Abstract
Background
Underuse of guideline‐recommended therapy in peripheral artery disease (PAD) in administrative and procedural databases has been described, but reports on medically managed patients and referral to supervised exercise therapy (SET) in PAD are lacking. We aimed to document the use of PAD guideline‐recommended therapy, including SET in patients with PAD symptoms consulting a specialty clinic across 3 countries.
Methods and Results
The 16‐center PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry enrolled 1275 patients with new or an exacerbation of PAD symptoms (2011–2015). We prospectively documented antiplatelet medications, statins, smoking cessation counseling and/or therapy, and referral to SET: “2 quality measures” referred to the use of both statin and antiplatelet medications; “4 quality measures” to receiving all 4 measures. Median odds ratios were calculated to quantify treatment variation across sites. A total of 89% patients were on antiplatelets, 83% on statins, and 23% had been referred to SET. Of 455 current smokers, 342 (72%) patients received smoking cessation therapy/counseling. Overall, 77.2% of patients received “2 quality measures” and 19.7% “4 quality measures.” The median odds ratio for 2 quality measures was 2.13 (95% CI, 1.61–3.56; P<0.001) and for 4 quality measures was 5.43 (95% CI, 2.84–17.91; P<0.001). Variability in adherence was not explained by country, except for referral to SET. The odds for SET referral in The Netherlands (70% referral rate) was nearly 100 times greater than in US sites (2% referral rate).
Conclusions
Not all patients who have undergone a PAD workup at a specialty care facility are treated with evidence‐based care, especially so for SET.
Keywords: medical management, peripheral artery disease, quality of care
Subject Categories: Vascular Disease, Quality and Outcomes
Clinical Perspective
What Is New?
When patients receive a workup for peripheral artery disease (PAD) symptoms in the specialty care setting, only 1 in 5 receive all eligible evidence‐based medical management quality measures for PAD, with high variability across institutions.
Especially a lack of referral to supervised exercise therapy stands out in the US context versus European specialty care settings.
What Are the Clinical Implications?
Structural barriers for realizing optimal quality PAD care in the specialty setting need to be explored and addressed as without realizing optimal quality care, patients suffering from PAD remain disadvantaged in their ability to manage their PAD and cardiovascular risk.
Introduction
Worldwide, more than 200 million individuals suffer from peripheral artery disease (PAD).1 Lower‐extremity PAD is a burdensome condition caused by the underlying process of atherosclerosis that manifests itself in the leg arteries. It represents a spectrum of clinical manifestations that can include atypical lower‐extremity symptoms, intermittent claudication, rest pain, and tissue loss secondary to ischemia.2 Patients with PAD are at a significantly increased risk for morbidity and mortality attributable to cardiovascular events not involving the lower extremities.3, 4 As such, medical and lifestyle interventions constitute essential elements of evidence‐based PAD care, both from a perspective of improving cardiovascular mortality as well as limb‐related outcomes.
As part of the evidence‐based risk management of PAD, 4 noninvasive treatments are recommended by the American College of Cardiology/American Heart Association performance measures for PAD: (1) statin medications, (2) antiplatelet therapy, (3) smoking cessation therapy and/or counseling, and (4) referral to a PAD‐specific supervised exercise training (SET) program.2, 5, 6 These quality metrics for PAD care have since then been retained in several guideline statements, including the recent 2016 PAD treatment guidelines.2 Several studies have demonstrated low adherence rates to these performance measures for patients with PAD as compared with patients with other forms of cardiovascular disease.7, 8, 9, 10, 11 This evidence came from retrospective analyses using administrative data11 or from procedure‐based databases only.12 There is a lack of prospective studies describing adherence rates to PAD treatment guidelines, and, in particular, evidence on referral patterns for SET is missing. Little is known about the variability in adherence to evidence‐based PAD care across treatment sites and healthcare settings.
To address this gap in knowledge, we aimed to quantify rate of adherence to PAD performance measures as it relates to medical therapy and SET in subspecialty vascular centers for patients presenting with new‐onset or an exacerbation of PAD symptoms. Next, we examined how adherence to these measures varies across sites.
Methods
Because of the sensitive nature of the data collected for this study, requests to access a de‐identified data set from qualified researchers trained in human subject confidentiality protocols may be considered on an individual basis by contacting the PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) group on the website.13
Study Population
The PORTRAIT study is a multicenter, prospective study of patients presenting for specialty vascular care with new‐onset, or an exacerbation of, lower‐extremity symptoms. The methods have been described previously.14 Briefly, patients with new onset or worsening of PAD symptoms were screened for enrollment at the time of referral to subspecialty vascular care sites. Patients with a resting ankle brachial index ≤0.90 or drop in postexercise ankle pressure of ≥20 mm Hg were eligible for inclusion. Patients with a noncompressible ankle brachial index (≥1.30), patients presenting with ischemic rest pain, ulceration, or gangrene (Rutherford grade II–III), or those who had undergone a lower‐limb surgical or endovascular procedure in the past year in the ipsilateral leg were excluded. Other exclusion criteria were non‐English, Spanish, or Dutch speaking, hard of hearing, unable to provide informed consent, or currently a prisoner. The participating centers consisted of 10 sites in the United States, 5 sites in The Netherlands, and 1 site in Australia (16 total). Institutional review board approval was obtained for the study at each participating site. Diagnostic enrollment criteria for PAD consisted of an ankle brachial index with PAD defined as an ankle brachial index ≤0.90 or a postexercise drop in ankle blood pressure ≥20 mm Hg. Before the initiation of subspecialty treatment, baseline demographic, socioeconomic, and clinical data were obtained by trained data collectors.
Primary Outcomes
The primary outcome of the study was the adherence rate to 4 noninvasive interventions first recommended by the 2010 American College of Cardiology/American Heart Association PAD performance measures, which mirror the recommendations of the current 2016 treatment guidelines15: (1) antiplatelet therapy, (2) statin therapy, (3) SET, and (4) smoking cessation counseling.5, 15, 16 Adherence rates were collected after the PAD workup that patients received at the subspecialty clinic as part of their routine care to reflect contemporary subspecialty treatment practices.
Antiplatelet therapy was defined as the use of aspirin at a dose of 75 to 325 mg daily or clopidogrel at a dose of 75 mg daily. Statin therapy was defined as the prescription of any available 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor. For both medications, patients were excluded from the denominator if there were documented medical reasons or patient reasons as to why patients would not be eligible to receive this metric.2, 5 The smoking cessation performance measure included verbal instructions, referral to a special program or formal counseling, as well as pharmacological therapy.5 For the purposes of our study, we considered achieving either of these criteria as a successful effort to meet the recommendation. Nonsmokers were excluded from the analysis of the smoking cessation measure. SET was defined as referral to a formal supervised exercise program. Unstructured and home‐based exercise therapy was not considered to have met the recommendation, given that this was not supported by the 2010 American College of Cardiology/American Heart Association performance measures and is currently not a Class I recommendation.2, 5 Per the American College of Cardiology/American Heart Association performance measures, patients were excluded from the denominator if there were documented medical reasons for not offering SET (eg, heart failure).2, 5 If a patient had a contraindication or if the measure did not apply to them (such as smoking), they were marked as having achieved that quality measure.
We further analyzed adherence to combinations of the measures: (1) adherence to the 2 pharmacological measures (being on a statin and antiplatelet therapy or “2 quality measures”) and (2) adherence to all measures (4 performance measures for smokers and participation in 3 performance measures [antiplatelets, statins, and SET] for nonsmokers or “4 quality measures”). Variability in achieving rates of “2 quality measures” and “4 quality measures” across sites were calculated using median odds ratios (MORs). The MOR represents the median value of the odds for receiving the performance measures for 2 statistically identical patients being treated at 1 random clinic versus another. For example, an MOR of 1.5 suggests that, on average, a patient treated at 1 clinic has a 50% higher odds of receiving the treatment than if they had presented at another clinic.17
Statistical Analysis
Descriptive statistics for continuous variables are expressed as means with SD and for categorical variables as frequencies and percentages. Comparisons were performed by the receipt of 2 quality measures as well as by 4 quality measures using Student t tests or Mann–Whitney U tests for continuous variables and chi‐square tests or Fisher's exact tests for categorical variables.
Achievement of each of the individual performance measures was calculated by study site, and then adherence rates were compared using multivariable, hierarchical, logistic regression models with site as a random effect. The odds of achieving the study variable of interest is expressed as odds ratios plus 95% CIs. To compare practice patterns across subspecialty sites, MORs were calculated for each of the study variables to quantify variations in treatment and rates of achieving “2 quality measures” and “4 quality measures.”
All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC). A 2‐sided P value of <0.05 was considered to be statistically significant for all analyses.
Results
A total of 1275 patients with new or an exacerbation of symptoms prompting referral to a vascular specialty clinic were enrolled in the study. Patient baseline demographics are represented in Table. After the first PORTRAIT subspecialty site visit, 1104 (89.0%) patients with new or worsening symptoms of PAD were prescribed antiplatelet medications, 1055 (82.7%) were prescribed a statin, and 280 (23.3%) were referred to a supervised exercise program. There were 455 (35.7%) current smokers; of those patients, 342 (75.2%) had been referred to or counseled about smoking cessation (Table). Patients receiving 2 quality measures (antiplatelet therapy and statin) were more likely to be male and more likely to have ischemic cardiovascular risk factors as compared with those who did not receive 2 quality measures. Patients receiving 4 quality measures (antiplatelet therapy, statin, smoking cessation, and supervised exercise therapy referral) were more likely to be from The Netherlands, white, married, lower education, had some money left over at the end of the month, and had less cardiovascular risk factors (Table).
Table 1.
Baseline Patient Characteristics for the Overall Sample and by Receipt of 2 Quality Measures and by 4 Quality Measures
| Overall Sample (n=1275) | 2 Quality Measures | P Value | 4 Quality Measures | P Value | |||
|---|---|---|---|---|---|---|---|
| Yes (n=984; 77%) | No (n=291; 23%) | Yes (n=251; 20%) | No (n=1024; 80%) | ||||
| Age, y | 67.6±9.4 | 67.6±9.3 | 67.7±10.1 | 0.88 | 66.3±8.4 | 67.9±9.7 | 0.019 |
| Female sex | 483 (37.9%) | 354 (36.0%) | 128 (44.0%) | 0.013 | 84 (33.5%) | 398 (38.9%) | 0.11 |
| Country | 0.11 | <0.001 | |||||
| (1) United States | 798 (62.5%) | 621 (63.1%) | 176 (60.5%) | 37 (14.7%) | 760 (74.2%) | ||
| (2) Netherlands | 384 (30.1%) | 298 (30.3%) | 85 (29.2%) | 198 (78.9%) | 185 (18.1%) | ||
| (3) Australia | 95 (7.4%) | 65 (6.6%) | 30 (10.3%) | 16 (6.4%) | 79 (7.7%) | ||
| White | 1047 (82.1%) | 810 (82.3%) | 237 (81.4%) | 0.73 | 237 (94.4%) | 810 (79.1%) | <0.001 |
| Hispanic | 17 (1.9%) | 16 (2.4%) | 1 (0.5%) | 0.14 | 2 (3.8%) | 15 (1.8%) | 0.27 |
| Married | 753 (59.4%) | 582 (59.4%) | 171 (59.2%) | 0.93 | 168 (67.2%) | 585 (57.5%) | 0.004 |
| High school education or above | 872 (69.0%) | 672 (68.9%) | 200 (69.4%) | 0.87 | 117 (48.0%) | 755 (74.1%) | <0.001 |
| Active working status | 303 (23.8%) | 231 (23.5%) | 72 (24.7%) | 0.67 | 67 (26.7%) | 236 (23.0%) | 0.23 |
| Finances at end of month | 0.79 | 0.05 | |||||
| Some money left over | 684 (55.1%) | 534 (55.5%) | 150 (54.0%) | 150 (62.0%) | 534 (53.5%) | ||
| Just enough to make ends meet | 423 (34.1%) | 328 (34.1%) | 95 (34.2%) | 71 (29.3%) | 352 (35.2%) | ||
| Not enough to make ends meet | 134 (10.8%) | 101 (10.5%) | 33 (11.9%) | 21 (8.7%) | 113 (11.3%) | ||
| Atrial fibrillation | 143 (11.2%) | 96 (9.8%) | 47 (16.2%) | 0.002 | 19 (7.6%) | 124 (12.1%) | 0.04 |
| Current smokers | 455 (35.7%) | 349 (40.4%) | 106 (44.4%) | 0.27 | 95 (44.0%) | 360 (40.6%) | 0.36 |
| Congestive heart failure | 128 (10.0%) | 103 (10.5%) | 24 (8.2%) | 0.27 | 8 (3.2%) | 119 (11.6%) | <0.001 |
| Dyslipidemia | 1016 (79.6%) | 836 (85.0%) | 179 (61.5%) | <0.001 | 182 (72.5%) | 833 (81.3%) | 0.001 |
| Hypertension | 1018 (79.7%) | 795 (80.8%) | 222 (76.3%) | 0.09 | 171 (68.1%) | 846 (82.6%) | <0.001 |
| History of TIA/CVA | 146 (11.4%) | 122 (12.4%) | 24 (8.2%) | 0.05 | 32 (12.7%) | 114 (11.1%) | 0.47 |
| History of angina pectoris | 178 (13.9%) | 148 (15.0%) | 30 (10.3%) | 0.040 | 36 (14.3%) | 142 (13.9%) | 0.85 |
| Previous myocardial infarction | 244 (19.1%) | 204 (20.7%) | 38 (13.1%) | 0.003 | 40 (15.9%) | 202 (19.7%) | 0.17 |
| History of PCI/CABG | 447 (35.0%) | 380 (38.6%) | 65 (22.3%) | <0.001 | 63 (25.1%) | 382 (37.3%) | <0.001 |
| Chronic kidney disease | 142 (11.1%) | 113 (11.5%) | 29 (10.0%) | 0.47 | 16 (6.4%) | 126 (12.3%) | 0.007 |
| Chronic lung disease | 218 (17.1%) | 162 (16.5%) | 56 (19.2%) | 0.27 | 47 (18.7%) | 171 (16.7%) | 0.44 |
| History of cancer | 128 (10.0%) | 96 (9.8%) | 32 (11.0%) | 0.54 | 24 (9.6%) | 104 (10.2%) | 0.78 |
| History of depression | 102 (8.0%) | 40 (4.1%) | 13 (4.5%) | 0.76 | 12 (4.8%) | 41 (4.0%) | 0.58 |
| Diabetes mellitus | 424 (33.2%) | 343 (34.9%) | 81 (27.8%) | 0.025 | 57 (22.7%) | 367 (35.8%) | <0.001 |
| ABI | 0.67±0.19 | 0.66±0.18 | 0.68±0.20 | 0.14 | 0.65±0.18 | 0.67±0.19 | 0.06 |
| Adherence to antiplatelet therapy | 1104 (89.0%) | 961 (100%) | 143 (51.3%) | <0.001 | 240 (100%) | 864 (86.4%) | <0.001 |
| Adherence to statin therapy | 1055 (82.7%) | 984 (100%) | 71 (24.4%) | <0.001 | 251 (100%) | 804 (78.5%) | <0.001 |
| Performance measure for supervised exercise therapy | 280 (23.3%) | 228 (24.6%) | 52 (18.8%) | 0.044 | 196 (100%) | 84 (8.3%) | <0.001 |
| Performance measure for smoking cessation | 342 (75.2%) | 262 (75.1%) | 80 (75.5%) | 0.93 | 95 (100%) | 247 (68.6%) | <0.001 |
| Primary PAD treatment | |||||||
| Medical therapy only | 932 (74.8%) | 725 (75.2%) | 207 (73.4%) | 0.45 | 193 (79.8%) | 739 (73.6%) | 0.13 |
| Endovascular intervention | 275 (22.1%) | 212 (22.0%) | 63 (22.3%) | 42 (17.4%) | 233 (23.2%) | ||
| Surgical intervention | 39 (3.1%) | 27 (2.8%) | 12 (4.3%) | 7 (2.9%) | 32 (3.2%) | ||
| Unsupervised exercise referral | 145 (17.0%) | 112 (17.1%) | 33 (16.5%) | 0.84 | 18 (36.0%) | 127 (15.8%) | <0.001 |
| Site location | 0.017 | <0.001 | |||||
| Suburban location | 130 (10.2%) | 101 (10.3%) | 29 (10.0%) | 24 (9.6%) | 106 (10.4%) | ||
| Urban location | 1103 (86.5%) | 843 (85.7%) | 260 (89.3%) | 206 (82.1%) | 897 (87.6%) | ||
| Urban/rural location | 42 (3.3%) | 40 (4.1%) | 2 (0.7%) | 21 (8.4%) | 21 (2.1%) | ||
| Site characteristics | 0.86 | <0.001 | |||||
| Nonacademic | 492 (38.6%) | 381 (38.7%) | 492 (38.6%) | 222 (88.4%) | 492 (38.6%) | ||
| University affiliated | 783 (61.4%) | 603 (61.3%) | 783 (61.4%) | 29 (11.6%) | 783 (61.4%) | ||
ABI indicates ankle‐brachial index; CABG, coronary artery bypass grafting; CVA, cerebrovascular attack; PAD, peripheral artery disease; PCI, percutaneous intervention; TIA, transient ischemic attack.
Across sites, there was a high degree of variability in the use of performance measures for high‐quality care (Figure 1A through 1D). For example, the rate of prescribing antiplatelet therapy across sites ranged from 63% to 100% (Figure 1A), with a median odds ratio of 2.93 (95% CI, 1.94–6.17; P<0.0001). The MOR for achieving the other 3 individual performance measures were also high: 2.11 (95% CI, 1.57–3.63; P<0.0001) for statin therapy; 3.06 (95% CI, 1.90–7.06; P<0.001) for smoking cessation therapy; and 4.98 (95% CI, 2.73–15.46; P<0.0001) for SET.
Figure 1.
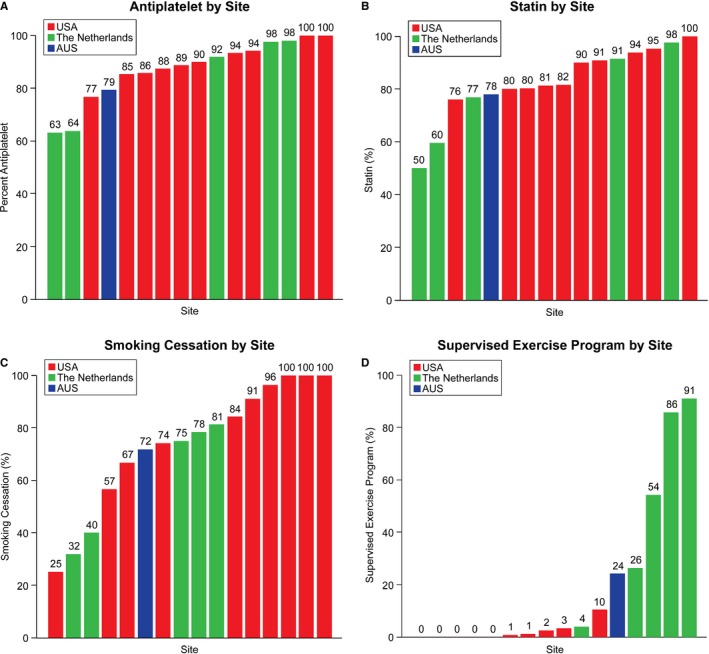
A through D, Adherence levels (%) to 4 peripheral artery disease performance measures by enrollment sites.
There was little difference between international sites and US sites in prescribing antiplatelets or statins or for achieving the smoking cessation measure. In contrast, the odds that patients in The Netherlands were referred to SET was nearly 100 times (odds ratio=97.42; 95% CI, 9.79–969.59; P=0.001) greater than its use in the US sites; and patients in Australia had a 27 times greater odds of referral to SET (odds ratio=27.38; 95% CI, 0.42–1771.47; P=0.11) than US sites (Figure 1D).
There was also a high degree of variability in achieving “2 quality measures” and “4 quality measures” (Figure 2). For the entire cohort, there were 984 (77.2%) patients who achieved “2 quality measures” and 251 (19.7%) who achieved “4 quality measures.” The MOR for achieving 2 quality measures was 2.13 (95% CI, 1.61–3.56; P<0.001) and for 4 quality measures was 5.43 (95% CI, 2.84–17.91; P<0.001). There was no difference in achieving 2 quality measures between patients in the United States (77.9%), The Netherlands (77.8%), and Australia (68.4%; P=0.107). However, there was a marked difference in the rate of achieving 4 quality measures in the United States (4.6%) versus The Netherlands (51.7%) and Australia (16.8%; P<0.001).
Figure 2.
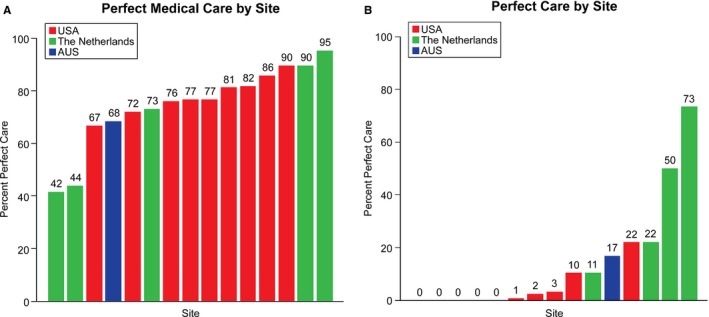
A and B, Adherence to combinations of PAD performance measures across enrollment sites. (A) Adherence to 2 quality measures, including antiplatelet and statin therapy. (B) Adherence to 4 quality measures, including antiplatelet and statin therapy, tobacco cessation, and supervised exercise therapy. PAD indicates peripheral artery disease.
Discussion
In the PORTRAIT registry, we found that although adherence rates to 2 pharmacological interventions—antiplatelet therapy and statin therapy—were high overall, low adherence rates were observed for the nonpharmacological interventions—smoking cessation and SET. Although 2 quality measures (antiplatelet and statin therapy) rates were higher in the United States, 4 quality measures (antiplatelet, statin therapy, smoking cessation, and SET) rates were higher in non‐US countries. A high degree of variability in quality of care between vascular specialty centers was observed, especially so for the nonpharmacological interventions offered to patients with PAD. The most extreme example of this variability was adherence to SET: As high as 90% adherence rates were observed at some sites, whereas other centers referred no patients to SET. Centers with the highest performance rates of adherence to SET were predominantly sites from The Netherlands. SET was rarely prescribed in the US sites.
Both statins and antiplatelet therapy have robust data to support their use in PAD. Statins and antithrombotic therapy improve morbidity and mortality in patients with PAD and cardiovascular disease and improve limb‐related outcomes in PAD.12, 18, 19, 20 Despite these compelling data, patients with PAD have historically been prescribed statins and antiplatelet therapy less often than patients with CAD or cerebrovascular disease.7, 9, 10, 11, 21, 22 Although underuse of guideline‐recommended therapy in PAD has been documented in several administrative databases,12, 18, 23, 24 PORTRAIT is the first study to have prospectively documented these measures in the specialty setting.
It is striking that up to 90% of patients with PAD in The Netherlands were prescribed SET and little use in the United States. The referral network to SET facilities in The Netherlands is well developed and the therapy is reimbursed by patients’ health insurance.25 In contrast, US providers have only since 2017 been given the option of prescribing SET as a Centers for Medicare and Medicaid Services reimbursable therapy.26 Given that reimbursement is an important criterion to successfully implement SET, an availability of specialized SET centers and trained staff as well as education and experience of providers on the benefits of SET are key ingredients of SET implementation success.26 Other barriers, such as availability and unawareness, may remain.25, 26, 27 Given that prevalence of PAD and healthcare costs to care for this population are both rising,1 barriers to SET access should be further studied, given that SET may be a potent instrument to cost‐effectively manage lower‐extremity symptoms in PAD.28
Another powerful, but underused, risk‐mitigation strategy in PAD is smoking cessation support for patients that are active smokers. Continued smoking in PAD comes with a high cost, both from the patient's and societal perspective.12, 29, 30, 31, 32 Only 75% of current smokers were counseled against tobacco use or referred for smoking cessation counseling. An issue in achieving these performance measures may be the role of insurance or other third‐party payers. For tobacco cessation therapies, patients are more likely to participate in tobacco cessation programs and adhere to physician recommendations if insurance companies cover nicotine gum, etc, and overall cessation rates improved when compared with patients for whom those therapies were not covered.21 Besides coverage issues, awareness about evidence‐based therapies for smoking cessation,29, 33, 34 chronic monitoring, and management strategies as well as health‐system–supported structural programs that facilitate easy referral to evidence‐based programs are critical to improve performance on this measure.
Adherence to pharmacotherapy measures was relatively high in this study. An 89% and 83% adherence rate was observed for antiplatelet and statin medications, respectively. These rates are higher than those documented from earlier databases, which did not specifically focus on the vascular specialty setting, but rather the outpatient setting in general.11 As such, our PAD performance adherence estimates for the vascular specialty setting add to our current understanding of how PAD is managed.
There are limitations that must be acknowledged in the PORTRAIT study. PORTRAIT data represent a selection of 16 vascular specialty centers, and it is unclear how their performance rates extend to other specialty centers that were not in our study. Next, enrollment sites came from 3 different countries, and policy differences as well as differences in the landscape of healthcare organization are factors that need to be taken into account when interpreting our findings. Another limitation is that we relied on abstracted information from medical records, and the potential for misclassification attributable to nondocumentation is a possibility. Finally, PORTRAIT data in the United States were collected at a time when SET was defined as a guideline‐recommended therapy but without reimbursement framework from the Centers for Medicare and Medicaid Services. This changed in 2017, and it is uncertain how this development has affected current SET prescription rates in the United States.
Conclusions
PAD performance measures that include pharmacotherapy (statins and antiplatelet therapy) seem to be relatively feasible and well adhered to by vascular specialists. Although key strategies to lower PAD risk and improve patients’ outcomes, adherence to nonpharmacological risk‐mitigation strategies, including smoking cessation referral and SET, is not well realized in the specialty care setting. Along with the great variability in performance rates across sites, this requires future deeper exploration as to why nonpharmacological guideline‐recommended risk‐prevention strategies are not successfully realized. Structural reasons, including coverage, availability, as well as patient, practice, and provider preferences, are factors that will need to be considered in our search to optimize key risk‐management strategies and outcomes for PAD.
Sources of Funding
Research reported in this article was partially funded through a Patient‐Centered Outcomes Research Institute (PCORI) Award (IP2 PI000753‐01; CE‐1304‐6677), The Netherlands Organization for Scientific Research (VENI Grant No. 916.11.179), and an unrestricted grant from W. L. Gore & Associates, Inc (Flagstaff, AZ).
Disclosures
Dr Smolderen is supported by an unrestricted research grant from Terumo, Boston Scientific, and Abbott Vascular. Dr Patel is supported by an unrestricted research grant from AstraZeneca, Bayer, Janssen and serves on the advisory board of Bayer and Janssen. Dr Mena‐Hurtado is a consultant for Abbott, Cardinal Health, Cook, Medtronic, Boston Scientific, Bard. The remaining authors have no disclosures to report.
Acknowledgments
The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. All manuscripts for the PORTRAIT study are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding organizations and sponsors of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr Smolderen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2020;9:e012541 DOI: 10.1161/JAHA.119.012541.)
References
- 1. Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. [DOI] [PubMed] [Google Scholar]
- 2. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. [DOI] [PubMed] [Google Scholar]
- 4. Ankle Brachial Index Collaboration , Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olin JW, Allie DE, Belkin M, Bonow RO, Casey DE Jr, Creager MA, Gerber TC, Hirsch AT, Jaff MR, Kaufman JA, Lewis CA, Martin ET, Martin LG, Sheehan P, Stewart KJ, Treat‐Jacobson D, White CJ, Zheng ZJ, Masoudi FA. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). Circulation. 2010;122:2583–2618. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. [DOI] [PubMed] [Google Scholar]
- 7. Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, Berger J, Torp‐Pedersen C, Fosbol EL. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower‐extremity peripheral artery disease, underuse remains. Circulation. 2012;126:1345–1354. [DOI] [PubMed] [Google Scholar]
- 8. Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, Bax JJ, van Sambeek MR, Poldermans D. Long‐term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–1596. [DOI] [PubMed] [Google Scholar]
- 9. Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hira RS, Cowart JB, Akeroyd JM, Ramsey DJ, Pokharel Y, Nambi V, Jneid H, Deswal A, Denktas A, Taylor A, Nasir K, Ballantyne CM, Petersen LA, Virani SS. Risk factor optimization and guideline‐directed medical therapy in US veterans with peripheral arterial and ischemic cerebrovascular disease compared to veterans with coronary heart disease. Am J Cardiol. 2016;118:1144–1149. [DOI] [PubMed] [Google Scholar]
- 11. Berger JS, Ladapo JA. Underuse of prevention and lifestyle counseling in patients with peripheral artery disease. J Am Coll Cardiol. 2017;69:2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong EJ, Chen DC, Westin GG, Singh S, McCoach CE, Bang H, Yeo KK, Anderson D, Amsterdam EA, Laird JR. Adherence to guideline‐recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3:e000697 DOI: 10.1161/JAHA.113.000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardiovascular Outcomes . Available at: https://cvoutcomes.org. Accessed October 13, 2019.
- 14. Smolderen KG, Gosch K, Patel M, Jones WS, Hirsch AT, Beltrame J, Fitridge R, Shishehbor MH, Denollet J, Vriens P, Heyligers J, Stone MN, Aronow H, Abbott JD, Labrosciano C, Tutein‐Nolthenius R, Spertus JA. PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): overview of design and rationale of an international prospective peripheral arterial disease study. Circ Cardiovasc Qual Outcomes. 2018;11:e003860. [DOI] [PubMed] [Google Scholar]
- 15. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2011 WRITING GROUP MEMBERS; 2005 WRITING COMMITTEE MEMBERS; ACCF/AHA TASK FORCE MEMBERS . 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. [DOI] [PubMed] [Google Scholar]
- 17. Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–88. [DOI] [PubMed] [Google Scholar]
- 18. Sigvant B, Kragsterman B, Falkenberg M, Hasvold P, Johansson S, Thuresson M, Nordanstig J. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J Vasc Surg. 2016;64:1009–1017.e3. [DOI] [PubMed] [Google Scholar]
- 19. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL; REACH Registry Investigators . Statin therapy and long‐term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones WS, Patel MR. Antithrombotic therapy in peripheral artery disease: generating and translating evidence into practice. J Am Coll Cardiol. 2018;71:352–362. [DOI] [PubMed] [Google Scholar]
- 21. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 22. McBride CL, Akeroyd JM, Ramsey DJ, Nambi V, Nasir K, Michos ED, Bush RL, Jneid H, Morris PB, Bittner VA, Ballantyne CM, Petersen LA, Virani SS. Statin prescription rates and their facility‐level variation in patients with peripheral artery disease and ischemic cerebrovascular disease: insights from the Department of Veterans Affairs. Vasc Med. 2018;23:232–240. [DOI] [PubMed] [Google Scholar]
- 23. Safley DM, Kennedy KF, Stansby G, Flather M, Cohen DJ, Spertus JA. Prevalence and predictors of persistent health status impairment in patients referred to a vascular clinic with intermittent claudication. Eur J Vasc Endovasc Surg. 2011;42:355–362. [DOI] [PubMed] [Google Scholar]
- 24. Ardati AK, Kaufman SR, Aronow HD, Nypaver TJ, Bove PG, Gurm HS, Grossman PM. The quality and impact of risk factor control in patients with stable claudication presenting for peripheral vascular interventions. Circ Cardiovasc Interv. 2012;5:850–855. [DOI] [PubMed] [Google Scholar]
- 25. Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home‐based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44:569–575; discussion, 576. [DOI] [PubMed] [Google Scholar]
- 26. US Centers for Medicare & Medicaid Services (CMS) . Decision Memo for Supervised Exercise Therapy (SET) for Symptomatic Peripheral Artery Disease (PAD) (CAG‐00449N). Baltimore, MD: CMS; 2017. [Google Scholar]
- 26. Hageman D, van den Houten MM, Spruijt S, Gommans LN, Scheltinga MR, Teijink JA. Supervised exercise therapy: it does work, but how to set up a program? J Cardiovasc Surg (Torino). 2017;58:305–312. [DOI] [PubMed] [Google Scholar]
- 27. Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds MR, Apruzzese P, Galper BZ, Murphy TP, Hirsch AT, Cutlip DE, Mohler ER III, Regensteiner JG, Cohen DJ. Cost‐effectiveness of supervised exercise, stenting, and optimal medical care for claudication: results from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) trial. J Am Heart Assoc. 2014;3:e001233 DOI: 10.1161/JAHA.114.001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duval S, Long KH, Roy SS, Oldenburg NC, Harr K, Fee RM, Sharma RR, Alesci NL, Hirsch AT. The contribution of tobacco use to high health care utilization and medical costs in peripheral artery disease: a state‐based cohort analysis. J Am Coll Cardiol. 2015;66:1566–1574. [DOI] [PubMed] [Google Scholar]
- 30. Hussain MA, Al‐Omran M, Mamdani M, Eisenberg N, Premji A, Saldanha L, Wang X, Verma S, Lindsay TF. Efficacy of a guideline‐recommended risk‐reduction program to improve cardiovascular and limb outcomes in patients with peripheral arterial disease. JAMA Surg. 2016;151:742–750. [DOI] [PubMed] [Google Scholar]
- 31. Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260. [PubMed] [Google Scholar]
- 32. Armstrong EJ, Wu J, Singh GD, Dawson DL, Pevec WC, Amsterdam EA, Laird JR. Smoking cessation is associated with decreased mortality and improved amputation‐free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565–1571. [DOI] [PubMed] [Google Scholar]
- 33. Hennrikus D, Joseph AM, Lando HA, Duval S, Ukestad L, Kodl M, Hirsch AT. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. J Am Coll Cardiol. 2010;56:2105–2112. [DOI] [PubMed] [Google Scholar]
- 34. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]


