Abstract
Background
Intravenous thrombolytic therapy (IVT) with tissue plasminogen activator for acute ischemic stroke is underutilized in many parts of the world. Randomized trials to test the effectiveness of thrombolysis implementation strategies are limited.
Methods and Results
This study aimed to test the effectiveness of a multicomponent, multidisciplinary tissue plasminogen activator implementation package in increasing the proportion of thrombolyzed cases while maintaining accepted benchmarks for low rates of intracranial hemorrhage and high rates of functional outcomes at 3 months. A cluster randomized controlled trial of 20 hospitals in the early stages of thrombolysis implementation across 3 Australian states was undertaken. Monitoring of IVT rates during the baseline period allowed hospitals (the unit of randomization) to be grouped into 3 baseline IVT strata—very low rates (0% to ≤4.0%); low rates (>4.0% to ≤10.0%); and moderate rates (>10.0%). Hospitals were randomized to an implementation package (experimental group) or usual care (control group) using a 1:1 ratio. The 16‐month intervention was based on behavioral theory and analysis of the steps, roles, and barriers to rapid assessment for thrombolysis eligibility and involved comprehensive strategies addressing individual and system‐level change. The primary outcome was the difference in tissue plasminogen activator proportions between the 2 groups postintervention. The absolute difference in postintervention IVT rates between intervention and control hospitals adjusted for baseline IVT rate and stratum was not significant (primary outcome rate difference=1.1% (95% CI −1.5% to 3.7%; P=0.38). Rates of intracranial hemorrhage remained below international benchmarks.
Conclusions
The implementation package resulted in no significant change in tissue plasminogen activator implementation, suggesting that ongoing support is needed to sustain initial modifications in behavior.
Clinical Trial Registration
URL: http://www.anzctr.org.au Unique identifiers: ACTRN12613000939796 and U1111‐1145‐6762
Keywords: health system change, implementation, ischemic stroke, quality improvement, thrombolysis
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Health Services, Quality and Outcomes
Clinical Perspective
What Is New?
There is limited evidence pointing to which strategies can potentially be used to boost stroke thrombolysis rates, across healthcare organizations.
The TIPS (Thrombolysis Implementation in Stroke) trial tested a multicomponent thrombolysis implementation package across 20 Australian hospitals but did not demonstrate a sustained significant increase in rates.
What Are the Clinical Implications?
Although a transient increase in thrombolysis rates was evident during the active phase of implementation support, the negative overall result of the TIPS trial confirms the recognized challenge of delivering and sustaining health systems change and suggests the need for further implementation research into novel strategies for thrombolysis implementation at scale.
With over 20 million new stroke events each year worldwide and over 5 million deaths, stroke remains a major burden on global healthcare systems.1, 2 Effective treatment for acute ischemic stroke remains limited to strategies promoting early reperfusion of the ischemic brain. Intravenous thrombolysis (IVT) using tissue plasminogen activator (tPA) is the only approved drug therapy and the only widely available treatment option. However, tPA is underutilized in most healthcare systems. One likely reason for undertreatment is that IVT is a complex intervention that involves interacting health system components requiring alignment of clinician behaviors, and collaboration among a number of medical discipline groups and/or organizational levels.3
A recent systematic review identified factors recognized to enhance IVT implementation4 including expert and coordinated multidisciplinary care, individual and team‐based advanced knowledge and skills, streamlined systems of care, and clinician experience, confidence, and acceptance of risk. Expert teams working in organized systems of care can deliver tPA to up to 30% of all acute ischemic stroke presentations5, 6, 7 and to up to 50% of cases presenting within the standard time window of 4.5 hours from symptom onset. However, IVT implementation is highly variable across regions,8, 9, 10 and, even in well‐resourced healthcare systems, implementation rates are not uncommonly well below recommended benchmarks, 11.4% in the United Kingdom11 and 3.4% in the United States.12 In Australia, IVT implementation had plateaued over the previous decade, and the TIPS (Thrombolysis Implementation in Stroke) trial ran at a time when the national average of ischemic strokes patients receiving IVT was 7%, giving emphasis to the magnitude of Australia's undertreatment problem.13
The TIPS trial aimed to address IVT undertreatment in the Australian healthcare system by testing whether a multicomponent, multidisciplinary collaborative intervention could:
Increase the proportion of all stroke patients receiving thrombolysis at intervention hospitals, compared with control hospitals.
Maintain best‐practice benchmarks for stroke outcomes.
Ensure that the adverse event rate for symptomatic intracranial hemorrhage did not rise above best‐practice benchmarks.
Methods
TIPS was a cluster‐randomized controlled trial conducted in 20 hospitals across 3 states of Australia (New South Wales, Victoria, and Queensland) between 2011 and 2015 that evaluated the effectiveness of a multicomponent, multidisciplinary collaborative intervention to improve implementation of IVT. Institutional review board approval was obtained from Hunter New England Health, University of Newcastle, Darling Downs Health Service, Sydney Adventist Hospital Group, Epworth HealthCare, LaTrobe Regional Hospital, Peninsula Health, and Melbourne Health Human Research Ethics Committees. The trial protocol (https://doi.org/10.1186/1748-5908-9-38) has been previously published14 with the trial designed in accordance with the CONSORT (Consolidated Standard for Reporting Trials) statement for cluster‐randomized trials.15 The data that support the findings of this study are available from the corresponding author on reasonable request.
Participants
Participating hospitals were identified from National Stroke Foundation audit records and in communication with New South Wales, Victoria, and Queensland Stroke Unit Networks. Hospitals were potentially eligible if they fulfilled the following criteria:
On‐site stroke care unit and staffing equivalent of a stroke physician and stroke nurse.
Early‐stage implementation (<10% thrombolysis implementation rate or had commenced intravenous thrombolysis delivery within ≈5 years previously), or about to commence IVT implementation.
Agreement to participate in ongoing continuous audit of IVT processes of care and outcomes.
The clinical lead of the research team contacted potentially eligible hospitals to invite participation. Hospitals expressing interest were then visited by the research team to confirm eligibility and to develop and sign a Memorandum of Understanding to confirm their participation throughout the study duration regardless of whether they were allocated to the intervention or control group. Patient consent was waived for obtaining deidentified data on stroke patients. Following hospital enrollment, staff at participating centers completed surveys at baseline and follow‐up to describe attitudes and practices relating to IVT. Staff provided implied consent by completing the survey.
Trial Design and Time Lines
The baseline IVT rate observation period averaged 32 months, during which time the intervention components and intervention rollout strategy were developed. Following randomization (see below), the intervention commenced with a launch at a collaborative intervention site meeting. The intervention was then actively maintained by the TIPS support team over a 16‐month period. At the end of the 16‐month active intervention period, intervention hospitals were required to continue running the intervention components under self‐direction for a further 12 months. This additional 12‐month period was undertaken to examine the sustainability of any initial treatment effect (see Figure 1).
Figure 1.
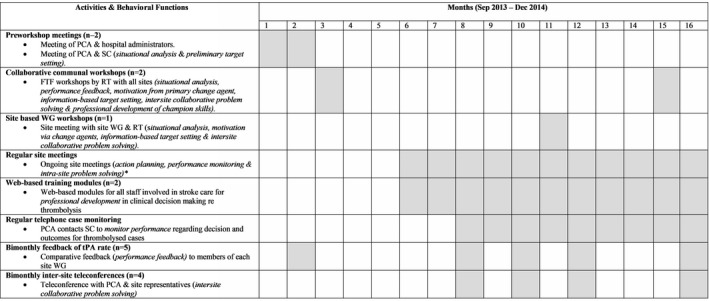
Intervention elements and time frames. *Data collected from March 2014 onwards. NB: Gray boxes indicate that the activity was undertaken in that month. Functions are included in brackets. PCA is 1 or more members of the RT. FTF indicates face to face; PCA, primary change agent; RT, research team; SC, site champions (usually lead nurse and lead clinician); tPA, tissue plasminogen activator; WG, working group.
Randomization and Masking
Monitoring of IVT rates during the baseline period allowed hospitals (the unit of randomization) to be grouped into 3 baseline IVT strata—very low rates (0% to ≤4.0%); low rates (>4.0% to ≤10.0%); and moderate rates (>10.0%). Randomization of hospitals to intervention or control was then performed as a single event by a statistician using a 1:1 ratio within strata. All sites were informed by the clinical lead of the research team of their group allocation and, at the same time, by an email to site principal investigators and coordinators, as blinding of clinicians was not possible once intervention activities commenced.
Intervention Components
Intervention components were developed in accordance with a behavior‐change wheel method and strategies.16 The behavior‐change wheel emphasizes the importance of ensuring that staff involved in change have the capability, opportunity, and motivation to perform the desired behavior; behavior‐change techniques include education, training, environmental restructuring, modeling, and enablement. Information regarding the study intervention has been published elsewhere.14 During the baseline and postintervention periods, interviews and surveys were completed with intervention hospital staff to describe the IVT patients’ experience in each facility, local practice, and system of care along with perceived barriers and facilitators to thrombolysis. This allowed construction of a situation analysis, an assessment of readiness for change along with an understanding of likely engagement with intervention elements. Seven intervention components were delivered over 16 months via a suite of activities. Briefly, these activities included preworkshop meetings, collaborative communal workshops, site‐based working groups, web‐based training modules, regular telephone case monitoring, bimonthly feedback of IVT rate, and bimonthly intersite teleconferences; the timing and content of each are summarized in Figure 1. The intervention elements were primarily aimed at stroke clinicians, but some also incorporated collaboration with emergency department clinicians as outlined in Figure 1. The majority of activities and elements were provided by the clinical lead and other members of the research team at a hospital or interhospital collaborative group level involving site champions and representatives from emergency or other departments. Online training modules and regular case monitoring were provided at an individual level. Collaborative workshops were held at a metro‐based training venue, and site meetings were held at each hospital. Further details are available in Table S1.
Control Site Activities, Blinding, and Avoidance of Contamination
No IVT implementation support was provided to control sites. The stroke unit director, stroke care coordinator, and hospital general manager for each individual intervention and control hospital were the only local staff aware of the hospital's allocation status. These cluster guardians were instructed not to discuss trial hospital allocation status; however, this could not be mandated. Staff at intervention hospitals could not effectively be blinded to their allocation because they were aware of their involvement in intervention activities. At state or national levels there was no organized program of hospital‐based IVT support during the course of the trial. Ambulance Service New South Wales established a prehospital stroke assessment and prenotification program in 201317; however, this program was launched across all New South Wales stroke centers, thereby not creating any differential bias. No organized large‐scale telestroke programs were initiated in TIPS hospitals in New South Wales or Queensland during the intervention or postintervention phases. An established telestroke system covered the TIPS intervention and postintervention phases in Victoria. Small‐scale local initiatives stroke care quality improvement occurred during the study period with no indication of differential effects (anecdotal data only).
Procedures
Data Collection
Data on all patients treated with tPA in both the intervention and control sites were recorded in a secure, purpose‐built online audit tool hosted by the National Stroke Foundation. Data included details of eligibility assessment and tests undertaken, demographics, National Institutes of Health Stroke Scale score, risk factor profiles, hemorrhagic transformation, processes of care, and 3‐month modified Rankin Scale score (mRS). Data entry was performed by stroke unit staff as part of each unit's routine stroke thrombolysis audit procedure. Deidentified data were available to the central TIPS trial data manager allowing individual sites to be prompted to collect any missing data element and 3‐month follow‐up on each treated patient.
The state‐level health information service in each state provided the number of all stroke cases with a primary discharge code of stroke (International Classification of Diseases‐10th Revision codes I61, I62.9, I63, I64) for each trial hospital.
Outcomes
The primary outcome measure was the proportion of stroke cases in each hospital that were treated with tPA within each month, defined as the number of cases entered in the hospital tPA data set divided by the total number of stroke cases (as outlined above per month). Hereafter we refer to the baseline, intervention, and postintervention periods as study periods. Because monthly rates were small and unstable, they were aggregated in 4‐month blocks; hence, there were 8 4‐month blocks during the baseline period (32 months total), 4 4‐month blocks in the intervention period (16 months total), and 3 4‐month blocks in the postintervention period (12 months total) for a grand total trial duration of 60 months. We refer to the 4‐month blocks as time points.
Secondary outcomes were the proportion of patients treated with IVT experiencing (1) favorable 3‐month outcomes (mRS score 0‐1) and (2) symptomatic intracranial hemorrhage.
Process measures included intervention involvement at each intervention site and change in staff attitudes. Intervention involvement was assessed by the health behavior change expert of the research team against each intervention component using a scoring rubric of 0=low‐level engagement, 1=medium‐level engagement, 2=high‐level engagement, according to the proportion of eligible staff participating in intervention components included as assessment of executive support for IVT; attendance at meetings, workshops, and teleconferences; and uptake of online training modules. Staff attitudes were assessed using a cross‐sectional pen‐and‐paper survey, which was distributed to medical and nursing staff at all 20 study sites who were involved in assessment of potential stroke cases and stroke care during both the baseline phase and follow‐up phase of the trial. These data will be reported separately.
Sample Size
From the baseline data, we estimated that participating hospitals (not all equal in size) would have an average of 150 stroke patients per year, that 5% of stroke patients in the control group would receive tPA, and that the average coefficient of variation across strata would be ≈0.4. With 10 hospitals per treatment group, and data collected for 12 months postintervention, the study would have 80% power with a 5% significance level to detect an absolute difference of 7% to 10% in the IVT rate.
Statistical Analyses
Primary Outcome
We modeled the thrombolysis rates in a number of ways. In the primary, prespecified analysis, the absolute difference between the intervention and control group thrombolysis rates during the postintervention phase was compared using a linear regression model adjusted for baseline thrombolysis rate and strata. As a secondary, posthoc analysis, we modeled thrombolysis rates at each time point relative to the thrombolysis rate for the full baseline period. We used a generalized linear mixed‐effect model under a binomial distributional assumption with a log‐link function, a site‐level random intercept, and fixed effects for time points, intervention groups, and their interaction. Parameter estimates from this model, when exponentiated, reflect the relative increase/decrease in the change from baseline thrombolysis rates for intervention and control sites and the difference between them. Analyses of the primary outcome were intention‐to‐treat in that the numerator included all individuals administered tPA, and the denominator was obtained from hospital separations data.
Secondary Outcomes
The proportion of thrombolysed cases with favorable outcomes and the proportion with symptomatic intracranial hemorrhages at each hospital were compared with benchmarks of 30% (for favorable mRS outcomes), and 6% (for symptomatic intracranial hemorrhage) using a 1‐tailed hypothesis test in a manner analogous to stopping rules for randomized clinical trials; thereby monitoring for any use of thrombolysis causing a decrease in good outcomes or an increase in adverse outcomes.
Patients who died within 3 months of admission had missing mRS replaced with a 6 (death). Due to a large fraction of additional missing data on the mRS, complete case analysis and multiple imputation analyses (chained regression equations imputing missing data based on hospital site, stroke severity [National Institutes of Health Stroke Scale], age, and sex, and 20 imputed data sets) were performed. Multiple imputation was not performed for sites/times with fewer than 10 thrombolysed cases due to instability in parameter estimates. Normal approximations using the asymptotic standard errors were used to calculate the 95% CI and P‐values for the multiple imputation proportions.
Data manipulation, summary statistics, figures, and mixed model estimates were generated using R version 3.3.1 (2016‐06‐21; Vienna, Austria), Stata 14.1 (StataCorp, College Station, TX), and SAS v9.4 (SAS Institute, Cary, NC).
The first author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Twenty‐two hospitals were identified that fulfilled eligibility criteria, and 20 hospitals agreed to participate, signing memoranda of understanding. All 20 hospitals (4 in Victoria, 3 in Queensland, and 13 in New South Wales) remained in the trial from commencement of the baseline period in January 2011 to completion of the intervention period in December 2015. The trial profile including numbers of stroke cases in the baseline, intervention, and postintervention phase is provided in Figure 2.
Figure 2.
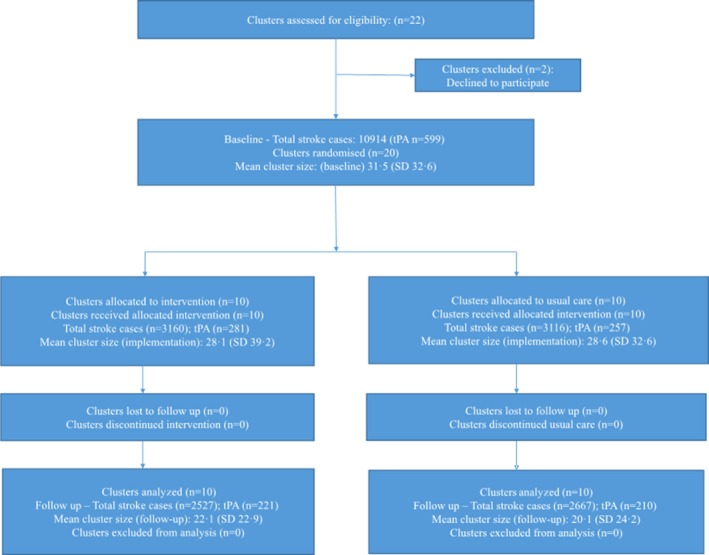
Trial profile. Baseline, January 2011 to August 2013; intervention, September 2013 to December 2014; follow‐up, January to December 2015. tPA indicates tissue plasminogen activator.
The characteristics of the 20 hospitals are illustrated in Table 1, 18 The hospitals ranged from 65 to 716 stroke cases per year at baseline. The majority of hospitals serviced regional cities and adjacent rural populations with a catchment radius of up to 300 km and an average population base of 40 000 people. There were 6 outer metropolitan hospitals situated in each of the state capitals serving urban and regional communities of over 100 000; 2 metropolitan academic private hospitals, and 2 metropolitan academic public hospitals. For the duration of the trial there was limited access to endovascular reperfusion therapies in the metropolitan centers and no access from regional centers. The characteristics of the patients treated with IVT across the 2 groups at baseline are shown in Table 2.
Table 1.
Baseline Characteristics of the Thrombolysis Implementation in Stroke Trial Hospitals
| Hospital Characteristics | Intervention Hospitals (n=10) | Control Hospitals (n=10) |
|---|---|---|
| Average bed number (range)a | 416 (62‐750) | 384 (231‐534) |
| Average annual stroke separations (range)b | 232 (65‐452) | 236 (122‐716) |
| Academic/nonacademicc | 3/7 | 3/7 |
| Metropolitan/regionald | 5/5 | 7/3 |
| Average overall baseline thrombolysis rate (total treated cases)e | 5.06% (285) | 4.94% (314) |
ICD‐10 indicates International Statistical Classification of Diseases Tenth Revision; TIPS, Thrombolysis Implementation in Stroke.
Data extracted from hospital and local health district websites, access date April 6, 2017.
Averaged over 5‐y period 2011‐2015 and based on hospital ICD‐10 data 2011‐2013 (ICD‐10 codes I61.x, I62.9, I63.x, I64) and site data collected through the project.
2011‐2012 hospital data from Australian Institute of Health and Welfare 2015. Australian hospital peer groups. Health services series no. 66. Catalogue no. HSE 170. Canberra: Australian Institute of Health and Welfare.18
2011‐2012 hospital data from Australian Institute of Health and Welfare 2015. Australian hospital peer groups. Health services series no. 66. Catalogue no. HSE 170. Canberra: AIHW. Previous and current classification included. Classification of hospitals as private vs public in Acute Group A has been removed.18
Data from TIPS trial database 2011‐2013 and in‐hospital ICD‐10 data 2011‐2013 (ICD‐10 codes I61.x, I62.9, I63.x, I64) and site data collected through the project.
Table 2.
Characteristics of Patients Treated With Intravenous Thrombolysis in Baseline Period
| Control (N=314) | Intervention (N=285) | Total (N=599) | |
|---|---|---|---|
| Age, mean (SD) | 70.37 (13.81) | 71.78 (14.22) | 71.04 (14.01) |
| Weight, mean (SD) | 74.67 (19.79) | 75.51 (16.78) | 75.07 (18.41) |
| NIHSS score (admission), median (Q1, Q3) | 10.00 (7.00, 16.00) | 11.00 (6.00, 17.00) | 11.00 (6.00, 17.00) |
| Modified Rankin Score (admission), median (Q1, Q3) | 0.00 (0.00, 2.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) |
| Modified Rankin Score (3 mo posttreatment), median (Q1, Q3) | 2.00 (1.00, 4.00) | 2.00 (0.00, 3.00) | 2.00 (1.00, 4.00) |
| Sex, n (%) female | 138 (44) | 135 (47) | 273 (46) |
| Death, n (%) | 44 (14) | 59 (21) | 103 (17) |
| Diabetes mellitus, n (%)a | 67 (22) | 51 (19) | 118 (20) |
| Hypertension, n (%)a | 196 (66) | 179 (64) | 375 (65) |
| Hyperlipidemia, n (%)a | 128 (47) | 106 (40) | 234 (44) |
| Current smoker, n (%)a | 51 (20) | 37 (15) | 88 (17) |
| Previous smoker, n (%)a | 69 (43) | 46 (26) | 115 (34) |
NIHSS indicates National Institutes of Health Stroke Scale; Q1, quartile 1 around the median; Q3, quartile 3 around the median.
Numbers may not add to total sample size due to missing values.
The level of involvement with each intervention component at each intervention site is described in Table 3. As indicated in Table 3, there was a varying level of involvement with each intervention component, with site scores ranging from 11 up to 20 out of a maximum possible score of 22. Comparison of staff attitudes at baseline versus follow‐up found a significant positive change in attitude score for physicians (change in group mean score=1.4, 95% CI 0.3‐2.6; P<0.05) but not for nurses (P>0.5).
Table 3.
Level of Site Involvement With Each Intervention Component Measured by Participation Score
| Intervention Component | Intervention Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Executive support | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 2 |
| Preworkshop site meeting | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| Workshop 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 |
| Site visits | 1 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 1 |
| Case monitoring | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Feedback | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Teleconferences | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 1 | 2 | 1 |
| Workshop 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Medical training modules | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 2 | 2 |
| Nursing training modules | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Site‐based working group | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 1 |
| Overall score | 16 | 17 | 12 | 11 | 19 | 18 | 19 | 16 | 20 | 17 |
Score: 0=low‐level engagement; 1=medium‐level engagement; 2=high‐level engagement.
During the course of the trial (baseline and intervention periods) there were 11 018 stroke separations from intervention hospitals and 11 366 from control hospitals. Overall, during the baseline periods, 285 of 5331 stroke patients were treated with IVT in the intervention hospitals (5.3%, 95% CI 4.7% to 5.9%) compared with 314 of 5583 patients (5.6%, 95% CI 5.0% to 6.2%) in control hospitals. During the intervention study period, IVT rates increased in the intervention hospitals to an average of 8.9% (281 of 3160 strokes; 95% CI 7.9% to 9.9%). However, rates also increased over this time period in the control hospitals to an average of 8.2% (257 of 3116 strokes; 95% CI 7.3% to 9.2%). Finally, although the intervention hospitals maintained IVT rates over the postintervention period at an average of 8.7% (221 of 2527 strokes; 95% CI 7.6% to 9.8%), the IVT rates in the control hospitals declined to 7.9% (210 of 2667 strokes; 95% CI 6.9% to 8.9%).
The absolute difference in postintervention IVT rates between intervention and control hospitals adjusted for baseline IVT rate and stratum was 1.1% (95% CI −1.5% to 3.7%; P=0.38). Within strata differences between intervention and control hospitals, IVT rates were 1.1% (95% CI −2.8% to 5.1%) for the very‐low‐rate stratum, 1.8% (95% CI −2.5% to 6.2%) for the low‐rate stratum, and −2.4% (95% CI −12.6% to 7.8%) for the moderate‐rate stratum. The differences in IVT rates between intervention and control hospitals over time are summarized in Figure 3.
Figure 3.
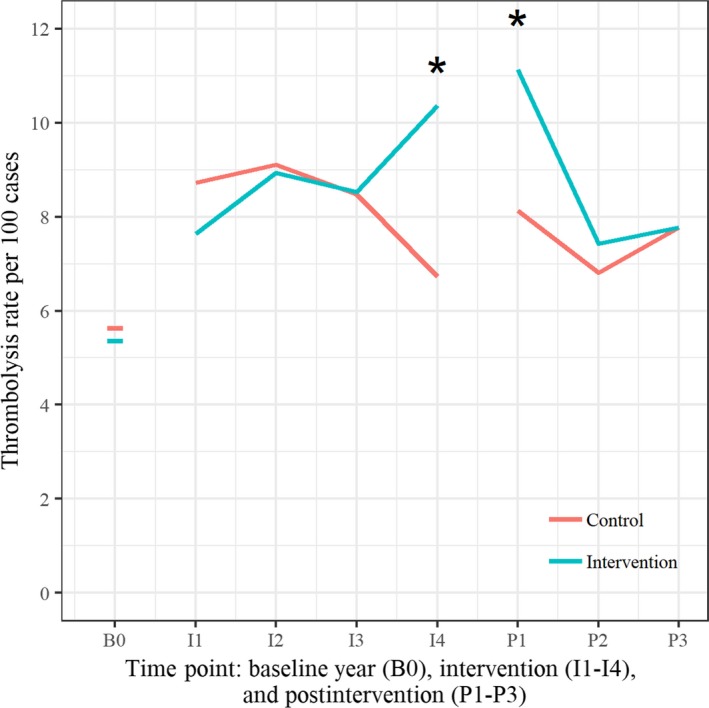
Differences in intravenous thrombolysis rates between intervention and control hospitals over time. (*rate difference intervention to control P < 0.05).
Table 4 and Figure 4 show the secondary analysis of the primary outcome, IVT rate ratios, and 95% CIs at each time point during the intervention and postintervention periods to the baseline IVT rates. The results indicate a statistically significant increase in IVT rate at the last time point of the intervention period and the first time point of the postintervention period. This effect appears to diminish over the rest of the postintervention period.
Table 4.
Intravenous Thrombolysis Rate Ratios (Relative to Baseline Time Point) and 95% CIs for Intervention and Control Hospitals Over Intervention Time Period
| Intervention | Postintervention | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | |
| Intervention | 1.442 | 1.619 | 1.611 | 1.923 | 2.016 | 1.35 | 1.482 |
| (1.103, 1.884) | (1.264, 2.074) | (1.257, 2.064) | (1.534, 2.411) | (1.618, 2.511) | (1.043, 1.746) | (1.15, 1.91) | |
| Control | 1.548 | 1.599 | 1.515 | 1.197 | 1.386 | 1.186 | 1.344 |
| (1.205, 1.988) | (1.244, 2.056) | (1.188, 1.932) | (0.903, 1.585) | (1.069, 1.797) | (0.917, 1.535) | (1.057, 1.71) | |
| Ratio (intervention/ control) | 0.931 | 1.012 | 1.063 | 1.607 | 1.454 | 1.138 | 1.103 |
| (0.646, 1.343) | (0.711, 1.44) | (0.751, 1.505) | (1.12, 2.306) | (1.035, 2.043) | (0.79, 1.637) | (0.777, 1.564) | |
An omnibus test suggested that there was negligible support for an interaction term, P=0.516.
Figure 4.
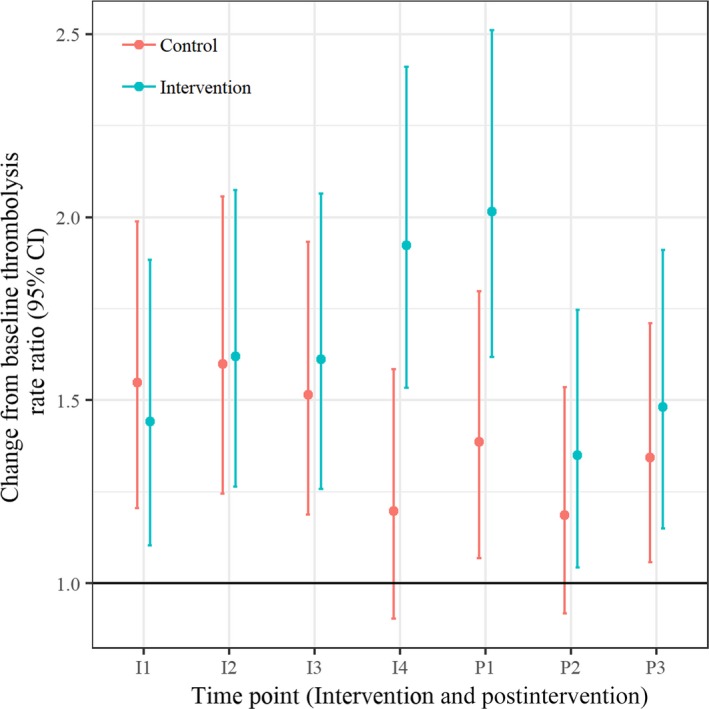
Intravenous thrombolysis rate ratios and 95% CIs over the intervention and postintervention time periods.
After adjustment for multiple comparisons, 1 control site did show a significantly lower rate of favorable outcome, as judged by mRS of 0 to 1, compared with the benchmark of 30%; some centers were performing significantly better (Table S2). Two intervention sites showed significantly better rates of intracranial hemorrhage postintervention, and no centers performed significantly worse on proportion of people with intracranial hemorrhage, compared with the benchmark of 6%; some centers were performing significantly better (Table S3).
Discussion
The TIPS multicomponent collaborative intervention resulted in a small but temporary improvement in IVT implementation rates across the 10 intervention hospitals. This rise was evident toward the end of the 16‐month intervention support period and dissipated over the 12 months following withdrawal of external support. The overall treatment effect of a 1.1% (95% CI −2.8% to 5.1%) absolute increase in thrombolysis rate, although comparable to the treatment effect in 2 previous implementation trials in IVT,19, 20 was not statistically significant when averaged over the entire course of the postintervention period (our a priori analysis). These 2 published randomized trials examined the effectiveness of complex multidimensional interventions, and both reported relatively modest increases in IVT rates. The INSTINCT (Increasing Stroke Treatment Through Interventional Change Tactics) trial19 used a cluster randomized design to test an IVT support intervention across 24 community hospitals in the US state of Michigan. Using a similar approach to TIPS with barrier identification and an interactive education intervention, INSTINCT showed a nonsignificant trend to higher IVT rates in intervention hospitals and overall low absolute differences in rates in the order of 1% to 1.5%. The PRACTISE (Promoting Acute Thrombolysis in Ischemic Stroke) trial20 used breakthrough methodology involving formation of local teams, identification of barriers, and setting of action plans and improvement goals along with a series of intervention site meetings. The PRACTISE intervention produced an average of 0.9% increase in IVT rates in 6 intervention hospitals for all ischemic stroke presentations but a statistically significant 5.2% increase in the subgroup of patients arriving in hospital within 4 hours of onset. A lower tendency for intervention hospital staff to reject administering tPA for relative contraindications or unconventional reasons was noted. A third published implementation trial21 conducted in 18 emergency departments in France tested a systems intervention in a randomized stepped‐wedge controlled design. The intervention targeted emergency physicians and nurses, used a train‐the‐trainer approach, and aimed to decrease in‐hospital management times and increase the proportion of ischemic stroke patients receiving thrombolysis. This trial showed a significantly higher overall thrombolysis proportion in the intervention group compared with the control group (38.6% versus 34.8%) and reported a significant association between the study intervention and the overall thrombolysis proportion after adjusting for confounding factors. However, thrombolysis was considered a primary outcome only once statistical power had been confirmed. A fourth trial completed in 2016 and recently published22, 23 used a cluster‐randomized controlled design to test a triage, treatment, and transfer care bundle intervention aiming to improve emergency care for patients with acute stroke. The T3 Trial (Triage, Treatment, and Transfer Implementation Cluster Randomized Controlled Trial) featured multidisciplinary workshops to assess barriers and identify strategies, educational material delivered face to face, online, and in written form, support from local and national clinical experts, and proactive site visits and teleconferences. Despite implementation of a nurse‐initiated evidence‐based practice change intervention previously demonstrated to be effective in stroke unit environments, the trial returned a neutral result, with no statistically significant differences evident at follow‐up for 90‐day mRS or secondary outcomes. The trialists suggested that substandard hospital performance on vital emergency department stroke processes of care may have contributed to the nonsignificant primary result, although they acknowledged that thrombolysis administration rates in both groups were above 13%, exceeding the national average. The TIPS intervention used a generally recommended combination of elements based around behavioral theory, addressing both health systems barriers and clinician behavior changes in an effort to support clinicians to develop greater stroke thrombolysis capability and improve hospital systems. However, the nonsustained improvement in IVT rates in previous studies and replicated here suggests that many currently deployed strategies are not sufficiently robust to overcome practice change barriers in a complex system that involves multiple individuals. It is noteworthy that we did not demonstrate any change in the moderate rate stratum, suggesting that these hospitals may have reached their maximum capability given the available resources. Our process measures indicated that some change in physician attitudes may have occurred during the trial and that 2 of the 10 intervention sites were only moderately engaged with the implementation activities. Overall, the study data suggest a number of potential lessons for system change generally, as enumerated and discussed below.
The formation and maintenance of site‐based quality improvement teams that aimed to lead local barrier identification, solution generation, solution implementation, and goal setting were notable deficiencies at many intervention sites. Establishment of these teams may have been compromised by workload pressure for the lead stroke physician, generally a visiting medical officer running a busy hospital service with limited middle‐grade medical staff support. A potential solution, recruitment of additional medical workforce focused on translation and systems improvement, was not a component of the TIPS intervention. Despite a focus in TIPS on developing advanced stroke nursing skills via web‐based education modules, the time required for the intervention hospital nurses to complete training and the limited existing stroke expert nursing capability in regional centers may have further compromised the formation of functional quality improvement teams. Similarly, lack of dedicated time appeared to be a factor in very few medical staff completing the purpose‐built TIPS interactive online training modules, despite baseline surveys and interviews indicating a need for training.
Hospitals are complex dynamic systems,24 and shifting behavior may take longer than expected. Despite multiple modalities targeting system and individual factors in an active and interactive way, it was only in the past 4 months of the 16‐month intervention period that a shift in implementation was evident.
Although the enhanced rates of IVT continued for the first time block of the postintervention period, rates thereafter declined in line with the removal of the active intervention. Perhaps lasting behavior change requires a much longer period of support and active intervention (possibly via additional workshops and more opportunities for peer‐to‐peer learning) than we currently allowed for. Additional challenges are likely to be turnover of staff and the fact that good working teams and trust are hard to develop and sustain and are sensitive to even minor changes in team structure. Complex decisions and interventions such as the IVT cannot easily be hardwired in a system through IT platforms. Trials such as PRACTISE20 and INSTINCT19 have reported positive effects on IV tPA rates following intervention phases of 24 and 12 months, respectively, although the latter was only relevant for a subgroup of study sites. The French trial21 did not report on the duration of the study phases; thus, it would be difficult to elaborate about the sustainability of the results of this trial.
In Australia especially, here has been some heated, long‐standing, and factional disagreement between neurologists and emergency physicians about the effectiveness of thrombolysis,25, 26 and some centers had to overcome hostile colleagues to facilitate IVT implementation. Such barriers can substantially hinder system change. Local champions, such as the stroke unit leaders who were present at every participating center in this trial, are not necessarily sufficient on their own to overcome such large barriers, particularly when an intervention such as IVT requires collaboration between emergency department teams and stroke teams.
It is clear that some centers rose to the challenge of system change, although others were unable to achieve much progress. It will be instructive to look at the characteristics of these centers (including leadership styles or skills and team climate) where the intervention fell on fertile soil compared with those where it fell on more rocky terrain.
Aspects that were out of scope for TIPS but are recognized to have potential impact on IVT implementation are the streamlining of prehospital systems of care and telemedicine support for tPA delivery in smaller regional centers that lack stroke expert workforce and are limited by the long travel times between patient residences and the hospitals. Acute stroke telemedicine in the emergency departments of regional hospitals was implemented in Victoria before commencement of TIPS,27 and a hospital bypass and prenotification system was rolled out across New South Wales in 2012‐2013. Confining larger‐scale prehospital systems reforms or telestroke models of care to intervention hospitals alone was not a feasible option and therefore was not included in the intervention package.
Further analyses will be undertaken to examine which components of the intervention may have had the greatest impact on clinician attitudes, systems changes, and IVT rates during the time periods when significant changes were evident. However, both implementation and evaluation of complex multifaceted IVT support interventions are challenging. Limitations of the TIPS trial include the need to use hospital‐collected rather than independent or objective data sources and the inability to blind sites to group allocation. The study power may also have been affected by the policy changes and resultant attention on tPA during the study period. Diffusion of intervention components to control sites was deemed unlikely due to the geographical dispersion of sites and implementation challenges in intervention hospitals. It was generally challenging to encourage intervention site cluster guardians and their staffs to uptake the intervention; thus, inadvertent implementation of strategies at control sites seems improbable. Policy changes and initiatives were not selectively offered to hospitals on the basis of their intervention allocation in the TIPS study. However, a differential effect of non‐TIPS initiatives by study arm cannot be completely ruled out. The TIPS results suggest that many of the barriers to achieving high rates of tPA delivery cannot be overcome solely using existing systems, existing workforce establishments, and clinical practice improvement methodology. Some of the intervention functions referenced within the behavior change wheel,16 including incentivization and restriction, were not able to be used and may be necessary to achieve substantial and sustained change. Our intervention had a strong focus on clinician capability and motivation but was less able to influence opportunity, that is, the capacity of clinicians to engage with the intervention, because of their high and diverse workloads. A longer intervention period (as suggested by the secondary analyses) and greater intensity of the TIPS intervention activities may also be required, such as additional workshops and more peer‐to‐peer interaction. The results have implications for both the redesign of the intervention in an effort to achieve greater and more sustainable change and the development of higher‐level policy for improvement in stroke thrombolysis implementation, addressing issues such as expert workforce capacity building, healthcare management accountability to benchmarks, and incentives for achieving benchmark performance in IVT.
Appendix
The TIPS (Thrombolysis Implementation in Stroke) Study Group
Professor Craig Anderson (The University of New South Wales, Sydney, New South Wales, Australia; The George Institute for Global Health, Sydney, New South Wales, Australia). Dr Tim Ang (The University of Newcastle, School of Medicine and Public Health, Callaghan, New South Wales, Australia; John Hunter Hospital, New Lambton Heights, New South Wales, Australia; Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia). Dr Andrew Bivard (The University of Newcastle, School of Medicine and Public Health, Callaghan, New South Wales, Australia; Hunter Medical Research Institute, New Lambton Heights, New South Wales, Australia; University of Melbourne, Faculty of Dentistry, Medicine and Health Sciences, Melbourne, Victoria, Australia). Mr Greg Cadigan (Healthcare Improvement Unit, Brisbane, Queensland, Australia). Ms Sherisse Celestino (Western Hospital, Footscray, Victoria, Australia). Mr Tim Coles (Central Gippsland Health, Sale, Victoria, Australia). Professor Alistair Corbett (Concord Repatriation General Hospital, Concord, New South Wales, Australia). Dr Lisa Dark (Tamworth Hospital, Tamworth, New South Wales, Australia). Ms Susan Day (Royal North Shore Hospital, Department of Neurology, St. Leonards, New South Wales, Australia). Ms Jennifer Dennett (Central Gippsland Health, Sale, Victoria, Australia). Professor Geoffrey Donnan (The University of Melbourne, Parkville, Victoria, Australia; The Florey Institute of Neuroscience and Mental Health, Parkville, Victoria, Australia). Mr Malcolm Evans (Hunter New England Health, New Lambton Heights, New South Wales, Australia; University of Newcastle, School of Medicine and Public Health, Callaghan, New South Wales, Australia). Dr Kavisha Fernando (Western Hospital, Footscray, Victoria, Australia). Dr Richard Geraghty (Redcliffe Hospital, Redcliffe, Queensland, Australia). Professor Richard Gerraty (Epworth HealthCare, Richmond, Victoria, Australia). Dr Alice Grady (The University of Newcastle, Callaghan, New South Wales, Australia; Hunter Medical Research Institute, New Lambton Heights, New South Wales, Australia; Hunter New England Local Health District, Population Health, Wallsend, New South Wales, Australia). Dr Rohan Grimley (The University of Queensland, Sunshine Coast Clinical School, Birtinya, Queensland, Australia). Dr Jason Gu (Sydney Adventist Hospital, Wahroonga, New South Wales, Australia; The Wollongong Hospital, Wollongong, New South Wales, Australia). Professor Graeme J. Hankey (The University of Western Australia, Crawley, Western Australia, Australia; Sir Charles Gairdner Hospital, Perth, Western Australia, Australia). Ms Kim Hoffman (Lismore Base Hospital, Lismore, New South Wales, Australia). Dr James Hughes (Tamworth Hospital, Tamworth, New South Wales, Australia). Dr Jerome Ip (Hornsby‐Ku‐ring‐gai Hospital, Hornsby, New South Wales, Australia). Dr Bronwyn Jenkins (Royal North Shore Hospital, St Leonards, New South Wales, Australia). Mr Mark Jones (The University of Newcastle, School of Medicine and Public Health, Callaghan, New South Wales, Australia; Hunter Medical Research Institute, New Lambton Heights, New South Wales, Australia). Dr Martin Jude (Wagga Wagga Base Hospital, Wagga Wagga, New South Wales, Australia; UNSW Rural Clinical School Wagga Wagga Campus, Wagga Wagga, New South Wales, Australia). Ms Lara Kaauwai (Hunter New England Health, New Lambton Heights, New South Wales, Australia). Mr Malcolm Kanard (Hornsby‐Ku‐ring‐gai Hospital, Hornsby, New South Wales, Australia). Dr Matthew Kinchington (Port Macquarie Base Hospital, Port Macquarie, New South Wales, Australia). Clinical Associate Professor Martin Krause (Royal North Shore Hospital, Northern Clinical School, University of Sydney, St. Leonards, New South Wales, Australia). Ms Sarah Kuhle (Redcliffe Hospital, Redcliffe, Queensland, Australia). Dr Stanley Levy (Campbelltown Hospital, Campbelltown, New South Wales, Australia). Mr Mark Longworth (Stroke Services NSW/ACI Stroke Care Network, Chatswood, New South Wales, Australia). Ms Beverley Macdonald (Campbelltown Hospital, Campbelltown, New South Wales, Australia). Ms Elizabeth Mackey (Western Hospital, Footscray, Victoria, Australia). Dr Krishna Mandaleson (Central Gippsland Health Service, Sale, Victoria, Australia). Dr Ferdi Miteff (Hunter New England Health, New Lambton Heights, New South Wales, Australia). Ms Katherine Mohr (Wagga Wagga Base Hospital, Wagga Wagga, New South Wales, Australia). Dr Stephen Moore (Lismore Base Hospital, Lismore, New South Wales, Australia). Ms Kristy Morris (Hunter New England Health, New Lambton Heights, New South Wales, Australia). Ms Elizabeth O'Brien (Royal North Shore Hospital, St. Leonards, New South Wales, Australia). Dr Christopher Oldmeadow (The University of Newcastle, School of Medicine and Public Health, Callaghan, New South Wales, Australia; Hunter Medical Research Institute, New Lambton Heights, New South Wales, Australia). Mr Bruce Paddock (Ambulance Service of NSW, Rozelle, New South Wales, Australia). Ms Kim Parrey (Port Macquarie Base Hospital, Port Macquarie, New South Wales, Australia). Ms Rachel Peake (Tamworth Hospital, Tamworth, New South Wales, Australia). Ms Angela Royan (Hunter New England Health, New Lambton Heights, New South Wales, Australia). Ms Michelle Russell (Hunter New England Health, New Lambton Heights, New South Wales, Australia). Ms Margaret Stevenson (Frankston Hospital, Frankston, Victoria, Australia). Dr Sanjeev Taneja (Wollongong Hospital, Wollongong, New South Wales, Australia). Ms Natalie Teasdale (Western Hospital, Footscray, Victoria, Australia). Dr Zoe Terpening (Sydney Adventist Hospital, Sydney Adventist Hospital Clinical School, The University of Sydney, Wahroonga, New South Wales, Australia). Ms Anne Van Berkel (Central Gippsland Health, Sale, Victoria, Australia). Professor John D. G. Watson (Sydney Adventist Hospital Clinical School, University of Sydney, Sydney Adventist Hospital, Wahroonga, New South Wales, Australia). Dr Yolande Weiner (Logan Hospital, Meadowbrook, Queensland, Australia). Associate Professor Tissa Wijeratne (The University of Melbourne, Parkville, Victoria, Australia; Western Hospital, Footscray, Victoria, Australia). Ms Alison Wilson (Port Macquarie Base Hospital, Port Macquarie, New South Wales, Australia). Dr Nigel Wolfe (Blacktown Mount Druitt Hospital, Blacktown, New South Wales, Australia).
Sources of Funding
This study was funded by a National Health and Medical Research Council Partnership Grant (569328), partially funded by a National Health and Medical Research Council Practitioner Fellowship (1043913) and National Health and Medical Research Translating Research into Practice Fellowship, and included Partnership Grant contribution funding from Boehringer Ingelheim, in‐kind support from the Agency for Clinical Innovation Stroke Care Network/Stroke Services New South Wales, the National Stroke Foundation, and New South Wales Cardiovascular Research Network‐National Heart Foundation, with cash contribution from the Victorian Stroke Clinical Network and infrastructure funding from Hunter Medical Research Institute and The University of Newcastle.
Disclosures
Sanson‐Fisher, Levi, Paul, D'Este, Parsons, Bladin, Lindley, and Attia declare the receipt of support from the following third parties: National Health and Medical Research Council grant, cash contributions from Boehringer Ingelheim, the Victorian Stroke Clinical Network, and the New South Wales Cardiovascular Research Network‐National Heart Foundation, and in‐kind support from the Agency for Clinical Innovation Stroke Care Network/Stroke Services New South Wales, the National Stroke Foundation, and the New South Wales Cardiovascular Research Network‐National Heart Foundation. From the TIPS study group: C.A. acknowledges significant funding from National Health and Medical Research Council grants, modest fees for advisory board membership at AMGEN, and modest travel support and honoraria for speaking fees at Takeda. Graeme Hankey reports modest honoraria from Bayer for lecturing at sponsored scientific symposia about stroke prevention in atrial fibrillation. Jeremy Grimshaw reports modest nonfinancial support from Boehringer Ingelheim and modest personal fees from AbbVie. John Watson declares attendance at the Annual Australian Stroke Unit Heads meeting to which Boehringer Ingelheim contributes. Professor Richard Gerraty declares modest support from Boehringer Ingelheim in the form of travel support for educational meetings.
Supporting information
Table S1. Details Relating to the Thrombolysis Implementation in Stroke Intervention
Table S2. Proportion at Each Hospital With Good Functional Outcome Compared With 30% Benchmark
Table S3. Proportion at Each Hospital With Poor Functional Outcome Compared With 6% Benchmark
Acknowledgments
The authors would like to especially acknowledge the support from Professor Jeremy Grimshaw, A/Prof Andrew Lee, and Dr Heather Buchan who provided advice to the trial steering committee during trial establishment. The support from Professor Helen Dewey, Chair of the Victorian Stroke Clinical Network, is acknowledged. The role of the National Science Foundation in the provision of funding support and contribution to the development of the TIPS data collection tool is gratefully acknowledged. The study team would also like to acknowledge the following contributors for their support of the study: Dr Iain Bruce, Ms Camelia Burdusel, A/Prof Ernest Butler, Ms Michelle Doughty, Dr Frances Gearon, Dr Sumitha Gounden, Ms Amanda Jayakody, Ms Karen Longworth, A/Prof Michael Pollack, Ms Shiho Rose, Ms Rochelle Watson, Dr Jayantha Rupasinghe, Ms Fiona Ryan, Dr Andrew Searles, Ms Nicola Mitchell, Ms Sue Roberts, Ms Jennifer Goff, Ms Zoe Campbell, Ms Sue Huckson, Dr Paul Laird, Mr Christopher Price, Dr Chris Gavaghan, Ms Melissa Christof, Ms Michaela Plante, Mr Kelvin Hill, and Dr Erin Lalor. All staff and patients at participating hospitals are acknowledged. Boehringer Ingelheim is a collaborative partner in this project in accordance with National Health and Medical Research Council Partnership Grant rules and will have an interest in increasing the use of the tPA drug. They do not, however, have any rights to publish any results arising from this project.
(J Am Heart Assoc. 2020;9:e012732 DOI: 10.1161/JAHA.119.012732.)
Contributor Information
Christopher R. Levi, Email: christopher.levi@unsw.edu.au.
the TIPS (Thrombolysis Implementation in Stroke) Study Group:
Craig Anderson, Tim Ang, Andrew Bivard, Greg Cadigan, Sherisse Celestino, Tim Coles, Alistair Corbett, Lisa Dark, Susan Day, Jennifer Dennett, Geoffrey Donnan, Malcolm Evans, Kavisha Fernando, Richard Geraghty, Richard Gerraty, Alice Grady, Rohan Grimley, Jason Gu, Graeme J. Hankey, Kim Hoffman, James Hughes, Jerome Ip, Bronwyn Jenkins, Mark Jones, Martin Jude, Lara Kaauwai, Malcolm Kanard, Matthew Kinchington, Martin Krause, Sarah Kuhle, Stanley Levy, Mark Longworth, Beverley Macdonald, Elizabeth Mackey, Krishna Mandaleson, Ferdi Miteff, Katherine Mohr, Stephen Moore, Kristy Morris, Elizabeth O'Brien, Christopher Oldmeadow, Bruce Paddock, Kim Parrey, Rachel Peake, Michelle Russell, Margaret Stevenson, Sanjeev Taneja, Natalie Teasdale, Zoe Terpening, Anne Van Berkel, John D. G. Watson, Yolande Weiner, Tissa Wijeratne, Alison Wilson, and Nigel Wolfe
References
- 1. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJL, Forouzanfar MH; Global Burden of Diseases, Injuries, and Risk Factors Study 2013 and Stroke Experts Writing Group . Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 2. Thrift AG, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Feigin VL, Norrving B, Donnan GA, Cadilhac DA. Global stroke statistics. Int J Stroke. 2017;12:13–32. [DOI] [PubMed] [Google Scholar]
- 3. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M; Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. [reprint in Int J Nurs Stud. 2013;50:587‐592; pmid: 23159157]. BMJ. 2008;337:a1655. [DOI] [PubMed] [Google Scholar]
- 4. Paul CL, Ryan A, Rose S, Attia JR, Kerr E, Koller C, Levi CR. How can we improve stroke thrombolysis rates? A review of health system factors and approaches associated with thrombolysis administration rates in acute stroke care. Implement Sci. 2016;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quain DA, Parsons MW, Loudfoot AR, Spratt NJ, Evans MK, Russell ML, Royan AT, Moore AG, Miteff F, Hullick C. Improving access to acute stroke therapies: a controlled trial of organised pre‐hospital and emergency care. Med J Aust. 2008;189:429–433. [DOI] [PubMed] [Google Scholar]
- 6. Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in‐hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. [DOI] [PubMed] [Google Scholar]
- 7. Meretoja A, Weir L, Ugalde M, Yassi N, Yan B, Hand P, Truesdale M, Davis SM, Campbell BC. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology. 2013;81:1071–1076. [DOI] [PubMed] [Google Scholar]
- 8. Skolarus LE, Meurer WJ, Shanmugasundaram K, Adelman EE, Scott PA, Burke JF. Marked regional variation in acute stroke treatment among Medicare beneficiaries. Stroke. 2015;46:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scholten N, Pfaff H, Lehmann HC, Fink GR, Karbach U. Who does it first? The uptake of medical innovations in the performance of thrombolysis on ischemic stroke patients in Germany: a study based on hospital quality data. Implement Sci. 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scherf S, Limburg M, Wimmers R, Middelkoop I, Lingsma H. Increase in national intravenous thrombolysis rates for ischaemic stroke between 2005 and 2012: is bigger better? BMC Neurol. 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Royal College of Physicians . Sentinel stroke audit programme (SSNAP): results for clinical commissioning groups (CCG) in England and local health boards (LHB) in Wales. National Results. London; 2017. [Google Scholar]
- 12. Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue‐type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samsa GP, Reutter RA, Parmigiani G, Ancukiewicz M, Abrahamse P, Lipscomb J, Matchar DB. Performing cost‐effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol. 1999;52:259–271. [DOI] [PubMed] [Google Scholar]
- 14. Paul CL, Levi CR, D'Este CA, Parsons MW, Bladin CF, Lindley RI, Attia JR, Henskens F, Lalor E, Longworth M. Thrombolysis Implementation in Stroke (TIPS): evaluating the effectiveness of a strategy to increase the adoption of best evidence practice–protocol for a cluster randomised controlled trial in acute stroke care. Implement Sci. 2014;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 16. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agency for Clinical Innovation, Health Economics and Evaluation Team . NSW stroke reperfusion program evaluation report. Chatswood, NSW: ACI; 2015.
- 18. Australian Institute of Health and Welfare . Australian hospital peer groups. Health services series no. 66. Cat. no. HSE 170 Canberra, ACT: AIHW; 2013. [Google Scholar]
- 19. Scott PA, Meurer WJ, Frederiksen SM, Kalbfleisch JD, Xu Z, Haan MN, Silbergleit R, Morgenstern LB; INSTINCT Investigators . A multilevel intervention to increase community hospital use of alteplase for acute stroke (INSTINCT): a cluster‐randomised controlled trial. Lancet Neurol. 2013;12:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dirks M, Niessen LW, van Wijngaarden JD, Koudstaal PJ, Franke CL, van Oostenbrugge RJ, Huijsman R, Lingsma HF, Minkman MM, Dippel DW; Promoting Acute Thrombolysis in Ischemic Stroke (PRACTISE) Investigators . Promoting thrombolysis in acute ischemic stroke. Stroke. 2011;42:1325–1330. [DOI] [PubMed] [Google Scholar]
- 21. Haesebaert J, Nighoghossian N, Mercier C, Termoz A, Porthault S, Derex L, Gueugniaud P‐Y, Bravant E, Rabilloud M, Schott A‐M. Improving access to thrombolysis and in‐hospital management times in ischemic stroke: a stepped‐wedge randomized trial. Stroke. 2018;49:405–411. DOI: 10.1161/STROKEAHA.117.018335. [DOI] [PubMed] [Google Scholar]
- 22. Middleton S, Levi C, Dale S, Cheung NW, McInnes E, Considine J, D'Este C, Cadilhac DA, Grimshaw J, Gerraty R. Triage, treatment and transfer of patients with stroke in emergency department trial (the T 3 Trial): a cluster randomised trial protocol. Implement Sci. 2016;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Middleton S, Dale S, Cheung NW, Cadilhac DA, Grimshaw JM, Levi C, McInnes E, Considine J, McElduff P, Gerraty R, Craig LE, Schadewaldt V, Fitzgerald M, Quinn C, Cadigan G, Denisenko S, Longworth M, Ward J, D'Este C, Grimley R, Paolini R, Allen T, Jammali‐Blasi A, Phillips R, Pitkin J, Salama E, Sheridan T, McElduff B. Nurse‐initiated acute stroke care in emergency departments. Stroke. 2019;50:1346–1355. [DOI] [PubMed] [Google Scholar]
- 24. Braithwaite J, Churruca K, Long JC, Ellis LA, Herkes J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med. 2018;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi CR. Tissue plasminogen activator (tPA) in acute ischaemic stroke: time for collegiate communication and consensus. Med J Aust. 2004;180:634–636. [PubMed] [Google Scholar]
- 26. Fatovich DM. Tissue plasminogen activator (tPA) in acute ischaemic stroke: time for collegiate communication and consensus. Med J Aust. 2005;182:44–45. [PubMed] [Google Scholar]
- 27. Bladin CF, Molocijz N, Ermel S, Bagot KL, Kilkenny M, Vu M, Cadilhac DA; VST program investigators . Victorian Stroke Telemedicine Project: implementation of a new model of translational stroke care for Australia. Intern Med J. 2015;45:951–956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details Relating to the Thrombolysis Implementation in Stroke Intervention
Table S2. Proportion at Each Hospital With Good Functional Outcome Compared With 30% Benchmark
Table S3. Proportion at Each Hospital With Poor Functional Outcome Compared With 6% Benchmark


