Abstract
Background
With the introduction of direct-acting antivirals (DAAs) for hepatitis C virus (HCV) infection, drug–drug interactions (DDIs) emerged as significant challenge. Since then, HCV therapy and the infected population have rapidly changed. So far, very limited data are available regarding the clinical relevance of DDIs when using most modern DAA regimens. We aimed to assess how the importance of DDIs has evolved over time.
Methods
From January 2014 to July 2018, 668 consecutive HCV patients were evaluated for their outpatient medication and assessed for DDIs with DAAs. Different time periods were defined based on market approval of key DAAs: A (01/2014–11/2014), B (11/2014–08/2016), and C (08/2016–07/2018).
Results
The frequency of patients with real-world DDIs was highest in period B (A: 37.1%, B: 49.6%, C: 38.8%). The recently approved DAAs (period C) theoretically showed a lower DDI risk profile. However, real-world DDIs were still comparable to period A, as HCV patients’ characteristics changed (eg, age ≥75 years: A: 3.1%, B: 9.8%, C: 5.6%; polypharmacy/patients with ≥8 drugs: A: 11.1%, B: 15.2%, C: 17.2%). Furthermore, although DDIs via CYP 3A4 became less important for some modern regimens, other mechanisms like an altered pH value in the stomach, causing reduced bioavailability, evolved. Relevant DDIs most frequently occurred with proton pump inhibitors, metamizole, statins, and carvedilol.
Conclusions
DDIs during antiviral treatment still affect about 40% of HCV patients. The lower DDI potential of modern DAA regimens is partly counteracted by changing patient characteristics. Therefore, DDIs should not be underestimated.
Keywords: direct-acting antivirals (DAAs), drug–drug interactions (DDIs), hepatitis C virus (HCV) infection, patient characteristics, polypharmacy
In 2015, ~71 million people worldwide suffered from chronic hepatitis C virus (HCV) infection [1]. Currently the World Health Organization (WHO) is calling for a reduction of new infections by 90% and mortality by 65% by 2030, with the ultimate aim of HCV elimination [1]. This ambitious goal is driven by the tremendous improvements in HCV therapy that have been achieved due to the development of direct-acting antivirals (DAAs).
The first DAAs, the protease inhibitors (PIs) boceprevir (BOC) and telaprevir (TVR), were approved in 2011 but had several limitations. Both PIs were accompanied by considerable adverse events like anemia or skin rash; their usage was mainly restricted to HCV genotype 1, and their efficacy was rather limited [2–5]. Combination with pegylated interferon α (PEG-IFN α) was still required [6]. Thus, a significant proportion of patients (eg, those with advanced cirrhosis, significant comorbidities) were not eligible for therapy, and many of those with mild disease chose to defer treatment [7–9]. Furthermore, drug–drug interactions (DDIs) emerged as a new important challenge [10, 11]. BOC and TVR are substrates and inhibitors of P-glycoprotein (P-gp) and cytochrome P450 (CYP) 3A4 pathways that are frequently involved in DDIs [10].
In various fields of medicine, DDIs are a common but often neglected cause of drug-associated adverse events. In general, ~1% of all hospital admissions are caused by DDIs, with a higher risk in patients on polypharmacy [12, 13].
Since 2011, several new DAAs have been developed. Approval of the polymerase inhibitor sofosbuvir (SOF) in January 2014 allowed for IFN-free therapy, which facilitated treatment eligibility [14]. In 2014–2015, the first fixed-dose combination of SOF and the NS5A inhibitor ledipasvir (LDV) was approved, as well as the combination of omb-itasvir + paritaprevir + ritonavir ± dasabuvir (OBV/PTV/r ± DSV), which increased the rates of sustained virological response (SVR) [15–19]. However, DDIs still had to be considered, especially in the ritonavir-based regimen [20–23]. When looking at ritonavir in particular, a strong inhibition of CYP 3A4 affects the metabolism of various concomitant medications, leading to multiple clinical relevant DDIs. Finally, in 2016–2017, velpatasvir + sofosbuvir (VEL/SOF), elbasvir + grazoprevir (ELB/GRZ), glecaprevir + pibrentasvir (GLE/PIB), and velpatasvir + sofosbuvir + voxilaprevir (VEL/SOF/VOX) were marketed, which resulted in further improvements such as pangenotypic efficacy and the re-treatment of DAA failures [24–30].
Until now, almost all previous limitations of HCV treatment have been overcome. Currently available DAA regimens achieve SVR in >98% of patients and are very well tolerated [24–30]. It is widely believed that with today’s treatment options the risk for DDI via, for example, CYP 3A4 or P-gp is significantly lower as compared with previously used regimens. However, it has to be considered that other interaction mechanisms might have become relevant [31]. Furthermore, as almost all HCV patients are now eligible for the pangenotypic regimens and HCV elimination has been declared as the ultimate goal, it is to be expected that the diversity of HCV patients who receive antiviral treatment has increased over the last years. This includes patients with significant comorbidities, older age, and polypharmacy [21]. Although some studies have investigated DDIs with older DAA regimens in the past, almost no data are available regarding the relevance and most important mechanisms of DDIs when using the most modern DAA regimens. Furthermore, no study has addressed how the changes in the HCV-infected and -treated populations, which were accompanied by rapid improvements in HCV therapy, affected the risk for significant DDIs.
The aim of this work was to analyze the relevance and most frequent mechanisms of DDIs in the rapidly changing field of HCV therapy. A particular focus was put on the current situation using the most modern DAA regimens in the remaining HCV population.
METHODS
Cohort
Overall, 668 consecutive patients with chronic HCV infection who were treated with a DAA regimen at the hepatitis outpatient clinic of Hannover Medical School from January 2014 to July 2018 were included. To analyze the changing epidemiology and frequency of potential DDIs within the evolving field of DAA therapies, 3 different time periods were defined based on the approval of key DAA regimens that significantly changed HCV therapy at our center: (A) January 22, 2014 (first patient treated with SOF, and therefore IFN-free therapy became available), to November 28, 2014; (B) November 29, 2014 (first patient treated with LDV/SOF or OBV/PTV/r ± DSV, and therefore fixed-dose regimens became available), to August 14, 2016; and (C) August 15, 2016 (first patient treated with velpatasvir [VEL], elbasvir/grazoprevir [ELB/GRZ], or glecaprevir/pibrentasvir [GLE/PIB], and therefore pangenotypic therapies as well as highly effective re-treatment options became available), to July 31, 2018.
Assessment of Baseline Characteristics and Patients’ Regular Outpatient Medications
Laboratory testing for albumin, bilirubin, and quick/international normalized ratio (INR) was performed due to standard procedures at Hannover Medical School. HCV genotype setting was performed with the INNO-LiPA by Innogenetics according to the manufacturer’s instructions. Liver cirrhosis in patients was calculated using transient elastography. The applied cutoff value for cirrhosis was >13 kPa [32]. In case of failed transient elastography, additional clinical and laboratory parameters (ie, albumin, bilirubin, quick, INR, sonographic proof of ascites, and stage of encephalopathy) were used to determine the stage of liver cirrhosis.
Before treatment, all patients included in this retrospective analysis were routinely asked for all their regular outpatient medication. All types of medication were documented, including over-the-counter drugs and other nonprescription drugs, herbal drugs, and nutritional supplements. Due to the fact that herbal drugs and nutritional supplements more often contain several (active) ingredients, these were only counted as 1 medication. In case of other combination preparations, for example, sitagliptin/metformin, each active ingredient was counted separately. In some cases, the regular outpatient medication was modified before treatment due to the risk of DDI with the selected DAA therapy. Concerning this matter, we included the unmodified outpatient medication in the analysis.
Evaluation of Drug–Drug Interactions
DDIs were evaluated using www.hep-druginteractions.org (as of July 2019). In case of missing information, a clinical pharmacist and clinical pharmacologist were consulted. Pharmacokinetic and pharmacodynamic interactions between the antiviral substances and the regular outpatient medications were considered. Interactions were classified into 4 different categories: (1) no interaction expected, (2) potential weak interaction (additional action unlikely to be required), (3) potential significant interaction (additional monitoring, dose adjustment or therapy adjustment required), and (4) do not coadminister/contraindicated.
Interaction mechanisms with the DAA regimens were identified and analyzed. Information about the mechanisms of interaction was provided by the latest prescribing information and www.hep-druginteractions.org (as of November 2019).
Analysis of Drug–Drug Interactions
The relevance of DDIs was analyzed in 2 different scenarios:
1. Potential of different antiviral medications for DDIs with the regular outpatient medication
Potential DDIs were assessed between all regular outpatient medications used in the total cohort and each individual antiviral that was available for the treatment of HCV infection in the last decade. Administration of a fixed-dose combination was analyzed as a single medication. The following DAAs were assessed: boceprevir (BOC), telaprevir (TVR), daclatasvir (DAC), simeprevir (SIM), sofosbuvir (SOF), ombitasvir + paritaprevir + ritonavir ± dasabuvir (OBV/PTV/r ± DSV), ledipasvir (LDV) + sofosbuvir (SOF), glecaprevir (GLE) + pibrentasvir (PIB), elbasvir (ELB) + grazoprevir (GRZ), velpatasvir (VEL) + sofosbuvir (SOF) ± voxilaprevir (VOX). DAAs already withdrawn from the market (BOC, TVR, DAC, and SIM) were only analyzed to compare the frequencies of potential DDIs with the more modern regimens. In addition, DDIs were analyzed for pegylated interferon α (PEG-IFN α) and ribavirin (RBV).
2. Real-world incidence of DDIs due to antiviral treatment
DDIs were assessed between the actual regular outpatient medication and the actually used antiviral regimen in the respective patient. If >1 interaction per patient was identified, the more severe interaction category was chosen.
Statistics
The data are reported as absolute numbers, means, medians, percentages, and ranges, always clearly labeled. Statistical analysis was conducted using the chi-square test, f test, t test, and calculation of the relative risk. P values <.05 were considered statistically significant. Microsoft Excel (2010) was used for data collection and quantification, and IBM SPSS (version 25) was used for further analysis.
Ethics
This retrospective analysis was performed according to the principles of good clinical practice and the declaration of Helsinki and approved by the local ethics committee of Hannover Medical School (Nr. 8132_BO_K_2018). All patients gave written informed consent.
RESULTS
Changing Epidemiology of the Treated HCV Population Since 2014
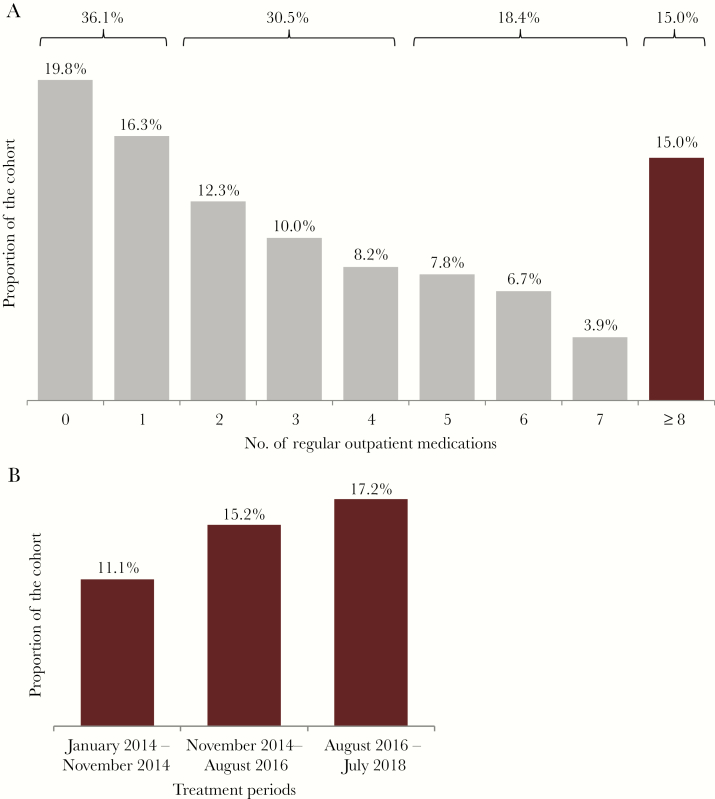
Overall, 668 patients were included in the analysis; 45.1% were female, and 45.1% had liver cirrhosis. The median number of drugs in the regular outpatient medication (range) was 3 (0–19). The mean age (range) was 55.5 (18–85) years. Furthermore, 44 patients (6.6%) were ≥75 years old. The mean age of the patients fluctuated over time, with a lower mean age of the patients in the most recent period (mean age in years: A: 55.3, B: 58.2, C: 52.9; A–B P = .011, B–C P < .001, and A–C P = .033). However, the proportion of patients aged ≥75 years increased from the first to the third observation period (A: 3.1%, B: 9.8%, C: 5.6%; A–B P = .010, B–C P = .079, and A–C P = .235). Of note, the frequency of cirrhosis among the patients receiving antiviral treatment declined significantly over the years (A: 80.2%, B: 44.1%, C: 23.2%; A–B P < .001, B–C P < .001, and A–C P < .001). In contrast, patients with kidney transplantation receiving sirolimus, everolimus, cyclosporine, or tacrolimus increased over time (A: 0.0%, B: 2.3%, C: 4.0%; A–C P = .008) (Table 1). Although there was no remarkable change in the median number of drugs over the different time periods (A: 3, B: 3, C: 2), we documented a numerical, continuous increase in the proportion of patients using ≥8 drugs as outpatient medications (A: 11.1%, B: 15.2%, C: 17.2%; A–B P = .231, B–C P = .549, and A–C P = .089) (Table 1, Figure 1). In this particular subgroup of patients, 32.0% and 9.0% were ≥65 years old and ≥75 years old, respectively. The frequency of cirrhosis was 52.0%.
Table 1.
Baseline Characteristics of the Cohort
| Total Cohort | Jan 2014–Nov 2014 | Nov 2014–Aug 2016 | Aug 2016–Jul 2018 | |
|---|---|---|---|---|
| No. of patients (%) | 668 (100.0) | 162 (24.3) | 256 (38.3) | 250 (37.4) |
| Sex, No. (%) | ||||
| Female | 301 (45.1) | 65 (40.1) | 122 (47.7) | 114 (45.6) |
| Male | 367 (54.9) | 97 (59.9) | 134 (52.3) | 136 (54.4) |
| Age, mean (range), y | 55.5 (18–85) [6.6% ≥75 y] | 55.3 (24–81) [3.1% ≥75 y] | 58.2 (24–85) [9.8% ≥75 y] | 52.9 (18–82) [5.6% ≥75 y] |
| Cirrhosis, No. (%) | 301 (45.1) | 130 (80.2) | 113 (44.1) | 58 (23.2) |
| Child A | 247 (82.1) | 108 (83.1) | 94 (83.2) | 45 (77.6) |
| Child B | 38 (12.6) | 19 (14.6) | 10 (8.8) | 9 (15.5) |
| Child C | 5 (1.7) | 2 (1.5) | 3 (2.7) | 0 (0) |
| N/A | 11 (3.7) | 1 (0.8) | 6 (5.3) | 4 (6.9) |
| HCV genotype, No. (%) | ||||
| 1 | 477 (71.4) | 101 (62.3) | 217 (84.8) | 159 (63.6) |
| 2 | 29 (4.3) | 13 (8.0) | 2 (0.8) | 14 (5.6) |
| 3 | 123 (18.4) | 42 (25.9) | 23 (9.0) | 58 (23.2) |
| 4 | 22 (3.3) | 4 (2.5) | 7 (2.7) | 11 (4.4) |
| 5 | 7 (1.0) | 1 (0.6) | 2 (0.8) | 4 (1.6) |
| 6 | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.4) |
| N/A | 9 (1.3) | 1 (0.6) | 5 (2.0) | 3 (1.2) |
| No. outpatient medications, median (range) | 3 (0–19) | 3 (0–18) | 3 (0–16) | 2 (0–19) |
| Kidney transplant patients receiving sirolimus, everolimus, cyclosporine, or tacrolimus | 16 (2.4) | 0 (0) | 6 (2.3) | 10 (4.0) |
Abbreviations: HCV, hepatitis C virus; N/A, not available.
Figure 1.
A, Number of outpatient medications at baseline. B, Patients taking ≥8 outpatient medications at baseline.
Potential of Different Antiviral Substances to Cause Drug–Drug Interactions
In total, 353 different substances were found in the regular outpatient medication of the cohort (Supplementary Table 1). In 28 patients (4.2%), some medication could not be clearly identified based on the description provided by the patient. Pantoprazole (22.0%), levothyroxine (14.4%), and spironolactone (12.3%) were used most frequently. Overall, the top most used medications did not change significantly over time (Supplementary Table 2).
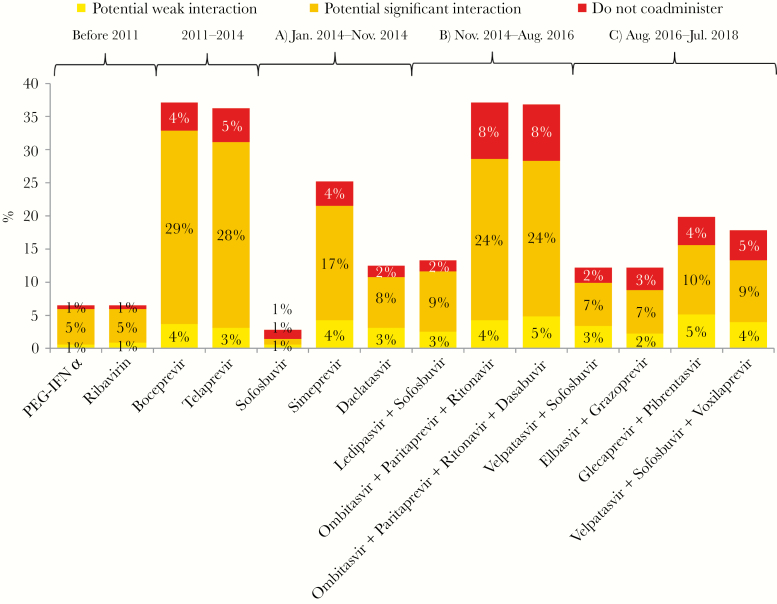
All outpatient medication was assessed with each individual antiviral drug/regimen for potential DDIs that might occur in case of coadministration. Among all treatment regimens, the combination of OBV/PTV/r and BOC (alone) showed the highest potential for DDI (ie, category 2, 3, or 4) with the analyzed concomitant medication (37.1%), whereas SOF (alone) showed the lowest (2.8%). When examining the different time periods in detail, it became obvious that in every treatment period DAAs with a considerable potential for DDIs were to be found. As expected, the first-wave PIs BOC and TVR had much higher potential for DDIs, affecting 37.1% and 36.3% of outpatient medications, respectively. In the following period, starting with the approval of SOF, the potential for DDIs widely differed between the individual drugs that were available. SOF, SIM, and DAC showed potential for DDIs with 2.8%, 25.2%, and 12.5% of the outpatient medication, respectively. Risk for DDIs increased in the era of the first DAA fixed-dose combinations. The IFN-free regimen OBV/PTV/r ± DSV showed contraindications because of DDIs (category 4) with 30 drugs from the outpatient medications of the patients (8.5%) and potential significant DDIs (category 3) with 86 out of 353 drugs (24.4%) (Figure 2; Supplementary Table 3).
Figure 2.
Possible drug–drug interactions with all outpatient medications from the cohort. Abbreviation: PEG-IFN, pegylated interferon.
Compared with previous treatment periods, the current most-used regimens, GLE/PIB, ELB/GRZ, VEL/SOF ± VOX, did not greatly differ in their potential for DDIs. The frequency of potential DDIs with outpatient medications ranged from 12.2% to 19.8%. Contraindications due to DDIs (category 4) appeared with up to 4.5% of the concomitant medications, up to 10.5% of the concomitant medications had a potential significant DDI (category 3) with the antivirals, and up to 5.1% showed a potential weak DDI (category 2). Overall, the potential for DDIs was lower compared with most of the older IFN-free regimens (Figure 2).
Among all 353 assessed regular outpatient medications, antiepileptic drugs (carbamazepine [5 patients], eslicarbazepine [1 patient], and oxcarbazepine [1 patient]), St. John’s wort (1 patient), and rifampicin (1 patient) were identified as the medications with the highest potential for DDIs, being contraindicated for coadministration with ≥10 of the 14 different antiviral regimens/substances (Supplementary Table 4). The main reason for the contraindications was a strong CYP induction by these substances, which would accelerate the metabolism of the antivirals and risk the efficacy of treatment when coadministered.
Real-World Incidence of Drug–Drug Interactions due to Antiviral Treatment
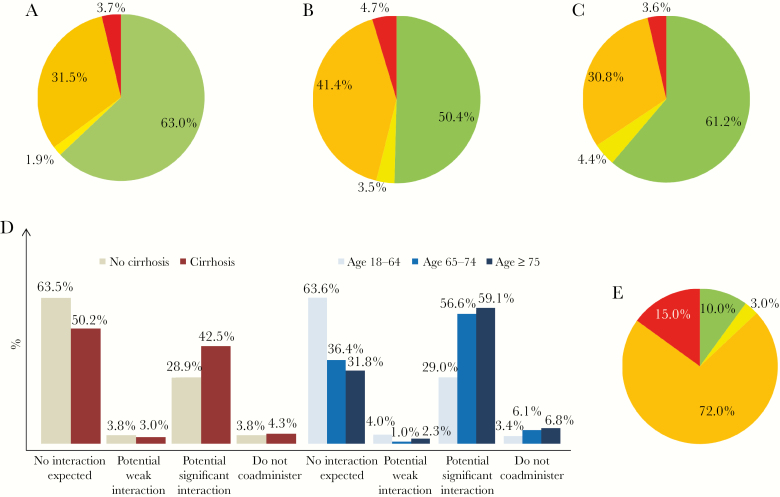
In the second step of our analysis, only the actually received HCV therapy and concomitant medications were considered for each patient in the assessment of DDIs: Overall, the frequency of DDIs in period B (49.6%) was significantly higher compared with periods A (37.1%; P = .012) and C (38.8%; P = .014). A similar picture was documented when exclusively focusing on potential significant DDIs (category ≥3). Moreover, the incidence of contraindications because of DDIs (category 4) stayed constant over all periods, with a slight increase in period B (A: 3.7%, B: 4.7%, C: 3.6%; A–B P = .629, B–C P = .540, and A–C P = .956) (Figure 3A–C). In the majority of these cases, contraindicated concomitant medications were discontinued and/or changed to a less interacting alternative. In some patients, medication was continued under individual clinical surveillance (Supplementary Table 5).
Figure 3.
Real occurring drug–drug interactions between outpatient medications and hepatitis C virus (HCV) regimens for each patient at baseline. A, Jan 2014–Nov 2014. B, Nov 2014–Aug 2016. C, Aug 2016–Jul 2018. D, Real occurring drug–drug interactions between outpatient medications and HCV regimens for each patient at baseline depending on the presence of cirrhosis and age. E, Real occurring drug–drug interactions between outpatient medications and HCV regimens for patients taking ≥8 drugs outpatient medications at baseline. Green: no interaction expected; yellow: potential weak interaction; orange: potential significant interaction; red: do not coadminister.
Patients with cirrhosis and those aged ≥65 years used significantly more concomitant medications (mean number of drugs, no cirrhosis vs cirrhosis: 3.2 vs 4.3, P < .001; <65 years vs ≥65 years: 3.4 vs 5.0, P < .001). Both of these subgroups showed an increased risk of DDIs (cirrhosis: relative risk [RR], 1.37; 95% confidence interval [CI], 1.14–1.63; age ≥65 years: RR, 1.79; 95% CI, 1.52–2.11). Among patients with cirrhosis (n = 301), 42.5% had potential significant DDIs (category 3) with the respective DAA therapy, in comparison with 28.9% of patients without cirrhosis (P < .001). The frequency of contraindicated outpatient medication was similar between both groups. Among patients with an age of 18–64 years (n = 525), 29.0% were at risk for potential significant DDIs (category 3), whereas this was the case in 56.6% and 59.1% (P < .001) of patients aged 65–74 and ≥75 years, respectively. The frequency of contraindicated concomitant medication was also higher in those aged ≥65 years (Figure 3D). Another particularly vulnerable group for DDIs was patients receiving polypharmacy. Overall, 9 out of 10 patients (90.0%) taking ≥8 drugs as outpatient medication showed at least 1 DDI (category 2–4) (Figure 3E).
Most Relevant Medications and Pharmacokinetic Mechanisms Involved in DDIs With Currently Used DAA Regimens
The most common outpatient medications involved in significant DDIs (category 3 or 4) with the most recently approved DAA regimens, VEL/SOF (n = 58), GLE/PIB (n = 46), and ELB/GRZ (n = 73), were proton pump inhibitors (PPIs), metamizole, statins, and carvedilol (Supplementary Table 6). More precise information on relevant DDIs and their respective mechanisms can be found in the Supplementary Data (Supplementary Table 7).
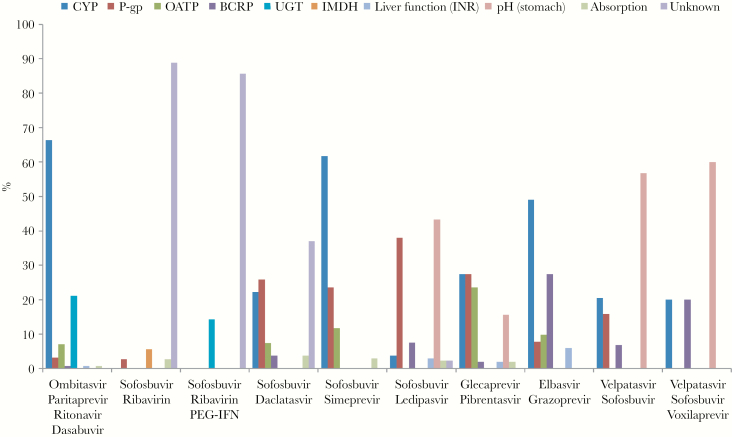
DDIs between regular outpatient medications and specific antiviral regimens were not uniform, but were dependent on the possible interaction mechanisms of each antiviral regimen and the concomitant medication. Overall, compared with the older regimens, interactions with the most modern DAAs were less frequently caused by CYP and P-gp pathways (Figure 4). GLE/PIB (n = 46) DDIs were distributed between DDIs facilitated by organic anion transporting protein (OATP; 23.5%), pH-change (15.7%), P-gp (27.5%), and the CYP pathways (27.5%). ELB/GRZ (n = 73) interactions were mediated via CYP enzymes (49.0%) and breast cancer resistance protein (BCRP; 27.5%) most of the time. This was mainly caused by interactions with statins. Some statins are metabolized by CYP enzymes and may also be transported via OATP1B1/3 and/or BCRP. ELB/GRZ is a weak CYP 3A inhibitor in vivo and blocks intestinal BCRP. Concomitant use may cause higher statin blood levels, and the risk for adverse events like rhabdomyolysis increases. In contrast to that, VEL/SOF ± VOX (without VOX: n = 58; with VOX: n = 9) interactions, in >50% of cases (VEL/SOF: 56.8%; VEL/SOF + VOX: 60.0%), were due to a PPI increasing gastric pH, thereby causing lower absorption rates of mainly VEL. DDIs between regular outpatient medications and VEL/SOF ± VOX via BCRP (6.8%; with VOX: 20.0%), P-gp (15.9%; with VOX: 0.0%), and CYP enzymes (20.5%; with VOX: 20.0%) also occurred but were represented less in this part of the cohort (Figure 4; Supplementary Table 8).
Figure 4.
Proportion of drug–drug interaction mechanisms between outpatient medications and HCV regimens. Abbreviations: BCRP, breast cancer resistance protein; CYP, cytochrome P450 enzyme; IMDH, inosine-5’-monophosphate dehydrogenase; INR, international normalized ratio; OATP, organic anion transporting polypeptide; PEG-IFN, pegylated interferon; P-gp, P-glycoprotein; UGT, UDP-glucuronosyltransferase.
Discussion
In the PEG-IFN α/RBV era, DDIs were of little concern. DDIs mainly emerged as a new challenge with the approval of DAAs for the treatment of HCV infection [11]. Although this work for the first time provides extensive data on DDIs with the most recent DAA regimens, it also allows a detailed comparison between each step of HCV therapy evolution over the last years. We here show that the different DAA regimens that were used over the last decade widely differ in their general potential to cause DDIs with the outpatient medications of HCV patients. Currently, the most widely used DAAs demonstrate a moderate DDI risk profile, which is significantly lower compared with first-generation PIs used from 2011 or the combinations with ritonavir booster used from 2014 [10, 20]. However, our work also demonstrates that despite these advantages, the overall frequency of DDIs in the real-world analysis remained more or less stable over the treatment periods, with about 40% of HCV patients affected. Advantages in HCV therapy were accompanied by a change in the epidemiology of HCV patients, mainly due to treatment eligibility and expansion [1, 14, 33]. Some of these changes (ie, higher frequency of polypharmacy) at least partly counteracted the lower potential risk for DDI with the newer DAA regimens. Thus, we conclude that DDIs remain relevant, should not be underestimated, and still need to be considered in the management of HCV patients.
Our analysis demonstrates that HCV patients’ characteristics have indeed changed during the different treatment periods. The frequency of treated patients with cirrhosis has significantly decreased. In addition, the mean age of the patients decreased, but, importantly, the proportion of patients aged ≥75 years or/and with polypharmacy increased. One may assume that this is a result of treatment policy over the last years. Patients with advanced stages of liver disease were treated first at our and several other centers [7, 14, 34]. Many of these patients were infected before the introduction of anti-HCV testing in the early 1990s. However, the most complicated cases (ie, severe contraindications, decompensated cirrhosis, and renal impairment) or those without advanced disease, and in particular those with older age, were frequently deferred [7, 14]. As a result, the overall HCV population we are now facing in HCV therapy has been getting younger (lower likelihood for advanced liver disease). However, at the same time, the proportion of the subgroup of highly complicated patients (eg, old age, polypharmacy) has been increasing, as modern DAA regimens have significantly improved on safety and efficacy in this group [21, 26, 35–37]. Putting the focus for example on immunosuppressive drugs, we recorded a significant increase due to new treatment options for renal transplant recipients (ie, glecaprevir/pibrentasvir and elbasvir/grazoprevir). The excellent treatment options available started an intensive debate about the use of HCV-positive donor organs for HCV-negative recipients. It seems likely that the treatment of transplant recipients may remain particularly relevant in the near future [38]. These patients often receive highly complex polypharmacy with the immunosuppressive drugs (ie, sirolimus, everolimus, cyclosporine, or tacrolimus) themselves being potential partners for relevant DDIs [39].
Changes in the epidemiology of the HCV population might furthermore affect the frequency of the most relevant mechanisms of DDIs. Additionally, the individual chemical and pharmacokinetic characteristics of each DAA play a substantial role as well. Indeed, the present work emphasizes that the pathways involved in DDIs in the real world are distinct for each regimen. Statins interact more frequently with ELB/GRZ, and VEL-containing regimens show a majority of DDIs via pH elevation in the stomach. When looking at the newer, mainly PPI-caused mechanisms, certainly more data are needed [40]. Due to the variety of DAAs available, DDI assessment has become more complex.
Individualized management, especially in complex patients, remains highly important. However, it also has to be noted that strict contraindications due to DDIs were quite rare when using modern DAAs and the remaining significant DDIs seemed very manageable by dose modifications or monitoring interventions. First of all, awareness of DDIs still represents the most important step. Tools like www.hep-druginteractions.org provide the involved health care professionals with reliable resources to identify and handle potential DDIs.
The present work has some limitations. First, it is a single-center analysis from a tertiary referral center. Thus, a higher frequency of patients with more advanced liver disease is likely, as indicated by the higher-than-average rate of patients with cirrhosis. However, it should be noted that it was not patients with cirrhosis but rather elderly patients who showed a comparably higher risk for DDIs, as shown in our analysis and as previously reported [21]. Second, with the restrictions for elderly patients for clinical trials and the low likelihood for liver transplantation, we would not expect that elderly patients were over-represented in our institution. The same affects patients with current intravenous drug use (PWID). For PWID, we would rather quote that they were under-represented due to the fact that active drug users are more frequently treated at other centers. Third, under-reporting of concomitant medication and nonadherence to recommendations on DDI management cannot be excluded, as the collection of medication data was based on patient self-report and letters of referral from physicians. Additionally, physicians may have changed the concomitant medication in anticipation of antiviral therapy. Therefore, we tracked changes to the concomitant medication back before initiation of antiviral therapy to include any changes due to suspected DDI.
Further research on DDIs in HCV therapy will be required to ensure a safe upscaling of HCV treatments, which is essential to meet World Health Organization goals and ultimately achieve HCV elimination. The HCV population will certainly see further changes over the next years that will affect the risk for DDIs, as well as the most relevant drugs and mechanisms involved. Unrestricted treatment of high-risk populations like PWID will require general considerations and strategies for how to handle the most relevant potential DDIs in this specific group, as adequate monitoring during therapy might not always be possible.
To our knowledge, this is the first work that assesses the risk of DDIs with various regimens in various time periods and also the first that considers the most recent DAA regimens as well as relevant DDI mechanisms. It shows that despite improved newer antiviral regimens, the frequency of significant real-world DDIs in a changing epidemiology of HCV patients has not declined, but remained stable. Therefore, careful assessment of patients’ regular outpatient medications and individual evaluation for potential DDIs seem as important as ever.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the participating patients.
Financial support. The work was partly funded by the DZIF (German Center for Infection Research).
Potential conflicts of interest. F.M. received financial support for www.hep-druginteractions.org from AbbVie, Merck, Gilead, and Janssen; honoraria for lectures or advisory boards were received from AbbVie, Merck, Gilead, and Janssen. M.P.M. received financial support as principal investigator/study from Bristol Myers Squibb, Gilead, Merck (MSD), and AbbVie. He also received grants from Bristol Myers Squibb, Gilead, Merck (MSD), and AbbVie. He also received personal fees for consulting, lectures and as travel support from Bristol Myers Squibb, Gilead, Merck (MSD), and AbbVie. D.B. received financial support for www.hep-druginteractions.org from AbbVie, Merck, Gilead, and Janssen; honoraria for lectures or advisory boards were received from AbbVie, Merck, Gilead, and Janssen. M.C. received personal fees from AbbVie, personal fees from Bristol-Myers Squibb, personal fees from Gilead Sciences, personal fees from Janssen-Cilag, grants and personal fees from Roche, personal fees from Merck (MSD), personal fees from Biogen, personal fees from Falk Foundation, personal fees from Boehringer Ingelheim, personal fees from Siemens, and personal fees from Spring Bank. C.H.z.S. received travel grants from Novartis and Gilead. B.M. received speaker and/or consulting fees from Abbott Molecular, Astellas, Intercept, Falk, AbbVie, Norgine, Bristol Myers Squibb, Fujirebio, Janssen-Cilag, Merck (MSD), and Roche. He also received research support from Abbott Molecular and Roche. All other authors report no potential conflicts.
Data availability. The data are not publicly available.
Author contributions. B.M., C.H.z.S., and B.S. designed the work. B.S. and M.W. collected and analyzed the data. All authors substantially contributed to the interpretation of the data. B.M., C.H.z.S., and B.S. drafted the manuscript. All authors critically revised the manuscript. All authors approved the manuscript to be published and therefore are accountable for all aspects of the work. B.M. and C.H.z.S. supervised the work.
References
- 1. World Health Organization. Global hepatitis report 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=6120C81F258231FE3F7294BE3E90786C?sequence=1. Accessed 2 March 2019.
- 2. McHutchison JG, Everson GT, Gordon SC, et al. . PROVE1 Study Team Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–38. [DOI] [PubMed] [Google Scholar]
- 3. Foster GR, Hézode C, Bronowicki JP, et al. . Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology 2011; 141:881–889.e1. [DOI] [PubMed] [Google Scholar]
- 4. Poordad F, McCone J Jr, Bacon BR, et al. . SPRINT-2 Investigators Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacon BR, Gordon SC, Lawitz E, et al. . HCV RESPOND-2 Investigators Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hézode C, Forestier N, Dusheiko G, et al. . PROVE2 Study Team Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–50. [DOI] [PubMed] [Google Scholar]
- 7. Maasoumy B, Port K, Markova AA, et al. . Eligibility and safety of triple therapy for hepatitis C: lessons learned from the first experience in a real world setting. PLoS One 2013; 8:e55285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hézode C, Fontaine H, Dorival C, et al. . CUPIC Study Group Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol 2013; 59:434–41. [DOI] [PubMed] [Google Scholar]
- 9. Maasoumy B, Port K, Deterding K, et al. . Limited effectiveness and safety profile of protease inhibitor-based triple therapy against chronic hepatitis C in a real-world cohort with a high proportion of advanced liver disease. Eur J Gastroenterol Hepatol 2014; 26:836–45. [DOI] [PubMed] [Google Scholar]
- 10. Maasoumy B, Port K, Calle Serrano B, et al. . The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther 2013; 38:1365–72. [DOI] [PubMed] [Google Scholar]
- 11. Kiser JJ, Burton JR Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol 2013; 10:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2014; 23:489–97. [DOI] [PubMed] [Google Scholar]
- 13. Patel N, Nasiri M, Koroglu A, et al. . A cross-sectional study comparing the frequency of drug interactions after adding simeprevir- or sofosbuvir-containing therapy to medication profiles of hepatitis C monoinfected patients. Infect Dis Ther 2015; 4:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Höner Zu Siederdissen C, Maasoumy B, Deterding K, et al. . Eligibility and safety of the first interferon-free therapy against hepatitis C in a real-world setting. Liver Int 2015; 35:1845–1852. [DOI] [PubMed] [Google Scholar]
- 15. Afdhal N, Zeuzem S, Kwo P, et al. . ION-1 Investigators Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 16. Afdhal N, Reddy KR, Nelson DR, et al. . ION-2 Investigators Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 17. Kowdley KV, Gordon SC, Reddy KR, et al. . ION-3 Investigators Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 18. Feld JJ, Kowdley KV, Coakley E, et al. . Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1594–603. [DOI] [PubMed] [Google Scholar]
- 19. Poordad F, Hezode C, Trinh R, et al. . ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–82. [DOI] [PubMed] [Google Scholar]
- 20. Höner Zu Siederdissen C, Maasoumy B, Marra F, et al. . Drug-drug interactions with novel all oral interferon-free antiviral agents in a large real-world cohort. Clin Infect Dis 2016; 62:561–567. [DOI] [PubMed] [Google Scholar]
- 21. Vermehren J, Peiffer KH, Welsch C, et al. . The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther 2016; 44:856–65. [DOI] [PubMed] [Google Scholar]
- 22. Marra F, Höner Zu Siederdissen C, Khoo S, et al. . Clinical impact of pharmacokinetic interactions between the HCV protease inhibitor simeprevir and frequently used concomitant medications. Br J Clin Pharmacol 2018; 84:961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondili LA, Gaeta GB, Ieluzzi D, et al. . Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS One 2017; 12:e0172159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gane E, Lawitz E, Pugatch D, et al. . Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 2017; 377:1448–55. [DOI] [PubMed] [Google Scholar]
- 25. Zeuzem S, Foster GR, Wang S, et al. . Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018; 378:354–69. [DOI] [PubMed] [Google Scholar]
- 26. Roth D, Nelson DR, Bruchfeld A, et al. . Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015; 386:1537–45. [DOI] [PubMed] [Google Scholar]
- 27. Jacobson IM, Lawitz E, Kwo PY, et al. . Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology 2017; 152:1372–1382.e2. [DOI] [PubMed] [Google Scholar]
- 28. Foster GR, Afdhal N, Roberts SK, et al. . ASTRAL-2 Investigators; ASTRAL-3 Investigators Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 29. Feld JJ, Jacobson IM, Hézode C, et al. . ASTRAL-1 Investigators Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373:2599–607. [DOI] [PubMed] [Google Scholar]
- 30. Bourlière M, Gordon SC, Flamm SL, et al. . POLARIS-1 and POLARIS-4 Investigators Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 2017; 376:2134–46. [DOI] [PubMed] [Google Scholar]
- 31. European Association for the Study of the Liver (EASL). EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 32. Friedrich-Rust M, Ong MF, Martens S, et al. . Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008; 134:960–74. [DOI] [PubMed] [Google Scholar]
- 33. Wedemeyer H, Dore GJ, Ward JW. Estimates on HCV disease burden worldwide - filling the gaps. J Viral Hepat 2015; 22(Suppl 1):1–5. [DOI] [PubMed] [Google Scholar]
- 34. Kittner JM, Weiss NM, Wiltink J, et al. . Defer or treat? Reasons for treatment decisions in patients with chronic hepatitis C genotype 1 in the early era of directly acting antiviral agents. Dig Liver Dis 2014; 46:67–71. [DOI] [PubMed] [Google Scholar]
- 35. Charlton M, Everson GT, Flamm SL, et al. . SOLAR-1 Investigators Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–59. [DOI] [PubMed] [Google Scholar]
- 36. Manns M, Samuel D, Gane EJ, et al. . SOLAR-2 Investigators Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016; 16:685–97. [DOI] [PubMed] [Google Scholar]
- 37. Reau N, Kwo PY, Rhee S, et al. . Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection. Hepatology 2018; 68:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez SA, Trotter JF. The rise of the opioid epidemic and hepatitis C-positive organs: a new era in liver transplantation. Hepatology 2018; 67:1600–8. [DOI] [PubMed] [Google Scholar]
- 39. Ortiz GA, Trivedi HD, Nader C. Pharmacokinetics and drug interactions of medications used to treat hepatitis C virus infection in the setting of chronic kidney disease and kidney transplantation. Hemodial Int 2018; 22(Suppl 1):22–35. [DOI] [PubMed] [Google Scholar]
- 40. Yu G, Zheng Y, Yu Y, et al. . Gastric-acid-mediated drug-drug interactions with direct-acting antiviral medications for hepatitis C virus infection: clinical relevance and mitigation strategies. Drug Discov Today 2019; 24:845–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.