Abstract
Systems combining photopolymerization and thermal polymerization have already been reported in the literature. Upon near-infrared (NIR) light exposure, this principle of polymerization is called photoinduced thermal polymerization or photothermal polymerization. Thanks to an NIR dye used as the light-to-heat convertor (called hereafter a heater), an alkoxyamine (e.g., BlocBuilder-MA) is dissociated upon NIR light irradiation, initiating the free-radical polymerization of methacrylates. In the present paper, a novel approach is presented for the first time to decompose the alkoxyamine through a direct heat generation upon mid-infrared irradiation by a CO2 laser at 10.6 μm. Compared with previous approaches, there is no additional heater used in this work, as the heat is directly generated by laser irradiation on the alkoxyamine/monomer system. The polymerization can be initiated for benchmark methacrylate monomers with spatial controllability, that is, only in the laser-irradiated area, opening the way for laser write or three-dimensional printing applications in the presence of fillers.
Introduction
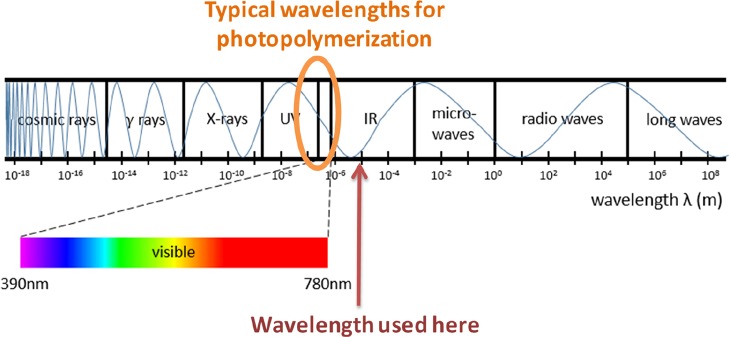
Photopolymerization of (meth)acrylates is reported widespread in the literature. In general, the polymerization process is possible with a photoinitiator, that is, a generator of active species (e.g., cations or free radicals) after light absorption. Traditionally, UV light is used for such polymerizations, but now, the use of visible light has been democratized.1,2 Thanks to the use of these longer wavelength light, curing of filled samples and more particularly composites is more and more promising. More particularly, the use of near-infrared (NIR) light for photopolymerization of (meth)acrylates is a good answer to such an issue.3−6 The higher the wavelength is, the deeper the penetration of light inside the media, especially in the presence of fillers, can be. In the present communication, we propose to use long-wavelength (mid-IR CO2 laser), rather than visible or even NIR, light for the polymerization process. Carbon dioxide lasers can deliver light with the wavelength in the mid-infrared region, as presented in Scheme 1.
Scheme 1. Classical Photopolymerization vs Polymerization Upon CO2 Laser Exposure.
Actually, the use of carbon dioxide lasers for polymer applications is not new. Indeed, carbon dioxide lasers are already extensively used in selective laser sintering.7−9 In such processes, blends of polymer powders are typically employed, and the carbon dioxide laser is used to melt the powders to construct the shape of the desired object in three dimensions (3D).
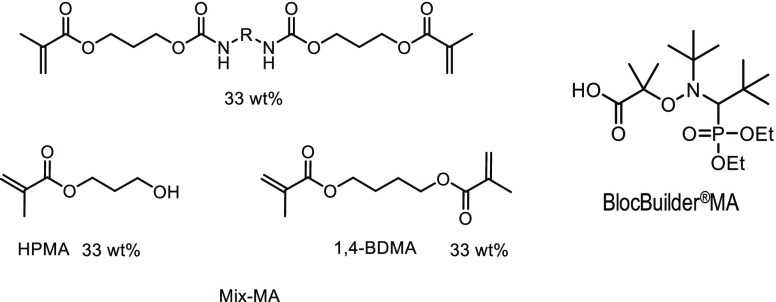
Herein, the principle is different: the heat delivered by the laser will be used for the first time to decompose a thermosensitive initiator, namely an alkoxyamine, dispersed in a liquid resin. To the best of our knowledge, very few studies have been devoted to this principle of polymerization, and all of these works are based on thermal initiators other than alkoxyamines.10−12 In particular, methyl methacrylate was successfully polymerized with a continuous-wave CO2 laser.10 Examples of frontal polymerization ignited by CO2 laser are given in refs (13) and (14). Therefore, we propose to polymerize a benchmark methacrylate monomer blend (Mix-MA, depicted in Scheme 2) containing an alkoxyamine as the thermal initiator (BlocBuilder-MA).
Scheme 2. Chemical Structures of the Monomers and the Alkoxyamine.
Results and Discussion
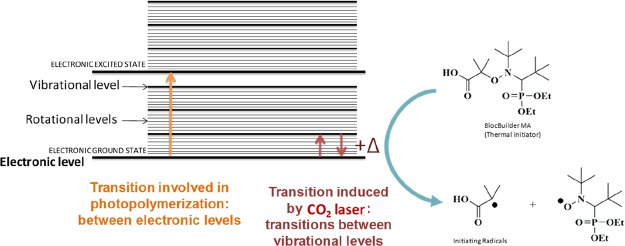
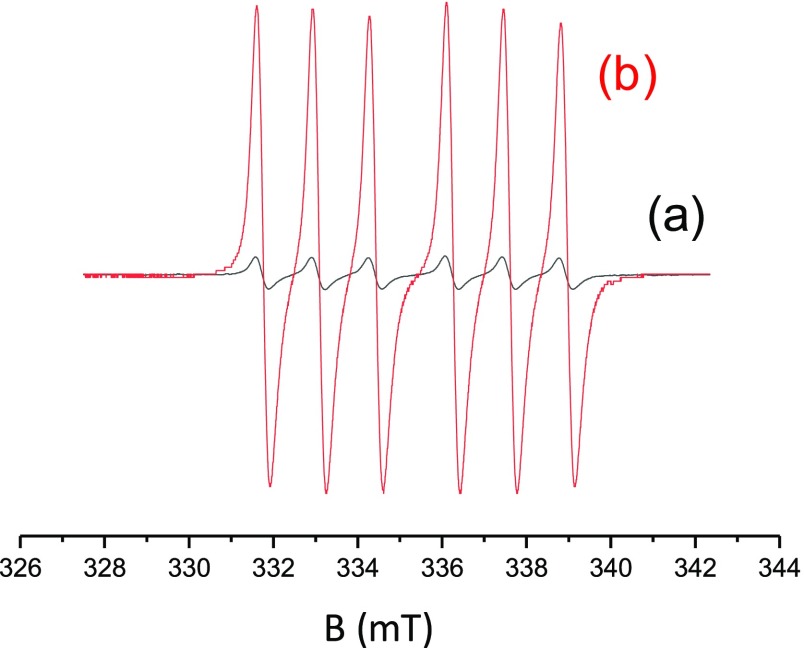
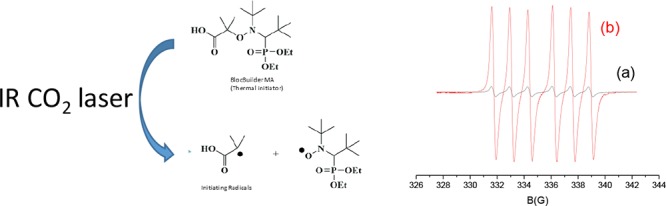
As specificity, in classical photopolymerization processes, the transitions involved during the excitation of the photoinitiator are between well-defined electronic levels (i.e., between its ground state and its excited state). To get this result, the wavelength of the light and thus the energy of the photons must be compatible with the energy gap between the ground state and the excited state to allow the occurrence of electronic transition. Therefore, a high energy is usually necessary to induce such an electronic transition. As the energy delivered by the light is inversely proportional to the wavelength, the photon energy is extremely low while using infrared light and not sufficient to enable the electrons to reach the higher electronic excited state. With such low-energy wavelengths, transitions are only observed between vibrational levels. Therefore, the loss of energy from the vibrationally excited levels is in the form of heat. Therefore, upon CO2 laser irradiation, the sample is locally heated, leading to a potential homolysis of the alkoxyamine (BlocBuilder-MA), enabling the initiation of free-radical polymerization. The mechanism is presented in Scheme 3. Such a behavior can be demonstrated through electron spin resonance (ESR) experiments. Indeed, using a solution of BlocBuilder-MA in tert-butylbenzene (10–4 M), almost no radical species were observed (Figure 1, curve a) before irradiation. Conversely, upon exposure to carbon dioxide laser, an important increase of the radical signal was detected (Figure 1, curve b). The ESR spectrum obtained clearly corresponded to the nitroxide radicals generated by the cleavage of BlocBuilder-MA, that is, the acyclic β-phosphorylated nitroxide SG1 (N-tert-butyl-N-[1-(diethoxyphosphoryl)-2,2-dimethylpropyl-N-oxyl nitroxide) radical characterized by the hyperfine coupling constants: aN = 13.7 G; aP = 45.7 G.2
Scheme 3. Mechanism of IR-Induced Decomposition of Alkoxyamines.
Figure 1.
ESR experiments on 10–4 M of Blocbuilder-MA dissolved into tert-butylbenzene (a) before and (b) after exposure to CO2 laser.
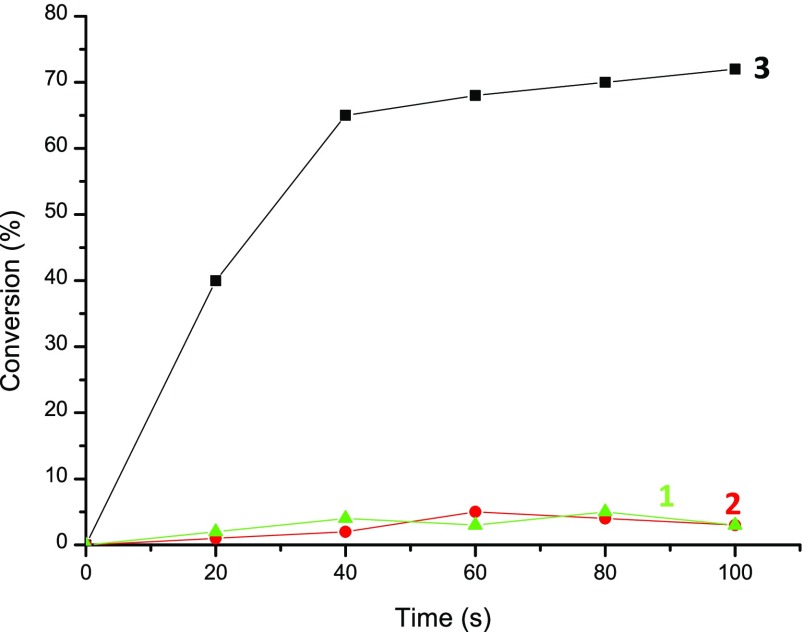
Considering that initiating carbon-centered radicals were also generated upon irradiation with carbon dioxide laser, polymerization experiments were carried out. A 2 wt % of BlocBuilder-MA was dissolved into the benchmark monomer blend and irradiated by the carbon dioxide laser. Remarkably, the polymerization of Mix-MA could be induced only in the simultaneous presence of BlocBuilder-MA and IR light (Figure 2, curve 3), that is, no polymerization occurs at the investigated timescale without IR irradiation (Figure 2, curve 1) or without BlocBuilder-MA (Figure 2, curve 2).
Figure 2.
Polymerization profiles of mix-MA (methacrylate function conversion vs irradiation time): (1) without irradiation; (2) upon CO2 laser irradiation but without BlocBuilder-MA; (3) upon CO2 laser irradiation with 2 wt % BlocBuilder-MA.
Using this approach in CO2 laser write experiments, transparent polymers were produced, but the shape was not satisfying as numerous defects could be detected on the different pieces (Figure 3), that is, with polymerization beyond the light-irradiated areas. For pure organic resins, the thermal effect is probably too high to really control the spatial resolution, that is, the heat diffusion beyond the irradiated areas also induced polymerization, leading to poorly resolved patterns.
Figure 3.
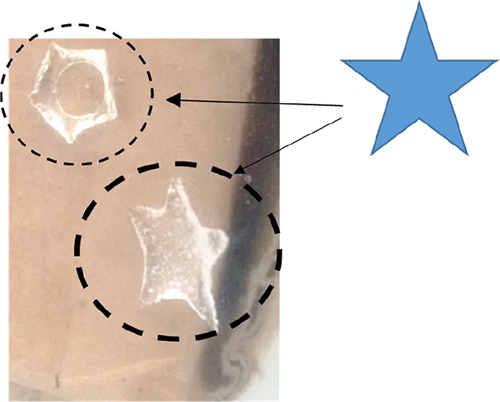
Laser write polymerization experiments upon CO2 laser using different powers of irradiation (shape expected: a star).
To avoid this diffusion of heat into the sample, fillers were introduced in the monomer blend in order to dissipate the heat released during polymerization. As a kind of fillers, high contents of silica were selected for good heat dissipation. This type of filler is also widely used in composite manufacturing. In particular, they are extensively used for manufacturing tools or electronic materials.
Remarkably, a mixture of 80/20 wt % fillers/Mix-MA resin containing 2% of BlocBuider-MA was prepared and has been successfully polymerized in CO2 laser write experiments with excellent spatial controllability, as shown in Figure 4. The shape is now very well resolved compared to that in Figure 3, and the polymerized parts can be easily removed. Without BlocBuilder-MA, no polymerization occurs, showing the crucial role of this compound to generate initiating radicals upon CO2 laser exposure.
Figure 4.
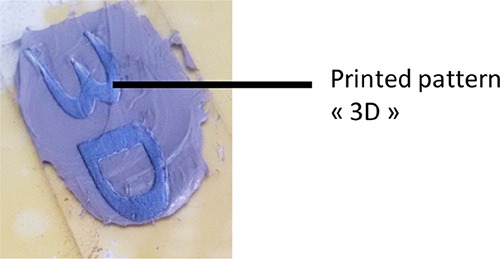
Polymerization upon CO2 laser of filled resin in laser write experiment.
Conclusions
In conclusion, alkoxyamines can be decomposed upon cheap and easily accessible CO2 lasers. A benchmark methacrylate resin has been successfully polymerized upon CO2 laser irradiation in the presence of an alkoxyamine. The patterns obtained in the CO2 laser write experiments for clear resins are not yet well defined. However, remarkably, the polymerization of filled samples is highly satisfying: a well-resolved polymerization is observed. Therefore, polymerization of composites and in particular highly filled samples is possible using this approach. Further applications in 3D printing experiments will be examined in the forthcoming works. The wavelength of excitation must be selected to ensure the light penetration in the filled samples.
Experimental Section or Computational Methods
Materials
The blend “Mix-MA”, used as a benchmark monomer, has been prepared with 33.3 wt % of (hydroxypropyl)methacrylate, 33.3 wt % of 1,4-butanediol dimethacrylate, and 33.3 wt % of a urethane dimethacrylate monomer, obtained from Sigma Aldrich and represented in Scheme 2. Mix-MA was already used in previous works.3,4 More particularly, its viscosity is well adapted for the preparation of formulations. In addition, interestingly, Mix-MA is not too viscous. Therefore, oxygen inhibition occurs, and only good-performance systems can polymerize it. BlocBuilder-MA was obtained from Arkema. tert-Butylbenzene was obtained from Sigma-Aldrich.
CO2 Laser and Laser Write Experiments
A 10 W CO2 laser derived from an engraving machine was used as the irradiation source. In laser write experiments, the engraving machine has been modified to work with an organic liquid and filled resins using a home-made resin tank.
ESR Experiments
A Magnettech MS400 ESR spectrometer was used.
Polymerization Experiments
The photosensitive formulations were deposited on a BaF2 pellet under air (thickness ≈ 1.4 mm for thick samples) and irradiated with a light source. The evolution of the C=C peak relative to the methacrylate function was continuously followed by Fourier transform infrared spectroscopy (JASCO FTIR 6600), as previously described, at about 6165 cm–1 for thick samples.
Acknowledgments
The authors gratefully acknowledge support from the Region Grand Est for the grant “MIPPI-4D”.
The authors declare no competing financial interest.
References
- Fouassier J. P.; Lalevée J.. Photoinitiators for Polymer Synthesis-Scope, Reactivity, and Efficiency; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, 2012. [Google Scholar]
- Lalevée J.; Fouassier J.-P.. How To Design Novel Photoinitiators for Blue Light. Photopolymerisation Initiating Systems; The Royal Society of Chemistry: London, 2018; Chapter 6, pp 179–199 [Google Scholar]
- Bonardi A.-H.; Bonardi F.; Morlet-Savary F.; Dietlin C.; Noirbent G.; Grant T. M.; Fouassier J.-P.; Dumur F.; Lessard B. H.; Gigmes D.; et al. Photoinduced Thermal Polymerization Reactions. Macromolecules 2018, 51, 8808–8820. 10.1021/acs.macromol.8b01741. [DOI] [Google Scholar]
- Bonardi A.-H.; Bonardi F.; Morlet-Savary F.; Dietlin C.; Noirbent G.; Grant T. M.; Fouassier J.-P.; Dumur F.; Lessard B. H.; Gigmes D.; et al. Photoinduced Thermal Polymerization Reactions. Macromolecules 2018, 51, 8808–8820. 10.1021/acs.macromol.8b01741. [DOI] [Google Scholar]
- Schmitz C.; Halbhuber A.; Keil D.; Strehmel B. NIR-Sensitized Photoinitiated Radical Polymerization and Proton Generation with Cyanines and LED Arrays. Prog. Org. Coat. 2016, 100, 32–46. 10.1016/j.porgcoat.2016.02.022. [DOI] [Google Scholar]
- Schmitz C.; Strehmel B. Photochemical Treatment of Powder Coatings and VOC-Free Coatings with NIR Lasers Exhibiting Line-Shaped Focus: Physical and Chemical Solidification. ChemPhotoChem 2017, 1, 26–34. 10.1002/cptc.201600009. [DOI] [Google Scholar]
- Goodridge R. D.; Tuck C. J.; Hague R. J. M. Laser Sintering of Polyamides and Other Polymers. Prog. Mater. Sci. 2012, 57, 229–267. 10.1016/j.pmatsci.2011.04.001. [DOI] [Google Scholar]
- Wudy K.; Lanzl L.; Drummer D. Selective Laser Sintering of Filled Polymer Systems: Bulk Properties and Laser Beam Material Interaction. Phys. Procedia 2016, 83, 991–1002. 10.1016/j.phpro.2016.08.104. [DOI] [Google Scholar]
- Greiner S.; Wudy K.; Lanzl L.; Drummer D. Selective Laser Sintering of Polymer Blends: Bulk Properties and Process Behavior. Polym. Test. 2017, 64, 136–144. 10.1016/j.polymertesting.2017.09.039. [DOI] [Google Scholar]
- Yue C.; Xu X.; Lu H.; Yue C.. A Study of Infrared Laser-Initiated Polymerization of Methyl Methacrylate. Integration of Fundamental Polymer Science and Technology; Springer Netherlands, 1988; Vol. 2, pp 75–80. [Google Scholar]
- Jawad H. A.; Jassim A. S.; Yousif Y. B. CW-CO2 Laser Photoinitiated Polymerization of Methyl Methacrylate. J. Edu. & Sci. 2010, 23, 24–36. [Google Scholar]
- Liu Y.; Wang C.-F.; Chen S. Facile Access to Poly(DMAEMA-Co-AA) Hydrogels via Infrared Laser-Ignited Frontal Polymerization and Their Polymerization in the Horizontal Direction. RSC Adv. 2015, 5, 30514–30521. 10.1039/C5RA01366F. [DOI] [Google Scholar]
- Zhou Z.-F.; Yu C.; Wang X.-Q.; Tang W.-Q.; Wang C.-F.; Chen S. Facile access to poly(NMA-co-VCL) hydrogels via long range laser ignited frontal polymerization. J. Mater. Chem. A 2013, 1, 7326–7331. 10.1039/c3ta11409k. [DOI] [Google Scholar]
- Li Q.; Zhang W.-C.; Wang C.-F.; Chen S. In situ access to fluorescent dual-component polymers towards optoelectronic devices via inhomogeneous biphase frontal polymerization. RSC Adv. 2015, 5, 102294–102299. 10.1039/c5ra19173d. [DOI] [Google Scholar]