Abstract
Background
Several trials have demonstrated protective effects from inhibition of sodium‐glucose cotransporter 2 among patients with type 2 diabetes mellitus. There is uncertainty about the consistency of the cardiovascular benefits achieved across patient subsets.
Methods and Results
We included 4 large‐scale trials of sodium‐glucose cotransporter 2 inhibition compared with placebo in patients with diabetes mellitus that reported effects on cardiovascular outcomes overall and for participant subgroups defined at baseline by cardiovascular disease, reduced kidney function, and heart failure. Fixed effects models with inverse variance weighting were used to estimate summary hazard ratios and 95% CIs. There were 38 723 patients from 4 trials, with a mean 2.9 years of follow‐up. Of the patients, 22 870 (59%) had cardiovascular disease, 7754 (20%) had reduced kidney function, and 4543 (12%) had heart failure. There were 3828 major adverse cardiac events. There was overall benefit for major adverse cardiac events (0.88; 95% CI, 0.82–0.94; P<0.001) and no evidence that the effects of sodium‐glucose cotransporter 2 inhibition varied across patient subgroups, defined by the presence of cardiovascular disease or heart failure at baseline (all P interaction >0.252; I2<25%). All patient subgroups benefited with respect to hospitalization for heart failure (all P interaction>0.302; I2<10%), cardiovascular death (all P interaction>0.167; I2<50%), and death from any cause (all P interaction>0.354; I2=0%). The only difference in effects across subgroups was for stroke, with protection observed among those with reduced kidney function but not those with preserved kidney function (P interaction=0.020; I2=81%).
Conclusions
Sodium‐glucose cotransporter 2 inhibitors protect against cardiovascular disease and death in diverse subsets of patients with type 2 diabetes mellitus regardless of cardiovascular disease history.
Keywords: cardiovascular disease, meta‐analysis, sodium‐glucose cotransporter 2 inhibition, type 2 diabetes mellitus
Subject Categories: Clinical Studies, Cardiovascular Disease, Secondary Prevention, Primary Prevention
Clinical Perspective
What Is New?
Sodium‐glucose cotransporter 2 inhibitors protect against cardiovascular disease and death in those with type 2 diabetes mellitus, irrespective of established cardiovascular disease history or kidney function.
Sodium‐glucose cotransporter 2 inhibitors may protect against stroke in individuals with reduced kidney function.
What Are the Clinical Implications?
Sodium‐glucose cotransporter 2 inhibition should be considered in all patients with type 2 diabetes mellitus and elevated risk of cardiovascular disease, even in the absence of established disease.
Introduction
Type 2 diabetes mellitus (T2DM) is a global pandemic, with an estimated 370 million people currently affected.1, 2 It is a major risk factor for both cardiovascular disease (CVD) and chronic kidney disease (CKD), with CVD the leading cause of death in people with T2DM.3 Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are a class of glucose‐lowering agent whose mechanism of action involves blockade of SGLT2 cotransporters on the luminal surface of the proximal renal tubule. The resultant increase in glycosuria and natriuresis contributes to a broad range of metabolic benefits,4 including reduction in glycosylated hemoglobin, body weight, blood pressure, and albuminuria.5
Large randomized control trials of SGLT2 inhibition in T2DM6, 7, 8, 9, 10, 11 have shown a clear reduction in CVD events among individuals with established atherosclerotic CVD.12, 13 There remains, however, significant uncertainty about the potential benefits of SGLT2 inhibition in those without established CVD, with recent reviews suggesting this is an area of uncertainty that needs further evaluation and clarification.14 North American guidelines currently recommend the use of SGLT2 inhibitors as second‐line therapy after metformin, specifically in those with established atherosclerotic CVD.15, 16
The comparative effects of SGLT2 inhibitors on cardiovascular outcomes in patients with and without reduced kidney function are also yet to be fully elucidated, with concerns that the renal mechanism of action might lead to attenuated efficacy in this population. The recent publication of the CREDENCE (The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation) trial, which reports on cardiovascular, renal, and safety outcomes in patients with CKD and high vascular risk, enables this subgroup to be examined in greater detail.10 In addition, people with concomitant T2DM and heart failure (HF) have a 10‐fold increase in mortality compared with those with T2DM without HF,17 and the comparative effects of SGLT2 inhibition in these individuals are of great interest.
The aim of this systematic review and meta‐analysis was to define the cardiovascular benefits and the effects on key safety outcomes of SGLT2 inhibition, overall and separately among participants with and without established CVD, reduced kidney function, or HF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
We performed a systematic review and meta‐analysis of randomized, placebo‐controlled, event‐driven, cardiovascular or renal outcome trials of SGLT2 inhibitors that reported on cardiovascular outcomes and serious adverse events (SAEs). This review was conducted in accordance with the Preferred Reporting Items of Systematic Reviews and Meta‐Analyses statement.18
Search Strategy, Study Selection, and Data Extraction
Medline via Ovid (from 1946 to February 2019) and EMBASE via Ovid (from 1980 to February 2019) were searched systematically for relevant trials (Table S1 and Figure S1). The search had no language restriction and used subject headings relevant to SGLT2 inhibition, T2DM, CVD, and randomized control trial design. In addition, reference lists from included trials, review articles, and other relevant reports were manually scanned to identify other potentially relevant data.
Our primary aim was to assess the effect of SGLT2 inhibitors on cardiovascular outcomes; thus, we only included event‐driven, randomized, placebo‐controlled cardiovascular or renal outcome trials that reported independently adjudicated cardiovascular outcomes as primary or secondary end points. The titles and abstracts of all identified articles were extracted and screened for an initial assessment of eligibility. Full‐text versions of potentially eligible studies were reviewed to reach a final decision on inclusion or exclusion. We excluded studies in people with type 1 diabetes mellitus and those not conducted in humans. Duplicate reports, trials that involved compound agents (eg, SGLT2 inhibitors in combination with metformin), trials that did not compare with placebo, and trials that did not report on efficacy outcomes of interest (cardiovascular, death, or the specified safety outcomes) were considered ineligible. Data were extracted into an electronic spreadsheet with a specific focus on the collection of information about treatment effects in patient subgroups defined by the presence or absence of CVD at baseline, the presence or absence of reduced kidney function at baseline (defined as estimated glomerular filtration rate [eGFR] >60 or <60 mL/min per 1.73 m2), and the presence or absence of HF at baseline. In DECLARE (Dapagliflozin Effect on Cardiovascular Events)‐TIMI 58 Trial, cardiovascular outcomes were reported for eGFR <60, 60 to 90, and >90 mL/min per 1.73 m2 separately, with the upper 2 categories pooled to determine the hazard ratio (HR) in those with eGFR ≥60 mL/min per 1.73 m2.
Study quality was judged for each included trial, according to evidence of the proper conduct of randomization, concealment of treatment allocation, similarity of treatment groups at baseline, the provision of a description of the eligibility criteria, completeness of follow‐up, and use of intention‐to‐treat analysis using the Cochrane Risk of Bias Tool19 (Table S2).
Review of trials for eligibility, data extraction, and quality assessment was conducted independently by 2 authors (C.A. and A.K.) using a standardized approach. Any disagreement was settled by consultation with a third author (B.N.).
Outcomes
The efficacy outcomes studied were as follows: (1) major adverse cardiac events (MACEs) comprising cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke; (2) cardiovascular death; (3) fatal or nonfatal myocardial infarction; (4) fatal or nonfatal stroke; (5) hospitalization for HF; (6) the composite of cardiovascular death or hospitalization for HF; and (7) all‐cause mortality. The safety outcomes studied were as follows: total SAEs, severe hypoglycemia, metabolic acidosis, amputation, and bone fracture. For each outcome, we sought to identify for each trial HRs and 95% CIs describing the effects of SGLT2 inhibition in the overall population and each patient subset of interest. If HRs were not available, we used in order of preference rate ratios or risk ratios to maximize the use of trial‐level data from included studies.
Data Synthesis and Statistical Analysis
We summarized effect estimates for each trial, overall and for the patient subsets of interest, defined according to baseline CVD, kidney function, and HF. Mean levels of baseline characteristics were obtained by weighting individual trial values by sample size and dividing through by the total number of participants. Meta‐analysis of treatment effects was done using fixed effects models and inverse variance weighting. A fixed effects method was selected because the effects on the efficacy and safety outcomes in each trial and each patient subgroup were a priori considered more likely to be consistent than inconsistent. The constancy of effects was evaluated by assessing the percentage of variability across the pooled estimates attributable to heterogeneity beyond chance using the I2 statistic and by calculating the P value for heterogeneity. An I2 statistic of 0% to 25% was considered to reflect a low likelihood; 26% to 75%, a moderate likelihood; and 76% to 100%, a high likelihood of differences beyond chance. P<0.05 for heterogeneity was also deemed likely to reflect a high likelihood of differences beyond chance. For safety outcomes, where HRs were more frequently available, we also did subsidiary analyses including only the effect estimates based on HRs and excluding those based on rate ratios or risk ratios. All reported effects of SGLT2 inhibition are relative effects. A 95% CI that did not span unity indicated a statistically significant result for an outcome. Statistical analyses were performed with Stata, version 15 (Stata, College Station, TX).
Results
Four trials were identified for inclusion: EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients)8 (n=7020), the CANVAS (Canagliflozin Cardiovascular Assessment) Program11 (n=10 142), DECLARE‐TIMI 589 (n=17 160), and CREDENCE10 (n=4401). The CANVAS Program trial reported integrated data from the CANVAS and CANVAS R (CANVAS‐ Renal) trials8, 9, 10, 11, 20 (Table). In addition to the primary reports for each trial, there were several secondary articles from which data were extracted.6, 7, 21, 22, 23, 24, 25, 26, 27, 28, 29 In total, 38 723 patients were included in the meta‐analyses, with 3828 MACE outcomes, 1192 hospitalizations for HF, 1506 cardiovascular deaths, and 2612 deaths from any cause.
Table 1.
Baseline Characteristics of Included Studies and Trial Participants
| Characteristics | EMPA‐REG OUTCOME8 | CANVAS Program11 | DECLARE‐TIMI 589 | CREDENCE10 |
|---|---|---|---|---|
| Trial characteristics | ||||
| Randomized treatment | Empagliflozin/placebo | Canagliflozin/placebo | Dapagliflozin/placebo | Canagliflozin/placebo |
| Dose(s) | 10 mg, 25 mg | 100 mg, 300 mg | 10 mg | 100 mg |
| Participants, n | 7020 | 10 142 | 17 160 | 4401 |
| Median follow‐up period, y | 3.1 | 2.4 | 4.2 | 2.6 |
| Participant characteristics | ||||
| Age, mean (SD), y | 63.1 (8.7) | 63.3 (8.3) | 63.9 (6.8) | 63.0 (9.2) |
| Women, n (%) | 2004 (28.5) | 3633 (35.8) | 6422 (37.4) | 1494 (33.9) |
| Race, n (%) | ||||
| White | 5081 (72.4) | 7944 (78.3) | 13 653 (79.6) | 2931 (66.6) |
| Asian | 1517 (21.6) | 1284 (12.7) | 2303 (13.4) | 877 (19.9) |
| Black or African American | 357 (5.1) | 336 (3.3) | 603 (3.5) | 224 (5.1) |
| Other/missing | 65 (0.9) | 578 (5.7) | 601 (3.5) | 369 (8.4) |
| Cardiovascular disease, n (%) | 7020 (100) | 6656 (65.6) | 6974 (40.6) | 2223 (50.5) |
| Heart failure, n (%) | 706 (10.1) | 1461 (14.4) | 1724 (10.0) | 652 (14.8) |
| Reduced kidney function, n (%) | 1818 (25.9) | 2039 (20.1) | 1270 (7.4) | 2631 (59.8) |
| Urine ACR ≥300 mg/g, n (%) | 7649 (11.1) | 762 (7.6) | 1169 (6.8) | 3874 (88.0) |
| Glycosylated hemoglobin, mean (SD), % | 8.1 (0.8) | 8.2 (0.9) | 8.3 (1.2) | 8.3 (1.3) |
| Baseline use of RAS blockade, n (%) | 5666 (80.7) | 8116 (80.0) | 13 950 (81.3) | 4395 (99.9) |
| Baseline use of β blocker, n (%) | 4554 (64.9) | 5421 (53.5) | 9030 (52.6) | 1770 (40.2) |
| Baseline use of statin/ezetimibe, n (%) | 5403 (77.0) | 7599 (74.9) | 12 868 (75.0) | 3036 (69.0) |
| Insulin, n (%) | 3387 (48.2) | 5095 (50.2) | 7013 (40.9) | 2884 (65.5) |
| Metformin, n (%) | 5193 (74.0) | 7825 (77.2) | 14 068 (82.0) | 2545 (57.8) |
| Sulfonylurea, n (%) | 3006 (42.8) | 4361 (43.0) | 7322 (42.7) | 1268 (28.8) |
| Thiazolidinedione, n (%) | 299 (4.3) | 492 (4.9) | 0 (0) | 136 (3.1) |
| GLP‐1 receptor agonist, n (%) | 196 (2.8) | 406 (4.0) | 750 (4.4) | 183 (4.2) |
| DPP‐4 inhibitor, n (%) | 796 (11.3) | 1261 (12.4) | 2888 (16.8) | 751 (17.1) |
ACR indicates albumin/creatinine ratio; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; RAS, renin‐angiotensin system.
Estimated glomerular filtration rate <60 mL/min per 1.73 m2 based on the Modification of Diet in Renal Disease equation in EMPA‐REG OUTCOME and the CANVAS Program and the Chronic Kidney Disease Epidemiology Collaboration equation in DECLARE‐TIMI 58 and CREDENCE.8, 9, 10, 11
CANVAS and CANVAS‐R indicates CANagliflozin cardioVascular Assessment Program; CREDENCE, The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation Trial; DECLARE‐TIMI 58, Dapagliflozin Effect on Cardiovascular Events Trial; EMPA‐REG Outcome, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patient.
Across the 4 studies, the mean age of participants ranged between 63.1 and 63.9 years, the proportion of women ranged between 28.5% and 37.4%, and the mean glycosylated hemoglobin ranged between 8.1% and 8.3%. The proportions with a baseline history of CVD extended from 40.6% to 100%, the proportions with a baseline history of reduced kidney function ranged from 7.4% to 59.8%, the proportions with baseline macroalbuminuria ranged from 7.5% to 88.0%, and the proportions with a baseline history of HF ranged from 10.1% to 14.8% (Table).
Overall Effect of SGLT2 Inhibition on Cardiovascular Outcomes
All 4 studies reported on MACE outcomes, with 3 reporting this as the primary outcome. SGLT2 inhibition was associated with an overall 12% proportional reduction in MACE (HR, 0.88; 95% CI, 0.82–0.94), with consistent effects across all studies (I2=0%; P for interaction=0.477). There was an overall 17% relative reduction in cardiovascular death (HR, 0.83; 95% CI, 0.75–0.92), with moderate heterogeneity in effects across the 4 studies (I2=70.7%; P for interaction=0.017). SGLT2 inhibition reduced the risk of myocardial infarction (HR, 0.88; 95% CI, 0.80–0.97; I2=0%; P=0.996) but had no overall effect on stroke risk (HR, 0.96; 95% CI, 0.86–1.09; I2=0%; P for interaction=0.785).
With respect to HF outcomes, there was a 32% proportional reduction in hospitalization for HF for those treated with an SGLT2 inhibitor compared with placebo (HR, 0.68; 95% CI, 0.60–0.76) with no evidence of heterogeneity between studies (I2=0; P for interaction=0.720) and a 24% reduction in the composite end point of cardiovascular death and hospitalization for HF (HR, 0.76; 95% CI, 0.70–0.82). There was moderate heterogeneity between studies on the magnitude of the relative effect for this composite end point (I2=41.9; P for interaction=0.160). All‐cause mortality was also reduced (HR, 0.85; 95% CI, 0.79–0.92), with some evidence of heterogeneity between the trials (I2=63.1%; P for interaction=0.044) (Figure 1 and Figure S2).
Figure 1.
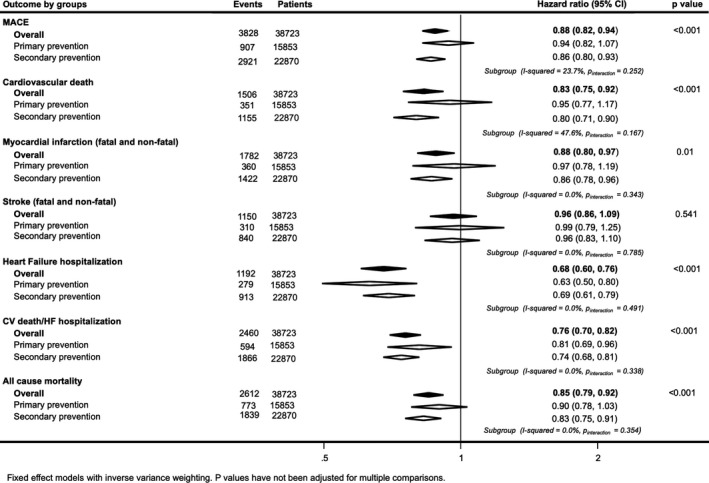
Effects of sodium‐glucose cotransporter 2 inhibition on death and cause‐specific cardiovascular (CV) events for patients with (secondary prevention) and without (primary prevention) CV disease at baseline. HF indicates heart failure; MACE, major adverse cardiac event.
A sensitivity analysis was performed excluding the EMPA‐REG OUTCOME trial to explore the effect of the outlying large reduction in cardiovascular death observed in that trial on the heterogeneity between study findings. Heterogeneity between the remaining studies was much reduced, with I2 values for all outcomes reduced to <20% and all corresponding P values for interaction being >0.312. A further analysis was performed for the composite end point of cardiovascular death and hospitalization for HF using data for the subset of patients with T2DM included in the DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) study30 (2139 patients). This resulted in no significant change in the outcome, with an HR of 0.75 (95% CI, 0.70–0.81) and P for interaction of 0.27.
Effect of SGLT2 Inhibition on Cardiovascular Outcomes in Patient Subgroups Defined by CVD, Reduced Kidney Function, and HF
A total of 22 870 of 38 723 participants (59%) had CVD at baseline. Point estimates of effect were to the left of unity for SGLT2 inhibition versus placebo for every efficacy outcome in those with and without CVD at baseline. The HR for MACE in the secondary prevention cohort was 0.86 (95% CI, 0.80–0.93) and 0.94 (95% CI, 0.82–1.07) in primary prevention. The HR for myocardial infarction in the secondary prevention cohort was 0.86 (95% CI, 0.78–0.96) and 0.97 (95% CI, 0.78–1.19) in primary prevention. There was no evidence of differences in the effects of SGLT2 inhibition in patients with or without CVD at baseline for any of the efficacy outcomes, except cardiovascular death, for which there was moderate evidence of greater protection with SGLT2 inhibition in those with CVD at baseline (HR, 0.80; 95% CI, 0.71–0.90) compared with those without (HR, 0.95; 95% CI, 0.77–1.17) (I2=47.6%; P for interaction=0.167). There was separately significant evidence of protection for HF (HR, 0.63; 95% CI, 0.50–0.80) and the composite outcome of cardiovascular death or HF (HR, 0.81; 95% CI, 0.69–0.96) among the primary prevention subset (Figure 1 and Figure S2).
Most patients had preserved kidney function, with 7754 participants (20%) with baseline eGFR <60 mL/min per 1.73 m2. There was separately significant evidence of protection with SGLT2 inhibition compared with placebo among patients with reduced kidney function for every efficacy outcome (Figure 2 and Figure S3). There was some evidence that patients with reduced kidney function (HR, 0.80; 95% CI, 0.70–0.90) achieved greater proportional risk reductions for MACE than patients with preserved kidney function (HR, 0.92; 95% CI, 0.85–0.99) (I2=73.4%; P for interaction=0.053). There was stronger evidence of different effects on stroke, with protection among those with reduced kidney function (HR, 0.75; 95% CI, 0.59–0.96) but not among those with preserved kidney function (HR, 1.05; 95% CI, 0.91–1.20; I2=81.4%) (P for interaction=0.020).
Figure 2.
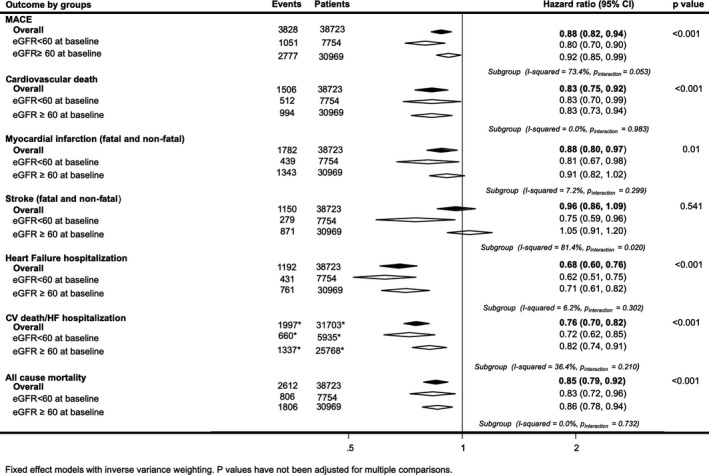
Effects of sodium‐glucose cotransporter 2 inhibition on death and cause‐specific cardiovascular (CV) events for patients with (estimated glomerular filtration rate [eGFR] <60 mL/min per 1.73 m2) and without (eGFR >60 mL/min per 1.73 m2) reduced kidney function at baseline. HF indicates heart failure; MACE, major adverse cardiac event. *Indicates subgroup event numbers not available for Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients (EMPA‐REG Outcome).
There were 4543 patients (12%) with a history of HF at baseline. SGLT2 inhibitors were associated with a reduction in risk of hospitalization for HF, irrespective of baseline HF (Figure 3 and Figure S4). Comparable proportional risk reductions in those with and without HF at baseline were achieved with SGLT2 inhibition for all other outcomes (all I2=0% and all P for interaction>0.354).
Figure 3.
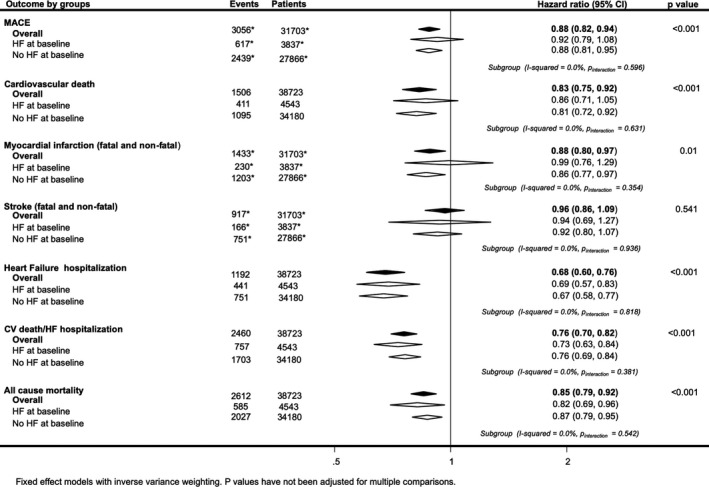
Effects of sodium‐glucose cotransporter 2 inhibition on death and cause‐specific cardiovascular (CV) events for patients with and without a history of heart failure (HF) at baseline. MACE indicates major adverse cardiac event. *Indicates subgroup event numbers not available for Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients (EMPA‐REG Outcome).
Effects of SGLT2 Inhibition on SAEs
SGLT2 inhibitors were associated with a lower relative risk of SAEs (HR, 0.91; 95% CI, 0.88–0.94) (Figure 4 and Figure S5). There were no differences in the rates of severe hypoglycemia or fracture but greater overall risks were observed for diabetic ketoacidosis (HR, 2.46; 95% CI, 1.43–4.24) and amputation (HR, 1.23; 95% CI, 1.05–1.44). There was moderate evidence of heterogeneity between the trial findings for amputation (I2=70.0%; P for interaction=0.019) attributable to an increase in amputation risk in the CANVAS Program trial but not the other trials. There were too few data describing safety outcomes by patient subsets to enable meaningful comparisons across groups defined by baseline CVD, kidney function, or HF.
Figure 4.
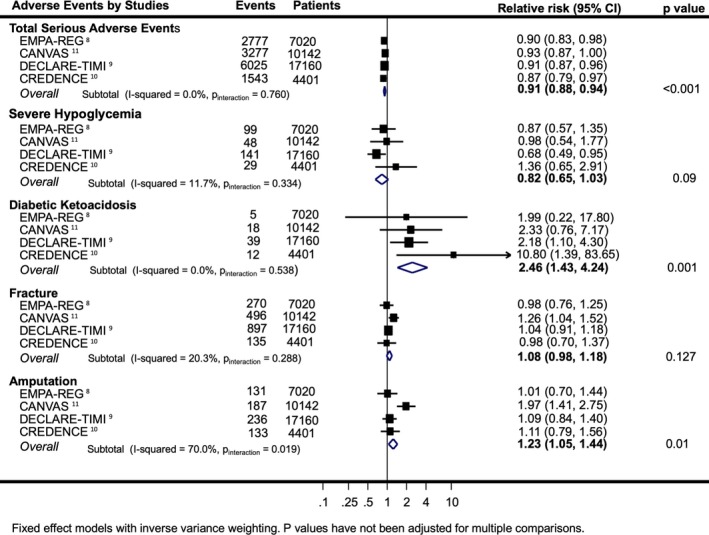
Effects of sodium‐glucose cotransporter 2 inhibition on serious adverse events. Relative risks are shown for Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patient (EMPA‐REG Outcome) total serious adverse events and hypoglycemia, and hazard ratios are shown for other included studies and outcomes. CANVAS and CANVAS‐RENAl indicates Canagliflozin Cardiovascular Assessment Program; CREDENCE, The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation; DECLARE‐TIMI 58.
Discussion
These data provide strong evidence of cardiovascular and mortality benefits with SGLT2 inhibition, with limited evidence that effects vary between patient subgroups or across completed trials. In conjunction with reductions in total SAEs in every individual trial, these findings indicate that a broad range of patients with T2DM are likely to achieve important net benefits from use of this drug class.
A key strength of these analyses is the inclusion of new data from the recently completed CREDENCE trial, which enrolled large numbers of individuals who were at high vascular risk but free of CVD at baseline. In contrast to previous reports suggesting that the benefits of SGLT2 inhibition on MACE are restricted to those with established CVD,12, 13 our updated analyses suggest comparable benefits for those with and without CVD. The prior interpretations were based on marginal evidence for an interaction by baseline CVD (P=0.0501),12 which was probably a chance finding. We found no evidence of an interaction in either our primary analysis or a series of sensitivity analyses and conclude that there is little evidence that effects in the primary versus secondary prevention subgroups differ by more than chance for the MACE outcome. Furthermore, with clear and separately significant evidence of protection against HF, and the composite of HF or vascular death, in the primary prevention subset, there is a strong case for the use of these agents in the primary prevention setting. Our results call for a reevaluation of current guideline recommendations for SGLT2 inhibitor therapy, with a view to include those with and without established CVD. A recent update to the European Guidelines (European Society of Cardiology/European Association for the Study of Diabetes)31 has partially addressed this issue, recommending that SGLT2 inhibition or glucagon‐like peptide‐1 receptor agonists may be used as first‐line therapy in those with T2DM and high risk of atherosclerotic CVD, irrespective of whether they are treatment naïve or already on metformin.
It is possible that the benefits for primary prevention observed in this overview could have been driven by the inclusion of participants with concomitant CKD in the primary prevention subset. CKD was an inclusion criterion for all CREDENCE trial participants, irrespective of the presence of CVD at baseline. Most participants in the CREDENCE trial had markedly increased albuminuria, and ≈60% had baseline eGFR <60 mL/min per 1.73 m2. However, the absence of heterogeneity of treatment effects by eGFR subgroups argues against eGFR as the reason why the conclusions for the primary prevention subset in this overview differ from that reported previously. In addition, previous data from the EMPA‐REG OUTCOME trial and CANVAS Program trial suggest that albuminuria does not modify the effects of SGLT2 inhibitors on major cardiovascular events.11, 25 To further explore a possible effect of CKD, we performed a supplementary analysis examining effects on MACE outcomes by albuminuria at baseline. This analysis identified no heterogeneity of treatment effects by baseline albuminuria (Figure S6).
The inclusion of data from the CREDENCE trial also greatly increased the capacity to explore effects of SGLT2 inhibition on cardiovascular outcomes among patients with preserved compared with reduced renal function. Lesser effects of SGLT2 inhibition on intermediate markers of cardiovascular risk, such as glycosylated hemoglobin and body weight, are established among patients with reduced eGFR21 and could reduce the magnitude of cardiovascular protection afforded. The present analyses identify proportional effects on cardiovascular events that are at least as large in participants with reduced kidney function compared with those with preserved kidney function. Alongside the clear evidence of renal safety and efficacy among those with CKD provided by the CREDENCE trial,10 these overview data provide compelling evidence for significant benefits among those with reduced kidney function.
The reason why greater protection against MACE may be achieved among those with reduced kidney function is unclear but appears to have been driven principally by a greater effect on stroke among those with reduced eGFR compared with those with preserved eGFR. The findings for stroke by baseline eGFR are somewhat inconsistent across the contributing studies and warrant further investigation. The risk of stroke in those with T2DM is twice that of those without the disease, and with the exception of pioglitazone for the secondary prevention of stroke,32 glucose‐lowering agents have shown limited efficacy for stroke prevention,27 although data to describe effects by level of renal function are absent for most prior trials.
This overview confirmed the large and well‐established benefits of SGLT2 inhibition on HF and showed consistent effects of SGLT2 inhibition on cardiovascular end points among those with and without a history of HF at baseline. The meta‐analyses also confirmed the known safety characteristics of this drug class, although data were not available to systematically evaluate SAEs with respect to each subgroup of interest. Overall risks of fracture were not increased, with little evidence of heterogeneity across the included studies, suggesting the increased risk of fracture observed within the CANVAS Program trial11 may have arisen by chance. Despite the absence of amputation risk in the CREDENCE trial, there remained an overall increased risk across all studies combined, with significant heterogeneity of effects between the constituent trials. The heterogeneity in effects between trials for amputation is driven by the CANVAS Program trial result, which differs from that of the other trial testing canagliflozin (CREDENCE), as well as trials testing empagliflozin (EMPA‐REG OUTCOME) and dapagliflozin (DECLARE‐TIMI 58). The reasons for these differences in trial findings remain unclear.
This meta‐analysis combines data from 4 large randomized studies, each with a robust design and low risk of bias, providing good power to explore the effects of SGLT2 inhibitors on cardiovascular outcomes both overall and for subgroups. The broad constancy of the findings across subgroups suggests wide clinical utility for the drug class and that the few differences in effects observed in the overview should be treated with caution. The statistical evidence of heterogeneity of effects between patient subsets in these analyses is in almost every case only moderate in strength and unless confirmed by future analyses the overall effect estimates provide the best current guide for clinical practice. The lack of individual patient data for some of the included studies is a limitation to the current analysis, but is unlikely to change the overall findings of this report.
The low absolute rates of events for those without a history of CVD at baseline meant that there was limited statistical power to define effects of SGLT2 inhibition separately in that patient subset. However, given comparable directions of effect and no evidence of heterogeneity between effects for any outcome between those with and without CVD, the overview findings support consideration of SGLT2 inhibition in primary, as well as secondary, prevention settings. Similarly, only a small proportion of patients had HF at baseline, and HF was defined by history rather than by rigorous validation based on imaging and natriuretic peptide assay. The recently published DAPA‐HF study30 in HF with reduced ejection fraction has provided additional insight into the role of SGLT2 inhibition in HF, with a clear reduction in hospitalization for HF and other key cardiovascular outcomes. The DEFINE‐HF (Dapagliflozin Effect on Symptoms and Biomarkers in Patients With Heart Failure) trial33 also demonstrated an improvement in HF‐related health status, as measured by Kansas City Cardiomyopathy Questionnaire in those with HF with reduced ejection fraction treated with SGLT2 inhibition. There remains some uncertainty with respect to the benefit in HF with preserved ejection fraction that will be addressed in ongoing large trials DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) [NCT03619213] and EMPEROR‐Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction) [NCT03057951].
In conclusion, SGLT2 inhibitors protect against CVD and death in diverse subsets of patients with T2DM. The magnitude of protection achieved may vary across patients, but among those studied to date, the available evidence does not identify clearly a patient group that is unlikely to achieve significant cardiovascular protection from use of this drug class.
Sources of Funding
Dr Perkovic is receiving research support from the Australian National Health and Medical Research Council (Program Grant). Dr Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship. Dr Neal receives research support from an Australian National Health and Medical Research Council Principal Research Fellowship. Dr Kang is supported by a National Health and Medical Research Council Postgraduate Scholarship via the University of New South Wales and an Australian Government Research Training Program Fees Offset.
Disclosures
Dr Neuen has received travel support from Janssen. Dr Lam has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; and has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, WebMD Global LLC, Radcliffe Group Ltd, and Corpus. Dr Cannon has received research grants (all >$10 000) from Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Merck, Janssen, and Takeda; and has received consulting fees from Aegerion, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Corvidia, GlaxoSmithKline, Innovent, Eisai, Eli Lilly, Kowa, Merck, Pfizer, Regeneron*, and Sanofi* (* denotes >$10 000). Dr Mahaffey's financial disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. Dr Perkovic is serving on Steering Committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer; and is serving on advisory boards and/or speaking at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol‐Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. Dr Jardine is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly, and Merck; has served on advisory boards sponsored by Akebia, Baxter, and Boehringer Ingelheim; and has spoken at scientific meetings sponsored by Janssen, Amgen, and Roche, with any consultancy, honoraria, or travel support paid to her institution. Dr Neal has received research support from Janssen, Roche, Servier, and Merck Schering Plough; and is serving on advisory boards and/or has involvement in Continuing Medical Education (CME) programs for Abbott, Janssen, Novartis, Pfizer, Roche, and Servier, with any consultancy, honoraria, or travel support paid to his institution.
Supporting information
Table S1. Electronic Search Terms
Table S2. Risk of Bias Assessment
Figure S1. PRISMA flow chart: identification of eligible studies.
Figure S2A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S2B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S2C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S3A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patient with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S3B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S3C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S4A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patients with and without a history of heart failure at baseline, for each included study.
Figure S4B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with and without a history of heart failure at baseline, for each included study.
Figure S4C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with and without a history of heart failure at baseline, for each included study.
Figure S5. Sensitivity analysis for the outcome of serious adverse events without inclusion of risk ratios.
Figure S6. The effects of SGLT2 inhibition on major adverse cardiac outcomes for patients depending on urine albumin creatinine ratio at baseline.
Acknowledgments
All authors had full access to the study data and had final responsibility for the decision to submit for publication.
(J Am Heart Assoc. 2020;9:e014908 DOI: 10.1161/JAHA.119.014908.)
References
- 1. Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium‐glucose co‐transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 3. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:262–274. [DOI] [PubMed] [Google Scholar]
- 5. Abdul‐Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA‐REG OUTCOME study. Diabetes Care. 2016;39:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 9. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE‐TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 10. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099. [DOI] [PubMed] [Google Scholar]
- 12. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 13. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 14. Pocock SJ, Collier TJ. Statistical appraisal of 6 recent clinical trials in cardiology: JACC State‐of‐the‐Art Review. J Am Coll Cardiol. 2019;73:2740–2755. [DOI] [PubMed] [Google Scholar]
- 15. Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Rastogi Kalyani R, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Standl E, Schnell O, McGuire DK. Heart failure considerations of antihyperglycemic medications for type 2 diabetes. Circ Res. 2016;118:1830–1843. [DOI] [PubMed] [Google Scholar]
- 18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala‐Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group and Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carbone S, Dixon DL. The CANVAS Program: implications of canagliflozin on reducing cardiovascular risk in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Desai M, Li Q, Deng H, Rosenthal N, Jardine MJ, Bakris G, Perkovic V. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Z, Lindley RI, Radholm K, Jenkins B, Watson J, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Shaw W, Oh R, Desai M, Matthews DR, Neal B. Canagliflozin and stroke in type 2 diabetes mellitus. Stroke. 2019;50:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–2536. [DOI] [PubMed] [Google Scholar]
- 24. Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause‐Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub‐analysis from DECLARE TIMI‐58 trial. Circulation. 2019;139:2516–2527. [DOI] [PubMed] [Google Scholar]
- 25. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–129. [DOI] [PubMed] [Google Scholar]
- 26. Wanner C, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–1802. [DOI] [PubMed] [Google Scholar]
- 27. Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, Mattheus M, Woerle HJ, Broedl UC, Johansen OE, Albers GW, Diener HC; EMPA‐REG OUTCOME Investigators (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) . Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017;48:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME® trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation. 2019;139:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 31. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa‐Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019; pii: ehz486. DOI: 10.1093/eurheartj/ehz486. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32. Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for secondary stroke prevention: a systematic review and meta‐analysis. Stroke. 2017;48:388–393. [DOI] [PubMed] [Google Scholar]
- 33. Nassif ME, Windsor S, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire D, Pitt B, Scirica BM, Austin B, Drazner M, Fong M, Givertz MM, Gordon R, Jermyn R, Katz S, Lamba S, Lanfear D, LaRue S, Lindenfeld J, Malone M, Margulies KB, Mentz R, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M; DEFINE‐HF Investigators . Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation. 2019;140:1463–1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Electronic Search Terms
Table S2. Risk of Bias Assessment
Figure S1. PRISMA flow chart: identification of eligible studies.
Figure S2A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S2B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S2C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with (secondary prevention) and without (primary prevention) cardiovascular disease at baseline.
Figure S3A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patient with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S3B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S3C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and without (estimated glomerular filtration rate >60 mL/min per 1.73 m2) reduced kidney function at baseline, for each included study.
Figure S4A. Effects of SGLT2 inhibition on MACE, CV death, total MI and total stroke for patients with and without a history of heart failure at baseline, for each included study.
Figure S4B. Effects of SGLT2 inhibition on HF hospitalization, CV death/HF hospitalization and all cause mortality for patients with and without a history of heart failure at baseline, for each included study.
Figure S4C. Effects of SGLT2 inhibition on non‐fatal MI and stroke for patients with and without a history of heart failure at baseline, for each included study.
Figure S5. Sensitivity analysis for the outcome of serious adverse events without inclusion of risk ratios.
Figure S6. The effects of SGLT2 inhibition on major adverse cardiac outcomes for patients depending on urine albumin creatinine ratio at baseline.


