Abstract
Background
Postthrombotic syndrome is a common complication of deep vein thrombosis, with limited treatment options.
Methods and Results
ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) is a multicenter, single‐arm, prospective study evaluating patients with chronic deep vein thrombosis and postthrombotic syndrome (Villalta score ≥8) who received minimum 3 months of anticoagulation. Patients underwent percutaneous transluminal venoplasty and ultrasound‐accelerated thrombolysis, with data collected on clinical characteristics, postthrombotic syndrome, imaging, and quality of life to 1 year. The primary efficacy outcome was a reduction of ≥4 points in the Villalta score 30 days after procedure. The primary safety outcomes were major bleeding episodes within 72 hours and symptomatic pulmonary embolism during the index hospitalization. A total of 82 limbs (78 patients) were treated (age, 54.6±12.7 years; 32.1% women; mean Villalta score, 15.5±5.2). The primary end point was met in 64.6% (51/79). At 1 year, 77.3% (51/66) of limbs continued with a Villalta reduction ≥4. At 365 days, >90% of segments had patency with ultrasound flow present. Baseline to 1‐year Physical Component Summary mean score of the Short Form‐36 increased from 38.9±9.5 to 45.2±9.8 (P≤0.0001), and mean VEINES‐QOL (Venous Insufficiency Epidemiological and Economic Study–Quality of Life) increased from 61.9±19.7 to 82.6±20.8 at 1 year (P<0.0001). Iliofemoral venous stenting was performed in 42 patients, with similar improvements seen in all outcomes, regardless of stenting status. One patient developed severe bleeding within 72 hours of the intervention and died at 32 days after procedure (1.3% mortality rate).
Conclusions
Percutaneous transluminal venoplasty and ultrasound‐accelerated thrombolysis resulted in successful recanalization of chronic venous obstruction with improved postthrombotic syndrome severity and quality of life. Results were sustained at 1‐year after procedure.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02159521.
Keywords: deep vein thrombosis, pharmacomechanical thrombectomy, postthrombotic syndrome, ultrasound‐accelerated thrombolysis
Subject Categories: Thrombosis, Vascular Disease, Quality and Outcomes, Revascularization, Treatment
Clinical Perspective
What Is New?
Two recent studies that focused on pharmacomechanical intervention for acute deep vein thrombosis demonstrated the incidence of postthrombotic syndrome (PTS) remained high (37%–55%) despite intervention; and in the recently published ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter‐Directed Thrombolysis) Trial, 44% of patients with femoropopliteal deep vein thrombosis in both the pharmacomechanical and conservative therapy arms developed PTS at 24 months.
For those experiencing chronic veno‐occlusive disease and PTS, standard of care therapy is often not beneficial.
ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) demonstrates that endovascular intervention of chronic deep vein thrombosis can be safely and effectively performed, and that combined venoplasty with ultrasound‐accelerated thrombolysis with adjunctive therapies led to successful reestablishment of flow and clinical improvement in patients with PTS.
What Are the Clinical Implications?
For patients experiencing PTS from chronic venous obstruction, combined percutaneous transluminal venoplasty and ultrasound‐accelerated thrombolysis interventions resulted in improvement in clinical PTS, as measured by the Villalta scale and Venous Clinical Severity Score as well as durable venous patency.
ACCESS PTS intervention also resulted in significant improvement in quality of life, as measured by the VEINES‐QOL (Venous Insufficiency Epidemiological and Economic Study–Quality of Life) and Short Form‐36 Physical Component Summary.
Introduction
Deep vein thrombosis (DVT) affects 600 000 to 900 000 people annually in the United States.1, 2, 3 The most common long‐term complication of DVT is postthrombotic syndrome (PTS), which affects 25% to 60% of patients2 and can lead to significant limitations in quality of life (QOL).3, 4
Although the pathophysiological characteristics of PTS are not fully understood, venous hypertension is thought to play a central role.5, 6 Acutely treating venous occlusion has been shown to have the potential to reduce the risk of PTS‐related outcomes in appropriately selected patients.7, 8, 9 However, once PTS has developed, treatment options are limited. It has been postulated that recanalizing chronically obstructed venous segments, with a potential reduction in chronic venous hypertension, may reduce the severity of PTS‐related symptoms and signs; however, the literature supporting this strategy is lacking.10
Although pharmacomechanical endovenous techniques have been successful in treating acute thrombus,7, 11 the composition of chronic thrombus, consisting of collagen type I and III, may not be amenable to such treatment. Nonetheless, use of high‐frequency, low‐power ultrasound may alter the local architecture of the fibrin and collagen latticework, enhancing its permeability.12, 13, 14, 15, 16
The aim of this prospective study (ACCESS PTS ‐ Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) was to evaluate the effectiveness of combined percutaneous transluminal venoplasty (PTV) and ultrasound‐accelerated thrombolysis (USAT) to improve PTS‐related symptoms and venous disease–related QOL in subjects with PTS in the presence of chronic veno‐occlusive disease.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to EKOS Corporation at 11911 N Creek Pkwy S, Bothell, WA 98011.
This open‐label, single‐arm, multicenter, prospective trial (NCT021595211) was sponsored by EKOS Corporation, a BTG International group company. Patients were enrolled at 18 centers in the United States. Institutional review board approval was obtained at all sites, and written consent was obtained from all patients.
Study Population
Patients who were aged 18 to 75 years and had experienced an acute DVT episode in the femoral vein (FV) or more proximal vein(s) at least 6 months before the study procedure were eligible for inclusion. There was no limit to the maximum time from the acute DVT episode. The presence of chronic venous occlusion was confirmed within 60 days before the study procedure. In addition, patients were required to have a Villalta score ≥8 in the affected limb within 30 days before the procedure and failure of at least 3 months of conservative treatment, consisting of therapeutic anticoagulation and graduated elastic compression stockings, in improving PTS symptoms at any time before the procedure.
Exclusion criteria included mechanical thrombectomy within 2 weeks or treatment with thrombolytic therapy within 48 hours of the study procedure; body mass index >40 kg/m2; complete occlusion of the popliteal vein, as confirmed by ultrasonography; DVT isolated to the ipsilateral iliac vein; or thrombus extending ≥3 cm into the inferior vena cava. Additional exclusion criteria were active bleeding or a history of peptic ulcer disease; gastrointestinal bleeding in the past 3 months; severe liver dysfunction, bleeding diathesis, eye surgery, or hemorrhagic retinopathy within the past 3 months; major surgery, trauma, cardiopulmonary resuscitation, obstetrical delivery, or other invasive procedure within the past 10 days; a history of stroke or intracranial/intraspinal hemorrhage, tumor, vascular malformation, or aneurysm; use of clopidogrel, ticlopidine, or other thienopyridine antiplatelet agents within the past 7 days; and life expectancy considered to be <1 year. Systolic blood pressure ≥175 mmHg or diastolic blood pressure ≥110 mmHg resulted in postponement of the procedure until the blood pressure was controlled. Laboratory exclusion criteria before the procedure were as follows: hemoglobin <9 g/dL, international normalized ratio ≥1.5, platelet count <100×109/L or >700×109/L, and serum creatinine outside the normal range at the investigative site.
Patients with bilateral DVT could be enrolled if both limbs met the inclusion/exclusion criteria. Treatment of the second limb was done at least 30 days after treatment of the first limb. If one limb met enrollment criteria and the other did not, the limb that did not meet enrollment criteria was not treated until the subject was off study.
Patient disposition is shown in Figure 1.
Figure 1.
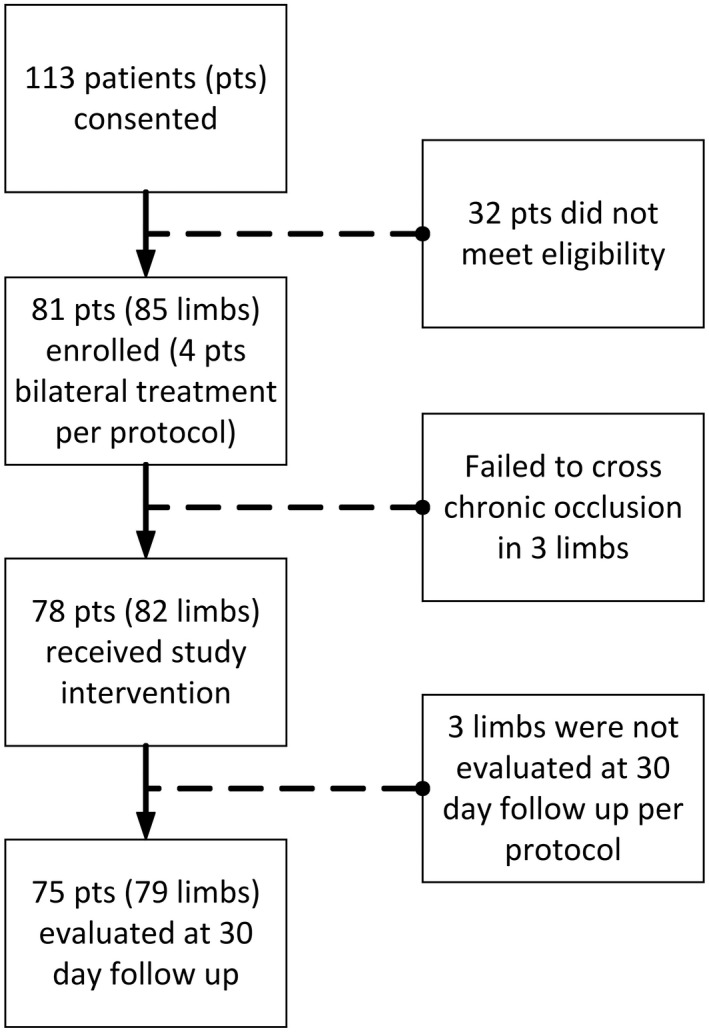
ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) patient enrollment.
Treatment Protocol
Patients were anticoagulated with enoxaparin (1 mg/kg twice a day) for at least 48 hours before the initiation of study treatment. The access site was determined by the extent of occlusive disease; access via the popliteal vein was preferred, unless significant popliteal disease was present, at which point tibial access was recommended but left to the discretion of the operator. After venous access was achieved, baseline venography was obtained of the entire limb to confirm the presence and extent of chronic thrombotic disease. Per protocol, imaging at each level was obtained from time of contrast appearance in the field of view, through contrast washout from the field of view or for 10 seconds, whichever came first. Standard catheter and wire techniques were used to cross the diseased venous segments. If the occlusion could not be crossed by the catheter/guidewire combination, the EkoSonic device (EKOS Corporation/BTG, Bothell, WA) could not be placed, or there was severe venous damage that could be further complicated by thrombolysis, such as perforation, the procedure was abandoned and considered a technical failure.
After the occlusion had been successfully crossed, venoplasty was performed by balloon dilatation of the diseased segments to create a lumen to allow passage of the EkoSonic catheter. Venoplasty was performed in each diseased segment to the expected size of a normal sized comparable segment (eg, the common FV, 10–12 mm; FV, 8–10 mm; popliteal, 6–8 mm; and tibial, 3–4 mm).
An EkoSonic catheter with the treatment zone length selected to cover as much of the entire occlusive segment as possible was placed over a guide wire. The guide wire was then removed, and the MicroSonic Device was inserted into the drug delivery catheter and Luer locked in place. USAT commenced with recombinant tPA (tissue‐type plasminogen activator) infusion at 0.5 to 1.0 mg/h (operator discretion), with coolant saline infusing at 35 to 120 mL/h, as tolerated by the patient. The thrombolytic infusion was continued for at least 12 hours and as needed up to a maximum of 48 hours. If the device ran for >24 hours, a new MicroSonic Device was placed as per the IFU (Instructions for Use) protocol. The recombinant tPA dose could be adjusted but did not exceed a rate of 1 mg/h or a total dose of 48 mg. After at least 12 hours of USAT infusion, the patient returned to the angiography laboratory, and venography was performed to determine the need for further lysis or if adjunctive therapy, such as stenting or further PTV, was required. While left to operator discretion, it was recommended that complete flow be restored. Pneumatic compression devices were placed on both lower extremities during the infusion procedure. Weight‐based enoxaparin, twice‐daily, injections continued throughout the procedure, as previously described.17
After discharge, patients were treated with enoxaparin (1 mg/kg twice a day) for minimum of 30 days. This was considered the initial anticoagulation period. They were also instructed to wear graded compression stockings, 20 to 30 or 30 to 40 mmHg, on both legs for 90 days. At the end of this initial anticoagulation period, enoxaparin could be substituted for an oral anticoagulant in those patients requiring long‐term anticoagulation. If limb swelling and discomfort did not allow standard graded compression stocking use, leg wraps, leg elevation, and pumps were used initially to reduce the swelling and pain. Patients were encouraged to ambulate early and often, as tolerated.
Contrast venography imaging was collected preprocedure, after percutaneous transluminal angioplasty, post‐USAT, and after adjunctive therapy. Analysis was conducted by a core laboratory (Syntactx, New York, NY). Venous duplex ultrasound imaging was collected post posture and at each follow‐up visit and analyzed by a vascular ultrasound core laboratory (VasCore, Boston, MA).
Study Outcomes
The primary efficacy end point was a reduction in the Villalta score of ≥4 points from baseline to 30 days. Secondary efficacy end points included the change in Villalta score from baseline to 90, 180, and 365 days; change from baseline to 30, 90, 180, and 365 days in Short Form‐36 Physical Component Summary score, VEINES (Venous Insufficiency Epidemiological and Economic Study)–QOL score, and Venous Clinical Severity Score (VCSS); as well as symptomatic PTS‐induced admission to an emergency department or an unplanned visit to a physician's office or hospitalization up to 365 days after procedure.
Efficacy is reported for each limb when: (1) chronic obstruction was successfully crossed and USAT treatment was activated and (2) Villalta scores were available at both baseline and 30 days after procedure. If an additional revascularization procedure was required within 30 days after procedure, this was regarded as a failure for the primary efficacy end point.
Predefined safety end points included the frequency of major bleeding episodes within 72 hours of treatment initiation and the rate of symptomatic pulmonary embolism (PE) during the hospitalization for the procedure. A major bleed was defined as fatal bleeding, symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a decrease in hemoglobin of ≥2 g/L or leading to transfusion of ≥2 units of whole blood or red cells, which correlates to the ISTH (The International Society on Thrombosis and Haemostasis) definitions of a major bleed.18
Safety outcomes were measured in patients for whom treatment was initiated (ie, venous access was attempted for catheter introduction).
Statistical Analysis
Study size was calculated on the basis of the primary efficacy end point. Study success was defined as at least 50% of subjects achieving this end point. Power analysis showed that 70 subjects will result in 83% power to achieve study success when the true proportion achieving the primary end point is at least 55% of subjects.
For efficacy end points, the analysis unit was the treated limb, as opposed to the patient (ie, 2 limbs were analyzed in patients with bilateral DVTs). QOL is reported per patient.
For changes from baseline to the follow‐up time points, continuous measures were summarized as mean, SD, median, minimum, and maximum. Categorical outcomes were summarized in 2‐way tables. All statistical analyses accounted for the repeated measures nature of multiple limbs per patient. With patient as a random effect, the SAS GLIMMIX procedure (SAS, Cary, NC) was used to evaluate the statistical significance for changes from baseline. Missing data were not imputed.
Results
Patient Characteristics
Eighty‐one patients were enrolled; of these, 78 patients (mean age, 54.6±12.7 years; 32.1% women; 82 limbs enrolled) underwent the procedure (3 patients experienced failure to cross the obstruction) and are included in the intent‐to‐treat population. Baseline demographic and clinical characteristics are shown in Table 1. Mean time since the most recent DVT was 13.2±4.4 months, and mean time since the most recent PE for the 25 patients who had a PE was 4.3±6.4 years. The maximum time from the acute DVT episode was 31.5 years (378.4 months). The mean Villalta score at baseline was 15.5±5.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | Value |
|---|---|
| Age, mean±SD, y | 54.6±12.7 |
| Women, n (%) | 25 (32.1) |
| White, n (%) | 58 (74.4) |
| Hypertension, n (%) | 40 (51.3) |
| Diabetes mellitus, n (%) | 12 (15.4) |
| Current or past tobacco use, n (%) | 30 (38.5) |
| Prior DVT, n (%) | 78 (100) |
| Prior PE, n (%) | 25 (32.1) |
| Presence of an inferior vena cava filter, n (%) | 9 (11.5) |
| Any thrombophilia,a | 20 (25.6) |
| Active cancer, n (%) | 0 (0) |
| BMI, mean±SD, kg/m2 | 32.1±5.9 |
| Villalta score, mean±SD | 15.5±5.2 |
BMI indicates body mass index; DVT, deep vein thrombosis; PE, pulmonary embolism.
Thrombophilia conditions in these 20 patients included lupus anticoagulant, factor V Leiden mutation, prothrombin gene mutation (20210a), decreased protein C and S activity, antithrombin deficiency, polycythemia, and factor II deficiency.
Procedural Characteristics
The mean dose of recombinant tPA was 18.5±7.6 mg (range, 6–46 mg). The mean duration of recombinant tPA infusion was 22.8±5.7 hours (range, 6–48 hours). The mean duration of EkoSonic treatment was 23.0±5.4 hours (range, 16–48 hours). Of the 82 limbs, 77 received adjunctive therapy on the second day of treatment after the USAT procedure (93.9%), which consisted of the following: PTV in 68 limbs (82.9%), stent placement in 40 limbs (48.8%), and additional percutaneous embolectomy or thrombectomy in 1 limb (1.2%). An inferior vena cava filter was inserted in 2 patients.
All patients were hospitalized for a mean of 3.4±6.1 days. Twenty‐five patients (32%) were admitted to the intensive care unit during USAT for 1.4±0.6 days.
Outcomes
Seventy‐five patients (79 limbs) completed 30‐day follow‐up and were included in the primary efficacy end point. The primary end point was met in 64.6% (51/79) of limbs at 30 days. At 1 year, 77.3% (51/66) of limbs had a reduction of ≥4 in the Villalta score.
Venographic results are reported on the basis of the Marder score. At baseline, the mean Marder score was 11.6±6.0 (median, 11.0). Compared with baseline, at postvenoplasty (day 1), Marder score changed by 2.3±3.4 (P<0.001), denoting improvement. Compared with baseline, at post‐USAT (day 2), Marder score changed by 3.7±3.9 (P<0.001). Compared with baseline, at postadjunctive treatment, Marder score changed by 7.0±4.0 (P<0.001).
The mean Villalta score at baseline was 15.5±5.2. Compared with baseline at 30, 90, 180, and 365 days after procedure, the Villalta scores changed by −5.9±5.8, −6.9±6.5, −7.8±6.1, and −8.2±6.4, respectively (P<0.0001 compared with baseline for all time points) (Figure 2A).
Figure 2.
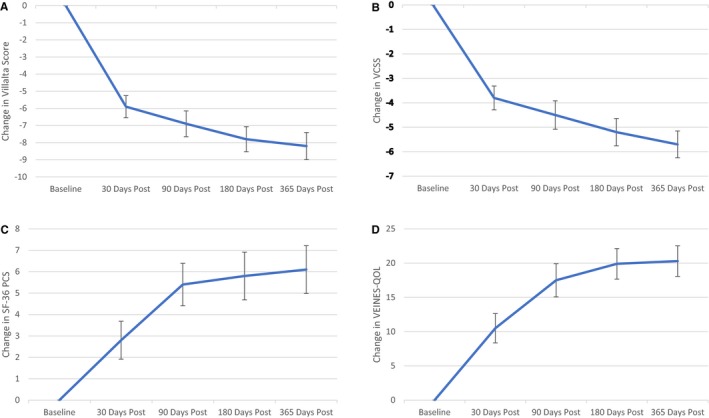
Results of the Villalta scale, Venous Clinical Severity Score (VCSS), Short Form‐36 (SF‐36) Physical Component Score (PCS), and VEINES‐QOL (Venous Insufficiency Epidemiological and Economic Study–Quality of Life) change from baseline through 365 days of follow‐up. A, Villalta score result change from baseline with SE. B, VCSS result change from baseline with SE. C, SF‐36 PCS result change from baseline with SE. D, VEINES‐QOL result change from baseline with SE.
The mean±SD VCSS at baseline was 12.0±5.3. Compared with baseline at 30, 90, 180, and 365 days, VCSS changed by −3.8±4.3, −4.5±5.0, −5.2±4.7, and −5.7±4.4, respectively, with P<0.0001 for change from baseline at each interval (Figure 2B).
The mean±SD Short Form‐36 Physical Component Summary score at baseline was 38.9±9.5. The scores improved from baseline by 2.8±0.9 (P=0.003), 5.4±1.0 (P<0.0001), 5.8±1.1 (P<0.0001), and 6.1±1.1 (P<0.0001) at 30, 90, 180, and 365 days, respectively (Figure 2C).
The mean±SD VEINES‐QOL score at baseline was 61.9±17.2. The scores improved from baseline by 10.5±16.32, 17.5±17.76, 19.9±16.1, and 20.3±15.7 at 30, 90, 180, and 365 days, respectively, with P<0.0001 for change from baseline for each interval time point (Figure 2D).
Venous patency at 365 days, as defined by any flow on duplex ultrasound, was present in 100%, 98.5%, 98.5%, 93.9%, 92.2%, and 98.5% in the common iliac vein, external iliac vein, common FV, proximal FV, distal FV, and popliteal vein, respectively (Figure 3). At that time interval, data were available in 55.7%, 72.2%, 82.3%, 83.5%, 79.7%, and 82.3% for the common iliac vein, external iliac vein, common FV, proximal FV, distal FV, and popliteal vein, respectively, as shown in Table 2, represented as the percentage of occluded vein segments.
Figure 3.
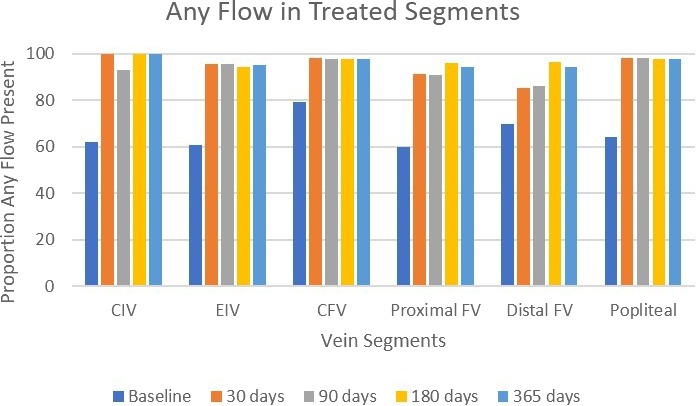
Venographic and Doppler flow over time per venous segment. Data represent comparison of any flow identified in venous segments that were treated during the procedure, from baseline venographic imaging through follow‐up duplex ultrasound imaging. CFV indicates common FV; CIV, common iliac vein; EIV, external iliac vein; FV, femoral vein.
Table 2.
Core Laboratory Adjudicated Occluded Venous Segments Over Time
| Segment | Occluded Segments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, % | No./Total | 30 d, % | No./Total | 90 d, % | No./Total | 180 d, % | No./Total | 365 d, % | No./Total | |
| CIV | 16.4 | 9/55 | 0 | 0/51 | 2.1 | 1/48 | 0 | 0/44 | 0 | 0/47 |
| EIV | 16.1 | 10/62 | 2.8 | 2/71 | 1.5 | 1/67 | 1.8 | 1/57 | 1.5 | 1/65 |
| CFV | 17.6 | 12/68 | 2.7 | 2/75 | 2.8 | 2/71 | 1.5 | 1/65 | 1.5 | 1/66 |
| Proximal FV | 35.7 | 25/70 | 9.5 | 7/74 | 8.7 | 6/69 | 4.5 | 3/66 | 6.1 | 4/66 |
| Distal FV | 36.2 | 25/55 | 17.1 | 12/70 | 15.2 | 10/66 | 6.3 | 4/63 | 7.8 | 5/64 |
| Popliteal | 29.8 | 17/57 | 2.7 | 2/75 | 4.3 | 3/69 | 3.1 | 2/65 | 1.5 | 1/65 |
Baseline status assessed by venography. Other time points reported by duplex ultrasonography. Occlusion was defined as total lack of flow in a vein segment. CIV indicates common iliac vein; EIV, external iliac vein; CFV, common FV; FV, femoral vein.
Patients could undergo central (iliofemoral) stenting for lesions identified by the operator. Stenting was not allowed below the common femoral bifurcation, per protocol. A subgroup analysis of stented (n=40) versus nonstented patients (n=42) was conducted. Improvements were seen in all measured outcomes, irrespective of stent status (Table 3 and Figures 4A through 4D).
Table 3.
Analysis of Outcome Measures Comparing Stented With Nonstented Subgroups
| Group | Baseline | 30 d | 90 d | 180 d | 365 d |
|---|---|---|---|---|---|
| Villalta stented | 17.0±5.17 | 10.7±5.49 | 9.3±6.49 | 8.7±6.13 | 8.4±5.51 |
| Villalta nonstented | 14.0±4.76 | 8.7±4.62 | 8.3±5.32 | 7.5±5.16 | 7.3±5.02 |
| VCSS stented | 12.6±5.39 | 8.4±4.64 | 7.6±4.98 | 7.3±4.59 | 6.8±3.50 |
| VCSS nonstented | 11.4±5.27 | 8.3±3.81 | 7.8±3.66 | 6.7±4.21 | 7.1±4.22 |
| VEINES‐QOL stented | 57.1±19.25 | 67.1±22.36 | 77.2±22.30 | 75.9±23.20 | 78.8±23.24 |
| VEINES‐QOL nonstented | 67.7±19.03 | 79.3±19.56 | 84.2±19.82 | 90.0±13.43 | 86.6±17.43 |
| SF‐36 PCS stented | 37.1±9.02 | 39.8±8.45 | 43.8±10.16 | 43.3±10.72 | 43.9±9.32 |
| SF‐36 PCS nonstented | 40.6±9.70 | 44.3±9.83 | 45.7±10.39 | 46.6±11.27 | 46.4±10.22 |
Data are given as mean±SD. At 365 days, P<0.001 for all measures, except SF‐36 PCS nonstented, for which P=0.0072. PCS indicates Physical Component Score; SF‐36, Short Form‐36; VCSS, Venous Clinical Severity Score; VEINES‐QOL, Venous Insufficiency Epidemiological and Economic Study–Quality of Life.
Figure 4.
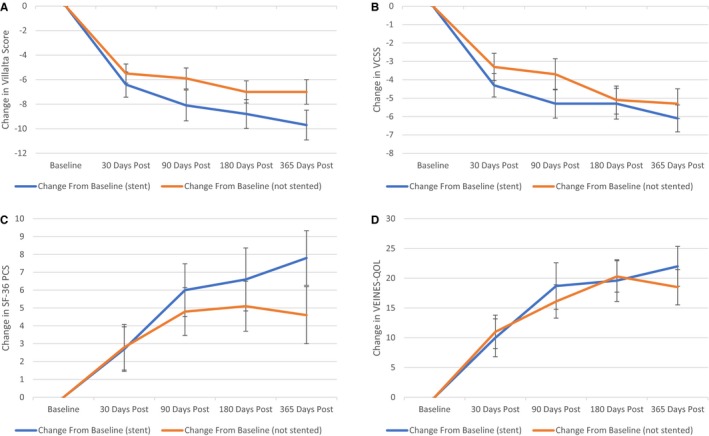
Stent vs nonstented subgroup results from baseline through 365 days of follow‐up of the Villalta scale, Venous Clinical Severity Score (VCSS), Short Form‐36 (SF‐36) Physical Component Score (PCS), and VEINES‐QOL (Venous Insufficiency Epidemiological and Economic Study–Quality of Life). A, Villalta score result stent vs nonstented subgroup change from baseline with SE. B, VCSS result stent vs nonstented subgroup change from baseline with SE. C, SF‐36 PCS result stent vs nonstented subgroup change from baseline with SE. D, VEINES‐QOL result stent vs nonstented subgroup change from baseline with SE.
Recurrent DVT or PE was diagnosed in 3 patients (3.8%) within 30 days and 1 additional patient (total, 5.1%) within 365 days.
Bleeding
Two major bleeding episodes occurred within the 72‐hour period after initiating USAT treatment. One patient developed epistaxis and ultimately died because of multisystem organ failure at 32 days after procedure. The second patient experienced an intra‐abdominal hemorrhage and recovered within 30 days of the procedure, and the event was adjudicated to be related to the procedure and anticoagulant. Total bleeding events of any kind within 30 days of the procedure were noted in 13 patients (16.7%). All patients were receiving therapeutic dose anticoagulation at the time of their bleed.
Mortality
One patient (1.3%) who developed epistaxis died because of respiratory distress and septic shock on day 39 after procedure.
PTS‐related healthcare use
In the 30 days after procedure, 6 patients (7.7%) visited an emergency department, 12 patients (15.4%) had an unplanned visit to a physician's office, and 3 patients (3.8%) were hospitalized for symptomatic PTS (defined as PTS‐related symptoms in a patient with a Villalta score ≥5). In the 365‐day period after procedure, 13 patients (16.7%) visited an emergency department, 18 patients (23.1%) had an unplanned visit to a physician's office, and 8 patients (10.3%) were hospitalized for symptomatic PTS.
Discussion
In this prospective, multicenter study, combined PTV and USAT for chronic venous obstruction after DVT resulted in a measurable and durable increase in venous patency as well as clinical gains over 1 year.
By 365 days after procedure, we observed sustained clinical improvements in measures related to PTS, including a clinically meaningful reduction in the Villalta score, with 77.3% of subjects having a reduction ≥4, as well as the mean Villalta score being reduced from 15.5±5.2 to 7.8±5.2. In addition, VCSS as well as measures of generic and venous disease–specific QOL all demonstrated statistically significant improvements from baseline to 365 days.
The postthrombotic sequelae of DVT can vary from minor pain and/or swelling to severe pain and venous stasis ulcerations affecting 5% to 10% of patients.19 In 2 recent studies that focused on pharmacomechanical intervention for acute DVT, the incidence of PTS remained high (37%–55%) despite intervention.7, 9, 11 Furthermore, although intervention showed some evidence of benefit for proximal (iliofemoral) DVT,8 PTS‐related symptoms were also noted in patients presenting with more distal (femoral‐popliteal) clot.20 In the recently published ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter‐Directed Thrombolysis) Trial, 44% of patients with femoropopliteal DVT in both the pharmacomechanical and conservative therapy arms developed PTS at 24 months.7
It has been postulated that PTS develops because of reduced venous flow, resulting in causing venous hypertension. It has been previously demonstrated that the degree of PTS is directly related to the degree of venous hypertension.6 Accordingly, treating chronic venous obstruction in patients who present with PTS is done with the intent of relieving venous hypertension and subsequently the sequelae of the PTS. Indeed, there are data to suggest that relieving chronically obstructed lower extremity veins may benefit patients who experience PTS. In a single‐center retrospective registry of patients with severe PTS, the combination of common FV stenting and great saphenous vein stripping resulted in complete relief of pain and swelling in 73% and 47% of 97 limbs at 5.5 years follow‐up, respectively.20 QOL improved as well. In a retrospective comparison of iliofemoral venous stenting (n=116) to graded compression stockings alone (n=94) in patients with PTS, intervention with stenting resulted in improved symptoms in patients with severe PTS but not in those with moderate PTS over a median follow‐up of 21 months.21 Similarly, short‐term relief of pain and swelling was achieved in 80% of 45 patients treated with iliac vein stenting and/or saphenous vein ablation.22
A specific challenge of treating chronic venous obstruction is the composition of the obstructing material. Once the acute thrombus organizes, actual thrombus is no longer present, and the obstruction and resultant chronic venous changes are related to collagen type I and III fibers.23 It is believed that thrombolysis with recombinant tPA has no effect in the dissolution of collagen‐rich chronic DVT. The scarring associated with the chronic venous changes may also lead to an atretic, occluded segment obstructing flow. Thus, the classic teaching has been that thrombolytic therapy is ineffective in relieving chronic thrombotic occlusions.24 However, in a single‐center retrospective registry using USAT and PTV in patients with chronic DVT experiencing PTS, significant improvement in symptoms was reported in 89 (93%) of 96 limbs.17 The results of the ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) study corroborate these findings and suggest that USAT in conjunction with adjunctive intervention can meaningfully improve clinical symptoms of PTS, even in the long‐term setting.
Our protocol called for full‐dose enoxaparin (1 mg/kg) during USAT rather than low‐dose heparin. We recognize that the SIR (Society of Interventional Radiology) position statement on CDT (catheter directed thrombolysis) recommends reduced heparin for CDT during PE treatment to reduce bleeding risk25; however, the SIR position is related to PE thrombolysis, whereas this article is about chronic lower extremity DVT. In a previous publication, we found our choice for enoxaparin twice‐daily dosing to be safe and more effective than low‐dose heparin.17 Specifically, in our original experience, it was observed that subtherapeutic heparin led to more acute intraprocedural thrombosis after the initial PTV. In addition, in the ATTRACT Trial protocol,7 physicians were given the option of either subtherapeutic heparin or weight‐based low‐molecular‐weight heparin. In that trial, there were no fatal or intracranial bleeding events in either arm.
The findings of this study also address 2 historical concerns of chronic veno‐occlusive disease: the benefit of thrombolytic therapy in infrainguinal disease as well as intervention in veins that have loss of valvular function. Inclusion into the ACCESS PTS study required at minimum FV disease. Given the lack of data evaluating endovascular intervention for chronic venous occlusions in the infrainguinal region, it has long been believed that intervention is ineffective in this population. This study, however, demonstrates that endovascular intervention in the femoropopliteal region can be undertaken safely and is effective in restoring patency and obtaining clinical benefit, as determined by various PTS and QOL outcome measures.
For venous valves, it has also been accepted that chronically diseased venous segments have lost valve function. In addition, there are those who argue that restoring flow in chronically diseased or occluded deep veins will worsen the PTS because of incompetent valves and increasing deep venous reflux. In this current study, information about superficial or deep venous reflux was not measured. Although deep venous insufficiency was not measured, if one assumes these segments all contained incompetent valves, then one can postulate from the overwhelmingly positive data that by restoring venous patency, the open vein hypothesis correlates with PTS improvement by improving flow and reducing venous hypertension.
Strengths of the current study include prospective enrollment of patients at multiple centers, standardization of the interventional approach, inclusion of patient‐reported outcomes as well as of safety end points, and independent adjudication of imaging outcomes. Nonetheless, this study has certain limitations. First, this was a single‐arm study. However, we aimed to compensate for this design by the prerandomization treatment requirements. All patients included had already failed 3 months of conservative therapy (therapeutic anticoagulation and compression garments) before enrollment. Thus, in practice, if patients were randomized to a control arm consisting of standard of care therapy, they would have been enrolled in a treatment arm they had already failed. Nonetheless, outcome adjudication in matched patients who did not receive intervention is lacking. The primary end point was not independently adjudicated, which could have resulted in exaggerated report of benefit. Nonetheless, venous patency, as assessed by duplex ultrasound at 1 year, was excellent. Also, although PTS may continue to evolve long after the index DVT event, some patients were included <2 years from their DVT. However, all patients had already developed PTS at the time of inclusion after failing conservative treatment. On average, most patients were recruited 13 months after their DVT episode and so by the end of the trial the average follow‐up exceeded 2 years, which is at least as long as previously suggested as necessary.26
Conclusions
For patients experiencing PTS from chronic venous obstruction, combined PTV and USAT interventions result in improvement in clinical PTS, as measured by the Villalta scale and VCSS as well as durable venous patency. Endovascular intervention also resulted in significant improvement in QOL. In a subgroup analysis of stented (n=40) versus nonstented patients (n=42), improvements were seen in all measured outcomes irrespective of stent status. These promising results should lead to further investigation.
ACCESS PTS Investigators
Kevin Herman, MD Holy Name Medical Center; Daniel Leung, MD Christiana Care; David Dexter, MD Sentara Norfolk General Hospital; David Williams, MD University of Michigan Medical Center; Paul Gagne, MD Norwalk Hospital; Rahul Razdan, MD Catholic Health Initiatives St Elizabeth; Ronald Winokur, MD Weill Cornell Medical Center; Amit Dwivedi, MD University of Louisville Research Foundation/Norton Hospital; Akhilesh Sista, MD New York University Hospital; Brett Butler, MD Aultman Hospital; David Johnson, MD University of Colorado Hospital; Paul Kim, MD Maine Medical Center; Clifford Davis, MD Tampa General Hospital; Robert Feldtman, MD Methodist Dallas Medical Center; Stephen Kee, MD UCLA Medical Center; Luis Leon, MD Tucson Medical Center; Krishna Mannava, MD Fairfield Medical Center.
Sources of Funding
The ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) study was funded by the EKOS Corporation, a BTG International group company.
Disclosures
Dr Garcia was compensated as a consultant for BTG/EKOS for the ACCESS PTS (Accelerated Thrombolysis for Post‐Thrombotic Syndrome Using the Acoustic Pulse Thrombolysis Ekosonic Endovascular System) study, as well as a paid consultant and received research support from BTG/EKOS, as well as a paid consultant for Boston Scientific. Dr Sterling has been financially compensated as a consultant and has received research support from BTG/EKOS, is a paid consultant for Boston Scientific, and has received research support from Angiodynamics. Dr Ouriel is employed by and holds equity in Syntactx. Syntactx receives research funding from EKOS/BTG. Dr Kahn is a Tier 1 Canada Research Chair and is an investigator of the Canadian Institutes of Health Research–funded CanVECTOR Network (Funding Reference: CDT‐142654). She was compensated as a consultant for BTG/EKOS for the ACCESS PTS study and has participated in advisory boards for Pfizer, Sanofi, and Aspen. Dr Comerota was compensated as a consultant for BTG/EKOS for the ACCESS PTS study. Dr Jaff is a noncompensated advisor and is the vice president, clinical affairs, innovation and technology, peripheral interventions for Boston Scientific, January 2020. He has been a compensated advisor for Abbott Vascular, Biotronik, Medtronic, Philips/Volcano, and Sanofi. He is also an equity investor in Embolitech. Dr Weinberg has no disclosures to report.
Acknowledgments
We wish to thank Lynn Allen, the sponsor study project lead; Timothy Keo, the sponsor study project manager; and Thomas Hughes, PharmD, the medical project manager for their insight, dedication, and assistance in seeing this study through to its completion.
(J Am Heart Assoc. 2020;9:e013398 DOI: 10.1161/JAHA.119.013398.)
The authors of the ACCESS PTS Investigators are listed at the end of the article.
Contributor Information
Mark J. Garcia, Email: Markmd@evcde.com.
the ACCESS PTS Investigators:
Kevin Herman, Daniel Leung, David Dexter, David Williams, Paul Gagne, Rahul Razdan, Ronald Winokur, Amit Dwivedi, Akhilesh Sista, Brett Butler, David Johnson, Paul Kim, Clifford Davis, Robert Feldtman, Stephen Kee, Luis Leon, and Krishna Mannava
References
- 1. Goldhaber S, Morrison R. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106:1436. [DOI] [PubMed] [Google Scholar]
- 2. Ashrani AA, Heit JA. Incidence and cost burden of post‐thrombotic syndrome. J Thromb Thrombolysis. 2009;28:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johri M, Ginsberg JS. Determinants of health‐related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. [DOI] [PubMed] [Google Scholar]
- 4. vanKorlaar IM, Vossen CY, Rosendaal FR, Bovill EG, Cushman M, Naud S, Kaptein AA. The impact of venous thrombosis on quality of life. Thromb Res. 2004;114:11–18. [DOI] [PubMed] [Google Scholar]
- 5. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long‐term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindner DJ, Edwards JM, Phinney ES, Taylor LM Jr, Porter JM. Long‐term hemodynamic and clinical sequelae of lower extremity deep vein thrombosis. J Vasc Surg. 1986;4:436–442. [PubMed] [Google Scholar]
- 7. Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Duncan JR, Nieters P, Derfler MC, Filion M, Gu CS, Kee S, Schneider J, Saad N, Blinder M, Moll S, Sacks D, Lin J, Rundback J, Garcia M, Razdan R, VanderWoude E, Marques V, Kearon C; ATTRACT Trial Investigators . Pharmacomechanical catheter‐directed thrombolysis for deep‐vein thrombosis. N Engl J Med. 2017;377:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, Jaff MR, Razavi MK, Kindzelski AL, Bashir R, Patel P, Sharafuddin M, Sichlau MJ, Saad WE, Assi Z, Hofmann LV, Kennedy M, Vedantham S; ATTRACT Trial Investigators . Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139:1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO, Njaaastad AM, Sandbæk G, Sandset PM; CaVenT Study Group . Post‐thrombotic syndrome after catheter‐directed thrombolysis for deep vein thrombosis (CaVenT): 5‐year follow‐up results of an open‐label, randomised controlled trial. Lancet Haematol. 2016;3:e64–e71. [DOI] [PubMed] [Google Scholar]
- 10. Garcia MJ. Endovascular options for chronic DVT. Endovascular Today. 2012;54–58. [Google Scholar]
- 11. Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbæk G, Sandset PM; CaVenT Study Group . Long‐term outcome after additional catheter‐directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–38. [DOI] [PubMed] [Google Scholar]
- 12. Kudo S. Thrombolysis with ultrasound effect. Tokyo Jikeikai Med J. 1989;104:1005. [Google Scholar]
- 13. Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound‐potentiated fibrinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]
- 14. Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78:1063–1068. [PubMed] [Google Scholar]
- 15. Tachibana K, Tachibana S. Ultrasound energy for enhancement of fibrinolysis and drug delivery: special emphasis on the use of a transducer‐tipped ultrasound system In: Siegel RJ, ed. Ultrasound Angioplasty. Boston, MA: Kluwer; 1996:121–133. [Google Scholar]
- 16. Atar S, Luo H, Nagai T, Sahm RA, Fishbein MC, Siegel RJ. Arterial thrombus dissolution in vivo using a transducer‐tipped, high frequency ultrasound catheter and local low dose urokinase delivery. J Endovasc Ther. 2001;8:282–290. [DOI] [PubMed] [Google Scholar]
- 17. Grilli CJ, McGarry M, Ali MMDJ, Agriantonis D, Thompson SA, Goodman C, Wrigley JM, Lee D, Leung G, Kimbiris M, Horvath MJ, Garcia MJ. Aggressive management of chronic deep venous thrombosis: technical and clinical outcomes. J Vasc Interv Radiol. 2012;23:S117–S118. Abstract No. 290. [Google Scholar]
- 18. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 19. Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Int Med. 2008;149:698–707. [DOI] [PubMed] [Google Scholar]
- 20. Kearon C, Gu CS, Julian JA, Goldhaber SZ, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Kahn SR, Kindzelski AL, Slater D, Geary R, Winokur R, Natarajan K, Dietzek A, Leung DA, Kim S, Vedantham S. Pharmacomechanical catheter‐directed thrombolysis in acute femoral‐popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thromb Haemost. 2019;119:633–644. [DOI] [PubMed] [Google Scholar]
- 21. Yin M, Shi H, Ye K, Lu X, Li W, Huang X, Lu M, Jiang M. Clinical assessment of endovascular stenting compared with compression therapy alone in post‐thrombotic patients with iliofemoral obstruction. Eur J Vasc Endovasc Surg. 2015;50:101–107. [DOI] [PubMed] [Google Scholar]
- 22. Nayak L, Hildebolt CF, Vedanthan S. Postthrombotic syndrome: feasibility of a strategy of imaging‐guided endovascular intervention. J Vasc Interv Radiol. 2012;23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 23. Comerota AJ, Oostra C, Favad Z, Gunning W, Henke P, Luke C, Lynn A, Lurie F. A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb Res. 2015;135:882–887. [DOI] [PubMed] [Google Scholar]
- 24. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter‐directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiol. 1999;211:39–49. [DOI] [PubMed] [Google Scholar]
- 25. Kuo WT, Sista AK, Faintuch S, Dariushnia SR, Baerlocher MO, Lookstein RA, Haskal ZJ, Nikolic B, Gemmete JJ. Society of interventional radiology position statement on catheter‐directed therapy for acute pulmonary embolism. J Vasc Interv Radiol. 2018;29:293–297. [DOI] [PubMed] [Google Scholar]
- 26. Vedanthan S, Kahn SR, Goldhaber SZ, Comerota AJ, Parpia S, Meleth S, Earp D, Williams R, Sista AK, Marston W, Rathbun S, Magnuson EA, Razavi MK, Jaff MR, Kearon C. Endovascular therapy for advanced post‐thrombotic syndrome: proceedings from a multidisciplinary consensus panel. Vasc Med. 2016;21:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]


