Abstract
Background
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy. Current guidelines endorse management in expert centers, but patient socioeconomic status can affect access to specialty care. The effect of socioeconomic status and specialty care access on HCM outcomes has not been examined.
Methods and Results
We conducted a retrospective cohort study that examined outcomes among HCM patients receiving care in the Yale New Haven Health System between June 2011 and December 2017. Patients were assigned to lower or higher socioeconomic status groups (LSES/HSES) based on medical insurance provider and to receivers of specialty care (SC) at Yale's Inherited Cardiomyopathy clinic or general cardiology care (GC). The primary outcome was all‐cause death, and the secondary outcome was all‐cause hospitalization. We identified 953 HCM patients; 820 (86%) were HSES and 133 (14%) were LSES. Forty‐three (4.5%) patients died from cardiac and noncardiac causes. LSES patients within the general cardiology care cohort had significantly higher all‐cause mortality compared with HSES patients (adjusted hazard ratio, [95% CI]=10.06 [4.38–23.09]; P<0.001). This was not noted in the specialty care cohort (adjusted hazard ratio, [95% CI]=2.87 [0.56–14.73]; P=0.21). The moderator effect of specialty care on mortality difference between LSES versus HSES, however, did not reach statistical significance (hazard ratio, 0.29 [0.05–1.77]; P=0.18). Specialist care was associated with increased hospitalization (adjusted hazard ratio, [95% CI]=3.28 [1.11–9.73]; P=0.03 for LSES; 2.19 [1.40–3.40]; P=0.001 for HSES).
Conclusions
Socioeconomically vulnerable HCM patients had higher mortality when not referred to specialty care. Further study is needed to understand the underlying causes.
Keywords: hypertrophic cardiomyopathy, socioeconomic disadvantage, health outcomes, cardiomyopathy specialty care
Subject Categories: Race and Ethnicity, Quality and Outcomes, Cardiomyopathy, Mortality/Survival
Clinical Perspective
What Is New?
Socioeconomically disadvantaged patients with hypertrophic cardiomyopathy had increased mortality when not involved in care at a specialty center.
Specialty care of hypertrophic cardiomyopathy patients leads to more‐consistent guideline‐directed testing and treatments.
What Are the Clinical Implications?
Patients with hypertrophic cardiomyopathy should be considered for referral to specialty care, especially among those who are socioeconomically disadvantaged.
Further study of specialty care should be undertaken to better assess for the drivers of patient benefit and evaluate for improved implementation strategies.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy, affecting >600 000 people in the United States alone.1 It is morphologically characterized by left ventricular hypertrophy in the absence of increased afterload, and pathologically characterized by myocyte hypertrophy and disarray, as well as interstitial fibrosis and abnormal myocardial fiber twitch and relaxation times.2 Clinically, the disease causes heart failure (HF), atrial fibrillation, and sudden cardiac death, with age‐adjusted mortality of 1.5 to 3.0 times that of the general population.3
Prognosis is affected by several risk factors, including age of symptom onset, genotypic status, family history of sudden death, and pathological features (wall thickness, fibrosis).3 Current society guidelines emphasize the importance of team‐based comprehensive specialty care (SC) for optimal treatment of HCM.4 Recent work has demonstrated differences in resource utilization between specialty and nonspecialty centers.5 Socioeconomic status (SES) is known to be an important driver of healthcare access, outcomes, and resource utilization6, 7, 8 and may also critically influence access to SC.9 The combined effect of team‐based SC access and SES on HCM outcomes has not been previously examined.
The Yale New Haven Health System (YNHHS) is a large multihospital medical system in southern New England serving ≈1.5 million patients. We compared outcomes for HCM patients of different socioeconomic background, receiving care in either a team‐based specialty HCM care center or followed by general cardiologists. The purpose of this study was to observationally assess for differences in outcomes and guideline adherence.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Sample
This was a retrospective cohort study that included all patients with a diagnosis of HCM receiving care in the YNHHS anytime between June 2011 and December 2017. This included patients cared for by the Yale Inherited Cardiomyopathy program, the only specialty HCM care program in the state of Connecticut. This program offers a team‐based holistic approach to diagnosis, risk stratification, and treatment of HCM patients, including psychosocial support from a dedicated social worker.
Eligible patients were identified by querying the YNHHS’ electronic medical record (EMR) system (Epic Systems Corp., Verona, WI). Inclusion criteria was age ≥18 years, the keywords “hypertrophic cardiomyopathy” (including any variations such as “hypertrophic obstructive cardiomyopathy” or “apical variant hypertrophic cardiomyopathy”) or nonstandard/disused HCM diagnostic nomenclature (“idiopathic hypertrophic subaortic stenosis”) on the patient's medical problem list as captured on EMR, and at least 1 documented cardiology visit in the YNHHS. Exclusion criteria were the existence of diagnostic confounders for HCM (uncontrolled hypertension, moderate or severe aortic stenosis, subaortic membrane, cardiac amyloid, myopathy, or storage disease).
EMR query captured demographics (eg, age, sex, race/ethnicity, and health insurance), medical history, surgeries, cardiology visits, and HCM‐related medical resource utilization (echocardiography, cardiac magnetic resonance imaging [MRI], Holter monitoring, and cardiopulmonary exercise tests). Eligible subjects were designated to 1 of 2 groups, using their medical insurance as a proxy for income/SES. Patients with Medicaid/no insurance were designated to the lower socioeconomic group (LSES), whereas patients with any other insurance were designated to the higher socioeconomic group (HSES). In addition, patients were categorized depending on having access to SC or being cared for by general cardiology practitioners alone (GC). Access to SC was defined as at least 1 visit to Yale's Inherited Cardiomyopathy program.
The primary outcome of the study was death from all causes. Morbidity was examined by tracking hospitalizations for any cause after the first visit to a cardiology practice. For patients who met the primary outcome, we conducted a detailed chart review to determine cause of death.
Statistical Analyses
We describe patient characteristics as mean and SD for continuous variables and as frequency and percent for categorical variables. Student t tests, chi‐square tests, or Fisher's exact tests were used to compare characteristics and clinical outcomes between groups, as appropriate. A Cox proportional hazards model was built to examine the effect of SES on all‐cause mortality and hospitalization. Models were adjusted for potential confounders, including age, sex, and race, and comorbidities, including coronary artery disease, diabetes mellitus, and hypertension. Patients were followed until death or the last day of follow‐up in our study (December 31, 2017). The moderation effect of SC was determined by including an interaction term between socioeconomic group and SC/GC in the model. All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC), with 2‐sided statistical tests and an alpha of 0.05. The study protocol was approved by the Yale Institutional Review Board and granted a waiver for informed consent.
Results
Study Cohort Characteristics
A total of 1062 patients within the YNHHS were identified with the diagnosis of HCM. One hundred nine patients were excluded either because they received cardiology care outside the YNHHS (EMR failing to capture at least 1 in‐system cardiology clinic visit) or were identified as also having a possible diagnostic confounder for HCM, leaving 953 subjects available for analysis (Figure 1). Mean age was 58.6±18.6 years. Fifty‐eight percent were males, with 75% being white, 12% black, 1% Asian, and 12% of other/unknown race. Age‐related comorbidities were common, with 12% of study patients having a history of coronary artery disease, 14% of diabetes mellitus, and 55% of hypertension (Table 1). Regarding SES, 133 patients (14%) had either Medicaid as their medical insurance or were uninsured and were thus assigned to the LSES group, whereas the rest (n=820; 86%) were assigned to the HSES group. Seventy‐four LSES patients (55.6%) and 314 HSES patients (38.3%) received care at the specialty center (SC). The remaining 59 (44.4%) LSES and 506 (61.7%) HSES patients received GC care only.
Figure 1.
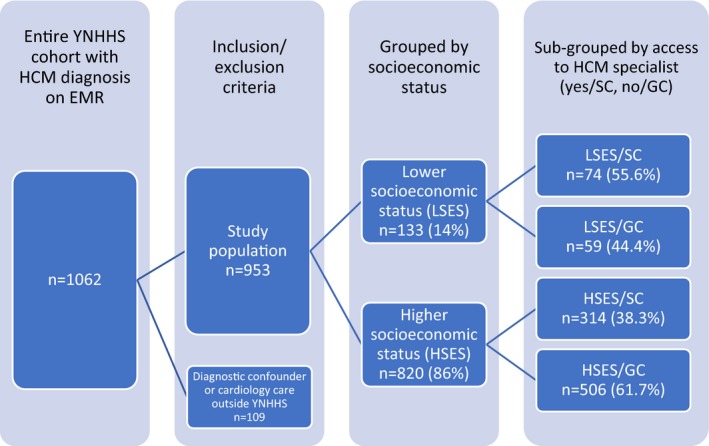
Breakdown of enrolled subjects. EMR indicates electronic medical record; GC, general cardiology care; HCM, hypertrophic cardiomyopathy; HSES indicates high socioeconomic status; LSES, low socioeconomic status; SC, specialty care; YNHHS, Yale New Haven Health System.
Table 1.
Characteristics of Hypertrophic Cardiomyopathy Populations: Patient Groups by Socioeconomic Status and Access to Specialty Care
| Higher Socioeconomic Status (HSES) | Lower Socioeconomic Status (LSES) | P Value (between totals) | |||||
|---|---|---|---|---|---|---|---|
| SC | GC | Total | SC | GC | Total | ||
| n (%) | 314 (38) | 506 (62) | 820 | 74 (56) | 59 (44) | 133 | |
| Age, y (SD) | 54.67 (15.6) | 65.2 (17.3) | 61.1 (17.4)a | 45.7 (15.5) | 40.2 (21.0) | 43.3 (18.3) | <0.001a |
| Sex, n (%) | |||||||
| Male | 199 (63) | 273 (54) | 472 (58)a | 49 (66) | 33 (45) | 82 (62) | <0.37a |
| Female | 115 (37) | 223 (46) | 348 (42)a | 25 (34) | 26 (55) | 51 (38) | |
| Race, n (%) | |||||||
| White | 251 (80) | 392 (77) | 643 (78) | 38 (51) | 29 (49) | 67 (50) | <0.001a |
| Black | 31 (10) | 48 (10) | 79 (10) | 20 (27) | 16 (27) | 36 (28) | |
| Asian | 3 (1) | 5 (1) | 8 (1) | 2 (3) | 1 (2) | 3 (2) | |
| Other/unknown | 29 (9) | 61 (12) | 90 (11) | 14 (19) | 13 (22) | 27 (20) | |
| Comorbidities, n (%) | |||||||
| CAD | 33 (11) | 75 (15) | 108 (13) | 3 (4) | 3 (5) | 6 (5) | 0.004a |
| DM | 31 (10) | 87 (17) | 118 (14)a | 11 (15) | 6 (10) | 17 (13) | 0.62 |
| Hypertension | 159 (51) | 313 (62) | 472 (56)a | 34 (46) | 25 (42) | 59 (44) | 0.005a |
CAD indicates coronary artery disease; DM, diabetes mellitus; GC, general cardiology care; SC, specialty care.
Denotes statistically significant differences (P<0.05) between SC and GC within HSES and LSES groups.
Primary Outcome (All‐Cause Mortality)
During the follow‐up period (mean 3.6±1.8 years), 43 (4.5%) patients died from cardiac and noncardiac causes (Table 2). Specifically, LSES patients within the GC cohort had significantly higher all‐cause mortality compared with HSES patients after adjustment for age, sex, race, and comorbidities (adjusted hazard ratio, 10.06 [4.38–23.09]; P<0.001; Table 3 and Figure 2A). This difference in mortality between LSES and HSES was not noted in the SC cohort (adjusted hazard ratio=2.87 [0.56–14.73]; P=0.21; Table 3 and Figure 2B). The moderator effect of SC on the mortality difference of LSES versus HSES, however, did not reach statistical significance (adjusted hazard ratio, 0.29 [0.05–1.77]; P=0.18), suggesting that, in our cohort, access to SC alone was not the only driver of better outcomes for LSES patients.
Table 2.
Characteristics of Hypertrophic Cardiomyopathy Patients Who Died During the Study Period
| LSES | HSES | |||
|---|---|---|---|---|
| Specialist Care | General Cardiology | Specialist Care | General Cardiology | |
| Total deaths, % | 2 (3%) | 8 (14%) | 6 (2%) | 27 (5%) |
| Primary cardiac death, % | 1 (50%) | 4 (50%) | 2 (33%) | 5 (19%) |
| Sepsis/infection, % | 0 | 1 (12%) | 1 (17%) | 3 (11%) |
| Cancer, % | 0 | 1 (12%) | 0 | 2 (7%) |
| Stroke, % | 0 | 1 (12%) | 0 | 3 (11%) |
| Unknown, % | 1 (50%) | 1 (12%) | 3 (50%) | 14 (52%) |
| Average age at death, y (SD) | 69 (1) | 53 (19) | 73 (9) | 73 (16) |
| Ethnicity, % | ||||
| White | 2 (100%) | 8 (100%) | 4 (67%) | 24 (89%) |
| Black | 0 | 0 | 0 (0%) | 1 (4%) |
| Other | 0 | 0 | 2 (33%) | 2 (7%) |
| Male sex, % | 1 (50%) | 5 (63%) | 3 (50%) | 9 (33%) |
| Atrial fibrillation, % | 1 (50%) | 5 (63%) | 3 (50%) | 12 (44%) |
HSES indicates high socioeconomic status; LSES, low socioeconomic status.
Table 3.
Cox Regression Adjusting For Age, Sex, Race, Diabetes Mellitus, and Coronary Artery Disease to Examine Moderation of Specialist Care on the Effect of Socioeconomic Status on Mortality
| HR (95% CI) | P Value | |
|---|---|---|
| Specialist care cohort | ||
| LSES | 2.87 (0.56–14.73) | 0.21 |
| HSES | 1.00 | |
| General cardiology cohort | ||
| LSES | 10.06 (4.38–23.09) | <0.001a |
| HSES | 1.00 | |
| Interaction (moderation), HRR | 0.29 (0.05–1.77) | 0.18 |
| Age, y | 1.06 (2.81–2.97) | <0.001a |
| Male | 0.79 (1.51–4.60) | 0.49 |
| Race | ||
| Black (vs white) | 0.16 (1.02–3.24) | 0.071 |
| Other (vs white) | 0.40 (1.10–5.57) | 0.22 |
| Unknown (vs white) | 1.19 (1.32–156.65) | 0.81 |
| DM | 2.30 (3.05–116.28) | 0.024a |
| CAD | 1.51 (2.02–25.53) | 0.29 |
CAD indicates coronary artery disease; DM, diabetes mellitus; HR, hazard ratio; HRR, Hazard Ratio's Ratio; HSES, high socioeconomic status; LSES, low socioeconomic status.
Reached statistical significance.
Figure 2.
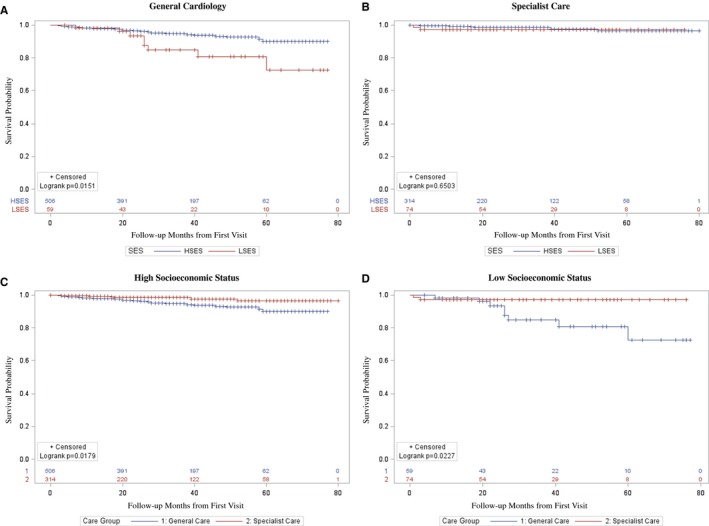
Kaplan–Meier curve showing unadjusted mortality differences between subgroups (LSES, HSES, SC, and GC). A, Survival among all HCM patients within general cardiology care comparing survival of those within HSES and LSES. B, Survival among all HCM patients within specialty care comparing survival of those within HSES and LSES. GC indicates general cardiology care; HCM, hypertrophic cardiomyopathy; SES, socioeconomic status; HSES, high socioeconomic status; LSES, low socioeconomic status; SC, specialty care.
Causes of Death
For deceased patients with documented causes of death, cardiovascular death (sudden cardiac death or fatal HF) was most frequent in all groups regardless of SES or access to SC. Notably, the LSES/GC subgroup had a substantially lower average age at death of 53 years, compared with all other groups, which had an average age of death between 69 and 73 years (Table 2).
Hospitalizations and Resource Utilization
During follow‐up, 112 HCM patients were hospitalized for any cause. Time‐to‐event analysis showed significantly higher risk for hospitalization during follow‐up for both LSES and HSES patients receiving SC (adjusted hazard ratio, 3.28 [1.11–9.73], P=0.03 for LSES; 2.19 (1.40–3.40), P=0.001 for HSES; Table 4). The moderator effect of SC on rate of hospitalization of LSES versus HSES, however, was not significant, again suggesting other factors at play.
Table 4.
Both LSES and HSES Patients Were at Higher Risk of Being Hospitalized If They Received Specialty Care
| HR (95% CI) | P Value | |
|---|---|---|
| LSES | ||
| Specialist care | 3.28 (1.11–9.73) | 0.032a |
| General cardiology | 1 | |
| HSES | ||
| Specialist care | 2.19 (1.40–3.40) | 0.001a |
| General cardiology | 1 | |
| Interaction (moderation), HRR | 1.50 (0.47–4.85) | 0.50 |
| Age, y | 1.02 (2.73–2.79) | 0.014a |
| Male | 0.66 (1.56–2.68) | 0.042 |
| Race | ||
| Black (vs white) | 0.99 (1.69–6.54) | 0.98 |
| Other (vs white) | 1.38 (1.89–19.47) | 0.41 |
| Unknown (vs white) | 1.12 (1.36–55.15) | 0.87 |
| Ethnicity | ||
| Hispanic (vs non‐Hispanic) | 1.16 (1.68–13.71) | 0.71 |
| Unknown (vs non‐Hispanic) | 0.12 (1.02–2.10) | 0.023a |
| DM | 1.08 (1.86–6.48) | 0.79 |
| CAD | 1.75 (2.82–19.38) | 0.036a |
CAD indicates coronary artery disease; DM, diabetes mellitus; HR, hazard ratio; HRR, Hazard Ratio's Ratio; HSES, high socioeconomic status; LSES, low socioeconomic status.
Reached statistical significance.
When focusing on the most common hospitalization causes for HCM patients (atrial fibrillation, syncope, and HF), there was no difference between LSES/HSES groups for atrial fibrillation or syncope (1.5% versus 2.8% and 2.3% versus 0.7%, respectively), but LSES patients were more likely to be hospitalized for HF (4.5% versus 0.9%; P<0.001). Thirteen patients were hospitalized with HF, of whom 12 were in the SC subgroup. A focused chart review of HF hospitalizations showed that, regardless of SES, most HF exacerbations requiring admission occurred while already under SC (in 8 of the 13 patients). Notably, of the 5 patients with HF hospitalizations while under GC care, 4 were subsequently referred to the HCM center (within a short period of time) and thus became part of the SC cohort in our study.
Clinic visits and resource utilization data are presented in Figure 3. LSES patients had fewer clinic visits and were more likely to undergo septal reduction therapy, specifically myectomy, compared with HSES patients (6.36±6.61 versus 7.71±8.62 for visits, 9 [6.8%] versus 26 [3.2%] for myectomies) without significant differences in alcohol septal ablation. There were no significant differences between LSES and HSES patient groups regarding ECGs, echo, Holter, cardiac MRI, cardiopulmonary stress tests, and implantable cardiac defibrillators.
Figure 3.
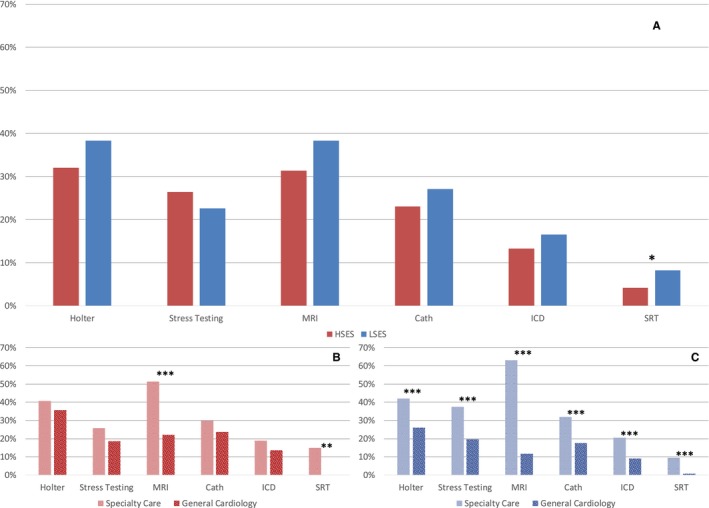
Comparison of guideline recommended testing for hypertrophic cardiomyopathy (HCM) comparing high and low socioeconomic groups as well as specialty care and general cardiology subgroups. A, Comparison of HSES and LSES groups. B, Comparison of HCM patients with LSES between specialty care and general cardiology subgroups. C, Comparison of HCM patients with HSES between specialty care and general cardiology subgroups. *P<0.05; **P<0.01; ***P<0.001. Septal reduction therapy includes alcohol ablation procedures and myectomies. Cath indicates cardiac catheterization; HSES, high socioeconomic status; ICD, implantable cardiac defibrillator; LSES, low socioeconomic status; MRI, magnetic resonance imaging; SRT, septal reduction therapy.
Advanced testing and interventions were not associated with SES, but rather with access to SC. Septal reduction therapies (myectomy and alcohol ablation) and cardiopulmonary stress tests for both LSES and HSES patients were almost exclusively performed at group S (41 septal reduction therapies and 62 cardiopulmonary exercise tests in SC patients versus 4 and 0, respectively, in GC). Furthermore, SC patients (in both LSES and HSES groups) were more likely to have cardiac MRIs (for LSES: 38 [51.4%] versus 13 [22.0%]; P<0.001; for HSES: 198 [63.1%] versus 59 [11.7%]; P<0.001). An exception in similar findings for patients of different SES status was observed for implantable cardiac defibrillator implantation. Implantation was more frequent for HSES patients in the SC versus GC groups (48 [15.3%] versus 33 [6.5%]; P<0.001), whereas no difference was found for LSES patients (7 [9.5%] versus 5 [8.5%]; P=0.68).
Discussion
We found that HCM patients with a background of lower SES suffered higher all‐cause mortality compared with patients with a higher SES when treated exclusively in general cardiology clinics. We did not find a similar mortality difference for patients referred to a specialized HCM care team, with comparable survival rate between different SES. These differences in mortality could not be explained by differences in age, sex, race, or comorbidities of referred patients.
Outcome Disparities
Differences in outcomes associated with SES is an almost universal finding in medical care.6, 7, 8, 10, 11 Specifically, worse outcomes have been persistently observed for those with lower SES. We found this also to be the case for LSES HCM patients without access to SC, although in our cohort the moderation effect of specialist care on mortality did not reach statistical significance. Regardless of effect size, SC influence on outcomes is likely to be driven, at least in part, by a team‐based approach to care. In this approach, care of HCM patients does not rest on the shoulders of a single physician, who may or may not have experience in HCM, but more closely resembles the Heart Team paradigm promoted by society guidelines in other areas of cardiovascular medicine.12, 13, 14 Supporting this, we and others have found substantive differences in resource utilization for HCM patients prereferral and postreferral to an expert center,5 as well as between expert and nonexpert centers overall.15 Case review by an “HCM team,” that consists of an in‐house network of clinical cardiologists, electrophysiologists, cardiac surgeons, genetic counselors, and social workers, is the care model most often used by centers specializing in HCM. These common program features may address some of the drivers of the poorer outcomes observed among LSES populations described in literature, including reduced access to care, lower medical compliance, and a higher prevalence of cardiovascular risk factors and other comorbidities.7, 8, 10, 16
Our study assigned patients to SC for any single referral visit to that clinic. Surprisingly, a great proportion of LSES patients (55.6%) were referred for SC than HSES patients (38.3%). We also noted that among HCM patients experiencing hospitalization, many referrals to SC occurred immediately following the hospitalization event, and most of the hospitalizations occurred in the LSES group. It is possible that LSES patients were sicker or more likely to have a sentinel event that prompted referral to SC. Given that more‐substantial barriers to access are typically noted among LSES patient populations, it may be that there is a lack of perceived need for specialist care in stable patients, especially if incentive structures make referral of some patients (HSES) less attractive. A larger sample size and longer duration of follow‐up, potentially in the setting of a prospective longitudinal study, are needed to further explore these issues.
Care Strategies and Compliance With Guidelines
Current society HCM guidelines propose routine annual clinic visits with transthoracic echo and/or 24‐hour ECG monitoring every 1 to 2 years, along with cardiac MRI, stress echo, and/or cardiopulmonary exercise tests at first contact.4 In our study, access to specialty care influenced availability and frequency of guideline directed clinical testing and procedures. Although patients in both the SC and GC groups where, on average, within the recommended time frame for clinic visits and transthoracic echocardiogram exams, SC group patients were more likely to undergo cardiac MRI, stress echo, or cardiopulmonary exercise tests. In addition, the majority of hospitalizations and implantable cardiac defibrillator implantations, and the vast majority of septal reduction procedures, took place in SC group patients (Figure 3).
Limitations of the Study
This single‐health‐system retrospective review took advantage of the concurrent implementation of a broad‐based, system‐wide EMR and the development of a highly structured disease specialty program embedded within that system. Although this allowed for identification and comparison between separate treatment groups within a single health system, the study was limited by the length and breadth of EMR data and the retrospective nature of the review. Additionally, it remains possible that some older patients with Medicare as their primary insurance transitioned from Medicaid or none.
Conclusions
We found that socioeconomically vulnerable HCM patients had higher mortality when not involved in SC. Additionally, SC provided more‐consistent guideline‐driven testing and treatment strategies. Although a causative link cannot be established, our findings do suggest that team‐based, guideline‐driven care may be particularly important for at‐risk populations with implications for healthcare policy. Given the cost of SC derived from multiprovider involvement and aggressive testing and treatment strategies, further study would be useful to assess the drivers of benefit and interrogate implementation strategies supporting broader access.
Sources of Funding
This research was supported by the Joshua C. Gibson, MD, Memorial Fund for Heart Research.
Disclosures
None.
Acknowledgments
We express our gratitude to Jeptha P. Curtis, MD, and Michael Singer, MD, PhD for their advice and support.
(J Am Heart Assoc. 2020;9:e014095 DOI: 10.1161/JAHA.119.014095.)
References
- 1. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. [DOI] [PubMed] [Google Scholar]
- 2. Sewanan LR, Campbell SG. Modelling sarcomeric cardiomyopathies with human cardiomyocytes derived from induced pluripotent stem cells. J Physiol. 2019 DOI: 10.1113/JP276753. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, Cirino AL, Fox JC, Lakdawala NK, Ware JS, Caleshu CA, Helms AS, Colan SD, Girolami F, Cecchi F, Seidman CE, Sajeev G, Signorovitch J, Green EM, Olivotto I. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (share). Circulation. 2018;138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. The Journal of thoracic and cardiovascular surgery. 2011;142:1303–1338. [DOI] [PubMed] [Google Scholar]
- 5. Beale A, Macciocca I, Olaussen A, Marasco SF, Mariani JA, Ellims AH. Clinical benefits of a specialised clinic for hypertrophic cardiomyopathy. Intern Med J. 2015;45:255–260. [DOI] [PubMed] [Google Scholar]
- 6. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA, Sperling LS. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. [DOI] [PubMed] [Google Scholar]
- 8. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the united states. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 9. Fjaer EL, Balaj M, Stornes P, Todd A, McNamara CL, Eikemo TA. Exploring the differences in general practitioner and health care specialist utilization according to education, occupation, income and social networks across Europe: findings from the European social survey (2014) special module on the social determinants of health. Eur J Public Health. 2017;27:73–81. [DOI] [PubMed] [Google Scholar]
- 10. Agarwal S, Garg A, Parashar A, Jaber WA, Menon V. Outcomes and resource utilization in ST‐elevation myocardial infarction in the United States: evidence for socioeconomic disparities. J Am Heart Assoc. 2014;3:e001057 DOI: 10.1161/JAHA.114.001057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackenbach JP, Stirbu I, Roskam AJ, Schaap MM, Menvielle G, Leinsalu M, Kunst AE; European Union Working Group on Socioeconomic Inequalities in Health . Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–2481. [DOI] [PubMed] [Google Scholar]
- 12. Bavaria JE, Tommaso CL, Brindis RG, Carroll JD, Deeb GM, Feldman TE, Gleason TG, Horlick EM, Kavinsky CJ, Kumbhani DJ, Miller DC, Seals AA, Shahian DM, Shemin RJ, Sundt TM III, Thourani VH. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:340–374. [DOI] [PubMed] [Google Scholar]
- 13. The American College of Obstetricians and Gynecologists . ACOG practice bulletin no. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 14. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 15. Mirabel M, Damy T, Donal E, Huttin O, Labombarda F, Eicher JC, Cervino C, Laurito M, Offredo L, Tafflet M, Jouven X, Giura G, Desnos M, Jeunemaitre X, Empana JP, Charron P, Habib G, Reant P, Hagege A; REMY working group of the French Society of Cardiology . Influence of centre expertise on the diagnosis and management of hypertrophic cardiomyopathy: a study from the French register of hypertrophic cardiomyopathy (REMY). Int J Cardiol. 2019;275:107–113. [DOI] [PubMed] [Google Scholar]
- 16. Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


