Abstract
Aims
Clonal haematopoiesis of indeterminate potential (CHIP), defined as the presence of an expanded somatic blood cell clone without other haematological abnormalities, was recently shown to increase with age and is associated with coronary artery disease and calcification. The most commonly mutated CHIP genes, DNMT3A and TET2, were shown to regulate inflammatory potential of circulating leucocytes. The incidence of degenerative calcified aortic valve (AV) stenosis increases with age and correlates with chronic inflammation. We assessed the incidence of CHIP and its association with inflammatory blood cell phenotypes in patients with AV stenosis undergoing transfemoral aortic valve implantation (TAVI).
Methods and results
Targeted amplicon sequencing for DNMT3A and TET2 was performed in 279 patients with severe AV stenosis undergoing TAVI. Somatic DNMT3A- or TET2-CHIP-driver mutations with a VAF ≥ 2% were detected in 93 out of 279 patients (33.3%), with an age-dependent increase in the incidence from 25% (55–69 years) to 52.9% (90–100 years). Patients with DNMT3A- or TET2-CHIP-driver mutations did not differ from patients without such mutations in clinical parameters, concomitant atherosclerotic disease, blood cell counts, inflammatory markers, or procedural characteristics. However, patients with DNMT3A- or TET2-CHIP-driver mutations had a profoundly increased medium-term all-cause mortality following successful TAVI. Differential myeloid and T-cell distributions revealed pro-inflammatory T-cell polarization in DNMT3A-mutation carriers and increased pro-inflammatory non-classical monocytes in TET2-mutation carriers.
Conclusion
This is the first study to show that acquired somatic mutations in the most commonly mutated CHIP-driver genes occur frequently in patients with severe degenerative AV stenosis, are associated with increased pro-inflammatory leucocyte subsets, and confer a profound increase in mortality following successful TAVI.
Keywords: Aortic valve disease, TAVI, Clonal haematopoiesis, Inflammation
See page 940 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz752)
Introduction
Clonal haematopoiesis of indeterminate potential (CHIP), defined as the presence of an expanded somatic blood cell clone in subjects without other haematological abnormalities,1 has recently shown to increase with age and is associated with coronary artery disease (CAD) and coronary calcification.2,3 Mechanistically, the most common mutated genes in CHIP, DNMT3A and TET2, were experimentally shown to regulate the inflammatory potential of circulating leucocytes.3–5 The incidence of calcified degenerative aortic valve (AV) stenosis increases with age6 and correlates with chronic inflammation.7 Moreover, the outcome of transcatheter aortic valve implantation (TAVI) was previously shown to be unfavourably influenced by a systemic inflammatory response.8 Therefore, it was the aim of the present study to assess the incidence of DNMT3A- and TET2-CHIP-driver mutations in patients with severe calcified AV stenosis and, more importantly, to associate its presence with the clinical outcome following TAVI.
Methods
Study cohort
A total of 287 consecutive patients undergoing TAVI for severe calcified AV stenosis at the University Hospital of the Goethe University, Frankfurt, Germany, between February 2017 and March 2019, were studied. Eight patients were excluded due to previous haematological diseases. Thus, the final cohort consisted of 279 patients. All patients provided written informed consent for both, the TAVI procedure as well as genetic testing of blood samples. The ethics review board approved the protocol, and the study complies with the Declaration of Helsinki.
Clinical data, echocardiographic findings, and laboratory data were prospectively collected. The single clinical endpoint was all-cause mortality.
Laboratory measurements
Peripheral venous blood samples were obtained pre-procedurally and analysed for standard inflammatory parameters, including total leucocyte counts (n = 279), high-sensitive C-reactive protein (n = 273), and serum interleukin-6 (IL-6) levels (n = 261). Serum high-sensitive troponin T and N-terminal pro brain natriuretic peptide (NT-proBNP) were assessed in 240 and 243 patients, respectively, prior to TAVI.
Enumeration of leucocyte subsets
Multiparameter flow cytometric [fluorescence activated cell sorting (FACS)] analysis was performed in a total of 82 patients. Monocyte subset (classical, intermediate, and non-classical) counts were determined using a 6-colour TruCount Assay (BD Biosciences) as previously described.9 T-cell subpopulations were determined using eight-colour (T-cell effectors, ‘CD4 Panel 1’) and five-colour (Tregs, ‘CD4 Panel 2’) flow cytometric assays. T-cell gating and subclassification was performed based on established models.10
Next-generation sequencing
Next-generation sequencing was commercially performed by MLLDxGmbH, München, Germany. In brief, DNA was isolated with the MagNaPure System (Roche Diagnostics, Mannheim, Germany) from mononuclear cells after lysis of erythrocytes. The patients’ libraries were generated with the Nextera Flex for enrichment kit (Illumina, San Diego, CA, USA) and sequences for DNMT3A and TET2 enriched with the IDT xGen hybridization capture of DNA libraries protocol and customized probes (IDT, Coralville, IA, USA). The libraries were sequenced on an Illumina NovaSeq 6000 with a mean coverage of 2147× and a minimum coverage of 400×, reaching a sensitivity of 2%. Reads were mapped to the reference genome (UCSC hg19) using Isaac aligner (v2.10.12) and a small somatic variant calling was performed with Pisces (v5.1.3.60). Protein truncating variants were classified as mutation. Non-synonymous changes were included, if they were well annotated (several definite submissions to COSMIC, IRAC, or ClinVAR). Other non-protein truncating variants were defined as variants of uncertain significance.
Statistical analysis
Continuous variables are presented as mean (SD) unless otherwise noted. Analysis of variance testing was used for comparison of continuous variables between groups. Categorical variables were compared by the χ2 test or Fisher’s exact test as appropriate. Bonferroni correction was used for multiple testing as appropriate. For survival analysis within the individual groups, the Kaplan–Meier analyses were used. Log-rank testing was applied to compare survival analysis and a stepwise multivariable Cox proportional regression analysis was performed to account for the potential effect of confounding variables. Statistical analysis was performed with SPSS statistical software package, version 24.0.
Results
Patient characteristics and detection of mutations
The clinical and echocardiographic characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics and echocardiographic findings in patients with DNMT3A/TET2 and without DNMT3A/TET2 CHIP-driver mutations
| Total cohort (n = 279) | DNMT3A/TET2 (n = 93) | No-DNMT3A/TET2 (n = 186) | P-value | |
|---|---|---|---|---|
| Age (years) (n = 279) | 83.0 (79.3–86.1) | 83.2 (80.1–86.7) (n = 93) | 82.9 (79.1–85.8) (n = 186) | 0.179 |
| Sex (female) (%) (n = 279) | 42.7% (n = 119) | 51.6% (n = 48) | 38.2% (n = 71) | 0.032 |
| BMI (kg/m2) (n = 279) | 26.2 (23.4–29.4) | 25.5 (22.7–29.4) (n = 93) | 26.5 (23.9–29.4) (n = 186) | 0.147 |
| Hypertension (%) (n = 279) | 88.2% (n = 246) | 91.4% (n = 85) | 86.5% (n = 161) | 0.238 |
| Diabetes (%) (n = 279) | 31.2% (n = 87) | 30.1% (n = 28) | 31.7% (n = 59) | 0.784 |
| On insulin (%) (n = 279) | 9.3% (n = 26) | 10.7% (n = 10) | 8.6% (n = 16) | 0.560 |
| Previous myocardial infarction (%) (n = 279) | 16.5% (n = 46) | 17.2% (n = 16) | 16.1% (n = 30) | 0.820 |
| Previous PCI (%) (n = 279) | 40.9% (n = 114) | 38.7% (n = 36) | 41.9% (n = 78) | 0.605 |
| Previous CABG (%) (n = 279) | 9.3% (n = 26) | 10.7% (n = 10) | 8.6% (n = 16) | 0.560 |
| Previous stroke (%) (n = 279) | 15.4% (n = 43) | 19.3% (n = 18) | 13.4% (n = 25) | 0.197 |
| Previous TIA (%) (n = 279) | 3.6% (n = 10) | 3.2% (n = 3) | 3.8% (n = 7) | 1.0 |
| Carotid artery disease (%) (n = 279) | 18.3% (n = 51) | 17.2% (n = 16) | 18.8% (n = 35) | 0.742 |
| Peripheral artery disease (%) (n = 279) | 11.5% (n = 32) | 10.7% (n = 10) | 11.8% (n = 22) | 0.790 |
| COPD (%) (n = 279) | 19.7% (n = 55) | 18.3% (n = 17) | 20.4% (n = 38) | 0.670 |
| Atrial fibrillation (%) (n = 279) | 47.3% (n = 132) | 50.5% (n = 47) | 45.7% (n = 85) | 0.445 |
| NYHA III (n = 279) | 68.8% (n = 192) | 72.0% (n = 67) | 67.2% (n = 125) | 0.411 |
| NYHA IV (n = 279) | 12.2% (n = 34) | 15.0% (n = 14) | 10.7% (n = 20) | 0.301 |
| LVEF (n = 279) | 60 (50–60) | 60 (45–60) (n = 93) | 60 (50–60) (n = 186) | 0.237 |
| Pmean (mmHg) (n = 260) | 43 (32–53) | 41 (30–53) (n = 88) | 45 (34–53) (n = 172) | 0.171 |
| Mitral valve insufficiency (>II) (n = 274) | 7.6% (n = 21) | 8.7% (n = 8) (n = 92) | 7.1% (n = 13) (n = 182) | 0.648 |
Continuous variables are shown as mean (SD) and median (interquartile range). Categorical variables are shown as frequency (%). Statistically significant difference is shown in bold.
BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; Pmean, mean transvalvular pressure gradient; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Patients had a median age of 83 (79.3–86.1) years. Pre-dilation of the stenotic valve was performed in the majority of patients. Seventy-two patients received a balloon-expandable prosthesis and 206 a self-expandable valve, transfemorally. One patient underwent a balloon-valvuloplasty.
Ninety-three of the 279 (33.3%) patients were carriers of a DNMT3A- (n = 53) or a TET2- (n = 40) CHIP-driver mutation with a variant allele frequency (VAF) ≥ 2%, with 10 patients harbouring mutations in both genes. A detailed list including the type of mutation and VAF is provided in the Supplementary material online, Tables S1 and S2. In accordance with previous reports in the general population,2 the prevalence of DNMT3A- and TET2-CHIP-driver mutations in the present cohort with severe AV stenosis increased with patient age from 25% (2 out of 8 patients) in the age group of 55–69 years, to 25.4% (17 out of 67) at age 70–79 years, to 34.7% (65 out of 187) at age 80–89 years, and finally to 52.9% (9 out of 17) at age 90–99 years. However, compared to published cohorts of unselected populations2 as well as patients with coronary heart disease,3 the frequency of the identified DNMT3A- and TET2-CHIP-driver mutations with a variant allele frequency ≥ 2% was considerably higher in all age groups. Thus, DNMT3A- and TET2-CHIP-driver mutations are enriched in patients with severe calcified AV stenosis undergoing TAVI.
Association between CHIP-driver mutations and baseline clinical and procedural characteristics
Tables 1and 2 summarize the baseline pre-TAVI clinical characteristics and laboratory values for carriers and non-carriers of DNMT3A- and TET2-CHIP-driver mutations. As illustrated, there were no significant differences except for the slightly higher frequency of female patients in the DNMT3A- and TET2-CHIP-driver mutations group. However, neither risk factors for nor the presence of clinically manifest atherosclerotic vascular disease were different. Likewise, procedural characteristics as well as laboratory values did not differ between the two groups. NT-proBNP serum levels were slightly, but non-significantly elevated in carriers of DNMT3A/TET2-CHIP-driver mutations.
Table 2.
Laboratory parameters in patients with DNMT3A/TET2 and without DNMT3A/TET2 CHIP-driver mutations
| Total cohort (n = 279) | DNMT3A/TET2 (n = 93) | No-DNMT3A/TET2 (n = 186) | P-value | |
|---|---|---|---|---|
| C-reactive protein (mg/dL) (n = 273) | 0.36 (0.15–0.99) | 0.41 (0.16–0.98) (n = 92) | 0.34 (0.14–1.01) (n = 181) | 0.638 |
| Leucocytes (/nL) (n = 279) | 7.1 (5.9–8.2) | 6.8 (5.8–8.0) (n = 93) | 7.2 (5.9–8.3) (n = 186) | 0.254 |
| Interleukin 6 (pg/mL) (n = 261) | 5.8 (3.5–13.0) | 5.7 (3.8–12.0) (n = 88) | 6.1 (3.4–13.1) (n = 173) | 0.875 |
| Haemoglobin (g/dL) (n = 279) | 11.9 ± 1.9 | 11.7 ± 1.9 (n = 93) | 12 ± 1.9 (n = 186) | 0.246 |
| Haematocrit (%) (n = 279) | 35.9 (31.7–38.9) | 35.3 (31.1–38.8) (n = 93) | 35.9 (32.0–39.0) (n = 186) | 0.411 |
| Platelets (/nL) (n = 279) | 214 (169–259) | 210 (162–254) (n = 93) | 218 (179–262) (n = 186) | 0.316 |
| Creatinine (mg/dL) (n = 279) | 1.15 (0.91–1.53) | 1.18 (0.93–1.60) (n = 93) | 1.12 (0.89–1.50) (n = 186) | 0.164 |
| Urea (mg/dL) (n = 277) | 46 (34–63) | 50 (35–65) (n = 91) | 46 (34–62) (n = 186) | 0.149 |
| NT-proBNP (pg/mL) (n = 243) | 2069 (956–5095) | 2244.5 (1139–6998) (n = 84) | 1933 (880–4503) (n = 159) | 0.058 |
| CK (U/L) (n = 275) | 71 (48–104) | 84 (45–111) (n = 91) | 69 (49–100) (n = 184) | 0.460 |
| CK-MB (U/L) (=261) | 17 (14–22) | 17 (14–21) (n = 82) | 17 (13–23) (n = 179) | 0.634 |
| Hs Troponin (pg/mL) (n = 240) | 24 (15–45) | 26 (17–57) (n = 79) | 24 (14–40) (n = 161) | 0.094 |
Continuous variables are shown as mean (SD) and median (interquartile range).
Prognostic significance of DNMT3A- and TET2-CHIP-driver mutations following transcatheter aortic valve implantation
During the first 30 days after TAVI, 6 out of 186 no-DNMT3A/TET2-CHIP-driver mutation carriers (3.2%) and 2 (1 DNMT3A and 1 TET2) out of 93 DNMT3A/TET2-CHIP-driver mutation carriers (2.1%) died due to procedure-related complications.
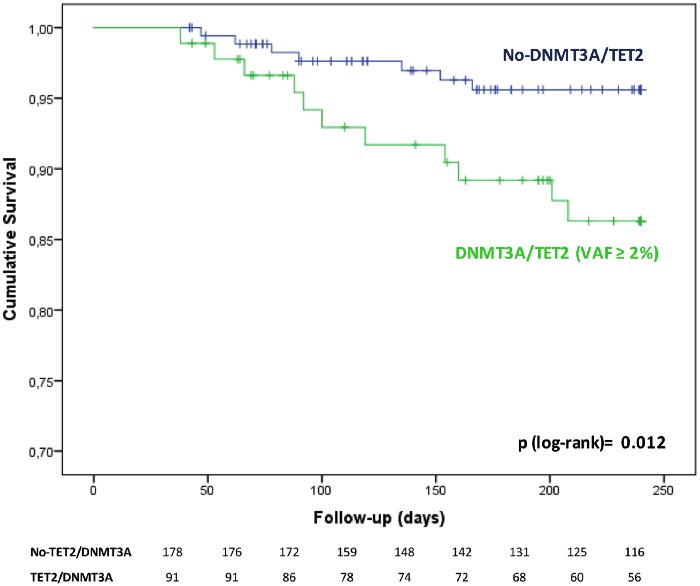
In order to assess medium-term clinical outcome following TAVI, patients dying within the first 30 days after the procedure were excluded to avoid potential confounding effects of intra- or early post-TAVI complications leading to death. Take home figure illustrates that patients carrying a DNMT3A- or TET2-CHIP-driver mutation experienced a significantly worse clinical outcome for death during the first 8 months after TAVI. Since carriers of DNMT3A/TET2-CHIP-driver mutations were significantly more frequently female, we separately analysed the Kaplan–Meier survival curves for women and men. However, as illustrated in Supplementary material online, Figure 1, there was no difference between female and male patients with respect to outcome and the log rank P-value adjusted for gender was 0.016. Thus, the difference in gender does not appear to confound our results that DNMT3A/TET2-CHIP-driver mutations are associated with increased mortality following TAVI. Likewise, when a stepwise multivariable Cox proportional regression analysis was performed to account for the potential effect of both age or sex, carrying a DNMT3A- or TET-2-CHIP-driver mutation remained independently associated with increased death in the medium-term follow-up after TAVI (HR 3.1, 95% CI 1.17–8.08; P = 0.022). Finally, further adjustment for NT-proBNP serum levels at baseline in addition to age and sex confirmed the independent association between the presence of DNMT3A/TET2-CHIP-driver mutations and death (HR 4.81, 95% CI 1.49–15.57; P = 0.009).
Take home figure.
Overall survival of patients with DNMT3A- or TET2-CHIP-driver mutations with a variant allele frequency ≥ 2% vs. patients without DNMT3A or TET2 mutations. *Patients with follow-up <30 days have been excluded in order to remove mortality due to peri-procedural complications.
Interestingly, although patients carrying a DNMT3A/TET2-mutation with a VAF > 0.1 numerically showed a reduced 8-months survival (85.3%) compared to those with a VAF ≤ 0.1 (89.5%) and the no-CHIP patients (96.1%), the overall number of events is insufficient to firmly establish a dose–response relationship between VAF and subsequent death following successful TAVI.
In order to assess, whether the increase in the observed mortality may be confounded by the presence of concomitant atherosclerotic disease, atrial fibrillation or increased New York Heart Association (NYHA) class, we tested all individual components as well as the extent of atherosclerotic disease defined as involving one vascular bed (=CAD), two vascular territories [defined as CAD plus peripheral arterial occlusive disease (PAOD) or cerebrovascular disease], and three vascular territories (CAD, PAOD, and cerebrovascular disease). As summarized in Table 3, neither the presence of atrial fibrillation or increased NYHA class nor the extent of polyvascular atherosclerosis were significantly associated with clinical outcome. Moreover, there was no difference with respect to the prevalence of polyvascular disease between carriers of a DNMT3A/TET2-CHIP-driver mutation and no-CHIP patients, with 52.8% in no-CHIP vs. 47.2% in DNMT3A/TET2-CHIP-driver mutation carriers (P = 0.44) in one bed, 15.7% vs. 21.9% (P = 0.24) in two territories, respectively, and 5.6% vs. 3.3% (P = 0.55), respectively, in three territories affecting polyvascular atherosclerotic disease. Thus, in the present cohort with severe aortic stenosis, patients harbouring a DNMT3A/TET2-CHIP-driver mutation do not appear to be enriched for polyvascular atherosclerosis nor did the different manifestations of atherosclerosis affect outcome in the mid-term following TAVI.
Table 3.
Association of CHIP-mutations, polyvascular bed disorders and risk factors with clinical outcome
| 95% CI for HR |
|||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wald | P-value | HR | Lower | Upper | |
| DNMT3A/TET2 | 1.146 | 0.484 | 5.617 | 0.018 | 3.145 | 1.219 | 8.114 |
| Vascular beds | |||||||
| 1 | −0.818 | 0.532 | 2.360 | 0.124 | 0.441 | 0.155 | 1.253 |
| 2 | −0.099 | 0.636 | 0.024 | 0.876 | 0.906 | 0.260 | 3.152 |
| 3 | 0.091 | 1.029 | 0.008 | 0.930 | 1.095 | 0.146 | 8.231 |
| NYHA (III/IV) | 0.711 | 0.750 | 0.899 | 0.343 | 2.037 | 0.468 | 8.862 |
| Previous atrial fibrillation | 0.118 | 0.471 | 0.063 | 0.802 | 1.126 | 0.447 | 2.836 |
Statistically significant difference is shown in bold.
1 vascular bed = coronary artery disease.
2 vascular beds = coronary artery disease and cerebrovascular disease or peripheral arterial occlusive disease.
3 vascular beds = coronary artery disease, cerebrovascular disease and peripheral arterial occlusive disease.
NYHA, New York Heart Association.
Potential mechanistic insights into increased mortality of CHIP carriers after transcatheter aortic valve implantation
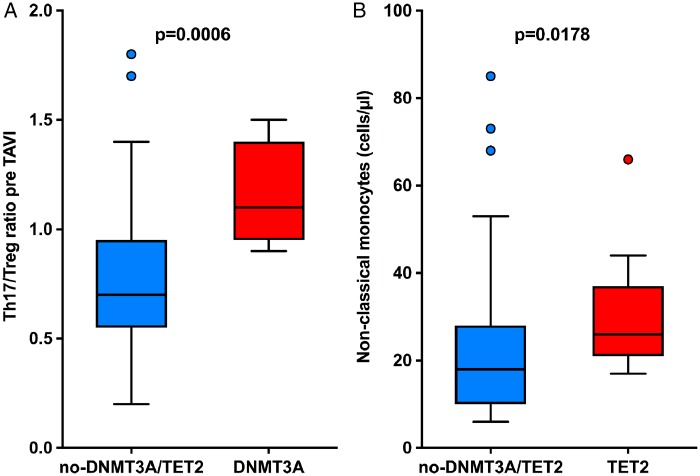
Differential myeloid and T-cell distributions were analysed pre-TAVI by FACS in a subset of 82 patients, of whom 15 carried a DNMT3A- and 13 a TET2-CHIP mutation. FACS analysis was performed without knowing the CHIP status of the patients. There were no differences between the three groups with respect to total counts of neutrophils, monocytes, total T cells, and the CD4+ T-cell subset. However, as illustrated in Figure 1, patients harbouring a DNMT3A-CHIP-driver mutation demonstrated a significantly increased Th17/T reg ratio indicating a pro-inflammatory T-cell polarization, whereas patients harbouring a TET2-CHIP-driver mutation exhibited increased levels of circulating non-classical monocytes (CD14dimCD16++), which are known to secrete high levels of pro-inflammatory cytokines. While the increased Th17/Treg ratio remained statistically significant after Bonferroni correction to account for multiple testing with a P-value of 0.023, the difference in non-classical monocytes was no longer statistically significant most likely due to the limited number of patients studied in this subanalysis. Thus, these exploratory analyses may indicate that both mutations confer a heightened inflammatory state, albeit by different mechanisms.
Figure 1.
(A) Th17/T reg ratio in carriers of a DNMT3A-CHIP-driver mutation and patients without DNMT3A/TET2-mutations. (B) Number of non-classical monocytes in carriers of TET2-CHIP-driver mutations and patients without TET2/DNMT3A mutations. TAVI, transcatheter aortic valve implantation.
Discussion
This is the first study to investigate the occurrence of acquired somatic mutations in the most commonly mutated CHIP-driver genes DNMT3A and TET2 in patients with severe degenerative AV stenosis. Our results demonstrate that DNMT3A- or TET2-CHIP-driver mutations occur frequently in this patient population with advanced age, appear to be associated with increases in pro-inflammatory subsets of circulating leucocytes, and confer a profound increase in mortality even after successful correction of the AV stenosis by TAVI.
The results of the present study significantly extend previous reports showing that CHIP-driver mutations increase with age in healthy subjects,2 are associated with the risk and prognosis of patients with CAD,3 and may contribute to the progression of chronic heart failure.11 Importantly, when using the classical definition of CHIP as a variant allele frequency of the mutation ≥ 2%,1 the age-adjusted prevalence of DNMT3A- or TET2-CHIP-driver mutations appears to be significantly higher in the present study cohort compared with previously published data in age-matched patients with CAD3 as well as compared to our own report in patients with chronic heart failure.11 Since both TET2- and DNMT3A loss-of-function in murine models of heart disease were experimentally shown to activate the inflammasome complex3 and to promote fibrosis development,12 both of which are of fundamental importance for developing degenerative AV stenosis,13,14 it is tempting to speculate that DNMT3A or TET2-CHIP-driver mutations have contributed to the development of severe AV stenosis.
Our extensive profiling for circulating inflammatory serum markers did not identify any differences between carriers of a DNMT3A- or TET2-CHIP-driver mutation and non-carriers, which is in agreement with previous studies in patients with CAD3 or chronic heart failure.11 However, when we assessed the inflammatory phenotype of specific circulating subsets of leucocytes in a subset of our patient population, it became apparent that patients carrying a DNMT3A-CHIP-driver mutation demonstrated a significantly elevated ratio of the pro-inflammatory Th17 cells over the anti-inflammatory regulatory T cells, whereas patients carrying a TET2-CHIP-driver mutation showed increased levels of so-called non-classical monocytes, which accumulate under various chronic inflammatory conditions and secrete high levels of pro-inflammatory cytokines, including tumour necrosis factor (TNF) α, IL-1β, and IL-8.15 Indeed, very recent studies in subjects with CHIP disclosed that single DNMT3A or TET2 mutated individuals had different lineage restriction patterns, with DNMT3A mutations affecting all haematopoietic lineages including T cells, whereas TET2 mutation was associated with myeloid restriction and mostly affected monocytes, but not T cells.16,17 These observations may also reconcile the seemingly contradictory findings that the DNA methyltransferase DNMT3A exerts similar pro-inflammatory effects as the DNA demethylase TET2. However, given that profiling of specific subsets of leucocytes was performed only in a subset of our patient population, these results should be regarded as hypothesis-generating and need to be confirmed in larger patient cohorts.
Strikingly, patients carrying either a DNMT3A- or a TET2-CHIP-driver mutation demonstrated profoundly increased mortality during medium-term follow-up even after successful replacement of the stenotic AV by TAVI and excluding the first 30 days after the procedure to eliminate potential confounding factors introduced by the TAVI procedure itself, e.g. vascular complications, renal impairment, major bleeding, and ischaemia during rapid pacing and balloon-expandable valve implantation. Since DNMT3A- and TET2-CHIP-driver mutation carriers did not differ from non-DNMT3A/TET2-mutation carriers with respect to the presence and extent of atherosclerotic disease, risk factors for atherosclerosis, and other co-morbidities, the increased mortality cannot be attributed to those potentially confounding factors. Thus, the present study extends previous observations in CAD3 as well as chronic heart failure11 and further establishes DNMT3A- and TET2-CHIP-driver mutations as an important risk factor for the progression of cardiovascular disease.
Conclusion
Our data support the hypothesis that acquired somatic mutations in haematopoietic cells due to mutations in the most common CHIP-driver genes DNMT3A and TET2 may be significantly associated with the progression of degenerative AV stenosis and a worse clinical mid-term outcome even after successful valve replacement by TAVI. Future studies will have to validate our findings in larger cohorts and test whether a targeted anti-inflammatory therapy may be a valuable treatment strategy in carriers of DNMT3A- or TET2-CHIP-driver mutations with severe degenerative AV stenosis undergoing TAVI.
Supplementary Material
Acknowledgements
We thank Graziella Pergola for her support in the Biobanking and Alexander Berkowitsch for his valuable help in the statistical analysis.
Funding
The study was supported by the German Research Foundation (SFB 834), the Excellence Cluster of Cardiopulmonary Systems, and by the German Centre for Cardiovascular Research, Berlin, Germany, partner site Frankfurt Rhine-Main.
Conflict of interest: A.M.Z. reports grants from the German Research Foundation and BMBF/DZHK, and personal fees from Sanofi, Pfizer, Amgen, and Boehringer Ingelheim. S.D. reports grants from BMBF/DZHK. M.V.-N. is proctor for Medtronic, Boston Scientific and Abbott. S.F. is proctor and reports consultancy activities for Abbott and Edwards Lifesciences. Michael Rieger reports grants from the German Research Foundation. All other authors have no conflicts of interest related to the subject of the article.
References
- 1. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL.. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL.. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL.. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C-L, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K.. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K.. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 2018;123:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.. Burden of valvular heart diseases: a population-based study. Lancet (Lond, Engl) 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 7. Lindman BR, Clavel M-A, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P.. Calcific aortic stenosis. Nat Rev Dis Prim 2016;2:16006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinning J-M, Scheer A-C, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, Müller C, Vasa-Nicotera M, Grube E, Nickenig G, Werner N.. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J 2012;33:1459–1468. [DOI] [PubMed] [Google Scholar]
- 9. Liebetrau C, Hoffmann J, Dörr O, Gaede L, Blumenstein J, Biermann H, Pyttel L, Thiele P, Troidl C, Berkowitsch A, Rolf A, Voss S, Hamm CW, Nef H, Möllmann H.. Release kinetics of inflammatory biomarkers in a clinical model of acute myocardial infarction. Circ Res 2015;116:867–875. [DOI] [PubMed] [Google Scholar]
- 10. Mahnke YD, Beddall MH, Roederer M.. OMIP-017: human CD4(+) helper T-cell subsets including follicular helper cells. Cytometry A 2013;83:439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA.. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K.. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhan Q, Song R, Zeng Q, Yao Q, Ao L, Xu D, Fullerton DA, Meng X.. Activation of tlr3 induces osteogenic responses in human aortic valve interstitial cells through the NF-κB and ERK1/2 pathways. Int J Biol Sci 2015;11:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathieu P, Boulanger M-C.. Basic mechanisms of calcific aortic valve disease. Can J Cardiol 2014;30:982–993. [DOI] [PubMed] [Google Scholar]
- 15. Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B.. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep 2015;5:13886.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buscarlet M, Provost S, Zada YF, Bourgoin V, Mollica L, Dubé M-P, Busque L.. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood 2018;132:277–280. [DOI] [PubMed] [Google Scholar]
- 17. Arends CM, Galan-Sousa J, Hoyer K, Chan W, Jäger M, Yoshida K, Seemann R, Noerenberg D, Waldhueter N, Fleischer-Notter H, Christen F, Schmitt CA, Dörken B, Pelzer U, Sinn M, Zemojtel T, Ogawa S, Märdian S, Schreiber A, Kunitz A, Krüger U, Bullinger L, Mylonas E, Frick M, Damm F.. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia 2018;32:1908–1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.