Abstract
Toxoplasma gondii is a zoonotic protozoan parasite that can cause morbidity and mortality in humans, domestic animals, and terrestrial and aquatic wildlife. The environmentally robust oocyst stage of T. gondii is fundamentally critical to the parasite's success, both in terms of its worldwide distribution as well as the extensive range of infected intermediate hosts. Despite the limited definitive host species (domestic and wild felids), infections have been reported on every continent, and in terrestrial as well as aquatic environments. The remarkable resistance of the oocyst wall enables dissemination of T. gondii through watersheds and ecosystems, and long-term persistence in diverse foods such as shellfish and fresh produce. Here, we review the key attributes of oocyst biophysical properties that confer their ability to disseminate and survive in the environment, as well as the epidemiological dynamics of oocyst sources including domestic and wild felids. This manuscript further provides a comprehensive review of the pathways by which T. gondii oocysts can infect animals and people through the environment, including in contaminated foods, water or soil. We conclude by identifying critical control points for reducing risk of exposure to oocysts as well as opportunities for future synergies and new directions for research aimed at reducing the burden of oocyst-borne toxoplasmosis in humans, domestic animals, and wildlife.
Keywords: Toxoplasma gondii, Oocyst, Transmission, Water, Soil, Food
Highlights
-
•
Oocyst ingestion constitutes a significant proportion of T. gondii infections.
-
•
Oocyst attributes facilitate T. gondii transmission in terrestrial and aquatic habitats.
-
•
Oocysts are persistent and prevalent in water, soil and foods.
-
•
Mitigating oocyst-borne infections requires transdisciplinary international efforts.
1. Introduction: the importance of oocysts in Toxoplasma gondii transmission
Toxoplasma gondii is an apicomplexan protozoan parasite that infects birds and mammals, including humans (Dubey and Beattie, 1988). Although infections are often asymptomatic, T. gondii can cause serious disease and death in humans and animals (Carme et al., 2002; Kreuder et al., 2003; Jones et al., 2012). The three primary transmission routes for T. gondii include vertical transmission from mother to fetus, ingestion of tissue cysts in infected animal tissues, and ingestion of oocysts from contaminated water, soil, or foods (Tenter et al., 2000). Since the parasite's discovery in 1908 (Ferguson, 2009), environmental transmission has arguably been the least studied route, likely due to the logistical constraints of safely producing large numbers of oocysts under laboratory conditions (Fritz et al., 2012a) and lack of standardized methods for detection of oocysts in complex environmental matrices (Dumetre and Darde, 2003).
Oocysts, the environmentally robust stage of T. gondii, play an important role in the epidemiology of this zoonotic parasite. Attributes of oocysts' biology may further explain the global distribution of T. gondii and the means by which it has evolved to be one of the most prevalent infectious agents of animals and humans (Dubey, 2004; Dumetre et al., 2012). While oocysts are exclusively deposited on land due to definitive hosts being solely terrestrial animals, the prevalent nature of infections observed in aquatic animals demonstrates a significant role for waterborne transmission. High prevalences of T. gondii exposure in marine species (up to 100% in some populations (Dubey et al., 2003)) further suggests that oocyst transport to, and accumulation in, nearshore or open ocean habitats is possible and epidemiologically significant (Miller et al., 2018).
Reports documenting the presence of T. gondii in diverse environmental matrices, including water, soil, vegetables and seafood have been increasing. New methods that can discriminate the route of T. gondii acquisition have demonstrated that, in some populations, a significant proportion of infections are caused by oocyst ingestion (Hill et al., 2011). Despite these findings, mainstream thinking in exposure mitigation for both humans and animals often neglects a comprehensive understanding and management of factors that are important to reducing the risk of exposure to oocysts. Therefore, this review aims to (i) summarize critical aspects of oocyst biology, environmental resistance, and felid dynamics of oocyst shedding; (ii) review the importance of oocyst-borne infections in human and animal populations; (iii) synthesize current knowledge on oocyst contamination of water, soil, fresh produce and seafoods; and (iv) identify critical gaps in current knowledge where further research and collaborative efforts should be directed to reduce T. gondii infections in animals and humans.
2. Felid dynamics of oocyst shedding
Although Toxoplasma gondii infects diverse species of warm-blooded animals, domestic cats (Felis catus) and wild felids are the only known definitive hosts capable of shedding environmentally hardy oocysts in their feces (Dubey et al., 1970; Hutchison et al., 1969; (Jewell et al., 1972; Miller et al., 1972). Domestic cats exist in close association with most human settlements throughout the world (Liberg et al., 2000), and both pet cats and free-ranging stray or feral domestic cats contribute to environmental oocyst burden. One or more of the 36 species of wild felids inhabit every continent except Australia and Antarctica (Macdonald and Loveridge, 2010), with multi-species assemblages present in many areas. Toxoplasma oocyst shedding has been identified microscopically and molecularly confirmed in free-ranging individuals from diverse wild felid species in North, Central and South America, and Asia (as reviewed in (Dubey, 2009; VanWormer et al., 2013a)), and likely occurs in all wild felids.
Oocyst contributions to the environment begin with infection of a felid host. Domestic cats and wild felids can be infected with T. gondii by consuming the tissues of an infected intermediate host (bradyzoite cysts), ingesting oocysts, or through congenital transmission (Dubey and Jones, 2008). The majority of felid infections are thought to be acquired through infected prey (Dubey and Jones, 2008), and the prevalence of oocyst shedding was higher in cats experimentally-infected with bradyzoites compared to those infected with tachyzoites or oocysts (Dubey, 2009) (Dubey and Frenkel, 1976). Following parasite ingestion, asexual and sexual reproduction of T. gondii occur in the felid's small intestine (Dubey, 2009). Genetic recombination and re-assortment during sexual reproduction has the potential to produce new strains of T. gondii (Elmore et al., 2010). Oocysts produced through sexual reproduction are shed in felid feces and sporulate one to five days later in the environment, becoming infective to other intermediate or definitive hosts (Elmore et al., 2010). When infected by ingesting tissue cysts, the natural exposure route for felids consuming infected prey, domestic cats can shed up to one billion oocysts typically over a 1–2 week period (Dubey and Frenkel, 1972; Dubey, 1995; Fritz et al., 2012a). The number of oocysts shed by experimentally-infected wild felids has not been quantified. However, a naturally-infected, free-ranging mountain lion in Canada shed quantities of oocysts similar to those shed by naturally-infected domestic cats (1.25 × 106 per gram of feces; (Aramini et al., 1998)). Young cats may shed higher numbers of oocysts, and higher prevalence of oocyst shedding was observed in pet and feral kittens relative to adult domestic cats (Ruiz and Frenkel, 1980; Dubey and Beattie, 1988) (Dubey and Beattie, 1988; Ruiz and Frenkel, 1980). However, oocyst shedding was reported in naturally-infected adult domestic cats (1–18 years of age) in Europe and the United States (Schares et al., 2008; Herrmann et al., 2010; Berger-Schoch et al., 2011; VanWormer et al., 2013b).
The strain of T. gondii infecting a domestic or wild felid may impact both the number of oocysts shed by an individual cat as well as the prevalence of oocyst shedding. Domestic cats experimentally infected with certain strains of T. gondii shed higher numbers of oocysts per cat, but limited parasite genotypes were tested (Dubey, 1995). Experimental infection of domestic cats and bobcats (Lynx rufus) with an identical strain of T. gondii isolated from domestic sheep resulted in lower oocyst shedding prevalence in the wild felids (Dubey et al., 1970; Miller et al., 1972). However, wild felid shedding prevalence may increase with exposure to wild or atypical strains of T. gondii. Asian leopard cats (Prionailurus bengalensis) experimentally infected with T. gondii strains isolated from domestic or wild animals only shed oocysts when exposed to the wild strain (Miller et al., 1972). In naturally-infected pet and feral domestic cat populations, reported shedding prevalence for molecularly or bioassay confirmed T. gondii oocysts ranged from 0 to 20%, with higher levels detected in feral cats when geographically overlapping populations of pet and feral cats were compared (Herrmann et al., 2010; Jones and Dubey, 2010; Berger-Schoch et al., 2011; Lilly and Wortham, 2013; VanWormer et al., 2013b; Mancianti et al., 2015; Veronesi et al., 2017; Nabi et al., 2018). The reported prevalence of cats shedding oocysts in naturally-infected, free-ranging wild felid populations varied widely (0–37%; (Jokelainen et al., 2013; Simon et al., 2013a; VanWormer et al., 2013b). In a field study on the west coast of the USA that compared oocyst shedding among sympatric wild and domestic felids, smaller wild felids (bobcats) and feral domestic cats feeding primarily on wild prey had higher levels of oocyst shedding than larger wild felids (mountain lions; Puma concolor) or feral domestic cats being fed by humans (VanWormer et al., 2013b). Reported levels of T. gondii-like and molecularly confirmed oocyst shedding also differed among wild felids in other areas of the USA, with higher prevalences observed in bobcats than mountain lions (Marchiondo et al., 1976).
Domestic and wild felid contributions to environmental oocyst load may also be impacted by the frequency of oocyst shedding. While many studies focus on young cats and the period of shedding following an animal's initial infection, experimental and field evidence supports the potential for repeated episodes of oocyst shedding over the course of a felid's life. In experimental settings, chronically infected domestic cats re-shed oocysts following glucocorticoid-induced immunosuppression, co-infection with another common feline parasite, Cystoisospora felis, and when infected with a new strain of T. gondii (Chessum, 1972; Dubey and Frenkel, 1974; Dubey, 1976; Zulpo et al., 2018). Repeat shedding occurred experimentally in both malnourished and well-nourished domestic cats (Ruiz and Frenkel, 1980). Captive wild felids naturally exposed to T. gondii in raw meat also repeatedly shed oocysts (Lukesova and Literak, 1998). Diet, which can vary drastically among felid species as well as within populations of a single species living in different environments (Hass, 2009; Plantinga et al., 2011), plays an important role in potential repeat shedding in free-ranging felids by influencing immune status along with exposure to C. felis and novel strains of T. gondii. Mixed infections with more than one strain of T. gondii have been identified in naturally-infected domestic and wild felids (VanWormer et al., 2014; Verma et al., 2017; Valenzuela-Moreno et al., 2019). Domestic cats fed by humans or scavenging from foods discarded by people as well as wild felids that consume fewer, larger prey animals or prey species with lower prevalence of T. gondii infection may have lower risk of repeat shedding and lower observed infection and oocyst shedding prevalences (Afonso et al., 2007; VanWormer et al., 2013b). It is currently unknown what proportion of naturally-infected, free-ranging domestic and wild felid populations may be re-shedding oocysts at a given time. Longitudinal studies are needed to enhance our understanding of repeat shedding in these groups. Oocyst shedding has long been associated with young cats, but due to the possibility of re-shedding, all cats should be considered in the dissemination of oocysts.
While both domestic and wild felids shed T. gondii oocysts, the number of felids present in a given area, prevalence of oocyst shedding, and numbers of oocysts shed impact their relative contributions to environmental oocyst load. Multiple factors, including human presence, land use, habitat, and prey availability, shape the numbers and species of felids present in diverse environments. Due to these diverse influences on felid presence and T. gondii oocyst shedding, field studies are critical to evaluate the relative contributions of domestic and wild felids to environmental T. gondii oocyst burden on local, regional, and global scales. Molecular epidemiology studies of parasite genotypes in geographically overlapping felids, intermediate and paratenic hosts illustrated that both domestic and wild felids can play a role in archetypal and atypical T. gondii infections (Miller et al., 2008; VanWormer et al., 2014; Shapiro et al., 2015). Statistical and geographic information system (GIS)-based modeling approaches have been used to assess relative oocyst contributions of domestic and wild felids in landscapes where multiple felid species exist, illustrating that both groups contribute to environmental T. gondii contamination (VanWormer et al., 2016). Interestingly, higher levels of T. gondii infection in populations of marine hosts were associated with proximity to areas with greater human presence where oocyst contributions were estimated to be higher for domestic cats than wild felids (VanWormer et al., 2016; Burgess et al., 2018). Infection in terrestrial and marine intermediate hosts, as well as paratenic hosts, in areas like Hawaii and New Zealand that lack wild felids underscore the importance of domestic cat contributions to environmental oocyst load (Roe et al., 2013; Barbieri et al., 2016; Coupe et al., 2018).
3. Structural, molecular, and biophysical attributes of oocysts
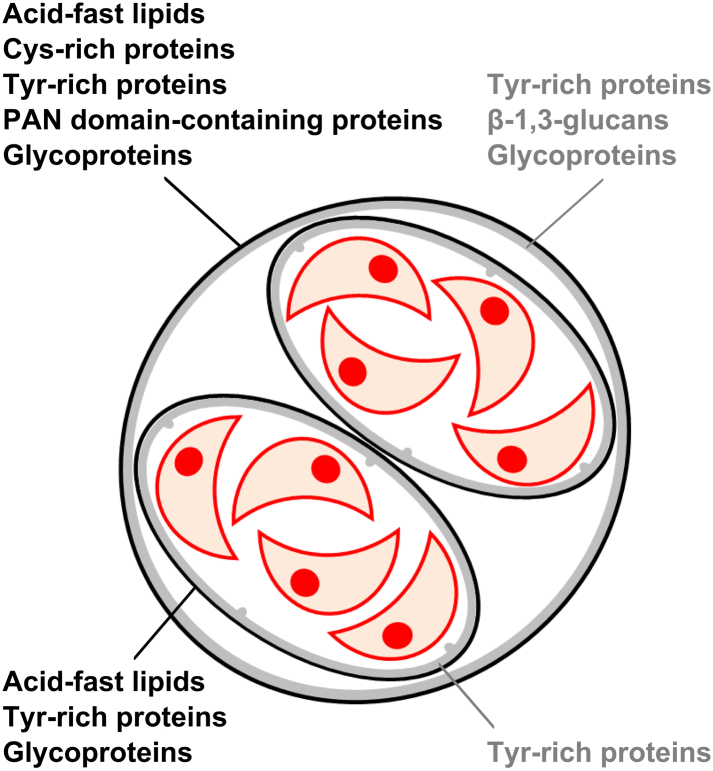
Excreted oocysts become infective following sporulation. This process results in the formation of two sporocysts each with four sporozoites (Figure 1). It is usually completed within 7 days at 20–25 °C under appropriate aerobic and humidity conditions, however it can be delayed at lower temperatures (4–11 °C) (Dubey et al., 1970), and even abrogated following constant freezing at −21 °C for 1 day or − 6 °C for 7 days (Frenkel and Dubey, 1973). Sporulated oocysts are considered to be more resistant to environmental insults than non-sporulated oocysts. This increase in resistance may be due to the additional presence of the sporocyst wall and the putative capacity of sporozoites to protect themselves against temperature variations, desiccation and ultraviolet radiation (Fritz et al., 2012b).
Fig. 1.
Structure of a sporulated Toxoplasma gondii oocyst and molecular composition of the walls enclosing the sporozoites (red crescent structures). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The structural, molecular, and biophysical properties of the oocyst and sporocyst walls play a key role in the environmental transmission of the parasite. These properties appear very conserved across the different parasite genotypes (Dumetre and Darde, 2007; Fritz et al., 2012b; Possenti et al., 2013). Both walls are bilayered and mainly proteinaceous (Mai et al., 2009). The outer oocyst wall layer (20 nm thick) contains cysteine-rich proteins of the oocyst wall protein (OWP) family and tyrosine-rich proteins (Possenti et al., 2010; Fritz et al., 2012b), which are prone to form robust polymeric structures through disulphide (Possenti et al., 2010) or dityrosine crosslinking (Fritz et al., 2012b), respectively. The latter are believed to be responsible for the natural blue fluorescence of oocyst and sporocyst walls. The additional presence of PAN domain-containing proteins could contribute to the stabilization of the architecture of the outer oocyst wall layer through disulfide bridging. Acid-fast lipids coat the oocyst surface and render the oocyst wall almost impermeable to water soluble molecules (Bushkin et al., 2013). The inner oocyst wall layer (30–70 nm thick) contains crosslinked Tyr-rich proteins and fibrils of β-1,3-glucans (Bushkin et al., 2012; Fritz et al., 2012b), which have a structural role. Several glycoproteins have been identified in both oocyst wall layers by using lectins (Samuelson et al., 2013; Harito et al., 2016), however their role is uncertain. The sporocyst wall appears to serve as a second level of protection. The outer sporocyst wall layer (15–20 nm thick) resembles the outer oocyst wall in structure and molecular composition except that it appears to lack OWP proteins. The inner sporocyst wall layer (40–50 nm) is made of four curved plates joined together by thick sutures. The unique structure of the inner sporocyst wall layer would provide additional mechanical resistance. Given the autofluorescence of both sporocyst wall layers, Tyr-rich proteins appear as the most abundant molecules in the sporocyst wall whereas β-1,3-glucans are absent (Freppel et al., 2019).
Due to their polymeric nature, the oocyst walls are resistant to mechanical perturbations (Dumetre et al., 2013) and almost hermetic to chemical inactivation agents, in particular strong acids, detergents, and chlorinated disinfectants (Jones and Dubey, 2010). For instance, diluted household bleach solutions can destroy the outer oocyst wall but do not significantly alter the structure, mechanics, and permeability of the inner oocyst wall, the sporocyst wall, nor the sporozoite infectivity (Dumetre et al., 2013). Oocyst walls similarly confer resistance to chlorine dioxide and chloramine at concentrations used in the water industry. In this regard, oocysts can pose health hazards in areas where people drink unfiltered chlorinated tap water (Jones and Dubey, 2010). Oocyst tolerance to other environmental stressors including salinity and enzymatic digestion, could also rely on the robust nature, molecular content and lack of permeability of the oocyst walls.
In addition to providing protection, the oocyst wall can mediate the retention or transport of oocysts in soils and waters in conjunction with environmental factors (Dumetre et al., 2012). The oocyst surface is hydrophilic, faintly adhesive and negatively charged in low-ionic strength solutions, suggesting that oocysts can mobilize from soils following heavy rainfalls and disperse in freshwaters (Dumetre et al., 2012, Dumetre et al., 2013; Shapiro et al., 2009). In contrast, oocyst surface charge approaches neutral in high-ionic strength solutions mimicking estuarine or marine waters (Shapiro et al., 2009), which can enhance oocyst interactions with marine biofilm and algae growing in coastal areas (Shapiro et al., 2014). This process results in oocyst incorporation into marine food webs, with exposure implications to higher tropic level animals as well as people. The oocyst wall properties, therefore, play a key role in the transmission dynamics of T. gondii across landscapes, as well as from land to aquatic habitats, facilitating exposure to numerous host species living in different biotopes worldwide (Dumetre et al., 2012).
The potential effect of T. gondii genotype on oocyst transport and persistence patterns in the environment is relatively unexplored. Some studies have demonstrated similar oocyst wall molecules via proteomic analyses performed on oocysts derived from genotypes II and III (Fritz et al., 2012a, Fritz et al., 2012b and Possenti et al. (2013), respectively); however, a more recent investigation by Zhou et al. (2017) identified differing expression of proteins between oocysts derived from a virulent phenotype (ToxoDB#9) vs those belonging to the less virulent Type II genotype. Additional investigations are warranted to evaluate if parasite genotype is associated with transport behavior or resistance to variable environmental stressors.
4. Importance of oocyst-borne infections
4.1. Oocyst-borne infections in humans
The epidemiologic importance of whether human toxoplasmosis is transmitted by oocysts or tissue cysts was debated even before the life cycle was determined in the 1970s (Jackson and Hutchison, 1989). The early observation in the 1950s that vegetarians and non-vegetarians had similar prevalence rates (Jacobs, 1957; Rawal, 1959) suggested that carnivorism could not be the only source of infection. Presently, the relative importance of oocyst vs tissue cyst ingestion for Toxoplasma gondii transmission in humans remains unknown for the majority of endemically infected populations.
Transmission patterns of T. gondii oocysts to people have been mostly characterized in toxoplasmosis outbreaks (Teutsch et al., 1979; Benenson et al., 1982; Coutinho et al., 1982; Bowie et al., 1997; de Moura et al., 2006; Ekman et al., 2012). Specifically, Brazil has experienced several oocyst-borne outbreaks, with water or produce implicated as the common source of exposure (Ferreira et al., 2018). Several factors that contribute to oocyst transmission patterns in Brazil are likely representative of other epidemic and/or endemic T. gondii regions, including precarious infrastructure for water and sewage treatment, large segments of the population that are poor and underserved, and inadequate access to healthcare. Endemic regions tend to include low-middle income countries, with detrimental consequences particularly evident in poor populations due to high rates of congenital and acquired toxoplasmosis (Bahia-Oliveira et al., 2017; El Bissati et al., 2018). The importance of T. gondii oocyst transmission in Brazil has been evaluated for both urban and peri-urban regions. Peri-urban regions are prevalent in this country and can be defined as the landscape interface between town and country. Specific factors that contribute to oocyst-borne infections in these regions include: 1) the high levels of environmental contamination with oocysts; 2) presence of diverse T. gondii genotypes and rich feline biodiversity in peri-urban, rural and forested areas; and 3) the lack of control of stray domestic cats living in urban and peri-urban areas. Combined, these features imply that exposure to oocysts likely contributes to the high prevalence of T. gondii infection in many populations in Brazil. The presence of diverse and atypical T. gondii strains in this region is also likely to contribute to acute disease outbreaks, as atypical genotypes were characterized as more virulent in several studies (Darde et al., 1998; Carme et al., 2002; Carme et al., 2009). Additional information on the role of parasite genotype and disease outcome is addressed in a separate manuscript in this Special Issue (Galal et al., 2019 FAWPAR, Present Special Issue on Toxoplasmosis & One Health).
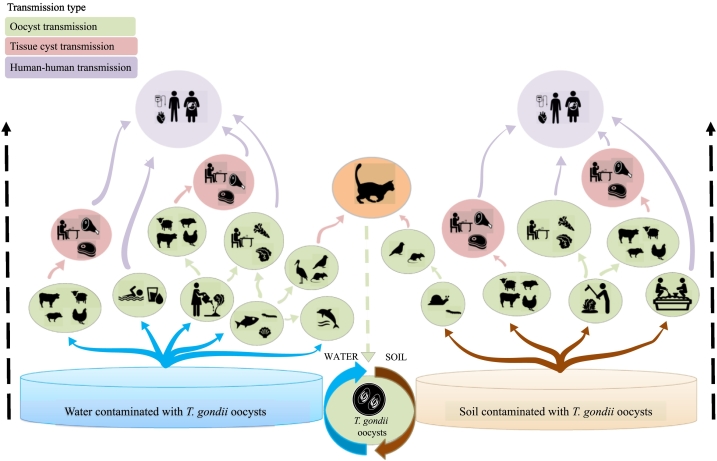
Ferreira et al. (2018) reported that from 25 outbreaks recorded in Brazil over the past 50 years, 56% (14/25) occurred between 2010 and 2018. Seventy two percent (18/25) had ingestion of oocysts in food, soil or water as the main risk factors; 24% (6/25) were associated with ingestion of tissue cysts from undercooked or raw meat; and 4% (1/25) were associated with tachyzoites ingested through unpasteurized milk. The low socioeconomic and educational circumstances in Brazil were identified as additional important risk factors for T. gondii infections in humans, and were associated with ingestion of oocysts in untreated water, consuming contaminated vegetables, or via contact with soil (Amendoeira et al., 2003; Bahia-Oliveira et al., 2003; Spalding et al., 2005; Cavalcante et al., 2006; Heukelbach et al., 2007; Boia et al., 2008; Sroka et al., 2010; Dattoli et al., 2011; Dias et al., 2011; Carellos et al., 2014). In terms of probability, exposure to oocysts in water or other matrices has been proposed to comprise a major risk for human infection both in developed and developing countries, largely due to the oocysts' long persistence in the environment and the diversity of paratenic hosts that can be ingested as food (e.g., shellfish, as represented in Fig. 2 (Bahia-Oliveira et al., 2017)).
Fig. 2.
A Toxoplasma gondii oocyst transmission ‘tree’. The flux of environmental transport and infection with T. gondii starts with oocysts shed in cat feces that contaminate soil and/or water, and are subsequently transmitted to hosts (intermediate, paratenic and definitive). Green ovals and arrows represent different sources/scenarios of contamination/infections caused by ingestion of oocysts; pink ovals and arrows represent scenarios of transmission via bradyzoites (tissue cysts); and purple ovals and arrows represent human-to-human transmission caused by vertical (congenital), transfusional, or organ transplantation infections. Water is represented in blue with blue arrows depicting infection or contamination transmitted directly from water sources to hosts. Soil is represented in brown with brown arrows depicting infection or contamination transmitted directly from sources of soil to hosts. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Adapted from Bahia-Oliveira et al. (2017).
An important methodological advancement for discriminating oocyst-borne infections has recently aided epidemiological investigations that aim to clarify the importance of different routes (oocysts vs. tissue cysts) of T. gondii transmission in people. These serological assays utilize a new generation of recombinant protein antigens that have been incorporated in ELISA systems and can detect immunoglobulins that recognize the sporozoite-specific embryogenesis-related protein (TgERP) (Hill et al., 2011), or the CCp5A, a recombinant protein derived from T. gondii sporozoites (Santana et al., 2015). When used together, both antigens, can further assist with establishing time since infection. Namely, antibodies against TgERP remain detectable in individuals' sera for 8 months after exposure, while antibodies against CCp5A are only detectable within 2 months of infection. In the USA and Chile, serological data regarding T. gondii sporozoite antigens indicate that oocysts were responsible for 78% and 45% of acute infections in pregnant woman, respectively (Boyer et al., 2011; Hill et al., 2011). The high rate of oocyst-borne infections in the USA is especially intriguing given that the overall seroprevalence for T. gondii has been declining in this country since the 1990s. The reduced burden of T. gondii infections in the USA has been largely attributed to improvements in biosecurity of farm animal management and meat preparation (e.g., thorough cooking), as well as an increase in hygiene-related safety guidelines (hand washing after gardening, safe handling of cat feces, etc.) (Jones et al., 2014). A similar pattern of declining T. gondii seroprevalence and associated explanatory variables has been observed in Europe (Pappas et al., 2009; Nogareda et al., 2014). However, increasing awareness and consumer demands for animal welfare may impact future patterns of toxoplasmosis in Europe as animals raised in free-ranging settings are more likely to be infected (Kijlstra and Jongert, 2009).
In Brazil, two studies investigated antibodies against TgERP in sera and saliva of persons exposed to drinking water contaminated with T. gondii oocysts and demonstrated that IgG was positive in 50% of individuals older than 20 years (Vieira et al., 2015), and that salivary IgA was detectable in 60% of patients 15 to 21 years of age (Mangiavacchi et al., 2016). Both studies raise important questions regarding the possibility of continuous reinfection in patients living in endemic settings under high pressure of environmental contamination with T. gondii oocysts. At the time of this review preparation, the largest T. gondii outbreak in Brazil had started in February 2018, and was still ongoing more one year after the initial cases were detected, with >700 serologically and clinically confirmed cases of acute toxoplasmosis. When applying the sporozoite antigen CCp5A-based serological assay in 36 of these confirmed cases, 78% (28/36) were positive, supporting the epidemiological finding implicating drinking water from faucets and eating vegetables washed with water from faucets as the main risk factors associated with T. gondii infection during this outbreak (Menegolla et al., 2018).
In terms of the relative epidemiologic importance of oocysts versus tissue cysts in the global T. gondii burden of infections, the new generation of recombinant sporozoite-specific antigens could serve as a valuable tool complementing epidemiological studies where often a single serological sample is available to estimate parameters such as prevalence (using conventional serological tests), mode of infection (oocysts versus tissue cysts) and the incidence (temporal rate of infection) in a given population. Additional studies validating the use of sporozoite-specific seroassays are needed to better understand the epidemiological value of these assays in discriminating the dynamics and route of T. gondii transmission in other geographical regions. These methodologies will thus facilitate logistics and cost efficiency of field investigations, as well as aid with determining the most appropriate local prevention measures against exposure to T. gondii, especially for pregnant women and immunocompromised patients.
4.2. Oocyst-borne infections in animals
The importance of oocysts as the primary infective stage responsible for transmission of Toxoplasma gondii to intermediate hosts such as rodents or birds, as well as herbivorous meat-producing animals, has been recognized since the life cycle of the parasite was initially described in 1969 (Jacobs et al., 1960; Hutchison et al., 1969; Dubey et al., 1970). In domestic farm animals, exposure to T. gondii is more likely if cats are present on farm premises and when animals are allowed greater access to free-roaming pastures (Waltnertoews et al., 1991; Jones and Dubey, 2012; Guo et al., 2015). In fact, free-roaming chickens have been used as an indicator of environmental oocyst contamination due to their feeding behavior in close proximity to contaminated soil (More et al., 2012). Of note are several reports demonstrating that husbandry operations with outdoor access result in higher likelihood of T. gondii infections in pigs (van der Giessen et al., 2007; Dubey et al., 2012) and chickens (Dubey, 2010) as compared with conventional operations, most likely resulting from greater access to natural outdoor habitats and free-ranging management strategies that lead to greater likelihood of exposure to contaminated soil or water. Higher burden of T. gondii-infected livestock is relevant for human public health not only for meat-producing animals, but also livestock producing dairy products. Several papers have documented the presence of T. gondii DNA (likely tachyzoites) in milk from goats and sheep (Mancianti et al., 2013; de Santana Rocha et al., 2015; Luptakova et al., 2015; Saad et al., 2018), and toxoplasmosis outbreak in people have been linked to consumption of unpasteurized milk in Brazil and the US (Sacks et al., 1982; Ferreira et al., 2018). As consumer demands are rising for improved animal welfare and more natural farming conditions, there is a potential for increasing proportions of T. gondii-infected meat- and/or dairy-producing livestock, with cascading public health implications.
In wildlife, oocyst-borne T. gondii infections have been proposed to occur in numerous species including freshwater-dwelling mammals (Ribas et al., 2018), ungulates (Dubey et al., 2017), and birds (Frenkel et al., 1995). In the past decade, several studies have specifically focused on the curious finding of high prevalence of T. gondii in marine mammals (Fayer et al., 2004). A thorough review on the global prevalence and distribution of T. gondii infections in marine mammals was recently compiled by Miller et al. (2018). Clinical toxoplasmosis has been documented in cetaceans, phocids, otariids, walruses, sirenians and sea otters. Serological exposure has been further documented in species sampled from coastal habitats to the deep sea, and across all continents including arctic locales and Antarctica where felids are completely absent.
The mechanism of land-to-sea transport of T. gondii oocysts and incorporation into marine food webs has been addressed in several investigations. In temperate regions, rainfall can mobilize the transport of fecally-deposited oocysts into watersheds that drain into coastal waters (Miller et al., 2002; VanWormer et al., 2016), while in colder locales early snowmelt may also provide a means for mobilizing oocysts deposited the previous year (Simon et al., 2013b). In coastal waters, entry of T. gondii oocysts into the marine food chain is facilitated through their accumulation in organic flocs that serve as food for fish and invertebrates (Shapiro et al., 2012; Shapiro et al., 2014). While these cold-blooded animals may not serve as true intermediate hosts, T. gondii oocysts can be ingested and retained by fish (Massie et al., 2010), bivalves (Arkush et al., 2003; Lindsay et al., 2004), and marine snails (Krusor et al., 2015; Schott et al., 2016). Ingestion of these invertebrates has been epidemiologically linked to increased risk of T. gondii infections in higher trophic animals such as marine mammals (Johnson et al., 2009) or people consuming seafood (Jones et al., 2009).
5. Environmental transmission of oocysts
5.1. Oocysts in water
Humans and susceptible animal hosts can be exposed to Toxoplasma gondii oocysts in the environment through drinking water contaminated with felid feces. Oocysts can survive various inactivation procedures especially those using chemical reagents (Dubey, 2004). Oocysts remain viable in water even after exposure to aqueous 2% sulfuric acid for at least 18 months at 4 °C; they also resist detergents or disinfectant solutions such as sodium hypochlorite. Drinking-water treatment plants using chlorination as the sole method of disinfection could therefore supply water containing infective oocysts.
Interest in detecting T. gondii oocysts in the environment is emerging due to recent outbreaks of waterborne toxoplasmosis in humans. Until recently, Toxoplasma was not considered to be a significant waterborne pathogen like other protozoan parasites, such as Cryptosporidium and Giardia which have been linked with numerous waterborne outbreaks, including a very large Cryptosporidium outbreak reported in Milwaukee, Wisconsin, USA (MacKenzie et al., 1995). The first T. gondii waterborne outbreak was associated with exposure to water from a jungle stream in Panama in 1979, resulting in infection of 39 soldiers who used three water sources for drinking water (Benenson et al., 1982). An epidemiological investigation identified the source as creek water contaminated by oocysts excreted by wild felids. A prominent outbreak was then reported in British Columbia, Canada in 1995, with 100 cases of human acute Toxoplasma infection (Bowie et al., 1997). A municipal water system that used unfiltered and chloraminated surface water was the likely source of contamination by cougar and/or domestic cat feces (Aramini et al., 1999). In 1999, drinking water was also reported as the vehicle of infection among Jains, a community of strict vegetarians in India (Hall et al., 1999).
Waterborne toxoplasmosis has been reported most often from Brazil, in both epidemic as well as endemic transmission patterns. The largest outbreak in the published literature, with 290 human cases, was reported in Brazil and involved an unfiltered water reservoir (Keenihan et al., 2002). Moreover, a high T. gondii prevalence related to drinking unfiltered water was found in Brazilian communities where endemic toxoplasmosis is prevalent (Bahia-Oliveira et al., 2003). Likewise, a large toxoplasmosis outbreak in Brazil in 2001–2002 was associated with the consumption of contaminated water from a municipal reservoir, which was vulnerable to infiltration due to its precarious management state. This outbreak was thought to be responsible for infection of 155 persons served by an underground tank reservoir delivering unfiltered water (de Moura et al., 2006).
While most T. gondii waterborne outbreaks have been described from low-middle income countries, epidemiological investigations have also linked water with exposure to T. gondii in people living in high-income regions. In the USA, the NHANES survey data (1999–2004; 2009–2010) demonstrated that among US-born participants, individuals drinking well water, as well as public/private company-provided tap water with no additional at-home water treatment devices, were significantly more likely to be seropositive for T. gondii as compared with participants who used home treatment devices (Krueger et al., 2014). Despite limitations in the NHANES cross-sectional survey, the association between water and T. gondii infection in the USA warrants further research.
Detection methods for T. gondii oocysts in the environment are underdeveloped in comparison to other protozoan parasites (i.e., Cryptosporidium spp. and Giardia duodenalis). Detection of oocysts has relied on a combination of bioassays, microscopy, and molecular assays as reviewed by Dumetre and Darde (2003) and more recently by Bahia-Oliveira et al. (2017). Although PCR does not demonstrate infectivity, it is widely applied for estimating the presence of water contamination with pathogenic protozoa. Immunofluorescence assays commonly employed to detect other waterborne protozoa, namely Cryptosporidium and Giardia oo(cysts), are described (Dumetre and Darde, 2005, Dumetre and Darde, 2007), but are not commercially available for T. gondii (because of a lack of suitable monoclonal antibodies) and they have not been extensively tested in field conditions. One approach recently described in the literature for capture of T. gondii oocysts from concentrated water utilized lectin-coated magnetic beads (Harito et al., 2017), however additional application of this methodology is needed to evaluate feasibility and sensitivity when applied to large volumes of diverse water types.
Similar to detection of other protozoan parasites, an initial concentration step is required due to the relatively dilute distribution of T. gondii oocysts in environmental water sources. Concentration methods include membrane filtration, capsule filtration, hollow fiber ultrafiltration, flocculation, and centrifugation. For direct visualization of T. gondii, microscopy-based methods were employed based on the autofluorescence nature of T. gondii oocyst and sporocyst walls (Lindquist et al., 2003). While cost effective, methods that rely on microscopy-based detection require molecular confirmation to definitively identify oocysts as T. gondii, because other apicomplexan parasites have an identical morphology (e.g., Hammondia, Besnoitia, and Neospora oocysts).
Isolation of T. gondii from water using mouse bioassays provides definitive identification of viable parasites, but this approach is laborious, costly, and time-consuming. Interestingly, this approach was successfully applied in the 2001 Brazil outbreak, where water collected from the suspected reservoir was filtered and multiple bioassays were performed in cats, chickens and pigs fed with the membrane filters, leading to parasite isolation and genotyping (de Moura et al., 2006). To date, this report represents the sole outbreak in which detection of T. gondii in the implicated water source was achieved. Multilocus DNA sequencing has identified a nonarchetypal strain of T. gondii as the causal agent of this waterborne outbreak. The strain, isolated from a water supply epidemiologically linked to the outbreak, was virulent to mice, and it has previously been identified as BrI (Vaudaux et al., 2010). Because no parasites were recovered from infected individuals, no direct determination of the responsible strain type(s) could be performed. Moreover, Vaudaux et al. (2010) used a serologic strain-typing assay to determine “serotypes” for a group of such individuals. There was a dominant serotype in the majority (65%) of individuals, which was indistinguishable from the serotype found in serum from mice that had been infected with isolates from the implicated water supply. Furthermore, the outbreak strains were genotyped at multiple polymorphic loci and found to be clonotypic and nonarchetypal. Hence, data support prior evidence that the municipal cistern was the point source of this clonal outbreak.
Due to ethical concerns, cost and the time-consuming nature of bioassays, rapid and sensitive pathogen detection methods are essential for public health and water quality industries. PCR has already been described as more rapid, sensitive, and specific for Cryptosporidium detection in environmental water (Fontaine and Guillot, 2003). Therefore, molecular detection has been proposed for sensitive and rapid detection of T. gondii oocysts, and assays include both conventional and real-time, quantitative PCR. Villena et al. (2004) have previously compared bioassays and molecular approaches and reported better performance of PCR, with sensitivity varying from <10 to >1000 oocysts/l, depending on water type. Using this PCR method in environmental samples, Aubert and Villena (2009) reported detection of DNA in raw surface water, underground water and public drinking water, while isolation of T. gondii by bioassay was unsuccessful (Aubert and Villena, 2009). Additional molecular approaches using loop-mediated isothermal amplification (LAMP) have also been described with better results than nested PCR and applied to T. gondii detection in environmental samples (Sotiriadou and Karanis, 2008).
Application of PCR for detection of T. gondii in water has been applied in numerous studies worldwide, and recently reviewed by Bahia-Oliveira et al. (2017). In Colombia, the prevalence of T. gondii DNA in 46 samples of drinking water was 58.6% (Trivino-Valencia et al., 2016). Similar prevalences were reported in raw and treated water in Bulgaria at 48% (Sotiriadou and Karanis, 2008) and in Poland at 37.5% (Sroka et al., 2006). In comparison, lower prevalences of Toxoplasma in water have been reported via real-time PCR in Scotland at 8.7% (N = 1411) (Wells et al., 2015), and in France's Champagne-Ardenne region at 7.7% (N = 482), where some of the positive samples were obtained from public drinking water (Aubert and Villena, 2009).
It is imperative that any molecular detection technique applied to environmental samples be followed by sequence analysis for definitive confirmation of T. gondii DNA due to a potential for non-target amplification by flora and biota commonly present in environmental habitats, even when using primers shown to be specific for T. gondii when compared with other coccidian or enteric pathogens (Shapiro et al., 2015). Moreover, as certain molecular approaches do not easily lend themselves to downstream sequence analysis (e.g., qPCR or LAMP), additional conventional PCR may be necessary as an intermediary step. Thus, although multiple methods for oocyst detection are described, the lack of standardized assays for detection of Toxoplasma oocysts in the environment has likely contributed to an underestimation of the role that oocysts play in the epidemiology of T. gondii in human populations. Additional research on the burden of environmental contamination with T. gondii oocysts including different water sources is necessary to accurately estimate the risk of oocyst ingestion by people and animals.
5.2. Oocysts in soil
Toxoplasma gondii oocysts can contaminate soil after infected felids shed the parasite in their feces (Gilot-Fromont et al., 2012). Because of the limited definitive host species for T. gondii (felids only), oocysts are not randomly distributed in soil, but rather tend to concentrate in or near cat defecation sites (Afonso et al., 2007) (Table 1). However, oocysts can be further dispersed within the soil column or to other matrices by wind, earthworms and arthropods, as well as by rain (Dumetre and Darde, 2003). Exposure to soil contaminated with T. gondii oocysts is one of the main risk factors for infection in people (Cook et al., 2000; Spalding et al., 2005; Jones et al., 2009; Egorov et al., 2018) and is likely the main route of transmission for domestic food-producing animals and many other intermediate hosts including rodents and birds (Gilot-Fromont et al., 2012). Table 1 summarizes published reports that have focused on detection and prevalence of T. gondii in soil. Overall, the reported prevalence of T. gondii in soil from various locations ranges from 0% (Hawaii, USA) to nearly 50% (Northeastern France).
Table 1.
Reports documenting the detection and prevalence of Toxoplasma gondii in soil.
| Country/region (reference) | Location (urban/rural) | Oocyst recovery method | Detection method (gene target) | Sample size and prevalence | Sequence confirmation | Key findings |
|---|---|---|---|---|---|---|
| France/Lyon (Afonso et al. 2008) |
Urban | Sucrose flotation | Real-time PCR (529-bp RE) |
N = 117 9.4% |
NR | T. gondii DNA most commonly detected in cat latrines |
| Poland/Tricity (Lass et al., 2009) |
Urban | Sodium nitrate flotation | Conventional PCR (B1) |
N = 101 17.8% |
Yes (two random samples) | Type I and II genotypes detected |
| China/Hubei Province (Du et al., 2012b) |
Urban | NR | Conventional PCR (B1 and 529-bp RE); LAMP (MIC3) |
N = 252 PCR:16.3% LAMP: 23.02% |
Yes (four random samples) | Significantly lower T. gondii DNA detected in Fall and Winter |
| China/Hubei Province (Du et al., 2012a) |
Rural | NR | Conventional PCR (B1); LAMP (MIC3) |
N = 95 PCR: 21.1% LAMP: 37.9% |
NR | |
| France/Northeastern (Gotteland et al., 2014) |
Rural | Modified sucrose flotation (Lélu et al., 2011): sugar-water interface used | Real-time PCR (529-bp RE) |
N = 243 29.2% |
NR | T. gondii DNA was not restricted to areas of high cat density |
| China/Nanjing Region (Liu et al., 2017) |
Rural | NR | Conventional PCR (ITS1) |
N = 700 1% |
Yes (all) | T. gondii only detected during Fall (3.3%) and Winter (0.56%) |
| China/Gansu province (Wu et al., 2017) |
Urban | Suspension in distilled water | Nested PCR (B1); LF-RPA (B1) |
N = 35 Nested PCR: 14.3% LF-RPA: 14.3% |
Yes (all) | |
| France/Northeastern (Simon et al., 2017) |
Rural | Modified sucrose flotation (Lélu et al., 2011): entire supernatant used | Real-time PCR (529-bp RE) |
N = 558 49.82% |
NR | T. gondii detected near core cat colony areas; and associated with latrines and scattered feces, as compared with random soil samples |
| USA/O'ahu (Davis et al., 2018) |
Urban and rural | Modified sucrose flotation method (Lélu et al., 2011) | Conventional PCR (GRA6) |
N = 120 0% |
NR | T. gondii not detected in soil samples. |
| USA/California (de Wit et al., in preparation 2019) |
Urban | Modified sucrose flotation method (Lélu et al., 2011) | Nested PCR (ITS-1) | N = 482 5.6% |
Yes (all) | T. gondii detection associated with wet season, coastal sites, and small cat colony size. |
NR = not reported; PCR = polymerase chain reaction; LAMP = loop-mediated isothermal amplification; LF-RPA = lateral flow recombinase polymerase amplification.
Oocyst viability and persistence in soil can be influenced by environmental factors such as humidity, temperature, vegetation and soil characteristics. Oocysts lose their ability to sporulate when exposed to freezing conditions (−21 °C for 1 day or − 6 °C for 7 days), extreme heat (50 °C for 10 min) or extreme solar radiation. However, once sporulated, oocysts are highly resistant and can persist in moist soil for up to 18 months when exposed to temperatures ranging from −20 °C to 35 °C (Dumetre and Darde, 2003). For example, the prevalence of T. gondii in soil tends to be higher in wet or moist seasons characterized by mild temperatures (Du et al., 2012b) (Table 1). In addition, soil with high concentrations of clay and sand can interfere with molecular methods of detection given their high content in organic and abrasive particles, respectively; however, whether these characteristics also affect retention and viability of oocysts in the soil column is unknown.
Despite the known risk of exposure to T. gondii oocysts through contact with soil (e.g., gardening or playing in sandboxes), confirming the presence and estimating the load of T. gondii in soil has proven difficult due to limitations in currently available methods of detection (Bahia-Oliveira et al., 2017). Given the likely heterogeneous dispersion of oocysts in soil, accurate estimations of T. gondii prevalence and load require large sample sizes, many of which may contain small quantities of oocysts, as well as oocysts of different ages, which may hinder the sensitivity of current detection methods (Lélu et al., 2011; Robert-Gangneux & Dardé, 2012). Commonly used methods for oocyst detection in soil include microscopy and molecular assays (Bahia-Oliveira et al., 2017; Dumetre and Darde, 2003). For both methodological approaches, initial concentration and purification of oocysts from soil is required. To accomplish these steps, most studies have applied flotation techniques using sucrose (specific gravity ≥ 1.15), cesium chloride, or sodium nitrate saturated solutions (Dumetre and Darde, 2003; Lass et al., 2009; Lélu et al., 2011) (Table 1). Molecular techniques for detection of T. gondii DNA in soil include conventional PCR, nested PCR, real-time quantitative PCR, loop-mediated isothermal amplification (LAMP), and to a lesser extent, lateral flow recombinase polymerase amplification (LF-RPA) (Table 1). Spiking experiments using various numbers of oocysts aliquoted into soil samples are commonly used to validate the sensitivity and limits of detection of molecular assays (e.g., (Lélu et al., 2011; Liu et al., 2017; Wu et al., 2017). Molecular assays are currently the most sensitive and efficient methods for detecting T. gondii in soil; nevertheless, it is important to confirm amplification results through sequence analysis (Bahia-Oliveira et al., 2017)
An intriguing route of T. gondii oocyst transmission that is not often discussed is the role of mechanical vectors in disseminating oocysts to true intermediate hosts in terrestrial habitats. For example, invertebrates including cockroaches (Wallace, 1973), earthworms, and flies (Frenkel et al., 1975) have been shown to act as paratenic or mechanical hosts through which oocysts can pass inertly. In addition, dogs have been found to defecate T. gondii oocysts in their feces, presumably from passive gastrointestinal transport of oocysts that they ingest through coprophagy (feeding on cat feces) (Lindsay et al., 1997); mechanical transmission of oocysts on dog fur following rolling in cat feces has also been proposed as a mechanism for domestic dogs to disseminate oocysts, especially to children (Frenkel et al., 2003).
6. Foodborne transmission of oocysts
6.1. Oocysts on fresh produce
For decades, foodborne transmission of Toxoplasma gondii has traditionally referred to the ingestion of tissue cysts in raw or poorly cooked meats (Guo et al., 2015). However, it has become increasingly evident that ingestion of oocysts on fresh produce and other foods is under recognized, and the significance of this route of transmission to humans is not entirely clear. Unlike other foodborne protozoan parasites, which have been implicated in numerous illness outbreaks worldwide, there have been only two reported outbreaks of toxoplasmosis associated with the consumption of fresh produce or juice. Ekman et al. (2012) described an outbreak of acute toxoplasmosis at an industrial plant in Brazil, which was associated with the consumption of green vegetables. In a second outbreak in Brazil, Morais et al. (2016) identified 73 cases of acute toxoplasmosis which were associated with the consumption of açaí juice (Morais et al., 2016). Robertson (2016) concluded that the scarcity of reported toxoplasmosis cases associated with the consumption of salad vegetables might be due to the many potential routes of infection for this parasite, and the fact that infections are often asymptomatic.
While numerous surveillance studies have been performed worldwide on the presence of foodborne parasites (e.g., Giardia, Cryptosporidium, Cyclospora, and others) on fresh produce (Dixon, 2015), fewer such studies have been reported on T. gondii. In addition to the under recognition of this mode of transmission for T. gondii, a major reason for the paucity of surveillance studies on fresh produce is the lack of standard detection methods for this parasite. In the past decade, there have only been several reports on the occurrence of T. gondii on fresh produce, based on different elution methods and a variety of microscopy- and molecular-based assays (Table 2). An additional approach utilizing LAMP was recently validated for detection of T. gondii on salads (Lalle et al., 2018), but this method has not been applied in surveillance investigations to date. The application of recently developed methods that can discriminate oocyst viability is essential for further discerning whether detected T. gondii DNA on produce is derived from infectious versus non-viable parasites (Hohweyer et al., 2016; Travaille et al., 2016).
Table 2.
Reports documenting the presence of Toxoplasma gondii on fresh produce.
| Country (reference) | Produce type | Oocyst recovery method | Detection method (gene target) | Sample size and prevalence | Sequence confirmation | Molecular characterization (method) |
|---|---|---|---|---|---|---|
| Saudi Arabia (Al-Megrin, 2010) |
Leafy vegetables | Wash, passive | Microscopy |
N = 470 6.6% |
No | |
| Poland (Lass et al., 2012) |
Radish, carrots, lettuce | Flocculation | qPCR (B1, nested PCR SAG2) |
N = 216 9.7% |
No | Types I and II (RFLP) |
| Pakistan (Shafa-ul-Haq et al., 2014) |
Market vegetables | Wash – sedimentation or flotation | Microscopy |
N = 500 1.9% |
No | |
| Canada (Lalonde and Gajadhar, 2016) |
Retail leafy greens | Wash, orbital shaking | qPCR - melting curve analysis (18S rDNA) |
N = 1171 0.26% |
Yes (98–99% identity) | |
| Brazil (Marchioro et al., 2016) |
Leafy greens | Wash, manual | PCR (B1, 529 bp RE) |
N = 238 3.8% |
No | |
| Italy (Caradonna et al., 2017) |
RTE salads | Wash, orbital shaking | Microscopy; qPCR - melting curve analysis (B1) |
N = 648 0.8% |
Yes | Type I (Sequencing) |
| Brazil (Ferreira et al., 2018) |
Organic leafy greens | Wash, manual | PCR (529 bp RE) |
N = 83 9.5% |
Attempted, not successful |
Contamination of fresh produce with T. gondii oocysts may occur at primary production sites on farms (e.g., cultivation in soil contaminated with cat feces) or by means of contact with fecally-contaminated water used in irrigation, washing or processing (Fig. 2). These direct and indirect sources of contamination are particularly prevalent in low- and middle-income countries where hygiene, sanitation and water quality may be sub-optimal (see Section 3.1; (Dixon, 2016)). Largely as a result of the global food trade, particularly the importation of exotic and out-of-season fruits and vegetables, risk of exposure to T. gondii-contaminated produce is also present in high-income countries. With the increasing domestic cat population in many regions worldwide, there will consequently be more T. gondii oocysts released into the environment resulting in a greater risk of food and waterborne transmission to humans and other animals (Bahia-Oliveira et al., 2017).
As fresh produce is often consumed raw, effective control measures to minimize parasite contamination, inactivate or reduce parasite viability, or physically remove oocysts, are imperative to reduce the risk of T. gondii exposure to consumers. At the preharvest level, control measures to reduce the likelihood of contamination of produce with T. gondii include mainly the use of treated water for irrigation, and restricted access to gardens and croplands by cats. However, once crops are contaminated, the resistance of T. gondii oocysts to environmental stressors should be taken into consideration with respect to their survival. T. gondii oocysts are very robust and likely persist on produce for weeks to months (Robertson, 2016; Bahia-Oliveira et al., 2017). Kniel et al. (2002) demonstrated that sporulated oocysts could attach to and remain infectious on berries for at least 8 weeks under refrigeration. Although there are little supporting data, desiccation may be responsible for significant inactivation of parasite stages on fresh produce in the field or during storage, and on surfaces and equipment.
Postharvest control measures include primarily the use of treated water for washing and processing produce, and for cleaning equipment. The use of chemical and physical disinfectants, either directly on foods, or on surfaces and equipment, represent other potential barriers to the foodborne transmission of parasites (Erickson and Ortega, 2006). As discussed in previous sections of this review, T. gondii oocysts are very resistant to chemical treatments and sanitizing regimens used in the water and food industries. In terms of physical disinfection, a variety of technologies have been shown to be effective in the destruction of parasites on fresh produce. For example, gamma irradiation of protozoa has been shown to be an effective means of decontaminating fresh fruits and vegetables. Dubey et al. (1998) reported that sporulated T. gondii oocysts inoculated onto raspberries were inactivated at 0.4 kilogray (kGy) and concluded that 0.5 kGy would be effective in killing coccidian oocysts on fruits and vegetables. More recently, Lacombe et al. (2017) (Lacombe et al., 2017) demonstrated that the viability of T. gondii oocysts could be significantly reduced following irradiation treatment of just 0.2 kGy. High hydrostatic pressure (or high-pressure processing, HPP) has also shown some promise in the inactivation of protozoan parasites on fresh produce and in juices. Lindsay et al. (2008) reported that T. gondii oocysts inoculated onto raspberries were rendered noninfectious to mice when the berries were exposed to 340 MPa for 60 s in a commercial HPP unit. Although studies have been performed on the efficacy of UV treatment in the inactivation of T. gondii oocysts in water, results have been inconsistent (Bahia-Oliveira et al., 2017).
At the consumer level, peeling of fruits and vegetables whenever possible will also reduce the risk. As with other foods, the adherence to safe food-handling practices, such as hand washing and the use of separate cutting boards, knives and other utensils will help to reduce the likelihood of cross-contamination of fresh produce. Treatments such as cooking and freezing may be used as final barriers against transmission of T. gondii but, since fresh produce is very often consumed raw, these methods are not always relevant. Household freezing should not be recommended as the sole means of inactivating parasites in foods because sporulated T. gondii oocysts have been shown to tolerate temperatures as low as −20 °C for up to 28 days (Frenkel and Dubey, 1973).
6.2. Oocysts in seafood
Compared with meat-producing and poultry animals, research on seafood species contaminated with Toxoplasma gondii represents a relatively new field of study. For example, while literature on cystic T. gondii in food animals can be dated as far back as the 1960s (Jacobs et al., 1960), the first published study reporting natural T. gondii contamination in shellfish was published in 2008 where the atypical Type X genotype was detected in a single mussel from central California, USA (Miller et al., 2008). Between 2008 and 2015, eight additional studies describing the presence of T. gondii in diverse seafood species were reviewed by Bahia-Oliveira et al. (2017). Overall, T. gondii contamination has been detected in wild and commercial seafoods such as clams, mussels, oysters, and fishes from several countries including Brazil, China, Turkey, and the USA (Miller et al., 2008; Esmerini et al., 2010; Putignani et al., 2011; Aksoy et al., 2014; Zhang et al., 2014; Shapiro et al., 2015; Staggs et al., 2015). Since 2015, two additional studies reported the presence of T. gondii in supermarket-purchased mussels in New Zealand (Coupe et al., 2018), and in wild clams from Tunisia (Ghozzi et al., 2017).
Methods for detecting T. gondii in seafoods remain inconsistent across studies, which hinders direct comparison of prevalence and distribution among studies. Common approaches utilize whole tissue homogenates, gills, digestive tissues, or hemolymph (from shellfish) as the testing matrix and apply different molecular assays based on conventional or real-time PCR. The ability of shellfish to bioaccumulate oocysts from contaminated waters renders these species as efficient biosentinels for T. gondii contamination of aquatic habitats, both in marine (Arkush et al., 2003; Lindsay et al., 2004) as well as freshwater habitats (Palos Ladeiro et al., 2014). The presence of T. gondii in shellfish and fish is not only a risk to susceptible marine wildlife (as discussed in Section 4.2), but also to people. Jones et al. (2009) (Jones et al., 2009) reported that eating raw shellfish is a significant risk of T. gondii infection in humans. With anthropogenic influences and climate variability scenarios that forecast increased pathogen contamination of aquatic ecosystems (VanWormer et al., 2016), the extent of T. gondii presence and load is likely to rise in seafood species consumed by wildlife or people. Method standardization focusing on affordable, rapid and accurate assays for T. gondii detection is imperative, as well as application of molecular approaches for distinguishing presence of viable from non-viable oocysts. Ultimately, the most sustainable approach for reducing the risk of T. gondii exposure through consumption of seafood should focus on reducing T. gondii contamination at its source (e.g., domestic cat management strategies), as well as mitigating the flow of contaminated runoff to water bodies.
7. Managing oocyst-borne infections
Reducing the risk of oocyst-borne Toxoplasma gondii infections in animals and people should target three distinct but not mutually exclusive factors: 1) reducing felid contributions of oocysts into the environment; 2) preventing oocyst contamination of water, soil, and foods; and 3) physically removing or inactivating oocysts in water and foods such as shellfish and produce. Detailed recommendations to achieve these steps have been further outlined by Bahia Oliveira et al., (2018), and are summarized below:
-
•
Domestic cats should be kept indoors, and efforts made to reduce the likelihood of intermediate hosts entering the household (e.g., rodents, etc.).
-
•
Domestic cats should be neutered or spayed to help reduce stray cat populations.
-
•
Domestic cats should be fed by a specially formulated diet (canned or dry food), not human food.
-
•
Feces from pet cat litter boxes should be collected daily, sealed in a bag, and disposed of in the garbage (not flushed in the toilet). Litter box must be cleaned with hot water (to avoid oocysts dissemination).
-
•
Sand boxes and play areas should be covered to restrict cat defecation in the area.
-
•
Private and community gardens should likewise restrict access to cats, via fencing or hazing tools such as water sprinklers.
-
•
Gloves should be worn when gardening, and hands thoroughly washed afterwards.
-
•
Filtered or bottled water should be consumed if living or travelling in an endemic region.
-
•
Persons at-risk (e.g., pregnant women and immunocompromised individuals seronegative for toxoplasmosis) should avoid recreating in fresh or marine waters in endemic regions, or in non-endemic regions if in close proximity to overland runoff from heavily populated zones.
-
•
Seafood should be thoroughly cooked to inactivate oocysts. Heating oocysts at 80 °C for 2 min has been shown to render them nonviable (Travaille et al., 2016).
-
•
Produce should be washed with drinking water (or with filtered or bottled water if living or travelling in an endemic region); peeling fruits and vegetables for at-risk persons is also recommended.
-
•
Common household products such as detergents, antimicrobial soaps, and bleach are not effective at killing oocysts, and their use for this purpose is not recommended.
-
•
Municipal and ecosystem-level management strategies should be implemented to reduce the overall flux of oocysts mobilized to nearshore waters through runoff. Specific recommendations include wetland preservation and restoration (Shapiro et al., 2010), replacement of impermeable surfaces such as asphalt with alternative permeable paving options (Newman et al., 2013), and storm-water treatment processes including bioswales and raingardens (Virahsawmy et al., 2014).
8. Future risk and opportunities for research
Future projections of human population growth coupled with climate variability scenarios will likely lead to greater likelihood of environmental contamination with Toxoplasma gondii oocysts (Shapiro, 2012). Domestic cat density is associated with human density (Liberg et al., 2000), thus more people often mean more pets, and in this case, more definitive hosts for T. gondii. Concurrently, projections of weather patterns in many regions of the world forecast increase in intense rainfall events interspersed with longer periods of drought (Heim, 2015). Given the robust nature of T. gondii oocysts and their ability to persist over long durations in feces and soil, a greater force of intense rainfall events will lead to enhanced mobilization of oocysts that have accumulated during dry periods. The risk of oocyst-borne infections for humans and animals may, therefore, rise in coming decades. For this reason, it is imperative that collaborative, multidisciplinary expert teams from around the world tackle applied research on the biology, detection, epidemiology and ecology of T. gondii oocysts in the environment. Specific opportunities for future research are outlined below:
-
•
The current lack in standardized T. gondii oocyst detection and quantification methods hinders effective establishment of surveillance programs for monitoring oocyst presence in water, soil and foods. This also prevents accurate comparison of T. gondii contamination across studies that often use different approaches for oocyst purification, nucleic acid extraction and amplification, as well as sequence analysis and confirmation. Establishing a consensus among laboratories and regulatory agencies regarding optimized protocols for oocyst detection should facilitate enhanced surveillance for the parasite. Early and accurate detection of T. gondii in water and foods is essential for reducing the occurrence of toxoplasmosis outbreaks in endemic geographical regions.
-
•
In addition to recommended protocols for T. gondii DNA detection, it is imperative that methods for distinguishing oocyst viability are continuously improved and increasingly applied in investigations on T. gondii prevalence in the environment. While oocysts are remarkably persistent, some decay over time under diverse environmental conditions must certainly occur (Lélu et al., 2012). Thus, viability discrimination assays are essential for accurately characterizing the risk that T. gondii poses to exposed populations when its DNA is identified in water, soil or foods (Rousseau et al., 2019).
-
•
Innovative and reliable inactivation methods are needed for application on contaminated water and foods for removing T. gondii oocysts or reducing their infectivity. Filtration systems (sand filtration, ultra- and nano-filtration) appear to be the most effective methods for removing oocysts from drinking water prior to its chemical disinfection and distribution to consumers. Disinfection of fresh produce is more problematic as treatments have to kill oocysts without altering the organoleptic properties of food. In this regard, pulsed light is an attractive technology for inactivating bacterial spores and C. parvum oocysts on berries (Le Goff et al., 2015; Rousseau et al., 2018), however this approach requires experimental validation for T. gondii.
-
•
Investigations are warranted to explore the frequency of oocyst re-shedding in nature, particularly in feral domestic cats and wild felids that are exposed repeatedly and to diverse genotypes of the parasite. Oocyst shedding data (prevalence and number of oocysts shed) for more populations of free-ranging domestic and wild felids will enhance predictions of T. gondii load and distribution in the environment. An enhanced understanding of when, where and how many oocysts are present in soil will help target intervention measures for reducing the risk of exposure to oocysts through recreation (e.g. children playing in sand boxes), gardening, or ingestion of contaminated fresh produce.
-
•
Transport and fate questions remain regarding the ability of T. gondii to disseminate in the environment, and a particularly puzzling finding is the presence of T. gondii in deep ocean dwelling mammals or in remote regions devoid of cats, such as Antarctica. Mechanisms that allow for oocyst movement via ocean currents or within mechanical hosts such as fish should be further explored. In addition, the effect of habitat change and climate variability on oocyst transport and fate warrant further research. Insight on oocyst transport can reveal how food resources including marine mammals, harvested for sustenance by indigenous populations (Sharma et al., 2018), or shellfish become contaminated – which is key for managing T. gondii infections in the people consuming them.
-
•
Safe and efficacious vaccines to protect livestock against oocyst-borne T. gondii infections are needed. The currently available TOXOVax® is used in some countries (e.g., New Zealand and the UK) but prohibited in others (USA and Canada) due to its modified-live nature and subsequent risk of accidental zoonotic transmission. T. gondii is a leading cause of abortions in sheep and goats in Canada (Hazlett et al., 2013), and a vaccine is urgently needed to reduce economic losses, improve animal welfare, and reduce the burden of infected meat or contaminated dairy products that are sold to consumers.
-
•
Finally, it is noteworthy to conclude that regions where T. gondii is most prevalent, and subsequently people and animals suffer greatest morbidity and mortality due to this parasite, are also low- and middle-income countries, where resources for research are limited, and the need for improving water and food sanitation are greatest. It is imperative, therefore, that international collaborations form to support high caliber investigations in regions that need it most, and that dissemination of expertise and resources for T. gondii research among countries is supported.
Conflict of interest
The authors report no conflict of interest.
Acknowledgments
A.D. is supported by the Institut Hospitalo-Universitaire (IHU), the National Research Agency (grants 10-IAHU-03 and 17-CE21-0005-07), the Région Provence Alpes Côte d'Azur and European funding FEDER PRIMI. Laboratory and field investigations targeting Toxoplasma gondii transmission in Brazil (LBH) were supported by grant number FAPERJ E-26/210.740/2014.
References
- Afonso E., Thulliez P., Pontier D., Gilot-Fromont E. Toxoplasmosis in prey species and consequences for prevalence in feral cats: not all prey species are equal. Parasitology. 2007;134:1963–1971. doi: 10.1017/S0031182007003320. [DOI] [PubMed] [Google Scholar]
- Aksoy U., Marangi M., Papini R., Ozkoc S., Bayram Delibas S., Giangaspero A. Detection of Toxoplasma gondii and Cyclospora cayetanensis in Mytilus galloprovincialis from Izmir province coast (Turkey) by real time PCR/High-Resolution Melting analysis (HRM) Food Microbiol. 2014;44:128–135. doi: 10.1016/j.fm.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Al-Megrin W.A.I. Prevalence of intestinal parasites in leafy vegetables in Riyadh, Saudi Arabia. Int. J. Zool. Res. 2010;6:190–195. [Google Scholar]
- Amendoeira M.R., Sobral C.A., Teva A., de Lima J.N., Klein C.H. Serological survey of Toxoplasma gondii infection in isolated Amerindians, Mato Grosso. Rev. Soc. Bras. Med. Trop. 2003;36:671–676. doi: 10.1590/s0037-86822003000600005. [DOI] [PubMed] [Google Scholar]
- Aramini J.J., Stephen C., Dubey J.P. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): serology and oocyst shedding. J. Parasitol. 1998;84:438–440. [PubMed] [Google Scholar]
- Aramini J.J., Stephen C., Dubey J.P., Engelstoft C., Schwantje H., Ribble C.S. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 1999;122:305–315. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkush K.D., Miller M.A., Leutenegger C.M., Gardner I.A., Packham A.E., Heckeroth A.R. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) Int. J. Parasitol. 2003;33:1087–1097. doi: 10.1016/s0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Aubert D., Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne Region, France. Mem I Oswaldo Cruz. 2009;104:290–295. doi: 10.1590/s0074-02762009000200023. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira L.M., Jones J.L., Azevedo-Silva J., Alves C.C., Orefice F., Addiss D.G. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia-Oliveira L., Gomez-Marin J., Shapiro K., Jiménez-Cisneros a. Toxoplasma gondii. In: Rose J.B., B., editors. Global Water Pathogens Project. Michigan State University, E. Lansing, MI, UNESCO; 2017. [Google Scholar]
- Barbieri M.M., Kashinsky L., Rotstein D.S., Colegrove K.M., Haman K.H., Magargal S.L. Protozoal-related mortalities in endangered Hawaiian monk seals Neomonachus schauinslandi. Dis. Aquat. Org. 2016;121:85–95. doi: 10.3354/dao03047. [DOI] [PubMed] [Google Scholar]
- Benenson M.W., Takafuji E.T., Lemon S.M., Greenup R.L., Sulzer A.J. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N. Engl. J. Med. 1982;307:666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- Berger-Schoch A.E., Herrmann D.C., Schares G., Muller N., Bernet D., Gottstein B., Frey C.F. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet. Parasitol. 2011;177:290–297. doi: 10.1016/j.vetpar.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Boia M.N., Carvalho-Costa F.A., Sodre F.C., Pinto G.M., Amendoeira M.R. Seroprevalence of Toxoplasma gondii infection among indian people living in Iauarete, Sao Gabriel da Cachoeira, Amazonas, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2008;50:17–20. doi: 10.1590/s0036-46652008000100004. [DOI] [PubMed] [Google Scholar]
- Bowie W.R., King A.S., Werker D.H., Isaac-Renton J.L., Bell A., Eng S.B., Marion S.A. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–177. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- Boyer K., Hill D., Mui E., Wroblewski K., Karrison T., Dubey J.P. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin. Infect. Dis. 2011;53:1081–1089. doi: 10.1093/cid/cir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T.L., Tim Tinker M., Miller M.A., Bodkin J.L., Murray M.J., Saarinen J.A. Defining the risk landscape in the context of pathogen pollution: Toxoplasma gondii in sea otters along the Pacific Rim. R. Soc. Open Sci. 2018;5 doi: 10.1098/rsos.171178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushkin G.G., Motari E., Magnelli P., Gubbels M.J., Dubey J.P., Miska K.B. Beta-1,3-Glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. Mbio. 2012;3 doi: 10.1128/mBio.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushkin G.G., Motari E., Carpentieri A., Dubey J.P., Costello C.E., Robbins P.W., Samuelson J. Evidence for a structural role for acid-fast lipids in oocyst walls of Cryptosporidium, Toxoplasma, and Eimeria. Mbio. 2013;4 doi: 10.1128/mBio.00387-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna T., Marangi M., Del Chierico F., Ferrari N., Reddel S., Bracaglia G. Detection and prevalence of protozoan parasites in ready-to-eat packaged salads on sale in Italy. Food Microbiol. 2017;67:67–75. doi: 10.1016/j.fm.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Carellos E.V., de Andrade G.M., Vasconcelos-Santos D.V., Januario J.N., Romanelli R.M., Abreu M.N. Adverse socioeconomic conditions and oocyst-related factors are associated with congenital toxoplasmosis in a population-based study in Minas Gerais, Brazil. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carme B., Bissuel F., Ajzenberg D., Bouyne R., Aznar C., Demar M. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J. Clin. Microbiol. 2002;40:4037–4044. doi: 10.1128/JCM.40.11.4037-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carme B., Demar M., Ajzenberg D., Darde M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 2009;15 doi: 10.3201/eid1504.081306. (656-658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante G.T., Aguilar D.M., Camargo L.M., Labruna M.B., de Andrade H.F., Meireles L.R. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. J. Parasitol. 2006;92:647–649. doi: 10.1645/GE-774R.1. [DOI] [PubMed] [Google Scholar]
- Chessum B.S. Reactivation of Toxoplasma oocyst production in the cat by infection with Isospora felis. Br. Vet. J. 1972;128:33–36. [PubMed] [Google Scholar]
- Cook A.J.C., Gilbert R.E., Buffolano W., Zufferey J., Petersen E., Jenum P.A. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. Br. Med. J. 2000;321:142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe A., Howe L., Burrows E., Sine A., Pita A., Velathanthiri N. First report of Toxoplasma gondii sporulated oocysts and Giardia duodenalis in commercial green-lipped mussels (Perna canaliculus) in New Zealand. Parasitol. Res. 2018;117:1453–1463. doi: 10.1007/s00436-018-5832-8. [DOI] [PubMed] [Google Scholar]
- Coutinho S.G., Lobo R., Dutra G. Isolation of Toxoplasma from the soil during an outbreak of toxoplasmosis in a rural area in Brazil. J. Parasitol. 1982;68:866–868. [PubMed] [Google Scholar]
- Darde M.L., Villena I., Pinon J.M., Beguinot I. Severe toxoplasmosis caused by a Toxoplasma gondii strain with a new isoenzyme type acquired in French Guyana. J. Clin. Microbiol. 1998;36 doi: 10.1128/jcm.36.1.324-324.1998. (324-324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattoli V.C.C., Veiga R.V., Cunha S.S., Pontes-de-Carvalho L., Barreto M.L., Alcantara-Neves N.M. Oocyst ingestion as an important transmission route of Toxoplasma gondii in Brazilian urban children. J. Parasitol. 2011;97:1080–1084. doi: 10.1645/GE-2836.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.A., Lepczyk C.A., Haman K.H., Morden C.W., Crow S.E., Jensen N., Lohr M.T. Toxoplasma gondii detection in fecal samples from domestic cats (Felis catus) in Hawai'i. Pac. Sci. 2018;72:501–511. [Google Scholar]
- van der Giessen J., Fonville M., Bouwknegt M., Langelaar M., Vollema A. Seroprevalence of Trichinella spiralis and Toxoplasma gondii in pigs from different housing systems in the Netherlands. Vet. Parasitol. 2007;148:371–374. doi: 10.1016/j.vetpar.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Dias R.C., Lopes-Mori F.M., Mitsuka-Bregano R., Dias R.A., Tokano D.V., Reiche E.M. Factors associated to infection by Toxoplasma gondii in pregnant women attended in Basic Health Units in the city of Rolandia, Parana, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:185–191. doi: 10.1590/s0036-46652011000400002. [DOI] [PubMed] [Google Scholar]
- Dixon B.R. Transmission dynamics of foodborne parasites on fresh produce. In: Gajadhar A.A., editor. Foodborne Parasites in the Food Supply Web: Occurrence and Control. Woodhead Publishing Ltd; Cambridge, UK: 2015. pp. 317–353. [Google Scholar]
- Dixon B.R. Parasitic illnesses associated with the consumption of fresh produce - an emerging issue in developed countries. Curr. Opin. Food Sci. 2016;8:104–109. [Google Scholar]
- Du F., Zhang Q.L., Yu Q., Hu M., Zhou Y.Q., Zhao J.L. Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet. Parasitol. 2012;187:53–56. doi: 10.1016/j.vetpar.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Du F., Feng H.L., Nie H., Tu P., Zhang Q.L., Hu M. Survey on the contamination of Toxoplasma gondii oocysts in the soil of public parks of Wuhan, China. Vet. Parasitol. 2012;184:141–146. doi: 10.1016/j.vetpar.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Reshedding of Toxoplasma oocysts by chronically infected cats. Nature. 1976;262:213–214. doi: 10.1038/262213a0. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J. Parasitol. 1995;81:410–415. [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis - a waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009;39:877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57:60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Beattie C.P. CRC Press; Boca Raton, FL: 1988. Toxoplasmosis of Animals and Man. [Google Scholar]
- Dubey J.P., Frenkel J.K. Cyst-induced toxoplasmosis in cats. J. Protozool. 1972;19:155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Immunity to feline toxoplasmosis: modification by administration of corticosteroids. Vet. Pathol. 1974;11:350–379. doi: 10.1177/030098587401100407. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Feline toxoplasmosis from acutely infected mice and the development of Toxoplasma cysts. J. Protozool. 1976;23:537–546. doi: 10.1111/j.1550-7408.1976.tb03836.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jones J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Miller N.L., Frenkel J.K. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 1970;132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Thayer D.W., Speer C.A., Shen S.K. Effect of gamma irradiation on unsporulated and sporulated Toxoplasma gondii oocysts. Int. J. Parasitol. 1998;28:369–375. doi: 10.1016/s0020-7519(97)83432-7. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Zarnke R., Thomas N.J., Wong S.K., Van Bonn W., Briggs M. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003;116:275–296. doi: 10.1016/s0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hill D.E., Rozeboom D.W., Rajendran C., Choudhary S., Ferreira L.R. High prevalence and genotypes of Toxoplasma gondii isolated from organic pigs in northern USA. Vet. Parasitol. 2012;188:14–18. doi: 10.1016/j.vetpar.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Brown J., Verma S.K., Cerqueira-Cezar C.K., Banfield J., Kwok O.C.H. Isolation of viable Toxoplasma gondii, molecular characterization, and seroprevalence in elk (Cervus canadensis) in Pennsylvania, USA. Vet. Parasitol. 2017;243:1–5. doi: 10.1016/j.vetpar.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Dumetre A., Darde M.L. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol. Rev. 2003;27:651–661. doi: 10.1016/S0168-6445(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Dumetre A., Darde M.L. Immunomagnetic separation of Toxoplasma gondii oocysts using a monoclonal antibody directed against the oocyst wall. J. Microbiol. Methods. 2005;61:209–217. doi: 10.1016/j.mimet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Dumetre A., Darde M.L. Detection of Toxoplasma gondii in water by an immunomagnetic separation method targeting the sporocysts. Parasitol. Res. 2007;101:989–996. doi: 10.1007/s00436-007-0573-0. [DOI] [PubMed] [Google Scholar]
- Dumetre A., Aubert D., Puech P.H., Hohweyer J., Azas N., Villena I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 2012;78:905–912. doi: 10.1128/AEM.06488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumetre A., Dubey J.P., Ferguson D.J., Bongrand P., Azas N., Puech P.H. Mechanics of the Toxoplasma gondii oocyst wall. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11535–11540. doi: 10.1073/pnas.1308425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov A.I., Converse R., Griffin S.M., Styles J., Klein E., Sams E. Environmental risk factors for Toxoplasma gondii infections and the impact of latent infections on allostatic load in residents of Central North Carolina. BMC Infect. Dis. 2018;18 doi: 10.1186/s12879-018-3343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman C.C.J., Chiossi M.F.D.V., Meireles L.R., Andrade Júnior H.F.D., Figueiredo W.M., Marciano M.A.M., Luna E.J.D.A. Case-control study of an outbreak of acute toxoplasmosis in an industrial plant in the state of São Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2012;54:239–244. doi: 10.1590/s0036-46652012000500001. [DOI] [PubMed] [Google Scholar]
- El Bissati K., Levigne P., Lykins J., Adlaoui E., Barkat A., Berraho A. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerg. Microbes. Infec. 2018;7 doi: 10.1038/s41426-018-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S.A., Jones J.L., Conrad P.A., Patton S., Lindsay D.S., Dubey J.P. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Erickson M.C., Ortega Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006;69:2786–2808. doi: 10.4315/0362-028x-69.11.2786. [DOI] [PubMed] [Google Scholar]
- Esmerini P.O., Gennari S.M., Pena H.F.J. Analysis of marine bivalve shellfish from the fish market in Santos city, Sao Paulo state, Brazil, for Toxoplasma gondii. Vet. Parasitol. 2010;170:8–13. doi: 10.1016/j.vetpar.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Fayer R., Lindsay D., Olson M.E., Appelbee A., Measures L., Cole R.A. Zoonotic protozoa in the marine environment: a threat to aquatic mammals and public health. Vet. Parasitol. 2004;125:131–135. doi: 10.1016/j.vetpar.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ferguson D.J.P. Toxoplasma gondii: 1908–2008, homage to Nicolle, Manceaux and Splendore. Mem I Oswaldo Cruz. 2009;104:132–147. doi: 10.1590/s0074-02762009000200003. [DOI] [PubMed] [Google Scholar]
- Ferreira F.P., Caldart E.T., Freire R.L., Mitsuka-Bregano R., de Freitas F.M., Miura A.C. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Rev. Bras. Parasitol. Vet. 2018;27:327–337. doi: 10.1590/S1984-296120180050. [DOI] [PubMed] [Google Scholar]
- Fontaine M., Guillot E. An immunomagnetic separation-real-time PCR method for quantification of Cryptosporidium parvum in water samples. J. Microbiol. Methods. 2003;54:29–36. doi: 10.1016/s0167-7012(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K., Dubey J.P. Effects of freezing on viability of Toxoplasma oocysts. J. Parasitol. 1973;59:587–588. [PubMed] [Google Scholar]
- Frenkel J.K., Ruiz A., Chinchilla M. Soil survival of Toxoplasma oocysts in Kansas and Costa-Rica. Am. J. Trop. Med. Hyg. 1975;24:439–443. doi: 10.4269/ajtmh.1975.24.439. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K., Hassanein K.M., Hassanein R.S., Brown E., Thulliez P., Quinteronunez R. Transmission of Toxoplasma gondii in Panama City, Panama - a 5-year prospective cohort study of children, cats, rodents, birds, and soil. Am. J. Trop. Med. Hyg. 1995;53:458–468. doi: 10.4269/ajtmh.1995.53.458. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K., Lindsay D.S., Parker B.B., Dobesh M. Dogs as possible mechanical carriers of Toxoplasma, and their fur as a source of infection of young children. Int. J. Infect. Dis. 2003;7:292–293. doi: 10.1016/s1201-9712(03)90112-3. [DOI] [PubMed] [Google Scholar]
- Freppel W., Ferguson J.P., Shapiro K., Dubey J.P., Puech P.H., Dumètre A. Structure, composition, and roles of the Toxoplasma gondii oocyst and sporocyst walls. Cell Surf. December 2019;5:100016. doi: 10.1016/j.tcsw.2018.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H., Barr B., Packham A., Melli A., Conrad P.A. Methods to produce and safely work with large numbers of Toxoplasma gondii oocysts and bradyzoite cysts. J. Microbiol. Methods. 2012;88:47–52. doi: 10.1016/j.mimet.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H.M., Bowyer P.W., Bogyo M., Conrad P.A., Boothroyd J.C. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal L., Hamidović A., Dardé M.-L., Mercier A. Diversity of Toxoplasma gondii strains at the global level and its determinants. Food Waterborne Parasitol. 2019 doi: 10.1016/j.fawpar.2019.e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghozzi K., Marangi M., Papini R., Lahmar I., Challouf R., Houas N. First report of Tunisian coastal water contamination by protozoan parasites using mollusk bivalves as biological indicators. Mar. Pollut. Bull. 2017;117:197–202. doi: 10.1016/j.marpolbul.2017.01.057. [DOI] [PubMed] [Google Scholar]
- Gilot-Fromont E., Lélu M., Dardé M.-L., Richomme C., Aubert D., Afonso E. The life cycle of Toxoplasma gondii in the natural environment. In: Djaković O.D., editor. Toxoplasmosis. IntechOpen; London, UK: 2012. [Google Scholar]
- Gotteland C., Gilot-Fromont E., Aubert D., Poulle M.L., Dupuis E., Darde M.L. Spatial distribution of Toxoplasma gondii oocysts in soil in a rural area: influence of cats and land use. Vet. Parasitol. 2014;205:629–637. doi: 10.1016/j.vetpar.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Guo M., Dubey J.P., Hill D., Buchanan R.L., Gamble H.R., Jones J.L., Pradhan A.K. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 2015;78:457–476. doi: 10.4315/0362-028X.JFP-14-328. [DOI] [PubMed] [Google Scholar]
- Hall S.M., Pandit A., Golwilkar A., Williams T.S. How do Jains get toxoplasma infection? Lancet. 1999;354:486–487. doi: 10.1016/s0140-6736(99)02587-8. [DOI] [PubMed] [Google Scholar]
- Harito J.B., Campbell A.T., Prestrud K.W., Dubey J.P., Robertson L.J. Surface binding properties of aged and fresh (recently excreted) Toxoplasma gondii oocysts. Exp. Parasitol. 2016;165:88–94. doi: 10.1016/j.exppara.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Harito J.B., Campbell A.T., Tysnes K.R., Robertson L.J. Use of lectin-magnetic separation (LMS) for detecting Toxoplasma gondii oocysts in environmental water samples. Water Res. 2017;127:68–76. doi: 10.1016/j.watres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Hass C.C. Competition and coexistence in sympatric bobcats and pumas. J. Zool. 2009;278:174–180. [Google Scholar]
- Hazlett M.J., McDowall R., DeLay J., Stalker M., McEwen B., van Dreumel T. A prospective study of sheep and goat abortion using real-time polymerase chain reaction and cut point estimation shows Coxiella burnetii and Chlamydophila abortus infection concurrently with other major pathogens. J. Vet. Diagn. Investig. 2013;25:359–368. doi: 10.1177/1040638713484729. [DOI] [PubMed] [Google Scholar]
- Heim R.R. An overview of weather and climate extremes - products and trends. Weather Clim. Extreme. 2015;10:1–9. [Google Scholar]
- Herrmann D.C., Pantchev N., Vrhovec M.G., Barutzki D., Wilking H., Frohlich A. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. Int. J. Parasitol. 2010;40:285–292. doi: 10.1016/j.ijpara.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Meyer-Cirkel V., Moura R.C., Gomide M., Queiroz J.A., Saweljew P., Liesenfeld O. Waterborne toxoplasmosis, northeastern Brazil. Emerg. Infect. Dis. 2007;13:287–289. doi: 10.3201/eid1302.060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D., Coss C., Dubey J.P., Wroblewski K., Sautter M., Hosten T. Identification of a sporozoite-specific antigen from Toxoplasma gondii. J. Parasitol. 2011;97:328–337. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohweyer J., Cazeaux C., Travaille E., Languet E., Dumetre A., Aubert D. Simultaneous detection of the protozoan parasites Toxoplasma, Cryptosporidium and Giardia in food matrices and their persistence on basil leaves. Food Microbiol. 2016;57:36–44. doi: 10.1016/j.fm.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Hutchison W.M., Dunachie J.F., Siim J.C., Work K. Life cycle of Toxoplasma gondii. Br. Med. J. 1969;4:806. doi: 10.1136/bmj.4.5686.806-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.H., Hutchison W.M. The prevalence and source of Toxoplasma infection in the environment. Adv. Parasitol. 1989;28:55–105. doi: 10.1016/s0065-308x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Jacobs L. The interrelation of toxoplasmosis in swine, cattle, dogs, and man. Public Health Rep. 1957;72:872–882. [PMC free article] [PubMed] [Google Scholar]
- Jacobs L., Remington J.S., Melton M.L. A survey of meat samples from swine, cattle, and sheep for the presence of encysted Toxoplasma. J. Parasitol. 1960;46:23–28. [PubMed] [Google Scholar]
- Jewell M.L., Frenkel J.K., Johnson K.M., Reed V., Ruiz A. Development of Toxoplasma oocysts in neotropical felidae. Am. J. Trop. Med. Hyg. 1972;21:512–517. doi: 10.4269/ajtmh.1972.21.512. [DOI] [PubMed] [Google Scholar]
- Johnson C.K., Tinker M.T., Estes J.A., Conrad P.A., Staedler M., Miller M.A. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2242–2247. doi: 10.1073/pnas.0806449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen P., Deksne G., Holmala K., Nareaho A., Laakkonen J., Kojola I., Sukura A. Free-ranging Eurasian lynx (Lynx lynx) as host of Toxoplasma gondii in Finland. J. Wildl. Dis. 2013;49:527–534. doi: 10.7589/2011-12-352. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dubey J.P. Waterborne toxoplasmosis—recent developments. Exp. Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dubey J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012;55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dargelas V., Roberts J., Press C., Remington J.S., Montoya J.G. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009;49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- Jones K.H., Wilson F.D., Fitzgerald S.D., Kiupel M. A natural outbreak of clinical toxoplasmosis in a backyard flock of guinea fowl in Mississippi. Avian Dis. 2012;56:750–753. doi: 10.1637/10226-043012-Case.1. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Kruszon-Moran D., Rivera H.N., Price C., Wilkins P.P. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am. J. Trop. Med. Hyg. 2014;90:1135–1139. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenihan S.H., Schetters T., Taverne J. Toxoplasmosis in Brazil. Trends Parasitol. 2002;18:203–204. [Google Scholar]
- Kijlstra A., Jongert E. Toxoplasma-safe meat: close to reality? Trends Parasitol. 2009;25:18–22. doi: 10.1016/j.pt.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Kniel K.E., Lindsay D.S., Sumner S.S., Hackney C.R., Pierson M.D., Dubey J.P. Examination of attachment and survival of Toxoplasma gondii oocysts on raspberries and blueberries. J. Parasitol. 2002;88:790–793. doi: 10.1645/0022-3395(2002)088[0790:EOAASO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kreuder C., Miller M.A., Jessup D.A., Lowenstine L.J., Harris M.D., Ames J.A. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. J. Wildl. Dis. 2003;39:495–509. doi: 10.7589/0090-3558-39.3.495. [DOI] [PubMed] [Google Scholar]
- Krueger W.S., Hilborn E.D., Converse R.R., Wade T.J. Drinking water source and human Toxoplasma gondii infection in the United States: a cross-sectional analysis of NHANES data. BMC Public Health. 2014;14:10. doi: 10.1186/1471-2458-14-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusor C., Smith W.A., Tinker M.T., Silver M., Conrad P.A., Shapiro K. Concentration and retention of Toxoplasma gondii oocysts by marine snails demonstrate a novel mechanism for transmission of terrestrial zoonotic pathogens in coastal ecosystems. Environ. Microbiol. 2015;17:4527–4537. doi: 10.1111/1462-2920.12927. [DOI] [PubMed] [Google Scholar]
- Lacombe A., Breard A., Hwang C.A., Hill D., Fan X.T., Huang L.H. Inactivation of Toxoplasma gondii on blueberries using low dose irradiation without affecting quality. Food Control. 2017;73:981–985. [Google Scholar]
- Lalle M., Possenti A., Dubey J.P., Pozio E. Loop-Mediated Isothermal Amplification-Lateral-Flow Dipstick (LAMP-LFD) to detect Toxoplasma gondii oocyst in ready-to-eat salad. Food Microbiol. 2018;70:137–142. doi: 10.1016/j.fm.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Lalonde L.F., Gajadhar A.A. Detection of Cyclospora cayetanensis, Cryptosporidium spp., and Toxoplasma gondii on imported leafy green vegetables in Canadian survey. Food Waterborne Parasitol. 2016;2:8–14. [Google Scholar]
- Lass A., Pietkiewicz H., Modzelewska E., Dumetre A., Szostakowska B., Myjak P. Detection of Toxoplasma gondii oocysts in environmental soil samples using molecular methods. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:599–605. doi: 10.1007/s10096-008-0681-5. [DOI] [PubMed] [Google Scholar]
- Lass A., Pietkiewicz H., Szostakowska B., Myjak P. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1101–1108. doi: 10.1007/s10096-011-1414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff L., Hubert B., Favennec L., Villena I., Ballet J.J., Agoulon A. Pilot-scale pulsed UV light irradiation of experimentally infected raspberries suppresses Cryptosporidium parvum infectivity in immunocompetent suckling mice. J. Food Prot. 2015;78:2247–2252. doi: 10.4315/0362-028X.JFP-15-062. [DOI] [PubMed] [Google Scholar]
- Lélu M., Gilot-Fromont E., Aubert D., Richaume A., Afonso E., Dupuis E. Development of a sensitive method for oocyst extraction in soil. Vet. Parasitol. 2011;183:59–67. doi: 10.1016/j.vetpar.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Lélu M., Villena I., Darde M.L., Aubert D., Geers R., Dupuis E. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl. Environ. Microbiol. 2012;78:5127–5132. doi: 10.1128/AEM.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg O., Sandell M., Pontier D., Natoli E. Density, spatial organization and reproductive tactics in the domestic cat and other felids. In: Turner D.C., Bateson P., editors. The Domestic Dat: The Biology of Its Behavior. Cambridge University Press; 2000. pp. 119–147. [Google Scholar]
- Lilly E.L., Wortham C.D. High prevalence of Toxoplasma gondii oocyst shedding in stray and pet cats (Felis catus) in Virginia, United States. Parasit. Vectors. 2013;6:4. doi: 10.1186/1756-3305-6-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist H.D., Bennett J.W., Hester J.D., Ware M.W., Dubey J.P., Everson W.V. Autofluorescence of Toxoplasma gondii and related coccidian oocysts. J. Parasitol. 2003;89:865–867. doi: 10.1645/GE-3147RN. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P., Butler J.M., Blagburn B.L. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet. Parasitol. 1997;73:27–33. doi: 10.1016/s0304-4017(97)00048-4. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Collins M.V., Mitchell S.M., Wetch C.N., Rosypal A.C., Flick G.J. Survival of Toxoplasma gondii oocysts Eastern oysters (Crassostrea virginica) J. Parasitol. 2004;90:1054–1057. doi: 10.1645/GE-296R. [DOI] [PubMed] [Google Scholar]
- Lindsay D.S., Holliman D., Flick G.J., Goodwin D.G., Mitchell S.M., Dubey J.P. Effects of high pressure processing on Toxoplasma gondii oocysts on raspberries. J. Parasitol. 2008;94:757–758. doi: 10.1645/GE-1471.1. [DOI] [PubMed] [Google Scholar]
- Liu X.C., He Y., Han D.G., Zhang Z.C., Li K., Wang S. Detection of Toxoplasma gondii in chicken and soil of chicken farms in Nanjing region, China. Infect. Dis. Poverty. 2017;6 doi: 10.1186/s40249-017-0277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukesova D., Literak I. Shedding of Toxoplasma gondii oocysts by Felidae in zoos in the Czech Republic. Vet. Parasitol. 1998;74:1–7. doi: 10.1016/s0304-4017(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Luptakova L., Benova K., Rencko A., Petrovova E. DNA detection of Toxoplasma gondii in sheep milk and blood samples in relation to phase of infection. Vet. Parasitol. 2015;208:250–253. doi: 10.1016/j.vetpar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Macdonald D., Loveridge A. Oxford University Press; 2010. The Biology and Conservation of Wild Felids. [Google Scholar]
- MacKenzie W.R., Schell W.L., Blair K.A., Addiss D.G., Peterson D.E., Hoxie N.J. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin. Infect. Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- Mai K., Sharman P.A., Walker R.A., Katrib M., De Souza D., McConville M.J. Oocyst wall formation and composition in coccidian parasites. Mem I Oswaldo Cruz. 2009;104:281–289. doi: 10.1590/s0074-02762009000200022. [DOI] [PubMed] [Google Scholar]
- Mancianti F., Nardoni S., D'Ascenzi C., Pedonese F., Mugnaini L., Franco F., Papini R. Seroprevalence, detection of DNA in blood and milk, and genotyping of Toxoplasma gondii in a goat population in Italy. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/905326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancianti F., Nardoni S., Mugnaini L., Zambernardi L., Guerrini A., Gazzola V., Papini R.A. A retrospective molecular study of select intestinal protozoa in healthy pet cats from Italy. J. Feline Med. Surg. 2015;17:163–167. doi: 10.1177/1098612X14533549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi B.M., Vieira F.P., Bahia-Oliveira L.M., Hill D. Salivary IgA against sporozoite-specific embryogenesis-related protein (TgERP) in the study of horizontally transmitted toxoplasmosis via T. gondii oocysts in endemic settings. Epidemiol. Infect. 2016;144:2568–2577. doi: 10.1017/S0950268816000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiondo A.A., Duszynski D.W., Maupin G.O. Prevalence of antibodies to Toxoplasma gondii in wild and domestic animals of New Mexico, Arizona and Colorado. J. Wildl. Dis. 1976;12:226–232. doi: 10.7589/0090-3558-12.2.226. [DOI] [PubMed] [Google Scholar]
- Marchioro A.A., Tiyo B.T., Colli C.M., de Souza C.Z., Garcia J.L., Gomes M.L., Falavigna-Guilherme A.L. First detection of Toxoplasma gondii DNA in the fresh leafs of vegetables in South America. Vector Borne Zoonotic Dis. 2016;16:624–626. doi: 10.1089/vbz.2015.1937. [DOI] [PubMed] [Google Scholar]
- Massie G.N., Ware M.W., Villegas E.N., Black M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Menegolla I., Difante C., Ramos L., Dorneles D., Streb A., Mello L. IV Simpósio Brasileiro de Toxoplasmose. Brasilia, Brazil. 2018. Investigação de surto de toxoplasmose em Santa Maria/RS, 2018. [Google Scholar]
- Miller N.L., Frenkel J.K., Dubey J.P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 1972;58:928–937. [PubMed] [Google Scholar]
- Miller M.A., Gardner I.A., Kreuder C., Paradies D.M., Worcester K.R., Jessup D.A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Miller W.A., Conrad P.A., James E.R., Melli A.C., Leutenegger C.M. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Shapiro K., Murray M., Haulena M.J., Raverty S. Protozoan parasites of of marine mammals. In: Gulland Frances M.D., Dierauf Leslie A., Whitman K.L., editors. CRC Handbook of Marine Mammal Medicine. CRC Press; 2018. pp. 425–470. [Google Scholar]
- Morais R.d.A.P.B., Freire A.B.C., Barbosa D.R.L., Silva L.d.C.T.d., Pinheiro A.F., Costa S.S.d. Surto de toxoplasmose aguda no Município de Ponta de Pedras, Arquipélago do Marajó, Estado do Pará, Brasil: características clínicas, laboratoriais e epidemiológicas. Revista Pan-Amazônica de Saúde. 2016;7:143–152. [Google Scholar]
- More G., Maksimov P., Pardini L., Herrmann D.C., Bacigalupe D., Maksimov A. Toxoplasma gondii infection in sentinel and free-range chickens from Argentina. Vet. Parasitol. 2012;184:116–121. doi: 10.1016/j.vetpar.2011.09.012. [DOI] [PubMed] [Google Scholar]
- de Moura L., Bahia-Oliveira L.M., Wada M.Y., Jones J.L., Tuboi S.H., Carmo E.H. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 2006;12:326–329. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi H., Rashid M.I., Islam S., Bajwa A.A., Gul R., Shehzad W. Prevalence of Toxoplasma gondii oocysts through Copro-PCR in cats at Pet Center (UVAS), Lahore, Pakistan. J. Pak. Med. Assoc. 2018;68:115–118. [PubMed] [Google Scholar]
- Newman A.P., Aitken D., Antizar-Ladislao B. Stormwater quality performance of a macro-pervious pavement car park installation equipped with channel drain based oil and silt retention devices. Water Res. 2013;47:7327–7336. doi: 10.1016/j.watres.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Nogareda F., Le Strat Y., Villena I., De Valk H., Goulet V. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol. Infect. 2014;142:1661–1670. doi: 10.1017/S0950268813002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palos Ladeiro M., Aubert D., Villena I., Geffard A., Bigot A. Bioaccumulation of human waterborne protozoa by zebra mussel (Dreissena polymorpha): interest for water biomonitoring. Water Res. 2014;48:148–155. doi: 10.1016/j.watres.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Pappas G., Roussos N., Falagas M.E. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Plantinga E.A., Bosch G., Hendriks W.H. Estimation of the dietary nutrient profile of free-roaming feral cats: possible implications for nutrition of domestic cats. Brit. J. Nutr. 2011;106:S35–S48. doi: 10.1017/S0007114511002285. [DOI] [PubMed] [Google Scholar]
- Possenti A., Cherchi S., Bertuccini L., Pozio E., Dubey J.P., Spano F. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int. J. Parasitol. 2010;40:1639–1649. doi: 10.1016/j.ijpara.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Possenti A., Fratini F., Fantozzi L., Pozio E., Dubey J.P., Ponzi M. Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle. BMC Genomics. 2013;14:183. doi: 10.1186/1471-2164-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignani L., Mancinelli L., Del Chierico F., Menichella D., Adlerstein D., Angelici M.C. Investigation of Toxoplasma gondii presence in farmed shellfish by nested-PCR and real-time PCR fluorescent amplicon generation assay (FLAG) Exp. Parasitol. 2011;127:409–417. doi: 10.1016/j.exppara.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Rawal B.D. Toxoplasmosis; a dye-test survey on sera from vegetarians and meat eaters in Bombay. Trans. R. Soc. Trop. Med. Hyg. 1959;53:61–63. doi: 10.1016/0035-9203(59)90084-7. [DOI] [PubMed] [Google Scholar]
- Ribas M.P., Almeria S., Fernandez-Aguilar X., De Pedro G., Lizarraga P., Alarcia-Alejos O. Tracking Toxoplasma gondii in freshwater ecosystems: interaction with the invasive American mink (Neovison vison) in Spain. Parasitol. Res. 2018;117:2275–2281. doi: 10.1007/s00436-018-5916-5. [DOI] [PubMed] [Google Scholar]
- Robertson L.J. Parasitic protozoa in salad vegetables. In: Kotzekidou P., editor. Food Hygiene and Toxicology in Ready-to-eat Foods. Elsevier; New York: 2016. [Google Scholar]
- Robert-Gangneux F., Darde M.L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe W.D., Howe L., Baker E.J., Burrows L., Hunter S.A. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector's dolphins (Cephalorhynchus hectori) Vet. Parasitol. 2013;192:67–74. doi: 10.1016/j.vetpar.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Rousseau A., La Carbona S., Dumetre A., Robertson L.J., Gargala G., Escotte-Binet S. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite. 2018;25 doi: 10.1051/parasite/2018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau A., Villena I., Dumetre A., Escotte-Binet S., Favennec L., Dubey J.P. Evaluation of propidium monoazide-based qPCR to detect viable oocysts of Toxoplasma gondii. Parasitol. Res. 2019;118:999–1010. doi: 10.1007/s00436-019-06220-1. [DOI] [PubMed] [Google Scholar]
- Ruiz A., Frenkel J.K. Toxoplasma gondii in Costa Rican cats. Am. J. Trop. Med. Hyg. 1980;29:1150–1160. doi: 10.4269/ajtmh.1980.29.1150. [DOI] [PubMed] [Google Scholar]
- Saad N.M., Hussein A.A.A., Ewida R.M. Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in upper Egypt. Vet. World. 2018;11:1262–1265. doi: 10.14202/vetworld.2018.1262-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks J.J., Roberto R.R., Brooks N.F. Toxoplasmosis infection associated with raw goat's milk. JAMA. 1982;248:1728–1732. doi: 10.1001/jama.1982.03330140038029. [DOI] [PubMed] [Google Scholar]
- Samuelson J., Bushkin G.G., Chatterjee A., Robbins P.W. Strategies to discover the structural components of cyst and oocyst walls. Eukaryot. Cell. 2013;12:1578–1587. doi: 10.1128/EC.00213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santana Rocha D., de Sousa Moura R.L., Maciel B.M., Guimaraes L.A., O'Dwyer H., Munhoz A.D., Albuquerque G.R. Detection of Toxoplasma gondii DNA in naturally infected sheep's milk. Genet. Mol. Res. 2015;14:8658–8662. doi: 10.4238/2015.July.31.14. [DOI] [PubMed] [Google Scholar]
- Santana S.S., Gebrim L.C., Carvalho F.R., Barros H.S., Barros P.C., Pajuaba A.C. CCp5A protein from Toxoplasma gondii as a serological marker of oocyst-driven infections in humans and domestic animals. Front. Microbiol. 2015;6:1305. doi: 10.3389/fmicb.2015.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Vrhovec M.G., Pantchev N., Herrmann D.C., Conraths F.J. Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet. Parasitol. 2008;152:34–45. doi: 10.1016/j.vetpar.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schott K.G., Krusor C., Tinker M.T., Moore J., Conrad P.A., Shapiro K. Concentration and retention of Toxoplasma gondii surrogates from seawater by red abalone (Haliotis rufescens) Parasitology. 2016;143:1703–1712. doi: 10.1017/S0031182016001359. [DOI] [PubMed] [Google Scholar]
- Shafa-ul-Haq, Maqbool A., Khan U.J., Yasmin G., Sultana R. Parasitic contamination of vegetables eaten raw in Lahore. Pak. J. Zool. 2014;46:1303–1309. [Google Scholar]
- Shapiro K., Largier J., Mazet J.A.K., Bernt W., Ell J.R., Melli A.C., Conrad P.A. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Appl Environ Microbiol. 2009;75:1185–1191. doi: 10.1128/AEM.02109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K. Climate and coastal habitat change: a recipe for a dirtier ocean. Mar. Pollut. Bull. 2012;64:1079–1080. doi: 10.1016/j.marpolbul.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Shapiro K., Conrad P.A., Mazet J.A.K., Wallender W.W., Miller W.A., Largier J.L. Effect of estuarine wetland degradation on transport of Toxoplasma gondii surrogates from land to sea. Appl. Environ. Microbiol. 2010;76:6821–6828. doi: 10.1128/AEM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K., Silver M.W., Largier J., Conrad P.A., Mazet J.A.K. Association of Toxoplasma gondii oocysts with fresh, estuarine, and marine macroaggregates. Limnol. Oceanogr. 2012;57:449–456. [Google Scholar]
- Shapiro K., Krusor C., Mazzillo F., Conrad P.A., Largier J.L., Mazet J.A.K., Silver M.W. Aquatic polymers can drive pathogen transmission in coastal ecosystems. Proc. R. Soc. B. 2014;281:20141287. doi: 10.1098/rspb.2014.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K., VanWormer E., Aguilar B., Conrad P.A. Surveillance for Toxoplasma gondii in California mussels (Mytilus californianus) reveals transmission of atypical genotypes from land to sea. Environ. Microbiol. 2015;17:4177–4188. doi: 10.1111/1462-2920.12685. [DOI] [PubMed] [Google Scholar]
- Sharma R., Loseto L.L., Ostertag S.K., Tomaselli M., Bredtmann C.M., Crill C. Qualitative risk assessment of impact of Toxoplasma gondii on health of beluga whales, Delphinapterus leucas, from the Eastern Beaufort Sea, Northwest Territories. Arch. Sci. 2018;4:321–337. [Google Scholar]
- Simon A., Bigras Poulin M., Rousseau A.N., Dubey J.P., Ogden N.H. Spatiotemporal dynamics of Toxoplasma gondii infection in Canadian lynx (Lynx canadensis) in western Quebec, Canada. J. Wildl. Dis. 2013;49:39–48. doi: 10.7589/2012-02-048. [DOI] [PubMed] [Google Scholar]
- Simon A., Rousseau A.N., Savary S., Bigras-Poulin M., Ogden N.H. Hydrological modelling of Toxoplasma gondii oocysts transport to investigate contaminated snowmelt runoff as a potential source of infection for marine mammals in the Canadian Arctic. J. Environ. Manag. 2013;127:150–161. doi: 10.1016/j.jenvman.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Simon J.A., Kurdzielewicz S., Jeanniot E., Dupuis E., Marnef F., Aubert D. Spatial distribution of soil contaminated with Toxoplasma gondii oocysts in relation to the distribution and use of domestic cat defecation sites on dairy farms. Int. J. Parasitol. 2017;47:357–367. doi: 10.1016/j.ijpara.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Sotiriadou I., Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT) Diagn. Microbiol. Infect. Dis. 2008;62:357–365. doi: 10.1016/j.diagmicrobio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Spalding S.M., Amendoeira M.R., Klein C.H., Ribeiro L.C. Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev. Soc. Bras. Med. Trop. 2005;38:173–177. doi: 10.1590/s0037-86822005000200009. [DOI] [PubMed] [Google Scholar]
- Sroka J., Wojcik-Fatla A., Dutkiewicz J. Occurrence of Toxoplasma gondii in water from wells located on farms. Ann. Agric. Environ. Med. 2006;13:169–175. [PubMed] [Google Scholar]
- Sroka S., Bartelheimer N., Winter A., Heukelbach J., Ariza L., Ribeiro H. Prevalence and risk factors of toxoplasmosis among pregnant women in Fortaleza, Northeastern Brazil. Am. J. Trop. Med. Hyg. 2010;83:528–533. doi: 10.4269/ajtmh.2010.10-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs S.E., Keely S.P., Ware M.W., Schable N., See M.J., Gregorio D. The development and implementation of a method using blue mussels (Mytilus spp.) as biosentinels of Cryptosporidium spp. and Toxoplasma gondii contamination in marine aquatic environments. Parasitol. Res. 2015;114:4655–4667. doi: 10.1007/s00436-015-4711-9. [DOI] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch S.M., Juranek D.D., Sulzer A., Dubey J.P., Sikes R.K. Epidemic toxoplasmosis associated with infected cats. N. Engl. J. Med. 1979;300:695–699. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- Travaille E., La Carbona S., Gargala G., Aubert D., Guyot K., Dumetre A. Development of a qRT-PCR method to assess the viability of Giardia intestinalis cysts, Cryptosporidium spp. and Toxoplasma gondii oocysts. Food Control. 2016;59:359–365. [Google Scholar]
- Trivino-Valencia J., Lora F., Zuluaga J.D., Gomez-Marin J.E. Detection by PCR of pathogenic protozoa in raw and drinkable water samples in Colombia. Parasitol. Res. 2016;115(5):1789–1797. doi: 10.1007/s00436-016-4917-5. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Moreno L.F., Rico-Torres C.P., Cedillo-Pelaez C., Luna-Pasten H., Mendez-Cruz S.T., Lara-Martinez G. Mixed Toxoplasma gondii infection and new genotypes in feral cats of Quintana Roo, Mexico. Acta Trop. 2019 Mar 6;193:199–205. doi: 10.1016/j.actatropica.2019.03.006. [DOI] [PubMed] [Google Scholar]
- VanWormer E., Fritz H., Shapiro K., Mazet J.A., Conrad P.A. Molecules to modeling: Toxoplasma gondii oocysts at the human-animal-environment interface. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:217–231. doi: 10.1016/j.cimid.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWormer E., Conrad P.A., Miller M.A., Melli A.C., Carpenter T.E., Mazet J.A.K. Toxoplasma gondii, source to sea: higher contribution of domestic felids to terrestrial parasite loading despite lower infection prevalence. ECOHEALTH. 2013;10:277–289. doi: 10.1007/s10393-013-0859-x. [DOI] [PubMed] [Google Scholar]
- VanWormer E., Miller M., Conrad P., Grigg M., Rejmanek D., Carpenter T., Mazet J. Using molecular epidemiology to track Toxoplasma gondii from terrestrial carnivores to marine hosts: implications for public health and conservation. PLoS Negl. Trop. Dis. 2014;8:e2852. doi: 10.1371/journal.pntd.0002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWormer E., Carpenter T.E., Singh P., Shapiro K., Wallender W.W., Conrad P.A. Coastal development and precipitation drive pathogen flow from land to sea: evidence from a Toxoplasma gondii and felid host system. Sci. Rep. 2016;6:9. doi: 10.1038/srep29252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux J.D., Muccioli C., James E.R., Silveira C., Magargal S.L., Jung C. Identification of an atypical strain of Toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. J. Infect. Dis. 2010;202:1226–1233. doi: 10.1086/656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.K., Sweeny A.R., Lovallo M.J., Calero-Bernal R., Kwok O.C., Jiang T. Seroprevalence, isolation and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA. Int. J. Parasitol. 2017;47:297–303. doi: 10.1016/j.ijpara.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Santoro A., Milardi G.L., Diaferia M., Morganti G., Ranucci D., Gabrielli S. Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol. Res. 2017;116:1063–1069. doi: 10.1007/s00436-017-5388-z. [DOI] [PubMed] [Google Scholar]
- Vieira F.P., Alves Mda G., Martins L.M., Rangel A.L., Dubey J.P., Hill D., Bahia-Oliveira L.M. Waterborne toxoplasmosis investigated and analysed under hydrogeological assessment: new data and perspectives for further research. Mem. Inst. Oswaldo Cruz. 2015;110:929–935. doi: 10.1590/0074-02760150262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena I., Aubert D., Gomis P., Ferte H., Inglard J.C., Denis-Bisiaux H. Evaluation of a strategy for Toxoplasma gondii oocyst detection in water. Appl Environ Microbiol. 2004;70:4035–4039. doi: 10.1128/AEM.70.7.4035-4039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virahsawmy H.K., Stewardson M.J., Vietz G., Fletcher T.D. Factors that affect the hydraulic performance of raingardens: implications for design and maintenance. Water Sci. Technol. 2014;69:982–988. doi: 10.2166/wst.2013.809. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. Intermediate and transport hosts in natural history of Toxoplasma gondii. Am. J. Trop. Med. Hyg. 1973;22:456–464. doi: 10.4269/ajtmh.1973.22.456. [DOI] [PubMed] [Google Scholar]
- Waltnertoews D., Mondesire R., Menzies P. The seroprevalence of Toxoplasma gondii in Ontario sheep flocks. Can. Vet. J. 1991;32:734–737. [PMC free article] [PubMed] [Google Scholar]
- Wells B., Shaw H., Innocent G., Guido S., Hotchkiss E., Parigi M. Molecular detection of Toxoplasma gondii in water samples from Scotland and a comparison between the 529bp real-time PCR and ITS1 nested PCR. Water Res. 2015;87:175–181. doi: 10.1016/j.watres.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Wu Y.D., Xu M.J., Wang Q.Q., Zhou C.X., Wang M., Zhu X.Q., Zhou D.H. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for detection of Toxoplasma gondii in the environment. Vet. Parasitol. 2017;243:199–203. doi: 10.1016/j.vetpar.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Zhang M., Yang Z., Wang S., Tao L.F., Xu L.X., Yan R.F. Detection of Toxoplasma gondii in shellfish and fish in parts of China. Vet. Parasitol. 2014;200:85–89. doi: 10.1016/j.vetpar.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Zhou D.H., Wang Z.X., Zhou C.X., He S., Elsheikha H.M., Zhu X.Q. Comparative proteomic analysis of virulent and avirulent strains of Toxoplasma gondii reveals strain-specific patterns. Oncotarget. 2017;8:80481–80491. doi: 10.18632/oncotarget.19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulpo D.L., Sammi A.S., dos Santos J.R., Sasse J.P., Martins T.A., Minutti A.F. Toxoplasma gondii: a study of oocyst re-shedding in domestic cats. Vet. Parasitol. 2018;249:17–20. doi: 10.1016/j.vetpar.2017.10.021. [DOI] [PubMed] [Google Scholar]