Abstract
Background
The trajectory of ischemic stroke patients attributable to large vessel occlusion is fundamentally altered by endovascular thrombectomy. This study aimed to develop a nomogram for predicting 3‐month mortality risk in patients with ischemic stroke attributed to artery occlusion in anterior circulation who received successful endovascular thrombectomy treatment.
Methods and Results
Patients with successful endovascular thrombectomy (modified Thrombolysis in Cerebral Infarction IIb or III) were enrolled from a multicenter registry as the training cohort. Step‐wise logistic regression with Akaike information criterion was utilized to establish the best‐fit nomogram. The discriminative value of the nomogram was tested by concordance index. An additional 224 patients from 2 comprehensive stroke centers were prospectively recruited as the test cohort for validating the new nomogram. Altogether, 417 patients were enrolled in the training cohort. Age (odds ratio [OR], 1.07; 95% CI, 1.03−1.10), poor pretreatment collateral status (OR, 2.13; 95% CI, 1.18−3.85), baseline blood glucose level (OR, 1.12; 95% CI, 1.04−1.21), symptomatic intracranial hemorrhage (OR, 9.51; 95% CI, 4.54−19.92), and baseline National Institutes of Health Stroke Scale score (OR, 1.08; 95% CI, 1.03−1.12) were associated with mortality and were incorporated in the nomogram. The c‐index of the nomogram was 0.835 (95% CI, 0.785–0.885) in the training cohort and 0.758 (95% CI, 0.667–0.849) in the test cohort.
Conclusions
The nomogram, composed of age, pretreatment collateral status, baseline blood glucose level, symptomatic intracranial hemorrhage, and baseline National Institutes of Health Stroke Scale score, may predict risk of mortality in patients with ischemic stroke and treated successfully with endovascular thrombectomy.
Keywords: ischemic stroke, endovascular thrombectomy, mortality, nomogram, recanalization
Subject Categories: Mortality/Survival, Ischemia, Clinical Studies, Revascularization
Clinical Perspective
What Is New?
Our study developed and validated a nomogram for predicting 90‐day mortality risk in ischemic stroke patients attributed to artery occlusion in anterior circulation and received successful endovascular thrombectomy treatment.
What Are the Clinical Implications?
The nomogram, composed of age, pretreatment collateral status, baseline blood glucose level, symptomatic intracranial hemorrhage, and baseline National Institutes of Health Stroke Scale score may be useful for mortality risk stratification in ischemic stroke patients treated with endovascular thrombectomy despite successful recanalization.
Introduction
Randomized controlled trials have validated the efficacy of endovascular thrombectomy (EVT) in patients with acute ischemic stroke caused by large vessel occlusion in anterior circulation.1, 2, 3, 4, 5 Recanalization status (successful or unsuccessful) is the dominating factor determining functional outcomes.6, 7 Patients with successful recanalization have much better prognoses than those with unsuccessful recanalization. However, only half of patients with successful recanalization achieve favorable outcomes (90‐day modified Rankin Scale ≥2). The other half of patients with successful recanalization, but with unfavorable outcomes, is usually defined as “futile recanalization.”8, 9, 10 The fact that a large proportion of patients with successful recanalization will die is frustrating to accept. Pooled analysis of the MERCI (Mechanical Embolus Removal in Cerebral Ischemia) and Multi MERCI trials indicated that 90‐day mortality in patients with successful recanalization is as high as 28%.11 Therefore, besides increasing the recanalization rates by skill training and improvement of equipment, determining other influencing factors for functional outcomes is of vital importance for continuously improving the prognosis of stroke patients with EVT.
In recent years, several prognostic models based on clinical and radiological pretreatment variables have been applied in predicting outcomes after EVT for acute anterior circulation stroke,10, 12, 13 such as the HIAT2 (Houston Intra‐Arterial Therapy 2) score and THRIVE (Totaled Health Risks in Vascular Events) score. However, performance of these scores for individualized prediction of outcome is limited by categorization of continuous variables, such as age, blood glucose level, and National Institutes of Health Stroke Scale (NIHSS) score. The nomogram is a graphical statistical instrument that incorporates variables to develop a continuous scoring system and calculates the precise risk probability of a particular outcome for an individual patient. This instrument is an important component of modern medical decision making, which has been used in an extensive array of applications including cancer, surgery, and other specialties.14, 15, 16, 17 To date, a nomogram model with adequate power to detect probability of mortality in ischemic stroke patients treated with EVT despite successful recanalization has yet to be designed.
This study aimed to determine risk factors associated with mortality in patients with successful EVT (modified Thrombolysis in Cerebral Infarction, IIb or III) and establish a novel nomogram for predicting mortality with external validation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Participants
Eligible patients with successful EVT in the training cohort were selected from the multicenter ACTUAL (Endovascular Treatment for Acute Anterior Circulation) ischemic stroke registry between January 2014 and June 2016. ACTUAL is a retrospective registry of EVT for acute anterior circulation ischemic stroke in 21 centers across 10 provinces in China.18 Between June 2016 and March 2019, an additional 224 anterior circulation stroke patients who underwent EVT despite successful recanalization, admitted to Jinling Hospital and Nanjing First Hospital, were prospectively recruited for the test cohort. Successful EVT was defined as a modified Thrombolysis in Cerebral Infarction score of IIb or III.8 Patients were recruited to this study if they: (1) were treated with successful EVT modified Thrombolysis in Cerebral Infarction (IIb or III); (2) were aged ≥18 years; (3) had a prestroke modified Rankin Scale score ≤2; (4) were treated with EVT within 6 hours of stroke onset; (5) had a baseline NIHSS score ≥6; (6) had an Alberta Stroke Program Early Computed Tomography Score ≥6; and (7) had occlusion at the internal carotid artery or middle cerebral artery confirmed by computed tomographic angiography, magnetic resonance angiography, or digital subtracted angiography. Patients with malignant tumor, autoimmune disease, severe renal insufficiency, hepatic disease, or heart failure were excluded. The ACTUAL registry was approved by the Central Ethics Committee at Jinling Hospital and the ethics committee of each participating center. The test cohort was approved by the Ethics Committee of Jinling Hospital and Nanjing First Hospital. Informed consents was obtained from participants or legal representatives.
Data Collection
A standardized case‐report form was used to collect demographics, clinical data, and procedural characteristics. Stroke subtype was classified according to the criteria of the Trial of ORG 10172 in Acute Stroke Treatment.19 Symptomatic intracranial hemorrhage (sICH) was defined as that detected within 72 hours after EVT, according to the criteria of the Heidelberg Bleeding Classification.20 Collateral status was assessed based on digital subtracted angiography using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system, with grade 0 to 1 representing poor collateral status and grade 2 to 4 representing moderate to excellent.21
Follow‐up of modified Rankin Scale at 3 months was conducted at each center, either by telephone or outpatient visit. Mortality at 3 months was recorded.
Statistical Analysis
Statistical analysis was performed using SPSS (version 22.0; IBM, New York, NY) and R statistical software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were expressed as n (%) and continuous variables as means (SD) or medians (interquartile range). Differences in baseline characteristics between groups were analyzed using independent sample t tests, Mann–Whitney U tests for continuous variables, and the chi‐squared test or Fisher's exact test for categorical variables, as appropriate.
To construct the nomogram, multivariate regression analysis with Akaike information criterion was applied to select the significant predictors of mortality using a backward‐selection method that included variables with a P<0.1 in the univariate analysis. The nomogram was trained using the ACTUAL data and externally validated using the test cohort. The model was also internally validated using all data of the training and test cohorts by 10‐fold cross‐validation. Discriminative performance was measured by concordance index (c‐index). Calibration was tested using a calibration plot with bootstraps of 1000 resamples, which described the degree of fit between actual and nomogram‐predicted mortality.
Decision curve analysis was used to evaluate the validity of the nomogram. Detailed descriptions of the decision curve analysis were previously reported.22 Results were considered statistically significant at P<0.05.
Results
The flow chart outlining the patient inclusion process is shown in Figure 1. The training and test cohorts included 417 (mean age, 65.7±11.9 years; 62.1% male) and 224 (mean age, 69.2±11.6 years; 60.7% male) eligible patients, respectively. Baseline characteristics between the training and test cohorts are shown in Table 1. No significant differences regarding mortality were detected between cohorts (20.4% versus 15.6%; P=0.141). Patients in the training cohort were younger (65.7±11.9 versus 69.2±11.6 years; P=0.003), had a lower proportion of diabetes mellitus (18.5% versus 28.1%; P=0.005) and atrial fibrillation (41.5% versus 54.5%; P=0.002), and had higher levels of systolic blood pressure (145.6±25.7 versus 140.8±23.2 mm Hg; P=0.001), hs‐CRP (hypersensitive C‐reactive protein; median, 8.1 versus 6.6 mg/L; P=0.026), and baseline Alberta Stroke Program Early Computed Tomography Score (9 versus 8; P=0.002) than those in the test cohort.
Figure 1.
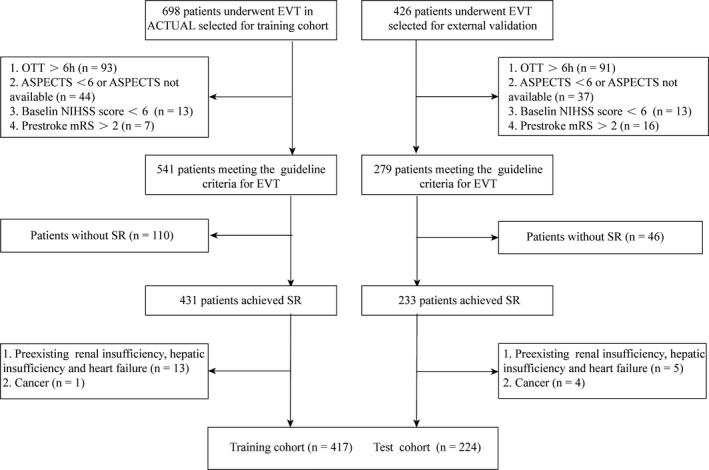
Flow chart outlining the patient inclusion process. ACTUAL indicates Endovascular Treatment for Acute Anterior Circulation; ASPECTS, the Alberta Stroke Program Early Computed Tomography Score; EVT, endovascular thrombectomy; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; OTT, onset to treatment time; SR, sinus rhythm.
Table 1.
Demographics and Clinical Characteristics of the Training and Test Cohorts
| Variable | Training Cohort (n=417) | Test Cohort (n=224) | P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 65.7±11.9 | 69.2±11.6 | 0.003 |
| Male, n (%) | 259 (62.1) | 136 (60.7) | 0.729 |
| Vascular risk factors, n (%) | |||
| Hypertension | 276 (66.2) | 165 (73.7) | 0.058 |
| Diabetes mellitus | 77 (18.5) | 63 (28.1) | 0.005 |
| Hyperlipidemia | 34 (8.2) | 19 (8.5) | 0.885 |
| Atrial fibrillation | 173 (41.5) | 122 (54.5) | 0.002 |
| Smoking | 111 (26.2) | 61 (27.2) | 0.867 |
| Coronary heart disease | 108 (25.9) | 48 (21.4) | 0.209 |
| Clinical data | |||
| Systolic blood pressure, mm Hg | 145.6±25.7 | 140.8±23.3 | 0.001 |
| Diastolic blood pressure, mm Hg | 84.3±14.8 | 82.3±15.9 | 0.118 |
| Time from onset to puncture, min | 240.0 (185.0, 299.0) | 227.0 (170.0, 283.0) | 0.149 |
| Time from puncture to recanalization, min | 69.0 (49.0, 103.0) | 73.0 (57.0, 105.0) | 0.084 |
| Baseline NIHSS, score | 16.0 (13.0, 21.0) | 16 (12.0, 20.0) | 0.490 |
| Baseline ASPECTS, score | 9.0 (8.0, 10.0) | 8.0 (7.0, 9.0) | 0.002 |
| Cause of stroke, n (%) | |||
| Atherosclerotic | 183 (43.9) | 79 (35.3) | 0.109 |
| Cardioembolic | 214 (51.3) | 131 (58.5) | |
| Others | 20 (4.8) | 14 (6.3) | |
| Poor collateral status | 198 (47.5) | 98 (43.8) | 0.366 |
| Procedural modes, n (%) | |||
| Stent retriever only | 194 (46.5) | 122 (50.0) | 0.645 |
| Stent retriever with rescue therapya | 191 (45.8) | 94 (42.0) | |
| Other modes without stent retrieverb | 32 (7.7) | 18 (8.0) | |
| Passes of stent retriever | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.295 |
| Vascular occlusion site, n (%) | |||
| ICA | 164 (39.3) | 82 (36.6) | 0.499 |
| MCA | 253 (60.7) | 142 (63.4) | |
| Previous IVT, n (%) | 153 (36.7) | 87 (38.8) | 0.592 |
| sICH, n (%) | 53 (12.7) | 21 (9.4) | 0.208 |
| Mortality at 3 mo, n (%) | 85 (20.4) | 35 (15.6) | 0.141 |
| Laboratory data | |||
| Total cholesterol, mmol/L | 4.2±1.1 | 4.3±1.6 | 0.367 |
| Triglyceride, mmol/L | 1.1 (0.7, 1.5) | 1.0 (0.7, 1.5) | 0.414 |
| Low‐density lipoprotein, mmol/L | 2.4 (1.8, 3.0) | 2.4 (1.8, 2.9) | 0.963 |
| High‐density lipoprotein, mmol/L | 1.2±0.3 | 1.1±0.3 | 0.157 |
| Baseline blood glucose level, mmol/L | 7.5±3.3 | 7.2±3.0 | 0.115 |
| hs‐CRP, mg/L | 8.1 (2.5, 22.6) | 6.6 (2.5, 11.4) | 0.026 |
ASPECTS indicates the Alberta Stroke Program Early Computed Tomography Score; hs‐CRP, hypersensitive C‐reactive protein; ICA, internal carotid artery; IVT, intravenous thrombolysis; MCA, middle cerebral artery; NIHSS, National Institute of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Rescue therapy includes balloon angioplasty, permanent implantation of stent, intra‐arterial thrombolysis, or intra‐arterial tirofiban infusion.
Other modes include balloon angioplasty with or without stent implantation.
The results of uni‐ and multivariate analyses for risk factors associated with 3‐month mortality in the training cohort are shown in Tables 2 and 3. According to univariate analysis in the training cohort, age, atrial fibrillation, systolic blood pressure, baseline NIHSS score, poor collateral status, passes of stent retriever, and blood glucose level were potential predictors for mortality (P<0.1; Table 2). Age (odds ratio [OR], 1.07; 95% CI, 1.03−1.10; P=0.001), poor pretreatment collateral status (OR, 2.13; 95% CI, 1.18−3.85; P=0.012), baseline blood glucose level (OR, 1.12; 95% CI, 1.04−1.21; P=0.003), sICH (OR, 9.51; 95% CI, 4.54−19.92; P=0.001), and baseline NIHSS score (OR, 1.08; 95% CI, 1.03−1.12; P=0.001) were detected by Akaike information criterion as predictors of mortality (Table 3). Higher total points based on the sum of the assigned number of points for each predictor in the nomogram were associated with an increased risk of mortality (Figure 2). For example, a patient aged 60 years with a blood glucose level of 13.0 mmol/L, a baseline NIHSS score of 20, poor collateral status, and sICH would have a total of 176.5 points (60 points for age, 30 points for blood glucose level, 23 points for baseline NIHSS score, 16 points for poor collateral status, and 47.5 points for sICH). The predicted 3‐month mortality is 71.0% for this patient.
Table 2.
Comparison of Baseline Characteristics of Patients With and Without Mortality in the Training Cohort
| Variable | Death (n=85) | Survival (n=332) | P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 70.9±10.1 | 63.5±11.8 | 0.001 |
| Male, n (%) | 47 (55.3) | 212 (63.9) | 0.147 |
| Vascular risk factors, n (%) | |||
| Hypertension | 65 (70.6) | 216 (65.1) | 0.336 |
| Diabetes mellitus | 16 (8.8) | 61 (18.4) | 0.924 |
| Hyperlipidemia | 5 (5.9) | 29 (8.7) | 0.391 |
| Atrial fibrillation | 46 (54.1) | 127 (38.3) | 0.008 |
| Smoking | 17 (20.0) | 94 (28.3) | 0.122 |
| Coronary heart disease | 24 (28.2) | 84 (25.3) | 0.582 |
| Clinical data | |||
| Systolic blood pressure, mm Hg | 153.3±25.4 | 144.9±25.6 | 0.007 |
| Diastolic blood pressure, mm Hg | 86.5±14.8 | 83.7±14.8 | 0.123 |
| Time from onset to puncture, min | 250.0 (202.0, 307.0) | 239.0 (184.0, 295.0) | 0.195 |
| Time from puncture to recanalization, min | 65.0 (49.0, 116.0) | 68.0 (49.0, 100.0) | 0.353 |
| Baseline NIHSS, score | 19.0 (15.0, 24.0) | 16 (12.0, 19.0) | 0.001 |
| Baseline ASPECTS, score | 9.0 (8.0, 10.0) | 9.0 (8.0, 10.0) | 0.121 |
| Cause of stroke, n (%) | |||
| Atherosclerotic | 33 (38.8) | 150 (45.2) | 0.210 |
| Cardioembolic | 50 (58.8) | 164 (49.2) | |
| Others | 2 (2.4) | 18 (5.4) | |
| Poor collateral status | 59 (69.4) | 139 (41.8) | 0.001 |
| Procedural modes, n (%) | |||
| Stent retriever only | 44 (51.8) | 150 (45.2) | 0.218 |
| Stent retriever with rescue therapya | 38 (44.7) | 153 (46.1) | |
| Other modes without stent retrieverb | 3 (3.5) | 29 (8.7) | |
| Passes of stent retriever | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.013 |
| Vascular occlusion site, n (%) | |||
| ICA | 42 (49.4) | 122 (36.7) | 0.033 |
| MCA | 43 (50.6) | 210 (63.3) | |
| Previous IVT, n (%) | 27 (31.8) | 126 (38.0) | 0.291 |
| sICH, n (%) | 31 (36.5) | 22 (6.6) | 0.001 |
| Laboratory data | |||
| Total cholesterol, mmol/L | 4.1±1.2 | 4.2±1.0 | 0.429 |
| Triglyceride, mmol/L | 1.0 (0.6, 1.6) | 1.1 (0.7, 1.5) | 0.125 |
| Low‐density lipoprotein, mmol/L | 2.2 (1.7, 2.9) | 2.4 (1.9, 3.0) | 0.164 |
| High‐density lipoprotein, mmol/L | 1.2±0.4 | 1.2±0.3 | 0.416 |
| Baseline blood glucose level, mmol/L | 9.2±5.1 | 7.2±2.6 | 0.001 |
| hs‐CRP, mg/L | 11.0 (3.1, 28.6) | 8.0 (2.5, 22.5) | 0.173 |
ASPECTS indicates, the Alberta Stroke Program Early Computed Tomography Score; hs‐CRP, hypersensitive C‐reactive protein; ICA, internal carotid artery; IVT, intravenous thrombolysis; MCA, middle cerebral artery; NIHSS, National Institute of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Rescue therapy includes balloon angioplasty, permanent implantation of stent, intra‐arterial thrombolysis, or intra‐arterial tirofiban infusion.
Other modes include balloon angioplasty with or without implantation of stent.
Table 3.
Multivariate Logistic Regression Analysis for the Risk Factors Associated With Mortality in the Training Cohort
| Variable | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Age | 1.07 (1.06−1.09) | 0.001 | 1.07 (1.03−1.10) | 0.001 |
| Atrial fibrillation | 1.90 (1.18−3.08) | 0.009 | ||
| Systolic blood pressure | 1.01 (1.00−1.02) | 0.010 | ||
| Baseline NIHSS, score | 1.10 (1.60−1.14) | 0.001 | 1.08 (1.03−1.12) | 0.001 |
| Poor collateral status | 3.15 (1.89−5.24) | 0.001 | 2.13 (1.18−3.85) | 0.012 |
| Passes of stent retriever | 1.23 (1.09−1.55) | 0.003 | ||
| ICA vs MCA | 1.68 (1.04−2.72) | 0.034 | ||
| sICH | 8.09 (4.36−15.01) | 0.001 | 9.51 (4.54−19.92) | 0.001 |
| Baseline blood glucose level | 1.17 (1.09−1.25) | 0.001 | 1.12 (1.04−1.21) | 0.003 |
ICA indicates internal carotid artery; MCA, middle cerebral artery; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio; sICH, symptomatic intracranial hemorrhage.
Figure 2.
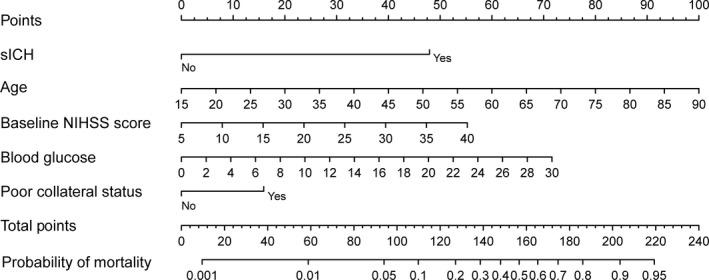
Nomogram for predicting the probability of 3‐month mortality. Points were assigned for sICH, age, baseline score, blood glucose level, and poor collateral status by drawing a line upward from the corresponding values to the “points line.” The “total points” are calculated as the sum of the individual score of each of the 5 variables included in the nomogram. NIHSS indicates National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Discrimination of the nomogram was measured by calculating the c‐index, which was 0.835 (95% CI, 0.785–0.885), indicating good predictive power. Figure 3 shows a calibration plot. This compares the prediction of mortality between the nomogram prediction and actual observation. The calibration plot revealed good predictive accuracy of the nomogram (Figure 3A).
Figure 3.
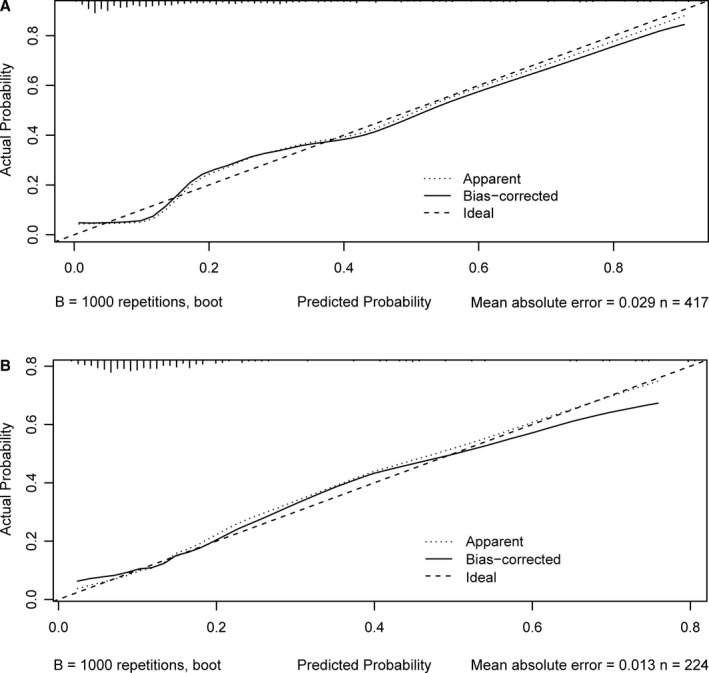
Calibration plot of the nomogram in the training (A) and test cohorts (B). The dotted line represents the performance of the nomogram, whereas the solid line corrects for any bias in the nomogram. The dashed line represents the reference line where an ideal nomogram would lie.
After 10‐fold cross‐validation was performed on all data of the training and test cohorts, the c‐index was 0.803 (95% CI, 0.725–0.881). Furthermore, the model was externally validated using the test cohort with a c‐index of 0.758 (95% CI, 0.667–0.849). Given that a c‐index >0.75 is generally considered to indicate reliable discrimination, this nomogram performed well using both the training and test cohorts.23 The satisfactory calibration of the nomogram was also confirmed by the test cohort (Figure 3B).
Decision curve analysis can estimate the net benefit of a model based on the difference between the number of true‐ and false‐positive results and is widely used in assessing whether the nomogram‐assisted decision would improve patient outcome. As shown in Figure 4, the decision curve analysis indicated that when the threshold probabilities ranged between 3.9% and 83.5% in the training cohort, and between 6.4% and 79.1% in the test cohort, the use of the nomogram to predict 3‐month mortality provided greater net benefit than the “treat all” or “treat none” strategies, which indicates the clinical usefulness of the nomogram. For example, if the personal threshold probability of a patient is 40% (the patient would opt for treatment if the probability of mortality were >40%), then the net benefit is 0.050 in the training cohort and 0.0375 in the test cohort.
Figure 4.
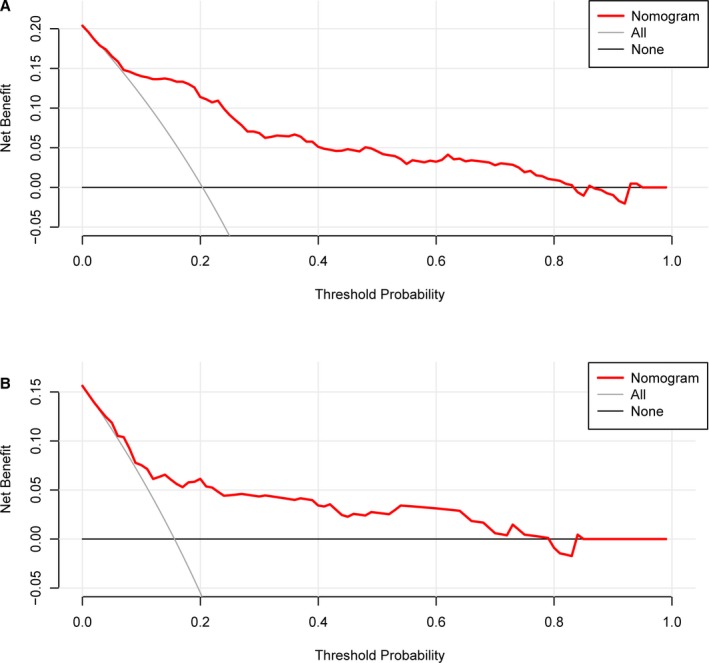
Decision curve analysis of the nomogram in the training (A) and test cohorts (B). The x‐axis indicates the threshold probability. The y‐axis measures the net benefit. The gray line displays the net benefit of the strategy of treating all patients. The black line illustrates the net benefit of the strategy of treating no patients. The red line indicates the nomogram. Decision curve analysis is a specific method developed for evaluating the prognostic value of nomogram strategies. This nomogram was developed to assess the probability of the post‐EVT death of a given patient. A patient with a high risk of death after EVT may need “further treatment,” such as hematoma removal or decompressive craniectomy; a patient with a low risk of death after EVT may not need “further treatment.” Distinguishing patients with a high and low risk of death is the main purpose of this nomogram. In the present study, the reference risk was calculated by assuming that all patients need further treatment for preventing death, whereas zero net benefit was defined as no patients needing further therapy. The threshold probability is when the expected benefit of further therapy is equal to the expected benefit of avoiding further therapy. For any given probability threshold, the nomogram with the greatest net benefit would be the most preferred model. EVT indicates endovascular thrombectomy.
Discussion
In this study, we presented a precise nomogram, based on age, baseline NIHSS score, blood glucose level, collateral status, and sICH, to predict the probability of 3‐month mortality for anterior circulation stroke patients with successful EVT. The good discrimination and calibration of the nomogram were demonstrated in the training cohort and were further confirmed in external validation. Given that the sICH was evaluated within 72 hours of EVT treatment, this nomogram could be applied in the acute stage of stroke for predicting mortality at 3 months.
Compared with previous studies, 3‐month mortality in our training and test cohorts were relatively lower at 20.4% and 15.6%, respectively. This may be attributed to differences in inclusion criteria and thrombectomy devices. We rigorously selected patients according to the 2018 guidelines for the early management of patients with acute ischemic stroke,24 including baseline NIHSS score ≥6, Alberta Stroke Program Early Computed Tomography Score ≥6, and onset to treatment time ≤6 hours. Moreover, most of our patients received EVT using the Solitaire FR device (Covidien, Irvine, CA), which has been reported to have fewer endovascular complications than MERCI device treatment, particularly with respect to symptomatic cerebral hemorrhage.25
Current prognostic scores for predicting clinical outcomes of stroke following EVT have limitations.10, 12, 13 The PREDICT score,10 THRIVE score,12 and HIAT2 score13 are points‐based risk scores based on cut‐off values of discrete and continuous variables, such as age, baseline NIHSS score, and blood glucose level. However, this may reduce predictive accuracy, given that they do not fully utilize within‐category information. The Pittsburg Response to Endovascular therapy score included age, baseline NIHSS score, and Alberta Stroke Program Early Computed Tomography Score score as continuous variables and had improved predictive accuracy compared with THRIVE and HIAT2 scores in a first validation cohort of anterior large vessel occlusion stroke.26 By converting the total score into a continuum of individual scores through a logarithmic formula, we developed a precise nomogram (c‐index in the training cohort, 0.835; c‐index in the test cohort, 0.758) for predicting probability from 0.1% to 95% of 3‐month mortality following successful EVT. Therefore, our nomogram may serve as a more precise and reliable predictive tool for mortality in patients with large vessel occlusion stroke after EVT, which may aid patient management.
Advanced age and high NIHSS score on admission have previously been reported to be associated with poor outcomes in EVT patients.8, 9, 10 Consistent with previous reports,8, 9, 10 age and baseline NIHSS score in our nomogram were significant predictors of mortality despite recanalization. These data seem to indicate that the task of reversing elderly patients with a severe neurological deficit at presentation is difficult to accomplish by EVT. Except for nonmodifiable variables (age and baseline NIHSS score) of the individual patient, hyperglycemia on admission was the strongest predictor in the nomogram. Hyperglycemia may have deleterious effects on neurological function by several mechanisms, including endothelial damage, intracellular acidosis, and blood–brain barrier disruption,27 all of which may lead to severe neurological deficit and higher risk of mortality. Our study further confirmed the importance of blood glucose management in anterior large vessel occlusion stroke after EVT.
Angiographic grade of collateral flow may affect the risk of hemorrhagic transformation28 and prognosis29 after therapeutic recanalization for acute ischemic stroke. After adjusting for potential confounders in multivariate regression analysis, we also found that sICH and poor collateral status (American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology <2) were independent predictors of 3‐month mortality in successful recanalization patients. Some characteristics, such as longer delay to treatment and passes of stent retriever, were not found to be associated with mortality, probably attributable to the differences in sample size, study population, and study methods.
Several limitations should be addressed when interpreting the results of this study. First, we only included patients of Asian ethnicity who strictly met the criteria of the current guideline for EVT (American Heart Association/American Stroke Association, 2018), which may limit generalizability of the results. Second, collaborating with multiple centers undoubtedly led to a lack of standard diagnostic or treatment approaches. Nevertheless, the multicenter nature of the present study was also an advantage because it improves the generalizability of our findings. Third, the present study used the modified Thrombolysis in Cerebral Infarction score to assess degree of recanalization. Other definitions have been used, including the Thrombolysis in Myocardial Ischemia scale as well as the Thrombolysis in Brain Ischemia scale.30 Despite these limitations, our analysis has a few strengths. We included a large sample of participants and first established a precise nomogram with external validation. In addition, the nomogram was based on variables that can be easily abstracted and used in a real‐world setting.
In summary, the nomogram, composed of age, pretreatment collateral status, baseline blood glucose level, sICH, and baseline NIHSS score, may predict risk of mortality in patients with ischemic stroke and treated successfully with EVT. Further studies are warranted to validate the effectiveness of this nomogram in other populations.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (Grant No.: 81901248).
Disclosures
None.
Acknowledgments
We express our gratitude to all the researchers and patients who participated in this study.
(J Am Heart Assoc. 2020;9:e014899 DOI: 10.1161/JAHA.119.014899.)
Contributor Information
Xinfeng Liu, Email: gelinxu@nju.edu.cn.
Gelin Xu, Email: xfliu2@vip.163.com.
References
- 1. Jovin T, Chamorro A, Cobo E, de Miquel M, Molina C, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López‐Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez‐Pérez M, Goyal M, Demchuk A, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 2. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND‐IA Investigators . Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 4. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg‐Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 5. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators . Stent‐retriever thrombectomy after intravenous t‐PA vs. T‐PA alone in stroke. N Engl J Med. 2015;137:2285–2295. [DOI] [PubMed] [Google Scholar]
- 6. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta‐analysis. Stroke. 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 7. Fields JD, Lutsep HL, Smith WS; MERCI Multi MERCI Investigators . Higher degrees of recanalization after mechanical thrombectomy for acute stroke are associated with improved outcome and decreased mortality: pooled analysis of the MERCI and Multi MERCI trials. AJNR Am J Neuroradiol. 2011;32:2170–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Z, Liebeskind D, Xiang B, Ge S, Feng L, Albers G, Budzik R, Devlin T, Gupta R, Jansen O, Jovin T, Killer‐Oberpfalzer M, Lutsep H, Macho J, Nogueira R, Rymer M, Smith W, Wahlgren N, Duckwiler G; Multi MERCI, TREVO, and TREVO 2 Investigators . Predictors of functional dependence despite successful revascularization in large‐vessel occlusion strokes. Stroke. 2014;45:1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linfante I, Starosciak AK, Walker GR, Dabus G, Castonguay AC, Gupta R, Sun CH, Martin C, Holloway WE, Mueller‐Kronast N, English JD, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi MA, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Abou‐Chebl A, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Nogueira RG, Zaidat OO. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016;8:224–229. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Zhang M, Hao Y, Zi W, Yang D, Zhou Z, Geng Y, Wang Z, Li H, Xu G, Hankey G, Xiong Y, Liu X. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg. 2018;115:e312–e321. [DOI] [PubMed] [Google Scholar]
- 11. Nogueira R, Liebeskind D, Sung G, Duckwiler G, Smith WS; MERCI; Multi MERCI Writing Committee . Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: Pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke. 2009;40:3777–3783. [DOI] [PubMed] [Google Scholar]
- 12. Flint AC, Xiang B, Gupta R, Nogueira RG, Lutsep HL, Jovin TG, Albers GW, Liebeskind DS, Sanossian N, Smith WS; TREVO‐2 Trialists . THRIVE score predicts outcomes with a third‐generation endovascular stroke treatment device in the TREVO‐2 trial. Stroke. 2013;44:3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarraj A, Albright K, Barreto AD, Boehme AK, Sitton CW, Choi J, Lutzker SL, Sun CH, Bibars W, Nguyen CB, Mir O, Vahidy F, Wu TC, Lopez GA, Gonzales NR, Edgell R, Martin‐Schild S, Hallevi H, Chen PR, Dannenbaum M, Saver JL, Liebeskind DS, Nogueira RG, Gupta R, Grotta JC, Savitz SI. Optimizing prediction scores for poor outcome after intra‐arterial therapy in anterior circulation acute ischemic stroke. Stroke. 2013;44:3324–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim Y, Margonis G, Prescott J, Tran T, Postlewait L, Maithel S, Wang T, Evans D, Hatzaras I, Shenoy R, Phay J, Keplinger K, Fields R, Jin L, Weber S, Salem A, Sicklick J, Gad S, Yopp A, Mansour J, Duh Q, Seiser N, Solorzano C, Kiernan C, Votanopoulos K, Levine E, Poultsides G, Pawlik T. Nomograms to predict recurrence‐free and overall survival after curative resection of adrenocortical carcinoma. JAMA Surg. 2016;151:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jehi L, Yardi R, Chagin K, Tassi L, Russo G, Worrell G, Hu W, Cendes F, Morita M, Bartolomei F, Chauvel P, Najm I, Gonzalez‐Martinez J, Bingaman W, Kattan M. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14:283–290. [DOI] [PubMed] [Google Scholar]
- 16. Cappellari M, Mangiafico S, Saia V, Pracucci G, Nappini S, Nencini P4, Konda D, Sallustio F, Vallone S, Zini A, Bracco S, Tassi R, Bergui M, Cerrato P, Pitrone A, Grillo F, Saletti A, De Vito A, Gasparotti R, Magoni M, Puglielli E, Casalena A, Causin F, Baracchini C, Castellan L, Malfatto L, Menozzi R, Scoditti U, Comelli C, Duc E, Comai A, Franchini E, Cosottini M, Mancuso M, Peschillo S, De Michele M, Giorgianni A, Delodovici ML, Lafe E, Denaro MF, Burdi N, Internò S, Cavasin N, Critelli A, Chiumarulo L, Petruzzellis M, Doddi M, Carolei A, Auteri W, Petrone A, Padolecchia R, Tassinari T, Pavia M, Invernizzi P, Turcato G, Forlivesi S, Ciceri EFM, Bonetti B, Inzitari D, Toni D; Listing of IER Collaborators . IER‐SICH nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50:909–916. [DOI] [PubMed] [Google Scholar]
- 17. Cappellari M, Turcato G, Forlivesi S, Zivelonghi C, Bovi P, Bonetti B, Toni D. STARTING‐SICH nomogram to predict symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke. Stroke. 2018;49:397–404. [DOI] [PubMed] [Google Scholar]
- 18. Zi W, Wang H, Yang D, Hao Y, Zhang M, Geng Y, Lin M, Wan Y, Shi Z, Zhou Z, Wang W, Xu H, Tian X, Lv P, Wang S, Liu W, Wang Z, Liu X, Guo F, Zheng D, Li H, Tu M, Jin P, Xiao G, Liu Y, Xu G, Xiong Y, Liu X; ACTUAL Investigators . Clinical effectiveness and safety outcomes of endovascular treatment for acute anterior circulation ischemic stroke in china. Cerebrovasc Dis. 2017;44:248–258. [DOI] [PubMed] [Google Scholar]
- 19. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 20. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, San Román L, Saver JL, Strbian D, Whiteley W, Hacke W. The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. [DOI] [PubMed] [Google Scholar]
- 21. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vickers A, Elkin E. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Agostino R, Nam B. Evaluation of the performance of survival analysis models: discrimination and calibration measures In: Balakrishnan N, Rao CR, eds. Handbook of Statistics v23: Advances in Survival Analysis. Amsterdam, the Netherlands: Elsevier; 2004. [Google Scholar]
- 24. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. 2018 Council American Heart Association Stroke guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 25. Akins P, Amar A, Pakbaz R, Fields J. Complications of endovascular treatment for acute stroke in the swift trial with solitaire and merci devices. AJNR Am J Neuroradiol. 2014;35:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rangaraju S, Aghaebrahim A, Streib C, Sun C, Ribo M, Muchada M, Nogueira R, Frankel M, Gupta R, Jadhav A, Jovin T. Pittsburgh response to endovascular therapy (pre) score: optimizing patient selection for endovascular therapy for large vessel occlusion strokes. J Neurointerv Surg. 2015;7:783–788. [DOI] [PubMed] [Google Scholar]
- 27. Ennis S, Keep R. Effect of sustained‐mild and transient‐severe hyperglycemia on ischemia‐induced blood‐brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–1582. [DOI] [PubMed] [Google Scholar]
- 28. Bang O, Saver J, Kim S, Kim G, Chung C, Ovbiagele B, Lee K, Liebeskind D. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. [DOI] [PubMed] [Google Scholar]
- 29. Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von Kummer R, Sugg RM, Zaidat OO, Hussain SI, Goyal M, Menon BK, Al Ali F, Yan B, Palesch YY, Broderick JP; IMS III Investigators . Collaterals at angiography and outcomes in the interventional management of stroke (IMS) III trial. Stroke. 2014;45:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol. 2007;28:382–384. [PMC free article] [PubMed] [Google Scholar]


