Abstract
Background
Social skills programmes (SSP) are treatment strategies aimed at enhancing the social performance and reducing the distress and difficulty experienced by people with a diagnosis of schizophrenia and can be incorporated as part of the rehabilitation package for people with schizophrenia.
Objectives
The primary objective is to investigate the effects of social skills training programmes, compared to standard care, for people with schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group’s Trials Register (November 2006 and December 2011) which is based on regular searches of CINAHL, BIOSIS, AMED, EMBASE, PubMed, MEDLINE, PsycINFO, and registries of clinical trials. We inspected references of all identified studies for further trials.
A further search for studies has been conducted by the Cochrane Schizophrenia Group in 2015, 37 citations have been found and are currently being assessed by review authors.
Selection criteria
We included all relevant randomised controlled trials for social skills programmes versus standard care involving people with serious mental illnesses.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated risk ratios (RRs) and their 95% confidence intervals (CI) on an intention‐to‐treat basis. For continuous data, we calculated mean differences (MD) and 95% CIs.
Main results
We included 13 randomised trials (975 participants). These evaluated social skills programmes versus standard care, or discussion group. We found evidence in favour of social skills programmes compared to standard care on all measures of social functioning. We also found that rates of relapse and rehospitalisation were lower for social skills compared to standard care (relapse: 2 RCTs, n = 263, RR 0.52 CI 0.34 to 0.79, very low quality evidence), (rehospitalisation: 1 RCT, n = 143, RR 0.53 CI 0.30 to 0.93, very low quality evidence) and participants’ mental state results (1 RCT, n = 91, MD ‐4.01 CI ‐7.52 to ‐0.50, very low quality evidence) were better in the group receiving social skill programmes. Global state was measured in one trial by numbers not experiencing a clinical improvement, results favoured social skills (1 RCT, n = 67, RR 0.29 CI 0.12 to 0.68, very low quality evidence). Quality of life was also improved in the social skills programme compared to standard care (1 RCT, n = 112, MD ‐7.60 CI ‐12.18 to ‐3.02, very low quality evidence). However, when social skills programmes were compared to a discussion group control, we found no significant differences in the participants social functioning, relapse rates, mental state or quality of life, again the quality of evidence for these outcomes was very low.
Authors' conclusions
Compared to standard care, social skills training may improve the social skills of people with schizophrenia and reduce relapse rates, but at present, the evidence is very limited with data rated as very low quality. When social skills training was compared to discussion there was no difference on patients outcomes. Cultural differences might limit the applicability of the current results, as most reported studies were conducted in China. Whether social skills training can improve social functioning of people with schizophrenia in different settings remains unclear and should be investigated in a large multi‐centre randomised controlled trial.
Keywords: Adult; Female; Humans; Male; Social Skills; Assertiveness; Communication; Cultural Characteristics; Patient Readmission; Patient Readmission/statistics & numerical data; Program Evaluation; Psychotic Disorders; Psychotic Disorders/rehabilitation; Quality of Life; Randomized Controlled Trials as Topic; Recurrence; Schizophrenia; Schizophrenia/rehabilitation; Schizophrenic Psychology; Stress, Psychological; Stress, Psychological/rehabilitation
Plain language summary
Social skills programmes for people with schizophrenia
Social skills programmes (SSP) use behavioural therapy and techniques for teaching individuals to communicate their emotions and requests. This means they are more likely to achieve their goals, meet their needs for relationships and for independent living as well as getting on with other people and socially adjusting. Social skills programmes involve 'model learning' (role playing) which was introduced to improve general 'molecular' skills (eye contact, fluency of speech, gestures) and 'molar' skills (managing negative emotions, giving positive feedback). Social skills programmes enhance social performance and reduce the distress and difficulty experienced by people with schizophrenia. Social skills programmes can be incorporated as part of a rehabilitation package for people with schizophrenia.
The main objective of this review is to investigate the effectiveness of social skills programmes, compared to standard care or discussion groups, for people with schizophrenia. Based on searches carried out in 2006 and 2011, this review includes 13 trials with a total of 975 participants. Authors chose seven main outcomes of interest, all data for these outcomes were rated to be very low quality. The review found significant differences in favour of social skills programmes compared to standard care on all measures of social functioning. Rates of relapse were lower for social skills compared to standard care and there was a significant difference in favour of social skills on people’s mental state. Quality of life was also improved in the social skills programme compared to standard care. However, when social skills programmes were compared to discussion groups, there were no significant differences in people’s social functioning, relapse rates, mental state or quality of life.
Compared to standard care, social skills programmes may improve the social skills of people with schizophrenia and reduce relapse rates. However, at the moment evidence is very limited with data only of very low quality available. Cultural differences might also limit the relevance of current results, as most reported studies were conducted in China. Whether social skills programmes or training can improve the social functioning of people with schizophrenia in different settings remains unclear and should be further investigated in a large multi‐centre randomised controlled trial.
Ben Gray, Senior Peer Researcher, McPin Foundation.http://mcpin.org/
Summary of findings
Summary of findings for the main comparison. SOCIAL SKILLS versus STANDARD CARE for schizophrenia.
| SOCIAL SKILLS versus STANDARD CARE for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: Intervention: SOCIAL SKILLS versus STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SOCIAL SKILLS versus STANDARD CARE | |||||
| Social functioning Various scales Follow‐up: 8‐52 weeks | See comment | See comment | Not estimable | 585 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | The four RCTs that provided data for this comparison used different scales to measure social functioning and so data were not pooled for this comparison. |
| Relapse Follow‐up: 6‐12 months | 351 per 1000 | 183 per 1000 (119 to 277) | RR 0.52 (0.34 to 0.79) | 263 (2 studies) | ⊕⊝⊝⊝ very low4,6,7 | |
| Rehospitalisation Follow‐up: 12 months | 366 per 1000 | 194 per 1000 (110 to 340) | RR 0.53 (0.3 to 0.93) | 143 (1 study) | ⊕⊝⊝⊝ very low4,8 | |
|
Mental state: general symptoms (BPRS) Follow‐up: 12 weeks |
The mean mental state: general symptoms in the intervention groups was 4.01 lower (7.52 to 0.50 lower) | 91 (1 study) | ⊕⊝⊝⊝ very low4,8 | |||
| Global state: not clinically improved. CGI Follow‐up: 6 months | 529 per 1000 | 153 per 1000 (63 to 360) | RR 0.29 (0.12 to 0.68) | 67 (1 study) | ⊕⊝⊝⊝ very low4,5 | |
| General functioning MRSS Follow‐up: 8 weeks | The mean General functioning: average endpoint score (various scales) ‐ MRSS in the intervention groups was 10.6 lower (17.47 to 3.73 lower) | 112 (1 study) | ⊕⊝⊝⊝ very low4,5 | |||
| Quality of life GWB. Scale from: 10 to 40. Follow‐up: 8 weeks | The mean Quality of life in the intervention groups was 7.6 lower (12.18 to 3.02 lower) | 112 (1 study) | ⊕⊝⊝⊝ very low4,5 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: very serious. Only one of the trials had adequate sequence generation. Allocation concealment was unclear in all trials and none were blinded. Two trials addressed incomplete data adequately and three were free from selective reporting. It was unclear in all trials whether they were free from other biases. 2 Inconsistency: serious. See comment. 3 Imprecision: very serious. Data were not combined, but for each single study the 95% confidence intervals around the pooled effect estimate are very wide. 4 Publication bias: strongly suspected. Less than five studies provided data for this outcome. 5 Risk of bias: very serious. The RCT that provided data for this outcome did not have adequate sequence generation, allocation concealment and blinding. It was unclear whether there were other biases. 6 Risk of bias: very serious. Neither of the two RCTs were blinded, and it was unclear in both if there was adequate allocation concealment. Only one had adequate sequence generation and one addressed incomplete data adequately. It was unclear in both if they were free from other biases. 7 Inconsistency: serious. There was moderate heterogeneity for this outcome. This may be explained by differences in type of social skills training. Ma 2003 included sessions on medication and symptom management as well as social skills, whereas in Saren 2004 the focus was on interpersonal interaction. 8 Risk of bias: very serious. The RCT that provided data for this outcome had unclear allocation concealment and was not blinded. It was unclear if it was free from other biases.
Summary of findings 2. SOCIAL SKILLS versus DISCUSSION CONTROL for schizophrenia.
| SOCIAL SKILLS versus DISCUSSION CONTROL for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: Intervention: SOCIAL SKILLS versus DISCUSSION CONTROL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SOCIAL SKILLS versus DISCUSSION CONTROL | |||||
| Social functioning Various scales Follow‐up: 6 months | See comment | See comment | Not estimable | 63 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | The two RCTs that provided data for this comparison used different scales to measure social functioning and so data were not pooled for this comparison. |
| Relapse Follow‐up: 6 months | 111 per 1000 | 235 per 1000 (49 to 1000) | RR 2.12 (0.44 to 10.1) | 35 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | |
| Global state GAS Follow‐up: 6 months | The mean Global state: average endpoint score in the intervention groups was 4.5 higher (1.2 lower to 10.2 higher) | 63 (1 study) | ⊕⊝⊝⊝ very low2,3,5 | |||
|
Mental state: general symptoms Follow‐up: 6 months |
The mean mental state: general symptoms in the intervention groups was 0.22 higher (4.05 lower to 4.49 higher) | 99 (2 studies) |
⊕⊝⊝⊝ very low1,3,4 | |||
| Quality of life QLS Follow‐up: 6 months | The mean Quality of life: average endpoint score in the intervention groups was 3.7 higher (6.47 lower to 13.87 higher) | 63 (1 study) | ⊕⊝⊝⊝ very low2,3,5 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: serious. Only one of the RCTs had adequate sequence generation and allocation concealment, neither were blinded nor addressed incomplete data adequately. Only one was free from selective reporting it was unclear if either was free from selective reporting. 2 Imprecision: serious. The 95% confidence intervals are very wide and include both significant benefit and harm of the intervention. 3 Publication bias: strongly suspected. Less than three studies provided data for this outcome. 4 Risk of bias: serious. The RCT that provided data for this outcome was not blinded. It was unclear whether incomplete data were adequately addressed and whether it was free from other biases. 5 Risk of bias: very serious. The RCT that provided data fro this outcome was not blinded, did not adequately address incomplete data and was not free from selective reporting. It was unclear if the sequence generation and allocation concealment was adequate and whether it was free from other biases.
Background
Description of the condition
Schizophrenia can occur as a single episode of illness. By far the greater proportion of sufferers, however, have remission and relapses; for up to 41% of those who develop schizophrenia it becomes a chronic and often disabling illness (Prudo 1987). Antipsychotic medications are commonly used for management of symptoms. However, the conclusions reached by meta‐analytical methods are that treatment with antipsychotic medication 'should be combined and coordinated with other interventions involving the patient's family, and social psychological and psychotherapeutic support' (MSPI 1997). These treatments are subsumed under the general term 'rehabilitation'.
Preceding the movement of care into the community, the rehabilitation process was mostly provided by the large psychiatric hospitals in which sufferers often spent many years (Wing 1961; Wing 1970). This pattern of care has now changed (Hume 1995). During the 1980s many psychiatric hospitals were closed, community‐based services were developed, and psychiatric units in general hospitals were established. Currently, few chronically mentally ill people spend longer than a few weeks per year in hospital. The rest of their care takes place in the community. Up to 75% of people with chronic schizophrenia are maintained in the community in the United Kingdom ‐ chronic in this case is defined as lasting more than 2.5 years (Davies 1990). Relative to other chronic illnesses, the personal and economic costs of schizophrenia are considerable (Knapp 1994). It is therefore important to offer rehabilitation treatments that are both clinically effective and cost‐effective.
Description of the intervention
As a part of the rehabilitation package for people with schizophrenia, social skills programmes (SSP) aim to utilise behaviour therapy principles and techniques for teaching individuals to communicate their emotions and requests, so they are more likely to achieve their goals and meet their needs for relationships and roles required for independent living and social competence (Kopelowicz 2006).
SSP involves 'model learning' (role playing) which was introduced to improve general 'molecular' skills (eye contact, fluency of speech, gestures, etc) and 'molar' skills (managing negative affects, giving positive feedback, etc.) (Brenner 1994). A problem‐solving model was later incorporated and rehabilitation topics that are particularly relevant for people with schizophrenia were introduced (Liberman 1993). The application of these modules appears far more effective than control conditions, particularly in terms of generalisation of skills and social adjustment (Marder 1996; Wallace 1998).
How the intervention might work
Learning‐based procedures used in SSP include identifying the problems and setting the goals in collaboration with the client. Through role play or behavioural rehearsal, participants demonstrate the required skills and positive or corrective feedback is given to them accordingly. By social modelling and behavioural practice, participants observe and repeat the skills until the communications reach a level of quality tantamount to success in the real‐life situation. Homework assignments are then given to motivate participants to implement these communications in real‐life situations.
Why it is important to do this review
This review focuses on social skills programmes, which are some of the most established psychosocial treatments for schizophrenia (Liberman 1986; Liberman 1986a) but vary in use across the world. Essentially, social skills programmes are treatment strategies aimed at enhancing the social performance and reducing the distress and difficulty experienced by people with a diagnosis of schizophrenia. Few reviews exist (Kopelowicz 2006) and none are maintained.
Objectives
To investigate the effects of social skills training programmes, compared to standard care, for people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. If a trial had been described as 'double blind' but implied randomisation, we planned to include such trials in a sensitivity analysis. If their inclusion did not result in a substantive difference, they would have remained in the analyses. If their inclusion did result in statistically significant differences, we would not have added the data from these lower quality studies to the results of the better trials, but presented such data within a subcategory. We excluded quasi‐randomised studies, such as those allocating by alternate days of the week. Where people were given additional treatments within a social skills trial, we only included data if the adjunct treatment was evenly distributed between groups and it was only the social skills programme that was randomised.
Types of participants
Adults, however defined, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis.
We were interested in making sure that information is as relevant to the current care of people with schizophrenia as possible so proposed to clearly highlight the current clinical state (acute, early post‐acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Social skills training programmes
Defined as any structured psychosocial intervention, whether group or individual, aimed at enhancing the social performance and reducing the distress and difficulty in social situations. The key components are; i. a careful behavioural‐based assessment of a range of social and interpersonal skills; ii. An importance placed on both verbal and non‐verbal communication; as well as the individual's ability to; i. perceive and process relevant social cues; and ii. to respond to and provide appropriate social reinforcement. This approach has the goal of building up individual behavioural elements into complex behaviours. The aim is to develop more effective social communication. There is considerable emphasis not just on clinic‐based interventions (including modelling, role‐play and social reinforcement) but also the setting of homework tasks and the applicability of the treatment.
Programmes where social skills training are a component part of a more complex rehabilitation intervention were excluded, as were token economies, life skills programmes and other similar milieu‐based interventions which may include an element of social skills training in a broader programme.
Programmes of five sessions and less are considered as 'brief ', and six or more as 'long'. Place of residence is defined as either 'hospital' or 'community' for the purposes of this review. For example, if people are in hospital at time of attending a day‐hospital based programme they are considered to be receiving 'hospital‐based' care. If, on the other hand, they attend the day hospital from home then they are considered to be receiving' community‐based' care. Trained staff are those personnel who hold a professionally recognised health care qualification.
2. The control treatment
Defined as standard care without a dedicated programme of the type described above.
Types of outcome measures
If possible we divided outcomes into short term (less than six months), medium term (seven to 12 months) and long term (over one year).
Primary outcomes
1. Social functioning
No clinically important change in general social functioning
2. Relapse
3. Mental state
No clinically important change in general mental state
Secondary outcomes
1. Social functioning
1.2 Average endpoint general social skills score 1.3 Average change in general social skills scores 1.4 No clinically important change in specific social skills 1.5 Average endpoint specific social skills score 1.6 Average change in specific social skills scores
2. Global state
2.1 No clinically important change in global state (as defined by individual studies) 2.2 Average endpoint global state score 2.3 Average change in global state scores
3. Service outcomes
3.1 Hospitalisation 3.2 Time to hospitalisation
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia)
4.1 Average endpoint general mental state score 4.2 Average change in general mental state scores 4.3 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 4.4 Average endpoint specific symptom score 4.5 Average change in specific symptom scores
5. General functioning
5.1 No clinically important change in general functioning 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 Employed
6. Behaviour
6.1 No clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 No clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‐ general and specific
7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects 7.7 Death ‐ suicide and natural causes
8. Engagement with services
9. Satisfaction with treatment
9.1 Leaving the studies early 9.2 Recipient of care not satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer not satisfied with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Quality of life
10.1 No clinically important change in quality of life 10.2 Average endpoint quality of life score 10.3 Average change in quality of life scores 10.4 No clinically important change in specific aspects of quality of life 10.5 Average endpoint specific aspects of quality of life 10.6 Average change in specific aspects of quality of life
11. Economic outcomes
11.1 Direct costs 11.2 Indirect costs
12. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADE 2004) to import data from RevMan 5 (Review Manager 2008) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes was rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the Table 1 and Table 2:
1. Social functioning ‐ Clinically significant response on social skills ‐ as defined by each of the studies
2. Clinical response
Clinically significant response in global state ‐ as defined by each of the studies
Healthy days
3. Service utilisation outcomes
Hospital admission
Days in hospital
4. Adverse effect ‐ Any important adverse event
5. Quality of life ‐ Improved to an important extent
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Trials Search Co‐ordinator (TSC) searched the Cochrane Schizophrenia Group’s Register of Trials (November 2006, December 21, 2011) using the following search strategy:
((*social* OR *personal*) AND (*skill* OR *program* OR *training*)) in Title, Abstract and Indexing Terms Fields of REFERENCE and (*social skill* OR *social support* OR *sociotherapy* OR *socioenvironmental* OR *interpersonal*) in Intervention Field of STUDY
The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors Muhammad Okba Al Marhi (MOA), (Mohamad Alsabbag (MA) and Muhammad Jawoosh (MJ) independently inspected citations from the November 2006 search and identified relevant abstracts. A random 20% sample was independently re‐inspected by Muhammad Qutayba Almerie (MQA) to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. Full reports of the abstracts meeting the review criteria were obtained and inspected by MOA and MS. Again, a random 20% of reports were re‐inspected by MQA in order to ensure reliable selection.
For the June 2010 search, review authors Nicola Maaya (NM) and Hanna Bergman (HB) independently inspected citations, and identified and inspected relevant abstracts. A random 20% sample was independently re‐inspected by Karla Soares Weiser (KSW) to ensure reliability. Full reports were obtained and inspected by NM and HB and a random 20% of reports were re‐inspected by KSW. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
For the November 2006 search, review authors MOA, MA and MJ extracted data from included studies. Where further clarification was needed, the authors' of trials were contacted to provide missing data. Any disagreements were discussed and the decisions documented.
For the June 2012 search, review author NM extracted data from all included studies. In addition, to ensure reliability, HB independently extracted data from a random sample of these studies, comprising 10% of the total. Again, any disagreement was discussed, decisions documented and, if necessary, authors of studies were contacted for clarification. With remaining problems KSW helped clarify issues and these final decisions were documented. Data presented only in graphs and figures were extracted whenever possible, but included only if two review authors independently had the same result. Attempts were made to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible, we extracted data relevant to each component centre separately. The data from the Chinese papers were extracted by a speaker of that language (JX); but these Chinese language studies were not re‐inspected by review authors.
2. Management
2.1 Forms
Data were extracted onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, in Description of studies we have noted if this is the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. Endpoint and change data were combined in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2009, Chapter 9.4.5.2 ).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations SDs) and means were reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS, Kay 1986), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied; however, we did not find any skewed endpoint data. When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. Skewed data from studies of less than 200 participants would have been entered as other data within the data and analyses section rather than into a statistical analysis. Skewed data pose less of a problem when looking at means if the sample size is large and would have been entered into statistical syntheses.
2.5 Common measure
To facilitate comparison between trials, if necessary we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, efforts were made to convert outcome measures to dichotomous data. This was done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this can be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for social skills training.
Assessment of risk of bias in included studies
Again review authors NM and HB worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, the final rating was made by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. Non‐concurrence in quality assessment was reported, but if disputes arose as to which category a trial is to be allocated, again, resolution was made by discussion.
The level of risk of bias is noted in both the text of the review and in the Table 1 and Table 2.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). For statistically significant results we had planned to calculate the number needed to treat to provide benefit /to induce harm statistic (NNTB/H), and its 95% confidence interval (CI) using Visual Rx (http://www.nntonline.net/) taking account of the event rate in the control group. This, however, has been superseded by Table 1 and Table 2, and the calculations therein.
2. Continuous data
For continuous outcomes we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
We did not have any data from cluster trials. Had there been data from these types of trials, and where clustering was not accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error and contacted first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and adjusted for this by using accepted methods (Gulliford 1999). If clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted the data for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1 + (m‐1)*ICC] (Donner 2002). If the ICChad not been reported, it would have been assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we would have only used data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, the additional treatment arms were presented in comparisons. If data were binary these were simply added and combined within the two‐by‐two table. If data were continuous we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions. Where the additional treatment arms were not relevant, these data were not reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table/s by down‐rating quality. Finally, we also downgraded quality within the 'Summary of findings' table/s where loss was 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50%, we presented data for the total number of participants randomised for studies that used an intention‐to‐treat (ITT) analysis; where studies did not use an ITT analysis, we presented completer‐only data.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and completer‐only data were reported, we reproduced these.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we first tried to obtain the missing values from the authors. If not available, where there are missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either P value or T value available for differences in mean, we can calculate them according to the rules described in the Handbook (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Handbook (Higgins 2011) present detailed formulae for estimating SDs from P values, T or F values, confidence intervals, ranges or other statistics. If these formulae do not apply, we can calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We planned to examine the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, if LOCF data had been used in the trial, if less than 50% of the data had been assumed, we planned to reproduce these data and indicate that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, these were fully discussed.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, these were fully discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2). an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2009). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. As we did not have more than 10 studies providing data for outcomes, we did not need to use funnel plots. If future versions of this review do require funnel plots, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. Therefore, we chose the fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
We planned to perform subgroup analyses to assess the impact of the following possible sources of heterogeneity for any of the social skills training programmes: brief versus long programmes, clinical state, stage or problem. However, there were not enough data to undertake these analyses.
2. Investigation of heterogeneity
If inconsistency was high, this was reported. First, we investigated whether data had been entered correctly. Second, if data were correct, the graph was visually inspected and outlying studies were successively removed to see if heterogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, data were presented. If not, data were not pooled and issues discussed. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
We also planned to conduct sensitivity analyses for the main outcomes for trials with the implication of randomisation, assumptions made for lost binary data, trials with high risk of bias, imputed values and fixed‐effect versus random‐effects models. However, all of the outcomes had less than 10 trials that reported data.
Results
Description of studies
Please see Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
The November 2006 search identified 1283 references and a search in December 2011 identified a further 555 references. Agreement about which reports may have been randomised was 100%. See also Figure 1.
1.
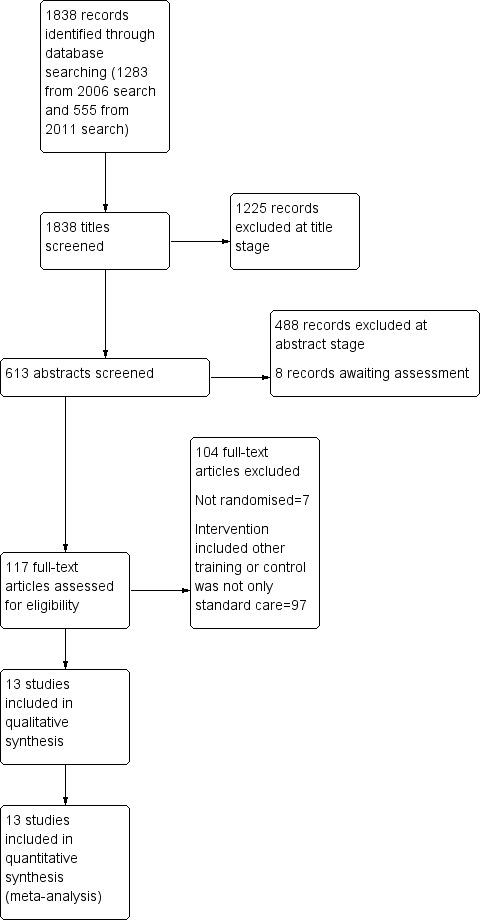
Study flow diagram.
Included studies
The current review includes 17 reports describing 13 studies involving 975 participants.
1. Methods
All studies were stated to be randomised. Dobson 1995, Hayes 1995, Huang 2005 and Ng 2006 all stated that the assessors were blinded to the participant's treatment allocation; it was unclear in Potelunas 1982 whether all raters were blinded. In Tsang 2001, the raters were blinded, but it was unclear whether the participants were. Chien 2003, Cui 2004, Kopelowicz 1998, Lu 2004, Ma 2003, Mayang 1990 and Saren 2004 did not report that blinding was attempted. For further details please see sections below on Allocation and Blinding in Risk of bias in included studies.
2. Duration
Five trials were undertaken for no longer than three months: Chien 2003 four weeks; Cui 2004 12 weeks; Kopelowicz 1998 three months, Lu 2004 eight weeks; Mayang 1990 four sessions with a four‐week follow‐up (it did not report the frequency of the sessions); and Potelunas 1982 six weeks. Seven trials were longer than three months: Dobson 1995 involved nine weeks of training with three months of follow‐up for all participants, although the follow‐up period lasted up to one year for the social skills group; Hayes 1995 lasted for 18 weeks, but had an additional period of six months where the patients received decreasing frequency of training; the duration of Huang 2005 was six months; Ma 2003 had eight weeks of intervention and 26 weeks follow‐up; Ng 2006 was eight weeks with a six‐month follow‐up; Saren 2004 was 10 weeks with 12 months follow‐up; and Tsang 2001 was 10 weeks with a three‐month follow‐up period.
3. Participants
All participants were people with a chronic mental illness, mostly with schizophrenia and schizoaffective disorders. One of the studies randomised only men (Cui 2004) and two studies randomised only women (Mayang 1990 and Potelunas 1982); the others included both sexes. Data on mean age were available from seven trials. The average age was between 33 and 48 years. Ma 2003 did not report any demographic information about the participants.
All studies included patients with schizophrenia and schizoaffective psychoses. Three studies used the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) criteria, one used the third edition (DSM‐III), two used the third version of the Chinese Classification of Mental Disorders (CCMD‐III), three used the second version (CCMD‐II), and four studies did not report the diagnostic criteria.
4. Setting
Nine studies used a hospital setting (Chien 2003; Cui 2004; Dobson 1995; Huang 2005; Kopelowicz 1998; Lu 2004; Mayang 1990; Ng 2006; Tsang 2001); in four studies (Hayes 1995; Ma 2003; Potelunas 1982; Saren 2004) the participants were outpatients. Three of the included trials were conducted in USA, five in China, one in Taiwan, two in Hong Kong, one in Canada and one in Australia.
5. Interventions
5.1 Experimental treatment
In Chien 2003, the social skills training focused on conversation and assertiveness skills. Participants attended a one‐hour session twice a week for four weeks. In Dobson 1995, the focus was on communication and assertiveness for four one‐hour sessions per week over a nine‐week period.
The social skills training programme used in Cui 2004 was the UCLA Social and independent Living Skills programme, which includes: i) general social interpersonal skills training, using video demonstration, role play and discussion; ii) educating patients of the purpose and function of antipsychotic medication; iii) medication management; iv) how to recognise a drug adverse effect; v) opportunity to discuss issues relating to drug therapy. The training lasted 90 to 120 minutes per session, and there were three sessions per week for 12 weeks.
Ma 2003 also used the UCLA Social and independent Living Skills programme as the social skills training (90 to 120 minutes per session, one session a week for eight weeks) and Kopelowicz 1998 used an adapted version, which was translated into Spanish (participants attended three months of training four days a week, although the length of each session was not reported).
The social skills training in Hayes 1995 emphasised interpersonal skills, social problem solving, positive time use skills, generalisation enhancement techniques, and used instructions, modelling, behaviour rehearsal, feedback, and structured homework. The training consisted of 36 sessions of 75 minutes over 18 weeks, followed by a six‐month period in which patients received decreasing frequency of training.
The social skills training in Huang 2005 was delivered in groups of eight to nine people, and each session was two hours per weeks for 24 weeks.The social skills group also received routine drug therapy, recreational activities and work in the wards, which was the standard care for all patients.
In Lu 2004, the training included targeted problem solving training to improve patients' attention and planning skills with the aim of gradually improving patient's social function. Training was 60 minutes per session and two sessions per week for eight weeks.
In Mayang 1990and Ng 2006, the social skills training focused on problem solving skills (receiving skills, processing skills and sending skills) and involved instructions, modelling, feedback, role playing, social reinforcement. The training consisted of four sessions of 45 minutes of training in Mayang 1990 and 30 to 32 hours of treatment within eight weeks in Ng 2006.
In Potelunas 1982, the social skills training consisted of four one‐hour sessions over four weeks of videotaped presentation of eight problem situations, videotaped modelling, behaviour rehearsal, coaching and feedback, which occurred within a work adjustment programme.
In Saren 2004, the training focused on making eye contact, facial expressions, tone of voice, language fluency, posture and gestures and overall enthusiasm. Training was provided in groups of between 10 to 12 people, for 60 to 90minutes per session, three sessions per week for 10 weeks. In Tsang 2001, social skills training also consisted of basic social skills (facial expression, gestures etc.) and basic social survival skills (personal appearance, tidiness), but also included core work‐related skills. In this study there were two intervention groups: one received social skills training plus follow‐up support, which consisted of contact with group members and the trainer; the other group received only the social skills training and no follow‐up support.The training consisted of 10 weekly group sessions lasting one and a half to two hours, with approximately six to eight people in each group.
5.2 Control treatment
In five of the studies (Chien 2003; Cui 2004; Huang 2005; Kopelowicz 1998; Tsang 2001), the comparison treatment was standard psychiatric care. However, three of the studies (Dobson 1995; Hayes 1995; Ng 2006) used a discussion group as the control treatment to control for any confounding effects that arise from interacting with the therapist during social skills training. Dobson 1995 used a social milieu, which consisted of structured activities including supportive discussion groups, exercise groups and activity groups, and occurred at same time of day and same length of time as social skills training group. Hayes 1995 used a discussion group condition, which focused on interpersonal relations and purposeful use of time using open ended questions, paraphrasing, reflecting, and summarising the participants' comments to promote self‐disclosure. In Ng 2006, the control treatment was supportive group discussion with topics that included practical tips on money management and information on application for social security allowances, with special attention paid to avoid discussions on interpersonal skills issues and use of behavioural techniques. Two studies (Mayang 1990 and Potelunas 1982) had both standard care and a discussion group as their control treatments. In Mayang 1990, the interaction group condition consisted of non‐treatment interaction with trainers, and in Potelunas 1982, the discussion control was four one‐hour sessions over four weeks of videotaped presentation and discussion of eight problem situations.
7. Outcomes scales
7.1 Social functioning
i) Disabilities Assessment Schedule ‐ DAS (WHO 1988) This scale covers six domains: cognition – understanding and communicating; mobility; self‐care – hygiene, dressing, eating and staying alone; interacting with other people; life activities – domestic responsibilities, leisure, work and school; participation – joining in community activities. Higher scores indicate a worse outcome. Ma 2003 reported data from this scale.
ii) Social Disability Schedule ‐ SDSS (WHO 1988) The SDSS is a Chinese simplified version of the World Health Organization’s Disability Assessment Schedule and assesses 10 different aspects of social functioning (WHO 1988). Higher scores indicate a worse outcome. Lu 2004 and Saren 2004 reported data from this scale.
iii) Social avoidance and disability scale ‐ SAD (Watson 1969) The Social Avoidance and Distress Scale (SAD) is a 28‐item, self‐rated scale used to measure various aspects of social anxiety including distress, discomfort, fear, anxiety, and the avoidance of social situations. Higher scores indicate a worse outcome. Saren 2004 reported data from this scale.
iv) Scale of Social Skills for Psychiatric Inpatients ‐ SSPI (Guo 1995) This scale is a Chinese rating scale, commonly used for assessing response to antipsychotic treatment. Higher scores indicate a worse outcome. Huang 2005 reported data from this scale.
v) Social Situations questionnaire ‐ SSQ (Bryant 1974) Measures self‐perception of social difficulty; participants rate their anxiety and avoidance of 30 different social situations from zero “no difficulty” to four “avoidance if possible”. Higher scores indicate a worse outcome. It is not clear whether this is a validated scale. Hayes 1995 reported data from this scale.
vi) Social Behaviour Schedule ‐ SBS (Wykes 1986) The scale covers 21 behaviour areas such as destructive behaviours, personal appearance and hygiene, measured on a five‐point Likert scale from zero (no problem or acceptable behaviour) to four (serious problem). Higher scores indicate a worse outcome. Hayes 1995 reported data from this scale.
vii) Social functioning scale ‐ SFS (Birchwood 1990) This scale is an 81‐item self‐administered questionnaire covering several behaviour areas: employment, social withdrawal, pro‐social activities, recreation, interpersonal functioning, perceived independence, competence and perceived independence performance. Higher scores indicate a better outcome. Ng 2006 reported data from this scale.
viii) Conversation with a stranger task ‐ SCON (Wallace 1985) This assesses the participants’ skill in initiating a conversation in an unstructured five‐minute interaction with a stranger. It is scored on a rating scale from one “skill not evident” to five “very high level of skill”. Coding of the SCON used the system described by Bellack 1990. It is unclear whether the SCON is a validated scale. Hayes 1995 reported data from this scale.
7.2 Mental state
i) Scale for the Assessment of Negative Symptoms ‐ SANS (Andreasen 1983) This scale allows a global rating of the following negative symptoms: alogia (impoverished thinking), affective blunting, avolition‐apathy, anhedonia‐asociality, and attention impairment. Assessments are made on a six‐point scale from zero (not at all) to five (severe). Higher scores indicate more symptoms. Hayes 1995 and Ng 2006 reported data from this scale.
ii) Scale for the Assessment of Positive Symptoms – SAPS (Andreasen 1983). This is a six‐point scale providing a global rating of positive symptoms such as delusions, hallucinations and disordered thinking. Higher scores indicate more symptoms. Ma 2003 reported data from this scale.
iii) Brief Psychiatric Rating Scale ‐ BPRS (Overall 1962) This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a seven‐point scale varying from 'not present' to 'extremely severe', scoring from zero to six or one to seven. Scores can range from zero to 126, with high scores indicating more severe symptoms. Cui 2004, Hayes 1995 and Ng 2006 reported data from this scale.
7.3 General Functioning
i) Morningside Rehabilitation Status Scale ‐ MRSS (Affleck 1984) In this scale four individual areas are rated on an 8‐point scale: dependency, occupation and leisure activity, social isolation, and current symptoms. Higher scores indicate a worse outcome. Lu 2004 reported data from this scale.
7.4 Global state
i) Global Assessment Scale ‐ GAS (Endicott 1976) This is an observer‐rated scale for evaluating the overall functioning of a patient during a specified time period on a continuum from psychological or psychiatric sickness to health. Score ranges from zero to 100, where higher score indicates a better outcome. Hayes 1995 reported data from this scale.
ii) Clinical Global Impression Scale ‐ CGI (Guy 1976) This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven‐point scoring system is usually used with low scores showing decreased severity and/or overall improvement. Huang 2005 reported data from this scale.
7.5 Quality of Life
i) Quality of Life Scale ‐ QLS (Heinrich 1984) This six‐point quality of life scale has been designed as an outcome instrument for schizophrenic deficit syndrome as well as to measure impaired functioning in studies of chronic schizophrenia, to assess the deficit syndrome's impact on the patient's life. There are seven severity steps (zero to six, six being adequately functioning and zero being deficient). The time frame is one month. Four item categories have been identified by factor analysis 1) interpersonal relationships (seven items), 2) instrumental role (four items), 3) intrapsychic function (seven items) and 4) commonplace objects and activities. Hayes 1995 reported data from this scale.
ii) General Well‐Being Scale ‐ GWB (Dupuy 1977) This is a self‐administered questionnaire consisting of 18 items covering six dimensions of anxiety, depression, general health, positive well‐being, self‐control and vitality. There is a total score running from zero to 110, higher scores indicate less distress. Lu 2004 reported data from this scale.
iii) Rosenberg Self‐esteem scale ‐ SES (Rosenberg 1965) The Rosenberg Self‐Esteem Scale is a 10‐item self‐report measure of global self‐esteem. It consists of 10 statements related to overall feelings of self‐worth or self‐acceptance. The items are answered on a four‐point scale ranging from strongly agree to strongly disagree. The SES has also been administered as an interview. Scores range from 10 to 40, with higher scores indicating higher self‐esteem. Lu 2004 reported data from this scale.
Excluded studies
We excluded 104 studies. Of these, seven studies were not randomised. The other 97 studies were randomised but either the experimental groups were allocated to a programme that had some elements of social skills but also incorporated other training interventions, for example life skills and cognitive behavioural therapies, or the control group was not only standard care, but for example, holistic therapy. We did, however, include studies that used a discussion group as an attention control.
Awaiting assessment
Eight studies are awaiting assessment.
Ongoing studies
We found eight ongoing studies, three have social skills training programmes as the intervention (NCT00338975 2006, NCT00791882 2008, NCT00183625 2005), two include health management training with the social skills training (NCT00069433 2003, Pratt 2008), one has cognitive behavioural social skills training as the intervention (NCT00237796 2005), another social cognition and interaction training (NCT00601224 2008) and one attention shaping procedures (NCT00391677 2006).
Risk of bias in included studies
We prepared a 'Risk of bias' assessment for each trial. Our judgments regarding the overall risk of bias in individual studies is illustrated in Figure 2 and Figure 3. Overall, we felt the risk of bias in the included studies to be high.
2.
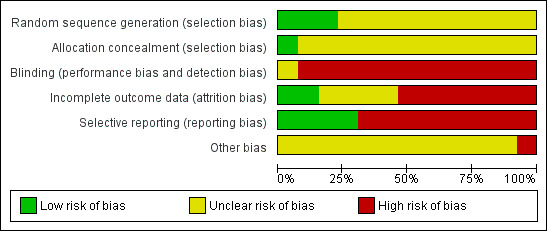
Risk of bias Summary for included studies
3.
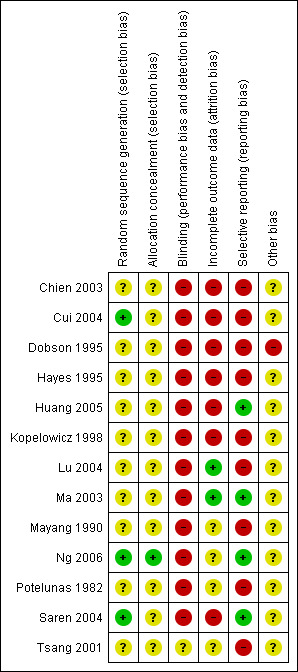
Risk of bias Summary for included studies
Allocation
All studies were stated to be randomly assigned. However, only three of the studies described how allocation to intervention was undertaken: Cui 2004, Ng 2006 and Saren 2004 each used a random numbers table. Only Ng 2006 described the allocation concealment; it was unclear in the remaining studies.
Blinding
Only Tsang 2001 was stated to be double blind, although it was unclear if participants in the control group were blinded. In five of the studies the assessors were blinded (Dobson 1995, Hayes 1995, Huang 2005, Ng 2006 and Potelunas 1982), although in Potelunas 1982 it was unclear whether all the raters were blinded.
Incomplete outcome data
Two studies did not have any incomplete data (Lu 2004 and Ma 2003), seven studies did not address incomplete outcome data adequately and it was unclear in four of the studies (Mayang 1990, Potelunas 1982, Ng 2006 and Tsang 2001).
Selective reporting
Four of the studies were free from selective reporting (Huang 2005; Ma 2003; Ng 2006 and Saren 2004), the remaining nine studies were rated as high risk of bias for selective reporting.
Other potential sources of bias
It was unclear in all of the trials except Dobson 1995 whether they were free from other biases. In Dobson 1995 there was bias from allowing the control group to begin social skills training before the end of the follow‐up period and we downgraded this trial to high risk for other potential sources of bias.
Effects of interventions
For dichotomous data we calculated risk ratios (RR) and their 95% confidence intervals (CIs). For continuous data, we calculated mean differences (MD) and 95% CIs.
1. COMPARISON 1: SOCIAL SKILLS versus STANDARD CARE
1.1 Social functioning
1.1.1 Social functioning: Average endpoint score
We found significant differences between social skills and standard care on all scales that measured social functioning (Analysis 1.1). Lu 2004 reported data on the Social Disability Schedule (SDSS) at eight weeks (1 RCT, n = 112, MD ‐1.50 CI ‐2.39 to ‐0.61) and Saren 2004 at 12 months (1 RCT, n = 143, MD ‐10.00 CI ‐11.35 to ‐8.65). Saren 2004 also reported data on the Social Avoidance and Distress Scale (SAD) at 12 months follow‐up (1 RCT, n = 143, MD ‐16.00 CI ‐17.04 to ‐14.96) and Huang 2005 reported data on the Scale of Social‐skills for Psychiatric Inpatients (SSPI) at six months follow‐up (1 RCT, n = 67, MD ‐6.06 CI ‐7.17 to ‐4.95). In addition, Ma 2003 reported data for general functioning on the Disability Assessment Scale (DAS) at 26 weeks follow‐up and also found a significant difference in favour of the social skills training group (1 RCT, n = 120, MD ‐6.8 CI ‐10.52 to ‐3.08).
1.1. Analysis.
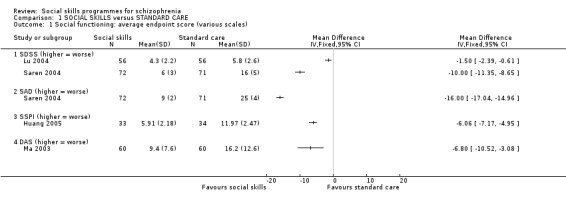
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 1 Social functioning: average endpoint score (various scales).
1.2 Relapse
Two studies reported on the number of participants relapsing, with follow‐up between six months and 12 months. A significant difference was found in favour of the social skills group (2 RCTs, n = 263, RR 0.52 CI 0.34 to 0.79; Analysis 1.2). The results showed moderate heterogeneity (I2 = 40%).
1.2. Analysis.
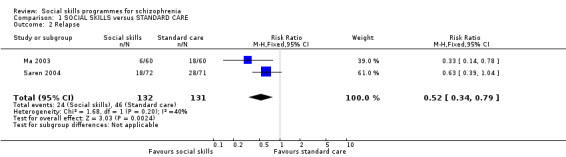
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 2 Relapse.
1.3 Global state: not clinically improved
Huang 2005 reported data for this outcome at six months follow‐up and found that significantly more participants showed a clinical improvement in global state in the social skills training group compared to the standard care group (1 RCT, n = 67, RR 0.29 CI 0.12 to 0.68; Analysis 1.3).
1.3. Analysis.
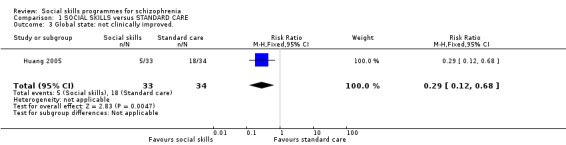
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 3 Global state: not clinically improved..
1.4 Service outcome: re‐hospitalisation
Saren 2004 reported data on re‐hospitalisation at 12 months follow‐up and showed a significant difference in favour of the social skills group (1 RCT, n = 143, RR 0.53 CI 0.30 to 0.93; Analysis 1.4).
1.4. Analysis.
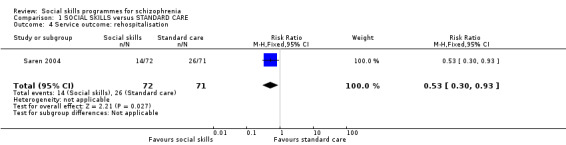
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 4 Service outcome: rehospitalisation.
1.5 Mental state
1.5.1 Average endpoint score in negative and positive symptoms
Hayes 1995 and Ma 2003 measured negative symptoms on the SANS scale with eight and 18 weeks follow‐up, respectively, and found a significant difference in favour of the social skills training group (2 RCTs, n = 187, MD ‐8.92 CI ‐10.46 to ‐7.38). Ma 2003 also measured positive symptoms on the SAPS scale, and also found a significant difference favouring social skills training (1 RCT, n = 120, MD ‐1.90 CI ‐3.37 to ‐0.43; Analysis 1.5).
1.5. Analysis.
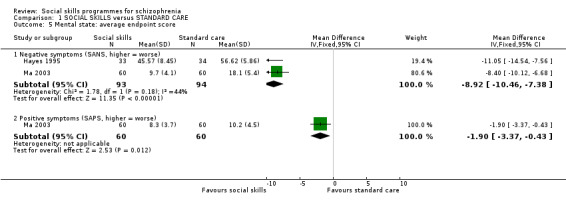
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 5 Mental state: average endpoint score.
1.5.2 Average change in general and negative symptoms
Cui 2004 reported the average change in mental state at 12 weeks follow‐up (Analysis 1.6). General symptoms were measured using the BPRS (1 RCT, n = 91, MD ‐4.01 CI ‐7.52 to ‐0.50) and negative symptoms were measured using the SANS (1 RCT, n = 91, MD ‐7.70, CI ‐12.33 to ‐3.17), both of which showed a significant improvement in symptoms in the social skills group compared with standard care.
1.6. Analysis.
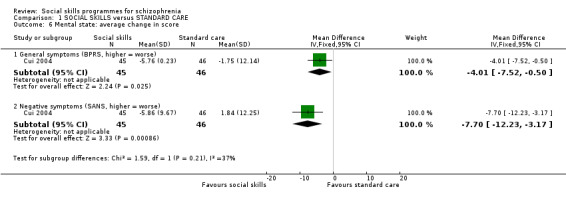
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 6 Mental state: average change in score.
1.6 General functioning: average endpoint score
Lu 2004 reported data on the Morningside Rehabilitation Status Scale (MRSS) at eight weeks follow‐up and found a significant difference in favour of the social skills training for the participants rehabilitation status (1 RCT, n = 112, MD ‐10.60 CI ‐17.47 to ‐3.73; (Analysis 1.8).
1.8. Analysis.
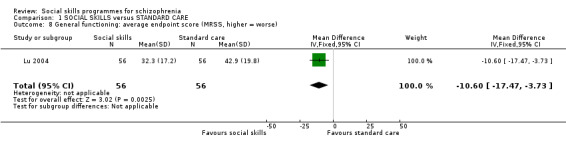
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 8 General functioning: average endpoint score (MRSS, higher = worse).
1.7 General functioning: employment
(Kopelowicz 1998) reported whether participants were employed at the end of the study and found no difference between treatment groups (n = 46, RR 5.43 CI 0.28 to 107.33) Analysis 1.9.
1.9. Analysis.
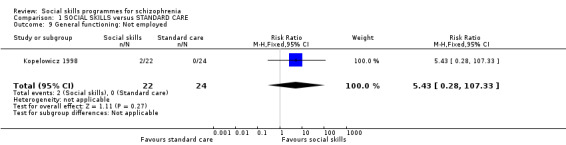
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 9 General functioning: Not employed.
1.8 Leaving the study early
We found no significant differences between social skills training and standard care for the number of participants leaving the study early with follow‐up between eight weeks and 12 months (9 RCTs, n = 719, RR 2.04 CI 1.00 to 4.16; Analysis 1.10). In Kopelowicz 1998, two people in the social skills group dropped out because they had found employment, but despite this there was very low heterogeneity for these results (I2 = 10%).
1.10. Analysis.
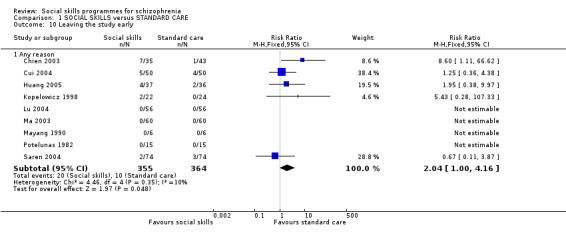
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 10 Leaving the study early.
1.9 Quality of Life: average endpoint score
Lu 2004 measured quality of life at 8 weeks follow‐up on two scales, the General Well‐Being scale (n = 112, MD ‐7.60 CI ‐12.18 to ‐3.02) and the Self‐Esteem scale (n = 112, MD ‐8.30 CI ‐10.07 to 6.53), both of which showed a significant difference in favour of the social skills training (Analysis 1.11).
1.11. Analysis.
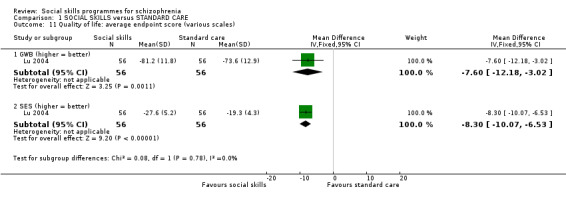
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 11 Quality of life: average endpoint score (various scales).
2. COMPARISON 2: SOCIAL SKILLS versus DISCUSSION CONTROL
2.1 Social functioning: average endpoint score
Two studies reported data for this outcome at six months follow‐up, each on two different scales, none of which showed a significant difference between social skills training and a discussion control (Analysis 2.1). Hayes 1995 measured social functioning on the Social situations questionnaire (SSQ) (n = 63, MD ‐1.10 CI ‐11.14 to 8.94) and the conversation with a stranger task (SCON) (n = 63, MD ‐2.50 CI ‐5.52 to 0.52). Ng 2006 used the Social Functioning Scale (SFS) (n = 36, MD 4.60 CI ‐7.54 to 16.74) and the Social behaviour schedule (SBS) (n = 36, MD 0.50 CI ‐‐2.56 to 3.56).
2.1. Analysis.
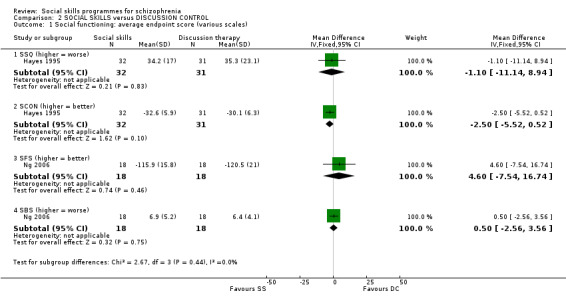
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 1 Social functioning: average endpoint score (various scales).
2.2 Relapse
This outcome was reported by Ng 2006 at six months follow‐up and no significant difference was found between the treatment groups (1 RCT, n = 33, RR 2.12 CI 0.44 to 10.10; Analysis 2.2).
2.2. Analysis.
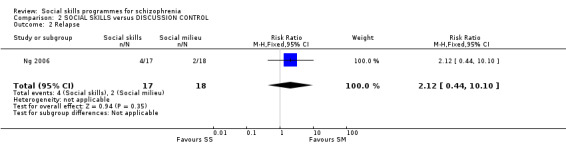
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 2 Relapse.
2.3 Global state: average endpoint score
Hayes 1995 reported data for global state on the Global Assessment Scale at six months follow‐up and found no significant difference between the social skills training and the discussion control (1 RCT, n = 63, MD 4.50 CI ‐1.20 to 10.20) (Analysis 2.3).
2.3. Analysis.
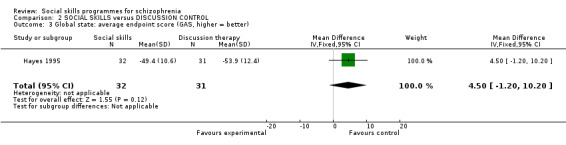
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 3 Global state: average endpoint score (GAS, higher = better).
2.4 Mental state: average endpoint score
Two trials reported the average endpoint score for general symptoms at six months follow‐up on the BPRS scale and found no significant difference between the treatment groups (2 RCTs, n = 99, MD 0.22 CI ‐4.05 to 4.49; Analysis 2.4). The results showed high heterogeneity (I2 = 54%). In addition, the two trials also found no significant difference in negative symptoms on the SANS scale (2 RCTs, n = 99, MD 2.89 CI ‐4.43 to 10.22; Analysis 2.4). These results were moderately heterogeneous (I2 = 19%).
2.4. Analysis.
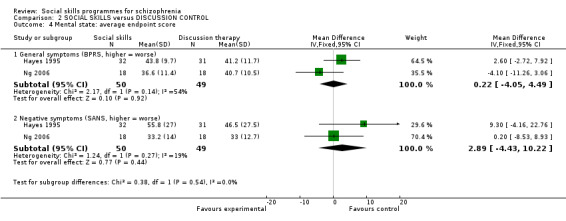
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 4 Mental state: average endpoint score.
2.5 Leaving the study early
Two trials reported data for this outcome at three months and six months follow‐up and showed no significant difference between the social skills training and discussion control (2 RCTs, n = 69, RR 1.58 CI 0.37 to 6.68; (Analysis 2.5). The results showed no heterogeneity (I2 = 0%).
2.5. Analysis.
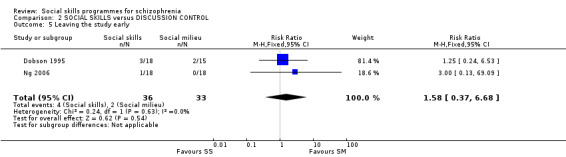
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 5 Leaving the study early.
2.6 Quality of life
Hayes 1995 reported data on quality of life at six months follow‐up and found no significant difference between the treatment groups (Analysis 2.6). (1 RCT, n = 63, MD 3.70 CI ‐6.47 to 13.87).
2.6. Analysis.
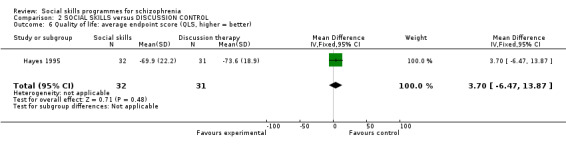
Comparison 2 SOCIAL SKILLS versus DISCUSSION CONTROL, Outcome 6 Quality of life: average endpoint score (QLS, higher = better).
3. Missing outcomes
None of the studies evaluated general behaviour, engagement with services, satisfaction with treatment and economic outcomes.
Discussion
Summary of main results
The summary below reflects the outcomes chosen for the 'Summary of findings' tables chosen to be of clinical importance before seeing the data. These are considered to be the main findings of this review. For all outcomes, we judged the quality of evidence to be very low ‐ randomisation was not well described, observation bias was impossible to exclude and few attempts were made to minimise it, and outcomes were poorly reported.
1. COMPARISON 1: SOCIAL SKILLS versus STANDARD CARE
1.1 Social functioning
We found it surprising that ‐ for this comparison ‐ social functioning was just so poorly reported. The use of various scales made any potential analysis prone to problems from the very many assumptions that would have had to be made. Four studies reported on 585 people but different scales were used in each with little help for the reader in the interpretation of what outcomes would mean in everyday life.
1.2 Relapse/rehospitalisation
Two trials suggest (n = 263) that the risk of relapse is halved in between six and 12 months follow‐up. The low‐quality data are also moderately heterogeneous ‐ but this is an encouraging finding ‐ mirroring that of the risk of rehospitalisation. If only rehospitalisation had some suggestion of decrease and not relapse then it may have been argued that the putative increase in social skills would have helped avoid admission. However, this is not the case and there is a suggestion that the social skills programmes help offset the relapse as well. This finding, as others, need replicated in larger studies.
1.3 Mental state: general symptoms
Mental state outcomes were few and is it not clear that the social skills programmes have any effect.
1.4 Global state: not clinically improved.
The global state outcome, as measured by the CGI, was also favouring social skills programmes. This is in keeping with the relapse and rehospitalisation outcomes but is only based on one very small study (n = 67).
1.5 General functioning
One small study (n = 112) reported on general functioning. We have not been able to find explanation of what a 10‐point decline would mean in everyday life, but the decline did favour the social skills programme group.
1.6 Quality of life
Finally, the quality of life measure also favoured the social skills programme group in one study (n = 112).
1.7 Overall
The overall impression that we are left with is that social skills programmes, in the hands of expert and committed practitioners ‐ who these trialists were ‐ may have some valuable effects (please see Implications for research).
2. COMPARISON 2: SOCIAL SKILLS versus DISCUSSION CONTROL
As stated above, we thought that is would not be right to summate a social skills programme versus standard care comparison with that comparing the social skills programme to a more active discussion group control. However, data were more thin and inconclusive.
2.1 Social functioning
Two studies (total n = 63 people) reported on two different scales. There did not seem to be any difference between the social skills programme and the discussion group control.
2.2 Relapse
One tiny study (n = 35) reported on relapse with no differences between groups. Such outcomes should be reported as a matter of routine ‐ but it would seem likely that much larger studies are needed to highlight differences with any degree of meaningful confidence.
2.3 Global state, mental state. quality of life
In the one study of 63 people global state was unchanged. This applies to mental state (2 RCTs, n = 99) and quality of life 1 RCT, 63 people).
2.4 Overall
When social skills training was compared to this discussion control, no evidence was found to suggest that social skills training was superior to the control group for improving social functioning, employment, relapse rates, mental state, global state, quality of life and leaving the study early.
Overall completeness and applicability of evidence
1. Completeness
Even for outcomes for which we identified usable data, because all studies were of a small sample size (range 18 to 148) we feel that no outcomes could be labelled as complete. We did not find studies with any data for the following outcomes: general behaviour, engagement with services, satisfaction with treatment and economic outcomes.
2. Applicability
The definition of social skills training varied between studies, and we have tried to be as consistent as possible when screening potential studies. All programmes had an element of social problem solving and used techniques of modelling, role‐play and social reinforcement. Some trials focused on interpersonal communication such as conversation and assertiveness skills, whereas others were more diverse and included symptom and medication management. The focus of the studies also differed, with the aim of some to reduce stress and the risk of relapse and others focusing on employment.
Five of the studies were conducted in China and reported in Chinese (Cui 2004; Huang 2005; Lu 2004, Ma 2003; Saren 2004), a further two were conducted in Hong Kong and reported in English (Ng 2006 and Tsang 2001) and one was in Taiwan and also reported in English (Chien 2003).Of the remaining five studies, three were conducted in the USA, one in Canada and one in Australia. Most of the data for the results are from the Chinese studies and those conducted in Hong Kong.
Should we have been able to synthesise more data across the inevitable varied definitions of 'social skills programme' and different care cultures we might have been able to see if, despite these differences, findings were consistent ‐ and in being so, supported the idea of wide applicability. More data are needed.
Quality of the evidence
1. Methods
Most studies did not address incomplete data adequately as they did not use an intention‐to‐treat analysis. Only three of the 13 included studies described an adequate sequence generation, and only one described the methods used to conceal allocation. Twelve of the studies were not blinded and in one (Tsang 2001), it was unclear. Four studies were free from selectively reporting the outcomes. Dobson 1995 was not free from other biases and it was unclear in the remaining studies. All the studies were small.
2. Reporting
Reporting was very poor. Many of the trials presented data that we were not able to use, mostly because authors had not reported either means or SDs, or data were not from a validated scale. For two included trials we were not able to use any data (Mayang 1990 and Potelunas 1982) and for a further four, we could only use data for one of the outcomes.
2. Consistancy of measure
For the outcomes for which data were available, with the exception of leaving the study early, only one or two small trials provided data. The studies used different assessment scales and we were unable to pool the divergent outcome data, which further hinders the detection of potential treatment effects.
Potential biases in the review process
We have worked only with published reports. By doing this we may be perpetuating a reporting and publishing bias. In addition, it was not possible to tell if there was publication bias, as most of the 13 included trials did not provide useful data, and a funnel plot could not be performed. Five of the reports were written in Chinese. These studies were assessed and data extracted by one person who is fluent in Mandarin (JX).
We do not think that we embarked on this review with prior biases as to the effects of the programme of care ‐ and have tried to keep to the protocol guidance we stipulated before seeing any data.
We realise the search date is out of date and new studies may have been published which could alter the results of this review. We are currently assessing results of a new search and aim to update this review within the next six months.
Agreements and disagreements with other studies or reviews
We do not know of any other relevant quantitative review in this topic.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
Studies are still so limited that this package of care has to be thought of as experimental. There is no indication that these social skills programmes do harm and some that they do good. If asked to join a social skills programme, the person could be encouraged that randomisation occurs to add to the body of useful knowledge.
2. For clinicians
Whether social skills programme do any more good than other interventions is unproven. Less sophisticated approaches may be just as effective. Until more evidence is available, social skills programmes should be thought of as experimental and not fully tested.
3. For policy makers
Compared with standard care, social skills training may improve the social skills of schizophrenic patients and reduce relapse rates, but at present, the evidence is very limited. In addition, there is no evidence to suggest that social skills training is superior to the act of discussing problems in a group. Currently, because of lack of good, consistent, applicable data social skills programme have to be seen to be vulnerable to replacement by other approaches with more robust evidence.
Implications for research.
1. General
Only four of the included studies predate CONSORT (Begg 1996; Moher 2001) but many had not followed guidance in their reporting. Clear and strict adherence to the CONSORT statement may well have resulted in this review having more data. Full availability of all data from each study could greatly help future reviewers (AllTrials).
2. Specific
2.1 Reviews
Many excluded trials could find a place in new or existing systematic reviews. It is difficult to categorise all these studies for presentation in a table but we have assigned them broad categories and suggest Cochrane reviews that may cover these areas (Table 3).
1. Broad areas for review suggested by excluded studies.
2.2 Trials
If social skills training programmes are to be incorporated into the treatment of people living with schizophrenia, more reliable evidence is needed. Future trials should involve all consumers of care, including the patients, families and carers, organisations that provide care for those with schizophrenia and not only focus on research outcomes. There is certainly a need for a large multi‐centre randomised controlled trial to show if social skills training is affected by cultural differences. Interpretation of social skills programmes research would be enhanced if future trials made use of validated measures ‐ perhaps even only those used in routine care and preferably agreed upon by a COMET‐type approach.
We are aware that enormous effort and care goes into the design of a trial and that we can only suggest some thoughts about design from our understanding of what has gone on before (Table 4). We have, however, looked at these trials very carefully and do think that there are some lessons to be learnt about gaining valuable information from simplicity of design. After all, with the complexity of design that has gone before, we are left in the dark about the most basic effects of this approach.
2. Suggested design of study.
| Methods | Allocation: randomised, clearly described and concealed. Duration: 1 year or more. |
| Participants | Diagnosis: schizophrenia. N = 300. Age: adults. Sex: men or women. History: perhaps once an episode of moderate severity has subsided and after a period of stability. |
| Interventions | 1. Social skills programme: delivered in a way that is possible in routine care with minimal additional resource. 2. Standard care. |
| Outcomes | Healthy days.
Mental state: improved to important degree.
Global state: improved to important degree, relapse.
Service use: admission, time in hospital.
Social functioning: employment status, relationships.
Quality of life: improved to important degree.
Economic outcomes: cost. Adverse effect: Any important adverse event |
What's new
| Date | Event | Description |
|---|---|---|
| 28 April 2015 | Amended | Author added to byline. |
Acknowledgements
Many thanks must go to Dr. Adib Essali who first introduced us to The Cochrane Collaboration. Thanks also go to Damascus School of Medicine/Syria for its encouragement. The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
Our thanks also go to Jun Xia (JX) for her translation of all Chinese studies and to Karla Soares Weiser (KSW) for her assistance with the selection of studies and data extraction.
Data and analyses
Comparison 1. SOCIAL SKILLS versus STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Social functioning: average endpoint score (various scales) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 SDSS (higher = worse) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 SAD (higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 SSPI (higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 DAS (higher = worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relapse | 2 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.79] |
| 3 Global state: not clinically improved. | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.68] |
| 4 Service outcome: rehospitalisation | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.30, 0.93] |
| 5 Mental state: average endpoint score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Negative symptoms (SANS, higher = worse) | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐8.92 [‐10.46, ‐7.38] |
| 5.2 Positive symptoms (SAPS, higher = worse) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.37, ‐0.43] |
| 6 Mental state: average change in score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 General symptoms (BPRS, higher = worse) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐4.01 [‐7.52, ‐0.50] |
| 6.2 Negative symptoms (SANS, higher = worse) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐7.7 [‐12.23, ‐3.17] |
| 7 Behaviours: Rated as worse | 1 | 48 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7.1 aggressive behaviour | 1 | 12 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 7.2 interaction with staff | 1 | 12 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 7.3 interaction with patients | 1 | 12 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 7.4 problem solving ability | 1 | 12 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.27, 0.27] |
| 8 General functioning: average endpoint score (MRSS, higher = worse) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐10.60 [‐17.47, ‐3.73] |
| 9 General functioning: Not employed | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.28, 107.33] |
| 10 Leaving the study early | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Any reason | 9 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.00, 4.16] |
| 11 Quality of life: average endpoint score (various scales) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 GWB (higher = better) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐12.18, ‐3.02] |
| 11.2 SES (higher = better) | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐8.3 [‐10.07, ‐6.53] |
1.7. Analysis.
Comparison 1 SOCIAL SKILLS versus STANDARD CARE, Outcome 7 Behaviours: Rated as worse.
Comparison 2. SOCIAL SKILLS versus DISCUSSION CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Social functioning: average endpoint score (various scales) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 SSQ (higher = worse) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐11.14, 8.94] |
| 1.2 SCON (higher = better) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐5.52, 0.52] |
| 1.3 SFS (higher = better) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 4.60 [‐7.54, 16.74] |
| 1.4 SBS (higher = worse) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐2.56, 3.56] |
| 2 Relapse | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.44, 10.10] |
| 3 Global state: average endpoint score (GAS, higher = better) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 4.50 [‐1.20, 10.20] |
| 4 Mental state: average endpoint score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 General symptoms (BPRS, higher = worse) | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐4.05, 4.49] |
| 4.2 Negative symptoms (SANS, higher = worse) | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | 2.89 [‐4.43, 10.22] |
| 5 Leaving the study early | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.37, 6.68] |
| 6 Quality of life: average endpoint score (QLS, higher = better) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐6.47, 13.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chien 2003.
| Methods | Allocation: randomised. Blinding: no. Duration: 4 weeks (follow‐up 1 month). Setting: inpatients. Design: parallel. Country: Taiwan. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N = 84.* Sex: M 43, F 35. Age: mean ˜ 42 years. History: illness (1‐35 years; mean ˜ 17 years), history of social skills training (19 patients). | |
| Interventions | 1. Social skills training: Group treatment phase ‐ 60‐minute social skills training course twice a week for 4 weeks to improve patients conversation and assertiveness skills (N = 35). 2. Routine nursing care treatment (N = 43). | |
| Outcomes | Leaving the study early.* Unable to use ‐ Mental state: PANSS (only pre‐treatment results reported). Social functioning: Social anxiousness scale (IAS) (only pre‐treatment results reported). Social functioning: Interpersonal communication satisfaction scale (data not reported for control group). Social functioning: The Assertive skills scale (data not reported for control group). |
|
| Notes | *The number randomised is stated as 84. Eight losses to follow‐up are reported, but the final number in the analysis is 78. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A table of random numbers was used to select 28 subjects from the first subgroup and randomly assigned 14 of them to the experimental group or control group. This same procedure was repeated with the second and the third subgroups of subjects". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Two subjects in the experimental group withdrew from the study before treatment one because of medical disease that required an inter‐hospital transfer and the other who stabilized discharge from the hospital. Two subjects in the experimental group withdrew because they refused to participate. One subject in each of the groups withdrew during the training because of being transferred to another ward. Two subjects in the experimental group were excluded from the study because they were able to participate in less than half of the training sessions (four times)". |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Cui 2004.
| Methods | Allocation: randomised. Blinding: no. Duration: 12 weeks. Setting: inpatients. Design: parallel. Country: China. | |
| Participants | Participants: schizophrenia (CCMD‐2‐R). N = 100. Sex: M 100. Age: average ˜44 ±7 years History: average length of illness ˜ 20 ± 6 years. | |
| Interventions | 1. Social skills training + routine drug therapy. Training is delivered in groups of 12 or 13, employing UCLA Social and independent Living Skills programme. Training contents include: 1) general social interpersonal skills training, using video demonstration, role play and discussion; 2) educate patients of the purpose and function of antipsychotic medication; 3) medication management; 4) how to recognise drug adverse effect; 5) opportunity to discuss issues relating to drug therapy. Frequency of intervention is 90‐120 minutes per session, 3 sessions per week for 12 weeks (N = 50). 2. Control group: routine drug therapy (N = 50). | |
| Outcomes | Leaving the study early.
Mental state: BRPS and SANS ("decreased rate" reported = (before treatment score ‐ after treatment score)/before treatment score X 100%). Unable to use ‐ Social functioning: Social disability screening schedule (SDSS) (no mean and SD). |
|
| Notes | Study in Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were five dropouts from the social skills training group and four from the control group. People who left the study early were not included in the final analysis. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Dobson 1995.
| Methods | Allocation: randomised. Blinding: no (raters blinded). Duration: 11 weeks (3 months follow‐up).* Setting: inpatients. Design: parallel. Country: Canada. | |
| Participants | Diagnosis: schizophrenia (structured clinical interview for DSM‐III‐R). N = 33. Sex: M 21, F 12. Age: 18‐ 55 years. History: schizophrenic, 18 to 55 years, low‐average intelligence or higher. | |
| Interventions | 1. Social skills training: first three weeks ‐ basic communication skills; second three weeks ‐ assertiveness training; third three weeks ‐ individual communication and assertive goal setting. Four one‐hour sessions per week over nine week period (N = 18).
2. Social milieu: structured activities including supportive discussion groups, exercise groups and activity groups. Occurred at same time of day and same length of time as social skills training group (N = 15). Neuroleptic medication for all patients. |
|
| Outcomes | Leaving the study early. Unable to use ‐ Mental state: PANSS (no SDs). Relapse and re‐hospitalisation (confounding effect of social milieu participants beginning social skills training*) |
|
| Notes | * One week of assessments before the treatment, nine weeks of treatment, and one week of assessments after the treatment. Three month follow‐ups were completed for all patients, at that point social milieu participants were able to participate in social skills training. The social skills group received additional assessment at 6 months follow‐up. Rehospitalisation data and relapse data were collected on all patients at 6 months and 1 years after treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" no further information given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blind: "None of the assessments were completed by the group therapists for subjects in the study, and none of the treatment groups were led by the investigators". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 5 dropouts: 2 from the social milieu group and 3 from the social skills group. "The reasons they gave for dropping out were unrelated to the treatment". |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | High risk | Protocol not available. The follow‐up period for social milieu group was limited mid‐study so they could also receive social skills training, which they had expressed interest in to the investigators. |
Hayes 1995.
| Methods | Allocation: randomised. Blinding: no (raters blinded). Duration: 18 weeks (plus 6 months booster period*). Setting: outpatients. Design: parallel. Country: Australia. | |
| Participants | Diagnosis: schizophrenia. N = 63. Sex: M 47, F16. Age: mean ˜ 36 years (18‐65 years). History: psychotic symptoms, significant residual impairment, deficit on social and skills and conversation tasks. | |
| Interventions | 1. Social skills training: emphasised interpersonal skills, social problem solving, positive time use skills, generalisation enhancement techniques, and used instructions, modelling, behaviour rehearsal, feedback, and structured homework (N = 32).**
2. Discussion group condition: focused on interpersonal relations and purposeful use of time using open ended questions, paraphrasing, reflecting, and summarising the participants' comments to promote self‐disclosure (N = 31).** Both interventions consisted of 36 sessions each of 75 minutes duration, over 18 weeks in small groups between 5 and 7 participants. Neuroleptic medication maintenance doses for all patients. |
|
| Outcomes | Social functioning: Simulated Social Interaction Test (SSIT), conversation with a stranger task (SCON) and Social Situations Questionnaire (SSQ).
Mental state: BPRS, negative symptoms (SANS).
Global state (GAS).
Quality of Life (QLS). Unable to use ‐ Leaving the study early (not reported which groups dropouts had been assigned to). Relapse (number relapsing from each group not reported). Social functioning: Time Diary (not reported for 6‐month follow‐up). Quality of life: Adapted Pleasant Events Schedule (APES) (total scores not reported). Satisfaction with treatment: Evaluation of therapist (data not reported). |
|
| Notes | * During this period patients received decreasing frequency of training.
** Number randomised to each group not reported. We have assumed that N = 32 for the social skills training group and N = 31 the discussion group. 22 participants (35%) attended less than half the scheduled sessions and classed as non completers. 45 participants (71%) were reassessed at post‐treatment; 37 of these were treatment completers. At follow‐up, 34 participants were assessed; 32 were treatment completers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects were assigned randomly" no further information. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. "All research assistants conducting interviews and rating measures were uninformed to the treatment condition of participants". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18 dropouts at post treatment assessment, another 11 had dropped out at follow‐up assessment. Dropouts were reported as either in hospital, unable to be reached or declined to participate, it was not reported to which groups they had been assigned. |
| Selective reporting (reporting bias) | High risk | Reported insufficient funds to code time diary data for follow‐up assessment. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. Source of funding: National Health and Medical Research Council of Australia and University Queensland. |
Huang 2005.
| Methods | Allocation: randomised. Blinding: single blind (assessor blind). Duration: 6 months. Setting: outpatients. Design: parallel. Country: China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 73. Sex: M 48 F19*. Age: mean˜41years, SD˜7 years. History: current length of hospitalisation is at least 2 years, overall length of illness at least 10 years. | |
| Interventions | 1. Social skills training + routine drug medication, recreational activities and work in the wards: training is delivered in groups of 8‐9 people, 2 hours per weeks for 24 weeks. (N = 37) 2. Standard care: Routine drug therapy, recreational activities and work in the wards (N = 36). |
|
| Outcomes | Leaving the study early. Mental state: SANS. Social functioning: Scale of Social Skills for Psychiatric Inpatients (SSPI). Global state: No clinically important change (CGI). | |
| Notes | In Chinese. * number who completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Raters were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four participants dropped out of the social skills training group and two from the control group. Dropouts were excluded from the final analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Kopelowicz 1998.
| Methods | Allocation: randomised. Blinding: no. Duration: 3 months. Setting: inpatients. Design: parallel. Country: USA. | |
| Participants | Diagnosis: schizophrenia and schizoaffective disorders (DSM‐IV). N = 46. Sex: M 30, F 16. Age: mean ˜ 33.9 years. History: in hospital (mean ˜ 2 years) prior to the study. | |
| Interventions | 1. Social skills training: Spanish language version of the UCLA Social and Independent Living Series. Three months of skills training four days a week. (N = 22). 2. Customary care: monthly visits to a psychiatrist who prescribed antipsychotic medication (N = 24). | |
| Outcomes | Leaving the study early.** Unable to use ‐ Social functioning: Skill acquisition and generalisation* (no mean and SD). Mental state: UCLA Expanded BPRS (no mean and SD). |
|
| Notes | * Generalisation of skills to home environment. ** Two participants dropped out due to finding employment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No information given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Two patients dropped out of skills training because they obtained full time employment and were thus unable to participate." No further details given. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Lu 2004.
| Methods | Allocation: randomised. Blinding: no. Duration: 8 weeks. Setting: inpatients. Design: parallel. Country: China | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N = 112. Sex: male and female. Age: 18‐60 years. History: mean duration of illness ˜9.9 years, SD˜7.1 years. | |
| Interventions | 1. Social skills training + routine antipsychotic medication: training included targeted problem solving training ‐ breaking down complicated daily problems into small and simple parts, teaching patients through repeated explanation and demonstration to eventually solve the complex problems. Training to improve patients' cognitive skills (attention, planning) with the aim of gradually improving patient's social function. Training was 60 minutes per session and 2 sessions per week for 8 weeks (N = 56) 2. Routine antipsychotic medication only (N = 56) |
|
| Outcomes | Leaving the study early. General functioning: Morningside rehabilitation status scale (MRSS). Social functioning: Social Disability screening schedule (SDSS). Quality of Life: General well‐being schedule (GWB). Self‐esteem scale (SES). | |
| Notes | In Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method is not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Ma 2003.
| Methods | Allocation: randomised. Blind: no. Duration: 8 weeks intervention + 26 weeks follow‐up. Setting: outpatients. Design: parallel. Country: China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 120. Sex: not reported. Age: not reported. History: not reported. | |
| Interventions | 1. Social skills training group: UCLA Social and independent Living Skills programme guidelines, including three major elements: 1) independent social living skills training, e.g. draft plans on reintegrate to society and cope with stressful life events; 2) medication management, e.g. getting to know the effect and side effects of antipsychotic drugs, learn to recognise and deal with medication side effect; 3) symptom self‐monitoring. Means of delivering the training including the use of video, role play, problem solving discussions. Frequency of the session is 90 to 120minutes per session, one session a week for 8 weeks (N = 60). 2. Control group: conventional community care (N = 60). | |
| Outcomes | Leaving the study early. Mental state: positive symptoms (SAPS) and negative symptoms (SANS). General functioning: Disability Assessment Scale (DAS). Relapse. | |
| Notes | In Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised with random number tables. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Mayang 1990.
| Methods | Allocation: randomised. Blinding: no. Duration: 4 sessions of training, follow‐up 4 weeks. Setting: inpatients. Design: parallel. Country: USA. | |
| Participants | Diagnosis: schizophrenia. N = 18. Sex: F 18. Age: early 20s to mid‐50s. History: able to express themselves verbally, a recent history of physical and/or verbal aggression, deficient in adaptive problem solving skills. | |
| Interventions | 1. Treatment group: problem solving skills training (receiving skills, processing skills and sending skills) involving instructions, modelling, feedback, role playing, social reinforcement. Four sessions of 45 minutes of training (N = 6). 2. Interaction group: non‐treatment interaction with trainers (N = 6). 3. Control group: did not receive any training or interaction with trainers (N = 6). |
|
| Outcomes | Unable to use ‐ Social functioning: problem solving skills and number of seclusion hours (no SDs). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned", no further details given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample sizes not calculated |
Ng 2006.
| Methods | Allocation: randomised. Blinding: No (raters were blinded). Duration: 8 weeks + 6 months follow‐up. Setting: Inpatients. Design: Parallel. Country: Hong Kong. | |
| Participants | Diagnosis: Schizophrenia, DSM‐IV criteria. N = 36. Sex: M 18, F 18. Age: 18‐ 65 years. History: All participants were already admitted at a rehabilitation ward for chronic psychiatric patients, diagnosed with schizophrenia, no organic central nervous system disorder or history of substance misuse in the previous 12 months. | |
| Interventions | 1. Social skills training (SST): training in receiving skills, processing skills and sending skills using behavioural techniques including instruction, taped modelling, role‐play practise, verbal feedback, social reinforcement, coaching, prompting, and homework assignment. 30 to 32 hours of treatment within eight weeks (N = 18).* 2. Supportive group discussion: topics included practical tips on money management and information on application for social security allowances. Special attention was paid to avoid discussions on interpersonal skills issues and use of behavioural techniques (N = 18). |
|
| Outcomes | Leaving the study early.
Social functioning: Social behaviour Schedule, Social Functioning Scale.
Psychopathology: BPRS and SANS.
Relaspe. Unable to use‐ Role Play Test (total scores not reported). |
|
| Notes | * Both groups received 2 sessions per week for 8 weeks, and standard care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a random number table, and stratified for gender, medication type and hospitalisation duration. |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done by an administrator...The administrator was only aware of the code number, name, date of birth, and stratification criteria for each patient." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Raters were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Three participants allocated to SST and 2 allocated to supportive group discussion dropped out before completion of the trial." "All patients who relapsed and were withdrawn from the trial during the treatment phase were included as treatment failures. All missing data were given mean values of that particular variable at that particular assessment point." |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Unclear risk | Protocol not published a priori. Funding: not reported. |
Potelunas 1982.
| Methods | Allocation: randomised ‐ table of random numbers. Blindness: no (unclear if all raters were blinded). Duration: 6 weeks. Setting: outpatients*. Design: parallel. Country: USA. | |
| Participants | Diagnosis: schizophrenia. N = 45. Sex: F 45. Age: mean ˜ 33 years (21‐25 years). History: volunteers, females, previous hospitalisation under the diagnosis of schizophrenia, participation in the program beyond five weeks. | |
| Interventions | 1. Social skills training: four one‐hour sessions over four weeks of videotaped presentation of eight problem situations, videotaped modelling, behaviour rehearsal, coaching and feedback (N = 15). 2. Discussion control: four one‐hour sessions over four weeks of videotaped presentation of eight problem situations, with discussion (N = 15). 3. No treatment control (N = 15). | |
| Outcomes | Leaving the study early. Unable to use ‐ Overall rating form (invalidated due to problems in the administration of the form).** Supervisee Self Efficacy Test (not validated scale). Supervisee Social Competency Test (not validated scale). Nonverbal assertiveness scale (no overall score given). |
|
| Notes | * Psychiatrically disabled who are well enough to have been discharged from the hospital, but still needs some time in a supportive environment. ** Many of the supervisors were changed for the patients between pre and post tests, thus most of the forms were not filled out by the same supervisor for each patient. Social skills training was given as part of a work adjustment programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A table of random numbers was used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The primary experimenter was not seen by participants on a regular basis, but it is not stated whether they were blind to group allocation. Three research assistants blind to treatment condition independently rated the videotapes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Sample size calculation done. Source of funding not reported. Protocol not available. |
Saren 2004.
| Methods | Allocation: randomised using random number table. Blinding: no. Duration: 10 weeks intervention + 12 months follow‐up. Setting: outpatients. Design: parallel. Country: China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R, ICD‐10). N = 148. Sex: male and female. Age: mean ˜48 years, SD˜16 years. History: mean length of illness ˜18 years, SD˜13 years. | |
| Interventions | 1. Social skills training + routine drug treatment: 1) training in making eye contact ‐ when and where is appropriate and how to make eye contact ; 2) facial expression ‐ promote positive facial expression, e.g. smile and nod head from time to time when converse with others; 3) tone of voice ‐ avoid flat tone of voice and try to appear enthusiastic; 4) language fluency, using logical expressions and avoid short sentences without adjectives; 5) posture and gesture,how to coordinate body language; 6) overall enthusiasm. Training is provided in groups of between 10‐12 people, 60to 90minutes per session, 3 sessions per week for 10 weeks. (N = 74) 2. Routine drug treatment (N = 74). |
|
| Outcomes | Leaving study early. Relapse. Rehospitalisation. Social avoidance and disability scale (SAD). Social disability screening schedule (SDSS). | |
| Notes | In Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants dropped out of the social skills training group due to a change of address; In the control group 3 participants left dropped out, also due to a change of address. These were not included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Protocol not available. Sample size not calculated. |
Tsang 2001.
| Methods | Allocation: randomised. Blinding: unclear. Duration: 10 weeks (3 months follow‐up). Setting: inpatients. Design: parallel. Country: Hong Kong. | |
| Participants | Diagnosis: schizophrenia. N = 97. Sex: M 60, F 37. Age: 18‐ 50 years. History: unemployed patients, have at least fifth grade education. | |
| Interventions | 1. Socail skills training with follow‐up support: basic social skills (facial expression, gestures etc.), basic social survival skills (personal appearance, tidiness) and core work‐related skills. Follow‐up support consisted of contact with group members and the trainer. Ten weekly group sessions lasting 1.5 to 2 hours, with approximately 6 to 8 people in each group (N = 30). 2. Social skills training without follow‐up support: same programme as described above but no follow‐up support (N =6). 3. Standard psychiatric care (N = 41). | |
| Outcomes | Employment. Unable to use ‐ Social functioning measures ‐ self‐administered check‐list score, role‐play test score, nonverbal skills (no means and SDs). |
|
| Notes | The purpose of the training was to help mentally ill persons to find and keep a job. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned by residential facility, not individually. |
| Allocation concealment (selection bias) | Unclear risk | Not addressed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "During the entire process of data collection, all participants and those responsible for rating participants' performance were blind to the group status of the participants, and participants did not know that there were groups with and without follow up." It is unclear if the control group was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no dropouts reported from the two intervention groups, but no information whether there were dropouts in the control group. |
| Selective reporting (reporting bias) | High risk | Not all expected outcomes reported. |
| Other bias | Unclear risk | Protocol not available. Sample sizes not calculated. |
Scales BPRS = Brief Psychiatric Rating Scale CGI = Clinical Global Impression GAS = Global Assessment Scale PANSS = Positive and Negative Syndrome Scale QLS = Quality of Life Scale SANS = Scale for the Assessment of Negative Symptoms SAPS = Scale for the Assessment of Positive Symptoms Other CCMD = Chinese Classification of Mental Disorders DSM = Diagnostic and Statistical Manual of Mental Disorders SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12609000317291 | Ongoing study. Allocation: randomised. Participants: people with psychosis and personality disorders. Intervention: Collaborative therapy which provides education and coping strategies to enhance self management of mental illness versus treatment as usual. |
| Alter 1993 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders. Intervention: Coping skills training versus no training. |
| Armstrong 1991 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders. Interventions: life skills program (SST, life management, nutrition, problem solving, self‐control skills) versus supportive psychotherapeutic milieu (social milieu, assistance with life skills, supportive environment). |
| Barrowclough 2001 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders (ICD‐10, DSM‐IV), and substance abuse or dependence (DSM‐IV). Interventions: integrated intervention programme (motivational intervention, CBT, family intervention) and routine care versus routine care alone. |
| Beal 1977 | Allocation: randomised. Diagnosis: schizophrenia (diagnostic criteria not reported). Intervention: remotivation therapy and activities versus remotivation therapy and social living groups versus remotivation therapy. |
| Brown 1983 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III). Interventions: life skills program ( interpersonal, nutrition, health, finance, time management, and community networks) versus rehabilitation condition (recreation, art, occupational therapy). |
| Browne 1996 | Allocation: unclear. Participants: people with schizophrenia (DSM‐III‐R). Interventions:psychosocial and educative program versus conventional rehabilitation. |
| Bruns 1992 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III‐R). Interventions: psychotherapeutic treatment versus leisure time activities. |
| Buchbauer 1986 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III). Interventions: positive assertion training versus negative assertion training versus discussion control versus complete assertion (positive and negative). |
| Cannon 1985 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorder. Interventions: psychosocial rehabilitation model (everyday skills, work skills, communication skills, medication) versus control group (occupational therapy, work therapy, family services). |
| Cerniglia 1978 | Allocation: randomised. Participants: schizophrenic patients. Intervention: treatment programme of patient decision making and self‐management versus routine care. |
| Chabannes 2008 | Allocation: randomised ‐ no further details given. Participants: participants meeting criteria for a schizophrenia spectrum disorder according to the DSM‐IV criteria. Interventions: “Soleduc” psychoeducational program (8 module, 7 session presentations, presented three times) versus non‐specific psychosocial group training (sessions of oral information about schizophrenia and its treatment according to the standard of each participating centre). |
| Chan 2009 | Allocation: randomly assigned ‐ no further description. Participants: Individuals diagnosed with schizophrenia according to the criteria of the DSM‐IV. Interventions: Psychoeducation program (teaches clients and their families about disorders, treatments, coping techniques, and available resources) 10 sessions conducted over 3 months on a weekly basis versus control group. Both groups received the same access to routine care. |
| Chandler 2000 | Allocation: randomised. Participants: 40% had a diagnosis of schizophrenia, 23% had diagnoses of schizoaffective disorder and 14% of bipolar disorder. Interventions: Social skills training modules as an adjunct to an assertive community treatment program versus assertive community treatment only. |
| Choi 2006 | Allocation: random allocation ‐ no further details. Participants: Persons with a DSM‐IV‐based diagnosis of schizophrenia or schizoaffective disorder and on stable antipsychotic medications. Interventions: Social cognition enhancement training and standard psychiatric rehabilitation training vs. standard psychiatric rehabilitation training only. |
| Cormier 1995 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: psychoeducational program versus leisure activities versus usual follow‐up. |
| Daniels 1998 | Allocation: randomised. Participants: people with schizophrenia or schizoaffective disorder (DSM‐IV). Intervention: Interactive behavioral therapy (IBT): an approach to social skills training with a combined focus on cognitive‐behavioural techniques and group process strategies versus waiting list control group (N = 20). |
| DeCarlo 1985 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia or depression. Interventions: verbal therapy versus activities group versus normal milieu therapy. Outcome: results were not broken down for each group (schizophrenia or depression). |
| Deng 2007 | Intervention: Social skills training +routine care versus routine care alone. Training including: 1) interpersonal communication training; 2) confidence training; 3) recreational activities with token economy for good behaviour. |
| Denicola 1980 | Allocation: randomised. Diagnosis: schizophrenia. Intervention: job training using three modelling conditions versus videotaped didactic presentation of instruction and control group. |
| Dincin 1982 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐II). Interventions: comprehensive rehabilitation program (individual casework, vocational rehabilitation, social rehabilitation, residential facilities, prevention of rehospitalisation) versus supportive treatment program. |
| Eisler 1978 | Allocation: randomised. Diagnosis: schizophrenia. Intervention: Social skills training using the Behavioural Assertiveness Test‐Revised (BAT‐R) versus social skills training with modeling using the BAT‐R versus rehearsal of scenes from the BAT‐R. |
| Falloon 1977 | Allocation: randomised ‐ no further description. Participants: people with social skills deficits (social phobia, personality disorders, inadequate, depressive neurosis, schizophrenia,drug/alcohol abuse, obsessive compulsive neurosis, sexual deviation). Interventions: cohesive group discussion versus modelling and role‐rehearsal versus modelling, role‐rehearsal and daily social homework. |
| Finch 1977 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: interpersonal skills training versus normal hospital routine. Outcome: unusable data. |
| Fiorillo 2004 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: training program (psychoeducation, communication and problem solving training) versus augmented program (coping skills, meetings, exercises on the application of psychoeducation sessions). |
| Foxx 1985 | Allocation: randomised (matched on the basis of their pre‐assessment). Participants: people with schizophrenia (most of the participants). Interventions: game based social skills versus game based social skills. |
| Fuentes 2007 | Allocation: randomised. Participants: people with schizophrenia (ICD‐10). Intervention: Social perception module of IPT (integrated therapy program for schizophrenic patients) versus standard care control group. |
| Galderisi 2009 | Allocation: randomised. Participants: clinical diagnosis of chronic schizophrenia or schizoaffective disorder according to the DSM‐IV criteria, stable pharmacological treatment. Interventions: Integrated rehabilitation program, including individualised cognitive and social skills training versus standard structured leisure activities. |
| Gao 2006 | Allocation: quasi‐randomised according to odd and even number (possibly hospital admission number, but does not specify). Intervention including 1) independent living skills training, 2) social skills training; 3) team work training; 4) social activities training. |
| Glynn 1999 | Allocation: randomised. Participants: individuals with DSM‐IV diagnosis of schizophrenia or schizoaffective disorder on the basis of interviews with the Structured Clinical Interview for DSM‐IV. Interventions: clinic‐based skills training supplemented with manual‐based generalisation sessions in the community, or clinic‐based skills training alone. |
| Granholm 2002 | Allocation: randomised ‐ no further description. Participants: old people with schizophrenia, schizoaffective disorder (DSM‐IV). Interventions: cognitive behavioural social skills training versus treatment as usual. |
| Gudeman 1981 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia with minimum life daily skills. Interventions: Quraterway rehabilitation program (general integrative rehabilitation system) versus inpatients care. |
| Hayes 1991 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III‐R). Interventions: activity therapy and social skills training versus activity therapy and social skills training (initiation time is different). |
| Hogarty 1973 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐II). Interventions: (factorial design 2 X 2) major role therapy versus control, chlorpromazine versus placebo. |
| Hogarty 2001 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorder with cognitive disability. Interventions: cognitive enhancement therapy versus enriched supportive therapy (social skills, psychoeducation, stress avoidance skills). |
| Horan 2009 | Allocation: randomly assigned ‐ no further description. Participants: patients meeting DSM‐IV criteria for schizophrenia or schizoaffective disorder. Interventions: social cognitive intervention: affect perception, social perception, attributional style, Theory of Mind versus Control intervention: illness self‐management and relapse prevention skills training, a module of the UCLA Social and Independent Living Skills Program. |
| Jaffe 1976 | Allocation: randomisation unclear. Participants: psychosis (mainly schizophrenia), retardation. Interventions: modelling treatment versus instructions versus attention treatment. |
| Jenner 2004 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders, psychotic disorder (not otherwise specified). Interventions: hallucination focused integrative treatment (coping skills, CBT, psychoeducation, rehabilitation, and medication) versus routine care. |
| Jeppesen 2001 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia spectrum disorder (ICD‐10) and recent onset. Interventions: integrated treatment (AST, Psychoeducational multi family groups, SST) versus standard care. |
| Jerrell 1996 | Allocation: randomised, URN randomisation method. Participants: people with psychotic disorder or major affective disorders (DSM‐IIIR), substance abuse. Interventions: behavioural skills training (CBT, reinforcement, social skills and independent living skills) versus intensive case management versus 12‐step recovery model. |
| Kern 2005 | Allocation: randomised. Participants: people with schizophrenia or schizoaffective disorder (DSM‐IV criteria). Interventions: Social skills training: errorless learning versus symptom management module of UCLA Social and Independent Living Skills. |
| Kim 1997 | Allocation: unclear. Participants: people with schizophrenia (DSM‐IV). Interventions: family psychoeducation, patient psychoeducation , SST and routine care versus routine care alone. |
| Lecomte 1999 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders (DSM‐III and SCID). Interventions: empowerment and self‐esteem treatment (increase in self‐esteem, self‐worth, competence, as well as the ability to set goals and use active coping strategies) versus control. |
| Lee 1994 | Allocation: quasi‐randomised. |
| Lehman 2002 | Allocation: randomised ‐ no further description. Participants: people with severe mental illnesses. Interventions: Individual Placement and Support program (work therapy) versus psychosocial rehabilitation program. |
| Li 2008 | Intervention: rehabilitation training + routine care versus routine care alone. Rehabilitation training including 1) independent living skills training; 2) social skills training; 3) cognitive behavioural training. |
| Li 2008a | Intervention: social skills training versus routine antipsychotic treatment. Main components of the training including 1) independent living skill training; 2) behavioural correction, e.g. return a borrowed book to library and basis social manners; 3) simple exercises and leisure activities; 4) outings ‐ day trips. |
| Liang 2006 | Intervention: social skills training + routine care versus routine care. Training including 1) independent living skills training; 2) introducing new activities and broaden patients hobbies; 3) social skills training; 4) psychoeducation. |
| Liberman 1981 | Allocation: randomised. Participants: schizophrenia (DSM‐III, PES, CATEGO criteria). Intervention: social skills training versus holistic therapy. |
| Liberman 1998 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: psychosocial occupational therapy versus social and independent living‐skills program. |
| Liberman 2002 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: (factorial design 2 X 2) haloperidol versus resperidone , social living skills versus social living skills and IVAST (in vivo amplified skills training). |
| Lindsay 1980 | Allocation: randomised. Diagnosis: schizophrenia. Study took place on a token economy ward. |
| Linszen 1994 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia or related disorder (DSM‐III‐R): 55% schizophrenia, 13% schizophreniform, 21% schizoaffective disorder, 11% other schizophrenia‐related disorders. Interventions: individual oriented psychosocial intervention versus individual and family oriented psychosocial intervention. |
| Lu 2003 | Allocation: randomised ‐ no further description. Participants: inpatient with chronic inpatient schizophrenia (CCMD). Interventions: hospitalised occupational rehabilitation and routine care versus ordinary treatment (psychotherapy, work and amusement, music, drugs, and behavioural correction). |
| Lundin 1990 | Allocation: randomised. Participants: people with schizophrenia. Interventions: Social skills as part of a psychosocial rehabilitation programme versus control group with maintenance drug treatment and one afternoon of social training per week. |
| Malm 2003 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia spectrum disorder (DSM‐IV). Interventions: integrated care (CBT, 24‐hour crisis response, stress management, clinical decision making) versus rational rehabilitation. |
| Mausbach 2008 | Allocation: randomised. Participants: persistent psychotic disorders. Intervention: Functional Adaptation and Skills training (FAST) verus PEDAL (culturally tailored version of FAST) versus control support group. |
| May 1985 | Allocation: randomisation unclear. Participants: people with mental diseases. Interventions: life skills training (problem solving, interpersonal communication and fitness training) versus group counseling and occupational therapy. |
| McLatchie 1981 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (most of the participants). intervention : interpersonal problem solving therapy versus relaxation therapy. |
| Medalia 2000 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorder (DSM‐IV). Interventions: problem‐solving remediation group (using micro‐computerised exercises) versus memory remediation group versus control group. All patients were treated with medications before and during the study. |
| Moriana 2006 | Allocation: not randomised. Participants: people with schizophrenia according to DSM‐IV criteria. Interventions: adapted version of UCLA Social and independent living skills program versus control group, receiving conventional outpatient treatment for schizophrenia. |
| Mueller 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: cognitive social skills training for vocational functioning versus conventional unspecific social skills training. |
| Mueser 2001 | Allocation: randomised ‐ no further description. Participants: people with severe mental illness. interventions: Individual Placement and Support (work therapy) versus psychiatric rehabilitation program versus standard services. |
| Munjiza 1999 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: psychopharmacotherapy in a milieu therapy versus group therapy and pharmacotherapy. |
| Munroe 1995 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia and their relatives. Interventions: social skills training program (time limited) versus psychoeducation for relatives versus both treatments in parallel. outcomes: unusable data. |
| NCT00071591 2003 | Ongoing trial. Intervention: Functional Adaptation Skills Training (FAST) versus psychosocial support group. |
| Ni 2008 | Intervention: social skills training versus care as usual. Three main components: 1) 10 weeks were spent solely social skills training; 2) then 10 months on occupational training, e.g. cooking, gardening, cleaning, being shop assistant, craft work; 3 )finally 10 months on return to community skills training, including training on medication management, symptom self‐monitoring, managing finances, how to utilise community services. |
| Nordentoft 1999 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizophrenia‐like psychosis (criteria of international classification of disease). Interventions: integrative treatment (psychoeducation, social skills training and family involvement) versus standard treatment. |
| Norman 2002 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III‐R, SCID). Interventions: stress management versus social activities. |
| Nuechterlein 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (recent onset). Interventions: individual placement and supportive and work fundamental modules versus traditional vocational rehabilitation. |
| Nuttbrock 1997 | Allocation: randomised ‐ no further description. Participants: people with mental illness (DSM‐III‐R), substance abuse. Interventions: community residence versus therapeutic community. |
| O'Keefe 2003 | Allocation: unclear. Participants: people with schizophrenia, schizoaffective disorder. Interventions: workplace fundamental modules versus supported employment. |
| Odhner 1970 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia nonparanoid. Interventions: performance tasks versus verbal tasks. |
| Oei 1981 | Allocation: randomised ‐ no further description. Participants: people with chronic schizophrenia ‐ females only. Interventions: no visit to patient versus 1 visit/week versus 2 visits/week versus 3 visits/week. |
| Patterson 2005 | Allocation: randomised. Participants: Schizophrenia or schizoaffective disorder. Intervention: "PEDAL" Program for Training and Development of Skills in Latinos, which is culturally tailored and in Spanish to enhance the functioning of Latino patients with schizophrenia who live in the community versus "FAST" Equivalent skills training program with no cultural tailoring versus time equivalent support group |
| Patterson 2006a | Allocation: randomised. Diagnosis: DSM‐IV‐based chart diagnosis of schizophrenia or schizoaffective disorder. Intervention: Functional Adaptation and Skills training (FAST) versus a time‐equivalent attention‐control program. |
| Paul 1977 | Allocation: not randomised, matched groups design. Participants: people with schizophrenia. Interventions: social learning programme and token economy versus milieu environmental therapy verus traditional hospital therapy. |
| Petersen 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia spectrum (ICD‐10) (first episode). Interventions: integrated treatment (psychoeducation, SST, psychoeducational family treatment) versus standard care. |
| Prentice 2003 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia. Interventions: social skills training versus decision‐making training. |
| Razali 2000 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM IV). Interventions: behavioural family therapy versus cultured modified family therapy. |
| Rosenbaum 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenic spectrum disorder (ICD‐10). Interventions: supportive psychodynamic psychotherapy versus integrated treatmant (assertive, psychosocial and educational treatment program) versus TAU. |
| Rossotto 2003 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders. Interventions: psychoeducational treatment (the community re‐entry program) versus standard educational classes. |
| Smith 1999 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders (DSM‐III‐R). Interventions: skills training (community re‐entry program) and medication versus supportive psychotherapy and medication. |
| Tang 2007 | Allocation: randomised. Participants: schizophrenic patients. Intervention: experimental intervention is social skills training, including independent living skills training; 2) medication self‐management training; 3) symptom self‐monitoring training. |
| Tarrier 2000 | Allocation: randomised. Participants: people with schizophrenia. Interventions: cognitive behavioural therapy versus supportive counselling and routine care. |
| Test 1989 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia and schizotypal personality disorder (research diagnostic criteria), schizotypal personality disorders DSMIII. Interventions: training community living (TCL) (system management, optimally supportive environment) versus Dane models system (bio/psycho/social services similar to TCL but organised in different way) invoked depending on individuals needs. |
| Torres 2002 | Allocation: randomly assigned ‐ no further description. Participants: People meeting DSM‐IV criteria for schizophrenia, all receiving antipsychotic medication. Interventions: 1) Playing “El Tren” (team problem solving board game emphasizing positive reinforcement, repetition and procedural learning, designed to overcome negative symptoms), social skills training, psychomotor skills training and occupational therapy. 2) Social skills training, psychomotor skills training and occupational therapy. 3) Occupational therapy only. |
| Tsang 2009 | Allocation: randomly assigned using SPSS. Participants: People suffering from SMI (operationally defined as schizophrenia, schizo‐affective disorder, bipolar disorder, recurrent major depression, or borderline personality disorder) following DSM‐IV criteria. Interventions: Integrated supported employment (ISE) program, which augments Individual Placement & Support (IPS) with social skills training (SST), IPS only, and traditional vocational rehabilitation (TVR). |
| Urioste 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia, schizoaffective disorders. Interventions: psychosocial tool (GAIN Acceptance Approach) versus approach as usual in supporting acceptance of long‐acting risperidone. |
| Vauth 2005 | Allocation: randomised ‐ no further description. Participants:people with schizophrenia (DSM‐IV). Interventions: computer‐assisted cognitive strategy training versus self‐management skills for negative symptoms (time scheduling, mastery, pleasure techniques). |
| Ventegodt 2001 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia,delusional disorder, paranoiac state. Interventions: integrative treatment (assertive community treatment, psychoeducation and social skills training) versus standard treatment. |
| Walker 1969 | Allocation: randomisation not efficiently described. Participants: people with mental diseases. Interventions: community hospital industrial rehabilitation placement (CHIRP) versus employment without community hospital industrial rehabilitation. |
| Weinman 1974 | Allocation: not randomised, control group is matched on the basis of age and length of hospitalisation. Participants: people with chronic psychotic disorders. Interventions: community treatment versus socioenvironmental therapy versus traditional hospital treatment. |
| Weng 2005 | Allocation: randomised ‐ no further description. Participants: people with schizophrenia (DSM‐III‐R). Interventions: rehabilitation program (SST, daily living skills, relaxation therapy, occupational therapy, educational program, and medication) versus control group. |
| Whetstone 1986 | Allocation: quasi‐randomised. Participants: people with schizophrenia (American Psychiatric Association). Interventions: social dramatic scenarios versus control. |
| Wiersma 2004 | Allocation: randomised ‐ no further description. Participants: people with chronic schizophrenia with hallucination. Interventions: integrated treatment condition (rehabilitation, coping skills, family treatment, cognitive behavioural intervention) versus standard care. |
| Wirshing 1991 | Allocation: randomised. Participants: schizophrenic patients. Intervention: Medication management and symptom management versus standard supportive psychotherapy. |
| Xiang 2002 | Allocation: randomised. Participants: schizophrenic patients Intervention: skills training vs routine care. Training package includes elements of independent living skills training, medication management training, symptom self‐monitoring training. |
| Xiang 2007 | Allocation: randomised. Participants: schizophrenic (DSM IV). Interventions: community re‐entry module of the UCLA Social and independent living skills programme versus group psycho‐education,. |
| Xu 2008 | Intervention: social skills training versus routine care. Training including 1) medication management training; 2) social skills training; 3) occupational skills training |
| Yang 2003 | Allocation: not randomised. |
| Yue 1998 | Intervention: target behaviour therapy versus care as usual. |
| Zhang 2002 | Allocation: randomised. Participants: 67 schizophrenia patients and 59 affective disorder patients; Intervention: psychoeducation versus care as usual |
| Zheng 2006 | Allocation: randomised. Participants: schizophrenic patients. Intervention: life skills training including 1) independent living skills training; 2) social skills training; 3) art appreciation and other recreational activities versus routine care. |
CBT = cognitive behavioural therapy CCMD = Chinese Classification of Mental Disorders DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition ICD‐10 ‐ International Classification of Diseases, tenth revision.
Characteristics of studies awaiting assessment [ordered by study ID]
Bellack 1986.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | In German, awaiting translation. |
Piegari 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting more information |
卢春爱 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
唐仕友 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
康建华 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
樊献丽 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
蒋德政 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
陈世珍 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | to be translated |
Characteristics of ongoing studies [ordered by study ID]
NCT00069433 2003.
| Trial name or title | Skills training for schizophrenia: enhancing outcomes |
| Methods | Randomised controlled trial. |
| Participants | People with schizophrenia. |
| Interventions | 1. Intensive symptom management and social skills training. 2. Group therapy. |
| Outcomes | Not reported. |
| Starting date | Not reported. |
| Contact information | Not reported. |
| Notes | ClinicalTrials.gov Identifier: NCT00069433 2003. Trial suspended. |
NCT00183625 2005.
| Trial name or title | The effectiveness of supplementing supported employment with behavioral skills training in schizophrenia patients taking risperidone or olanzapine |
| Methods | Randomised controlled trial. |
| Participants | People with schizophrenia. |
| Interventions | 1. Social skills training 2. Individual placement and support Drug intervention1. Olanzapine Drug intervention 2. Risperidone |
| Outcomes | Functional outcome. Quality of life. |
| Starting date | June 2000. |
| Contact information | |
| Notes | ClinicalTrials.gov Identifier: NCT00183625 2005. |
NCT00237796 2005.
| Trial name or title | Functional rehabilitation of older patients with schizophrenia. |
| Methods | Randomised controlled trial. |
| Participants | Older patients with schizophrenia. |
| Interventions | 1. Cognitive behavioural social skills training. 2. Supportive contact. |
| Outcomes | Social functioning (at baseline); End of treatment (9‐month follow‐up); 9‐month post‐treatment (18‐month follow‐up); additionally, at midtreatment (4.5 months). Secondary outcomes: Neuropsychological functioning, cognitive insight, psychotic symptoms, and health services utilisation, will be conducted at baseline, end of treatment (9‐month follow‐up), and 9‐month post‐treatment (18‐month follow‐up). |
| Starting date | February 2005. |
| Contact information | Jody Delapena, BA 858‐552‐8585 Ext. 2743 jodelapena@popmail.ucsd.edu Sherry Edwards, BA 858‐552‐8585 Ext. 2275 sedwards@ucsd.edu |
| Notes | ClinicalTrials.gov identifier NCT00237796 2005. |
NCT00338975 2006.
| Trial name or title | Cognitive behavioral social skills training for improving social functioning in people with schizophrenia |
| Methods | Randomised controlled trial. |
| Participants | People with schizophrenia. |
| Interventions | 1. Cognitive behavioural therapy. 2. Social skills training. 3. Goal setting. 4. Active supportive contact. |
| Outcomes | Primary outcomes: Measured at months 9, 15, and 21: Social functioning. Secondary outcomes: Measured at months 9, 15, and 21: Neuropsychological functioning; Cognitive insight; Psychotic symptoms; Health services utilisation. |
| Starting date | June 2005. |
| Contact information | Sherry Edwards 858‐552‐8585 Ext. 2275 sedwards@ucsd.edu. |
| Notes | ClinicalTrials.gov identifier NCT00338975 2006. |
NCT00391677 2006.
| Trial name or title | Attention shaping procedures for improving psychosocial skills among adults with schizophrenia |
| Methods | Randomised controlled trial. |
| Participants | People with schizophrenia. |
| Interventions | 1. Attention shaping procedures plus basic conversation skills training (BCS). 2. BCS alone. |
| Outcomes | Primary outcomes: Measured 6 months post‐intervention: Observational ratings of in‐group attentiveness; observational ratings of in‐group attentiveness in non‐study groups; changes in knowledge of information about social skills taught in the study; changes in ability to demonstrate behavioural skills taught in the study; level of social functioning. Secondary outcomes: Measured 6 months post‐intervention: Self‐efficacy; working alliance; satisfaction with treatment. |
| Starting date | December 2006. |
| Contact information | Steven M. Silverstein, PhD 732‐235‐5149 silvers1@umdnj.edu. Igor Malinovski 732‐235‐5148 malinovsky01@icqmail.com. |
| Notes | ClinicalTrials.gov identifier NCT00391677 2006. |
NCT00601224 2008.
| Trial name or title | Social cognition and interaction training for improving social functioning in people with schizophrenia |
| Methods | Randomised controlled trial. |
| Participants | People with schizophrenia. |
| Interventions | 1. Social cognition and interaction training (SCIT) 2. Treatment as usual (TAU) |
| Outcomes | Face emotion identification task (FEIT). Face emotion discrimination task (FEDT). The hinting task. The awareness of social inference test (TASIT). Ambiguous intentions hostility questionnaire (AIHQ). |
| Starting date | June 2007. |
| Contact information | Piper S. Meyer, PhD 919‐843‐5262 psmeyer@email.unc.edu. |
| Notes | ClinicalTrials.gov identifier: NCT00601224 2008 |
NCT00791882 2008.
| Trial name or title | Evaluation of social skills training in reducing negative symptoms in patients with refractory schizophrenia |
| Methods | Randomised controlled trial. |
| Participants | People with refractory schizophrenia. |
| Interventions | 1. Social skills training. 2. Control group. |
| Outcomes | Response: at least 20% decrease in PANSS ‐ negative subscale after 20 weeks, in comparison with baseline, maintained at 26 weeks follow‐up. |
| Starting date | February 2009. |
| Contact information | Helio Elkis, MD PhD + 55 11 30697531 helkis@usp.br Silvia Scemes, BSc + 55 11 30697808 silviascemes@gmail.com |
| Notes | NCT00791882 2008. |
Pratt 2008.
| Trial name or title | Helping older people experience success: an integrated model of psychosocial rehabilitation and health care management for older adults with serious mental illness. |
| Methods | Randomised controlled trial. |
| Participants | Older people with serious mental illness. |
| Interventions | 1. Social skills training and health management. 2. Standard care. |
| Outcomes | Not reported. |
| Starting date | Not reported. |
| Contact information | Sarah Pratt, Ph.D., Assistant Professor of Psychiatry, Dartmouth Psychiatric Research Center, 105 Pleasant Street, Main Building, Concord, NH 03301, USA. E‐mail: sarah.i.pratt@dartmouth.edu. |
| Notes |
PANSS = Positive and Negative Syndrome Scale
Contributions of authors
Muhammad Qutayba Almerie ‐ helped write protocol, selection of studies and data extraction (based on 2006 search)
Muhammad Okba Al Marhi ‐ helped write protocol, selection of studies and data extraction (based on 2006 search) .
Mohamad Jawoosh ‐ helped write protocol, screened the search results, appraised the papers, extracted data, managed them and then entered them into RevMan (based on 2006 search).
Mohamad Alsabbagh ‐ helped write protocol, selection of studies and data extraction (based on 2006 search)
Hosam Matar ‐ helped write protocol, selection of studies and data extraction (based on 2006 search)
Nicola Maayan ‐ selection of studies, data extraction (based on 2010 search) completion of the 'Summary of findings' tables, risk of bias tables, completion of report.
Hanna Bergman ‐ selection of studies, data extraction (based on 2010 search).
Sources of support
Internal sources
Faculty of Medicine, Damascus University, Syrian Arab Republic.
External sources
No sources of support supplied
Declarations of interest
All review authors have no conflict of interest.
Hanna Bergman and Nicola Maayan ‐ work for Enhance Review Ltd, a company that carries out systematic reviews mostly for the public sector. We currently do not provide services for the pharmaceutical industry.
New
References
References to studies included in this review
Chien 2003 {published data only}
- Chien H, Ku C, Lu R, Chu H, Tao Y, Chou K. Effects of social skills training on improving social skills of patients with schizophrenia. Archives of Psychiatric Nursing 2003;17(5):228‐36. [CINAHL: 2004042315] [DOI] [PubMed] [Google Scholar]
Cui 2004 {published data only}
- Cui Y, Yang W, Weng Y. Effectiveness of social skills training in patients with chronic schizophrenia. Chinese Mental Health Journal 2004;18(11):798‐801. [MEDLINE: ] [Google Scholar]
- Cui Y, Yang WY, Yao FX, Hu YD, Wang HJ. Self‐management with drugs in the training of inpatients with chronic schizophrenics. Zhongguo Linchuang Kangfu 2006;10(10):57‐60. [EMBASE: 2006191712] [Google Scholar]
Dobson 1995 {published data only}
- Dobson DJG, McDougall G, Busheikin J, Aldous J. Effects of social skills training and social milieu treatment on symptoms of schizophrenia. Psychiatric Services 1995;46(4):376‐80. [CINAHL: 1995039452] [DOI] [PubMed] [Google Scholar]
Hayes 1995 {published data only}
- Hayes RL, Halford WK, Varghese FT. Social skills training with chronic schizophrenic patients ‐ effects on negative symptoms and community functioning. Behavior Therapy 1995;26(3):433‐49. [PsycINFO: 60‐09769] [Google Scholar]
Huang 2005 {published data only}
- Huang Q, Wen Y, Tang Y, Li H, Zhao S. Social vocational training of schizophrenia in decline period. Chinese Journal of Health Psychology 2005; Vol. 13, issue 6:442‐3.
Kopelowicz 1998 {published data only}
- Kopelowicz A. Adapting social skills training for Latinos with schizophrenia. International Review of Psychiatry 1998;10(1):47‐50. [PsycINFO: 1998‐02756‐007] [Google Scholar]
- Kopelowicz A, Zarate R, Gonzalez V, Tripodis K. Social skills training for Latinos with schizophrenia. Schizophrenia Research 1999;1‐3:327‐8. [PsycINFO: 64‐13154] [Google Scholar]
Lu 2004 {published data only}
- Lu SC, Liu L, Fu FZ. Effect of social skill training on social functions and happiness degree of patients with schizophrenia. Journal of Practical Nursing 2004;20(2A):46‐7. [MEDI0404] [Google Scholar]
Ma 2003 {published data only}
- Ma ZY, Zhang PW, Chen F. The effect of returning to community through skill training to improve social function of the patients with schizophrenia. Chinese Journal of Clinical Rehabilitation 2003;7(24):3320‐1. [CAJ: MEDI0401] [Google Scholar]
Mayang 1990 {published data only}
- Mayang A. The Effects of Problem‐Solving Skills Training with ChronicSschizophrenic Patients. Michigan, USA: University of Western Michigan, 1990. [Google Scholar]
Ng 2006 {published data only}
- Ng RMK, Cheung MSL. Social skills training in Hong Kong chinese patients with chronic schizophrenia. Hong Kong Journal of Psychiatry 2006;16:14‐20. [Google Scholar]
Potelunas 1982 {published data only}
- Potelunas‐Campbell M. The development and evaluation of a social skills training program for supervisor/supervisee dyad interactions in a work adjustment program with female schizophrenic outpatients. University of New York. New York, USA, 1982.
Saren 2004 {published data only}
- Saren TN, Xiang YQ, Weng YZ, Feng YC. Social skill training for rehabilitating social skill disability of schizophrenics in the community. Modern Rehabilitation 2003;7(5):734‐5, 745. [CAJ: MEDI0306] [Google Scholar]
- Saren TN, Xiang YQ, Weng YZ, Feng YC, Chen L. Effect of social skill training for rehabilitating social skill disability of schizophrenics in the community. Chinese Journal of Clinical Rehabilitation 2004;8(36):8170‐2. [EMBASE: 2004428997] [Google Scholar]
Tsang 2001 {published data only}
- Tsang HW. Rehab rounds: Social skills training to help mentally ill persons find and keep a job. Psychiatric Services 2001;52(7):891‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tsang HW, Pearson V. Work‐related social skills training for people with schizophrenia in Hong Kong. Schizophrenia Bulletoin 2001;27:139‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12609000317291 {published data only}
- ACTRN12609000317291. A randomised control trial of people with psychosis taking risperdal consta compared to people with psychosis taking risperdal consta and receiving collaborative therapy (a psychoeducational program) in a naturalistic setting. Australian New Zealand Clinical Trials Registry 2008.
Alter 1993 {published data only}
- Alter B. Preferred Styles of Coping with Schizophrenia Effects of Matching Treatment with Coping Style (Psychosis Relapse). New Jersey, USA. University of Fairleigh Dickinson, 1993.
Armstrong 1991 {published data only}
- Armstrong HE, Cox GB, Short BA, Allmon DJ. A comparative evaluation of two day treatment programs. Psychosocial Rehabilitation Journal 1991;14(4):53‐67. [PsycINFO: 78‐28541] [Google Scholar]
Barrowclough 2001 {published data only}
- Barrowclough C, Haddock G, Tarrier N, Lewis SW. A randomised, controlled trial of a psychosocial intervention in dual diagnosis. Schizophrenia Research 2002;53(3 Suppl 1):227. [CINAHL: 2009245707] [Google Scholar]
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O'Brien. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. American Journal of Psychiatry 2001;158(10):1706‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haddock G, Barrowclough C, Tarrier N, Moring J, O'Brien R, Schofield N, et al. Cognitive‐behavioural therapy and motivational intervention for schizophrenia and substance misuse. 18‐month outcomes of a randomised controlled trial. British Journal of Psychiatry 2003;183:418‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beal 1977 {published data only}
- Beal D, Duckro P, Elias J, Hecht E. Graded group procedures for long term regressed schizophrenics. Journal of Nervous and Mental Disease 1977;164(2):102‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 1983 {published data only}
- Brown MA, Munford AM. Life skills training for chronic schizophrenics. Journal of Nervous and Mental Disease 1983;171(8):466‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Browne 1996 {published data only}
- Browne S, Roe M, Lane A, Gervin M, Morris M, Kinsella A, et al. The effect of psychosocial rehabilitation on quality of life and symptomatology in schizophrenia. Schizophrenia Research 1996;18(2,3):240. [Google Scholar]
Bruns 1992 {published data only}
- Bruns U, Franzen U, Hornung WP, Klingberg S, Wiesemann C. Effects of an ambulant group therapy program on psychosocial adaptation of chronic schizophrenic outpatients. Schizophrenia Research 1992;6(2):175. [Google Scholar]
Buchbauer 1986 {published data only}
- Buchbauer M. The Effects of Positive and Negative Assertion Training on Chronic Schizophrenic Outpatients. University of Seton Hall. New Jersey, USA, 1986:203. [Dissertation Abstracts (order number) AAC 8709627]
Cannon 1985 {published data only}
- Cannon N. A Cost Effectiveness Analysis of a Controlled Experiment Comparing Treatment Alternatives for Chronically Mentally Ill Patients. University of Brandeis. Waltham, Massachusetts, 1985.
Cerniglia 1978 {published data only}
- Cerniglia RP, Horenstein D, Christensen EW. Group decision‐making and self‐management in the treatment of psychiatric patients. Journal of Clinical Psychology 1978;34:489‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chabannes 2008 {published data only}
- Chabannes JP, Bazin N, Leguay D, Nuss P, Peretti CS, Tatu P, et al. Two‐year study of relapse prevention by a new education program in schizophrenic patients treated with the same antipsychotic drug. European Psychiatry 2008;23(1):8‐13. [DOI] [PubMed] [Google Scholar]
Chan 2009 {published data only}
- Chan SW, Yip B, Tso S, Cheng BS, Tam W. Evaluation of a psychoeducation program for Chinese clients with schizophrenia and their family caregivers. Patient Education and Counseling 2009;75(1):67‐76. [DOI] [PubMed] [Google Scholar]
Chandler 2000 {published data only}
- Chandler D, Quinlivan R. Social skills training modules in an intensive community support program. Administration and Policy in Mental Health 2000;27(4):211‐20. [DOI] [PubMed] [Google Scholar]
Choi 2006 {published data only}
- Choi KH, Kwon JH. Social cognition enhancement training for schizophrenia: a preliminary randomized controlled trial. Community Mental Health Journal 2006;42(2):177‐87. [DOI] [PubMed] [Google Scholar]
Cormier 1995 {published data only}
- Cormier H, Leblanc G, Picher F, Lachance L. A French‐Canadian trial of the symptom and medication modules. Proceedings of the150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, California, USA. 1997.
- Cormier H, Leblanc G, Picher F, Lachance L. Results of a clinical trial of the symptom and medication modules in schizophrenia. Schizophrenia Research 1995;15(1, 2):146. [CAJ: MEDI0212] [Google Scholar]
Daniels 1998 {published data only}
- Daniels L. A group cognitive‐behavioural and process‐oriented approach to treating the social impairment and negative symptoms associated with chronic mental illness. Journal of Psychotherapy Practice and Research 1998;7:167‐76. [CAJ: MEDI0212] [PMC free article] [PubMed] [Google Scholar]
DeCarlo 1985 {published data only}
- DeCarlo JJ, Mann WC. The effectiveness of verbal versus activity groups in improving self perceptions of interpersonal communication skills. American Journal of Occupational Therapy 1985;39(1):20‐7. [ 72‐31756] [DOI] [PubMed] [Google Scholar]
Deng 2007 {published data only}
- Deng H‐Y, Shao Y‐F, Xu X‐P. The effect of comprehensive social skills training on chronic inpatients of schizophrenia. Chinese Journal of Behavioral Medical Science 2007;16(3):227‐9. [Google Scholar]
Denicola 1980 {published data only}
- Denicola J. The Effects of Method of Presentation on Acquisition of Job Interview Skills in Schizophrenic Patients. University of South Florida. Florida, USA, 1980.
Dincin 1982 {published data only}
- Dincin J, Witheridge TF. Psychiatric rehabilitation as a deterrent to recidivism. Hospital and Community Psychiatry 1982;33(8):645‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eisler 1978 {published data only}
- Eisler RM, Blanchard EB, Fitts H, Williams JG. Social skill training with and without modeling for schizophrenic and non psychotic hospitalized psychiatric patients. Behavior Modification 1978;2(2):147‐72. [CAJ: MEDI0306] [Google Scholar]
Falloon 1977 {published data only}
- Falloon IR, Lindley P, McDonald R, Marks IM. Social skills training of out‐patient groups: a controlled study of rehearsal and homework. British Journal of Psychiatry 1977;131:599‐609. [PsycINFO: 60‐09769] [DOI] [PubMed] [Google Scholar]
Finch 1977 {published data only}
- Finch BE, Wallace CJ. Successful interpersonal skills training with schizophrenic inpatients. Journal of Consulting and Clinical Psychology 1977;45(5):885‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fiorillo 2004 {published data only}
- Fiorillo A, Magliano L, Malangone C, Rosa C, Maj M, The Working Group. Psychoedutraining study: the Italian experience. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
Foxx 1985 {published data only}
- Foxx RM, McMorrow MJ, Bittle RG, Fenlon SJ. Teaching social skills to psychiatric inpatients. Behaviour Research and Therapy 1985;23(5):531‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fuentes 2007 {published data only}
- Fuentes I, Garcia S, Ruiz JC, Soler MJ, Roder V. Social perception training in schizophrenia: A pilot study. International Journal of Psychology and Psychological Therapy 2007;7(1):1‐12. [Google Scholar]
Galderisi 2009 {published data only}
- Galderisi S, Piegari G, Mucci A, Acerra A, Luciano L, Rabasca AF, et al. Social skills and neurocognitive individualized training in schizophrenia: comparison with structured leisure activities. European Archives of Psychiatry and Clinical Neuroscience 2009;206(4):305‐15. [DOI: 10.1007/s00406-009-0078-1] [DOI] [PubMed] [Google Scholar]
Gao 2006 {published data only}
- Gao H, Zhang Z, Wang H, Bao Z. A controlled clinical trial of social skill training for inpatients with chronic schizophrenia. Medical Journal of Chinese People's Health 2006; Vol. 18, issue 3:225‐7.
Glynn 1999 {published data only}
- Glynn SM, Marder SR, Liberman RP, Blair K, Ross D, Mintz J, et al. Community skills training increases benefits accruing from clinic‐based behavioural psychiatric rehabilitation conference abstract. Schizophrenia Research 1999;1, 2 & 3:325. [Google Scholar]
- Glynn SM, Marder SR, Liberman RP, Blair K, Wirshing WC, Wirshing DA, et al. Supplementing clinic based skills training for schizophrenia with manualized community support: nine month follow‐up effects on social adjustment. Schizophrenia Research 2001;49(1, 2):261. [Google Scholar]
- Glynn SM, Marder SR, Liberman RP, Blair K, Wirshing WC, Wirshing DA, et al. Supplementing clinic‐based skills training with manual‐based community support sessions: Effects on social adjustment of patients with schizophrenia. American Journal of Psychiatry 2002;159:829‐37. [DOI] [PubMed] [Google Scholar]
- Glynn SM, Marder SR, Mintz J, Liberman RP, Blair K, Ross D. Patient‐family contact may improve outcomes of behaviorally‐based rehabilitation programs. Schizophrenia Research 2003;60(1):323. [Google Scholar]
Granholm 2002 {published data only}
- Granholm E, McQuaid JR, Auslander LA, McClure FS. Group cognitive‐behavioral social skills training for older outpatients with chronic schizophrenia. Journal of Cognitive Psychotherapy 2004;18(3):265‐79. [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle‐aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry 2005;162(3):520‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Pedrelli P, Jeste DV. A randomized controlled pilot study of cognitive behavioral social skills training for older patients with schizophrenia. Schizophrenia Research 2002;53(1‐2):167‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gudeman 1981 {published data only}
- Gudeman JE, Dickey B, Rood L, Hellman S, Grinspoon L. Alternative to the back ward: the quarterway house. Hospital and Community Psychiatry 1981;32(5):330‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hayes 1991 {published data only}
- Hayes RL, Halford WK, Varghese FN. Generalization of the effects of activity therapy and social skills training on the social behavior of low functioning schizophrenic patients. Occupational Therapy in Mental Health 1991;11(4):3‐20. [EMBASE: 1992096139] [Google Scholar]
Hogarty 1973 {published data only}
- Goldberg SC, Schooler NR, Hogarty GE, Roper M. Prediction of relapse in schizophrenic outpatients treated by drug and sociotherapy. Archives of General Psychiatry 1977;34(2):171‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC. Drug and sociotherapy in the aftercare of schizophrenic patients. One‐year relapse rates. Archives of General Psychiatry 1973;28(1):54‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR. Drug and sociotherapy in the aftercare of schizophrenic patients. III. Adjustment of nonrelapsed patients. Archives of General Psychiatry 1974;31(5):609‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Goldberg SC, Schooler NR, Ulrich RF. Drug and sociotherapy in the aftercare of schizophrenic patients. II. Two‐year relapse rates. Archives of General Psychiatry 1974;31(5):603‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Munetz MR. Pharmacogenic depression among outpatient schizophrenic patients: a failure to substantiate. Journal of Clinical Psychopharmacology 1984;4(1):17‐24. [MEDLINE: ] [PubMed] [Google Scholar]
- Hogarty GE, Ulrich RF. Temporal effects of drug and placebo in delaying relapse in schizophrenic outpatients. Archives of General Psychiatry 1977;34(3):297‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hogarty 2001 {published data only}
- Hogarty GE. Environmental‐personal treatment of schizophrenia. CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html ) (accessed 19 February 2001). [CRISP: 5R01MH30750‐24]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue‐Geile M, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2‐year randomized trial on cognition and behavior. Archives of General Psychiatry 2004;61(9):866‐76. [EMBASE: 2004382709; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Horan 2009 {published data only}
- Horan WP, Kern RS, Shokat‐Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophrenia Research 2009;107(1):47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffe 1976 {published data only}
- Jaffe PG, Carlson PM. Relative efficacy of modeling and instructions in eliciting social behavior from chronic psychiatric patients. Journal of Consulting and Clinical Psychology 1976;44:200‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jenner 2004 {published data only}
- Jenner JA, Nienhuis F, Wiersma D, Willige G. Integrative treatment significantly improves the burden of illness and controls symptoms in drug‐resistant schizophrenia. Schizophrenia Research 2002;53(3 Suppl 1):211. [Google Scholar]
- Jenner JA, Nienhuis FJ, Wiersma D, Willige G. Hallucination focused integrative treatment: a randomized controlled trial. Schizophrenia Bulletin 2004;30(1):133‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jenner JA, Willige G, Nienhuis F, Wiersma D. Hallucination focused integrative treatment induces statistically significant subjective and objective improvements in chronic schizophrenia patients with persistent voices. Schizophrenia Research 2004;67(1):145‐6. [ISI: 000188788100347] [Google Scholar]
Jeppesen 2001 {published data only}
- Jeppesen P, Nordentoft M, Abel M, Hemmingsen RP, Joergensen, Kassow P. Opus‐project: a RCT of integrated psychiatric treatment for recentonset psychotic patients. Schizophrenia Research 2001;49(1, 2):262. [Google Scholar]
- Jeppesen P, Petersen L, Thorup A, Abel MB, Oehlenschlaeger J, Christensen TØ, et al. Integrated treatment of first‐episode psychosis: effect of treatment on family burden: OPUS trial. British Journal of Psychiatry 2005;48(Suppl):S85‐90. [DOI] [PubMed] [Google Scholar]
Jerrell 1996 {published data only}
- Jerrell JM. Toward cost‐effective care for persons with dual diagnoses. Journal of Mental Health Administration 1996;23:329‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jerrell JM, Wilson JL. The utility of dual diagnosis services for consumers from nonwhite ethnic groups. Psychiatric Services 1996;47(11):1256‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kern 2005 {published data only}
- Kern RS, Green MF, Mitchell S, Kopelowicz A, Mintz J, Liberman RP. Extensions of errorless learning for social problem‐solving deficits in schizophrenia. American Journal of Psychiatry 2005;162(3):513‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 1997 {published data only}
- Kim C, Kang DH, Jang JH, Cho JS, Youn S, Shim KS. Two‐year effects of psychosocial treatment on relapse of chronic schizophrenia. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, California, USA. 1997.
Lecomte 1999 {published data only}
- Lecomte T, Cyr M, Lesage AD, Wilde J, Leclerc C, Ricard N. Efficacy of a self‐esteem module in the empowerment of individuals with schizophrenia. Journal of Nervous and Mental Disease 1999;187(7):406‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 1994 {published data only}
- Lee S, Yeh MY, Hung TM. Effects of social skill training on improving expression and communication of hospitalized psychotic patients. Nursing Research 1994;2(2):166‐79. [CINAHL: 1995038594] [Google Scholar]
Lehman 2002 {published data only}
- Lehman AF. Improving employment outcomes for persons with severe mental illness. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Lehman AF, Goldberg R, Dixon LB, McNary S, Postrado L, Hackman A, et al. Improving employment outcomes for persons with severe mental illnesses. Archives of General Psychiatry 2002;59(2):165‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li L, Wu F, Mai G, Zhu F. Effect of comprehensive rehabilitation training for the rehabilitation of adolescent schizophrenia patients. Modern Clinical Nursing 2008; Vol. 7, issue 1:9‐24.
Li 2008a {published data only}
- Li M, Wu D, Li F. Effect of social skills training on social functioning of patients with chronic schizophrenia. Modern Clinical Nursing 2008; Vol. 7, issue 2:4‐6.
Liang 2006 {published data only}
- Liang J, Ling L. Observation on effective outcome of social rehabilitation in the treatment of schizophrenic hospitalized long time. Journal of Qilu Nursing 2006; Vol. 12, issue 2A:199‐200.
Liberman 1981 {published data only}
- Liberman RP, Mueser KT, Wallace CJ. Social skills training for schizophrenic individuals at risk for relapse. American Journal of Psychiatry 1986;143:523‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liberman RP, Wallace CJ, Falloon IR, Vaughn CE. Interpersonal problem solving therapy for schizophrenics and their families. Comprehensive Psychiatry 1981;22(6):627‐30. [MEDLINE: ; PsycINFO: 68‐03930] [DOI] [PubMed] [Google Scholar]
- Lukoff D, Wallace CJ, Liberman RP, Burke K. A holistic program for chronic schizophrenic patients. Schizophrenia Bulletin 1986;12(2):274‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallace CJ, Liberman RP. Social skills training for patients with schizophrenia: a controlled clinical trial. Psychiatry Research 1985;15(3):239‐47. [MEDLINE: ; PsycINFO: 73‐20548] [DOI] [PubMed] [Google Scholar]
Liberman 1998 {published data only}
- Liberman RP, Wallace CJ, Blackwell G, Kopelowicz A, Vaccaro JV, Mintz J. Skills training versus psychosocial occupational therapy for persons with persistent schizophrenia. American Journal of Psychiatry 1998;155(8):1087‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liberman 2002 {published data only}
- Liberman RP, Glynn S, Blair KE, Ross D, Marder SR. In vivo amplified skills training: promoting generalization of independent living skills for clients with schizophrenia. Psychiatry 2002;65(2):137‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lindsay 1980 {published data only}
- Lindsay WR. The training and generalization of conversation behaviours in psychiatric in‐patients: a controlled study employing multiple measures across settings. British Journal of Social and Clinical Psychology 1980;19:85‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Linszen 1994 {published data only}
- Linszen D, Dingemans P, Lenior M. Early intervention and a five year follow up in young adults with a short duration of untreated psychosis: ethical implications. Schizophrenia Research 2001;51(1):55‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Linszen D, Dingemans P, Lenior M, Scholte W. Early individual and family intervention in schizophrenia. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid, Spain. 1996.
- Linszen D, Dingemans P, Does JW, Nugter A, Scholte P, Lenior R, et al. Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychological Medicine 1996;26(2):333‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Linszen D, Lenior M, Haan L, Dingemans P, Gersons B. Early intervention, untreated psychosis and the course of early schizophrenia. British Journal of Psychiatry Supplementum 1998;172(33):84‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME, Nugter MA, Scholte WF, Does AJ. Relapse criteria in schizophrenic disorders: different perspectives. Psychiatry Research 1994;54(3):273‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nugter A, Dingemans P, Does JW, Linszen D, Gersons B. Family treatment, expressed emotion and relapse in recent onset schizophrenia. Psychiatry Research 1997;72(1):23‐31. [MEDLINE: ; PsycINFO: 1997‐41313‐004] [DOI] [PubMed] [Google Scholar]
Lu 2003 {published data only}
- Lu L, Ma S, Weng Y, Cui R. Evaluating the effectiveness of hospitalized occupational rehabilitation on social functioning in chronic schizophrenic patients. Modern Rehabilitation 2003;7(1):158‐9. [CAJ: MEDI0307] [Google Scholar]
Lundin 1990 {published data only}
- Lundin L, Dencker SJ, Malm U. Community based rehabilitation of schizophrenia. Nordisk Psykiatrisk Tidsskrift 1990;44(1):81‐7. [PsycINFO: 77‐26061] [Google Scholar]
Malm 2003 {published data only}
- Malm U, Ivarsson B, Allebeck P, Falloon IR. Integrated care in schizophrenia: a 2‐year randomized controlled study of two community‐based treatment programs. Acta Psychiatrica Scandinavica 2003;107(6):415‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mausbach 2008 {published data only}
- Mausbach BT, Bucardo J, McKibbin CL, Goldman SR, Jeste DV, Patterson TL, et al. Evaluation of a culturally tailored skills intervention for Latinos with persistent psychotic disorders. American Journal of Psychiatric Rehabilitation 2008;11(1):61‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
May 1985 {published data only}
- May HJ, Gazda GM, Powell M, Hauser G. Life skill training: psychoeducational training as mental health treatment. Journal of Clinical Psychology 1985;41:359‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McLatchie 1981 {published data only}
- McLatchie L. Interpersonal Problem‐Solving Group Therapy an Evaluation of a Potential Method of Social Skills Training for the Chronic Psychiatric Patient. University of Pennsylvania State. Pennsylvania, USA, 1981.
Medalia 2000 {published data only}
- Medalia A, Revheim N, Casey M. Remediation of problem‐solving skills in schizophrenia: evidence of a persistent effect. Schizophrenia Research 2002;57(2‐3):165‐71. [EMBASE: 2002386495] [DOI] [PubMed] [Google Scholar]
- Medalia A, Revheim N, Casey M. The remediation of problem‐solving skills in schizophrenia. Schizophrenia Bulletin 2001;27(2):259‐67. [PsycINFO: 2001‐17483‐009] [DOI] [PubMed] [Google Scholar]
Moriana 2006 {published data only}
- Moriana JA, Alarcon E, Herruzo J. In‐home psychosocial skills training for patients with schizophrenia. Psychiatric Services 2006;57(2):260‐2. [DOI] [PubMed] [Google Scholar]
Mueller 2005 {published data only}
- Mueller DR, Roder V, Zorn P. Advantages of work ‐ related social skills training in comparison to unspecific social skills training in vocational rehabilitation of schizophrenia outpatients: a randomized controlled trial. Schizophrenia Bulletin 2005;31:529‐30. [Google Scholar]
Mueser 2001 {published data only}
- Mueser KT, Becker DR, Wolfe R. Supported employment, job preferences, job tenure and satisfaction. Journal of Mental Health 2001;10(4):411‐7. [MEDLINE: ] [Google Scholar]
Munjiza 1999 {published data only}
- Munjiza M, Mandi D, Kuzmanovi S, Vidojevi P, Lapevi D, Ljubomirovic N. The comparative analysis of group psychotherapy and pharmacotherapy of schizophrenia. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999; Vol. 2.
Munroe 1995 {published data only}
- Munroe‐Blum H, McCleary L. Predicting compliance with social treatments for schizophrenia. Schizophrenia Research 1995;15(1, 2):220. [Google Scholar]
- Munroe‐Blum H, McCleary L. RCT: social treatment for schizophrenia, 12 month findings. Schizophrenia Research 1995;15(1, 2):221. [Google Scholar]
NCT00071591 2003 {published data only}
- NCT00071591. Functional skills training for late life schizophrenia. http://www.clinicaltrials.gov 2003.
Ni 2008 {published data only}
- Ni Y, Jin Y. Social skills training for the rehabilitation of inpatient schizophrenia. Shanghai Nursing 2008;8(3):25‐7. [Google Scholar]
Nordentoft 1999 {published data only}
- Jørgensen P, Nordentoft M, Abel MB, Gouliaev G, Jeppesen P, Kassow P. Early detection and assertive community treatment of young psychotics: the Opus study rationale and design of the trial. Social Psychiatry and Psychiatric Epidemiology 2000;35(7):283‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Melau M, Bertelsen M, Jeppesen P, Krarup G, Nordentoft M, Thorup A, et al. A randomised clinical trial of the effect of five‐years versus two‐years specialised assertive intervention for first episode psychosis ‐ the opus‐II trial. Schizophr Res 2010; Vol. 117, issue 2‐3:526. [DOI] [PMC free article] [PubMed]
- Melau M, Jeppesen P, Thorup A, Bertelsen M, Petersen L, Gluud C, et al. The effect of five years versus two years of specialised assertive intervention for first episode psychosis ‐ opus ii: Study protocol for a randomized controlled trial. Trials 2011; Vol. 12:72. [DOI] [PMC free article] [PubMed]
- Melau M, Thorup A, Bertelsen M, Jeppesen P, Krarup G, Nordentoft M. Does extended specialized intervention for patients with first episode psychosis improve outcome in the critical period.The opus ii trial. Schizophr Bull 2011; Vol. 37:315.
- Nordentoft M, Bertelsen M, Albert N, Jeppesen P, Petersen L, Thorup A, et al. The opus trial: A randomized multicentre single‐blinded trial of specialized assertive early intervention (opus treatment) versus standard treatment for patients with a first episode of psychotic illness ‐ five‐year follow‐up. Early intervention in psychiatry 2010; Vol. 4, issue Suppl 1:24.
- Nordentoft M, Jeppesen P, Abel M, Kassow P, Petersen L, Thorup A, et al. OPUS study: suicidal behaviour, suicidal ideation and hopelessness among patients with first‐episode psychosis. One‐year follow‐up of a randomised controlled trial. British Journal of Psychiatry Supplementum 2002;181(Suppl 43):S98‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nordentoft M, Jeppesen P, Abel M, Petersen L, Thorup A, Christensen T, et al. Opus‐project: a randomised controlled trial of integrated psychiatric treatment in first‐episode psychosis ‐ clinical outcome improved. Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25‐28; Copenhagen, Denmark. 2002.
- Nordentoft M, Jeppesen P, Jørgensen P, Abel MB, Kassow P, Reisby N, et al. Opus ‐ project : a randomised controlled trial of first episode psychotic patients better compliance. Proceedings of the 2nd International Conference on Early Psychosis; 2000 Mar 31 ‐ Apr 2; New York, New York, USA. 2000.
- Nordentoft M, Jeppesen P, Kassow P, Abel M, Petersen L, Thorup A, et al. Opus‐project: a randomised controlled trial of integrated psychiatric treatment in first‐episode psychosis‐clinical outcome improved. Schizophrenia Research 2002;53(3 Suppl 1):51. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M, Ohlenschlaeger JK, et al. OPUS project: A randomised controlled trial of integrated psychiatric treatment in first episode psychosis. Schizophrenia Research 2003;60(1):297. [Google Scholar]
- Nordentoft M, Jeppesen P, Petersen L, Thorup a, Krarup G, Abel M, et al. The Danish opus‐trial: a randomised controlled trial of integrated treatment among 547 first‐episode psychotic patients. One and two years follow‐up. Schizophrenia Research 2004;67(1):35‐6. [ISI: 000188788100081] [Google Scholar]
- Nordentoft M, Jeppesen P, Ventegodt AT, Joergensen P, Abel M, Petersen L, et al. Opus‐project: a randomised controlled trial of first episode psychotic patients: patient satisfaction, depression and suicidal behaviour. Schizophrenia Research 2001;49(1, 2):265. [Google Scholar]
- Nordentoft M, Melau M, Jeppesen P, Petersen L, Thorup A, Ohlenschlager J, et al. The OPUS ‐ trial; a randomised single‐blinded trial of integrated versus standard treatment for patients with a first episode of psychotic illness ‐ results of five‐years follow‐up and presentation of a new trial. Schizophr Res 2010; Vol. 117, issue 2‐3:116.
- Nordentoft M, Reisby N, Jeppesen P, Abel M‐B, Kassow P, Jírgensen P. Opus‐project: differences in treatment outcome of a randomised controlled trial of integrated psychiatric treatment of first‐episode psychotic patients. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999; Vol. 2:165.
- Nordentoft M, Secher G, Bertelsen M, Thorup A, Austin S, Albert N, et al. Opus: Concept and recent findings. European archives of psychiatry and clinical neuroscience 2011; Vol. 261:S37‐S8.
- Secher RG, Austin SF, Ole Mors NP, Nordentoft M. The opus‐trial: Intensive, early, psycho‐social intervention versus treatment as usual for first‐episode psychosis patients. Results from the 10‐year follow‐up. European archives of psychiatry and clinical neuroscience 2011; Vol. 261:S59.
- Thorup A. Gender differences in first‐episode psychosis at five‐year followup ‐ results from the danish opus study gender differences have been found. Early intervention in psychiatry 2010; Vol. 4, issue Suppl 1:53.
- Thorup A, Nordentoft M, Petersen L, Oehlensschlaeger J, Abel M, Jeppesen P, et al. The Danish OPUS‐project: psychopathology and gender differences in first episode psychotic patients. Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25‐28; Copenhagen, Denmark. 2002.
- Thorup A, Petersen L, Jeppesen P, Nordentoft M. The quality of life among first‐episode psychotic patients in the opus trial. Schizophr Res. Netherlands, 2010; Vol. 116, issue 1:27‐34. [MEDLINE: ] [DOI] [PubMed]
Norman 2002 {published data only}
- Norman RM, Malla AK, McLean TS, McIntosh EM, Neufeld RW, Voruganti LP, et al. An evaluation of a stress management program for individuals with schizophrenia. Schizophrenia Research 2002;58(2‐3):293‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nuechterlein 2005 {published data only}
- Nuechterlein KH, Subotnik KL, Ventura J, Gitlin MJ, Green MF, Wallace CJ, et al. Advances in improving and predicting work outcome in recent ‐ onset schizophrenia. Schizophrenia Bulletin 2005;31:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuttbrock 1997 {published data only}
- Nuttbrock L, Rahav M, Rivera J, Ng‐Mak D, Struening E. Mentally ill chemical abusers in residential treatment programs: effects of psychopathology on levels of functioning. Journal of Substance Abuse Treatment 1997;14:269‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Keefe 2003 {published data only}
- O'Keefe C, Bailey EL, Edney M, Noordsy DL, Glynn SM, Marder SR. Helping persons with schizophrenia stay employed: challenges to implementing the workplace fundamentals module in the clinic. Schizophrenia Research 2003;60(1):327. [Google Scholar]
Odhner 1970 {published data only}
- Odhner F. A study of group tasks as facilitators of verbalization among hospitalized schizophrenic patients. American Journal of Occupational Therapy 1970;24:7‐11. [PsycINFO: 1993‐98432‐005] [PubMed] [Google Scholar]
Oei 1981 {published data only}
- Oei TP, Tan ES. Companion program by university students and behavioral change in female chronic schizophrenics. Journal of Clinical Psychology 1981;37:96‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patterson 2005 {published data only}
- Patterson TL, Bucardo J, McKibbin CL, Mausbach BT, Moore D, Barrio C, et al. Development and pilot testing of a new psychosocial intervention for older Latinos with chronic psychosis. Schizophrenia Bulletin 2005;31(4):922‐30. [DOI] [PubMed] [Google Scholar]
Patterson 2006a {published data only}
- Patterson TL, Mausbach BT, McKibbin C, Goldman S, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a randomized trial of a psychosocial intervention for middle‐aged and older patients with chronic psychotic disorders. Schizophrenia Research 2006;86(1‐3):291‐9. [DOI] [PubMed] [Google Scholar]
Paul 1977 {published data only}
- Paul GL, Lentz RJ. Psychosocial Treatment of Chronic Mental Patients: Milieu versus Social‐learning Programmes. Harvard University Press. Cambridge, USA, 1977. [EMBASE: 2000260213]
Petersen 2005 {published data only}
- Petersen L, Jeppesen P, Thorup A, Abel MB, Ohlenschlaeger J, Christensen TO, et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ 2005;331(7517):602‐8. [CINAHL: 2009245707; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Nordentoft M, Jeppesen P, Ohlenschaeger J, Thorup A, Christensen TO, et al. Improving 1‐year outcome in first‐episode psychosis: OPUS trial. British Journal of Psychiatry 2005;48(Suppl):S98‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Petersen L, Nordentoft M, Thorup A, Oehlenschlaeger J, Jeppesen P, Christensen T, et al. The opus trial: a randomised multi ‐ centre trial of integrated versus standard treatment for 547 first ‐ episode psychotic patients. Schizophrenia Bulletin 2005;31:531. [Google Scholar]
Prentice 2003 {published data only}
- Prentice KJ, Bellack AS, Gold JM, Carpenter WT. Improving decisional capacity for informed consent in schizophrenia. Schizophrenia Research 2003;60(1):327‐8. [MEDLINE: ] [Google Scholar]
Razali 2000 {published data only}
- Razali SM, Hasanah CI, Khan UA, Subramaniam M. Psychosocial interventions for schizophrenia. Journal of Mental Health 2000;9(3):283‐9. [EMBASE: 2000260213] [Google Scholar]
Rosenbaum 2005 {published data only}
- Rosenbaum B, Valbak K, Harder S, Knudsen P, Koster A, Lajer M, et al. The Danish national schizophrenia project: prospective, comparative longitudinal treatment study of first‐episode psychosis. British Journal of Psychiatry 2005;186:394‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossotto 2003 {published data only}
- Rossotto E, Wirshing D, Wirshing W, Boyd J, Liberman R, Marder S. Reducing rehospitalization rates for patients with schizophrenia: The community re‐entry supplemental intervention. Schizophrenia Research 2003;60(1):328. [Google Scholar]
Smith 1999 {published data only}
- Smith TE, Hull JW, Romanelli S, Fertuck E, Weiss KA. Symptoms and neurocognition as rate limiters in skills training for psychotic patients. American Journal of Psychiatry 1999;156(11):1817‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tang 2007 {published data only}
- Tang H, Fang C, Zhu H. Impact of skills training program on soical function of patients with schizophrenia in the community. Shanghai Nursing 2007; Vol. 7, issue 6:14‐6.
Tarrier 2000 {published data only}
- Tarrier N. A psychological intervention programme to reduce positive symptoms and prevent relapse in psychotic patients. National Research Register 2000. [National Research Register: N0226003215]
Test 1989 {published data only}
- Cohen LJ, Test MA, Brown RL. Suicide and schizophrenia: data from a prospective community treatment study. American Journal of Psychiatry 1990;147:602‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Test MA, Knoedler W, Allness D, Burke S, Brown R, Wallisch L. Community care of schizophrenia: two year findings. Proceedings of the 142nd Annual Meeting of the American Psychiatric Association; 1989 May 6‐11; San Francisco, California, USA 1989;3:1‐16. [Google Scholar]
- Test MA, Knoedler WH, Allness DJ, Burke SS, Brown RL, Wallisch LS. Long‐term community care through an assertive continuous treatment team. Advances in neuropsychiatry and psychopharmacology: Schizophrenia Research 1991;1:239‐46. [PsycINFO: 91‐032024‐023] [Google Scholar]
Torres 2002 {published data only}
- Torres A, Mendez LP, Merino H, Moran EA. Improving social functioning in schizophrenia by playing the train game. Psychiatric Services 2002;53(7):799‐801. [DOI] [PubMed] [Google Scholar]
Tsang 2009 {published data only}
- Tsang HWH, Chan A, Wong A, Liberman RP. Vocational outcomes of an integrated supported employment program for individuals with persistent and severe mental illness. Journal of Behavior Therapy and Experimental Psychiatry 2009;40(2):292‐305. [DOI] [PubMed] [Google Scholar]
Urioste 2005 {published data only}
- Urioste R, Lasser R, Gharabawi GM, Jarboe K, Litrell K, Miller AL, et al. Patient acceptance and long‐acting risperidone: start program, gain approach. Proceedings of the 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, Georgia, USA. 2005.
Vauth 2005 {published data only}
- Vauth R, Corrigan PW, Clauss M, Dietl M, Dreher‐Rudolph M, Stieglitz R‐D, et al. Cognitive strategies versus self‐management skills as adjunct to vocational rehabilitation. Schizophrenia Bulletin 2005;31:55‐66. [DOI] [PubMed] [Google Scholar]
Ventegodt 2001 {published data only}
- Ventegodt AT, Jeppesen P, Petersen L, Abel M, Nordentoft M, Kassow P, et al. Opus‐project: a randomised controlled trial of first episode psychotic patients: gender differences, social network and negative symptoms. Schizophrenia Research 2001;49(1, 2):267. [Google Scholar]
Walker 1969 {published data only}
- Walker R, Winick W, Frost ES, Lieberman JM. Social restoration of hospitalised psychiatric patients through a program of special employment in industry. Rehabilitation Literature 1969;30:297‐303. [MEDLINE: ] [PubMed] [Google Scholar]
Weinman 1974 {published data only}
- Weinman B, Kleiner R, Yu JH, Tillson VA. Social treatment of the chronic psychotic patient in the community. Journal of Community Psychology 1974;2(4):358‐65. [PsycINFO: 53‐10283] [Google Scholar]
Weng 2005 {published data only}
- Weng Y‐Z, Xiang Y‐Q, Liberman RP. Psychiatric rehabilitation in a Chinese psychiatric hospital. Psychiatric Services 2005;56(4):401‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Whetstone 1986 {published data only}
- Whetstone WR. Social dramatics: social skills development for the chronically mentally ill. Journal of Advanced Nursing 1986;11(1):67‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wiersma 2004 {published data only}
- Wiersma D, Jenner JA, Nienhuis FJ, Willige G. Hallucination focused integrative treatment improves quality of life in schizophrenia patients. Acta Psychiatrica Scandinavica 2004;109(3):194‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wirshing 1991 {published data only}
- Wirshing WC, Eckman T, Liberman RP, Marder SR. Management of risk of relapse through skills training of chronic schizophrenics. In: Tamminga CA, Schulz SC editor(s). Schizophrenia Research: Advances in neuropsychiatry and psychopharmacology. Vol. 1, New York, USA: Raven Press, 1991:255‐67. [Google Scholar]
Xiang 2002 {published data only}
- Xiang Y, Li W, Weng Y, Hou Y, Gao L, Chen G. The influence of skills training on social function of outpatients with schizophrenia. Chinese Journal of Clinical Rehabilitation 2002; Vol. 6, issue 23:3484‐5.
Xiang 2007 {published data only}
- Xiang YT, Weng YZ, Li WY, Gao L, Chen GL, Xie L, et al. Efficacy of the community re‐entry module for patients with schizophrenia in Beijing, China: outcome at 2‐year follow‐up. British Journal of Psychiatry 2007;190(1):49‐56. [DOI] [PubMed] [Google Scholar]
Xu 2008 {published data only}
- Xu W, Yuan G, Zhang Z, Zhang F, Zha Z, Wu E, et al. A study on social skills training for patients with chronic schizophrenia. Chinese Journal of Rehabilitaion Medicine 2008; Vol. 23, issue 3:241‐4.
Yang 2003 {published data only}
- Yang DN, Yue SY, Li SG. The improving effects of social life simulated training of male schizophrenia patients on their hospital‐dependent behaviors. Journal of Practical Nursing 2003;19(8):62‐3. [CAJ: MEDI0309] [Google Scholar]
Yue 1998 {published data only}
- Yue S, Xu L, Zhao X. Study on enhancement of the ability to control the impulsive action in female patients with psychosis. Shanxi Nursing Journal 1998;12(1):25‐6. [EMBASE: 1999051372] [Google Scholar]
Zhang 2002 {published data only}
- Zhang Y, Zhang M, Lv X. Effect of psychosocial intervention on social functioning of patients with mental illnesses. Chinese Journal of Clinical Rehabilitation 2002; Vol. 6, issue 15:2274‐5.
Zheng 2006 {published data only}
- Zheng F, Wang Y, Zhang D. Effects of living skill training on recovery of the social skill in psychiatric patients. Journal of Xinxiang Medical College 2006; Vol. 23, issue 5:481‐3.
References to studies awaiting assessment
Bellack 1986 {published data only}
- Bellack AS. Social skills training in the treatment of chronic schizophrenics [Das training sozialer fertigkeiten zur behandlung chronisch schizophrener]. In: Boeker W, Brenner HD editor(s). Bewaltigung der Schizophrenie. Bern, Switzerland: Verlag Hans Huber, 1986:121. [PSYNDEX 0020596] [Google Scholar]
Piegari 2011 {published data only}
- Piegari G, Galderisi S, Bucci P, Mucci A, Riso F, Campana T, et al. Social skills vs. Neurocognitive training in psychotic patients. European psychiatry 2011; Vol. 26, issue Suppl. 1:1476.
卢春爱 2010 {published data only}
- 卢春爱, 黄金茹, 吕申敏, 张月卿, 赵宝珠, 冯方. schizophrenic patients of social skills training integrated [Google Translate] [精神分裂症患者社交综合技能训练分析]. Chinese Journal of Misdiagnostics [中国误诊学杂志] 2010; Vol. 10, issue 14:3315.
唐仕友 2010 {published data only}
- 唐仕友, 许波, 吕天才, 程伟. Social skills training on social function, and the reemployment rate of patients with schizophrenia [社会技能训练对精神分裂症患者社会功能及再就业率的影响]. Guide of Chinese Medicine [中国医药指南] 2010; Vol. 8, issue 25:62‐3.
康建华 2010 {published data only}
- 康建华, 张帆. social skills training on the efficacy of chronic schizophrenia patients [Google Translate] [社会技能训练对慢性精神分裂症病人的疗效观察]. Modern Preventive Medicine 2010; Vol. 37, issue 8:1488‐90, 1506.
樊献丽 2011 {published data only}
- 樊献丽, 焦太林, 刘世兴. Social skills training in the rehabilitation of schizophrenic patients [社交技能训练在住院精神分裂症病人康复中的应用]. Shanxi Nursing Journal [Shanxi Nursing Journal; 护理研究] 2011; Vol. 25, issue 7B:1810‐1.
蒋德政 2010 {published data only}
- 蒋德政, 易崇贵, 王世荣, 刘会, 汪波. Social Skills Training on cognitive function in schizophrenia [Google Translate] [社会技能训练模式对精神分裂症认知功能的影响]. 中国当代医药 2010; Vol. 17, issue 5:11‐2.
陈世珍 2010 {published data only}
- 陈世珍, 蔡卓珊. Social skills training for the rehabilitation of schizophrenic patients the impact of social adaptability [Google Translate] [社会技能训练对康复期精神分裂症患者社会适应能力的影响]. Hainan Medical Journal [海南医学] 2010; Vol. 21, issue 11:52‐3.
References to ongoing studies
NCT00069433 2003 {published data only}
- NCT00069433. Skills training for schizophrenia: enhancing outcomes. http://www.clinicaltrials.gov 2003.
NCT00183625 2005 {published data only}
- NCT00183625. The effectiveness of supplementing supported employment with behavioral skills training. http://www.clinicaltrials.gov 2005.
NCT00237796 2005 {published data only}
- NCT00237796. Functional rehabilitation of older patients with schizophrenia. http://www.clinicaltrials.gov 2005.
NCT00338975 2006 {published data only}
- NCT00338975. Cognitive behavioral skills training for schizophrenia. http://www.clinicaltrials.gov 2006.
NCT00391677 2006 {published data only}
- NCT00391677. Effectiveness trial of attention shaping for schizophrenia. http://www.clinicaltrials.gov 2006.
NCT00601224 2008 {published data only}
- NCT00601224. Social cognition and interaction training for improving social functioning in people with schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00791882 2008 {published data only}
- NCT00791882. Social skills training in refractory schizophrenia. http://www.clinicaltrials.gov 2008.
Pratt 2008 {published data only}
- Pratt SI, Bartels SJ, Mueser KJ, Forester B. Helping older people experience success: an integrated model of psychosocial rehabilitation and health care management for older adults with serious mental illness. American Journal of Psychiatric Rehabilitation 2008;11(1):41‐60. [Google Scholar]
Additional references
Affleck 1984
- Affleck JW, McGuire RJ. The measurement of psychiatric rehabilitation status: a review of the needs and a new scale. British Journal of Psychiatry 1984;145:517‐25. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecing skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1983
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa: University of Iowa, 1983. [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Bellack 1990
- Bellack. An analysis of social competence in schizophrenia. British Journal of Psychiatry 1990;156:809‐16. [DOI] [PubMed] [Google Scholar]
Birchwood 1990
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The social functioning scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry 1990;157:853‐9. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Brenner 1994
- Brenner HD, Roder V, Hodel B, Kienzle N, Reed D, Liberman RP. Integrated Psychological Therapy For Schizophrenic Patients. Seattle, WA: Hogrefe & Huber, 1994. [Google Scholar]
Bryant 1974
- Bryant B, Trower PE. Social difficulty in a student sample. British Journal of Educational Psychology 1974;44:13‐21. [DOI] [PubMed] [Google Scholar]
Davies 1990
- Davies LM, Drummond MF. The economic burden of schizophrenia. Psychiatric Bulletin 1990;14:522‐5. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Dupuy 1977
- Dupuy HJ. The general well‐being schedule. In: McDowell I, Newell C editor(s). Measuring Health: A Guide to Rating Scales and Questionnaire. 2nd Edition. USA: Oxford University Press, 1977:206‐213. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33:766‐71. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
GRADE 2004 [Computer program]
- GRADE Working Group. GRADE Profiler. Version 3.2. GRADE Working Group, 2004.
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guo 1995
- Guo GY. Reliability and validity of the scale of social skills of chronic schizophrenic in‐patients. Chinese Journal of Neurology 1995;28(0):16‐8. [Google Scholar]
Guy 1976
- Guy U. Early Clinical Drug Evaluation (ECDEU) Assessment Manual for Psychopharmacology. Revised. Rockville, Maryland: National Institute of Mental Health, 1976:217. [Google Scholar]
Heinrich 1984
- Heinrich DW, Hanlon TE, Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 1984;10:388‐98. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org..
Hume 1995
- Hume C, Pullen I. Rehabilitation in Psychiatry. 2nd Edition. Edinburgh: Churchill Livingstone, 1995. [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Knapp 1994
- Knapp M, Beecham J, Koutsogeorgopoulou V, Hallam A, Fenyo A, Marks IM, et al. Service use and costs of home‐based versus hospital‐based care for people with serious mental illness. British Journal of Psychiatry 1994;165(2):195‐203. [DOI] [PubMed] [Google Scholar]
Kopelowicz 2006
- Kopelowicz A, Liberman RP, Zarate R. Recent advances in social skills training for schizophrenia. Schizophrenia Bulletin 2006;32 Suppl 1:S12‐23. [PUBMED: 16885207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberman 1986
- Liberman RP, Mueser KT, Wallace CJ. Social skills training for schizophrenic individuals at risk for relapse. American Journal of Psychiatry 1986;143(4):523‐6. [PUBMED: 2869704] [DOI] [PubMed] [Google Scholar]
Liberman 1986a
- Liberman RP, Mueser KT, Wallace CJ, Jacobs HE, Eckman T, Massel HK. Training skills in the psychiatrically disabled: learning coping and competence. Schizophrenia Bulletin 1986;12(4):631‐47. [PUBMED: 3810067] [DOI] [PubMed] [Google Scholar]
Liberman 1993
- Liberman RP, Wallace CJ, Blackwell G, Eckman TA, Vaccaro JV, Kuehnel TG. Innovations in skills training for the seriously mentally ill: the UCLA social and independent living skills modules. Innovations and Research 1993;2:43‐60. [Google Scholar]
Marder 1996
- Marder SR, Wirshing WC, Mintz J, McKenzie J, Johnston K, Eckman TA, et al. Two‐year outcome of social skills training and group psychotherapy for outpatients with schizophrenia. American Journal of Psychiatry 1996;153:1585‐92. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams CE, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001 1987;285:91. [DOI] [PubMed] [Google Scholar]
MSPI 1997
- Swedish Council on Health Technology Assessment. Antipsychotic drugs should be reserved for psychosis. Medical Science and Practice Information. Stockholm: Swedish Council on Health Technology Assessment, 1997:4‐5. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Prudo 1987
- Prudo R, Blurn HM. Five‐year outcome and prognosis in schizophrenia. British Journal of Psychiatry 1987;150:345‐54. [DOI] [PubMed] [Google Scholar]
Review Manager 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rosenberg 1965
- Rosenberg M. Society and The Adolescent Self‐Image. Princeton, N.J.: Princeton University Press, 1965. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Son, 2008:359‐83. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Wallace 1985
- Liberman RE, Massel HK, Mosk MD, Wong SE. Social skills training for chronic mental patients. Hospital Community Psychiatry 1985;36:369‐403. [DOI] [PubMed] [Google Scholar]
Wallace 1998
- Wallace CJ. Social skills training in psychiatric rehabilitation: recent findings. International Review of Psychiatry 1998;10:9‐19. [Google Scholar]
Watson 1969
- Watson D, Friend R. Measurement of social‐evaluative anxiety. Journal of Consulting and Clinical Psychology 1969;33:448‐57. [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization. Psychiatric Disability Assessment Scale (WHO/DAS). Geneva: WHO, 1988. [Google Scholar]
Wing 1961
- Wing JK, Brown GW. Social treatment of schizophrenia; a comparative study of three mental hospitals. Journal of Mental Science 1961;107:847‐61. [Google Scholar]
Wing 1970
- Wing JK, Brown GW. Institutionalisation and Schizophrenia; A Comparative Study of Three Mental Hospitals 1960‐1968. London: Cambridge University Press, 1970. [Google Scholar]
Wykes 1986
- Wykes T, Sturt E. The measurement of social behaviour in psychiatric patients: an assessment of the reliability and validity of the SBS schedule. British Journal of Psychiatry 1986;148:1‐11. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
References to other published versions of this review
Almerie 2011
- Almerie MQ, Al Marhi MO, Alsabbagh M, Jawoosh M, Matar HE, Maayan N. Social skills programmes for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD009006; PUBMED: 25414592] [DOI] [PMC free article] [PubMed] [Google Scholar]


