Abstract
Enteric protozoa infection among cattle may pose a threat to productivity and survival leading to negative impacts on the livestock industry. A number of these pathogens are also known to be zoonotic and are of public health concern. Despite the importance of these enteric protozoa to both animal and human health, there remains a paucity of published information on the epidemiological risk factors that may be associated with bovine cryptosporidiosis in Southeast Asia. The present study was undertaken to determine the molecular prevalence and associated risk factors for Cryptosporidium infection among beef and dairy cattle in Peninsular Malaysia. Faecal samples were collected from 824 cattle in 39 farms (526 beef and 298 dairy) situated in 33 locations throughout the country, and subjected to PCR detection for Cryptosporidium using primers targeting the 18S SSUrRNA gene. Epidemiological variables including host, environment and management factors were subjected to univariate and multivariate logistic regression analyses to determine the potential risk factors for infection. The prevalence of Cryptosporidium among the cattle was 12.5%, with significant difference in the infection rate among the various breeds. There was no significant effect of gender, and both the beef and dairy cattle were at similar odds for infection. The younger cattle had a significantly higher infection rate compared to the older animals. Multivariate analysis revealed that deworming practice, distance to human settlement, geographical location (zone) and farm management system were significant risk factors associated with Cryptosporidium infection. The cattle that were reared on farms located in the northeast of the country, closest (≤200 m) to human settlements, reared extensively, and dewormed every four months were at highest risk of infection. The present study constitutes the first attempt to analyze the multivariable epidemiological risk factors involved in bovine cryptosporidiosis in Malaysia and in Southeast Asia. It is envisaged that the data obtained will facilitate better control and prevention measures for Cryptosporidium infection among cattle in the region. Due to the potential zoonotic nature of the infection, serious steps should be instituted for animal treatment and biohazard waste management on local cattle farms.
Keywords: Cryptosporidium, Cattle, Risk factors, Peninsular Malaysia
Highlights
-
•
First multivariate risk factor analysis of cattle Cryptosporidium in Southeast Asia
-
•
Prevalence of Cryptosporidium was 12.5%; highest in young (≤1 year) cattle.
-
•
Farming system, deworming practice, and farm location were significant risk factors.
1. Introduction
Epidemiological studies including risk factor analysis for enteric protozoa infection among cattle are important as these parasites pose a threat to the productivity and survival of the animals (Casemore et al., 1997; Mohammed et al., 1999; Tung et al., 2012; Jacobson et al., 2018), and thus may exert negative impacts on the industry. In addition, a number of these enteric protozoa are of public health concern as zoonotic transmissions may occur to the animal handlers and those exposed to contaminated water, food, and animal products. One of the important causative agents of protozoa infections among animals is Cryptosporidium which is a widely distributed and infect a diversity of vertebrates including reptiles, birds, fish, mammals and humans (Fayer et al., 1997; Fayer, 2004; Xiao et al., 2004; Ryan et al., 2014). Cryptosporidium infects the gastrointestinal tract and is transmitted through the ingestion of oocysts found in contaminated food or water (Fayer, 2004; Robertson and Fayer, 2013). While sub-clinical infections are common, Cryptosporidium may cause enteritis leading to severe watery diarrhea and abdominal pain (Kosek et al., 2001; Chen et al., 2002; Kotloff et al., 2013). In addition, immuno-compromised hosts may suffer chronic infections which can be severe and fatal (Palit et al., 2005; Cohen et al., 2006; Siwila et al., 2007).
Cattle are known to be infected with a number of Cryptosporidium species, namely, C. parvum, C. bovis, C. ryanae, C. andersoni and C. ubiquitum (Ryan et al., 2014). Infected individuals may excrete large number of Cryptosporidium oocysts in their faeces resulting in contamination of drinking and recreational water, fruits and vegetables, leading to infections in humans and other animals (Del Coco et al., 2008). Cryptosporidiosis in calves and lambs are considered to be the main source for human cryptosporidiosis (Sari et al., 2009), a disease that is widely distributed in many countries. Previous studies on the prevalence of Cryptosporidium on livestock farms in Malaysia (Fatimah et al., 1995a, Fatimah et al., 1995b; Farizawati et al., 2005; Hisamuddin et al., 2016; Tan et al., 2017) have focused mainly on traditional microscopy detection using formalin ether concentration or faecal smears with acid fast staining. It is therefore important to employ more sensitive molecular detection techniques which have proven effective in determining the prevalence and co-infection status of enteric parasites among local ruminant livestock (Lim et al., 2013; Tan et al., 2013, Tan et al., 2014). With the exception of studies by Halim et al. (2008), Muhid et al. (2011) and Yap et al. (2016) on the molecular detection of Cryptosporidium in selected areas in Peninsular Malaysia, there remains a lack of information on the molecular epidemiology and spatial distribution of the infection among cattle throughout the country and in the Southeast Asian region. The present study therefore, aims to determine the molecular prevalence and epidemiological risk factors associated with Cryptosporidium infection among beef and dairy cattle over a widespread sampling area throughout Peninsular Malaysia.
2. Materials and methods
2.1. Study area
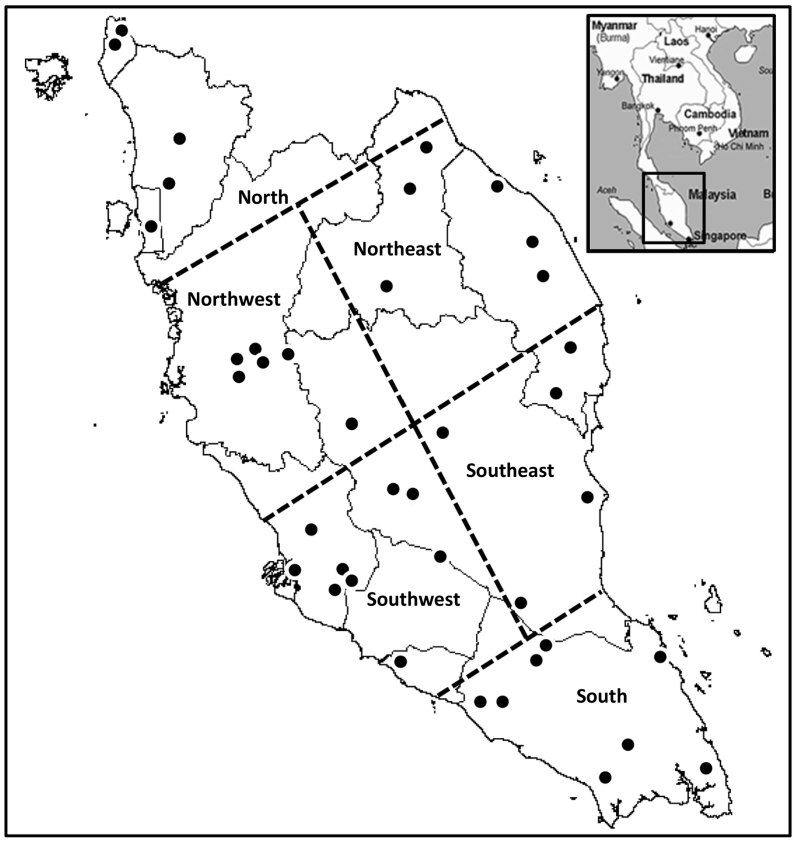
The study was conducted in Peninsular Malaysia, located at the southern-most tip of the Asian mainland, bordered by Thailand in the north, and separated from Singapore by the straits of Johor in the south. Peninsular Malaysia experiences a hot tropical climate year round with a relative humidity ranging from 70 to 90% and temperatures between 19.0 and 39.3 °C in the lowlands (Malaysian Meteorological Department, 2016). There is copious rainfall throughout the year (2000 mm average), and the maximum limits are reached between the months of November to January, with a short dry spell in June and July. For sampling purposes, the country was arbitrarily divided into six zones (Fig. 1) encompassing 39 beef and dairy farms in the following sampling locations: North (Bukit Mertajam, Perlis, Kuala Ketil and Sik), Northwest (Kintan Valley, Kampar, Air Papan, Gopeng, Raub and Cameron Highlands), Northeast (Gua Musang, Kuala Krai, Pasir Puteh, Permaisuri and Hulu Terengganu), Southwest (Serdang, Dengkil, Kajang, Shah Alam, Rawang, Temerloh, Melaka and Negeri Sembilan), Southeast (Jerantu, Rompin, Pekan, Dungun and Kemaman), and South (Muar, Labis, Mersing, Pontian and Kota Tinggi).
Fig. 1.
Schematic representation of the cattle farm sampling locations (filled circles) and zones in Peninsular Malaysia.
2.2. Sample population and faecal collection
A total of 824 heads of cattle (526 beef and 298 dairy) were sampled from 39 farms in 33 locations throughout Peninsular Malaysia (Fig. 1). Sampling was carried out using a cross-sectional study design and animals were selected by convenience and willingness of cattle owners. A range of 15–25 animals were sampled from each farm, representing the different age groups, gender and physiological status. Faecal samples were collected per rectum from each animal using sterile gloves, placed into clean plastic bags and transported on ice to the Parasitology Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia. Aliquots of faecal samples were stored at −20 °C for subsequent DNA extraction.
2.3. PCR detection of Cryptosporidium
Deoxyribonucleic acid (DNA) was extracted from each faecal sample using the Qiagen QIAamp® stool kit according to the manufacturers' protocol. Nested PCR amplification was performed on the extracted genomic DNA to amplify a partial fragment of the Cryptosporidium 18S SSUrRNA gene using oligonucleotide primers and thermocyclic profiles described by Yap et al. (2016). Amplifications were carried out in 25 μl reactions comprising 100–150 ng genomic DNA, 1× reaction buffer (Green GoTag Flexi Buffer, Promega Madison, USA), 5 mM of each deoxynucleoside triphosphate (Promega), 5 mM MgCl2 (Promega Madison, USA), 1 mM of each primer, and 0.5 units of Taq DNA polymerase (Promega Madison, USA). Negative controls (template DNA substituted with sterile Type-1+ purified water) and positive controls (positive samples confirmed by sequencing of amplicons) were included into each PCR run. The amplicons were electrophoresed on a 2% agarose gel at 100 V with TAE (Tris-acetic acid-EDTA) buffer, stained with ethidium bromide, and viewed under a UV transilluminator. In order to prevent cross-contamination, work areas were designated solely for DNA extraction, PCR reagent preparation, and PCR amplification. Reagent preparation was done in a dedicated biosafety cabinet which was UV illuminated at the end of each session. Representative positive amplicons were excised from the agarose gel with a sterile scalpel and purified using the QIAquick® Gel Extraction Kit (Qiagen, Germany) according to the manufacturers' protocol. The concentration of the gel purified DNA was determined by measuring the absorbance at 260 nm and subsequently sequenced using the BigDye® Terminator v3.1 cycle sequencing kit (Applied Biosystems, USA). To facilitate identification, the sequences obtained were compared with 18S SSUrRNA gene fragments of Cryptosporidium curated by the National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990).
2.4. Questionnaire and statistical analysis
To determine the risk factors associated with enteric protozoa of cattle, a structured questionnaire containing open-ended and closed ended (dichotomous or multiple choice) questions were designed to obtain information on the host (cattle) factors (breed, age, sex, physiological status and production type) environmental factors (location, state, zone, distance to body of water, and distance to human settlement), and management factors (herd owner, farm size, farm type, herd size, farm age, management system, quarantine period and deworming schedule). The questionnaires were filled by having a face to face interaction with the farm owners or managers. Geographical location from each participating farm was collected using a global positioning system (GPS) hand-held device. Epidemiological data were analyzed using the Statistical Package for Social Sciences version 22.0 (SPSS Inc. Chicago, Illinois). The prevalence of the parasite, the spatial distribution in each zone and risk factors associated with the parasite were statistically analyzed using the Chi Square test (univariable and multivariable models) for discrete variables at a 95% Confidence Interval.
3. Results
The prevalence of Cryptosporidium infection among the cattle examined in this study was 12.5%. There was a significant difference in the infection rate among the various breeds of cattle examined (Table 1). The highest prevalence (32.4%) was seen in the Mafriwal breed, followed by the Friesian x Jersey crosses (22.7%). Infection with this enteric protozoa was not detected in the indigenous cattle breed [Kedah-Kelantan (KK)] x Friesian and the Brahman cattle. Among the infected cattle, the KK had the lowest prevalence (2.5%) followed by the Brangus x Bradford crosses (3.9%), and the KK x Yellow China (4.0%). There was no significant effect (p = 0.99) of gender on the prevalence of Cryptosporidium, and both the beef and dairy cattle were at similar odds for infection with the parasite. The younger cattle (≤1 years) had a significantly higher (p = 0.04) infection rate compared to the older animals, where the odds of infection were more than twice that of cattle more than five years of age. The lactating cows harbored a significantly lower (p = 0.02) rate of infection (7.8%) compared to conspecifics in the other physiological status categories. Cattle reared on farms located in the northern region of the country showed the highest prevalence (29.0%) of Cryptosporidium, and were at significantly higher (p < 0.01) risk of being infected.
Table 1.
Molecular prevalence and epidemiological variables that were investigated as potential risk factors for bovine Cryptosporidium detection in Peninsular Malaysia.
| Variables | N | Positive (%) | OR (95% Cl) | p |
|---|---|---|---|---|
| Breed | ||||
| KKa | 122 | 3 (2.5) | 1.00 | |
| KK x Brahman | 191 | 32 (16.8) | 7.94 (2.62, 33.39) | <0.01⁎ |
| KK x Friesian | 4 | 0 (0.0) | – | |
| KK x LID | 58 | 6 (10.3) | 4.53 (1.09, 22.94) | 0.03⁎ |
| KK x Yellow China | 25 | 1 (4.0) | 1.65 (0.06, 16.12) | 0.66 |
| KK x Charolais | 14 | 1 (7.1) | 3.01 (0.11, 30.43) | 0.41 |
| Brahman | 19 | 0 (0.0) | – | |
| Brahman x LID | 37 | 8 (21.6) | 10.72 (2.75, 52.64) | <0.01⁎ |
| Friesian x Jersey | 75 | 17 (22.7) | 11.48 (3.50, 50.84) | <0.01⁎ |
| Friesian x Sahiwal | 125 | 10 (8.0) | 3.43 (0.97, 15.85) | 0.06 |
| Friesian x Charolais | 17 | 4 (23.5) | 11.78 (2.21, 69.4) | <0.01⁎ |
| Friesian x Mafriwal | 52 | 8 (15.4) | 7.12 (1.86, 34.46) | <0.01⁎ |
| Mafriwal | 34 | 11 (32.4) | 18.43 (5.03, 87.89) | <0.01⁎ |
| Brangus x Braford | 51 | 2 (3.9) | 1.61 (0.19, 11.16) | 0.62 |
| Gender | ||||
| Male | 200 | 25 (12.5) | 1.00 (0.61, 1.61) | 0.99 |
| Femalea | 624 | 78 (12.5) | 1.00 | |
| Age (years) | ||||
| <1 | 189 | 32 (16.9) | 2.05 (1.03, 4.29) | 0.04⁎ |
| 1–2 | 197 | 19 (9.6) | 1.08 (0.50, 2.36) | 0.86 |
| >2–5 | 305 | 40 (13.1) | 1.52 (0.78, 3.11) | 0.23 |
| >5a | 133 | 12 (9.0) | 1.00 | |
| Production type | ||||
| Dairy | 298 | 45 (15.1) | 1.44 (0.94, 2.18) | 0.09 |
| Beefa | 526 | 58 (11.0) | 1.00 | |
| Physiological status | ||||
| Immature | 354 | 45 (12.7) | 0.77 (0.45, 1.37) | 0.37 |
| Mating stock | 44 | 7 (15.9) | 1.01 (0.37, 2.50) | 0.97 |
| Pregnant | 108 | 15 (13.9) | 0.86 (0.41, 1.75) | 0.68 |
| Lactating | 179 | 14 (7.8) | 0.45 (0.22, 0.92) | 0.02⁎ |
| Drya | 139 | 22 (15.8) | 1.00 | |
| Geographical zone | ||||
| North | 69 | 20 (29.0) | 3.24 (1.49, 7.22) | <0.01⁎ |
| Northwest | 121 | 15 (12.4) | 1.13 (0.51, 2.54) | 0.76 |
| Northeast | 129 | 7 (5.4) | 0.46 (0.17, 1.19) | 0.11 |
| Southwest | 214 | 24 (11.2) | 1.01 (0.50, 2.12) | 0.99 |
| Southeast | 174 | 24 (13.8) | 1.28 (0.63, 2.70) | 0.51 |
| Southa | 117 | 13 (11.1) | 1.00 | |
| Herd size | ||||
| Small (≤100) | 401 | 61 (15.2) | 1.31 (0.80, 2.19) | 0.29 |
| Medium (>100–300) | 215 | 17 (7.9) | 0.63 (0.32, 1.20) | 0.16 |
| Large (>300)a | 208 | 25 (12.0) | 1.00 | |
| Farm owner | ||||
| Private | 564 | 64 (11.3) | 2.86 (0.97, 11.78) | 0.06 |
| Cooperative | 90 | 9 (10.0) | 2.47 (0.67, 11.70) | 0.19 |
| Semi-government | 100 | 27 (27.0) | 8.18 (2.60, 35.32) | 0.01⁎ |
| Governmenta | 70 | 3 (4.3) | 1.00 | |
| Management system | ||||
| Extensive | 102 | 14 (13.7) | 0.51 (0.23, 1.10) | 0.09 |
| Semi-intensive | 647 | 71 (11.0) | 0.39 (0.22, 0.72) | <0.01⁎ |
| Intensivea | 75 | 18 (24.0) | 1.00 | |
| Farm size (acres) | ||||
| ≤10 | 349 | 41 (11.7) | 1.39 (0.73, 2.77) | 0.33 |
| >10–20 | 123 | 21 (17.1) | 103.5 (24.87, 716.80) | <0.01⁎ |
| >20–50 | 124 | 14 (11.3) | 1.33 (0.59, 3.00) | 0.49 |
| >50–100 | 79 | 14 (17.7) | 2.25 (0.99, 5.14) | 0.05 |
| >100a | 149 | 13 (8.7) | 1.00 | |
| Farm age (years) | ||||
| ≤10 | 238 | 29 (12.2) | 2.56 (0.68, 16.54) | 0.20 |
| >10–20 | 341 | 43 (12.6) | 2.66 (0.72, 16.96) | 0.17 |
| >20–30 | 139 | 21 (15.1) | 3.28 (0.84, 21.57) | 0.10 |
| >30–40 | 67 | 8 (11.9) | 2.49 (0.54, 17.97) | 0.27 |
| >40a | 39 | 2 (5.1) | 1.00 | |
| Quarantine period | ||||
| None | 377 | 33 (8.8) | 0.51 (0.29, 0.88) | 0.01⁎ |
| ≤1 weeks | 107 | 7 (6.5) | 0.37 (0.14, 0.86) | 0.01⁎ |
| >1–≤2 weeks | 171 | 36 (21.1) | 1.40 (0.81, 2.45) | 0.23 |
| >2 weeksa | 169 | 27 (16.0) | 1.00 | |
| Deworming frequency | ||||
| 3 months | 133 | 23 (17.3) | 1.13 (0.63, 2.01) | 0.67 |
| 4 months | 75 | 3 (4.0) | 0.23 (0.05, 0.69) | <0.01⁎ |
| 5 months | 22 | 2 (9.1) | 0.54 (0.08, 2.12) | 0.45 |
| 6 months | 363 | 39 (10.7) | 0.65 (0.40, 1.07) | 0.09 |
| Nonea | 231 | 36 (15.6) | 1.00 | |
| Distance to human settlement | ||||
| ≤200 m | 107 | 13 (12.1) | 1.01 (0.51, 1.91) | 0.96 |
| >200–500 m | 104 | 9 (8.7) | 069 (0.31, 1.42) | 0.34 |
| >500–1000 m | 41 | 11 (26.8) | 2.67 (1.21, 5.60) | 0.01⁎ |
| >1000–1500 m | 174 | 22 (12.6) | 1.06 (0.61, 1.80) | 0.84 |
| >1500 ma | 398 | 48 (12.1) | 1.00 | |
| Distance to water bodies | ||||
| ≤200 m | 277 | 32 (11.6) | 0.52 (0.30, 0.88) | 0.02⁎ |
| >200–500 m | 134 | 21 (15.7) | 0.73 (0.39, 1.34) | 0.32 |
| >500–1000 m | 217 | 9 (4.1) | 0.17 (0.08, 0.36) | <0.01⁎ |
| >1000–1500 m | 38 | 9 (23.7) | 1.22 (0.50, 2.80) | 0.63 |
| >1500 ma | 158 | 32 (20.3) | 1.00 | |
Reference category.
Significant, OR = Odds Ratio, CI = Confidence Interval.
The herd size and the age of the farm did not exert significant effects on the prevalence of the parasite. However, the infection prevalence was significantly higher (27.0%, p = 0.01) in the semi-government owned cattle farms, but lower in the government (4.3%), private (11.3%) and cooperative (10.0%) owned farms. The intensively manage cattle exhibit a higher prevalence (24.0%) with Cryptosporidium, compared to the animals managed in the semi-intensive (11.0%) and extensive (13.7%) production systems.
Interestingly, the cattle that were not subjected to on-farm quarantine or those that were quarantined for less than one week had a significantly lower (p = 0.01) prevalence compared to those that were quarantined for longer periods. In addition, the cattle that were dewormed most regularly (every three months) showed the highest prevalence (17.3%) with Cryptosporidium. Infection with this protozoa was not significantly different among the cattle dewormed at other regular intervals or those that were not dewormed at all. The prevalence of Cryptosporidium was significantly higher (p = 0.01) on farms situated a distance of >500–1000 m from human settlement, although there was no distinct spatial trend of infection. In contrast, the farms that were situated at this distance from open water bodies had the lowest (4.1%) infection rates with the parasite.
A summary of the univariate model showed that factors including breed, age (years), physiological status, geographical location (zone), herd owner, management system, farm size, quarantine period, deworming frequency, distance to human settlement, and distance to water body were all significantly associated with the infection (Table 1). These putative risk factors were subjected to multivariable logistic regression (Table 2), which revealed that deworming practice, distance to human settlement, geographical location (zone) and farm management system were risk factors associated with Cryptosporidium infection. The animals that were dewormed every four months were at highest odds (5.45 times) of being infected while those that were dewormed at intervals of six months had the lowest odds (0.556) of being infected with this enteropathogen. Cattle farms that were located closest (≤200 m) to human settlements were at highest risk (OR = 1.333) of being infected with Cryptosporidium, while those located between 500 and 1500 m away were at significantly lower risk (OR = 0.131, p = 0.01). Higher odds of infection (OR = 2.209) was observed for cattle farms located in the northeast zone of the country. Cattle reared extensively (open pasture and free grazing) were at highest risk of infection, where they were over 2.5 times more likely to harbor this enteric protozoa compare to those reared in the intensive management system. The cattle reared semi-intensively (closed paddock pasture and feedlot) were the least likely to be infected with Cryptosporidium.
Table 2.
Multivariate association between epidemiological variables and bovine Cryptosporidium detection in Peninsular Malaysia.
| Variables | β | SE | p | OR | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Deworming frequency | ||||||
| 3 months | 0.312 | 0.494 | 0.527 | 1.366 | 0.519 | 3.596 |
| 4 months | 1.696 | 0.674 | 0.010⁎ | 5.450 | 1.453 | 20.440 |
| 5 months | 0.828 | 0.925 | 0.371 | 2.289 | 0.374 | 14.025 |
| 6 months | −0.587 | 0.351 | 0.095 | 0.556 | 0.279 | 1.107 |
| Nonea | 1.000 | |||||
| Distance to settlement | ||||||
| ≤200 m | 0.287 | 0.400 | 0.473 | 1.333 | 0.608 | 2.920 |
| >200–500 m | −0.412 | 0.480 | 0.391 | 0.663 | 0.259 | 1.697 |
| >500–1000 m | −2.036 | 0.544 | <0.010⁎ | 0.131 | 0.045 | 0.379 |
| >1000–1500 m | −1.339 | 0.406 | <0.010⁎ | 0.262 | 0.118 | 0.581 |
| >1500 ma | 1.000 | |||||
| Zones | ||||||
| North | −1.166 | 0.578 | 0.040⁎ | 0.312 | 0.100 | 0.967 |
| Northwest | −0.936 | 0.527 | 0.076 | 0.392 | 0.140 | 1.102 |
| Northeast | 0.792 | 0.545 | 0.146 | 2.209 | 0.760 | 6.422 |
| Southwest | −0.393 | 0.474 | 0.407 | 0.675 | 0.267 | 1.708 |
| Southeast | −0.916 | 0.472 | 0.052 | 0.400 | 0.159 | 1.009 |
| Southa | 1.000 | |||||
| Management system | ||||||
| Extensive | 0.950 | 0.474 | 0.045⁎ | 2.586 | 1.021 | 6.547 |
| Semi-intensive | −0.680 | 0.574 | 0.236 | 0.506 | 0.164 | 1.560 |
| Intensivea | 1.000 | |||||
Reference category.
Significant, OR = Odds Ratio, CI = Confidence Interval.
4. Discussion
This study constitutes the first attempt in identifying the epidemiological risk factors involved in Cryptosporidium infection among cattle in Peninsular Malaysia, and through multivariate epidemiological factor analysis, provides a more comprehensive representation of the infection risks for bovine cryptosporidiosis in Southeast Asia. Epidemiological information on parasitic diseases is an important facet in designing and implementing operational control procedures. It also facilitates clinical diagnosis, treatment, and informs on the possibility of the spread of a specific disease. Risk factors associated with gastrointestinal parasitic infection among ruminant livestock in Southeast Asia are known to depend on age and immunity levels of the animals, farm management, and climatic conditions (Tung et al., 2012; Tan et al., 2017). Environmental contamination is also of pivotal concern, especially in developing countries where proper farms waste treatment and disposal management are still lacking (Trotz-Williams et al., 2007). In the tropics, enteric parasite infections among ruminants occur all year round, with higher infection intensities observed after the rainy season (Jittapalapong et al., 1987). In spite of the high seasonal prevalence and public health concerns, there remains a paucity of published data on the molecular epidemiology of enteric protozoa infections among ruminant livestock in many Southeast Asian countries including Malaysia.
The overall prevalence of Cryptosporidium in this study (12.5%) is considered to be moderate. Previous studies focusing on Cryptosporidium infection among local ruminant livestock have demonstrated a microscopy prevalence ranging from 0.2 to 36.0% (Fatimah et al., 1995a, Fatimah et al., 1995b; Muhid et al., 2011; Hisamuddin et al., 2016; Tan et al., 2017). Recently, Yap et al. (2016) used PCR to detect and genetically characterize Cryptosporidium in faecal samples from a cohort of 215 asymptomatic cattle (of different ages) from six farms in five states of Peninsular Malaysia. They reported a molecular prevalence of 3.2%, which is lower than that detected in the present investigation. These discrepancies in the infection rates of Cryptosporidium among ruminant livestock in the country may be attributed to the sampling effort in terms of animal numbers and spatial representation, as well as possible challenges in diagnosis by microscopy.
This study has also revealed that the prevalence of Cryptosporidium was highest among cattle that were less than a year old. The present data is in agreement with a number of previous studies that demonstrated a significant association between age and Cryptosporidium prevalence, with the highest infection rates reported in the younger animals (Mohammed et al., 1999; Santin et al., 2004; Geurden et al., 2006; Trotz-Williams et al., 2007; Brook et al., 2008; Ranjbar-Bahadori et al., 2011; Hisamuddin et al., 2016; Yap et al., 2016). Molecular detection (Yap et al., 2016) revealed that 73% of the positive samples were from cattle that were ≥2 years of age. These younger cohort of animals are also known to play an important role in maintaining the infection in the herd.
Multivariate analysis revealed that the significant risk factors associated with Cryptosporidium infection among the cattle were the deworming frequency, distance to human settlement, geographical zones in the country, and the farm management system. Animals that were dewormed every four months were at higher odds of infection with Cryptosporidium as compare to others that were dewormed at other intervals or those that were not dewormed at all. While the exact reason for this finding cannot be determined without further investigation, it is possible that exposure to anthelminthic drugs at this frequency effectively reduces the nematode burden which may be beneficial in eliciting a protective immune response against certain gastrointestinal pathogens. It has been shown that intestinal nematodes encourage the presence of certain protective microbiota in the intestines, and that deworming treatment reduced certain beneficial microorganisms, while increasing certain pathogenic microbes (Ramanan et al., 2016).
This study has shown that cattle on farms that are situated ≤200 m from human settlement were more likely to be infected with Cryptosporidium. A number of studies (reviewed by Messenger et al., 2014) have indicated that enteric protozoal infection in animals may be anthropozoonotic, and have incriminated the close contact with animals and humans as a potential risk factor for infection in the former. These include animal infections with Cryptosporidium (Graczyk et al., 2001; Guk et al., 2004; Coklin et al., 2007; Dixon et al., 2011), Giardia (Graczyk et al., 2002a; Coklin et al., 2007; Teichroeb et al., 2009; Ash et al., 2010; Johnston et al., 2010; Dixon et al., 2011), Encephalitozoon (Graczyk et al., 2002b), and Blastocystis (Noel et al., 2005). The role of anthropozoonotic and zoonotic transmission of cryptosporidiosis in the ruminant livestock industry is of much concern as it has been known to cause economic losses, where infection in calves have been associated with considerable morbidity, mortality, and subsequent human reinfections (de Graaf et al., 1999).
The finding that animals raised in the northeast zone of the country were at highest risk of infection compared to the other zones cannot be explained without further investigation into other environmental and farm based causative factors. It is possible that subtle differences in farm management or hosts factors may contribute to the higher infection rate among the farms in the north. It is well established that farm management practices, contaminated feed, water and surrounding environment, introduction of new animals, immune status of the hosts and grazing in infected areas are risk factors for infection (Mohammed et al., 1999; Farizawati et al., 2005; Lim et al., 2005; Karanis et al., 2007; Lim et al., 2007; Manyazewal et al., 2018). It may be possible that farm management factors in the northeast (which constitute a large proportion of small-holder and rural farms) are not as adequate as that in other parts of the country and opens ground for more detailed epidemiological investigation.
The extensively reared cattle in this study showed the highest odds of being infected with Cryptosporidium. Our findings is contrary to that reported by Muhid et al. (2011), which revealed that there was no association between prevalence of infection and farm management type. In addition, previous studies have indicated that the infection rates of Cryptosporidium are lower in extensively farmed cattle (Ralston et al., 2003; Santin et al., 2004; Geurden et al., 2006; Manyazewal et al., 2018). However, it is not surprising that extensively reared cattle examined in this study possessed higher infection rates as transmission may occur from the contaminated grazing environment and adjacent water bodies that the animals may drink from. In addition, a majority of the extensively reared cattle in the country are allowed to graze freely in villages and agricultural areas like oil palm plantations. This exposes them to an environment that may be contaminated with the infective oocysts which may be of anthropogenic origin. This finding further supports our data that cattle closest to human settlements are also at higher risk of contracting the parasite, and corroborates earlier postulations on the anthropozoonotic nature of the infection (Graczyk et al., 2001; Guk et al., 2004; Coklin et al., 2007; Dixon et al., 2011). It is also important to note that most of the extensively reared animals belong to the small holder and rural farmers, and are of poor health and nutritional status. These animals may also need to compete for resources which could lead to increase in stress levels. Animals that experience chronic stress may be immuno-compromised (Yang and Glaser, 2002), and this may possibly lead to increased susceptibility to parasitic infection and other diseases (Tan, 2004; Zali et al., 2004). It is well established that farm management practices and environmental conditions play a vital role in the prevalence of cattle enteric protozoa (Noor Azian et al., 2007; Tan, 2008), which are linked to water and environmental contamination (Navarrete and Torres, 1993; Basualdo et al., 2000; Taamasri et al., 2000; Waikagul et al., 2002; Elshazly et al., 2007; Karanis et al., 2007; Li et al., 2007). It is almost certain that a farm without adequate hygienic practices, veterinary care, nutritional management, and biosecurity measures will lead to an increase in enteric protozoa infection among the cattle.
It is interesting that all the cattle in this study did not display any obvious clinical signs of infection like diarrhea. This may be due to the fact the sensitive molecular technique employed in this study was able to pick out sub-clinical levels of parasitaemia. Asymptomatic shedding of Cryptosporidium oocysts is not uncommon and has been reported in cattle (Lorenzo-Lorenzo et al., 1993; Scott et al., 1995; Razakandrainibe et al., 2018) and Red deer (Skerrett and Holland, 2001). While clinical manifestation may not be apparent, low-grade infection with Cryptosporidium could lead to suboptimal productivity, loss of weight, and inefficient feed conversion. As such, it is important that these sub-clinical infections be examined closer to determine if they are causing any negative impacts on the cattle industry in the country. Even though the zoonotic potential of Cryptosporidium detected in this study was not determined, the possibility of human infection from cattle excrement warrants further investigation. It is therefore imperative that veterinary and human health personnel be well acquainted with these zoonotic enteric pathogens in the country, and serious steps should be instituted for animal treatment and biohazard waste management on local and regional cattle farms.
Acknowledgments
Acknowledgements
We thank Pn Maizatul Akmal Moktar, En Abdul Rashid Ibrahim and Pn Amlizawaty Amzah of the Parasitology Laboratory, Faculty of Veterinary Medicine, Universiti Putra Malaysia for their assistance during the course of this project.
Declaration of interest
None.
References
- Altschul S., Gish W., Miller W., Myers E., Lipman D. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ash A., Lymbery A., Lemon J., Vitali S., Thompson R.C.A. Molecular epidemiology of Giardia duodenalis in an endangered carnivore - the African painted dog. Vet. Parasitol. 2010;174:206–212. doi: 10.1016/j.vetpar.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Basualdo J., Pezzani B., De Luca M., Córdoba A., Apezteguía M. Screening of the municipal water system of La Plata, Argentina, for human intestinal parasites. Int. J. Hyg. Environ. Health. 2000;203:177–182. doi: 10.1078/s1438-4639(04)70025-5. [DOI] [PubMed] [Google Scholar]
- Brook E., Hart C.A., French N., Christley R. Prevalence and risk factors for Cryptosporidium spp. infection in young calves. Vet. Parasitol. 2008;152:46–52. doi: 10.1016/j.vetpar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Casemore D.P., Wright S.E., Coop R. Cryptosporidiosis–human and animal epidemiology. In: Fayer R., editor. Cryptosporidium and Cryptosporidiosis. CRC Press; Boca Raton: 1997. pp. 65–92. [Google Scholar]
- Chen X.M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N. Engl. J. Med. 2002;346:1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Cohen S., Dalle F., Gallay A., Di Palma M., Bonnin A., Ward H.D. Identification of Cpgp40/15 Type Ib as the predominant allele in isolates of Cryptosporidium spp. from a waterborne outbreak of gastroenteritis in South Burgundy, France. J. Clin. Microbiol. 2006;44:589–591. doi: 10.1128/JCM.44.2.589-591.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coklin T., Farber J., Parrington L., Dixon B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet. Parasitol. 2007;150:297–305. doi: 10.1016/j.vetpar.2007.09.014. [DOI] [PubMed] [Google Scholar]
- de Graaf D.C., Vanopdenbosch E., Ortega-Mora L.M., Abbassi H., Peeters J.E. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 1999;29(8):1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Coco V.F., Córdoba M.A., Basualdo J.A. Cryptosporidium infection in calves from a rural area of Buenos Aires, Argentina. Vet. Parasitol. 2008;158:31–35. doi: 10.1016/j.vetpar.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Dixon B., Parrington L., Cook A., Pintar K., Pollari F., Kelton D., Farber J. The potential for zoonotic transmission of Giardia duodenalis and Cryptosporidium spp. from beef and dairy cattle in Ontario, Canada. Vet. Parasitol. 2011;175:20–26. doi: 10.1016/j.vetpar.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Elshazly A., Elsheikha H., Soltan D., Mohammad K., Morsy T. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2007;37:51–64. [PubMed] [Google Scholar]
- Farizawati S., Lim Y., Ahmad R., Fatimah C., Siti-Nor Y. Contribution of cattle farms towards river contamination with Giardia cysts and Cryptosporidium oocysts in Sungai Langat Basin. Trop. Biomed. 2005;22:89–98. [PubMed] [Google Scholar]
- Fatimah C., Lee C., Azri A., Rafie D., Fazlina B., Salim N., Azizah D., Safri A. The occurrence and epidemiology of enteropathogens and diarrhoea in neonatal lambs. J. Vet. Malays. 1995;7:27–29. [Google Scholar]
- Fatimah C., Lee C., Rafie D., Fazlina B., Salim N. Cryptosporidiosis and diarrhoea in goat kids. J. Vet. Malays. 1995;6:107–109. [Google Scholar]
- Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet. Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Fayer R., Speer C.A., Dubey J.P. The general biology of Cryptosporidium. In: Fayer R., editor. Cryptosporidium and Cryptosporidiosis. Taylor & Francis; Boca Raton, FL, USA: 1997. pp. 1–41. [Google Scholar]
- Geurden T., Goma F., Siwila J., Phiri I., Mwanza A., Gabriël S., Claerebout E., Vercruysse J. Prevalence and genotyping of Cryptosporidium in three cattle husbandry systems in Zambia. Vet. Parasitol. 2006;138:217–222. doi: 10.1016/j.vetpar.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., DaSilva A.J., Cranfield M.R., Nizeyi J.B., Kalema G.R., Pieniazek N.J. Cryptosporidium parvum genotype 2 infections in free-ranging mountain gorillas (Gorilla gorilla beringei) of the Bwindi Impenetrable National Park, Uganda. Parasitol. Res. 2001;87:368–370. doi: 10.1007/s004360000337. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., Bosco-Nizeyi J., Ssebide B., Thompson R.C.A., Read C., Cranfield M.R. Anthropozoonotic Giardia duodenalis genotype (assemblage) A infections in habitats of free-ranging human-habituated gorillas, Uganda. J. Parasitol. 2002;88:905–909. doi: 10.1645/0022-3395(2002)088[0905:AGDGAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K., Bosco-Nizeyi J., da Silva A.J., Moura I.N., Pieniazek N.J., Cranfield M.R., Lindquist H.D. A single genotype of Encephalitozoon intestinalis infects free-ranging gorillas and people sharing their habitats in Uganda. Parasitol. Res. 2002;88:926–931. doi: 10.1007/s00436-002-0693-5. [DOI] [PubMed] [Google Scholar]
- Guk S., Yong T., Park S., Park J., Chai J. Genotype and animal infectivity of a human isolate of Cryptosporidium parvum in the Republic of Korea. Korean J. Parasitol. 2004;42:85–89. doi: 10.3347/kjp.2004.42.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim N.A., Plutzer J., Bakheit M.A., Karanis P. First report of Cryptosporidium deer-like genotype in Malaysian cattle. Vet. Parasitol. 2008;152(3–4):325–329. doi: 10.1016/j.vetpar.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Hisamuddin N.H., Hashim N., Soffian S.N., Amin M.H., Wahab R.A., Mohammad M., Isa M.L., Yusof A.M. Identification of Cryptosporidium from dairy cattle in Pahang, Malaysia. Korean J. Parasitol. 2016;54(2):197–200. doi: 10.3347/kjp.2016.54.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C., Al-Habsi K., Ryan U., Williams A., Anderson F., Yang R., Abraham S., Miller D. Cryptosporidium infection is associated with reduced growth and diarrhoea in goats beyond weaning. Vet. Parasitol. 2018;260:30–37. doi: 10.1016/j.vetpar.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S., Jansawan W., Pinyopummin T. Survey of internal parasites of calves in Nongpho. Kasetsart Vet. 1987;8:124–132. [Google Scholar]
- Johnston A.R., Gillespie T.R., Rwego I.B., McLachlan T.L., Kent A.D., Goldberg T.L. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanis P., Plutzer J., Halim N.A., Igori K., Nagasawa H., Ongerth J., Liqing M. Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol. Res. 2007;101:1575–1580. doi: 10.1007/s00436-007-0681-x. [DOI] [PubMed] [Google Scholar]
- Kosek M., Alcantara C., Lima A.A., Guerrant R.L. Cryptosporidiosis: an update. Lancet Infect. Dis. 2001;1:262–269. doi: 10.1016/S1473-3099(01)00121-9. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., Faruque A.S., Zaidi A.K., Saha D., Alonso P.L., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ochieng J.B., Omore R., Oundo J.O., Hossain A., Das S.K., Ahmed S., Qureshi S., Quadri F., Adegbola R.A., Antonio M., Hossain M.J., Akinsola A., Mandomando I., Nhampossa T., Acacio S., Biswas K., O'Reilly C.E., Mintz E.D., Berkeley L.Y., Muhsen K., Sommerfelt H., Robins-Browne R.M., Levine M.M. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Li L.-H., Zhou X.-N., Du Z.-W., Wang X.-Z., Wang L.-B., Jiang J.-Y., Yoshikawa H., Steinmann P., Utzinger J., Wu Z. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 2007;56:281–286. doi: 10.1016/j.parint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lim Y.A.L., Rohela M., Sim B., Jamaiah I., Nurbayah M. Prevalence of cryptosporidiosis in HIV-infected patients in Kajang Hospital, Selangor. Southeast Asian J. Trop. Med. Public Health. 2005;36:30–33. [PubMed] [Google Scholar]
- Lim Y.A.L., Rohela M., Shukri M.M. Cryptosporidiosis among birds and bird handlers at Zoo Negara, Malaysia. Southeast Asian J. Trop. Med. Public Health. 2007;38:19–26. [Google Scholar]
- Lim Y.A.L., Mahdy M.A.K., Tan T.K., Goh X.T., Jex A.R., Nolan M.J., Sharma R.S.K., Gasser R.B. First molecular characterization of Giardia duodenalis from goats in Malaysia. Mol. Cell. Probes. 2013;27:28–31. doi: 10.1016/j.mcp.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Lorenzo M.J., Ares-Mazas E., Villacorta Martinez de Maturana I. Detection of oocysts and IgG antibodies to Cryptosporidium parvum in asymptomatic adult cattle. Vet. Parasitol. 1993;47:9–15. doi: 10.1016/0304-4017(93)90171-i. [DOI] [PubMed] [Google Scholar]
- Malaysian Meteorological Department . Ministry of Science, Technology and Innovation Malaysia. 2016. Annual Report 2016. 82 pp. [Google Scholar]
- Manyazewal A., Francesca S., Pal M., Gezahegn M., Tesfaye M., Lucy M., Teklu W., Getachew T. Prevalence, risk factors and molecular characterization of Cryptosporidium infection in cattle in Addis Ababa and its environs, Ethiopia. Vet. Parasitol. Reg. Stud. Rep. 2018;3:79–84. doi: 10.1016/j.vprsr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger A.M., Barnes A.N., Gray G.C. Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed H.O., Wade S.E., Schaaf S.L. Risk factors associated with Cryptosporidium parvum infection in dairy cattle in south-eastern New York State. Vet. Parasitol. 1999;83:1–13. doi: 10.1016/s0304-4017(99)00032-1. [DOI] [PubMed] [Google Scholar]
- Muhid A., Robertson I., Ng J., Ryan U. Prevalence of and management factors contributing to Cryptosporidium sp. infection in pre-weaned and post-weaned calves in Johor, Malaysia. Exp. Parasitol. 2011;127(2):534–538. doi: 10.1016/j.exppara.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Navarrete N., Torres P. Prevalence of infection by intestinal helminths and protozoa in school children from a coastal locality in the province of Valdivia, Chile. Bol. Chil. Parasitol. 1993;49:79–80. [PubMed] [Google Scholar]
- Noel C., Dufernez F., Gerbod D., Edgcomb V.P., Delgado-Viscogliosi P., Ho L.C., Singh M., Wintjens R., Sogin M.L., Capron M., Pierce R., Zenner L., Viscogliosi E. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 2005;43:348–355. doi: 10.1128/JCM.43.1.348-355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Azian M., San Y., Gan C., Yusri M., Nurulsyamzawaty Y., Zuhaizam A., Maslawaty M., Norparina I., Vythilingam I. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop. Biomed. 2007;24:55–62. [PubMed] [Google Scholar]
- Palit A., Sur D., MitraDhar K., Saha M.R. Asymptomatic cryptosporidiosis in a periurban slum setting in Kolkata, India––a pilot study. Jpn. J. Infect. Dis. 2005;58:110–111. [PubMed] [Google Scholar]
- Ralston B.J., Cockwill C., Guselle N., Van Herk F.H., McAllister T.A., Olson M.E. Prevalence of Giardia and Cryptosporidium andersoni and their effect on performance in feedlot beef calves. Can. J. Anim. Sci. 2003;83:153–159. [Google Scholar]
- Ramanan D., Bowcutt R., Lee S.C., Tang M.S., Kurtz Z.D., Ding Y., Honda K., Gause W.C., Blaser M.J., Bonneau R.A., Lim Y.A.L., Loke P., Cadwell K. Helminth infection promotes colonization resistance via Type 2 immunity. Science. 2016;352(6285):608–612. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar-Bahadori S., Sangsefidi H., Shemshadi B., Kashefinejad M. Cryptosporidiosis and its potential risk factor in children and calves in Babol, north of Iran. Trop. Biomed. 2011;28:125–131. [PubMed] [Google Scholar]
- Razakandrainibe R., Diawara E.H.I., Costa D., Le Goff L., Lemeteil D., Ballet J.J., Gargala G., Favennec L. Common occurrence of Cryptosporidium hominis in asymptomatic and symptomatic calves in France. PLoS Negl. Trop. Dis. 2018;29(12(3)) doi: 10.1371/journal.pntd.0006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L.J., Fayer R. Cryptosporidium. In: Robertson L.J., Smith H.V., editors. Foodborne Protozoan Parasites. Nova Science Publishers; Hauppauge, NY, USA: 2013. pp. 33–64. [Google Scholar]
- Ryan U., Fayer R., Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141(13):1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- Santin M., Trout J.M., Xiao L., Zhou L., Greiner E., Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Sari B., Arslan M.Ö., Gicik Y., Kara M., Taşçi G.T. The prevalence of Cryptosporidium species in diarrhoeic lambs in Kars province and potential risk factors. Trop. Anim. Health Prod. 2009;41:819–826. doi: 10.1007/s11250-008-9260-0. [DOI] [PubMed] [Google Scholar]
- Scott C.A., Smith H.V., Mtambo M.M.A., Gibbs H.A. An epidemiological study of Cryptosporidium parvum in two herds of adult beef cattle. Vet. Parasitol. 1995;57:277–288. doi: 10.1016/0304-4017(94)00694-8. [DOI] [PubMed] [Google Scholar]
- Siwila J., Phiri I.G., Vercruysse J., Goma F., Gabriel S., Claerebout E., Geurden T. Asymptomatic cryptosporidiosis in Zambian dairy farm workers and their household members. Trans. R. Soc. Trop. Med. Hyg. 2007;101:733–734. doi: 10.1016/j.trstmh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Skerrett H., Holland C. Asymptomatic shedding of Cryptosporidium oocysts by red deer hinds and calves. Vet. Parasitol. 2001;94:239–246. doi: 10.1016/s0304-4017(00)00405-2. [DOI] [PubMed] [Google Scholar]
- Taamasri P., Mungthin M., Rangsin R., Tongupprakarn B., Areekul W., Leelayoova S. Transmission of intestinal Blastocystosis related to the quality of drinking water. Southeast Asian J. Trop. Med. Public Health. 2000;31(1):112–117. [PubMed] [Google Scholar]
- Tan K.S. Blastocystis in humans and animals: new insights using modern methodologies. Vet. Parasitol. 2004;126:121–144. doi: 10.1016/j.vetpar.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Tan K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008;21:639–665. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.C., Tan P.C., Sharma R., Sugnaseelan S., Suresh K.G. Genetic diversity of caprine Blastocystis from Peninsular Malaysia. Parasitol. Res. 2013;112:85–89. doi: 10.1007/s00436-012-3107-3. [DOI] [PubMed] [Google Scholar]
- Tan T.K., Chandrawathani P., Low V.L., Lee S.C., Ngui R., Sharma R.S.K., Lim Y.A.L. Co-infection of Haemonchus contortus and Trichostrongylus spp. among livestock in Malaysia as revealed by amplification and sequencing of the internal transcribed spacer II DNA region. BMC Vet. Res. 2014;10:38. doi: 10.1186/1746-6148-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.K., Panchadcharam C., Low V.L., Bathmanaban P., Lee S.C., Chua K.H., Sharma R.S.K., Ngui R., Tay S.T., Quaza N.H.N., Lim Y.A.L. Occurrence of gastro-intestinal parasites among small ruminants in Malaysia: highlighting Dicrocoelium infection in goats. Trop. Biomed. 2017;34(4):963–969. [PubMed] [Google Scholar]
- Teichroeb J.A., Kutz S.J., Parkar U., Thompson R.C.A., Sicotte P. Ecology of the gastrointestinal parasites of Colobus vellerosus at Boabeng-Fiema, Ghana: possible anthropozoonotic transmission. Am. J. Phys. Anthropol. 2009;140:498–507. doi: 10.1002/ajpa.21098. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Martin S.W., Leslie K.E., Duffield T., Nydam D.V., Peregrine A.S. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007;82:12–28. doi: 10.1016/j.prevetmed.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung K.C., Huang C.C., Pan C.H., Yang C.H., Lai C.H. Prevalence of gastrointestinal parasites in yellow cattle between Taiwan and its Offshore Islands. Thai J. Vet. Med. 2012;42(2):219–224. [Google Scholar]
- Waikagul J., Krudsood S., Radomyos P., Radomyos B., Chalemrut K., Jonsuksuntigul P., Kojima S., Looareesuwan S., Thaineau W. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Health. 2002;33:218–223. [PubMed] [Google Scholar]
- Xiao L., Fayer R., Ryan U., Upton S.J. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E.V., Glaser R. Stress induced immunomodulation and the implication for health. J. Int. Immunopharmacol. 2002;2:315–324. doi: 10.1016/s1567-5769(01)00182-5. [DOI] [PubMed] [Google Scholar]
- Yap N.J., Koehler A.V., Ebner J., Tan T.K., Lim Y.A.L., Gasser R.B. Molecular analysis of Cryptosporidium from cattle from five states of Peninsular Malaysia. Mol. Cell. Probes. 2016;30:39–43. doi: 10.1016/j.mcp.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Zali M.R., Mehr A.J., Rezaian M., Meamar A.R., Vaziri S., Mohraz M. Prevalence of intestinal parasitic pathogens among HIV-positive individuals in Iran. Jpn. J. Infect. Dis. 2004;57:268–270. [PubMed] [Google Scholar]