Abstract
Zoonotic parasites of seals that are harvested for food may pose a health risk when seal meat or organ tissues of infected animals are eaten raw or undercooked. In this study, 124 tissue samples from 81 seals, comprising four species, were collected from northern and eastern Canada. Tissues from 23 ringed seals (Pusa hispida), 8 hooded seals (Cystophora cristata), 21 harp seals (Pagophilus groenlandicus), and 29 grey seals (Halichoerus grypus) were tested for parasites of the Sarcocystidae family including Toxoplasma gondii, Sarcocystis spp., and Neospora spp. using nested PCR followed by Sanger sequencing. Toxoplasma gondii DNA was present in 26% of ringed seals, 63% of hooded seals, 57% of harp seals, and 31% of grey seals. Sarcocystis sp. DNA was found in 9% of ringed seals, 13% of hooded seals, 14% of harp seals, and 4% of grey seals, while N. caninum-like DNA was present in 26% of ringed seals. While it is unclear how pinnipeds may become infected with these protozoans, horizontal transmission is most likely. However, one harp seal pup (4 days old) was PCR-positive for T. gondii, suggesting vertical transmission may also occur. Phylogenetic analysis of the 18S gene region indicates that Sarcocystis sp. in these seals belongs to a unique genotype. Furthermore, this study represents a new host report for T. gondii in harp seals, a new host and geographic report for N. caninum-like parasites in ringed seals, and four new hosts and geographic reports for Sarcocystis sp. These results demonstrate that parasites of the Sarcocystidae family are prevalent in northern and eastern Canadian seals. While the zoonotic potential of Sarcocystis sp. and the N. caninum-like parasite are unclear, consumption of raw or undercooked seal meat or organ tissues pose a risk of T. gondii infection to consumers.
Keywords: Toxoplasma, Sarcocystis, Neospora, Seals, Zoonotic, Canada
Highlights
-
•
Tissues from ringed, hooded, harp and grey seals in Canada were PCR-positive for Toxoplasma, Sarcocystis and Neospora.
-
•
Raw or undercooked seal meat may pose a risk for zoonotic transmission of T. gondii to consumers.
-
•
The risk for zoonotic transmission of Sarcocystis sp. and the Neospora caninum-like parasite is unknown.
1. Introduction
Seal meat and organs are important country foods of Inuit in Arctic and subarctic Canada and Greenland. In addition to subsistence harvests, some seal species are also harvested commercially in a government regulated sustainable harvest in eastern Canada (Hammill and Stenson, 2007; DFO, 2016), and seal meat is available at retail in this region. It is also offered in restaurants in metropolitan areas such as Toronto, Montreal and Quebec City, where the meat is often served as rare seal steak or as seal tartare. Seals are processed using specific guidelines for quality and food safety (Canadian Food Inspection Agency, 2014; Daoust and Stacey, 2014).
Seven pinniped species are hunted in Canada, including: walrus (Odobenus rosmarus), bearded seals (Erignathus barbutus), ringed seals (Pusa hispida), harbour seals (Phoca vitulina), hooded seals (Cystophora cristata), harp seals (Pagophilus groenlandicus), and grey seals (Halichoerus grypus). Most of these species are hunted for subsistence purposes by Inuit and others, but the latter three species are commercially hunted, with meat and by-products being sold to restaurants and exported. These pinnipeds have different distributions, abundance and, for some species, seasonal migrations which affect their availability to hunters. They also have different diets which affect their exposure to parasites and thus pose differential zoonotic risks to human consumers.
Ringed seals have a northern circumpolar distribution, are widespread in Arctic and subarctic Canada, and an estimated 100,000 are harvested annually for subsistence (Kingsley and Byers, 1998). Harp seals are separated into three populations based on specific pupping sites: Northwest Atlantic, Greenland Sea near Jan Mayen (West Ice) and White Sea/Barents Sea (East Ice). The abundant Northwest Atlantic harp seal population (DFO, 2012) is commercially harvested in Atlantic Canada from 307,000 (in 1956) to 40,000 (in 2011) annually (Stenson, 2014). Hooded seals are separated into two breeding herds (same genetic population) based on specific pupping sites: Northwest Atlantic and Greenland Sea (West Ice) (Coltman et al., 2007). The Northwest Atlantic population has subsistence and commercial harvests in Atlantic Canada ranging from 5905 (in 1946) to 0 (in 2006), and less than 400 annually since 1999 (Hammill and Stenson, 2006). Since 1964, commercial harvest of hooded seals is not permitted in the Gulf of St. Lawrence, and the majority of the harvest occurs in Greenland (Stenson et al., 2006). Grey seals, distributed on both sides of the North Atlantic, are found in the Gulf of St. Lawrence and coastal Nova Scotia and Newfoundland. There is a small commercial hunt in the Magdalen Islands and eastern shore of Nova Scotia of less than 1700 animals in 2016 (DFO, 2017).
Little is known about the risk to humans from the consumption of seal meat containing zoonotic pathogens and parasites that they may carry. However, various studies have identified pathogens and parasites in marine mammals of concern to human health (Tryland, 2000; Jenkins et al., 2013; Daoust and Stacey, 2014; Tryland et al., 2014). In this study, we tested ringed seals, harp seals, hooded seals, and grey seals for the presence of the protozoan parasites Toxoplasma gondii, Sarcocystis spp., and Neospora spp.
Toxoplasma gondii is the most prevalent parasite infecting humans and other warm-blooded animals worldwide (Hill and Dubey, 2002). Approximately one to two billion people are infected with this protozoan parasite (Bahia-Oliveira et al., 2017). The definitive hosts of T. gondii are felids, which shed oocysts in their feces. Humans may become infected by accidental ingestion of oocysts in contaminated soil, water, or food. Another transmission route is the consumption of raw or undercooked meats or organs from intermediate hosts infected with T. gondii tissue cysts (Jones and Dubey, 2010). In most healthy adult humans, the infection is asymptomatic. When a woman is exposed to T. gondii for the first time during pregnancy, the parasite may be vertically transmitted to the fetus (Hill and Dubey, 2002), and may result in death or severe illness Immunocompromised patients may develop toxoplasmic encephalitis (Hill and Dubey, 2002). Some Inuit communities show a high level of exposure to T. gondii, with almost 60% seroprevalence in Nunavik, Quebec (Messier et al., 2009). This high seroprevalence was associated with handling or consuming country foods (Messier et al., 2009). Most animal species that are harvested in the Canadian North as country foods, including various terrestrial and marine mammals, birds, and fish, have tested positive for T. gondii (see Reiling and Dixon, 2019). Traditionally, some country foods are eaten raw, which increases the chance of contracting toxoplasmosis.
Sarcocystis spp. typically have prey-predator life cycles involving herbivores and carnivores as intermediate and definitive hosts, respectively. These parasites primarily infect skeletal muscle, heart muscle, and lymph nodes of the intermediate host (Fayer, 2004). Humans can serve as definitive hosts for S. hominis and S. suihominis, which are acquired from eating undercooked beef and pork, respectively (Fayer et al., 2015). Humans can also serve as intermediate hosts for other Sarcocystis spp., likely acquired by ingesting sporocysts from contaminated food or water, or in the environment (Fayer et al., 2015). Infection in humans causes the disease sarcocystosis, which is generally asymptomatic. In Southeast Asia, muscular sarcocystosis in humans was found to be 21% (Wong and Pathmanathan, 1992). To our knowledge, no Sarcocystis infections in humans have been documented in Canada except for travel-related cases to Southeast Asia (Esposito et al., 2014).
Neospora caninum is closely related to T. gondii, and earlier reports confused the two species (Dubey et al., 2002a). Dogs and other canids are the definitive host of N. caninum and oocysts are shed in the canid's feces (Donahoe et al., 2015). Neospora caninum may cause severe neuromuscular disease in dogs, resulting in paraparesis of their hind limbs (Dubey et al., 2007). In cattle, which serve as intermediate hosts, N. caninum may cause encephalitis and abortions and can be transmitted vertically (Moré et al., 2009). Antibodies to N. caninum were reported in 7% of human serum samples in the USA (Tranas et al., 1999), and in 6% of healthy adults (Lobato et al., 2006). Neospora caninum seroprevalence is significantly higher in HIV-infected patients (38%) and in patients with neurological disorders (18%) (Lobato et al., 2006). However, this parasite has not been detected in human tissues, thus its zoonotic potential has not been clearly demonstrated (Dubey et al., 2007).
The objective of this study was to determine the prevalence of Toxoplasma, Sarcocystis, and Neospora infections in four species of seals that are harvested for food in northern and eastern Canada. Results from this study will aid in evaluating the risk of transmission of these parasites to humans through the consumption of seal meat or organ tissues.
2. Material and methods
2.1. Samples
Ringed seals, P. hispida, (n = 19), 12 young-of-the-year (YOY) or juvenile females (10 Age = 0 (YOY), 1 Age = 2, 1 Age = 4) and 7 YOY or juvenile males (6 Age = 0, 1 Age = 1), were collected by Inuit hunters and sampled in 1993 and 1994 at Salluit, Nunavik, Quebec (62°13′N, 75°39′W) (Table S1). In addition, tissues from four ringed seals were collected from Inukjuak, Nunavik, Quebec (58°26′N 78°06′W) but no information was available on the age or sex of these animals. Harp seals, P. groenlandicus, (n = 21), 19 adult females, one YOY male and one YOY female, and hooded seals, C. cristata, (n = 8), adult females only, were shot under scientific permit issued by Fisheries and Oceans Canada and sampled in 2005 from breeding ice floes located west of the Magdalen Islands (47°23′N, 61°52′W) in the Gulf of St. Lawrence, Québec. Grey seals, H. grypus, (n = 29), 14 adult females and 15 adult males, were shot under scientific permit and sampled in 2012 from breeding colonies on Saddle Island (45°48′N 63°15′W) and Pictou Island (45°49′N 62°33′W), Nova Scotia.
Canine teeth were extracted from lower jaws for age determination of ringed and grey seals only. Thin cross-sections of teeth were made and the number of dentinal annuli were counted with one growth layer group = one year of age. Hooded and grey seals were aged based on total length and sexual maturity (only adults are present on the breeding ice floes). Seals were classified as YOY, juvenile or adult as described in Measures et al. (2004). The sex was determined in 77 of 81 seals (Table S1); 54 (70%) were female and 23 (30%) were male.
A total of 124 tissue samples were collected from 81 seals and included diaphragm (n = 53), brain (n = 28), heart muscle (n = 20), lung (n = 19) and skeletal muscle (n = 4). Tissue samples were stored at −20 °C.
2.2. DNA extraction
Tissue samples were thawed, and 1 g subsample of each was divided into two 500 mg aliquots which were used for DNA extraction. The cell lysis protocol was adapted from Opsteegh et al. (2010). To each aliquot, 625 μl of cell lysis buffer containing 100 mM Tris-HCl pH 8.0, 50 mM EDTA pH 8.0, 100 mM NaCl, 1% SDS, 2% 2-mercaptoethanol, and 5 mg/ml proteinase K (Sigma-Aldrich, Oakville, ON, Canada), and 100 μl of 0.1 mm glass beads and 0.7 mm zirconia beads (BioSpec Products, Burlington, ON, Canada) were added. Samples were homogenized 6500 rpm for 3 × 20 s using the Precellys 24 homogenizer (Bertin Technologies, Rockville, MD, USA) before incubating overnight at 45 °C. Aliquots were pooled into 15 ml conical tubes and 1.25 ml cell lysis buffer was added and incubated for 2 h at 45 °C.
To each homogenized tissue sample, 625 μl of 5 M NaCl and 510 μl of cetyl trimethylammonium bromide (CTAB)/NaCl (10% CTAB in 0.7 M NaCl) were added. Samples were incubated at 65 °C for 15 min. An equal volume of phenol/chloroform/isoamyl-alcohol (25:24:1) was added to the sample, followed by a 1.5 h incubation at room temperature (RT) while mixing on a Revolver™ Rotator (Labnet International, Edison, NJ, USA). The solution was then centrifuged at 3000×g for 15 min at 12 °C. The supernatant was dispensed into a new 15 ml conical tube. An equal volume of chloroform/isoamyl-alcohol (24:1) was added, and the samples were placed on a revolver for 1 h at RT. Samples were then centrifuged as indicated above. The supernatant was collected and 2 vol of cold 100% ethanol were added to precipitate the DNA. Samples were stored overnight at 4 °C for complete precipitation.
The precipitated DNA was pelleted at 3000×g for 20 min at 4 °C. An equal volume of 70% ethanol was added to wash the DNA pellet before centrifugation at 1000×g for 10 min at 4 °C. This step was repeated twice. The pellet was then transferred to a 1.5 ml LoBind tube (Corning Inc., Corning, NY, USA) and air dried until translucent. The dry pellet was resuspended in 150 μl of EB Elution Buffer (Qiagen, Mississauga, ON, Canada) at 50 °C for 4 h. The extracted DNA was stored at −20 °C.
2.3. Nested PCR
The gene regions, primers, and their respective nucleotide sequences that were used in this study are listed in Table 1. All tissues available for testing in this study were tested with B1 and 18S primers. Sarcocystis-specific primers were used to confirm Sarcocystis sp. All PCR reactions were performed with a total reaction volume of 25 μl containing 1 × concentration of a 5 × Green GoTaq Reaction Buffer, 2 mM of MgCl2, 200 μM of dNTPs, 0.625 U GoTaq Polymerase (all from Promega, Madison, WI, USA), 300 nM of each primer (Sigma-Aldrich Canada, Oakville, ON, Canada), 1 μl of template DNA, and UltraPure water (Invitrogen, Carlsbad, CA. USA). The DNA concentration, quantified using Nanodrop (ThermoFisher Scientific, Waltham, MA, USA), was normalized to 500 ng per reaction. PCR reaction was performed using the Mastercycler Nexus X2 thermocycler (Eppendorf, Hamburg, Germany) for all samples. Cycling conditions for all samples were: 95 °C for 2 min, 35 cycles of 94 °C for 30 s, 50–68 °C for 30 s, and 72 °C for 60 s, following by a final extension at 72 °C for 10 min, and final hold temperature of 10 °C. The annealing temperatures varied between primers and are listed in Table 1. Negative controls were added to each PCR run. Positive controls consisted of DNA extracted from T. gondii oocysts kindly donated by Dr. J. P. Dubey, USDA. While positive controls were not available for Sarcocystis sp. or Neospora caninum, T. gondii positive control was used as a negative control for these parasites.
Table 1.
Primer sequences of the genes used for polymerase chain reactions.
| Gene | Annealing Temp. | Primer | Primer sequence (5′-3′) | Reference | |
|---|---|---|---|---|---|
| 18S (Sarcocystidae) | outer PCR | 68 °C | N-DIAGF2 | CAATTGGAGGGCAAGTCTGGTGCCAGC | Nichols et al. (2003) |
| N-DIAGR2 | CCTTCCTATGTCTGGACCTGGTGAGT | Nichols et al. (2003) | |||
| nested PCR | 59 °C | CPB-DIAGF | AAGCTCGTAGTTGGATTTCTG | Nichols et al. (2003) | |
| SJR Toxo2R | GTGCAGGAGAAGTCAAGCATGACG | Present study | |||
| 18S (Sarcocystis) | outer PCR | 50 °C | Sarc Back OutF | AGTAATGATTAATAGGGACAGTTG | Wassermann et al. (2017) |
| Sarc Back OutR | GTGAATGATCCTTCCGCAGGTTCA | Wassermann et al. (2017) | |||
| nested PCR | 50 °C | Sarc Back NestF | GCATTCGTATTTAACTGTCAGAGG | Wassermann et al. (2017) | |
| Sarc Back NestR | CTACGGAAACCTTGTTACGACTTC | Wassermann et al. (2017) | |||
| B1 (Toxoplasma) | outer PCR | 58 °C | B1outF | GGAACTGCATCCGTTCATGAG | Di Guardo et al. (2011) |
| B1outR | TCTTTAAAGCGTTCGTGGTC | Di Guardo et al. (2011) | |||
| nested PCR | 58 °C | B1intF | TGCATAGGTTGCAGTCACTG | Di Guardo et al. (2011) | |
| B1intR | GGCGACCAATCTGCGAATACACC | Di Guardo et al. (2011) |
Positive samples as determined by gel electrophoresis were purified using either the QIAquick PCR purification kit or the QIAquick Gel Extraction kit (Qiagen, Mississauga, ON, Canada) following manufacturer's instructions.
2.4. Sanger sequencing
The purified PCR products were prepared for, and subjected to, bi-directional, cycle sequencing using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) as recommended by the manufacturer. Amplified sequence products were purified using Wizard MagneSil green (Promega, Madison, WI, USA) sequencing reaction clean-up system, and capillary electrophoresis was performed on a 3500 Genetic Analyser (Applied Biosystems, Waltham, MA, USA). Sequences were assembled, edited and aligned using SeqScape v3 software (Applied Biosystems, Waltham, MA, USA). Resulting consensus sequences were aligned with representative GenBank 18S sequence data from T. gondii, Sarcocystis spp. and Neospora spp., and trimmed to identical lengths of 441bp using BioEdit (Hall, 1999). Sequences are available through GenBank accession numbers MH514961-MH514967 for the Sarcocystis-positive samples, and GenBank accession numbers MH595863-MH595890 for the Toxoplasma- or Neospora-positive samples.
2.5. Molecular phylogenetic analysis by Maximum Likelihood method
The evolutionary history was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model (Kimura, 1980). The tree with the highest log likelihood was used. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. Trees were drawn to scale, with branch lengths measured in the number of substitutions per site. All positions with less than 95% site coverage were eliminated. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
3. Results
3.1. Toxoplasma gondii and Sarcocystis sp. DNA was present in all seal species tested
Depending upon the seal species and collection method, different tissues were available for testing, including muscle, brain, heart, lung, and diaphragm (Supplementary Table S1). PCR was performed on all tissues using 18S and B1 primers as described in Table 1. 18S Sarcocystidae primers were used to detect all parasites of the Sarcocystidae family. All Sarcocystis sp. positive samples were confirmed using a second, Sarcocystis-specific, nested 18S primer. Toxoplasma gondii was detected using 18S and B1 primers, however, not all tissues were found to be positive for both primers (Supplementary Table S1).
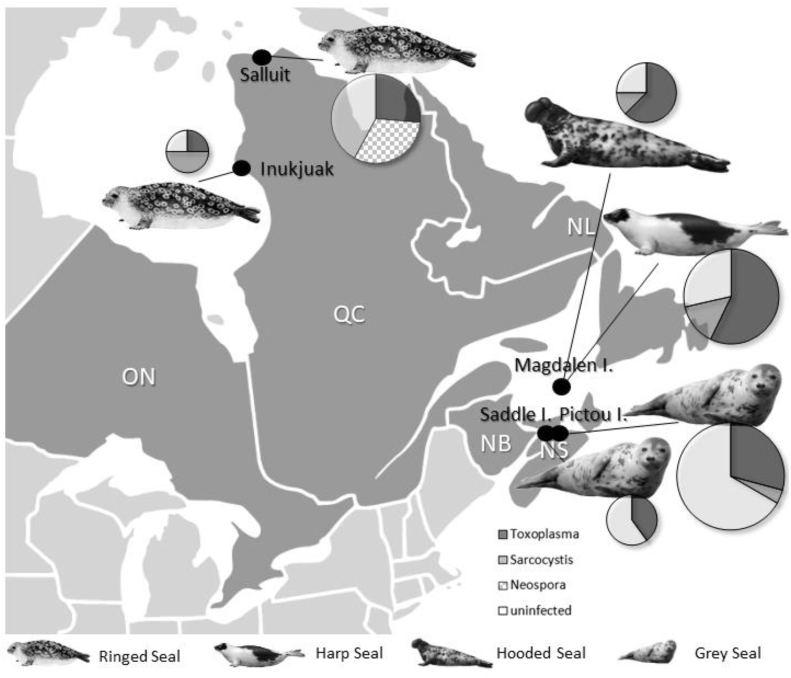
Toxoplasma gondii, Sarcocystis sp. and N. caninum-like DNA was detected in 40%, 9% and 7% of seals, respectively (Table 2). Analyses revealed T. gondii DNA in 26% of ringed seals, 63% of hooded seals, 57% of harp seals, and 31% of grey seals. Sarcocystis sp. DNA was found in 9% of ringed seals, 13% of hooded seals, 14% of harp seals, and 4% of grey seals. Neospora caninum-like DNA was only found in ringed seals from Salluit (26%).
Table 2.
Prevalence of Toxoplasma gondii, Sarcocystis sp. and Neospora caninum-like parasites in seals from northern and eastern Canada.
| Species | Locationa | No. animals tested | Toxoplasma gondii-positive (%) | Sarcocystis sp.-positive (%) | Neospora caninum-like-positive (%) |
|---|---|---|---|---|---|
| Ringed seals (Pusa hispida) | Inukjuak, QC | 4 | 1 (25) | 2 (50) | 0 (0) |
| Ringed seals (Pusa hispida) | Salluit, QC | 19 | 5 (26) | 0 (0) | 6 (32) |
| ringed seal total | 23 | 6 (26) | 2 (9) | 6 (26) | |
| Hooded seals (Cystophora cristata) | Magdalen Islands, QC | 8 | 5 (63) | 1 (13) | 0 (0) |
| Harp seals (Pagophilus groenlandicus) | Magdalen Islands, QC | 21 | 12 (57) | 3 (14) | 0 (0) |
| Grey seals (Halichoerus grypus) | Saddle Island, NS | 5 | 2 (40) | 0 (0) | 0 (0) |
| Grey seals (Halichoerus grypus) | Pictou Island, NS | 24 | 7 (29) | 1 (4) | 0 (0) |
| grey seal total | 29 | 9 (31) | 1 (4) | 0 (0) | |
| total | 81 | 32 (40) | 7 (9) | 6 (7) |
QC, Quebec; NS, Nova Scotia.
All Sarcocystis sp.-infected animals of known sex were female; however, 97% of the seals harvested from the Magdalen Islands were female and the sex of the seals from Inukjuak was unknown. All animals from the Salluit cohort were <5 years of age; of the 6 N. caninum-like positive animals, 4 (67%) were female and 2 (33%) were male, and 5 of the 6 were YOY and one was Age = 1. For T. gondii-infected animals, the sex was known for 31 of 32 animals, of which 19 (61%) were female and 12 (39%) were male, which included one 4-day-old male harp seal pup (Supplementary Table).
3.2. Parasite infections differed amongst regions
The prevalence of T. gondii, Sarcocystis sp., and N. caninum-like parasites in the seal populations differed by harvest location (Fig. 1, Table 2, Table S1). Toxoplasma gondii DNA was detected in all seal species from all harvest locations (Fig. 1). Sarcocystis sp. DNA was detected in all seal species but only from three of five harvest locations, namely ringed seals from Inukjuak, grey seals from Pictou Island, and harp and hooded seals from the Magdalen Islands. As Sarcocystis sp. DNA was predominantly found in skeletal muscle (Table 3), and this tissue was not available from all seal species and harvest locations, some Sarcocystis infections may have been undetected. Neospora caninum-like DNA was detected in ringed seals (32%) from Salluit but not in ringed seals from Inukjuak, nor in any other seal species.
Fig. 1.
Harvesting locations of seal species: Salluit, Quebec (ringed seals, n = 19), Inukjuak, Quebec (ringed seals, n = 4), Magdalen Islands, Quebec (hooded seals, n = 8; harp seals, n = 21), Saddle Island, Nova Scotia (grey seals, n = 5), Pictou Island, Nova Scotia (grey seals, n = 24). The size of the pie charts represents the number of seals sampled per harvesting location. NB, New Brunswick; NL, Newfoundland and Labrador; NS, Nova Scotia; ON, Ontario; QC, Quebec.
Table 3.
Distribution of Toxoplasma gondii, Sarcocystis sp. and Neospora caninum-like parasites in seal tissues.
| Tissuea | No. samples tested | Toxoplasma gondii-positive (%) | Sarcocystis sp.-positive (%) | Neospora caninum-like-positive (%) |
|---|---|---|---|---|
| Diaphragm | 53 | 17 (32) | 3 (6) | 0 (0) |
| Brain | 28 | 9 (32) | 1 (4) | 0 (0) |
| Heart muscle | 20 | 6 (30) | 1 (5) | 0 (0) |
| Lung | 19 | 5 (26) | 0 (0) | 6 (32) |
| Skeletal muscle | 4 | 1 (25) | 2 (50) | 0 (0) |
| total | 124 | 38 (31) | 7 (6) | 6 (5) |
Six individual seals were PCR-positive in more than one tissue.
3.3. Toxoplasma gondii was evenly distributed across all tissues
Tissues positive for T. gondii included diaphragm, brain, heart muscle, lung and skeletal muscle, in similar prevalences (Table S1, Table 3). Sarcocystis sp. appeared to have a preference for skeletal muscle compared to diaphragm, brain, heart muscle and lung. Neospora caninum-like DNA was detected only in lung tissues from ringed seals harvested in Salluit as it was the only tissue available from this particular seal cohort (Table S1).
3.4. Neospora caninum-like parasites are closely related to Toxoplasma gondii
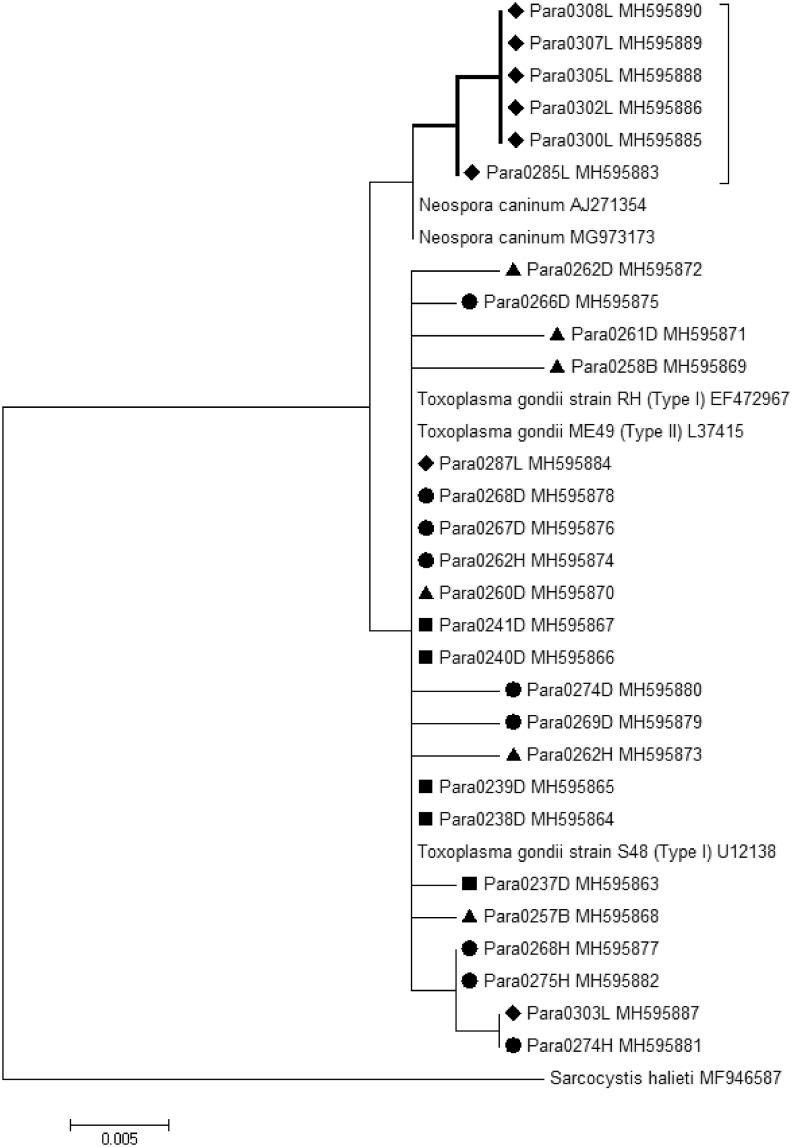
A phylogenetic tree was made for the 18S gene region of T. gondii and the N. caninum-like parasites. Five of the six N. caninum-like positive tissue samples were 100% identical by sequencing (Fig. 2), whereas the sixth shared 99.8% identity. For T. gondii, some animals harbored single nucleotide polymorphisms (SNPs) of the parasite (hooded seal Para0262H vs hooded seal Para0262D: 99.1% identity; harp seal Para0274H vs harp seal Para0274D: 99.1% identity) (Fig. 2). There was insufficient information to determine whether SNPs were due to infection with more than one T. gondii strain, or because 18S is a multi-copy gene that may contain SNPs in one or more of its copies.
Fig. 2.
Phylogenetic analysis of the 18S gene of Toxoplasma gondii and Neospora caninum-like parasites in seals from northern and eastern Canada. No distinctive phylogenetic pattern was observed between seal species and T. gondii infections. A cluster of six ringed seals (bold lines) was found to be most closely related to N. caninum. Symbols represent seal type: ◆ ringed seal, ● harp seal, ■ grey seal, ▲ hooded seal. Organisms shown with no symbol are GenBank 18S sequences with their respective accession numbers indicated. The letter following the ParaXXXX sample number represents the tissue of infection: D, diaphragm; B, brain; H, heart muscle; L, lung.
3.5. Sarcocystis sp. in seals belong to a unique genotype
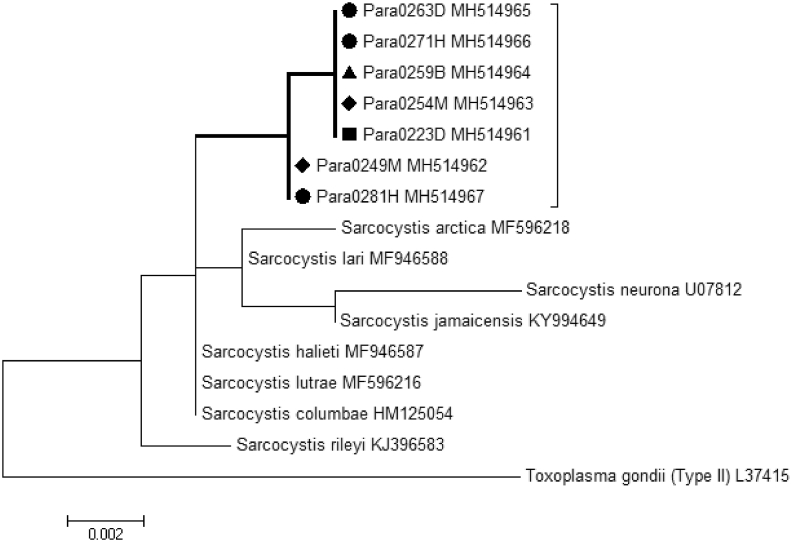
Five of the seven Sarcocystis sp.-positive tissues had a single nucleotide polymorphism (SNP) that was distinct from known Sarcocystis spp. reference sequences archived in GenBank (Fig. 3). Ringed seal Para0249M and harp seal Para0281D had four SNPs, of which only two were identical to the SNPs of the other five seal tissues. Moreover, ringed seal Para0249M had two nucleotide variants in two of the SNPs. While Sarcocystis spp. are haploid in their intermediate hosts, 18S is a multi-copy gene and allelic variations in Sarcocystis spp. have been described previously (Aleman et al., 2016).
Fig. 3.
Phylogenetic analysis of 18S gene of Sarcocystis sp. in seals from northern and eastern Canada. All seven seal samples were grouped in a cluster (bold lines) that was distinct from other known Sarcocystis species. Symbols represent seal type: ◆ ringed seal, ● harp seal, ■ grey seal, ▲ hooded seal. Organisms shown with no symbol are GenBank 18S sequences with their respective accession numbers indicated. The letter following the ParaXXXX sample number represents the tissue of infection: D, diaphragm; B, brain; H, heart muscle; M, skeletal muscle.
4. Discussion
Seal meat and organ tissues, including muscle, blubber, heart, liver, intestine, bones, cartilage, etc. (Pelly, 2001) have been consumed in Canada for thousands of years by indigenous people. They are generally eaten raw, rare, or undercooked depending on cultural habits, increasing the risk of parasites being transmitted to the consumer. Data from the present study suggests that Canadian seal meat and organ tissues may be a source of infection of T. gondii. As there is considerably less known about the infectivity and pathogenicity of Sarcocystis and Neospora in humans, the presence of these parasites in seals represents a lesser known risk to consumers.
In this study, we report the presence of DNA of Sarcocystidae parasites in all seal species tested. While DNA analysis may be less sensitive compared to serology, PCR and subsequent sequencing eliminates false-positive serological results due to cross-reaction between different species of the Sarcocystidae family (Gondim et al., 2017). Furthermore, serological tests should be carefully interpreted, depending on the antibodies used for testing, as some antibodies can be transmitted from mother to fetus (Montoya, 2002; Praet et al., 2010).
Toxoplasma gondii was reported in numerous wild otariid and phocid pinnipeds worldwide, including harbour seals, ringed seals, bearded seals, grey seals, hooded seals, and spotted seals (Phoca largha), but not ribbon seals (Histriophoca fasciata), in Canada and Alaska, USA (Lambourn et al., 2001; Dubey et al., 2003; Measures et al., 2004; Aguirre et al., 2007; Fujii et al., 2007; Simon et al., 2011; Rengifo-Herrera et al., 2012; Donahoe et al., 2014; Al-Adhami et al., 2016). Measures et al. (2004) reported seroprevalence of T. gondii in harbour, grey, and hooded seals, but not in harp seals, on the east coast of Canada. Oksanen and coworkers did not detect T. gondii in harp, ringed, and hooded seals from the Northeastern Atlantic using serology (Oksanen et al., 1998). Thus, we report harp seals as a new host for T. gondii.
Furthermore, we report evidence of vertical transmission (transplacental or transmammary) in one 4-day-old male harp seal pup (Para0262H). Vertical transmission may also have occurred in five T. gondii infected YOY ringed seals in our study but, as these animals were harvested by Inuit in September and eastern Arctic ringed seals are born mid-March to mid-April with two months of lactation, it is possible that they may have acquired infections via their diet which is initially pelagic crustaceans and later fish such as Arctic cod (Boreogadus saida) (see Smith, 1973). Muscle tissue of Arctic char (Salvelinus alpinus) and Atlantic salmon (Salmo salar) have recently been identified as T. gondii DNA-positive (Reiling and Dixon, 2019). Measures et al. (2004) reported one 10-day-old harbour seal pup and one 14-day-old grey seal pup seropositive to T. gondii, but they attributed seropositivity to maternal antibodies. Vertical transmission of T. gondii and S. neurona was documented in an aborted sea otter (Enhydra lutra) pup (Shapiro et al., 2016). Furthermore, co-infections with T. gondii and S. neurona have been reported (Gibson et al., 2011; Shapiro et al., 2016). Transmission of such protozoans to marine mammals, including cetaceans, in the marine environment is not fully understood and vertical transmission (exogenous or endogenous) may be one way to infect conspecifics or offspring (Worth et al., 2013; Donahoe et al., 2015; Iqbal et al., 2018).
Extralimital reports of ringed, grey, harp and hooded seals in southern waters such as southern Nova Scotia and New England (Lucas and McAlpine, 2002; Mignucci-Giannoni and Haddow, 2002), or even as far south as the Caribbean in the case of juvenile hooded seals (Mignucci-Giannoni and Odell, 2001), often involve seals that are sick and stranded. These seals may be taken into rehabilitation facilities, where they may be at greater risk of exposure to protozoans such as N. caninum, Sarcocystis spp. and T. gondii and where infected wild and domestic canids and felids contaminate coastal environments (Measures, 2004). For example, canids such as coyotes (Canis latrans) are known to venture onto ice in coastal environments to scavenge and predate seals (Way and Horton, 2004; Chubbs and Phillips, 2005). Canids, including coyotes, foxes (Vulpes vulpes) and wolves (C. lupus) are infected with Sarcocystis spp. and N. caninum (Dubey and Odening, 2001; Donahoe et al., 2015) but the relationship of these coccidians in canids with those in seals is unknown.
Sarcocystis spp. is reported in otariids [Guadalupe fur seals (Arctocephalus townsendi), northern fur seals (Callorhinus ursinus), and California sea lions (Zalophus californianus)] and phocids [harbour seals, northern elephant seals (Mirounga angustirostris) and Hawaiian monk seals (Monachus schauinslandi)] (Miller et al., 2001; Mylniczenko et al., 2008; Barbosa et al., 2015; Barbieri et al., 2016). Sarcocystis neurona DNA was detected in beach-cast otariids, phocids, sea otters and cetaceans in Oregon and Washington, USA and British Columbia, Canada (Gibson et al., 2011; Barbosa et al., 2015). Sarcocystis spp. has not been described in seals from Arctic Canada or eastern Canada. Thus, we report ringed seals, hooded seals, harp seals, and grey seals as new host reports in Canada.
Neospora caninum or N. caninum-like or “Coccidia C″ were reported in otariids (California sea lions, Guadalupe fur seals) and phocids (ringed seals, bearded seals, harbour seals, ribbon seals, Kuril harbour seals (P.v. stejnegeri), and spotted seals (Dubey et al., 2003; Fujii et al., 2007; Gibson et al., 2011). Because the genetic differences between N. caninum and Hammondia heydorni and their relationship to T. gondii have not been fully resolved (Mugridge et al., 1999; Mehlhorn and Heydorn, 2000; Dubey et al., 2002b), we consider N. caninum indistinguishable from H. heydorni. Furthermore, we consider H. hammondii indistinguishable from T. gondii in this study. In Alaska, seroprevalence of N. caninum was reported in harbour seals and ringed seals but not in bearded seals, spotted seals, or ribbon seals (Dubey et al., 2003). Our results provide new records of N. caninum-like parasites in Canadian ringed seals. It is not clear whether this suggests acute or systemic neosporosis as no histopathology was conducted on any of our samples. Furthermore, the 18S gene is conserved in some Neospora spp. and further sequencing will be needed to accurately identify the N. caninum-like parasites as N. caninum (Marsh et al., 1998). As noted above for T. gondii, N. caninum-like parasites were found in YOY ringed seals but we could not confirm transplacental infection for either parasite due to the date of collection.
Stranded marine mammals are often sick and do not represent the health of wild populations, thus prevalence of parasites and associated disease in carcasses or sick stranded animals, as frequently reported in the literature, may over-estimate the role of these parasites in wild populations. As S. neurona and other Sarcocystis spp. infections are associated with myositis, severe meningoencephalitis, and hepatitis in stranded marine mammals, the prevalence of Sarcocystis spp. in the wild population may be lower. In our study, apparently healthy seals were shot by Inuit hunters or under scientific permit and prevalence of protozoan infections may not be comparable to stranded animals. While some researchers described a novel S. neurona genotype in seals (Barbosa et al., 2015), we were unable to identify the closest relative to the genotype that was found in our study because many Sarcocystis spp. and Neospora spp. are identical in the 18S gene region that was analysed.
Our data also show that it is imperative to confirm PCR-positive results with sequencing. Because Toxoplasma, Sarcocystis and Neospora are very closely related to one another, it is possible to amplify more than one parasite species of the Sarcocystidae family with the same primers. Alternatively, more specific primers may be designed to eliminate false-positive PCR results, as was done in the present study using Toxoplasma B1 and 18S Sarcocystis-specific primers.
5. Conclusions
The results of this study demonstrate that DNA from parasites of the Sarcocystidae family, particularly T. gondii and Sarcocystis sp., is prevalent in tissues of northern and eastern Canadian seals. Although based only on the detection of parasite DNA, these findings nevertheless suggest that consumption of raw or undercooked seal meat or organ tissues can pose a risk of infection to consumers. For consumer safety, seal meat and other organ tissues should be thoroughly cooked or frozen. For example, freezing at −10 °C or lower for at least three days was shown to be sufficient for killing T. gondii and Sarcocystis spp. (Fayer, 1975; Srivastava et al., 1986; Kotula et al., 1991; El-Nawawi et al., 2008). The same protocols should be followed when feeding seal meat to dogs to prevent transmission and propagation of N. caninum or N. caninum-like parasites.
The limitations of this study are primarily due to sample size. With four different species of seals, varying in age, diet, behaviour and distribution, collected from five different harvest areas, an analysis of observed differences in prevalence of infection for each of three protozoan parasites is not possible. Moreover, there may be different modes of transmission or different exposure rates to the parasites because some species of seals (harp and hooded) undertake seasonal migrations to more southern waters. The types and numbers of tissues were also limited for some seals. Consequently, it is difficult to fully assess the risk to consumers except to state that these three parasites are present in Canadian pinnipeds and that further data are required to evaluate zoonotic risk.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Fellowship in Canadian Government Laboratories Program (S.J.R.). Research on ringed seals from Salluit was supported by an NSERC operating grant to L.M., and Fisheries and Oceans Canada (FOC) provided further research support to L.M. Ringed seal tissues from Inukjuak were kindly provided by Laurie Chan (University of Ottawa). We thank Mike Hammill (FOC) and the Canadian Coast Guard for assistance and helicopter support during field work in the Gulf of St. Lawrence. We also thank Salluit and Inukjuak hunters for samples from ringed seals.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fawpar.2019.e00067.
Declarations of interest
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aguirre A.A., Keefe T.J., Reif J.S., Kashinsky L., Yochem P.K., Saliki J.T. Infectious disease monitoring of the endangered Hawaiian monk seal. J. Wildl. Dis. 2007;43:229–241. doi: 10.7589/0090-3558-43.2.229. [DOI] [PubMed] [Google Scholar]
- Al-Adhami B.H., Simard M., Hernández-Ortiz A., Boireau C., Gajadhar A.A. Development and evaluation of a modified agglutination test for diagnosis of Toxoplasma infection using tachyzoites cultivated in cell culture. Food Waterborne Parasitol. 2016;2:15–21. [Google Scholar]
- Aleman M., Shapiro K., Sisó S., Williams D.C., Rejmanek D., Aguilar B. Sarcocystis fayeri in skeletal muscle of horses with neuromuscular disease. Neuromuscul. Disord. 2016;26:85–93. doi: 10.1016/j.nmd.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira L., Gomez-Marin J., Shapiro K. Toxoplasma Gondii. In: Rose J.B., Jiménez-Cisneros B., editors. 2017. http://www.waterpathogens.org (Global Water Pathogen Project). (Fayer, R., Jakubowski, W. (Eds), Part 3 Protists) http://www.waterpathogens.org/book/toxoplasma-gondii. Michigan State University, E. Lansing, MI, UNESCO. [Google Scholar]
- Barbieri M.M., Kashinsky L., Rotstein D.S., Colegrove K.M., Haman K.H., Magargal S.L. Protozoal-related mortalities in endangered Hawaiian monk seals Neomonachus schauinslandi. Dis. Aquat. Org. 2016;121:85–95. doi: 10.3354/dao03047. [DOI] [PubMed] [Google Scholar]
- Barbosa L., Johnson C.K., Lambourn D.M., Gibson A.K., Haman K.H., Huggins J.L. A novel Sarcocystis neurona genotype XIII is associated with severe encephalitis in an unexpectedly broad range of marine mammals from the northeastern Pacific Ocean. Int. J. Parasitol. 2015;45:595–603. doi: 10.1016/j.ijpara.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Food Inspection Agency . 2014. Code of practice for the harvest, transport, processing, and export of seal products intended for human consumption.http://www.inspection.gc.ca/food/food-exports/specific-requirements/code-of-practice/eng/1373640653113/1373640765807?chap=0 Available at. modified January 28, 2019. [Google Scholar]
- Chubbs T.E., Phillips F.R. Evidence of range expansion of eastern coyotes, Canis latrans. Labrador. Can. Field-Nat. 2005;119:381–384. [Google Scholar]
- Coltman D.W., Stenson G., Hammill M.O., Haug T., Davis C.S., Fulton T.L. Panmictic population structure in the hooded seal (Cystophora cristata) Mol. Ecol. 2007;16:1639–1648. doi: 10.1111/j.1365-294X.2007.03229.x. [DOI] [PubMed] [Google Scholar]
- Daoust P.Y., Stacey Z. Harvesting seal products of high quality for human consumption. DFO Can. Sci. Advis. Sec. Res. Doc. 2014:34. www.dfo-mpo.gc.ca/csas 2014/009. v 34p. Available at. [Google Scholar]
- DFO Current status of Northwest Atlantic harp seals, (Pagophilus groenlandicus) DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2012:15. www.dfo-mpo.gc.ca/csas 2011/070. Available at. [Google Scholar]
- DFO Statistics on the seal harvest. Seals and sealing. Fisheries and Oceans Canada. 2016. www.dfo-mpo.gc.ca/fm-gp/seal-phoque/seal-stats-phoques-eng.htm Available at.
- DFO Stock assessment of Canadian Northwest Atlantic grey seals (Halichoerus grypus) DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2017:14. www.dfo-mpo.gc.ca/csas 2017/045. Available at. [Google Scholar]
- Di Guardo G., Di Cesare A., Otranto D., Casalone C., Iulini B., Mignone W. Genotyping of Toxoplasma gondii isolates in meningo-encephalitis affected striped dolphins (Stenella coeruleoalba) from Italy. Vet. Parasitol. 2011;183:31–36. doi: 10.1016/j.vetpar.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Donahoe S.L., Rose K., Šlapeta J. Multisystemic toxoplasmosis associated with a type II-like Toxoplasma gondii strain in a New Zealand Fur seal (Arctocephalus forsteri) from New South Wales, Australia. Vet. Parasitol. 2014;205:347–353. doi: 10.1016/j.vetpar.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Donahoe S.L., Lindsay S.A., Krockenberger M., Phalen D., Šlapeta J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 2015;4:216–238. doi: 10.1016/j.ijppaw.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Odening K. Toxoplasmosis and related infections. In: Samuel W.M., Pybus M.J., Kocan A.A., editors. Parasitic Diseases of Wild Mammals. Iowa State University Press; Ames, U.S.A: 2001. pp. 478–519. [Google Scholar]
- Dubey J.P., Barr B.C., Barta J.R., Bjerkas I., Bjorkman C., Blagburn B.L. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 2002;32:929–946. doi: 10.1016/s0020-7519(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hill D.E., Lindsay D.S., Jenkins M.C., Uggla A., Speer C.A. Neospora caninum and Hammondia heydorni are separate species/organisms. Trends Parasitol. 2002;18:66–69. doi: 10.1016/s1471-4922(01)02172-9. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Zarnke R., Thomas N.J., Wong S.K., Van Bonn W., Briggs M. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 2003;116:275–296. doi: 10.1016/s0304-4017(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nawawi F.A., Tawfik M.A., Shaapan R.M. Methods for inactivation of Toxoplasma gondii cysts in meat and tissues of experimentally infected sheep. Foodb. Pathog. Dis. 2008;5:687–690. doi: 10.1089/fpd.2007.0060. [DOI] [PubMed] [Google Scholar]
- Esposito D.H., Stich A., Epelboin L., Malvy D., Han P.V., Bottieau E. Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011-2012. Clin. Infect. Dis. 2014;59:1401–1410. doi: 10.1093/cid/ciu622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R. Effect of refrigeration, cooking and freezing on Sarcocystis in beef from retail food stores. Proc. Helminthol. Soc. Wash. 1975;42:138–140. [Google Scholar]
- Fayer R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004;17:894–902. doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R., Esposito D.H., Dubey J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Kakumoto C., Kobayashi M., Saito S., Kariya T., Watanabe Y. Seroepidemiology of Toxoplasma gondii and Neospora caninum in seals around Hokkaido, Japan. J. Vet. Med. Sci. 2007;69:393–398. doi: 10.1292/jvms.69.393. [DOI] [PubMed] [Google Scholar]
- Gibson A.K., Raverty S., Lambourn D.M., Huggins J., Magargal S.L., Grigg M.E. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Neglected Trop. Dis. 2011;5:e1142. doi: 10.1371/journal.pntd.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondim L.F.P., Mineo J.R., Schares G. Importance of serological cross-reactivity among Toxoplasma gondii, Hammondia spp., Neospora spp., Sarcocystis spp. and Besnoitia besnoiti. Parasitology. 2017;144:851–868. doi: 10.1017/S0031182017000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hammill M.O., Stenson G. Abundance of Northwest Atlantic hooded seals (1960–2005) DFO Can. Sci. Advis. Sec. Res. Doc. 2006:19. www.dfo-mpo.gc.ca/csas 2006/068 iii . Available at. [Google Scholar]
- Hammill M.O., Stenson G.B. Application of the precautionary approach and conservation reference points to management of Atlantic seals. ICES J. Mar. Sci. 2007;64:702–706. [Google Scholar]
- Hill D., Dubey J.P. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Iqbal A., Measures L., Lair S., Dixon B. Toxoplasma gondii infection in stranded St. Lawrence Estuary beluga Delphinapterus leucas in Quebec, Canada. Dis. Aquat. Org. 2018;130:165–175. doi: 10.3354/dao03262. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Castrodale L.J., de Rosemond S.J., Dixon B.R., Elmore S.A., Gesy K.M. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013;82:33–204. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Dubey J.P. Waterborne toxoplasmosis-recent developments. Exp. Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kingsley M.C.S., Byers T.J. Failure of reproduction in ringed seals (Phoca hispida) in amundsen Gulf, northwest territories in 1984-1987. In: Heide-Jørgensen M.P., Lydersen C., editors. Ringed Seals in the North Atlantic. North Atlantic Marine Mammal Commission. 1998. pp. 197–210. Tromsø, Norway. [Google Scholar]
- Kotula A.W., Dubey J.P., Sharar A.K., Andrews C.D., Shen S.K., Lindsay D.S. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J. Food Prot. 1991;54:687–690. doi: 10.4315/0362-028X-54.9.687. [DOI] [PubMed] [Google Scholar]
- Lambourn D.M., Jeffries S.J., Dubey J.P. Seroprevalence of Toxoplasma gondii in harbor seals (Phoca vitulina) in southern Puget Sound. J. Parasitol. 2001;87:1196–1197. doi: 10.1645/0022-3395(2001)087[1196:SOTGIH]2.0.CO;2. Washington. [DOI] [PubMed] [Google Scholar]
- Lobato J., Silva D.A.O., Mineo T.W.P., Amaral J.D.H.F., Segundo G.R.S., Costa-Cruz J.M. Detection of immunoglobulin G antibodies to Neospora caninum in humans: high seropositivity rates in patients who are infected by human immunodeficiency virus or have neurological disorders. Clin. Vaccine Immunol. 2006;13:84–89. doi: 10.1128/CVI.13.1.84-89.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas Z.N., McAlpine D.F. Extralimital occurrences of ringed seals, Phoca hispida, on sable Island, Nova Scotia. Can. Field Nat. 2002;116:607. [Google Scholar]
- Marsh A., Bradd E., Barr C., Packham A.E., Conrad A.E. Description of a new Neospora species (protozoa: apicomplexa: sarcocystidae) J. Parasitol. 1998;84:983–991. [PubMed] [Google Scholar]
- Measures L.N. Marine mammals and ``wildlife rehabilitation'` programs. DFO Can. Sci. Advis. Sec. Res. Doc. 2004:35. www.dfo-mpo.gc.ca/csas 2004/112 ii 35p. Available at. [Google Scholar]
- Measures L.N., Dubey J.P., Labelle P., Martineau D. Seroprevalence of Toxoplasma gondii in Canadian pinnipeds. J. Wildl. Dis. 2004;40:294–300. doi: 10.7589/0090-3558-40.2.294. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H., Heydorn A. Neospora caninum: is it really different from Hammondia heydorni or is it a strain of Toxoplasma gondii? An opinion. Parasitol. Res. 2000;86:169. doi: 10.1007/s004360050028. [DOI] [PubMed] [Google Scholar]
- Messier V., Levesque B., Proulx J.F., Rochette L., Libman M.D., Ward B.J. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada) Zoonoses Public Hlth. 2009;56:188–197. doi: 10.1111/j.1863-2378.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- Mignucci-Giannoni A.A., Odell D.K. Tropical and subtropical records of hooded seals (Cystophora cristata) dispel the myth of extant Caribbean monk seals (Monachus tropicalis) Bull. Mar. Sci. 2001;68:47–58. [Google Scholar]
- Mignucci-Giannoni A.A., Haddow P. Wandering hooded seals. Science. 2002;295:627–628. doi: 10.1126/science.295.5555.627. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Sverlow K., Crosbie P.R., Barr B.C., Lowenstine L.J., Gulland F.M. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J. Parasitol. 2001;87:816–822. doi: 10.1645/0022-3395(2001)087[0816:IACOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Montoya J.G. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 2002;185:S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- Moré G., Bacigalupe D., Basso W., Rambeaud M., Beltrame F., Ramirez B. Frequency of horizontal and vertical transmission for Sarcocystis cruzi and Neospora caninum in dairy cattle. Vet. Parasitol. 2009;160:51–54. doi: 10.1016/j.vetpar.2008.10.081. [DOI] [PubMed] [Google Scholar]
- Mugridge N.B., Morrison D.A., Heckeroth A.R., Johnson A.M., Tenter A.M. Phylogenetic analysis based on full-length large subunit ribosomal RNA gene sequence comparison reveals that Neospora caninum is more closely related to Hammondia heydorni than to Toxoplasma gondii. Int. J. Parasitol. 1999;29:1545–1556. doi: 10.1016/s0020-7519(99)00150-2. [DOI] [PubMed] [Google Scholar]
- Mylniczenko N.D., Kearns K.S., Melli A.C. Diagnosis and treatment of Sarcocystis neurona in a captive harbor seal (Phoca vitulina) J. Zoo Wildl. Med. 2008;39:228–235. doi: 10.1638/2007-0141R.1. [DOI] [PubMed] [Google Scholar]
- Nichols R.A.B., Campbell B.M., Smith H.V. Identification of Cryptosporidium spp. oocysts in United Kingdom noncarbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl. Environ. Microbiol. 2003;69:4183–4189. doi: 10.1128/AEM.69.7.4183-4189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen A., Tryland M., Johnsen K., Dubey J.P. Serosurvey of Toxoplasma gondii in North Atlantic marine mammals by the use of agglutination test employing whole tachyzoites and dithiothreitol. Comp. Immunol. Microbiol. Infect. Dis. 1998;21:107–114. doi: 10.1016/s0147-9571(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Opsteegh M., Langelaar M., Sprong H., den Hartog L., De Craeye S., Bokken G. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int. J. Food Microbiol. 2010;139:193–201. doi: 10.1016/j.ijfoodmicro.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Pelly D.F. Greystone Books, Douglas and McIntyre Publishing Group; Vancouver: 2001. Sacred Hunt. [Google Scholar]
- Praet N., Rodriguez-Hidalgo R., Speybroeck N., Ahounou S., Benitez-Ortiz W., Berkvens D. Infection with versus exposure to Taenia solium: what do serological test results tell us? Am. J. Trop. Med. Hyg. 2010;83:413–415. doi: 10.4269/ajtmh.2010.10-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling S.J., Dixon B.R. Toxoplasma gondii: how an Amazonian parasite became an Inuit health issue. Can. Commun. Dis. Rep. 2019;45:183–190. doi: 10.4745/ccdr.v45i78a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengifo-Herrera C., Ortega-Mora L.M., Alvarez-Garcia G., Gomez-Bautista M., Garcia-Parraga D., Garcia-Pena F.J. Detection of Toxoplasma gondii antibodies in Antarctic pinnipeds. Vet. Parasitol. 2012;190:259–262. doi: 10.1016/j.vetpar.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Shapiro K., Miller M.A., Packham A.E., Aguilar B., Conrad P.A., Vanwormer E. Dual congenital transmission of Toxoplasma gondii and Sarcocystis neurona in a late-term aborted pup from a chronically infected southern sea otter (Enhydra lutris nereis) Parasitology. 2016;143:276–288. doi: 10.1017/S0031182015001377. [DOI] [PubMed] [Google Scholar]
- Simon A., Chambellant M., Ward B.J., Simard M., Proulx J.F., Levesque B. Spatio-temporal variations and age effect on Toxoplasma gondii seroprevalence in seals from the Canadian Arctic. Parasitology. 2011;138:1362–1368. doi: 10.1017/S0031182011001260. [DOI] [PubMed] [Google Scholar]
- Smith T.G. Population dynamics of the ringed seal in the Canadian eastern Arctic. Fish. Res. Board Can. Bull. 1973;181:55. [Google Scholar]
- Srivastava P.S., Saha A.K., Sinha S.R. Effects of heating and freezing on the viability of sarcocysts of Sarcocystis levinei from cardiac tissues of buffaloes. Vet. Parasitol. 1986;19:329–332. doi: 10.1016/0304-4017(86)90080-4. [DOI] [PubMed] [Google Scholar]
- Stenson G.B. Updated estimates of harp seal removals in the Northwest Atlantic. DFO Can. Sci. Advis. Sec. Res. 2014:35. www.dfo-mpo.gc.ca/csas Doc. 2014/015 v 35 p. Available at. [Google Scholar]
- Stenson G., Hammill M.O., Lawson J., Gosselin J.F. 2005 Pup production of hooded seals, Cystophora cristata, in the Northwest Atlantic. DFO Can. Sci. Advis. Sec. Res. Doc. 2006:40. www.dfo-mpo.gc.ca/csas 2006/067 ii 40 p. Available at. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranas J., Heinzen R.A., Weiss L.M., McAllister M.M. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 1999;6:765–767. doi: 10.1128/cdli.6.5.765-767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryland M. Zoonoses of arctic marine mammals. Infect. Dis. Rev. 2000;2:55–64. [Google Scholar]
- Tryland M., Nesbakken T., Robertson L., Grahek-Ogden D., Lunestad B.T. Human pathogens in marine mammal meat - a northern perspective. Zoonoses Public Hlth. 2014;61:377–394. doi: 10.1111/zph.12080. [DOI] [PubMed] [Google Scholar]
- Wassermann M., Raisch L., Lyons J.A., Natusch D.J.D., Richter S., Wirth M. Examination of Sarcocystis spp. of giant snakes from Australia and Southeast Asia confirms presence of a known pathogen – Sarcocystis nesbitti. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J.G., Horton J. Coyote kills harp seal. Canid News. 2004;7:1. http://canids.org/canidnews/7/Coyote_kills_harp_seal.pdf (Available at. [Google Scholar]
- Wong K.T., Pathmanathan R. High prevalence of human skeletal muscle sarcocystosis in south-east Asia. Trans. R. Soc. Trop. Med. Hyg. 1992;86:631–632. doi: 10.1016/0035-9203(92)90161-5. [DOI] [PubMed] [Google Scholar]
- Worth A.R., Lymbery A.J., Thompson R.C.A. Adaptive host manipulation by Toxoplasma gondii: fact or fiction? Trends Parasitol. 2013;29:150–155. doi: 10.1016/j.pt.2013.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.