrations. In addition, total cholesterol, high‐density lipoprotein cholesterol, LDL cholesterol, fibrinogen levels, and numbers of lesion vessels were positively associated with lipoprotein(a) levels, whereas ratios of current smokers (from 48.4% to 43.5%) and calcium‐channel‐blocker use at follow‐up (from 20.1% to 19.0%) were gradually decreased from the low to high lipoprotein(a) groups. There was no significant difference regarding family history of CAD and procedural characteristics, including target vessels, implanted stent numbers, bifurcation or occlusion lesions, and in‐stent restenosis, among the 3 groups.
Figure 2.
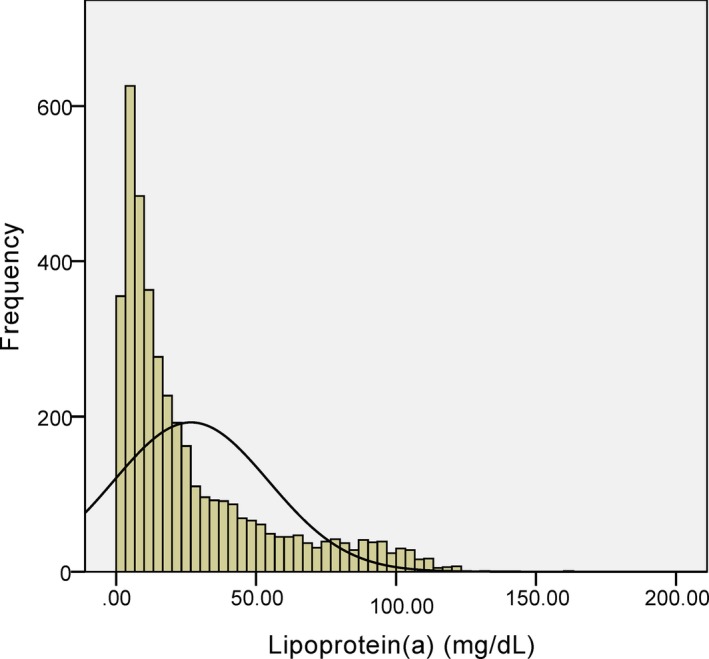
Distribution of lipoprotein(a) levels in the study population.
Table 1.
Clinical and Procedural Characteristics of the Study Participants According to Lipoprotein(a) Levels at Baseline
| Variable | Lipoprotein(a) Categories | |||
|---|---|---|---|---|
| Overall (n=4078) | <15 mg/dL (n=2005) | 15 to 30 mg/dL (n=826) | ≥30 mg/dL (n=1247) | |
| Age, y | 56.8±10.5 | 56.6±10.5 | 57.1±10.8 | 57.0±10.2 |
| Male, n (%) | 3120 (76.5) | 1594 (79.5) | 625 (75.7) | 901 (72.3) |
| BMI, kg/m2 | 26.04±3.69 | 26.21±4.12 | 25.83±3.40 | 25.92±3.10 |
| Hypertension, n (%) | 2557 (62.7) | 1295 (64.6) | 503 (60.9) | 759 (60.9) |
| Diabetes mellitus, n (%) | 1162 (28.5) | 581 (29.0) | 257 (31.1) | 324 (26.0) |
| Current smokers, n (%) | 1888 (46.3) | 970 (48.4) | 375 (45.5) | 543 (43.5) |
| Previous MI, n (%) | 1298 (31.8) | 615 (30.7) | 264 (32.0) | 419 (33.6) |
| Previous PCI, n (%) | 960 (23.5) | 465 (23.2) | 191 (23.1) | 304 (24.4) |
| Previous CABG, n (%) | 83 (2.0) | 30 (1.5) | 21 (2.5) | 32 (2.6) |
| Family history of CAD, n (%) | 591 (14.5) | 280 (14.0) | 121 (14.6) | 190 (15.2) |
| SBP, mm Hg | 127±17 | 127±17 | 127±18 | 126±17 |
| DBP, mm Hg | 78±11 | 78±11 | 78±12 | 77±11 |
| LVEF, % | 63.82±7.21 | 63.64±7.50 | 63.96±7.02 | 64.02±6.85 |
| FPG, mmol/L | 5.90±1.76 | 5.98±1.85 | 5.92±1.79 | 5.76±1.60 |
| HbA1c, % | 6.35±1.14 | 6.37±1.16 | 6.42±1.21 | 6.28±1.04 |
| TC, mmol/L | 4.10±1.11 | 3.98±1.06 | 4.15±1.15 | 4.28±1.13 |
| HDL‐C, mmol/L | 1.04±0.29 | 1.02±0.28 | 1.05±0.29 | 1.07±0.29 |
| LDL‐C, mmol/L | 2.48±0.96 | 2.35±0.90 | 2.55±0.97 | 2.66±1.01 |
| Triglyceride, mmol/L | 1.51 (1.14–2.13) | 1.56 (1.17–2.24) | 1.46 (1.08–2.05) | 1.47 (1.10–2.03) |
| Creatinine, μmol/L | 78.57±17.38 | 78.51±15.78 | 78.85±18.75 | 78.49±18.85 |
| Hs‐CRP, mg/L | 1.46 (0.79–2.97) | 1.39 (0.74–2.84) | 1.61 (0.85–3.30) | 1.47 (0.81–3.11) |
| Fibrinogen, g/L | 3.27±0.80 | 3.17±0.76 | 3.35±0.85 | 3.37±0.80 |
| No. of lesion vessels | ||||
| Single‐vessel, n (%) | 1046 (25.6) | 549 (27.4) | 214 (25.9) | 283 (22.7) |
| Double‐vessel, n (%) | 1398 (34.3) | 675 (33.7) | 293 (35.5) | 430 (34.5) |
| Multivessel, n (%) | 1597 (39.2) | 778 (38.8) | 285 (34.5) | 534 (42.8) |
| Target vessels | ||||
| LM, n (%) | 164 (4.0) | 78 (3.9) | 39 (4.7) | 47 (3.8) |
| LAD, n (%) | 2706 (66.4) | 1317 (65.7) | 571 (69.1) | 818 (65.6) |
| LCX, n (%) | 1616 (39.6) | 842 (42.0) | 334 (40.4) | 440 (35.3) |
| RCA, n (%) | 1945 (47.7) | 902 (45.0) | 378 (45.8) | 665 (53.3) |
| Grafts, n (%) | 60 (1.5) | 24 (1.2) | 12 (1.5) | 24 (1.9) |
| No. of target vessels | 1.20±0.44 | 1.21±0.46 | 1.19±0.44 | 1.19±0.42 |
| No. of stents implanted | 1.81±1.01 | 1.80±1.00 | 1.79±1.02 | 1.86±1.03 |
| Bifurcation lesion, n (%) | 1559 (38.2) | 756 (37.7) | 316 (38.3) | 487 (39.1) |
| Occlusion lesion, n (%) | 519 (12.7) | 232 (11.6) | 88 (10.7) | 199 (16.0) |
| In‐stent restenosis, n (%) | 189 (4.6) | 82 (4.1) | 50 (6.0) | 57 (4.6) |
| No. of predilations | 2 (1–4) | 2 (1–3) | 2 (1–5) | 2 (1–4) |
| No. of postdilations | 4 (2–6) | 4 (2–6) | 4 (2–5) | 4 (2–6) |
| Total stent length, mm | 40.03±24.32 | 39.27±20.50 | 41.74±23.53 | 42.19±24.72 |
| DES, n (%) | 3585 (87.9) | 1748 (87.2) | 736 (89.1) | 1101 (88.3) |
| Baseline medications | ||||
| Aspirin, n (%) | 3503 (85.9) | 1724 (86.0) | 693 (83.9) | 1086 (87.1) |
| ACEI/ARB, n (%) | 875 (21.5) | 419 (20.9) | 189 (22.9) | 267 (21.4) |
| β‐blockers, n (%) | 2032 (49.8) | 976 (48.7) | 401 (48.5) | 655 (52.5) |
| CCB, n (%) | 801 (19.6) | 403 (20.1) | 161 (19.5) | 237 (19.0) |
| Statins, n (%) | 2776 (68.1) | 1325 (66.1) | 546 (66.1) | 905 (72.6) |
| Medications at follow‐up | ||||
| Aspirin, n (%) | 4061 (99.6) | 2000 (99.8) | 822 (99.5) | 1238 (99.3) |
| ACEI/ARB, n (%) | 2154 (52.8) | 1059 (52.8) | 439 (53.2) | 656 (52.6) |
| β‐blockers, n (%) | 3434 (84.2) | 1672 (83.4) | 695 (84.2) | 1067 (85.6) |
| CCB, n (%) | 1668 (40.9) | 860 (42.9) | 312 (37.8) | 496 (39.8) |
| Statins, n (%) | 3829 (93.9) | 1870 (93.3) | 786 (95.1) | 1173 (94.1) |
Continuous values are summarized as mean±SD and median (interquartile range) and categorical variables as number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium‐channel blocker; DBP, diastolic blood pressure; DES, drug‐eluting stent; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; LDL‐C, low‐density lipoprotein cholesterol; LM, left main; LAD, left anterior descending; LCX, left circumflex; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; TC, total cholesterol.
Lipoprotein(a) Levels and Cardiovascular Outcomes
Over an average of 4.9 years of follow‐up, 315 end‐point events were recorded, including 83 cardiovascular deaths, 70 nonfatal MIs, and 162 strokes. As shown in Table 2, the events group had significantly higher lipoprotein(a) levels compared with the nonevents group (P=0.013). Meanwhile, patients in the events group were slightly older and more likely to be females compared with those in the nonevents group. Incidence of hypertension and DM and levels of systolic blood pressure, glycosylated hemoglobin, and hs‐CRP in the events group were also higher than the nonevents group. Additionally, subjects with CVEs had relatively more multivessel lesions and were less likely to be statin users at baseline.
Table 2.
Clinical and Procedural Characteristics of Patients With and Without Events
| Variables | Events (n=315) | Nonevents (n=3763) | P Value |
|---|---|---|---|
| Age, y | 61.5±9.2 | 56.5±10.5 | <0.001 |
| Male, n (%) | 221 (70.2) | 2899 (77.0) | 0.028 |
| BMI, kg/m2 | 25.89±3.17 | 26.05±3.72 | 0.570 |
| Hypertension, n (%) | 237 (75.3) | 2320 (61.7) | <0.001 |
| Diabetes mellitus, n (%) | 109 (34.8) | 1053 (28.0) | 0.041 |
| Current smokers, n (%) | 127 (40.2) | 1761 (46.8) | 0.047 |
| Previous MI, n (%) | 98 (31.1) | 1200 (31.9) | 0.956 |
| Previous PCI, n (%) | 85 (27.0) | 875 (23.2) | 0.205 |
| Previous CABG, n (%) | 12 (3.7) | 71 (1.9) | 0.083 |
| Family history of CAD, n (%) | 36 (11.5) | 555 (14.7) | 0.182 |
| SBP, mm Hg | 129±18 | 127±17 | 0.027 |
| DBP, mm Hg | 78±11 | 78±11 | 0.850 |
| LVEF, % | 63.39±7.08 | 63.85±7.22 | 0.404 |
| FPG, mmol/L | 5.99±1.70 | 5.90±1.77 | 0.520 |
| HbA1c, % | 6.59±1.14 | 6.34±1.14 | 0.003 |
| TC, mmol/L | 4.10±1.15 | 4.10±1.11 | 0.947 |
| HDL‐C, mmol/L | 1.04±0.29 | 1.04±0.29 | 0.897 |
| LDL‐C, mmol/L | 2.44±0.89 | 2.49±0.96 | 0.532 |
| Triglyceride, mmol/L | 1.57 (1.05–2.35) | 1.51 (1.14–2.12) | 0.634 |
| Lipoprotein(a), mg/dL | 21.95 (8.06–44.41) | 15.13 (6.75–37.20) | 0.013 |
| Creatinine, μmol/L | 81.20±19.81 | 78.43±17.23 | 0.063 |
| hs‐CRP, mg/L | 1.79 (0.96–3.80) | 1.45 (0.78–2.96) | 0.003 |
| Fibrinogen, g/L | 3.37±0.81 | 3.27±0.80 | 0.082 |
| No. of lesion vessels | |||
| Single‐vessel, n (%) | 66 (20.9) | 980 (26.0) | 0.035 |
| Double‐vessel, n (%) | 95 (30.1) | 1307 (34.6) | |
| Multivessel, n (%) | 151 (48.0) | 1491 (38.4) | |
| Target vessels | |||
| LM, n (%) | 17 (5.4) | 148 (3.9) | 0.926 |
| LAD, n (%) | 211 (66.9) | 2495 (66.3) | |
| LCX, n (%) | 126 (39.9) | 1490 (39.6) | |
| RCA, n (%) | 151 (48.0) | 1794 (47.7) | |
| Grafts, n (%) | 5 (1.7) | 55 (1.5) | |
| No. of target vessels | 1.14±045 | 1.20±0.44 | 0.500 |
| No. of stents implanted | 1.83±0.95 | 1.83±1.02 | 0.967 |
| Bifurcation lesion, n (%) | 127 (40.5) | 1432 (38.1) | 0.152 |
| Occlusion lesion, n (%) | 42 (13.2) | 477 (12.7) | 0.216 |
| In‐stent restenosis, n (%) | 15 (4.7) | 174 (4.6) | 0.714 |
| No. of predilations | 2 (1–5) | 2 (1–4) | 0.897 |
| No. of postdilations | 4 (2–6) | 4 (2–5) | 0.847 |
| Total stent length, mm | 41.83±26.49 | 39.57±19.86 | 0.348 |
| DES, n (%) | 278 (88.3) | 3307 (87.9) | 0.899 |
| Baseline medications | |||
| Aspirin, n (%) | 262 (83.1) | 3241 (86.1) | 0.473 |
| ACEI/ARB, n (%) | 61 (19.4) | 469 (25.5) | 0.583 |
| β‐blockers, n (%) | 164 (52.0) | 1868 (49.6) | 0.634 |
| CCB, n (%) | 66 (20.9) | 735 (19.5) | 0.706 |
| Statins, n (%) | 182 (57.8) | 2594 (68.9) | 0.001 |
| Medications at follow‐up | |||
| Aspirin, n (%) | 313 (99.3) | 3748 (99.6) | 0.183 |
| ACEI/ARB, n (%) | 186 (58.9) | 1972 (52.4) | 0.082 |
| β‐blockers, n (%) | 255 (81.1) | 3179 (84.5) | 0.219 |
| CCB, n (%) | 133 (42.2) | 1535 (40.8) | 0.430 |
| Statins, n (%) | 293 (93.0) | 3536 (94.0) | 0.583 |
Continuous values are summarized as mean±SD and median (interquartile range) and categorical variables as number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium‐channel blocker; DBP, diastolic blood pressure; DES, drug‐eluting stent; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; LDL‐C, low‐density lipoprotein cholesterol; LM, left main; LAD, left anterior descending; LCX, left circumflex; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SBP, systolic blood pressure; TC, total cholesterol.
Prevalence of total CVEs in the low and high lipoprotein(a) groups according to median of lipoprotein(a) levels was 6.4% and 9.0%, respectively, whereas in the low, medium, and high lipoprotein(a) groups, based on the cut‐off value of 15 and 30 mg/dL, prevalence was 6.3%, 7.7%, and 10.0%, respectively. As shown in Figure 3A, the Kaplan–Meier analysis with the log‐rank test showed that subjects with lipoprotein(a) levels above the median value had a significantly lower cumulative event‐free survival rate compared with those with lipoprotein(a) concentrations below the median level (P=0.028). Meanwhile, the lipoprotein(a) ≥30 mg/dL group also had significantly worse outcomes than the lipoprotein(a) <15 mg/dL group (P=0.003), whereas there was no significant difference of event‐free survival rates between the medium (15–30 mg/dL) and low lipoprotein(a) (<15 mg/dL) groups (P=0.196; Figure 3B). Furthermore, in 2 kinds of grouping methods, incidence of cardiovascular death (P=0.039 and 0.044, respectively) or stroke (P=0.008 and 0.037, respectively) was both significantly higher in patients with high lipoprotein(a) levels compared with those in the low lipoprotein(a) group, but the difference of nonfatal MI between the 2 groups did not reach statistical difference (Figure 3C and 3D).
Figure 3.
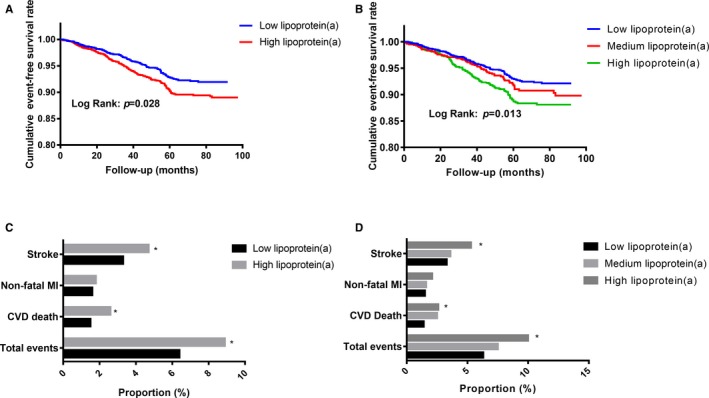
Difference of cardiovascular outcomes according to lipoprotein(a) levels. A, The cumulative event‐free survival analysis according to the median of lipoprotein(a); (B) the cumulative event‐free survival analysis according to lipoprotein(a) levels of 15 and 30 mg/dL; (C) the cardiovascular events incidence according to the median of lipoprotein(a); and (D) the cardiovascular events incidence according to lipoprotein(a) levels of 15 and 30 mg/dL. CVD indicates cardiovascular disease; MI, myocardial infarction. *P<0.05 compared with the low lipoprotein(a) group.
Adjusted HRs and 95% CI of CVEs according to the lipoprotein(a) levels are shown in Table 3. Patients with lipoprotein(a) ≥30 mg/dL had a 1.6‐fold higher risk of total end‐point events in the crude model compared with those with lipoprotein(a) <15 mg/dL. Additional adjustment for other potential covariates did not change this association (HR, 2.1; 95% CI, 1.5–3.0; P<0.001). Moreover, the association between lipoprotein(a) and CVEs remained essentially unchanged in a sensitivity analysis in which each of the other significant variables associated with CVEs was forced into the model with continuous lipoprotein(a) (per 1‐SD increase; Table 4). Additionally, the other 2 sensitivity analyses, by excluding subjects with extreme lipoprotein(a) levels or those developed CVEs during the first year, further demonstrated the relationship of lipoprotein(a) levels to CVE risk (Table 5). When CVEs were considered separately, we observed that lipoprotein(a) ≥30 mg/dL was associated with a 1.9‐fold (95% CI, 1.1–3.4; P=0.027) higher risk of cardiovascular death and a 2.0‐fold (95% CI, 1.3–2.9; P=0.001) higher risk of stroke, whereas there was an elevated, but nonsignificant, increased risk of nonfatal MI compared with lipoprotein(a) levels of <15 mg/dL. In further analysis, according to median of lipoprotein(a) levels, we observed similar results (HR, 1.6; 95% CI, 1.1–2.2 for all CVEs; HR, 1.7; 95% CI, 1.0–2.8 for cardiovascular death; HR, 1.6; 95% CI, 1.1–2.3 for stroke; Table 6). Moreover, per 1‐SD increment of lipoprotein(a) was associated with a 30.0% increased risk of total CVEs and a 29% increased risk of stroke (P=0.001, respectively), but a nonsignificant increased risk of cardiovascular death or nonfatal MI.
Table 3.
Cox Regression Models in Predicting Cardiovascular Outcomes According to Lipoprotein(a)p(a) Levels at Baseline
| End Point | Category | Events, n/Total | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Unadjusted Model | Model 1 | Model 2 | |||
| Total events | Lipoprotein(a) per 1‐SD increase | 315/4078 | 1.2 (1.0–1.3) | 1.2 (1.0–1.3) | 1.3 (1.1–1.5) |
| Low lipoprotein(a) | 126/2005 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| Medium lipoprotein(a) | 64/826 | 1.3 (0.9–1.8) | 1.2 (0.9–1.8) | 1.2 (0.7–1.8) | |
| High lipoprotein(a) | 125/1247 | 1.6 (1.2–2.2) | 1.6 (1.2–2.1) | 2.1 (1.5–3.0) | |
| CVD death | Lipoprotein(a) per 1‐SD increase | 83/4078 | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
| Low lipoprotein(a) | 29/2005 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| Medium lipoprotein(a) | 21/826 | 1.7 (1.0–3.0) | 1.6 (0.9–2.9) | 1.7 (0.9–3.3) | |
| High lipoprotein(a) | 33/1247 | 1.7 (1.0–2.8) | 1.7 (1.0–2.9) | 1.9 (1.1–3.4) | |
| Nonfatal MI | Lipoprotein(a) per 1‐SD increase | 70/4078 | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.1 (0.8–1.4) |
| Low lipoprotein(a) | 31/2005 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| Medium lipoprotein(a) | 13/826 | 1.0 (0.5–1.9) | 1.0 (0.5–1.8) | 1.0 (0.5–2.1) | |
| High lipoprotein(a) | 26/1247 | 1.3 (0.8–2.2) | 1.3 (0.8–2.2) | 1.4 (0.8–2.6) | |
| Stroke | Lipoprotein(a) per 1‐SD increase | 162/4078 | 1.2 (1.1–1.4) | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) |
| Low lipoprotein(a) | 66/2005 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | |
| Medium lipoprotein(a) | 30/826 | 1.1 (0.7–1.7) | 1.1 (0.7–1.6) | 1.0 (0.6–1.7) | |
| High lipoprotein(a) | 66/1247 | 1.6 (1.1–2.2) | 1.5 (1.1–2.1) | 2.0 (1.3–2.9) | |
Model 1 adjusted for age and sex; model 2 adjusted for age, sex, current smoking, hypertension, diabetes mellitus, systolic blood pressure, glycosylated hemoglobin, high‐sensitivity C‐reactive protein, numbers of lesion vessels, baseline statin use, triglyceride, and low‐density lipoprotein cholesterol. CVD indicates cardiovascular disease; MI, myocardial infarction.
Table 4.
Sensitivity Analysis of the Association of per 1‐SD Increase of Lipoprotein(a) With CVEs After Separate Adjustment for Each of the Other Significant Variables
| Adjustment Variable | Multivariate Analysis for CVEs | ||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Age | 1.2 | 1.0 to 1.3 | 0.035 |
| Sex | 1.1 | 1.0 to 1.3 | 0.044 |
| Hypertension | 1.2 | 1.0 to 1.3 | 0.026 |
| Diabetes mellitus | 1.2 | 1.0 to 1.4 | 0.023 |
| Current smoking | 1.2 | 1.0 to 1.3 | 0.042 |
| SBP | 1.2 | 1.0 to 1.4 | 0.028 |
| HbA1c | 1.2 | 1.0 to 1.3 | 0.030 |
| hs‐CRP | 1.2 | 1.0 to 1.3 | 0.036 |
| No. of lesion vessels | 1.2 | 1.0 to 1.3 | 0.041 |
| Baseline statin use | 1.2 | 1.1 to 1.4 | 0.008 |
CVEs indicates cardiovascular events; HbA1c, glycosylated hemoglobin; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; SBP, systolic blood pressure.
Table 5.
Sensitivity Analyses by Excluding Subjects With Extreme Lipoprotein(a) Levels or Participants With CVEs Developed During the First Year
| Category | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Unadjusted Model | Model 1 | Model 2 | |
| Sensitivity analysis 1 | |||
| Lipoprotein(a) per 1‐SD increase | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.3 (1.1–1.6) |
| Low lipoprotein(a) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| Medium lipoprotein(a) | 1.2 (0.8–1.7) | 1.1 (0.8–1.7) | 1.2 (0.7–1.8) |
| High lipoprotein(a) | 1.6 (1.1–2.2) | 1.5 (1.1–2.1) | 2.0 (1.4–3.0) |
| Sensitivity analysis 2 | |||
| Lipoprotein(a) per 1‐SD increase | 1.2 (1.0–1.3) | 1.2 (1.0–1.3) | 1.3 (1.1–1.5) |
| Low lipoprotein(a) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| Medium lipoprotein(a) | 1.2 (0.8–1.8) | 1.1 (0.7–1.7) | 0.9 (0.5–1.6) |
| High lipoprotein(a) | 1.6 (1.1–2.2) | 1.5 (1.1–2.2) | 1.9 (1.3–2.9) |
In sensitivity analysis 1, subjects with lipoprotein(a) levels in the top or the bottom 5% were excluded. In sensitivity analysis 2, participants with CVEs developed during the first year were excluded. CVEs indicates cardiovascular events.
Table 6.
Cox Regression Models in Predicting Cardiovascular Outcomes According to the Median of Lipoprotein(a)
| End Point | Category | Events, n/Total | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Unadjusted Model | Model 1 | Model 2 | |||
| Total events | Low Lipoprotein(a) | 131/2039 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High lipoprotein(a) | 184/2039 | 1.4 (1.1–1.7) | 1.4 (1.1–1.7) | 1.6 (1.1–2.2) | |
| CVD death | Low lipoprotein(a) | 31/2039 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High lipoprotein(a) | 52/2039 | 1.6 (1.0–2.5) | 1.6 (1.0–2.5) | 1.7 (1.0–2.8) | |
| Nonfatal MI | Low lipoprotein(a) | 33/2039 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High lipoprotein(a) | 37/2039 | 1.1 (0.7–1.8) | 1.1 (0.7–1.7) | 1.1 (0.6–1.9) | |
| Stroke | Low lipoprotein(a) | 67/2039 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| High lipoprotein(a) | 95/2039 | 1.4 (1.0–1.9) | 1.4 (1.0–2.1) | 1.6 (1.1–2.3) | |
Model 1 adjusted for age and sex; model 2 adjusted for age, sex, current smoking, hypertension, diabetes mellitus, systolic blood pressure, glycosylated hemoglobin, high‐sensitivity C‐reactive protein, number of lesion vessels, baseline statin use, triglyceride, and low‐density lipoprotein cholesterol. CVD indicates cardiovascular disease; MI, myocardial infarction.
Finally, we assessed whether evaluation of lipoprotein(a) levels in addition to established coronary risk factors could improve risk stratification for CVEs in patients with stable CAD under statin treatment after PCI. As presented in Table 7, the C‐statistic value of Cox prediction model consisting traditional risk factors was 0.668 (95% CI, 0.626–0.709). Addition of continuous (∆C‐statistic, 0.019 [0.005–0.039]; P=0.030) or categorical lipoprotein(a) (∆C‐statistic, 0.020 (0.005–0.041); P=0.030) to the original model showed significant improvements in C‐statistic. Furthermore, the addition of continuous lipoprotein(a) to the model resulted in a significant increase in net reclassification improvement (9.3%; 95% CI, 0.3–19.3; P=0.040) and integrated discrimination improvement (0.3%; 95% CI, 0.1–1.1; P=0.010), as did the addition of categorical lipoprotein(a) (net reclassification improvement, 12.8%; 95% CI, 1.0–21.3; P=0.030; integrated discrimination improvement, 0.5%; 95% CI, 0.1–1.4; P<0.001).
Table 7.
C‐statistic of Lipoprotein(a) for Predicting Cardiovascular Outcomes in Subjects Who Underwent Elective PCI
| C‐Statistic (95% CI) | ΔC‐Statistic (95% CI) | P Value | |
|---|---|---|---|
| Original model | 0.668 (0.626–0.709) | … | |
| Original model+continuous lipoprotein(a) | 0.686 (0.646–0.727) | 0.019 (0.005–0.039) | 0.030 |
| Original model+categorical lipoprotein(a) | 0.687 (0.645–0.730) | 0.020 (0.005–0.041) | 0.030 |
Original model included age, sex, current smoking, hypertension, diabetes mellitus, systolic blood pressure, glycosylated hemoglobin, high‐sensitivity C‐reactive protein, triglyceride, low‐density lipoprotein cholesterol, number of lesion vessels, and baseline statin use. PCI indicates percutaneous coronary intervention.
Discussion
This study is the first study on a sizable population with stable CAD treated with statins after PCI to demonstrate an association between higher baseline lipoprotein(a) levels and CVEs. Notably, after adjusting for potential confounding variables, the Cox regression analysis showed that patients with lipoprotein(a) >30 mg/L had a 2.1‐fold increased risk of CVEs compared with those with lipoprotein(a) <15 mg/dL, and that per 1‐SD increment of lipoprotein(a) was associated with a 30% increase of CVE risk. In addition, adding lipoprotein(a) to the model of established risk factors significantly improved the risk prediction for CVEs. Thus, the present study suggested a prognostic utility of lipoprotein(a) in statin‐treated patients with stable CAD undergoing PCI.
PCI has a considerable evidence base, and it is firmly established as the most common procedure used in the invasive treatment of individuals with CAD. Meanwhile, statin is the most widely used medical therapy for CAD patients. Effects of PCI and statins in reducing adverse CVEs have already been proven.1, 3, 4, 8, 14 Nevertheless, CAD is a multifactorial disease with multiple genetic variations and environmental factors resulting in phenotypic variability; therefore, factors that bring about CVEs in patients with CAD are complicated.8 Thus, cardiovascular risk remains high in CAD patients despite receiving PCI and intensive lipid‐lowering therapy with statins.5, 6, 7 The mechanisms underlying this residual risk are uncertain, and identification of these factors is important for more‐effective tailoring of risk‐reduction strategies.
Plasma lipoprotein(a) has been recently recognized as a novel predictor for cardiovascular risk. In fact, lipoprotein(a) is an inherited atherogenic lipoprotein, and >90% of the variance in concentrations can be explained by genetics.19 Circulating concentrations of lipoprotein(a) cannot be altered by diet or exercise; thus, high lipoprotein(a) levels might have lifelong effects on human health.19, 30 In addition, it has been reported that lipoprotein(a) concentrations are distinguished among different ethnicities.31 Levels are lowest in non‐Hispanic whites (median, 12 mg/dL; interquartile range, 5–32), Chinese (11; 4–22), and Japanese (13; 5–26), slightly higher in Hispanics (19; 8–43), and even higher in blacks (39; 19–69).31 Therefore, besides the categorization according to the median of lipoprotein(a) levels, we further divided the population into 3 groups based on lipoprotein(a) levels of 15 and 30 mg/dL rather than 30 mg/dL only, which is an established and widely used cut‐off value of lipoprotein(a).9, 15, 26
In the past decade, data in subjects without previous CVD from epidemiological studies,10, 11 meta‐analyses,32 genome‐wide association studies33, 34 and Mendelian randomization studies35 have provided substantial evidence that elevated lipoprotein(a) levels contribute to CVD risk. Furthermore, the predicting role of lipoprotein(a) for CVEs risk in patients with established CAD has also been well clarified. In the LIPID (Long‐Term Intervention with Pravastatin in Ischemic Disease) study with 7863 stable CAD patients, subjects with lipoprotein(a) levels in the highest decile (>73.7 mg/dL) had a 1.21‐fold higher risk for CVD events and a 1.23‐fold higher risk for CAD events compared with those in the lowest half of lipoprotein(a) levels (≤13.9 mg/dL).13 Another study of 6762 subjects with CAD from 3 studies, including 2 large statin trials, and then combined with 8 previously published studies for a total of 18 979 CAD patients, demonstrated that plasma lipoprotein(a) was significantly associated with risk of CVEs in patients with established CAD.16 More important, recent studies have suggested that circulating lipoprotein(a) concentration was an independent risk factor for CVD in spite of the levels of LDL cholesterol.13, 36, 37
More recently, the prognostic value of lipoprotein(a) in some special populations has been drawing increasing attention. For example, a study of 27 736 initially healthy postmenopausal women reported that lipoprotein(a) was a determinant of CVD risk among those free of hormone replacement therapy.17 Zhou et al21 and Mitsuda et al38 suggested that lipoprotein(a) on admission is an independent risk factor for subsequent adverse CVEs in patients with ACS. Additionally, our recent studies indicated that high levels of lipoprotein(a) also increased the risk of early‐onset CAD in patients with clinical familial hypercholesterolemia20 and subsequent CVEs in patients with pre–diabetes mellitus and DM.19 Moreover, measurement of lipoprotein(a) is recommended in patients with intermediate or high risk of CVD, particularly in those with premature CVD, familial hypercholesterolemia, a family history of premature CVD and/or elevated lipoprotein(a), and recurrent CVEs despite optimal lipid‐lowering therapy, according to European Society of Cardiology and European Atherosclerosis Society guidelines.39 The 2016 Canadian Cardiovascular Society Guidelines also suggested that lipoprotein(a) might aid risk assessment in subjects with intermediate Framingham Risk Score or with a family history of premature CAD.26 However, the relation of plasma lipoprotein(a) to clinical outcomes of patients undergoing PCI, a large population who remain at high risk of CVEs, has not been well established.
To our knowledge, there have been only a few studies with small sample size exploring the association between lipoprotein(a) and CVEs for patients after PCI. For example, 2 studies with slightly more than 1000 Japanese subjects, including both stable CAD and ACS patients, demonstrated that high lipoprotein(a) levels could be associated with advanced CVEs for patients with DM22 or under statin therapy14 after PCI. In Konishi et al's study,40 a high lipoprotein(a) value (≥30 mg/dL) was found to be associated with a poor prognosis after PCI in 411 patients (both stable CAD and ACS patients) who achieved target lipid levels. Meanwhile, with 600 patients after successful elective PCI and 8 months of follow‐up, Rahel et al23 found that higher levels of lipoprotein(a) were significantly associated with adverse CVEs. Igarashi et al41 and Mitsuda et al38 demonstrated that elevated baseline lipoprotein(a) concentration was a significant predictor for adverse outcomes in more than 100 acute MI patients treated with PCI. However, in another case‐control study with 520 ACS patients, the predicting role of lipoprotein(a) was not observed.24 Moreover, in Kardys et al's study,5 preprocedural lipoprotein(a) level was associated with 1‐year cardiovascular outcomes, but not 8‐year prognosis, in 161 complex patients undergoing PCI. Given the inconsistent results, heterogeneity of study subjects, small sample size, and/or short‐term follow‐up of previous studies, the association between circulating lipoprotein(a) and cardiovascular outcomes for pure stable CAD patients following PCI should be further determined. Thus, we performed the present study and found that after adjusting for those potential confounding factors and unbalanced variables among groups, subjects with elevated lipoprotein(a) levels had a 2.1‐fold increased risk of long‐term CVEs compared with those with low levels of lipoprotein(a), whereas per 1‐SD increase of lipoprotein(a) was associated with a 30% elevation of CVE risk. In the next subgroup analysis, elevated lipoprotein(a) levels were also significantly associated with incidence of cardiovascular death and stroke, respectively, whereas there was an elevated, but nonsignificant, increased risk of nonfatal MI, which may be attributable to the not long enough follow‐up time and the allocation of fatal MI to cardiovascular death. As previously mentioned, the significance level for the key association between plasma lipoprotein(a) levels and CVE in our study is low and just gets across the 5% mark somewhere. However, this phenomenon was consistent with previous studies. According to Tsimikas et al's study,15 the P value of the significant association between tertile 3 of lipoprotein(a) and cardiovascular outcomes was 0.02 (HR, 2.0; 95% CI, 1.1–3.7). In Konishi et al's study,22 the significance level between high lipoprotein(a) levels (>30 mg/dL) and composite end points was 0.04. Rahel et al23 suggested that lipoprotein(a) was significantly related to CVEs with a P value of 0.03. In addition, other relative studies on the association between lipoprotein(a) and clinical outcomes after PCI also showed a similar significance level. Moreover, the significant association of lipoprotein(a) with CVEs was further confirmed by sensitivity analysis. Besides, we also calculated C‐statistic, net reclassification improvement, and integrated discrimination improvement to investigate the value of adding lipoprotein(a) to the predicting model, including established risk factors of CVD, and observed that lipoprotein(a) could significantly improve CVEs risk prediction, strongly indicating a prognostic value of lipoprotein(a) in stable CAD patients receiving PCI.
The underlying mechanisms for the significant association between high plasma lipoprotein(a) levels and CVEs has not been fully understood. Nevertheless, its mediated atherogenic, proinflammatory, and thrombogenic effects might contribute to worse cardiovascular outcomes. Lipoprotein(a) quantitatively possesses all the atherogenic risk of LDL particles, including their tendency to oxidize after migrating into the arterial walls, producing highly proinflammatory and immunogenic oxidized LDL.15 Moreover, it is even more atherogenic than LDL given that it not only contains all the proatherogenic components of LDL, but also of apolipoprotein(a). It has been demonstrated that apolipoprotein(a) can enhance atherothrombosis by additional mechanisms, including inflammation through its content of oxidized phospholipids, whose presence of lysine binding sites allows accumulation in the vessel wall, and a potential antifibrinolytic role by inhibiting plasminogen activation.42 In addition, lipoprotein(a) may also have the ability to damage endothelial anticoagulant function by promoting endothelial dysfunction and increasing phospholipid oxidation.43, 44 In this study, we observed that lipoprotein(a) showed no effects on early post‐PCI events and its predicting role was mainly for long‐term prognosis. We deduced that the possible reason was that the acute damage of the PCI procedure and stent on the vessel endothelium were much stronger than plasma lipoprotein(a) in the early period after PCI, which may take the dominant position in the occurrence of early post‐PCI CVEs. On the other hand, the atherogenic, proinflammatory, and thrombogenic effects of lipoprotein(a) are chronic and persistent, which may mainly affect the long‐term prognosis.
Strong evidence has suggested a causal relationship of high concentrations of lipoprotein(a) to increased CVD risk. In contrast, its relationship with DM incidence is less clear. Previous prospective studies on this topic have shown an inverse association between lipoprotein(a) concentrations and incident DM.45, 46, 47, 48 In the Bruneck study, there was also an increased risk of DM in subjects with low lipoprotein(a) concentrations compared with those with the highest lipoprotein(a) levels. Nevertheless, there was no evidence of a linear association between lipoprotein(a) levels and DM prevalence.49 In the present study, we observed that incidence of diabetes mellitus was higher in the low and medium lipoprotein(a) groups, whereas it was lowest in the high lipoprotein(a) group. There was a significant difference between the medium and high lipoprotein(a) groups (P=0.009), whereas the difference between the low and high lipoprotein(a) groups did not reach statistical significance (P=0.077). Similar with the Bruneck study,49 the linear trend test between lipoprotein(a) concentrations and DM prevalence was not significant (P=0.103). The possible reasons of our findings may be attributable to the different categorical method of lipoprotein(a) and the cross‐sectional analysis of this association. Overall, the causality of the association between circulating lipoprotein(a) concentrations and risk of incident DM has not yet been sufficiently determined, and further related studies are necessary.49
However, our study has several limitations. First, lipoprotein(a) levels are known to vary with ethnicity, which might impact the generalizability of our findings. Second, we did not have the data of lipoprotein(a) levels at follow‐up, which may improve the significance of an association between lipoprotein(a) and CVD outcomes. Third, lipoprotein(a) was measured by an immunoturbidimetry method in our study, which was not apolipoprotein(a) isoform independent. However, a lipoprotein(a) protein‐validated standard was used to calibrate the examination, along with linking the results to the World Health Organization/International Federation of Clinical Chemistry and Laboratory Medicine International Reference Reagent, making the assay relatively isoform independent. Furthermore, for routine clinical care, currently available assays linked to the World Health Organization/International Federation of Clinical Chemistry and Laboratory Medicine standard are able to detect high‐risk patients with acceptable accuracy.15, 50 Fourth, recall bias in the clinics and with telephone calls is not perfect. However, we have around 10 years’ experience of follow‐up, well‐trained and specialized follow‐up staffs, and systemic propaganda and education work before discharge, which were pivotal for promoting the level of the quality of follow‐up. Fourth, the follow‐up time of this study needed to be longer in order to better examine the prognostic value of lipoprotein(a) in the long‐term outcomes.
In conclusion, in the present study, we first, with a large sample size and long‐term follow‐up, demonstrated that elevated lipoprotein(a) levels were significantly associated with CVEs in stable CAD patients after PCI in the contemporary era of statin therapy. Therefore, knowledge of an elevated baseline lipoprotein(a) level before PCI may provide novel information to help with risk stratification, clinical decision making in lipid‐lowering treatment intensification, and traditional modifiable risk factors targeting strategies. Moreover, changes in guidelines would probably need to occur in conjunction with further evidence from randomized controlled trials of lipoprotein(a) lowering.
Sources of Funding
This work was partially supported by the Capital Health Development Fund (201614035) and CAMS Major Collaborative Innovation Project (2016‐I2M‐1‐011) awarded to Dr Jian‐Jun Li, MD, PhD, and Shenzhen Science Fund (20170502165510880) awarded to Dr Xie‐Hui Chen, MD, PhD.
Disclosures
None.
Acknowledgments
The authors thank all the staff and participants of this study for their important contributions.
(J Am Heart Assoc. 2020;9:e014581 DOI: 10.1161/JAHA.119.014581.)
Contributor Information
Xie‐Hui Chen, Email: lijianjun938@126.com.
Jian‐Jun Li, Email: xhchen66@126.com.
References
- 1. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10 Suppl):S1–S42. [DOI] [PubMed] [Google Scholar]
- 2. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM; Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 4. Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, Juni P, Pijls NHJ, Hlatky MA, Investigators FT. Clinical outcomes and cost‐effectiveness of fractional flow reserve‐guided percutaneous coronary intervention in patients with stable coronary artery disease: three‐year follow‐up of the FAME 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation. 2018;137:480–487. [DOI] [PubMed] [Google Scholar]
- 5. Kardys I, Oemrawsingh RM, Kay IP, Jones GT, McCormick SP, Daemen J, Van Geuns RJ, Boersma E, Van Domburg RT, Serruys PW. Lipoprotein(a), interleukin‐10, C‐reactive protein, and 8‐year outcome after percutaneous coronary intervention. Clin Cardiol. 2012;35:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, Deedwania P, Kastelein JJ, Waters DD. Determinants of residual risk in secondary prevention patients treated with high‐ versus low‐dose statin therapy: the treating to new targets (TNT) study. Circulation. 2012;125:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reith C, Armitage J. Management of residual risk after statin therapy. Atherosclerosis. 2016;245:161–170. [DOI] [PubMed] [Google Scholar]
- 8. Li ZG, Li G, Zhou YL, Chen ZJ, Yang JQ, Zhang Y, Sun S, Zhong SL. Lack of association between lipoprotein(a) genetic variants and subsequent cardiovascular events in Chinese HAN patients with coronary artery disease after percutaneous coronary intervention. Lipids Health Dis. 2013;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholls SJ, Tang WH, Scoffone H, Brennan DM, Hartiala J, Allayee H, Hazen SL. Lipoprotein(a) levels and long‐term cardiovascular risk in the contemporary era of statin therapy. J Lipid Res. 2010;51:3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V. Lipoprotein(a) levels and risk of future coronary heart disease: large‐scale prospective data. Arch Intern Med. 2008;168:598–608. [DOI] [PubMed] [Google Scholar]
- 11. Kamstrup PR, Tybjaerg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 12. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–1370. [DOI] [PubMed] [Google Scholar]
- 13. Nestel PJ, Barnes EH, Tonkin AM, Simes J, Fournier M, White HD, Colquhoun DM, Blankenberg S, Sullivan DR. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:2902–2908. [DOI] [PubMed] [Google Scholar]
- 14. Suwa S, Ogita M, Miyauchi K, Sonoda T, Konishi H, Tsuboi S, Wada H, Naito R, Dohi T, Kasai T, Okazaki S, Isoda K, Daida H. Impact of lipoprotein (a) on long‐term outcomes in patients with coronary artery disease treated with statin after a first percutaneous coronary intervention. J Atheroscler Thromb. 2017;24:1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 16. O'Donoghue ML, Morrow DA, Tsimikas S, Sloan S, Ren AF, Hoffman EB, Desai NR, Solomon SD, Domanski M, Arai K, Chiuve SE, Cannon CP, Sacks FM, Sabatine MS. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gragnano F, Fimiani F, Di Maio M, Cesaro A, Limongelli G, Cattano D, Calabro P. Impact of lipoprotein(a) levels on recurrent cardiovascular events in patients with premature coronary artery disease. Intern Emerg Med. 2019;14:621–625. [DOI] [PubMed] [Google Scholar]
- 19. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Liu HH, Dong Q, Li JJ. Lipoprotein(a) and cardiovascular outcomes in coronary artery disease patients with prediabetes and diabetes. Diabetes Care. 2019;42:1312–1318. [DOI] [PubMed] [Google Scholar]
- 20. Li S, Wu NQ, Zhu CG, Zhang Y, Guo YL, Gao Y, Li XL, Qing P, Cui CJ, Xu RX, Sun J, Liu G, Dong Q, Li JJ. Significance of lipoprotein(a) levels in familial hypercholesterolemia and coronary artery disease. Atherosclerosis. 2017;260:67–74. [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Cui X, Jin X, Zhou J, Fu M, Zhong C, Sun A, Hu K, Fu M, Ge J. Association between lipoprotein (a) level on admission and the incidence of subsequent cardiovascular events in patients with acute coronary syndrome. Int J Cardiol. 2012;158:464–466. [DOI] [PubMed] [Google Scholar]
- 22. Konishi H, Miyauchi K, Shitara J, Endo H, Wada H, Doi S, Naito R, Tsuboi S, Ogita M, Dohi T, Kasai T, Okazaki S, Isoda K, Suwa S, Daida H. Impact of lipoprotein(a) on long‐term outcomes in patients with diabetes mellitus who underwent percutaneous coronary intervention. Am J Cardiol. 2016;118:1781‐1785. [DOI] [PubMed] [Google Scholar]
- 23. Rahel BM, Visseren FL, Suttorp MJ, Plokker TH, Kelder JC, de Jongh BM, Bouter KP, Diepersloot RJ. Preprocedural serum levels of acute‐phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res. 2003;60:136–140. [DOI] [PubMed] [Google Scholar]
- 24. Marcucci R, Brogi D, Sofi F, Giglioli C, Valente S, Liotta AA, Lenti M, Gori AM, Prisco D, Abbate R, Gensini GF. PAI‐1 and homocysteine, but not lipoprotein (a) and thrombophilic polymorphisms, are independently associated with the occurrence of major adverse cardiac events after successful coronary stenting. Heart. 2006;92:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao YX, Liu HH, Sun D, Jin JL, Xu RX, Guo YL, Wu NQ, Zhu CG, Li S, Zhang Y, Sun J, Li JJ. The different relations of PCSK9 and Lp(a) to the presence and severity of atherosclerotic lesions in patients with familial hypercholesterolemia. Atherosclerosis. 2018;277:7–14. [DOI] [PubMed] [Google Scholar]
- 26. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GB, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 27. Liu HH, Cao YX, Li S, Guo YL, Zhu CG, Wu NQ, Gao Y, Dong QT, Zhao X, Zhang Y, Sun D, Li JJ. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension. 2018;71:1039–1046. [DOI] [PubMed] [Google Scholar]
- 28. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 29. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57:1339–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg‐Hansen A; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emerging Risk Factors C, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 34.CARDIoGRAMplusC4D Consortium, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi‐Boehm S, Cox D, Dimitriou M, Do R, Consortium D, Consortium C, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco‐Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller‐Nurasyid M, Mu TC, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ; Wellcome Trust Case Control C , Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamstrup PR, Tybjaerg‐Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61:1146–1156. [DOI] [PubMed] [Google Scholar]
- 36. Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Response to letter regarding article, “lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the jupiter trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin)”. Circulation. 2014;130:e152. [DOI] [PubMed] [Google Scholar]
- 37. Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO Jr, Xu P, Marcovina SM. Relationship of apolipoproteins A‐1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM‐HIGH trial (atherothrombosis intervention in metabolic syndrome with low HDL/high triglyceride and impact on global health outcomes). J Am Coll Cardiol. 2013;62:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitsuda T, Uemura Y, Ishii H, Takemoto K, Uchikawa T, Koyasu M, Ishikawa S, Miura A, Imai R, Iwamiya S, Ozaki Y, Kato T, Shibata R, Watarai M, Murohara T. Lipoprotein(a) levels predict adverse vascular events after acute myocardial infarction. Heart Vessels. 2016;31:1923–1929. [DOI] [PubMed] [Google Scholar]
- 39. atapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 40. Konishi H, Miyauchi K, Kasai T, Tsuboi S, Ogita M, Naito R, Sai E, Fukushima Y, Katoh Y, Okai I, Tamura H, Okazaki S, Daida H. Impact of lipoprotein(a) as residual risk on long‐term outcomes in patients after percutaneous coronary intervention. Am J Cardiol. 2015;115:157–160. [DOI] [PubMed] [Google Scholar]
- 41. Igarashi Y, Aizawa Y, Satoh T, Konno T, Ojima K, Aizawa Y. Predictors of adverse long‐term outcome in acute myocardial infarction patients undergoing primary percutaneous transluminal coronary angioplasty: with special reference to the admission concentration of lipoprotein (a). Circ J. 2003;67:605–611. [DOI] [PubMed] [Google Scholar]
- 42. Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32:1550–1551. [DOI] [PubMed] [Google Scholar]
- 43. Schachinger V, Halle M, Minners J, Berg A, Zeiher AM. Lipoprotein(a) selectively impairs receptor‐mediated endothelial vasodilator function of the human coronary circulation. J Am Coll Cardiol. 1997;30:927–934. [DOI] [PubMed] [Google Scholar]
- 44. Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. [DOI] [PubMed] [Google Scholar]
- 45. Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye Z, Haycock PC, Gurdasani D, Pomilla C, Boekholdt SM, Tsimikas S, Khaw KT, Wareham NJ, Sandhu MS, Forouhi NG. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes. 2014;63:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamstrup PR, Nordestgaard BG. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2013;1:220–227. [DOI] [PubMed] [Google Scholar]
- 48. Onat A, Coban N, Can G, Yuksel M, Karagoz A, Yuksel H, Ademoglu E, Erginel‐Unaltuna N. Low, “quotient” Lp(a) concentration mediates autoimmune activation and independently predicts cardiometabolic risk. Exp Clin Endocrinol Diabetes. 2015;123:11–18. [DOI] [PubMed] [Google Scholar]
- 49. Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, Willeit J, Kiechl S, Willeit P. Lipoprotein(a) and incident type‐2 diabetes: results from the prospective Bruneck study and a meta‐analysis of published literature. Cardiovasc Diabetol. 2017;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]


