Abstract
The protozoan parasite Toxoplasma gondii is a zoonotic parasite that can be transmitted from animals to humans. Felids, including domestic cats, are definitive hosts that can shed oocysts with their feces. In addition to infections that occur by accidental oral uptake of food or water contaminated with oocysts, it is assumed that a large proportion of affected humans may have become infected by consuming meat or other animal products that contained infective parasitic stages of T. gondii. Since farm animals represent a direct source of infection for humans, but also a possible reservoir for the parasite, it is important to control T. gondii infections in livestock. Moreover, T. gondii may also be pathogenic to livestock where it could be responsible for considerable economic losses in some regions and particular farming systems, e.g. in areas where the small ruminant industry is relevant.
This review aims to summarize actual knowledge on the prevalence and effects of infections with T. gondii in the most important livestock species and on the effects of toxoplasmosis on livestock. It also provides an overview on potential risk factors favoring infections of livestock with T. gondii. Knowledge on potential risk factors is prerequisite to implement effective biosecurity measures on farms to prevent T. gondii infections. Risk factors identified by many studies are cat-related, but also those associated with a potential contamination of fodder or water, and with access to a potentially contaminated environment. Published information on the costs T. gondii infections cause in livestock production, is scarce. The most recent peer reviewed reports from Great Britain and Uruguay suggest annual cost of about 5–15 million US $ per country. Since these estimates are outdated, future studies are needed to estimate the present costs due to toxoplasmosis in livestock. Further, the fact that T. gondii infections in livestock may affect human health needs to be considered and the respective costs should also be estimated, but this is beyond the scope of this article.
Keywords: Zoonosis, Livestock, Prevalence, Natural infection, Experimental infection, Costs
Graphical abstract
Highlights
-
•
Brief overview on T. gondii prevalence in livestock
-
•
Natural and experimental T. gondii infections
-
•
Risk factors for T. gondii infection of livestock
-
•
Economic impact of toxoplasmosis in livestock
1. Introduction
Toxoplasma gondii is a zoonotic apicomplexan parasite that is able to infect probably all warm-blooded animals, including livestock (Dubey, 2010b; Schlüter et al., 2014). Domestic cats and other felids are definitive hosts of T. gondii. This implies that the parasite is only able to complete its sexual life cycle in these species, i.e. environmentally resistant oocysts can only be shed with the feces of infected felids (Dubey, 2010b). Oocysts are central in the life cycle of T. gondii. After one up to a few days of maturation (sporulation) in the environment, they become infective to a large variety of warm-blooded intermediate hosts (livestock, synanthropic and wild living animals such as rodents or birds, poultry or humans) if ingested (Dubey, 2010b). In addition to oocysts, there are two further stages of T. gondii, which are infective, i.e. tachyzoites and bradyzoites, the latter being present in tissue cysts. After infection, tachyzoites invade host cells, in which they multiply. This replication is strictly intracytoplasmatic in parasitophorous vacuoles formed by the parasite (Schlüter et al., 2014). In parallel, after several rounds of multiplication, the parasite establishes intracellular tissue cysts, which contain slowly or no longer replicating bradyzoites (Jerome et al., 1998; Radke et al., 2003; Watts et al., 2015). Tissue cyst formation preferentially occurs in brain tissue, the skeletal and heart muscle or also in the retina of infected intermediate hosts (Schlüter et al., 2014).
While the tachyzoite stages are repressed after the onset of the immune response of the host, the dormant tissue cysts ensure that T. gondii infections persist inside the host cells, where they are protected from the immune system, possibly for the rest of the life of the intermediate host (Rougier et al., 2017). Tissue cysts may contain hundreds of bradyzoites (Dubey, 2010b). If carnivorous or omnivorous animals feed on material that contains tissue cysts, encysted bradyzoites can survive the gastric passage and initiate parasite multiplication in the intestine of the infected intermediate or definitive host (Dubey, 2010b). In some regions of the world, in particular in Europe, risk factor studies suggested that most humans become infected by ingesting bradyzoites present in undercooked or not sufficiently inactivated meat (Cook et al., 2000; Kapperud et al., 1996).
Toxoplasma gondii is transmitted vertically in several intermediate host species including humans (Dubey, 2010b). The transplacental vertical transmission is facilitated by tachyzoites usually after the primary and during the acute phase of infection (Dubey, 2010b). Tachyzoites circulating after a reactivated chronic infection also seem to be able to facilitate vertical transmission in some livestock species, although experimental findings suggest that this might be a rare event in T. gondii (Dubey, 1982).
As farm animals represent a source of infection for humans and reservoirs of T. gondii for wildlife it has been proposed to reduce T. gondii infections in livestock as much as possible, particularly in pigs (Anonymous, 2011a, Anonymous, 2011b). Potential risk factors for T. gondii infections in livestock have been studied and were reviewed in recent years (Guo et al., 2015; Opsteegh et al., 2016), but since the knowledge on the epidemiology of toxoplasmosis in animals and humans is rapidly evolving, these reviews deserved an update. Therefore, the main objectives of this study were (i) to briefly summarize the recent gain in knowledge on the prevalence of T. gondii in the most important livestock species and on the importance of infection with this parasite in livestock production, (ii) to compile existing knowledge on the effect of natural and experimental T. gondii infections in the dominant livestock species, and finally (iii) to provide an up-to-date summary on potential risk factors and risk factor studies for T. gondii infection in livestock.
2. Toxoplasma gondii infections in livestock animals – importance for livestock production and prevalence
2.1. Pigs
2.1.1. Prevalence in pigs
Seroepidemiological surveys have provided evidence for a worldwide distribution of T. gondii in pigs, with prevalences varying according to age, pig categories, geography and management system. In general, a low prevalence of T. gondii infections (<1%) is observed in pigs reared in confinement with controlled management conditions, preventing access of rodents and cats, whereas higher prevalence values (>60%) can be found in farms with free-ranging pigs, farms without controlled conditions allowing outdoor access and in backyard holdings (De Berardinis et al., 2017).
Worldwide information on T. gondii infection in pigs up to 2009 was reviewed several times in the past (Dubey, 1986b, Dubey, 2009a; Dubey and Beattie, 1988). The situation in the USA was also reviewed more recently (Hill and Dubey, 2013). A systematic review, based on reports on a direct identification of T. gondii in pigs and pork identified a pooled T. gondii prevalence of 12.3% with a 95% prediction interval ranging from 0 to 55% (Belluco et al., 2017). In Europe, the seroprevalence for T. gondii-specific antibodies was reported to range from 0 to 64% in fattening pigs and from 3 to 31% in breeding sows (De Berardinis et al., 2017). In Africa, a systematic review and meta-data analysis of seroepidemiological studies reported seroprevalences from 9.3 to 40.6%, with an overall estimated T. gondii seroprevalence in pigs of 26% (Tonouhewa et al., 2017). In China, pigs are assumed to represent one of the farm animal species most frequently infected with T. gondii. It was estimated that 30 to 50% of the raised pigs are seropositive, reaching a prevalence of 70% in some areas and farms (Pan et al., 2017). There is quite a large number of further and more recent local studies on prevalence in pigs, but there is little information in addition to that what has been reported and summarized before.
2.1.2. Possible routes of infection in pigs
Most T. gondii infections in pigs are acquired postnatally, either by ingestion of sporulated oocysts in contaminated soil, feed and water, or by ingestion of cysts in the tissues of infected intermediate hosts (e.g. rodents, birds, meat and cannibalism). Pigs can also become infected prenatally by transplacental transmission of the parasite (Dubey, 2009a). The occurrence of galactogenic transmission of T. gondii from the sow to the piglets has been reported, but might be a rare event (Basso et al., 2017). It is presumed that infection through oocysts accounts for most infections in conventional pig breeding systems and especially in outbreaks of clinical toxoplasmosis involving several animals (Kim et al., 2009; Li et al., 2010; Okamoto et al., 1989; Weissenbock and Dubey, 1993).
2.1.3. Disease caused by T. gondii infections in pigs
Toxoplasma gondii infections in pigs are commonly subclinical; nevertheless, several cases of clinical disease after natural infection have been recorded worldwide (Dubey, 1986b, Dubey, 2009a; Dubey and Beattie, 1988). Clinical manifestations seem to occur more frequently in neonatal and weaned pigs, but also cases of clinical toxoplasmosis affecting sows have been described. Common signs observed in clinically infected pigs include anorexia, apathy, fever, ocular and nasal discharge, dyspnoea, cyanosis, diarrhea, limb weakness, neurological signs and sometimes death (Dubey, 1986b, Dubey, 2009a; Dubey and Beattie, 1988). None of these signs is pathognomonic for toxoplasmosis. Besides, T. gondii infections may be associated with reproductive failure in sows characterized by abortion, fetal mummification, stillbirth and neonatal mortality (Basso et al., 2015; Dubey, 1986b, Dubey, 2009a; Dubey and Beattie, 1988). Clinical disease is believed to occur only during the acute phase of infection as result of necrotic and inflammatory processes during tachyzoite multiplication in several tissues. Chronically infected animals do not have clinical signs, but they represent an important source of infection for humans, in particular, if undercooked pork or insufficiently treated meat products containing tissue cysts are consumed (Dubey, 2009a). Different factors are believed to influence the clinical presentation of T. gondii infection in pigs such as age and immune status of the host, co-infection with other agents, the parasitic stage of T. gondii (i.e. oocyst, tissue cyst), infection dose, and the strain or the genetic background of T. gondii. In some cases, viral infections such as Porcine Circovirus type 2 (Klein et al., 2010) and Porcine Parvovirus (Basso et al., 2015) were also associated with clinical manifestations of toxoplasmosis in pigs.
Reports prior to 2009 were summarized previously (Dubey, 1986b, Dubey, 2009a; Dubey and Beattie, 1988). Since then, further confirmed cases of clinical toxoplasmosis involving (i) suckling piglets in Brazil (Olinda et al., 2016), (ii) fattening pigs in China (Li et al., 2010), Germany (Klein et al., 2010) and Brazil (Olinda et al., 2016) and (iii) T. gondii infections associated with reproduction failure in sows in Switzerland (Basso et al., 2015) were published.
A severe outbreak of toxoplasmosis in fattening pigs was reported from Gansu Province, China, with morbidity affecting 549/960 (57%) fattening pigs (Li et al., 2010). The pigs had fever (40–42.2 °C), anorexia and depression, and 19 of the affected pigs died. Serological analysis of 154 clinically ill animals had T. gondii IgG or IgM positive ELISA results in 142 (92.2%) and 147 (95.4%) of the animals, respectively. Moreover, T. gondii could be isolated in mice by intraperitoneal inoculation of pooled heart, liver, spleen and brain tissues from two pigs which showed clinical signs. The source of infection was assumed to be feed contaminated with cat feces. A controlled feeding experiment administering randomly collected feed to five seronegative piglets lead to development of fever, depression and seroconversion of three of the animals. In another study (Klein et al., 2010), systemic toxoplasmosis was diagnosed in a 3.5-month-old fattening pig suffering from post-weaning multisystemic wasting syndrome associated with PCV-2 infection in Germany. The pig had severe respiratory signs and died suddenly. Immunohistochemically, T. gondii was detected associated with interstitial and necrotizing pneumonia, lymphadenitis and adrenal necrosis. It was assumed that immunosuppression caused by primary PCV-2 infection may have triggered secondary systemic toxoplasmosis (Klein et al., 2010).
Interestingly, many of these cases of clinical toxoplasmosis in pigs were registered in Asia (i.e. Japan, Taiwan, China, Korea, Thailand), although there are reports of clinical toxoplasmosis in pigs from several countries around the world (Dubey, 2009a; Dubey and Beattie, 1988). It is not known if specific T. gondii genotypes circulating in Asia may be more prone to cause clinical infections in pigs (Basso et al., 2017).
Recently, T. gondii parasites belonging to the Chinese 1 genotype (synonymous to ToxoDB#9), a frequent genotype in Asia and especially in China (Hou et al., 2018; Wang et al., 2017), were detected in apparently independent fatal cases of toxoplasmosis in two pigs in Brazil (Olinda et al., 2016). The animals (aged one and four months) showed apathy, dyspnoea, poor general condition and died after a few days. The main lesions in both pigs consisted of severe diffuse necrotizing bronchointerstitial pneumonia associated with numerous T. gondii tachyzoites present in the lesions. Interestingly, the cases occurred 3 months apart from each other and both animals derived from two different farms, showing that T. gondii resembling a ToxoDB#9-like genotype is circulating in Brazil (Olinda et al., 2016). This genotype was also identified in 16 out of 17 samples from infected pigs with high fever, dyspnoea, subcutaneous haemorrhage, abortion, enlargement and necrosis of liver and spleen suspected of having clinical toxoplasmosis in China (Jiang et al., 2013). In China, T. gondii infections in pigs are very common and outbreaks of clinical toxoplasmosis with death of numerous pigs have been reported on several occasions (summarized by Pan et al. (2017) and Li et al. (2010)). Moreover, there are reports of repeated outbreaks over 5 years in an individual pig farm in the Shandong Province (Li et al., 2010). These outbreaks of fatal toxoplasmosis were thought to be related to consumption of feed contaminated with oocysts from cat feces (Li et al., 2010). Unfortunately, no molecular characterization of the isolates involved in these outbreaks was performed. Studies in Jiangsu province, Eastern China, revealed high positive rates of T. gondii infection in sick pigs (showing poor mental state, fever, and/or dyspnoea) with 46.8% (66/141) PCR positive animals in various tissues (Hou et al., 2018). In 58 pigs, coinfection with other pathogens was observed but in seven animals T. gondii was the only agent detected, suggesting that it could be involved in the aetiology of sickness or death of pigs in that region. Molecular analysis of T. gondii from 17 sick pigs showed that T. gondii Chinese 1 (ToxoDB#9) was the most frequently (11/17) detected genotype (Hou et al., 2018). In China, parasites with this genotype were also isolated from one case of human toxoplasmosis, but in several American countries also from subclinically infected livestock animals (summarized in Olinda et al. (2016)).
Contrary to the vast knowledge about the importance of vertical transmission of T. gondii in small ruminants and humans, the role of T. gondii as cause of reproductive disorders in sows and the epidemiological significance of intrauterine and galactogenic infections in piglets, showing no clinical signs are less understood (Basso et al., 2017). Reports of reproductive failure due to toxoplasmosis and congenital infection in piglets are well documented, but the experimental reproduction of vertical transmission in pregnant sows is often not successful (Basso et al., 2017). In general, sows that abort or deliver infected offspring usually do not show further clinical signs, but fever, anorexia, neurological signs and even death were observed on some occasions in sows that aborted and transmitted the infection to the fetuses (Kim et al., 2009). In China, abortions caused by T. gondii in sows are considered common and assumed to cause economic losses (Pan et al., 2017). In Europe, reports of reproductive problems due to T. gondii infection in pigs are scarce. A large epidemiological study in 94 pig breeding farms in Germany suggested an association of T. gondii with reproductive failure in sows. The within-farm seroprevalence to T. gondii was significantly higher in farms experiencing reproductive disorders (repeat-breeders, abortion, neonatal mortality), than in farms without such problems, but the role of T. gondii in causing these reproductive problems was not further assessed (Damriyasa et al., 2004). Recently, T. gondii was detected in the placenta or in fetuses of 34 out of 113 sows that had aborted or delivered a high number of stillborn or weak piglets in Switzerland (Basso et al., 2015). By real time PCR, T. gondii DNA was detected in three placentas from one seropositive sow (abortion at 71 days of gestation [dg]), and in brain tissues from one fetus (abortion at 76 dg), one stillborn (116 dg) and one mummy (112 dg) originating from three further seropositive sows, but in no sample derived from the seronegative dams (Basso et al., 2015). By contrast, the examination of fetal tissues and fluids from 32 sow abortions in Romania by PCR did not yield any T. gondii positive samples (Iovu et al., 2010).
2.1.4. Effects of experimental infections in pigs
Pigs can be experimentally infected with any T. gondii stage (i.e. oocysts, tissue cysts, tachyzoites). Most experimentally inoculated pigs, including animals inoculated with very low infection doses (as few as 1 or 10 oocysts), seroconverted after 2–4 weeks and the parasite could be successfully recovered from different tissues. However, experimental reproduction of clinical toxoplasmosis, vertical transmission and congenital toxoplasmosis in pigs is considered difficult (Boch et al., 1965b; Dubey, 2009a; Dubey and Beattie, 1988; Dubey and Urban, 1990; Moller et al., 1970; Sanger and Cole, 1955; Work et al., 1970). Various parasite related factors (i.e. T. gondii stage, dose, infection route, virulence and the genetic background of the strain) and host related factors (i.e. breed, age, immune status and stage of gestation) may influence the outcome of an experimental infection (Dubey, 1986b, Dubey, 2009a). Weaned pigs fed oocysts or tissue cysts often developed transient clinical signs such as weight loss, anorexia and/or fever, independent of the T. gondii isolate in the inoculum and generally recovered by three weeks post inoculation (Basso et al., 2013; Dubey, 2009a).
Experimental infections with T. gondii in pigs were performed within the framework of numerous studies aiming to reveal different aspects of the biology of the parasite (pathogenesis, immune response, persistence of the infection in the tissues, reproduction of congenital toxoplasmosis, development and evaluation of diagnostic methods) or aiming to establish vaccines (Dubey, 2009a; Dubey and Beattie, 1988).
It seems that clinical toxoplasmosis in any pig category and vertical transmission of T. gondii in pregnant sows can be more frequently reproduced by intravenous inoculation of high doses of tachyzoites than by feeding tissue cysts or oocysts. Furthermore, the potential occurrence of vertical transmission may be influenced by the T. gondii isolate used in the inoculations (Basso et al., 2017; Dubey and Urban, 1990; Jungersen et al., 2001; Work et al., 1970). Oral inoculations with 103 oocysts of the GT-1 strain (Type I strain; ToxoDB#10) led to a transplacental infection in five out of 11 inoculated pregnant sows and to transient lethargy, anorexia and respiratory distress between 5 and 15 days post infection (dpi) (Dubey and Urban, 1990), while inoculations with 104 to105 oocysts of the CZ isolate (a European Type II isolate, Toxo DB#3) were not able to reproduce vertical transmission or other clinical signs in any of the 8 pregnant and infected sows (Basso et al., 2017). Likewise, feeding of 5 × 103 oocysts of the CZ isolate to six 4.5 week-old piglets caused infection in all animals but only transiently fever (in all animals); apathy, anorexia and soft feces (in only one piglet) were observed, suggesting a low virulence of this isolate for pigs (Basso et al., 2013). Nevertheless, some authors considered low pathogenic T. gondii strains as good candidates to reproduce vertical transmissions in sows as these parasites might produce a subclinical infection in the dam, having a better chance of establishing placental infections and congenital toxoplasmosis in the piglets before development of a limiting immune response in the sow (Jungersen et al., 2001).
Experimental infections of pigs have recently been performed to evaluate viability of T. gondii in meat after processing techniques. Twelve pigs were inoculated with 103 T. gondii oocysts of a type II field isolate from cat feces and slaughtered 4 months after inoculation. Clinical signs were not reported, but the pigs seroconverted post inoculation and PCR positive results were obtained from most thighs, both at slaughter and post curing (Genchi et al., 2017). In two further experimental studies conducted to test vaccination or to assess swine as an experimental model for human ocular toxoplasmosis, no clinical signs and also no ocular toxoplasmosis were reported after experimental infection with either 103 oocysts per animal or 103 tissue cysts per animal of the M4 strain (a T. gondii Type II strain) of pigs (Burrells et al., 2015; Garcia et al., 2017).
2.2. Sheep and goats
Sheep and goats are highly susceptible for infections with T. gondii and this protozoan parasite is considered a major cause of reproductive losses in small ruminants worldwide. While most descriptions and investigations have been carried out in sheep (Dubey, 2009b), toxoplasmosis has a similar or even greater importance as an abortive disease in goats (Dubey, 2010b). In addition, toxoplasmosis is a relevant zoonosis and infection in small ruminants may play a major role in its transmission to humans (Belluco et al., 2016; Opsteegh et al., 2016).
2.2.1. Prevalence in sheep and goats
Antibodies to T. gondii have been found in sheep and goats worldwide. More than 200 articles reported seroprevalence studies in these domestic ruminant species before 2010, as reviewed by Dubey, 2009b, Dubey, 2010b. At that time, areas of the world with a large number of seroprevalence reports were Brazil, Europe, North America, and the Middle East. From 2010 to 2018, further epidemiological studies in small ruminants have been published, including reports from areas where information was scarce and regions, where sheep and goats are relevant livestock species. These studies are from different parts of Asia (i.e. China, Pakistan, South East Asia), Sub-Saharan Africa and countries from the Mediterranean (Ahmed et al., 2016; Dong et al., 2018; Garcia-Bocanegra et al., 2013; Gazzonis et al., 2015; Kantzoura et al., 2013; Khan et al., 2017; Tilahun et al., 2018; Tzanidakis et al., 2012). Although differences in study design, purpose of the study, serological methodology and cut off points applied make it difficult or even impossible to compare data, these as well as the previous studies clearly show that T. gondii infections are highly prevalent in small ruminants (Dubey, 2010b). In the following, representative examples of recent studies conducted on different continents are summarized.
In Africa, in a recent meta-analysis, summarizing data from 1969 to 2016, the overall estimated prevalence was 26.1% (17.0–37.0%) for sheep and 22.9% (12.3–36.0%) for goats (Tonouhewa et al., 2017). In Egypt, antibody prevalence was higher in goats (62%) than in sheep (between 4.1 and 26%) (Al-Kappany et al., 2018). In Tunisia, antibodies to T. gondii were found in 40.2% sheep and 34.5% goats (Lahmar et al., 2015). In Ethiopia, the seroprevalence of T. gondii infection in sheep and goats was 33.7% and 27.6%, respectively (Tilahun et al., 2018). A further study from this country reported high flock (59.7%) and individual animal (31.8%) T. gondii seroprevalences associated with abortion in some districts (Gebremedhin et al., 2014). A lower seroprevalence was reported from South Africa with 8% in sheep (Hammond-Aryee et al., 2015).
In America, a systematic meta-analysis provided estimates on T. gondii infection in food animals produced in the United States, including small ruminants. T. gondii infection seroprevalence in goats (30.7%) was higher than in sheep or lambs (22.0%) (Guo et al., 2016). Further studies report T. gondii seroprevalences in sheep and goats from the Caribbean Islands Dominica (67%, 58%), Grenada (48%, 57%), Montserrat (89%, 80%) and St. Kitts and Nevis (57%, 42%) (Hamilton et al., 2014). In another study, antibodies to T. gondii (Modified Agglutination Test (MAT) titre 1:≥25) were found in 44.1% of sheep and 42.8% goats in Grenada and Carriacou (Chikweto et al., 2011). In Brazil, serum samples of 930 sheep were tested in two regions of Rio Grande do Norte (Northeastern Brazil), with different climatic conditions and the overall estimated prevalence was 22.1% (Andrade et al., 2013).
Regarding Asia, the situation of T. gondii infections has recently been reviewed for China. Seroprevalence for T. gondii in sheep has been estimated to be 11.8% (2305/19,565) and the overall estimated seroprevalence for T. gondii in goats was 17.6% (3260/18,556) (Dong et al., 2018). In Myanmar, an 11.4% seroprevalence has been reported in goats (Bawm et al., 2016). In other South Asian countries, reported prevalence in sheep and goats was 21.1% and 25.4%, respectively (Khan et al., 2017). In Pakistan, the results also showed higher seroprevalence of T. gondii in goats (42.8%) as compared to sheep (26.2%) (Ahmed et al., 2016). In Saudi Arabia, antibodies to T. gondii were found in 36.4% (325/891) of sheep and 35.3% (196/555) of goats (Alanazi, 2013).
In Europe, high prevalence values have been observed in both, sheep and goats in Mediterranean countries. In Greece, in one study, sheep had a higher seroprevalence (48.6% [729/1501]) for T. gondii than goats (30.7% [166/541]) (Tzanidakis et al., 2012). In Thessaly, a total of 540 sheep and goat serum samples were examined and the seroprevalence was 24.5% (Kantzoura et al., 2013). In another study, specific IgG against T. gondii were detected in 53.71% and 61.3% of the sheep and goats from mixed flocks (Diakoua et al., 2013). In Northern Italy, antibodies were found in 96.6% of goat farms and in 87.5% of sheep farms; 41.7% goats and 59.3% sheep had a positive result. The seroprevalence was significantly higher in sheep than in goats (Gazzonis et al., 2015). In Portugal, 33.6% of sheep and 18.5% of goats were seropositive by a modified agglutination test (MAT) (A.P. Lopes et al., 2013). In Southern Spain, 248 (49.3%) of 503 sheep, and 124 (25.1%) of 494 goats were seropositive. The herd seroprevalence was 84.7% (61/72), and 72.2% (52/72) for sheep and goats, respectively (Garcia-Bocanegra et al., 2013). In another study in the same region, the seroprevalence was 41.2% in sheep and 5.6% in goats (Almeria et al., 2018). In the northwestern part of Spain, individual (48%) and flock-level (74%) T. gondii seroprevalence values in goats were high; the within-flock prevalence was 53% (Diaz et al., 2016). In Eastern Europe as Poland, seroprevalences of 21% in goats and 47% in sheep have been reported (Moskwa et al., 2018). In Romania, the seroprevalence in sheep varied with the region, age and the serological methods from 6.9 to 71% (Dubey et al., 2014a). In the UK, of the 3539 sera collected from 227 sheep flocks, 2619 (74%) were found to be positive for T. gondii specific antibodies (Hutchinson et al., 2011). In France, applying a low cut off titre of 1:≥6 in MAT the overall estimate of the T. gondii seroprevalence was 17.7% (11.6–31.5%) for lambs and 89% (73.5–100%) for adult sheep (Halos et al., 2010). In a Scandinavian country (Norway), 55 of 73 flocks (75%) had one or more serologically positive animals, while 377 of 2188 (17%) of the individual samples tested positive for T. gondii antibodies (Stormoen et al., 2012).
In Oceania, 1917 out of 2254 (85%) sheep sera tested in New Zealand were positive, using a titre of 1:≥16, and 1384/2254 (61%) with a titre of 1:≥64 using a latex agglutination test. All 198 ewe flocks tested were seropositive for antibodies to T. gondii, at a titre of 1:≥16, and all but three were at a titre of 1:≥64 (Dempster et al., 2011).
Isolation of viable parasites from tissues of small ruminants corroborate serological findings and confirm that these species are important intermediate hosts. In sheep, viable T. gondii has been detected in brain, heart, diaphragm and different muscles (Dubey, 2010b; Opsteegh et al., 2016). Due to the fact that T. gondii readily disseminates into the edible tissues of sheep, this parasite represents a risk for consumers (Belluco et al., 2016; Opsteegh et al., 2016). In goats, brain and heart also rank high on the list of predilection organs and muscle tissues had high within study scores, and ranked first when combined in the meat/muscle category (Opsteegh et al., 2016). These results are corroborated by studies in different areas of the world. For instance, the proportion sheep carcasses in France carrying live parasites according to bioassay results was estimated at 5.4% (3–7.5%) (Halos et al., 2010). In the US, 53 isolates of T. gondii were obtained from 68 seropositive lambs sampled at the slaughterhouse (Dubey et al., 2008). In another study in this country, hearts of goats obtained from a local grocery store were examined for T. gondii infection and the parasite was isolated from 29 out of 112 animals (Dubey et al., 2011).
2.2.2. Possible routes of infection in sheep and goats
Horizontal transmission of T. gondii to small ruminants by the oral uptake of environmentally resistant oocysts through contaminated fodder or water is considered the most important route of transmission (Buxton and Losson, 2007; Dubey, 2010b). It is generally assumed that <2% of sheep become infected congenitally and <4% of the persistently infected sheep transmit the infection to their offspring (reviewed in Dubey (2010b) and Innes et al. (2009)). Recrudescence of a chronic infection and the endogenous trans-placental transmission of the parasite to offspring was described in goats (Dubey, 1982). In addition, it was proposed some years ago that a repeated transplacental transmission in sheep was more common than previously thought (Williams et al., 2005) and recent descriptions from Brazil seem to corroborate this hypothesis (Dos Santos et al., 2016; Klauck et al., 2016). Further studies are needed to assess the possibility that certain breeds are more susceptible to endogenous vertical transmission in chronically infected ewes or that vertical transmission is a trait of particular T. gondii strains or genotypes.
Possible alternative routes are venereal or galactogenic transmission. Several studies have identified T. gondii DNA in semen samples from rams and male goats, either from natural cases of infection (Bezerra et al., 2014) or from animals experimentally inoculated (W.D. Lopes et al., 2013; Santana et al., 2010). Furthermore, the transmission of the infection to sheep and goats through semen has also been proven, both under mating with experimentally infected rams (W.D. Lopes et al., 2013) or through artificial insemination with semen spiked with T. gondii tachyzoites (Consalter et al., 2017; Wanderley et al., 2013). On the other hand, the epidemiological significance of this route might be limited (Buxton, 1998). Similarly, milk may also pose a risk of infection to lambs or goat kids, as T. gondii DNA has been identified in milk samples from naturally infected ewes and goats (de Santana Rocha et al., 2015; Saad et al., 2018), and bioassay results in raw milk suggest its infective potential (Chiari and Neves, 1984; Dehkordi et al., 2013). However, it needs to be mentioned that the latter findings have been challenged and their epidemiological significance has been questioned (Dubey and Jones, 2014). Even if these alternative routes of transmission are possible in small ruminants, it still needs to be established, to which extent they contribute to infection.
2.2.3. Disease caused by T. gondii in naturally infected sheep and goats
It has been estimated that toxoplasmosis is responsible of 10 to 23% of ovine abortions in Europe or USA (Dubey, 2009b). Recent reports have shown that also in other regions of the world, as in the Middle East and South America, T. gondii infections are associated with 3 to 54% of ovine abortion (Table 1).
Table 1.
Reports of Toxoplasma gondii induced abortions in small ruminants since 2010.
| Country | No. of placentas, fetuses and stillborn lambs examined (sheep/goats) | No. farms tested (sheep/goats) | No. of submissions (sheep/goats) | % positive, total or ovine/caprine | Diagnostic methods |
Observations | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Other causes investigated | IHC | PCR | Fetal serology | |||||||
| Brazil | 35 | n.a. | n.a. | 14.3 | No | No | Yes | No | Ovine abortions | de Moraes et al. (2011) |
| Great Britain | n.a.a | n.a. | n.a. | 23.7 | Yesb | n.a.a | Yesb | Yesb | Ovine abortions | Carson and Group (2017) |
| Iran | 325 | n.a. | n.a. | 5 | No | No | No | Yes | Ovine abortions | Razmi et al. (2010) |
| 18 | n.a. | n.a. | 66 | No | No | Yes | No | Ovine abortions | Habibi et al. (2012) | |
| 200 | n.a. | n.a. | 13.5 | No | No | Yes | No | Ovine abortions | Rassouli et al. (2011) | |
| 37 | n.a. | n.a. | 54 | No | No | Yes | Yes | Ovine abortions | Danehchin et al. (2016) | |
| Ireland | 66 | n.a. | 17 | Yes | Yes | Yes | Yes | Ovine abortions | Gutierrez et al. (2012) | |
| Jordan | 106 (66/40) | n.a. | n.a. | 31 | No | No | Yes | No | Ovine/caprine abortions | Abu-Dalbouh et al. (2012) |
| Netherlands | n.a. | n.a. | 452 (282/170) | 10.6/5.9 | Yes | Yes | No | No | Ovine/caprine abortions | van den Brom et al. (2012) |
| n.a. | n.a. | 81 (57/24) | 16.7/14.0 | Yes | Yes | No | No | Ovine/caprine abortions | van Engelen et al. (2014) | |
| Spain | 100 (74/26) | n.a. | n.a. | 5.4/3 | Yes | No | Yes | No | Ovine/caprine abortions | Moreno et al. (2012) |
| Switzerland | 30 | n.a. | n.a. | 10 | Yes | No | Yes | No | Ovine/caprine abortions | Schnydrig et al. (2017) |
n.a. = not applicable.
Based on https://veterinaryrecord.bmj.com/content/vetrec/183/17/528.full.pdf (last accessed 2019-01-22).
The only evident clinical sign associated with acquired toxoplasmosis (horizontal transmission), is a brief episode of fever and lack of appetite from about 3–6 days after infection and lasting usually for about 4–5 days, but sometimes also for up to 10 days (Buxton et al., 1982; Buxton et al., 1988; Castano et al., 2016; Dubey, 1981; Esteban-Redondo et al., 1999; McColgan et al., 1988). In contrast, congenital transmission has severe consequences for the fetus. Whether the trans-placental transmission causes the death of the fetus partly depends on the time of gestation when infection occurs. If the dam was infected in early gestation, at a time when the immune system of the fetus is still immature, vertical transmission commonly results in fetal death and resorption. However, when infection occurs at mid gestation, abortion or the delivery of a stillborn lamb are the most common outcomes, while dams infected late in gestation may deliver a stillborn or congenitally infected weak or clinically normal offspring (Buxton and Losson, 2007). Macroscopic lesions in cases of abortion are restricted to the cotyledons, where multifocal areas of necrosis, macroscopically visible as white foci of variable size are suggestive for toxoplasmosis (Buxton and Finlayson, 1986; Buxton et al., 1982). Microscopically, multifocal necrosis, commonly associated with the infiltration of non-purulent lymphoid cells, could be found in placentomes or fetal organs, mainly brain, liver or lung (Buxton and Finlayson, 1986).
2.2.4. Effects of experimental infections in sheep and goats
The precise mechanisms responsible for T. gondii abortion in small ruminants are not yet fully understood. The most recent studies, employing the oral route for administering oocysts as infective parasitic stage are summarized in Table 2. The outcome of experimental infections might be affected by the viability of oocysts which needs to be confirmed prior to use (Dubey, 2010b). In addition, the T. gondii strain characteristics including the virulence, which seems to change after repeated passages (Saraf et al., 2017), need to be taken into account. Results of experimental infections have clearly shown that the gestational age, in particular the stage of maturation of the fetal immune system has an important effect on the pathogenesis (Buxton and Finlayson, 1986). In addition, the cellular immune response of the dam, mainly mediated by IFN-γ, is of importance in controlling the parasite multiplication (Innes et al., 1995). The experimental inoculation of sheep and goats has also helped to demonstrate that toxoplasmosis in small ruminants could also cause early abortion shortly after infection. In these early abortions, invasion and multiplication of the parasite in the placenta or fetus could not be demonstrated (Owen et al., 1998; Rosa et al., 2001). Although the cause of these early abortions was thought to be high fever or hormonal dysregulation (Dubey, 2010b), recent studies have shown that they are related to vascular lesions in the placenta and leukomalacia in the fetal brain (Castano et al., 2014). All together, these results suggest that the pathogenesis of early abortion is different from the classically described, which is based on the multiplication of the parasite and subsequent lesions in the placenta and target fetal organs. The mechanisms underlying the early abortions in this disease are still unknown. Bearing these observations in mind, there are still several gaps in the knowledge of small ruminant toxoplasmosis that warrant further characterization of the experimental models for ovine and caprine toxoplasmosis and investigation on how different variables, e.g. T. gondii strain or isolate virulence, previous immunization or individual susceptibility could affect the pathogenesis of this disease.
Table 2.
Experimental studies in sheep orally inoculated with Toxoplasma gondii oocysts or tissue cysts. Studies published since 2010.
| Designation of Toxoplasma gondii isolate | Dose | Numbers and age category | Remarks | Reference |
|---|---|---|---|---|
| M4 | 3000 | 28 sheep | Inoculated with sporulated oocysts at day 90 of gestation | Gutierrez et al. (2010) |
| 500,000; 5000 | 16 lambs | Inoculated with sporulated oocysts. | Benavides et al. (2011) | |
| 3000 | 9 sheep | Inoculated with sporulated oocysts at day 90 of gestation | O'Donovan et al. (2012) | |
| 3000 | 15 sheep | Inoculated with sporulated oocysts at day 90 of gestation | Marques et al. (2012) | |
| 2000; 500 | 24; 24 sheep | Inoculated with sporulated oocysts at day 90 of gestation (n = 24) and at day 120 (n = 24) | Castano et al. (2014) | |
| 500 | 33 lambs | Inoculated with sporulated oocysts | Katzer et al. (2014) | |
| 50 | 27 sheep | Inoculated with sporulated oocysts at three terms of gestation | Castano et al. (2016) | |
| PRU | 400; 400; 100 | 36; 54; 33 sheep | Inoculated with sporulated oocysts at mid-gestation | Mevelec et al. (2010) |
| 3000 | 13 lambs | Inoculated with tissue cysts | Verhelst et al. (2014) | |
| 3000 | 4 sheep | Inoculated with tissue cysts | Verhelst et al. (2015) | |
| P | 200,000 | 4 rams | Inoculated with sporulated oocysts. | Lopes et al. (2011) |
| ME49; VEG | 2500; 2500 | 20 sheep | Inoculated with sporulated oocysts at three terms of gestation of chronically infected ewes. | Dos Santos et al. (2016) |
| ME49 | 500; 50; 10 | 5; 5; 5 sheep | Inoculated with sporulated oocysts at day 90 of gestation | Sánchez-Sánchez et al. (2019) |
| TgShSp1 | 500; 50; 10 | 6; 6; 6 sheep | Inoculated with sporulated oocysts at day 90 of gestation | Sánchez-Sánchez et al. (2019) |
2.3. Chickens and other poultry
2.3.1. Prevalence in chickens and other poultry
Toxoplasma gondii infection in free-ranging poultry is an indicator of environmental contamination with T. gondii oocysts (Dubey, 2010b). Strains isolated from local poultry probably mirror the T. gondii genotypes prevailing in a region (Dubey, 2010a, Dubey, 2010b). The prevalence of T. gondii infections in poultry depends on a number of factors. The type of husbandry seems to be very important. In poultry originating from free-range or small backyard farms, the T. gondii prevalence is usually higher than in poultry kept indoors (Guo et al., 2015; Maksimov et al., 2011; Schares et al., 2017a; Yang et al., 2012).
In chickens, there is a number of recent articles summarizing the seroprevalence of antibodies to T. gondii (Bangoura et al., 2011; Deng et al., 2018; Dubey, 2010a; Guo et al., 2015; Shokri et al., 2017). Prevalence estimates are often not comparable among studies because different serological tests have been applied and sampled farms may differ for example in farm type and size, feed source, presence or absence of cats, rodent or bird control and water quality (Bangoura et al., 2011; Dubey, 2010a; Schares et al., 2017a). In some studies, a low specificity of the serological tests may have overestimated the seroprevalence or a low sensitivity may have led to an underestimation. Overall, the T. gondii seroprevalences in chickens ranged between 0 and 100% (Ayinmode and Olaosebikan, 2014; Bangoura et al., 2011; Deng et al., 2018; Dubey, 2010a; Matsuo et al., 2014).
Only few studies on T. gondii prevalence in turkeys have been published. Apparent seroprevalence varies largely between studies and ranges from 0% (Czech Republic), 11% (Brazil), 20% (Germany) to 59% (Egypt) (Bangoura et al., 2011; El-Massry et al., 2000; Harfoush and Tahoon Ael, 2010; Koethe et al., 2011; Sa et al., 2016).
The T. gondii seroprevalence in ducks and geese, as summarized for Poland, Czech Republic, Germany and Norway, varied between 1.7 and 21% in ducks and between 5.9 and 43% in geese (reviewed by Bangoura et al. (2011)). Only 3.5% of geese in Kazakhstan were seropositive (Bangoura et al., 2011). T. gondii seroprevalences reported for China in ducks and geese were in the range of 6.3–13.9% (Cong et al., 2012; Yang et al., 2012). The highest seroprevalences in ducks were reported from Egypt (50–55%) (El-Massry et al., 2000; Harfoush and Tahoon Ael, 2010) and Malaysia (30%) (Puvanesuaran et al., 2013).
2.3.2. Possible routes of infection in chickens and other poultry
Due to the ground feeding behavior of poultry, the oral ingestion of material or water contaminated with T. gondii oocysts is most likely the main route of infection (Dubey, 2010b). Water may be contaminated with T. gondii oocysts (Isaac-Renton et al., 1998). Thus, oocyst contaminations of water can be of particular importance as a source of infection for waterfowl. Infected rodents and other intermediate hosts on farms may serve as a reservoir (Schares et al., 2017a). Poultry such as chickens, turkeys, ducks and geese are omnivorous, i.e. they also may feed on earthworms, cockroaches and other insects, which may harbor, or could be contaminated with oocysts (Bettiol et al., 2000; Ruiz and Frenkel, 1980; Wallace, 1971, Wallace, 1972). In addition, poultry may predate on small rodents as putative carriers of T. gondii tissue cysts. In an experimental setting, turkeys became infected after inoculation with brains of chronically infected mice (Koethe et al., 2015) and also chickens fed tissue cysts became infected (summarized by Dubey (2010a)). There is, however, lack of information, to which extent such different routes of infection (i.e. infections via tissue cysts) are relevant under natural conditions. Vertical transmission of T. gondii in poultry has been discussed in the past, but extensive experiments in chickens indicated that this route of infection can be left outside the focus (Biancifiori et al., 1986; Boch et al., 1966).
2.3.3. Disease caused by T. gondii in naturally infected poultry
In general, chickens, turkeys, ducks and geese rarely develop clinical signs after infection with T. gondii (Dubey, 2010a). Worldwide, there are only few reports on clinical toxoplasmosis in naturally infected poultry (Dubey, 2010a, Dubey, 2010b). It has to be kept in mind, however, that some of the clinical cases reported as caused by T. gondii may have been triggered by other infections (e.g. viral) or complicated by other diseases (Dubey, 2010a).
No T. gondii genotype-dependent virulence in adult chickens has been recorded and even South-American strains, highly virulent to mice, seem to be avirulent in chickens (Hamilton et al., 2017; Vaudaux et al., 2010). However, it has to be mentioned here that young chickens (1-month-old), infected by oocysts of T. gondii Type I (GT1 strain) developed clinical toxoplasmosis, whereas those infected by oocysts of T. gondii Type II (ME49) did not develop any clinical signs (Dubey et al., 1993b). Chicken-line dependent differences in mortality after experimental inoculation of chicks with recombinant T. gondii clones suggested that, in addition to the parasites genotype, also genetic factors of the host may play an important role in the development of clinical toxoplasmosis in chickens (Schares et al., 2017b). Age of the chicken seems to be a very because also in another study, 10-day old chickens showed mortality in a dose-dependent fashion (S. Wang et al., 2015).
Reports of toxoplasmosis in magpie (Anseranas semipalmata) and Hawaiian geese (Branta sandvicensis) (Dubey et al., 2001; Work et al., 2015) suggest that there might be differences in susceptibility for T. gondii infection and toxoplasmosis between different species of Anseriformes. By contrast, we could not find reports on clinical toxoplasmosis in domestic geese (Anser anser).
2.3.4. Effects of experimental infections in livestock poultry
The susceptibility of poultry to experimentally induced toxoplasmosis depends on the infectious dose, the parasite strain, stage, the route of infection and, as mentioned above, the age of the animal (Dubey et al., 1993b; Schares et al., 2017b). In chickens, parenteral infection with T. gondii tachyzoites or oral infection with oocysts rarely cause clinical signs (Dubey, 2010a). However, in the case of intracranial infections using encysted T. gondii, the animals developed severe cerebral toxoplasmosis (Bickford and Saunders, 1966; Dubey, 2010a).
No clinical toxoplasmosis was reported in turkeys either after experimental oral infection with different doses of T. gondii oocysts (Bangoura et al., 2013; Dubey, 2010b; Dubey et al., 1993a), or intravenous inoculation with varying doses of tachyzoites (with strains representative for T. gondii clonal Types I, II and III) (Maksimov et al., 2018; Zöller et al., 2013).
The results of experimental infections in ducks and geese with oocysts (Bartova et al., 2004; Maksimov et al., 2011) and intravenous infections with tachyzoites (Maksimov et al., 2011) showed that also these animal species were resistant against clinical toxoplasmosis regardless of the parasite stage used for infection.
2.4. Cattle
2.4.1. Prevalence in cattle
Seroprevalence estimates in cattle, if obtained by highly specific tests, can be of value to monitor the exposure of cattle to T. gondii. However, these serological data must be interpreted with care as studies conducted with bioassay experiments suggest that in the vast majority of seropositive cattle there was no evidence for the presence of viable T. gondii infections. This has been also shown by analyses of naturally exposed animals, in some studies with very large populations of cattle (Boch et al., 1965a; Dubey et al., 2005; Dubey and Streitel, 1976; Fortier et al., 1990; Freyre et al., 1990; Jacobs and Moyle, 1963; Passos et al., 1984). There are only a few reports on naturally exposed cattle, in which positive T. gondii bioassays indicated viable infections (Arias et al., 1994; Catar et al., 1969; de Macedo et al., 2012; Dubey, 1992; Jacobs et al., 1960). This is in a sharp contrast to the findings in small ruminants as discussed above.
With the advent of new methodologies, i.e. genome detection by PCR, a number of studies utilizing these techniques yielded very high proportions (up to 10 or 20%) of T. gondii genome positive samples in cattle tissues (Amdouni et al., 2017; Azizi et al., 2014; Berger-Schoch et al., 2011; Campo-Portacio et al., 2014; Ergin et al., 2009; Hosein et al., 2016; Mahami-Oskouei et al., 2017; Opsteegh et al., 2011; Rahdar et al., 2012; Wyss et al., 2000). Keeping in mind the failure of many large-scale studies to find viable T. gondii in bovine tissues, the validity of these reports on the detection of T. gondii genome fragments has to be questioned. Detection of genome fragments of T. gondii in absence of positive bioassays should not be regarded as conclusive.
A recent meta-analysis revealed the possibility of geographic differences in the proportion of T. gondii-positive cattle. A significantly higher proportion of positive cattle was found in Central America as compared to North America (Belluco et al., 2016). This may indicate that the susceptibility of cattle to T. gondii is influenced by the genotype of the parasite, which largely varies in different regions of the world (Bertranpetit et al., 2017; Chaichan et al., 2017; Shwab et al., 2014). However, these considerations are hypothetical and need to be addressed in future studies. In addition, differences in husbandry conditions, hygienic situations, in climate and in other factors may affect the extent, to which cattle from different regions are exposed to T. gondii. Therefore, there is a need to confirm the detection of T. gondii genome positive samples in cattle by additional experiments, thus assessing the presence of viable parasites.
Over the past decades, numerous articles have been published on the seroprevalence of T. gondii-specific antibodies in taurine cattle. Many of these publications have been reviewed before, with a global scope (Dubey, 1986a, Dubey, 2010b; Tenter et al., 2000) or, more recently, by focusing on the situation in particular regions of the world like China (Deng et al., 2018), South Asia (Khan et al., 2017) and Africa (Tonouhewa et al., 2017). Overall, theses summaries show a large variation in the reported proportions of positive findings and the summarizing estimates were 9.5% for cattle in China (Deng et al., 2018), 27.9% in South Asia (Khan et al., 2017) or 12% in Africa (Tonouhewa et al., 2017).
2.4.2. Possible routes of infection in cattle
It is generally accepted that most cattle become infected orally, through ingestion of feed or water contaminated with T. gondii oocysts. Many experimental infections in cattle, especially earlier ones, used oocysts as the inoculum, thus demonstrating that cattle are susceptible to this infective stage (Burrells et al., 2018; Costa et al., 2011; de Oliveira et al., 2001; Dubey and Thulliez, 1993; Esteban-Redondo et al., 1999; Stalheim et al., 1980). However, usually large numbers of oocysts were administered, but we are not aware of any experiments that aimed at establishing the minimum infective dose for cattle.
There are also reports on bovine infections with viable T. gondii caused by oral inoculation with T. gondii tissue cysts (103) (Costa et al., 2011) or brains of chronically infected mice (Rommel et al., 1966). Although cattle are herbivores, infections through accidental ingestion of tissue cysts may occur, i.e. if cattle feed is contaminated with fresh tissue of an infected intermediate host.
In infection with Neospora caninum, an apicomplexan parasite closely related to T. gondii, vertical transmission after acute or chronic infection is of utmost importance in cattle (Dubey et al., 2017). However, for T. gondii the situation seems to be different. Although there are reports on the detection of T. gondii genome fragments in aborted bovine fetuses (Ellis, 1998; Gottstein et al., 1998), the isolation of viable T. gondii parasites from bovine fetuses was achieved only occasionally (Canada et al., 2002; Costa et al., 1977) or not at all (Conrad et al., 1993). Experimental inoculations with tachyzoites resulted in abortion or vertical transmission (Stalheim et al., 1980; Wiengcharoen et al., 2011), but the epidemiological significance of these findings is not clear, because the presence of T. gondii in the aborted fetuses was not confirmed. Overall, if vertical transmission of T. gondii naturally occurs in cattle, it seems to be a rare event. However, the large genetic variability between T. gondii populations worldwide should be kept in mind, which may result in a variety of biological traits that may also include differences in virulence in cattle. In the light of this variation, findings in North America and Europe with isolates prevailing in these regions should not be generalized without confirmation.
2.4.3. Disease caused by T. gondii infections in cattle
Reports on clinical toxoplasmosis in naturally infected cattle are rare. This suggests that cattle are resistant to infection and to clinical toxoplasmosis. Although clinical signs and histological alterations were recorded after experimental infection, natural cases of clinical toxoplasmosis in cattle comprised only of abortions in association with the isolation of T. gondii from the aborted fetuses (Canada et al., 2002). It is not clear, however, whether the infection with T. gondii had caused the abortions.
2.4.4. Effects of experimental infections in cattle
After experimental infection, febrile reactions starting 2 days post inoculation at the earliest and lasting up to 15 days p.i. have been regularly reported (Burrells et al., 2018; Costa et al., 1977; Esteban-Redondo et al., 1999; Munday, 1978; Rommel et al., 1966; Stalheim et al., 1980; Wiengcharoen et al., 2011). Bovine infection with T. gondii regularly leads to a parasitemia, which seems to be responsible for the elevated temperatures observed in inoculated animals shortly after infection (de Oliveira et al., 2001; Stalheim et al., 1980). In one study, the parasitemia was even recorded up to 62 days p.i. (Costa et al., 1977). In addition, respiratory distress, nasal discharge, and hyperemia of the conjunctivae were reported in the latter study (Costa et al., 1977).
Reports on mortality in inoculated animals are rare. It occurred in cases of calves inoculated with oocysts or intravenously with tachyzoites, but only in the latter the infection was confirmed (Stalheim et al., 1980). In another experiment, two out of four dams inoculated with T. gondii tachyzoites became recumbent and were euthanized (Wiengcharoen et al., 2011). In this case, adult cattle were affected a long time after inoculation (2 to 3 month p.i.) and this finding represented a surprising exception among a series of experiments, where inoculated cattle developed no or only mild clinical signs (Beverley et al., 1977; Burrells et al., 2018; Costa et al., 1977; Dubey, 1983; Esteban-Redondo et al., 1999; Munday, 1978; Rommel et al., 1966).
2.5. Horses and other equids
2.5.1. Prevalence in horses and other equids
A relatively large number of studies report on the seroprevalence of antibodies against T. gondii in horses, mules and donkeys world-wide. Most but not all of the publications have been reviewed previously (Dubey, 2010b; Dubey et al., 2014b; Tassi, 2007; Tenter et al., 2000). The study results are difficult to compare because different, not always validated serological methods and various cut-offs have been applied. In addition, the equids selected for the studies differed largely in number, age, origin and the purpose for keeping the animals. Currently, there is no reference standard available to validate serological tests in horses properly. A recent attempt to correlate serological test results (i.e. results by MAT and a commercially available ELISA) with those of T. gondii PCR on horse meat samples largely failed (Aroussi et al., 2015). There was almost no correlation between the serological data and T. gondii genome detection using a highly sensitive magnetic capture PCR (Aroussi et al., 2015). Nevertheless, there is no doubt that horses can harbor viable T. gondii, which could be isolated from tissues of both, naturally (Evers et al., 2013; Gennari et al., 2015; Klun et al., 2017; Shaapan and Ghazy, 2007) or experimentally infected animals (Al-Khalidi et al., 1980; Dubey, 1985). The results indicated that viable T. gondii can persist in edible tissues up to 476 days after infection (Dubey, 1985). In addition, imported meat from infected horses was suspected as cause of toxoplasmosis in France (Elbez-Rubinstein et al., 2009; Pomares et al., 2011). A recent example on isolation of viable T. gondii from horse shows that truly infected horse may remain seronegative or develop only a low specific antibody titre in particular serological tests such as the MAT (Klun et al., 2017). Currently, serological responses in equids do not seem to be reliable indicators for viable infections; this is similar to the situation in cattle. Nevertheless, positive antibody responses indicate the exposure of equids to T. gondii and could thus be used to identify risk factors for their exposure to T. gondii. Reported seroprevalence for equids range in South America from 3 to 90% (Cazarotto et al., 2016; Costa et al., 2012; Dangoudoubiyam et al., 2011; de Oliveira et al., 2013; Dubey, 2010b; Evers et al., 2013; Finger et al., 2013; Gennari et al., 2015; Portella et al., 2017; Ribeiro et al., 2016; Tassi, 2007; Venturi et al., 2017), in North America from 0 to 73% (Alvarado-Esquivel et al., 2015; Alvarado-Esquivel et al., 2012b; Dubey, 2010b; Dubey et al., 2014b; James et al., 2017; Schale et al., 2018; Tassi, 2007), in Europe from 0 to 55% (Bartova et al., 2015; Dubey, 2010b; Garcia-Bocanegra et al., 2012; Karatepe et al., 2010; Klun et al., 2017; Kouam et al., 2010; Machacova et al., 2014; Mancianti et al., 2014; Papini et al., 2015; Pastiu et al., 2015; Tassi, 2007; Zhou et al., 2017), in Asia from 0 to 71% (Aharonson-Raz et al., 2015; Alanazi and Alyousif, 2011; Dubey, 2010b; Hajialilo et al., 2010; Lee et al., 2014; Mancianti et al., 2014; Masatani et al., 2016; Matsuo et al., 2014; Miao et al., 2013; Raeghi et al., 2011; Razmi et al., 2016; Saqib et al., 2015; Tassi, 2007; Tavalla et al., 2015; J.L. Wang et al., 2015; Yang et al., 2013), in Africa from 14 to 45% (Ayinmode et al., 2014; Boughattas et al., 2011; Haridy et al., 2010) and 2% in Australia (Tassi, 2007).
2.5.2. Possible routes of infection in horses and other equids
In the case of equids, oral infection by oocysts is the most probable route as it has been confirmed by a number experimental infections using different doses of oocysts (Al-Khalidi et al., 1980; Altan et al., 1977; Dubey, 1985; Marques et al., 1995), i.e. doses of 105 (Al-Khalidi et al., 1980), 104 (Dubey, 1985), 106 (Altan et al., 1977), or up to 1.5 × 105 (Marques et al., 1995).
Rodents are intermediate hosts of T. gondii and regarded as a source of infection especially in omnivorous animals like pigs. Since it has been demonstrated that a large proportion of horses would eat meat and may become infected by Trichinella spiralis via this route (Murrell et al., 2004), it is tempting to speculate that the oral ingestion of carcasses of T. gondii infected rodents or other small intermediate hosts may represent another potential source of infection for equids.
Reports on transplacental T. gondii transmission in experimentally infected mares (Marques et al., 1995) have to be interpreted carefully and need further investigation because infections with other related parasites like Sarcocystis neurona or N. caninum need to be ruled out.
2.5.3. Disease caused by T. gondii infections in horses and other equids
The general view is that toxoplasmosis, i.e. disease caused by T. gondii infection, is rather rare in equids, after both natural and experimental infection (Dubey, 2010b; Tassi, 2007). T. gondii DNA has been detected in the eyes of an aborted foal (Turner and Savva, 1992) and in the placenta of a mare that foaled normally (Turner and Savva, 1990). Together with the transplacental transmission reported in an experimental study, these results may indicate that T. gondii could be occasionally involved in equine abortion (Marques et al., 1995), but further studies are necessary to clarify this issue. It was recently discussed if T. gondii is involved in equine protozoal myeloencephalitis (EPM) (James et al., 2017; Schale et al., 2018). A case control study conducted in California found an association between high levels of T. gondii IFAT titres (1:≥160 or 1:≥320) and the presence of neurologic signs compatible with EPM (James et al., 2017). Another study, not designed as a case-control study but thoroughly assessing EPM cases, revealed only low proportions of T. gondii (and also Neospora sp.) positive animals in this group of patients, contradicting an involvement of T. gondii in EPM. In this study, S. neurona was identified as the most probable cause of EPM.
An earlier study conducted in the UK reported on the presence of T. gondii DNA in the eye of a pony (Turner and Savva, 1991). The significance of this finding and the involvement of T. gondii as a possible cause of blindness in horse are unknown. However, after experimental infection of a pony with T. gondii, the infection was also observed in the eye (Dubey, 1985).
2.5.4. Effects of experimental infections in horses
In equids experimentally inoculated with large numbers of oocysts (104), mild fever was observed in few animals, while the others remained clinically normal (Dubey, 1985; Dubey and Desmonts, 1987). In ponies orally inoculated with a high number of oocysts and in addition immunosuppressed by corticosteroid, 8 out of 9 ponies developed fever between days 2 and 15 p.i. (Al-Khalidi et al., 1980). In an earlier study, ponies, not immunosuppressed but orally inoculated by 106 oocysts did not develop clinical signs (Altan et al., 1977). Horses inoculated intravenously with tachyzoites (i.e. 3.28 × 107 or 2.19 × 107, strain RH) developed fever between 4 to 8 days after inoculation and ocular discharge from 10 to 26 days post inoculation (Sposito Filha et al., 1992).
3. Potential risk factors for infection in livestock
Raw and undercooked meat are regarded as one of the main sources of T. gondii infections for humans. Knowledge on risk factors for the infection with T. gondii in livestock and an assessment of the importance of these risk factors is essential to ensure safe food and intervene effectively. This section is partially based on previous reviews (Guo et al., 2015; Opsteegh et al., 2016) and was extended including the most recent reports in the field. We present a brief overview on the existing literature, but no deeper meta-analysis. We chose to categorize the literature data on individual risk factors only into “statistically significant” and “not statistically significant”, not discriminating between the statistical testing methods used. Mainly reports were included, in which risk analysis was based on the seropositivity of livestock animals. In addition, the review was restricted to reports on the main livestock species, i.e. pigs, small ruminants (sheep and goats), cattle, equids (horses, donkey and mules) and poultry (chickens, ducks and geese).
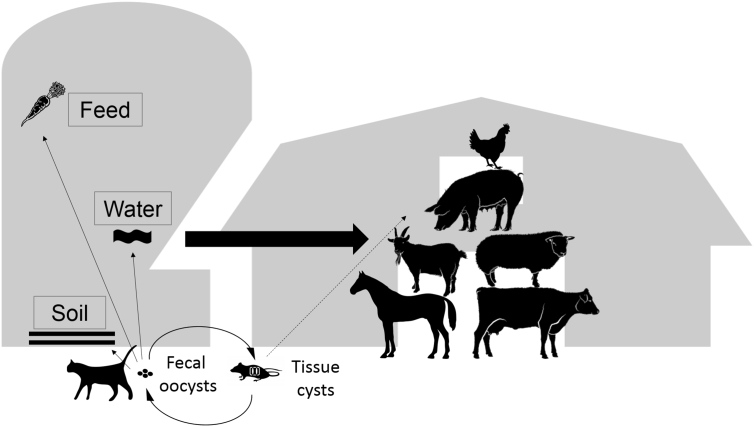
To identify specific risk factors for infection, it is crucial to know the most important routes, by which livestock can acquire the infection. These routes include oocyst contaminations of feed, water or the environment, and the ingestion of tissues of infected intermediate hosts like rodents (Fig. 1).
Fig. 1.
Toxoplasma gondii life cycle highlighting conditions of horizontal transmission concerning livestock infection.
The risk of infection and particularly of infection routes on individual farms (Fig. 1) are influenced by several indirectly acting factors. They include factors related to particular rearing systems of specific livestock groups. There are no general rules to define or to assess such risk factors, which makes a comparison of the results between studies difficult or even impossible. Although the spectrum of risk factors reported in the literature is very heterogenic, we tried to categorize them as much as possible to come to some more general conclusions. In our analysis, we looked for the basic, overarching factors and stratified the records by livestock species. If both, univariable and multivariable statistical analyses had been reported for a specific factor, we included only the results of multivariable statistical analyses, i.e. the results of analyses, in which the specific factor had been examined together with at least one other factor. Because almost all of the studies included were retrospective studies it has to be kept in mind that associations or statistical effects of factors identified by this type of studies are not entirely conclusive and only allow the generation of hypotheses.
3.1. General factors
3.1.1. Age
The association of age with the risk of a T. gondii infection has been examined in a number of studies (Table 3). Generally, the wide spread of T. gondii and the extremely broad host spectrum of the parasite leads to a time of exposure to the infective stages of the parasite that is proportional to the age of the animal. The age of the animals often depends on the production type, i.e. meat-producing animals are usually slaughtered at younger age, whereas animals reared for dairy production, reproduction or recreational purposes often live much longer. Overall, literature data confirm the expected association with age, i.e. in most studies age appeared as a risk factor for infection with T. gondii (Table 3). Due to its general importance, the factor “age” should be always included as one of the explanatory variables, if risk factor analyses on postnatal infection among animals with varying age are performed. Age is prone to act as a confounder or effect modifier in statistical analyses (Schares et al., 2017a).
Table 3.
The effect of age on the seropositivity for T. gondii in livestock species.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Higher age | Pigs | 17, 20, 25, 34, 33, 39, 53, 57 (p), 75, 80, 86, 94 | 6, 28, 73, 96 |
| Sheep | 13, 15, 23, 35, 36, 41, 42, 45, 46, 50, 57, 70, 85, 88, 90 | 7, 68, 72, 92, 95, 98 | |
| Goats | 3, 8, 19 (p), 24, 30, 35, 47, 57, 68, 88 | 92, 95 | |
| Cattle | – | 79, 95, 99 | |
| Equids | 102, 104 | 5, 11, 31, 40, 54, 100, 101, 103 | |
| Chicken | 4, 93 | – | |
| Finishing period | Pigs | 75 | – |
| Age below 12 months | Sheep | 16 (p) | – |
| Younger age | Cattle | 38, 79 | 53 |
| Age <24 months | Cattle | 48 (p) | – |
| Age >24 months–96 months + >120 months (dairy and mixed dairy) | Cattle | 48 | – |
| Age >48 months–72 months (beef and mixed beef) | Cattle | 48 | – |
(p) = protective factor; coding of references: 3 = Alvarado-Esquivel et al. (2011), 4 = Alvarado-Esquivel et al. (2012a), 5 = Alvarado-Esquivel et al. (2012b), 6 = Alvarado-Esquivel et al. (2014), 7 = Alvarado-Esquivel et al. (2013a), 8 = Alvarado-Esquivel et al. (2013b), 11 = Cazarotto et al. (2016), 13 = Andrade et al. (2013), 15 = Cosendey-KezenLeite et al. (2014), 16 = D'Alencar Mendonca et al. (2013), 17 = Damriyasa et al. (2004), 19 = de Moura et al. (2016), 20 = de Sousa et al. (2014), 23 = Deksne et al. (2017), 24 = Deng et al. (2016), 25 = Djokic et al. (2016), 28 = Esteves et al. (2014), 30 = Garcia et al. (2012), 31 = Garcia-Bocanegra et al. (2012), 33 = Garcia-Bocanegra et al. (2010a), 34 = Garcia-Bocanegra et al. (2010b), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 38 = Gilot-Fromont et al. (2009), 39 = Goerlich (2011), 40 = Guerra et al. (2018), 41 = Guimaraes et al. (2013), 42 = Hammond-Aryee et al. (2015), 45 = Holec-Gasior et al. (2015), 46 = Hutchinson et al. (2011), 47 = Iovu et al. (2012), 48 = Jokelainen et al. (2017), 50 = Katzer et al. (2011), 53 = Klun et al. (2006), 54 = Kouam et al. (2010), 57 = A.P. Lopes et al. (2013), 68 = Rego et al. (2016), 70 = Romanelli et al. (2007), 72 = Sakata et al. (2012), 73 = Santoro et al. (2017), 75 = Schulzig and Fehlhaber (2005), 79 = Tan et al. (2015), 80 = Tao et al. (2011), 85 = Vesco et al. (2007), 86 = Villari et al. (2009), 88 = Xu et al. (2015), 90 = Zhang et al. (2016), 92 = Zou et al. (2015), 93 = Schares et al. (2017a), 94 = Samico-Fernandes et al. (2017), 95 = Tilahun et al. (2018), 96 = Bawm et al. (2016), 98 = Yin et al. (2015), 99 = Schoonman et al. (2010), 101 = Bartova et al. (2017), 102 = Machacova et al. (2014), 103 = Miao et al. (2013), 104 = Ribeiro et al. (2016).
3.1.2. Gender
The gender of livestock animals as a putative risk factor has been studied only occasionally (Table 4). Experimental studies in mice and guinea pigs showed a higher susceptibility of females to infection with T. gondii (Kittas and Henry, 1979, Kittas and Henry, 1980; Roberts et al., 1995; Roberts et al., 2001). In most of the published studies on livestock recorded, a significant effect for female animals to be serologically positive for T. gondii was not detected. Nevertheless, with the exception of two studies, in which males showed an increased risk, females were more frequently seropositive in a few studies conducted with pigs, sheep and goats or equids (Table 4). Whether these apparent associations were in fact related to gender or to other underlying factors, e.g. the way animals of different gender are reared, needs to be questioned. It must be noted that gender frequently shows up as a confounder in epidemiological studies because “gender” may mask these underlying factors (Thrusfield, 2007).
Table 4.
The effect of female gender on the seropositivity for T. gondii in livestock species.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Female gender | Pigs | 73 | 6, 20, 28, 43, 53, 86, 94 |
| Sheep | 15 (p), 36, 45 (p), 68, 95 | 7, 23, 35, 41, 59, 72, 81, 88, 90, 92, 98 | |
| Goats | 19, 30, 68 | 8, 35, 81, 88, 92, 96 | |
| Cattle | – | 21, 53 | |
| Equids | 102, 103 | 5, 11, 31, 40, 54, 100, 101 |
(p) = protective factor; coding of references: 5 = Alvarado-Esquivel et al. (2012b), 6 = Alvarado-Esquivel et al. (2014), 7 = Alvarado-Esquivel et al. (2013a), 8 = Alvarado-Esquivel et al. (2013b), 11 = Cazarotto et al. (2016), 15 = Cosendey-KezenLeite et al. (2014), 19 = de Moura et al. (2016), 20 = de Sousa et al. (2014), 21 = de Souza et al. (2016), 23 = Deksne et al. (2017), 28 = Esteves et al. (2014), 30 = Garcia et al. (2012), 31 = Garcia-Bocanegra et al. (2012), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 40 = Guerra et al. (2018), 41 = Guimaraes et al. (2013), 43 = Herrero et al. (2016), 45 = Holec-Gasior et al. (2015), 53 = Klun et al. (2006), 54 = Kouam et al. (2010), 59 = Magalhaes et al. (2016), 68 = Rego et al. (2016), 72 = Sakata et al. (2012), 73 = Santoro et al. (2017); 81 = Tzanidakis et al. (2012), 86 = Villari et al. (2009), 88 = Xu et al. (2015), 90 = Zhang et al. (2016), 92 = Zou et al. (2015), 94 = Samico-Fernandes et al. (2017), 95 = Tilahun et al. (2018), 96 = Bawm et al. (2016), 98 = Yin et al. (2015), 100 = Almeida et al. (2017), 101 = Bartova et al. (2017), 102 = Machacova et al. (2014), 103 = Miao et al. (2013).
3.1.3. Geographic and regional characteristics
For all species taken into consideration in this review, there were studies reporting on significant differences in seroprevalence with respect to regions or geographic characteristics of the farm locations (Table 5). Many region- and geography-related variables that could possibly affect the survival and presence of T. gondii or the exposure of animals to the parasite must therefore be taken into account here. Few studies not only evaluated the differences between certain regions, but also looked into more details concerning the most likely underlying variables, such as mean temperatures, mean rainfall, humidity, altitude or terrain characteristics (Table 5). Most of them found a statistically significant influence of these parameters on the proportion of seropositivity in livestock. Consequently, it is important to take regional differences into consideration but the underlying true effectors such as climatic factors or variables related to differences in animal husbandry need to be included.
Table 5.
The effect of geographic parameters on the seropositivity for T. gondii in livestock species.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Region, province, municipality, prefecture or district | Pigs | 17, 20, 22, 53, 67 | 6 |
| Sheep | 15, 32, 35, 45, 53, 50, 59, 69, 83, 90, 92, 98, 106 | 23, 81, 88, 95 | |
| Goats | 8, 19, 26, 32, 35, 47, 92 | 81, 88, 95, 107 | |
| Cattle | 32, 48, 53, 95 | 78, 79 | |
| Equids | 40, 54, 101 | 31 | |
| Chicken | 4 | 89 | |
| Altitude | Pigs | 6, 86 | – |
| Sheep | 2, 7, 35, 36, 49, 77 | 14, 81 | |
| Goats | 8, 49 | 35, 81 | |
| Mean monthly temperatures | Pigs | 34 | 6 |
| Sheep | 7 | – | |
| Mean annual rainfall | Pigs | 34 | 6 |
| Sheep | 7 | – | |
| Climate | Pigs | 6 | – |
| Goats | 8 | – | |
| Relative humidity | Pigs | 34 | – |
| Hills relative to plains | Pigs | – | 80 |
| Generalized land cover | Sheep | 49 | – |
| Goats | 49 | – | |
| Distance to next village | Sheep | – | 81 |
| Goats | – | 81 | |
| Rural environment relative to urban environment | Sheep | – | 95 |
| Goats | – | 95 | |
| Cattle | – | 95 | |
| Equids | 5 | – | |
| Terrain waterlogged (versus rough and flat) | Sheep | 69 | – |
| Semi-desert | Goats | 3 | – |
Coding of references: 2 = Alvarado-Esquivel et al. (2013a), 3 = Alvarado-Esquivel et al. (2011), 4 = Alvarado-Esquivel et al. (2012a), 5 = Alvarado-Esquivel et al. (2012b), 6 = Alvarado-Esquivel et al. (2014), 7 = Alvarado-Esquivel et al. (2013b), 8 = Alvarado-Esquivel et al. (2013c), 14 = Condoleo et al. (2016), 15 = Cosendey-KezenLeite et al. (2014), 17 = Damriyasa et al. (2004), 19 = de Moura et al. (2016), 20 = de Sousa et al. (2014), 22 = Deksne and Kirjusina (2013), 23 = Deksne et al. (2017), 26 = Djokic et al. (2014), 31 = Garcia-Bocanegra et al. (2012), 32 = Garcia-Bocanegra et al. (2013), 34 = Garcia-Bocanegra et al. (2010b), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 40 = Guerra et al. (2018), 45 = Holec-Gasior et al. (2015), 47 = Iovu et al. (2012), 48 = Jokelainen et al. (2017), 49 = Kantzoura et al. (2013), 50 = Katzer et al. (2011), 53 = Klun et al. (2006), 54 = Kouam et al. (2010), 59 = Magalhaes et al. (2016), 67 = Pastiu et al. (2013), 69 = Rizzo et al. (2017), 77 = Skjerve et al. (1998), 78 = Sun et al. (2015), 79 = Tan et al. (2015), 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 83 = Verhelst et al. (2014), 86 = Villari et al. (2009), 88 = Xu et al. (2015), 90 = Zhang et al. (2016), 92 = Zou et al. (2015), 95 = Tilahun et al. (2018), 98 = Yin et al. (2015), 101 = Bartova et al. (2017), 106 = Jokelainen et al. (2010), 107 = Shuralev et al. (2018).
3.1.4. Farm management
3.1.4.1. Production system
The production systems, in which livestock is kept, may have a major impact on the risk for T. gondii infection (Table 6). However, the association of seropositivity in livestock within a particular production system provides no clear picture on the routes by which the animals became infected. Production systems are often related to specific conditions under which the animals are reared, fed or provided with water. These conditions may influence the likelihood of a contamination of feed, water or farmland with oocysts of T. gondii and the possibility of contact with the matrices mentioned above or with other infected intermediate hosts, e.g. rodents. In an intensive production system for example, the level of confinement is very high, at least for the respective livestock species, and thus, exposure of the animals to infective stages of the parasite is presumably lower as compared to extensive or other production system, where the animals have access to outdoor facilities. Intensive farming usually requires storing supplements. If these materials are stored open or in places where they may attract rodents or cats, additional routes of transmission may become relevant. Contaminated supplements may represent one explanation why intensive farming was not associated with a protective statistical effect in some studies (Table 6).
Table 6.
Production system as a putative risk factor for T. gondii seropositivity in livestock.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Intensive | Pigs | 9 (p), 20 (p), 28, 37 (p), 51 (p), 75 (p), 82 (p) | 94 |
| Sheep | 15 (p), 35 (p), 61, 69 (p), 81, 88 (p), 90 (p) | 18, 57 | |
| Goats | 35 (p), 61, 68 (p), 81 | – | |
| Semi-intensive | Sheep | 2, 35 (p), 68, 69, 81 | 36 |
| Goats | 30, 35, 81 | – | |
| Extensive/animal friendly/organic/transhumance | Pigs | 22, 51, 105 | 94 |
| Cattle | 59, 78 | 21, 97 | |
| Sheep | 35, 58, 68, 88 | 14, 56 | |
| Goats | 35 (p), 68, 88 | – | |
| Backyard | Pigs | 67 | – |
| Goats | 47 | – | |
| Chicken | 4, 89, 91 | – |
(p) = protective factor; coding of references: 2 = Alvarado-Esquivel et al. (2013a), 4 = Alvarado-Esquivel et al. (2012a), 9 = Assadi-Rad et al. (1995), 14 = Condoleo et al. (2016), 15 = Cosendey-KezenLeite et al. (2014), 18 = de Barros Correia et al. (2015), 20 = de Sousa et al. (2014), 21 = de Souza et al. (2016), 22 = Deksne and Kirjusina (2013), 28 = Esteves et al. (2014), 30 = Garcia et al. (2012), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 37 = Gebreyes et al. (2008), 47 = Iovu et al. (2012), 51 = Kijlstra et al. (2004), 56 = Liu et al. (2015), 57 = Lopes et al., 2013, Lopes et al., 2013, 58 = Lopes et al. (2010), 59 = Magalhaes et al. (2016), 61 = Mainar et al. (1996), 67 = Pastiu et al. (2013), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 75 = Schulzig and Fehlhaber (2005), 78 = Sun et al. (2015), 81 = Tzanidakis et al. (2012), 82 = van der Giessen et al. (2007), 86 = Villari et al. (2009), 88 = Xu et al. (2015), 89 = Xu et al. (2012), 90 = Zhang et al. (2016), 91 = Zhu et al. (2008), 94 = Samico-Fernandes et al. (2017), 97 = Fajardo et al. (2013), 105 = Wallander et al. (2016).
3.1.4.2. Specific farming conditions
It was observed in several studies that differences in farming conditions, which are often livestock species-specific, were statistically significantly associated with differences in the risk of infection with T. gondii for various livestock species (Table 7). However, many of these associations are difficult to explain. Like production systems, livestock species-specific farming conditions may influence the probability of contamination of feed, water or farmland with T. gondii oocysts. This holds also true for other potential sources of exposure to T. gondii, e.g. the presence of infected intermediate hosts such as rodents. Specific conditions related to more extensive farming may also represent risk factors for seropositivity in livestock (Table 7).
Table 7.
Livestock species-specific parameters as putative risk factors for T. gondii seropositivity.
| Species | Factor | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Complete production cycle performed on farm (farrow-to-finish) | 17 (p), 34, 55 (p), 86 (p) | – |
| Only part of production cycle performed on farm | 17, 53, 55 | – | |
| Origin of replacement sows, Source of pigs (own farm versus outside) | – | 17, 55 | |
| Cattle | Mixed farming | – | 78 |
| Beef farm (relative to dairy and mixed) | 48 (p) | 32, 78 | |
| Feeder/stocker/backgrounder (versus feeder/stocker) | 21 | – | |
| Sheep | Purpose subsistence (versus breeding/rebreeding/fattening) | 69 | – |
| Purpose (meat, milk, mixed) | – | 68 | |
| Mixed (milk, meat) | 14 | – | |
| Goats | Additional uses to dairy | 26 | – |
| Purpose meat (versus genetic enhancement) | 68 | – | |
| Purpose (milk/meat/mix) | – | 19, 68 | |
| Chicken | Breeders | 89 | – |
| Layers | 89 | – | |
| Broilers | 89 (p) | – | |
| Equids | Racing | – | 54 |
| Recreation | – | 54 | |
| Farming | 54 | – | |
| Use (breeding versus ceremonial, research or sports)a | 101 | – |
(p) = protective factor; coding of references: 14 = Condoleo et al. (2016), 17 = Damriyasa et al. (2004), 19 = de Moura et al. (2016), 21 = de Souza et al. (2016), 26 = Djokic et al. (2014), 32 = Garcia-Bocanegra et al. (2013), 34 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 48 = Jokelainen et al. (2017), 53 = Klun et al. (2006), 54 = Kouam et al. (2010), 55 = Limon et al. (2017), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 78 = Sun et al. (2015), 80 = Tao et al. (2011), 86 = Villari et al. (2009), 89 = Xu et al. (2012), 101 = Bartova et al. (2017).
Statistics not conclusive.
3.1.4.3. Herd and flock size
Herd or flock size is also related to the management and production system. Larger herds are more likely to be intensively managed. It can be assumed that farms with smaller herds often have a lower level of specialization and might be less confined. As farms applying animal welfare-oriented production techniques need more space to rear animals, this may restrict the size of the herd or flock. Again, herd or flock size may have an often un-explained link to conditions that can influence the probability of exposure of livestock to T. gondii due to contamination of feed, water or farmland with oocysts and contact to other infected intermediate hosts, e.g. rodents. There is a clear tendency showing that the smaller the herd or flock, the higher is the chance of seropositivity (Table 8).
Table 8.
The effect of herd size on the seropositivity for T. gondii in livestock species.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Herd/flock size (numbers of animals as continuous variable) | Pigs | – | 73 |
| Sheep | 35, 46, 81 | 14, 32, 45, 56 | |
| Goats | 35 (p), 81 (p) | 32, 56 | |
| Cattle | – | 78 | |
| Equids | 5 | – | |
| Small herd/flock size | Pigs | 9, 25, 43, 55, 65, 86 | |
| Sheep | 12, 36, 76, 88 | 95 | |
| Goats | 49, 88 | 95 | |
| Cattle | 38, 53, 95, 99 (p) | ||
| Chicken | 93 | ||
| Equids | 104 | 102 | |
| Large flock size | Sheep | 1, 18, 23 | – |
| Goats | 1 | ||
| Higher number of sows | Pigs | 25 | – |
| Increased breeding density | Pigs | 80 | – |
| Dairy herds with a size ≥40–105 and >384 animals versus herds with a size <40 animals | Cattle | 48 | – |
| Mixed beef herd with a size of <50–200 animals versus herds with a size of >400 animals | Cattle | 48 | – |
| Flock size 150–300 versus >300 animals | Sheep | 49 (p) | – |
| Low animal density in herd | Cattle | 32 | – |
(p) = protective factor; coding of references: 1 = Abu-Dalbouh et al. (2012), 5 = Alvarado-Esquivel et al. (2012b), 9 = Assadi-Rad et al. (1995), 12 = Cenci-Goga et al. (2013), 14 = Condoleo et al. (2016), 18 = de Barros Correia et al. (2015), 23 = Deksne et al. (2017), 25 = Djokic et al. (2016), 32 = Garcia-Bocanegra et al. (2013), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 38 = Gilot-Fromont et al. (2009), 43 = Herrero et al. (2016), 45 = Holec-Gasior et al. (2015), 46 = Hutchinson et al. (2011), 48 = Jokelainen et al. (2017), 49 = Kantzoura et al. (2013), 53 = Klun et al. (2006), 55 = Limon et al. (2017), 56 = Liu et al. (2015), 65 = Ortega-Pacheco et al. (2013), 73 = Santoro et al. (2017), 76 = Sechi et al. (2013), 78 = Sun et al. (2015), 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 86 = Villari et al. (2009), 88 = Xu et al. (2015), 93 = Schares et al. (2017a), 95 = Tilahun et al. (2018), 99 = Schoonman et al. (2010), 102 = Machacova et al. (2014), 104 = Ribeiro et al. (2016).
3.1.4.4. Presence of other animal species and contact to other herds
The presence of several animal species on a farm or other animals kept close to the livestock species may serve as an indicator of low farming intensity. Even contact to or mixing with other herds (of the same species, or from other farms) was established as a risk factor. As already discussed, low intensity was often related to a higher risk of T. gondii seropositivity (Table 6). The overall tendency that presence of or contact to other animal species may pose a risk for livestock to be T. gondii-seropositive (Table 9), could therefore be explained by the specific conditions in low-intensive farming. These conditions may be associated with an increased risk of exposure of livestock to T. gondii (e.g. through contamination of feed, water or farmland with oocysts and contact to rodents). On the other hand, the presence of other animal species could also have a direct effect on the risk of infection for livestock animals, as they may represent reservoirs for T. gondii and can thus be involved in the establishment of an on-farm multiplication of T. gondii (Table 9).
Table 9.
Parameters related to the presence of other animals (livestock, non-livestock) close to livestock species as putative risk factors for seropositivity to T. gondii.
| Species | Presence of other species, contact with other herds/flocks | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Presence of dairy cattle | – | 6, 25 |
| Presence of poultry | 86 | – | |
| Exposure to wild animals | – | 25 | |
| Other livestock | – | 6, 25, 55 | |
| Presence of dogs | 34, 43, 86 | 6 | |
| Cattle | Presence of sheep/goats/pigs/poultry/canids; number of additional species on farm | 97 | 78 |
| Sheep | Contact with other flocks | 50 | 14 |
| Grazing with other herds | 49 | 18, 81 | |
| Presence of different species | – | 14 | |
| Presence of animals (other than sheep) from other farms | 35 | 14, 18 | |
| Presence of wild animals | – | 14 | |
| Presence of cattle | 22, 46 | 14 | |
| Presence of goats | – | 14, 35 | |
| Presence of dogs | – | 14, 68, 81 | |
| Feeding of dogs with pet food (versus feeding food waste) | 68 | – | |
| Presence of wild dogs | – | 68 | |
| Presence of poultry | 23 (p) | 81 | |
| Presence of pigs | – | 14, 81 | |
| Goats | Common grazing with animals from other herds | 49 | 81 |
| Presence of sheep | 35 | – | |
| Presence of dogs | – | 81 | |
| Number of dogs ≤2 | 68 | – | |
| Feeding of the dogs with pet food/leftovers | – | 68 | |
| Dogs have access to water | 68 (p) | – | |
| Dogs have access to facilities | – | 68 | |
| Presence of poultry | – | 81 | |
| Presence of pigs | – | 81 | |
| Presence of wild dogs | – | 68 | |
| Presence of other species | 35, 96 | – | |
| Solely goats on the farm | 26 | 24 | |
| Chicken | Presence of sheep | 71 | – |
| Birth of sheep on property | 71 | – | |
| Reproductive disorders in sheep | 71 | – | |
| Equids | Presence of domestic ruminants | 31 | 102 |
(p) = protective factor; coding of references: 6 = Alvarado-Esquivel et al. (2014), 14 = Condoleo et al. (2016), 18 = de Barros Correia et al. (2015), 23 = Deksne et al. (2017), 24 = Deng et al. (2016), 25 = Djokic et al. (2016), 26 = Djokic et al. (2014), 31 = Garcia-Bocanegra et al. (2012), 34 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 35 = Gazzonis et al. (2015), 43 = Herrero et al. (2016), 46 = Hutchinson et al. (2011), 49 = Kantzoura et al. (2013), 50 = Katzer et al. (2011), 55 = Limon et al. (2017), 68 = Rego et al. (2016), 71 = Sa et al. (2017), 78 = Sun et al. (2015), 81 = Tzanidakis et al. (2012), 86 = Villari et al. (2009), 96 = Bawm et al. (2016), 97 = Fajardo et al. (2013), 102 = Machacova et al. (2014).
3.1.4.5. Biosecurity, farm buildings, staff hygiene, animal restocking
Biosecurity, the structural condition of farm buildings, staff hygiene and a restrictive policy of restocking seem to be associated with a lower risk of seropositivity in livestock (Table 10). In most cases, staff hygiene and biosecurity measures may have no direct effect on the exposure of animals to T. gondii, except for e.g. cleaning methods or the floor type (Table 10). However, the implementation of biosecurity and hygiene measures could represent an indirect indicator for the level of confinement under which livestock are reared. A restricted restocking policy by avoiding the purchase of animals from other farms may prevent the accidental incorporation of infected animals into the herd or flock.
Table 10.
Parameters related to biosecurity, structural condition of farm buildings, staff hygiene and restricted animal restocking as putative risk factors for T. gondii seropositivity in livestock.
| Species | Management, biosecurity and staff | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Biosecurity | – | 55 |
| Staff restriction | – | 55 | |
| Low level of staff hygiene | 39 | – | |
| Specialized boots, clothes | – | 25, 43 | |
| Proper maintenance of farm facilities | 43 (p) | – | |
| Control of mosquitos and flies | 34, 80 | 84 | |
| Bird proof nets | 34 (p) | – | |
| Removal of dead animals | 84 (p) | – | |
| Floor type | – | 17, 25, 84 | |
| Farm holdings (one or more sites) | – | 55 | |
| Danish entry | 25 | – | |
| Cattle | Presence of birds in stables | 78 | – |
| Farm neighborhood (isolation) | 38 | – | |
| Work clothes available | – | 78 | |
| Slaughter on property | – | 21 | |
| Cattle introduced from other farms | 78 | – | |
| Dirty floor versus concrete floor | 99 | – | |
| Sheep | State-owned farms (versus private) | 53 | – |
| Commercial (versus family) | 41 | – | |
| Large size | 70, 85 | – | |
| Agriculture is main occupation | 70 | – | |
| Lambing in paddocks or parks | – | 50 | |
| Slatted floor | 16, 68 | – | |
| Presence of pen | – | 41, 95 | |
| Cement | 68 (p) | – | |
| Dirt floor versus concrete floor | 68 | – | |
| Multiple boundaries | 50 | – | |
| Technified rearing | – | 18 | |
| Educational level of farmer | 49 | – | |
| Farm recently created | 61 | – | |
| Replacements during preceding year | 61 | 18 | |
| Use of exchanged or borrowed breeding males | 16 | – | |
| Leaving aborted fetuses on ground | 1 | 81 | |
| Predominantly external replacement | – | 81 | |
| Stocking rate (<1 versus ≥1) | 41 (p) | – | |
| Use of quarantine | 69 (p) | 81 | |
| Frequency of domestic slaughtering | – | 14 | |
| Availability of a special place for parturition | – | 81 | |
| Goats | Pen flooring dirt (to suspended slat, mix, cement) | 68 | – |
| Leaving aborted fetuses on ground | 1 | 81 | |
| Predominantly external replacement | – | 81 | |
| Purchase of spare breeding animals | – | 35 | |
| No replacements during preceding year | 61 | 24 | |
| Use of quarantine | – | 81 | |
| Availability of a special place for parturition | – | 81 | |
| Animals born on farm | 26 | – | |
| Educational level of farmer | 49 | – | |
| Farm recently created | 61 | – | |
| Chicken | Intake of fetal adnexa, fluids and placentas | 71 | – |
| Slaughter of animals on property | 71 | – | |
| Equids | Animals of replenishments from other districts or states | 29 | – |
| Use of studs from other stables | – | 29 | |
| Acquisition of female breeders in the last 5 years | – | 29 | |
| Introduced breeders in the last 5 years | – | 29 | |
| Treatment, cleaning and care area | 104 | – |
(p) = protective factor; coding of references: 1 = Abu-Dalbouh et al. (2012), 14 = Condoleo et al. (2016), 16 = D'Alencar Mendonca et al. (2013), 17 = Damriyasa et al. (2004), 18 = de Barros Correia et al. (2015), 21 = de Souza et al. (2016); 24 = Deng et al. (2016), 25 = Djokic et al. (2016), 26 = Djokic et al. (2014), 29 = Fonseca de Araujo Valenca et al. (2015), 34 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 35 = Gazzonis et al. (2015), 38 = Gilot-Fromont et al. (2009); 39 = Goerlich (2011), 41 = Guimaraes et al. (2013), 43 = Herrero et al. (2016), 49 = Kantzoura et al. (2013), 50 = Katzer et al. (2011), 53 = Klun et al. (2006), 55 = Limon et al. (2017), 61 = Mainar et al. (1996), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 70 = Romanelli et al. (2007), 71 = Sa et al. (2017), 78 = Sun et al. (2015); 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 84 = Veronesi et al. (2011), 85 = Vesco et al. (2007), 95 = Tilahun et al. (2018), 99 = Schoonman et al. (2010), 104 = Ribeiro et al. (2016).
3.1.4.6. Hygiene, cleaning and disinfection measures
Measures of hygiene and regimes of cleaning and disinfection applied at the farms may play an important role in the infection of livestock with T. gondii because cleaning reduces the probability of contamination of the facilities with oocysts and may also reduce exposure to infected intermediate hosts, e.g. rodents. There is a clear tendency that a high hygienic status and the implementation of cleaning and disinfection measures has a protective effect (Table 11).
Table 11.
Parameters related to hygiene, cleaning and disinfection measures as putative risk or protective factors for T. gondii seropositivity in livestock.
| Species | Hygiene, disinfection and cleaning measures | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Manual cleaning | 86 | – |
| Frequency of disinfection | – | 80 | |
| Empty period length (short versus long) | 39 | 25 | |
| All-in/all-out | 34 (p), 39 (p), 84 (p) | 43, 55 | |
| No cleaning and disinfection | 34 | – | |
| Cleaning method (only mechanical) | – | 17 | |
| Only removing manure | 39 | – | |
| Cattle | Cleaning method | – | 78 |
| Sheep | Poor hygiene conditions | 15, 56 | – |
| Hygiene level | – | 81 | |
| Disinfection of installations | 69 (p) | – | |
| Use of dunghill | 69 (p) | – | |
| Feces management | 69 (p) | – | |
| Goats | Feces management | – | 68 |
| Poor hygiene level | 56 | 81 | |
| Equids | No dunghill | 29 | – |
| Chicken | Service period prior to restocking | 93 | – |
(p) = protective factor; coding of references: 15 = Cosendey-KezenLeite et al. (2014), 17 = Damriyasa et al. (2004), 25 = Djokic et al. (2016), 29 = Fonseca de Araujo Valenca et al. (2015), 34 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 39 = Goerlich (2011), 43 = Herrero et al. (2016), 55 = Limon et al. (2017), 56 = Liu et al. (2015), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 78 = Sun et al. (2015), 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 84 = Veronesi et al. (2011), 86 = Villari et al. (2009), 93 = Schares et al. (2017a).
3.1.4.7. Disease and treatment parameters and other factors characterizing herd health and veterinary care
Disease and treatment parameters and other factors characterizing herd health and veterinary care are often unrelated to T. gondii seropositivity in livestock (Table 12). Even when parameters characterizing reproductive problems in small ruminants were assessed, statistically significant associations were hardly observed. However, a few studies that had specifically looked at abortion in general, serial abortions or neonatal mortality, revealed associations to T. gondii seropositivity (Table 12).
Table 12.
Disease and treatment parameters and other factors characterizing herd health and their association with T. gondii seropositivity in livestock.
| Species | Factors | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Deworming | – | 6, 55 |
| Cannibalism | 65 | 43 | |
| Cattle | Number of pregnancies (0 or 1 versus 3 or more pregnancies) | 79 | – |
| Reproductive disorder | – | 32, 21 | |
| Brucellosis status | – | 32 | |
| Veterinary service | – | 78 | |
| Sheep | No veterinary care | 15 | – |
| Vaccination status (multivariable testing including age) | 46 | – | |
| Anthelmintic treatment | 49 (p) | 68 | |
| Treatment with albendazoles (versus salicylanilides and imidazothiazoles) | 49 (p) | – | |
| Previous history of serial abortions | 61 | – | |
| Unusual episodes of neonatal mortality | 61 | – | |
| Proportion of abortions (high versus low) | 18 | 81 | |
| Occurrence of stillbirths | – | 18 | |
| Occurrence of death at weaning | – | 18 | |
| Neurological problems observed | – | 81 | |
| Number of abortion waves per year | – | 81 | |
| Laboratory investigation of causes of abortion | – | 81 | |
| Reproductive disorder | 15 | 32, 69, 81 | |
| Brucellosis status | – | 32 | |
| Goats | Proportion of abortions | – | 81 |
| Neurological problems observed | – | 81 | |
| Number of abortion waves per year | – | 81 | |
| Laboratory investigation of causes of abortion | – | 81 | |
| Reproductive disorder | – | 32, 81 | |
| Brucellosis status | – | 32 | |
| Anthelmintic treatment | 49 | – | |
| Deworming | 68 (p) | – | |
| Previous history of serial abortions | 61 | – | |
| Unusual episodes of neonatal mortality | 61 | – | |
| Equids | Vaccination (tetanus, influenza) | 104 (p) | – |
| Use of embryo transfer | 104 (p) | – |
(p) = protective factor; coding of references: 6 = Alvarado-Esquivel et al. (2014), 15 = Cosendey-KezenLeite et al. (2014), 18 = de Barros Correia et al. (2015), 21 = de Souza et al. (2016); 32 = Garcia-Bocanegra et al. (2013), 43 = Herrero et al. (2016); 46 = Hutchinson et al. (2011), 49 = Kantzoura et al. (2013), 55 = Limon et al. (2017), 61 = Mainar et al. (1996), 65 = Ortega-Pacheco et al. (2013); 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 78 = Sun et al. (2015); 79 = Tan et al. (2015); 81 = Tzanidakis et al. (2012), 104 = Ribeiro et al. (2016).
3.2. Factors related to the T. gondii life cycle
3.2.1. Definitive hosts (cats)
Cats as definitive hosts of T. gondii may shed oocysts with their feces. Excreted oocysts are environmentally resistant and become infective after a short period of sporulation. Thus, a large number of on-farm studies assessed cat-related factors in relation to seropositivity in livestock animals (Table 13). Surprisingly, about half of the studies which considered the presence of cats on the farms failed to find an association with seropositivity regarding T. gondii, especially in small ruminants, poultry and equids (Table 13). In addition, cat-related factors had a protective statistical effect in some investigations (Table 13). This shows that not just the presence of cats, but more specifically the realistic chance of cats to contaminate farmland, feed or water provided to livestock needs to be examined. A study in 12 pig farms in China indicated for example that the seroprevalence in pigs was higher in farms with a high cat density and with high soil contamination with T. gondii oocysts (as determined by PCR and loop-mediated isothermal amplification (LAMP)) than in those with low cat density (Du et al., 2012). If only a small number of pigs in the herd were infected, ingestion of tissue cysts, present in accidentally eaten intermediate hosts (e.g. birds or rodents), need to be taken into account as a potential source of infection. Although some research on serological methods to detect the source of infection (oocysts vs. cysts) in animals was reported (Hill et al., 2011; Munoz-Zanzi et al., 2012), such tools are hardly available or validated for epidemiological studies, unfortunately.
Table 13.
Cat-related parameters as putative risk factors for T. gondii seropositivity in livestock.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Presence of cats on farm | Pigs | 9, 20, 27, 33, 34, 39, 43, 65, 87 | 6, 17, 55, 84, 86, 94 |
| Cattle | 38, 59, 78 | 21, 32, 38, 99 | |
| Sheep | 1, 13, 16, 56, 58, 61, 77, 85, 90 | 14, 15, 32, 35, 36, 41, 72, 81 | |
| Goats | 1, 61, 24, 56, 96 | 32, 35, 81 | |
| Chicken | 60, 93 | 71 | |
| Equids | 102 | 11, 31, 40, 29 | |
| Number of cats on farm (as continuous variable or >2–3 cats/>2/>3) | Pigs | 27, 39, 63, 65 | – |
| Cattle | 59, 97 | – | |
| Sheep | 41, 68 | – | |
| Goats | 24 | 68 | |
| Chicken | 60 | – | |
| Contact/exposure to cats or cat feces (plus frequency of exposure) | Pigs | 63, 80 | 55 |
| Sheep | 59 | 95 | |
| Goats | 19, 30, 68 (p) | 24, 95 | |
| Cattle | – | 21, 95 | |
| Contact of cats with feed | Pigs | 55 | – |
| Cattle | 59 | – | |
| Sheep | 69, 70 | 68 | |
| Goats | 30 | – | |
| Equids | 102 | 29 | |
| Contact of cats with water | Pigs | – | 55 |
| Sheep | 12, 76, 68 | 41, 69 | |
| Goats | 68 (p) | – | |
| Equids | – | 102 | |
| Vaccination of cats on farm | Pigs | 62 (p) | – |
| Detection of oocysts in soil, cat feces, water | Pigs | 27, 87 | – |
| Cat-proof storage of feed supplements | Sheep | – | 81 |
| Goats | 81 | ||
| Cats seen in hay | Sheep | – | 81 |
| Goats | – | 81 | |
| Cats are used for rodent control | Cattle | 97 | – |
| Chicken | 93 | – | |
| Population control of cats | Sheep | 68 (p) | – |
| Goats | – | 68 | |
| Feeding of cats with pet food (versus food waste) | Sheep | 68 | – |
| Goats | – | 68 | |
| Cats feed on placenta remains | Sheep | – | 68 |
| Goats | 68 | – | |
| Presence of wild cats | Sheep | 68 (p) | – |
| Goats | 68 | – | |
| Stray cats or wild felids on farm or farm land | Sheep | 41 | – |
| Occurrence of birth of cats on property | Chicken | – | 71 |
(p) = protective factor; coding of references: 1 = Abu-Dalbouh et al. (2012), 6 = Alvarado-Esquivel et al. (2014), 9 = Assadi-Rad et al. (1995), 11 = Cazarotto et al. (2016), 12 = Cenci-Goga et al. (2013), 13 = Andrade et al. (2013), 14 = Condoleo et al. (2016), 15 = Cosendey-KezenLeite et al. (2014), 16 = D'Alencar Mendonca et al. (2013), 17 = Damriyasa et al. (2004), 19 = de Moura et al. (2016), 20 = de Sousa et al. (2014), 21 = de Souza et al. (2016), 24 = Deng et al. (2016), 27 = Du et al. (2012), 29 = Fonseca de Araujo Valenca et al. (2015), 30 = Garcia et al. (2012), 31 = Garcia-Bocanegra et al. (2012), 32 = Garcia-Bocanegra et al. (2013), 33 = Garcia-Bocanegra et al. (2010a), 34 = Garcia-Bocanegra et al. (2010b), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 38 = Gilot-Fromont et al. (2009), 39 = Goerlich (2011), 40 = Guerra et al. (2018), 41 = Guimaraes et al. (2013), 43 = Herrero et al. (2016), 55 = Limon et al. (2017), 56 = Liu et al. (2015), 58 = Lopes et al. (2010), 59 = Magalhaes et al. (2016), 60 = Magalhães et al. (2016), 61 = Mainar et al. (1996), 62 = Mateus-Pinilla et al. (1999), 63 = Meerburg et al. (2006), 65 = Ortega-Pacheco et al. (2013), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 71 = Sa et al. (2017), 72 = Sakata et al. (2012), 76 = Sechi et al. (2013), 77 = Skjerve et al. (1998), 78 = Sun et al. (2015), 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 84 = Veronesi et al. (2011), 85 = Vesco et al. (2007), 86 = Villari et al. (2009), 87 = Weigel et al. (1995), 90 = Zhang et al. (2016), 93 = Schares et al. (2017a), Samico-Fernandes et al. (2017), 95 = Tilahun et al. (2018), 96 = Bawm et al. (2016), 97 = Fajardo et al. (2013), 99 = Schoonman et al. (2010), 102 = Machacova et al. (2014).
Studies that looked in more detail at the routes, by which cats could expose livestock to T. gondii, detected more frequently statistically significant associations. In addition, the chance to find a significant association was increased, when the number of cats present on a farm was taken into account (Table 13).
3.2.2. Other on-farm intermediate hosts, such as rodents and their control
Rodents like mice and rats are intermediate hosts of T. gondii and may serve as a reservoir for the parasite on-farms. Cats, if allowed to ingest rodents that carry tissue cysts of T. gondii, may become infected and eventually shed oocysts. Omnivorous animals like pigs are at risk of getting infected through rodents. Overall, the recorded studies mainly showed that the presence of rodents and the absence of rodent control pose a risk for livestock to be T. gondii-seropositive (Table 14).
Table 14.
Parameters related to intermediate hosts (rodents) and their control on farms as putative risk factors for T. gondii seropositivity in livestock.
| Factor | Species | Statistically significant | Not statistically significant |
|---|---|---|---|
| Presence of rodents (mice, rats) | Pigs | – | 87 |
| Cattle | 78 | – | |
| Sheep | 15 | – | |
| Access of rodents to feed | Cattle | 59 | – |
| Sheep | 70 | – | |
| No rodent control measures | Pigs | 9, 34, 43, 52, 86 | 51 |
| Sheep | 77 | – | |
| Cats and dogs as a measure of rodent control/cats used for rodent control | Pigs | 39, 44 | – |
| Chickens | 93 | – | |
| Problems with mice and rats | Goats | – | 24 |
Coding of references: 9 = Assadi-Rad et al. (1995), 15 = Cosendey-KezenLeite et al. (2014), 24 = Deng et al. (2016), 34 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 39 = Goerlich (2011), 43 = Herrero et al. (2016), 44 = Hill et al. (2010), 51 = Kijlstra et al. (2004), 52 = Kijlstra et al. (2008), 59 = Magalhaes et al. (2016), 70 = Romanelli et al. (2007), 75 = Schulzig and Fehlhaber (2005), 77 = Skjerve et al. (1998), 78 = Sun et al. (2015), 86 = Villari et al. (2009), 87 = Weigel et al. (1995), 93 = Schares et al. (2017a).
3.2.3. Feed-related parameters
The uptake of infective stages of T. gondii with animal feed represents an important route, by which animals can get infected. Feed-related parameters are also influenced by the production system, which is in place on the farm. The evaluated studies suggest that open or less confined feed storage or feeding area represent an increased risk for exposure of livestock to the parasite (Table 15). In addition, feeding particular materials like goat whey may pose an infection risk as shown for pigs (Table 15). This suggests that goats excrete viable T. gondii stages in their milk, that may remain infective even after the whey has been produced (Table 15).
Table 15.
Feeding-related parameters as putative risk factors for T. gondii seropositivity in livestock.
| Species | Feeding characteristics | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Food storage open | 39 | 55, 65 |
| Food storage in owner's home | 6 | – | |
| Roughage not covered | 63 | – | |
| Manual feeder type (versus automatic feeder) | 39 | 25, 55, 65 | |
| Fluid feed (versus dry feed) | 39 (p) | – | |
| Trough | 39 | – | |
| Feeding human food | 6, 20 | – | |
| Ration, mixed versus human food waste | – | 94 | |
| Feeding goat whey | 63 | – | |
| Cattle | Use of silage | 38 | 53 |
| Raw milk consumption | – | 21 | |
| Sheep | Food storage uncovered | 69 | – |
| Food through covered trough | 69 (p) | – | |
| Feeding concentrate | 81 | – | |
| No mineral supplementation | 58, 70 | 18 | |
| Food source common | – | 41 | |
| Atypical grazing | 77 | – | |
| Goats | Feeding concentrate | 35, 81 | 19 |
| Use of mixer feeder | – | 24 | |
| Use of silo | – | 24 | |
| Chicken | Feeding from ground | 4 | – |
| Poultry feed indoors | 4 (p) | – | |
| Equids | Feed (with or without supplements) | – | 40 |
| Mix of collective and individual troughs | 29 (p) | – | |
| Ration consumption | – | 29 | |
| Ration storage location open | 29 | – | |
| Hay consumption | 29 | – | |
| Hay storage location open | 29 | – |
(p) = protective factor; coding of references: 4 = Alvarado-Esquivel et al. (2012a), 6 = Alvarado-Esquivel et al. (2014), 18 = de Barros Correia et al. (2015), 19 = de Moura et al. (2016), 20 = de Sousa et al. (2014), 21 = de Souza et al. (2016), 24 = Deng et al. (2016), 25 = Djokic et al. (2016), 29 = Fonseca de Araujo Valenca et al. (2015), 35 = Gazzonis et al. (2015), 38 = Gilot-Fromont et al. (2009), 39 = Goerlich (2011), 40 = Guerra et al. (2018), 41 = Guimaraes et al. (2013), 53 = Klun et al. (2006), 55 = Limon et al. (2017), 58 = Lopes et al. (2010), 63 = Meerburg et al. (2006), 65 = Ortega-Pacheco et al. (2013), 69 = Rizzo et al. (2017), 70 = Romanelli et al. (2007), 72 = Sakata et al. (2012), 77 = Skjerve et al. (1998), 81 = Tzanidakis et al. (2012), 94 = Samico-Fernandes et al. (2017).
3.2.4. Water-related parameters
Since oocysts of T. gondii can remain infective in water for a long time (i.e., under optimal conditions several months or even years (Dubey, 2010b)), it is hypothesized that the water supply for livestock may represent a risk factor for infection. Water can be supplied to the animals from a variety of sources such as tap water, wells or surface water and on different ways, which may depend on various factors like the production system or specific regional parameters. It is important to establish whether cats have access to the water at any stage before it reaches the livestock animals. Overall, from the recorded studies it is hard to quantify the risk for infection of the animals through contaminated water (Table 16). Often, the outcomes of the studies are contradicting. For example, well water was associated with an increased risk in some studies, while it seemed to have a protective statistical effect in others (Table 16).
Table 16.
Water-related parameters as putative risk factors for T. gondii seropositivity in livestock.
| Species | Water supply characteristics | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Water supply (various sources assessed) | – | 6, 55, 80, 84, 87 |
| Water from wells (versus municipal water) | 86 | – | |
| Cattle | Water supply from pond or well | 78 | – |
| Water point on pasture | 38 | – | |
| Water from reservoir | 59 | – | |
| Access to surface water | 99 | – | |
| Stagnant/pond water versus mixed water sources (river, stream, well) | 95 | – | |
| Tap water versus mixed water sources (river, stream, well) | 95 | – | |
| Sheep | Use of surface water | 85 | |
| Water from the public supply | 81 | 41 | |
| River water | 36 | 41 | |
| Tap water | 36 | – | |
| Water directly from the source (well) | 15 (p), 16 (p) | 69 | |
| Still water (versus running water) | 13 (p), 76 | 41 | |
| Water from deep well | 15, 16 (p) | – | |
| Water from sluice | 15 | – | |
| Stagnant/pond water versus mixed water sources (river, stream, well) | 95 | – | |
| Tap water versus mixed water sources (river, stream, well) | 95 | – | |
| Location of drinking trough | 68 | – | |
| Dogs and wild dogs have access to water | 68 | – | |
| Water sources in main grazing area | – | 14 | |
| Goats | Location of drinking trough | 68 | |
| Water source (various sources assessed) | 19, 24 | ||
| Water from river | 35 | – | |
| Water from the public supply | 81 | – | |
| Stagnant/pond water versus mixed water sources (river, stream, well) | 95 | – | |
| Tap water versus mixed water sources (river, stream, well) | 95 | – | |
| Chicken | Water source (dam) | 60 | – |
| Equids | Drinking from a mix of individual and collective troughs | 29 (p) | – |
| Water well versus public system | – | 100 | |
| Tank or river/stream versus public system | 100 | – |
(p) = protective factor; coding of references: 6 = Alvarado-Esquivel et al. (2014), 13 = Andrade et al. (2013), 14 = Condoleo et al. (2016), 15 = Cosendey-KezenLeite et al. (2014), 16 = D'Alencar Mendonca et al. (2013), 19 = de Moura et al. (2016), 24 = Deng et al. (2016), 29 = Fonseca de Araujo Valenca et al. (2015), 35 = Gazzonis et al. (2015), 36 = Gebremedhin et al. (2013), 38 = Gilot-Fromont et al. (2009), 41 = Guimaraes et al. (2013), 55 = Limon et al. (2017), 59 = Magalhaes et al. (2016), 60 = Magalhães et al. (2016), 68 = Rego et al. (2016), 69 = Rizzo et al. (2017), 76 = Sechi et al. (2013), 78 = Sun et al. (2015), 80 = Tao et al. (2011), 81 = Tzanidakis et al. (2012), 84 = Veronesi et al. (2011), 85 = Vesco et al. (2007), 86 = Villari et al. (2009), 87 = Weigel et al. (1995), 95 = Tilahun et al. (2018), 99 = Schoonman et al. (2010), 100 = Almeida et al. (2017).
3.2.5. Soil contact, outside access and pasturing
Exposure to contaminated soil or pastures seems to be another important factor (Table 17). Especially in pigs, extensive management or outdoor access seems to increase the chance for animals to get in contact with infective T. gondii stages (Table 17). Both, oocyst contaminations on farmland and, in the case of omnivorous livestock species, tissues of infected intermediate hosts represent likely sources of infection for livestock.
Table 17.
Soil-contact, outside access, pasturing and related parameters as putative risk factors for T. gondii seropositivity in livestock.
| Species | Soil-contact, outside access, pasturing | Statistically significant | Not statistically significant |
|---|---|---|---|
| Pigs | Outdoor facilities | 6, 9, 22, 33, 53, 55 | 43 |
| Detection of oocysts in soil, cat feces, water | 27, 87 | – | |
| Scavenging | 80 | – | |
| Pasture length month | 105 | – | |
| Sheep | Size of the grazing area | – | 14 |
| Frequency of grazing | – | 14 | |
| Pasture | 58 | 18, 41 | |
| Goats | Outdoor access | – | 24 |
| Grazing | 35 | – | |
| Cattle | Access to pasture (relative to stable only), grazing | 53, 99 | – |
| Equids | Shelter (in- or outdoor) | – | 31, 40 |
| Pasture versus stable | 101 | – | |
| Chicken | Size of chicken run per animal (≥10 sqm versus <10 sqm), multivariable analysis including age | 93 | – |
Coding of references: 6 = Alvarado-Esquivel et al. (2014), 9 = Assadi-Rad et al. (1995), 14 = Condoleo et al. (2016), 18 = de Barros Correia et al. (2015), 22 = Deksne and Kirjusina (2013), 24 = Deng et al. (2016), 27 = Du et al. (2012), 31 = Garcia-Bocanegra et al. (2012), 33 = Garcia-Bocanegra et al., 2010a, Garcia-Bocanegra et al., 2010b, 35 = Gazzonis et al. (2015), 40 = Guerra et al. (2018), 41 = Guimaraes et al. (2013), 43 = Herrero et al. (2016), 53 = Klun et al. (2006), 55 = Limon et al. (2017), 58 = Lopes et al. (2010), 80 = Tao et al. (2011); 87 = Weigel et al. (1995), 93 = Schares et al. (2017a), 99 = Schoonman et al. (2010), 101 = Bartova et al. (2017), 105 = Wallander et al. (2016).
4. Economic impact of toxoplasmosis in livestock
In this review, we focused on articles that assessed the costs of T. gondii in livestock animals. It is important to stress, however, that T. gondii infections in animals used for food production may also affect human health and cause costs in this respect. These aspects are very difficult to assess and beyond the scope of the article, but have been addressed by others (Buzby and Roberts, 1997; Todd, 1989).
As summarized in Section 2.2, T. gondii is considered a major cause of reproductive losses in the small ruminant industry worldwide and infections in small ruminants may play a major role in the transmission of the parasite to humans (Belluco et al., 2016; Opsteegh et al., 2016). Especially in China, abortions caused by T. gondii in sows seem to be common and may lead to huge losses (Pan et al., 2017). There is one report on severe clinical signs in a fattening pig farm in China (Li et al., 2010) indicating that toxoplasmosis is of economic importance on these farms.
The number of studies assessing the economic impact of T. gondii infection in livestock is scarce. To estimate the economic consequences of an infection exclusively for the affected livestock species, i.e. leaving out any potential effect on human health, the clinical consequences, their severity and impact on the performance of the animals have to be analyzed. Small ruminants may suffer from a T. gondii infection and the infection can therefore cause economic losses to farmers. Experimentally infected sheep showed a number of clinical signs, which included fever, diarrhea, abortion, stillbirth, and fetal mummification (Dubey, 2009b). There is also one report indicating that T. gondii infected rams may become infertile (Savvulidi et al., 2018). It has also been suggested that T. gondii could be transmitted via semen (de Moraes et al., 2010; W.D. Lopes et al., 2013).
In general, the economic impact of diseases has different aspects that need to be taken into account. Direct costs of a disease (C) include not only losses (L), but also costs for the treatment of animals (T) and costs for disease prevention (P). The first aspect is ‘the value of the loss in expected output and/or of resource wastage due to the disease (L)’ (Bennett et al., 1999). In sheep, for example, lambs, wool, milk and meat represent the main output of a flock. As an example, in the case of a primary infection of sheep with T. gondii, there is a high probability of abortion (Dubey, 2009b) and the loss is therefore at least the value of the newborn lamb. Moreover, in dairy flocks, fever after acute infection, but mainly the occurrence of abortion, is related to the complete or partial loss of milk production for that lactation, i.e. the main source of income for these farms.
‘The costs for treatment of affected animals (T)’ represent the second aspect in an economic analysis (Bennett, 2003; Dijkhuizen and Morris, 1997). In the case of toxoplasmosis, the treatment costs include costs for anti-inflammatory substances to reduce fever or other veterinary services (e.g. removing mummified lambs, treatment of fertility problems after abortion, costs of laboratory diagnosis etc.).
‘The costs associated with specific disease prevention (P)’ form the third aspect in the economic analysis (Bennett et al., 1999). In the case of toxoplasmosis, vaccination of sheep on a regular base may represent such a preventive measure to reduce T. gondii-associated abortion.
There are only two formally published studies on the economic impact of toxoplasmosis in sheep and they refer only to two countries, Great Britain and Uruguay. Both studies focused on the losses that were due to abortion. Freyre et al. (1997), who analyzed the situation in Uruguay, estimated that about 10 million lambs were born in 1993 and that 14,000–400,000 sheep fetuses were aborted due to toxoplasmosis. They assumed a loss of 10 to 12 US $ per fetus, resulting in a loss of 1.4–4.68 million US $. They took neither the retardation of the genetic progress, nor the costs for replacement animals nor for husbandry into consideration.
Bennett and colleagues published several studies on the direct costs associated with endemic diseases of livestock in Great Britain, including toxoplasmosis in sheep (Bennett, 2003; Bennett et al., 1997, Bennett et al., 1999; Bennett and Ijpelaar, 2005). In the first study, referring to the year 1996, annual costs of 12–18 million £ for output losses (L) and <1 million £ for treatment (T), but no costs for prevention (P) were estimated. An incidence of ovine toxoplasmosis in ewes of 1.2 and 2.2% was assumed. In an update of this study, the authors estimated that about 334,000 (range: 136,000–526,000) sheep were affected per year, with disease effects of 9.1 (range: 3.7–14.1) million £ due to abortion or stillbirth and 3.2 (range: 2.7–5.6) million £ for disease control. It was assumed that toxoplasmosis caused 50,000 (range 8000–116,000) severely affected sheep in Great Britain per year. According to a recent ABC News report (https://www.abc.net.au/news/rural/2017-02-07/toxoplasmosis-costs-south-australian-sheep-producers/8245676, assessed on 11/12/2018, 12:32), a study carried out by Ryan O'Handley in South Australia in 2017 estimated toxoplasmosis costs in sheep at 70 million Australian $.
In summary, there are only a few formally published studies that assessed the costs of toxoplasmosis in livestock. They focused on sheep and peer-reviewed reports on this topic are >20 years old.
To improve the assessment of the economic impact of T. gondii on animals, it is necessary to carry out further studies including all aspects of the infection (Perry and Randolph, 1999) and all cost categories that can lead to losses. Information at the country level is scarce. Estimates are often based on studies conducted on (heavily) affected farms. This may lead to an overestimation of the total costs. It also remains unclear, if all recorded abortions have been caused by infection with T. gondii, even if part of the aborted fetuses has tested positive for toxoplasmosis. The lack of information on the effect of potential co-infections, e.g. border disease or bacterial infections that may also cause abortion, represents another factor of uncertainty.
In many publications with statements on costs the parameters included in the cost calculations remain unclear. It is therefore difficult to compare these analyses. Furthermore, they may include only costs directly related to the animals, but often ignore potential consequences on trade, a possible decrease of the value of infected flocks or herds and any potential impact on human health, if products contaminated with infectious stages of T. gondii are placed on the market. Consequently, we suggest that such studies list all parameters in a given livestock production system that might be affected by infection with T. gondii and indicate how they were taken into account in the cost calculations. As example, we provide such information categorized as direct and indirect costs in Table 18. It has to be noted, however, that some factors (e.g. herd management, hygiene) may have a general influence on the herd health status. This will make it hard to allocate any potential costs related to these factors to specific infections, for example with T. gondii.
Table 18.
Factors possibly causing costs in livestock due to infection with T. gondii.
| Type of costs | Costs | Comments |
|---|---|---|
| Direct costs (production losses caused by disease) | Reduced milk yield | Only in dairy sheep and dairy goats |
| Weight loss in infected animals | Caused by fever and inappetence | |
| Reduced fertility | Cause of an increased replacement rate and retardation of the breeding progress | |
| Abortion/stillbirth | Cause of an increased replacement rate, loss of sells (e.g. lamb sells) and in retardation of the breeding progress | |
| Increased mortality | Reduced profit due to loss of animals and increased replacement rate | |
| Prolonged fattening periods in infected animals | Additional costs for feeding and reduced profit | |
| Weak or malformed progeny | Loss of progeny that causes additional costs, e.g. if a caesarian section has to be carried out or animals need veterinary service | |
| Indirect costs (reaction to disease) | Optimization of herd management | Improvements in biosecurity, hygiene and farm buildings |
| Treatment of diseased animals | Cost for veterinarian, drugs, etc. | |
| Control measures | For example vaccination | |
| Monitoring and diagnosis | For example costs for sampling and laboratory testing (also to achieve a differential diagnosis); testing of animals before housing | |
| Slaughter of infected animals | For example in animals with reduced fertility, increase in replacement costs | |
| Impact on trade (both national or international) | For example if meat of infected animals would be excluded from slaughter for specific meat products, or if international trade becomes restricted to avoid introduction of virulent types of T. gondii |
5. Conclusion and future prospects
Although many studies on T. gondii infections and toxoplasmosis in livestock are available in literature, there are still several important gaps in our current knowledge. The routes of infection seem to be clear in herbivores, as oocysts can be presumed to be the main source. Nevertheless, there is uncertainty regarding many aspects, e.g. about the role of contamination of water and pastures and concerning the intensity of farming. In pigs, it seems clear that outdoor access is an important risk factor for T. gondii infection. It is not clear, however, to which extent oocysts contamination or the presence or uptake of infected intermediate hosts such as rodents play a role. Overall, further epidemiological studies are necessary and the standards in these studies need to be raised to obtain a better knowledge and more confidence regarding the epidemiologically relevant routes of T. gondii infection in livestock. In addition, more prospective studies including the assessment of interventions are needed to confirm previous findings and to determine the feasibility and the efficiency of control measures. Furthermore, the economic impact of toxoplasmosis in livestock, e.g. in small ruminants, has never been assessed in most of the regions worldwide, although especially small ruminants are economically important species in many countries. There is a clear need for further assessments of economic consequences of T. gondii infections and toxoplasmosis in livestock.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
We wish to thank the FLI librarians (Viola Damrau, Mandy Nass) who supported our work tremendously. In addition, we thank Rob van Spronsen, Rijksinstituut voor Volksgezondheid en Milieu, Bilthoven, The Netherlands, for supporting our search for relevant literature. Luis-Miguel Ortega-Mora and Julio Benavides are supported by grant AGL2016-75935 from the Spanish Ministry of Science. Sandra Stelzer was funded by JIP1 ORION within the One Health EJP. One Health EJP has received funding from the European Union Horizon 2020 research and innovation program under grant agreement No 773830.
References
- Abu-Dalbouh M.A., Ababneh M.M., Giadinis N.D., Lafi S.Q. Ovine and caprine toxoplasmosis (Toxoplasma gondii) in aborted animals in Jordanian goat and sheep flocks. Trop. Anim. Health Prod. 2012;44:49–54. doi: 10.1007/s11250-011-9885-2. [DOI] [PubMed] [Google Scholar]
- Aharonson-Raz K., Baneth G., Lopes A.P., Brancal H., Schallig H., Cardoso L., Steinman A. Low seroprevalence of Leishmania infantum and Toxoplasma gondii in the horse population in Israel. Vector Borne Zoonotic Dis. 2015;15:726–731. doi: 10.1089/vbz.2015.1826. [DOI] [PubMed] [Google Scholar]
- Ahmed H., Malik A., Arshad M., Mustafa I., Khan M.R., Afzal M.S., Ali S., Mobeen M., Simsek S. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in north-eastern region of Pakistan. Korean J. Parasitol. 2016;54:439–446. doi: 10.3347/kjp.2016.54.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A.D. Determination of seropositivity for Toxoplasma gondii in sheep, goats and camels slaughtered for food and human consumptions in Riyadh municipal abattoirs, Saudi Arabia. J. Egypt. Soc. Parasitol. 2013;43:569–576. doi: 10.12816/0006414. [DOI] [PubMed] [Google Scholar]
- Alanazi A.D., Alyousif M.S. Prevalence of antibodies to Toxoplasma gondii in horses in Riyadh Province, Saudi Arabia. J. Parasitol. 2011;97:943–945. doi: 10.1645/GE-2677.1. [DOI] [PubMed] [Google Scholar]
- Al-Kappany Y.M., Abbas I.E., Devleesschauwer B., Dorny P., Jennes M., Cox E. Seroprevalence of anti-Toxoplasma gondii antibodies in Egyptian sheep and goats. BMC Vet. Res. 2018;14:120. doi: 10.1186/s12917-018-1440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khalidi N.W., Weisbrode S.E., Dubey J.P. Pathogaenicity of Toxoplasma gondii oocysts to ponies. Am. J. Vet. Res. 1980;41:1549–1551. [PubMed] [Google Scholar]
- Almeida J.C., Vidotto O., Ferreira E.P., Ribeiro L.P., Mongruel A.C., Vieira T.S., Freire R.L., Mota R.A., Vieira R.F. Serosurvey of anti-Toxoplasma gondii antibodies in sport horses from Paraiba state, Northeastern Brazil. Acta Parasitol. 2017;62:225–227. doi: 10.1515/ap-2017-0028. [DOI] [PubMed] [Google Scholar]
- Almeria S., Cabezon O., Paniagua J., Cano-Terriza D., Jimenez-Ruiz S., Arenas-Montes A., Dubey J.P., Garcia-Bocanegra I. Toxoplasma gondii in sympatric domestic and wild ungulates in the Mediterranean ecosystem. Parasitol. Res. 2018;117:665–671. doi: 10.1007/s00436-017-5705-6. [DOI] [PubMed] [Google Scholar]
- Altan Y., Heydorn A.O., Janitschke K. Zur Infektiosität von Toxoplasma-Oozysten für das Pferd [Infectivity of Toxoplasma oocysts for horses] Berl. Muench. Tieraerztl. Wschr. 1977;90:433–435. [Google Scholar]
- Alvarado-Esquivel C., Garcia-Machado C., Vitela-Corrales J., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii infection in domestic goats in Durango State, Mexico. Vet. Parasitol. 2011;183:43–46. doi: 10.1016/j.vetpar.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Gonzalez-Salazar A.M., Alvarado-Esquivel D., Ontiveros-Vazquez F., Vitela-Corrales J., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii infection in chickens in Durango State, Mexico. J. Parasitol. 2012;98:431–432. doi: 10.1645/GE-2979.1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Rodriguez-Pena S., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii infection in domestic horses in Durango State, Mexico. J. Parasitol. 2012;98:944–945. doi: 10.1645/GE-3174.1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Estrada-Malacon M.A., Reyes-Hernandez S.O., Perez-Ramirez J.A., Trujillo-Lopez J.I., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii in domestic sheep in Oaxaca State, Mexico. J. Parasitol. 2013;99:151–152. doi: 10.1645/GE-3220.1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Silva-Aguilar D., Villena I., Dubey J.P. Seroprevalence and correlates of Toxoplasma gondii infection in domestic sheep in Michoacán State, Mexico. Prev. Vet. Med. 2013;112:433–437. doi: 10.1016/j.prevetmed.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Silva-Aguilar D., Villena I., Dubey J.P. Seroprevalence of Toxoplasma gondii infection in dairy goats in Michoacan State, Mexico. J. Parasitol. 2013;99:540–542. doi: 10.1645/12-103.1. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Romero-Salas D., Garcia-Vazquez Z., Crivelli-Diaz M., Barrientos-Morales M., Lopez-de-Buen L., Dubey J.P. Seroprevalence and correlates of Toxoplasma gondii infection in domestic pigs in Veracruz State, Mexico. Trop. Anim. Health Prod. 2014;46:705–709. doi: 10.1007/s11250-014-0551-3. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Alvarado-Esquivel D., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in domestic donkeys (Equus asinus) in Durango, Mexico slaughtered for human consumption. BMC Vet. Res. 2015;11:6. doi: 10.1186/s12917-015-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdouni Y., Rjeibi M.R., Rouatbi M., Amairia S., Awadi S., Gharbi M. Molecular detection of Toxoplasma gondii infection in slaughtered ruminants (sheep, goats and cattle) in Northwest Tunisia. Meat Sci. 2017;133:180–184. doi: 10.1016/j.meatsci.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Andrade M.M.C., Carneiro M., Medeiros A.D., Neto V.A., Vitor R.W.A. Seroprevalence and risk factors associated with ovine toxoplasmosis in Northeast Brazil. Parasite. 2013;20 doi: 10.1051/parasite/2013019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous Scientific opinion on the public health hazards to be covered by inspection of meat (swine) EFSA J. 2011;9(10):2351. doi: 10.2903/j.efsa.2013.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous Technical specifications on harmonised epidemiological indicators for public health hazards to be covered by meat inspection of swine. EFSA J. 2011;9(10):2351. [Google Scholar]
- Arias M.L., Chinchilla M., Reyes L., Sabah J., Guerrero O.M. Determination of Toxoplasma gondii in several organs of cattle by carbon immunoassay (CIA) testing. Vet. Parasitol. 1994;55:133–136. doi: 10.1016/0304-4017(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Aroussi A., Vignoles P., Dalmay F., Wimel L., Darde M.L., Mercier A., Ajzenberg D. Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests. Parasite. 2015;22:14. doi: 10.1051/parasite/2015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi-Rad A.M., New J.C., Patton S. Risk factor associated with transmission of Toxoplasma gondii to sows kept in different management systems in Tennessee. Vet. Parasitol. 1995;57:289–297. doi: 10.1016/0304-4017(94)00677-5. [DOI] [PubMed] [Google Scholar]
- Ayinmode A.B., Olaosebikan R.I. Seroprevalence of Toxoplasma gondii infection in free ranged chicken from rural and urban settlements in Oyo State, Nigeria. Afr. J. Med. Med. Sci. 2014;43(Suppl):51–57. [PubMed] [Google Scholar]
- Ayinmode A.B., Oluwayelu D.O., Sule W.F., Obebe O.O. Seroprevalence of Toxoplasma gondii in recreational horses in two metropolitan cities of Southwestern Nigeria. Afr. J. Med. Med. Sci. 2014;43(Suppl):47–50. [PubMed] [Google Scholar]
- Azizi H., Shiran B., Boroujeni A.B., Jafari M. Molecular survey of Toxoplasma gondii in sheep, cattle and meat products in Chaharmahal va Bakhtiari Province, southwest of Iran. Iran. J. Parasitol. 2014;9:429–434. [PMC free article] [PubMed] [Google Scholar]
- Bangoura B., Zoller B., Daugschies A. Prevalence and relevance of avian Toxoplasma gondii infections in Europe. Berl. Munch. Tierarztl. Wochenschr. 2011;124:485–496. [PubMed] [Google Scholar]
- Bangoura B., Zoller B., Koethe M., Ludewig M., Pott S., Fehlhaber K., Straubinger R.K., Daugschies A. Experimental Toxoplasma gondii oocyst infections in turkeys (Meleagris gallopavo) Vet. Parasitol. 2013;196:272–277. doi: 10.1016/j.vetpar.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Bartova E., Dvorakova H., Barta J., Sedlak K., Literak I. Susceptibility of the domestic duck (Anas platyrhynchos) to experimental infection with Toxoplasma gondii oocysts. Avian Pathol. 2004;33:153–157. doi: 10.1080/03079450310001652068. [DOI] [PubMed] [Google Scholar]
- Bartova E., Machacova T., Sedlak K., Budikova M., Mariani U., Veneziano V. Seroprevalence of antibodies of Neospora spp. and Toxoplasma gondii in horses from southern Italy. Folia Parasitol. 2015:62. doi: 10.14411/fp.2015.043. [DOI] [PubMed] [Google Scholar]
- Bartova E., Sedlak K., Kobedova K., Budikova M., Joel Atuman Y., Kamani J. Seroprevalence and risk factors of Neospora spp. and Toxoplasma gondii infections among horses and donkeys in Nigeria, West Africa. Acta Parasitol. 2017;62:606–609. doi: 10.1515/ap-2017-0073. [DOI] [PubMed] [Google Scholar]
- Basso W., Hartnack S., Pardini L., Maksimov P., Koudela B., Venturini M.C., Schares G., Sidler X., Lewis F.I., Deplazes P. Assessment of diagnostic accuracy of a commercial ELISA for the detection of Toxoplasma gondii infection in pigs compared with IFAT, TgSAG1-ELISA and Western blot, using a Bayesian latent class approach. Int. J. Parasitol. 2013;43:565–570. doi: 10.1016/j.ijpara.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Basso W., Handke M., Sydler T., Borel N., Grimm F., Sidler X., Deplazes P. Involvement of Toxoplasma gondii in reproductive disorders in Swiss pig farms. Parasitol. Int. 2015;64:157–160. doi: 10.1016/j.parint.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Basso W., Grimm F., Ruetten M., Djokic V., Blaga R., Sidler X., Deplazes P. Experimental Toxoplasma gondii infections in pigs: humoral immune response, estimation of specific IgG avidity and the challenges of reproducing vertical transmission in sows. Vet. Parasitol. 2017;236:76–85. doi: 10.1016/j.vetpar.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Bawm S., Maung W.Y., Win M.Y., Thu M.J., Chel H.M., Khaing T.A., Wai S.S., Htun L.L., Myaing T.T., Tiwananthagorn S., Igarashi M., Katakura K. Serological survey and factors associated with Toxoplasma gondii infection in domestic goats in Myanmar. Scientifica (Cairo) 2016;2016:4794318. doi: 10.1155/2016/4794318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluco S., Mancin M., Conficoni D., Simonato G., Pietrobelli M., Ricci A. Investigating the determinants of Toxoplasma gondii prevalence in meat: a systematic review and meta-regression. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0153856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluco S., Simonato G., Mancin M., Pietrobelli M., Ricci A. Toxoplasma gondii infection and food consumption: a systematic review and meta-analysis of case-controlled studies. Crit. Rev. Food Sci. Nutr. 2017:1–12. doi: 10.1080/10408398.2017.1352563. [DOI] [PubMed] [Google Scholar]
- Benavides J., Maley S., Pang Y., Palarea J., Eaton S., Katzer F., Innes E.A., Buxton D., Chianini F. Development of lesions and tissue distribution of parasite in lambs orally infected with sporulated oocysts of Toxoplasma gondii. Vet. Parasitol. 2011;179:209–215. doi: 10.1016/j.vetpar.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Bennett R. The 'direct costs' of livestock disease: the development of a system of models for the analysis of 30 endemic livestock diseases in Great Britain. J. Agric. Econ. 2003;54:55–71. [Google Scholar]
- Bennett R., Ijpelaar J. Updated estimates of the costs associated with thirty four endemic livestock diseases in Great Britain: a note. J. Agric. Econ. 2005;56:135–144. [Google Scholar]
- Bennett R., Christiansen K., Clifton-Hadley R. An economic study of the importance of non-notifiable diseases of farm animals in Great Britain. Proceedings of the 8th Symposium of the International Society for Veterinary Epidemiology and Economics (ISVEE), Paris, France; 1997. [Google Scholar]
- Bennett R., Christiansen K., Clifton-Hadley R. Preliminary estimates of the direct costs associated with endemic diseases of livestock in Great Britain. Prev. Vet. Med. 1999;39:155–171. doi: 10.1016/s0167-5877(99)00003-3. [DOI] [PubMed] [Google Scholar]
- Berger-Schoch A.E., Herrmann D.C., Schares G., Muller N., Bernet D., Gottstein B., Frey C.F. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet. Parasitol. 2011;177:290–297. doi: 10.1016/j.vetpar.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Bertranpetit E., Jombart T., Paradis E., Pena H., Dubey J., Su C., Mercier A., Devillard S., Ajzenberg D. Phylogeography of Toxoplasma gondii points to a South American origin. Infect. Genet. Evol. 2017;48:150–155. doi: 10.1016/j.meegid.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Bettiol S.S., Obendorf D.L., Nowarkowski M., Milstein T., Goldsmid J.M. Earthworms as paratenic hosts of toxoplasmosis in eastern barred bandicoots in Tasmania. J. Wildl. Dis. 2000;36:145–148. doi: 10.7589/0090-3558-36.1.145. [DOI] [PubMed] [Google Scholar]
- Beverley J.K., Henry L., Hunter D., Brown M.E. Experimental toxoplasmosis in calves. Res. Vet. Sci. 1977;23:33–37. [PubMed] [Google Scholar]
- Bezerra M.J., Cruz J.A., Kung E.S., Albuquerque P.P., Kim P.C., Moraes E.P., Pinheiro Junior J.W., Mota R.A. Detection of Toxoplasma gondii DNA in fresh and frozen semen from rams in Brazil. Reprod. Domest. Anim. 2014;49:753–755. doi: 10.1111/rda.12361. [DOI] [PubMed] [Google Scholar]
- Biancifiori F., Rondini C., Grelloni V., Frescura T. Avian toxoplasmosis: experimental infection of chicken and pigeon. Comp. Immunol. Microbiol. Infect. Dis. 1986;9:337–346. doi: 10.1016/0147-9571(86)90046-9. [DOI] [PubMed] [Google Scholar]
- Bickford A.A., Saunders J.R. Experimental toxoplasmosis in chickens. Am. J. Vet. Res. 1966;27:308–318. [PubMed] [Google Scholar]
- Boch J., Janitschke K., Rommel M., Sommer R. Untersuchungen über das Vorkommen von Toxoplasma-Infektionen bei Schlachtrindern. Wien. Tierarztl. Monatsschr. 1965;12:1477–1480. [Google Scholar]
- Boch J., Rommel M., Janitschke K. Contributions on pig toxoplasmosis. 3. Studies on the possibility of connatal infection. Berl. Munch. Tierarztl. Wochenschr. 1965;78:115–120. [PubMed] [Google Scholar]
- Boch J., Rommel M., Weiland G., Janitschke K., Sommer R. Experimentelle Toxoplasma-Infektion bei Legehennen. Berl. Munch. Tierarztl. Wochenschr. 1966;79:352–356. [PubMed] [Google Scholar]
- Boughattas S., Bergaoui R., Essid R., Aoun K., Bouratbine A. Seroprevalence of Toxoplasma gondii infection among horses in Tunisia. Parasit. Vectors. 2011;4:218. doi: 10.1186/1756-3305-4-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells A., Benavides J., Canton G., Garcia J.L., Bartley P.M., Nath M., Thomson J., Chianini F., Innes E.A., Katzer F. Vaccination of pigs with the S48 strain of Toxoplasma gondii—safer meat for human consumption. Vet. Res. 2015;46:47. doi: 10.1186/s13567-015-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells A., Taroda A., Opsteegh M., Schares G., Benavides J., Dam-Deisz C., Bartley P.M., Chianini F., Villena I., van der Giessen J., Innes E.A., Katzer F. Detection and dissemination of Toxoplasma gondii in experimentally infected calves, a single test does not tell the whole story. Parasit. Vectors. 2018;11:45. doi: 10.1186/s13071-018-2632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet. Res. 1998;29:289–310. [PubMed] [Google Scholar]
- Buxton D., Finlayson J. Experimental infection of pregnant sheep with Toxoplasma gondii: pathological and immunological observations on the placenta and foetus. J. Comp. Pathol. 1986;96:319–333. doi: 10.1016/0021-9975(86)90052-6. [DOI] [PubMed] [Google Scholar]
- Buxton D., Losson B. Toxoplasmosis: general considerations. In: Ortega-Mora L.M., Gottstein B., Conraths F.J., Buxton D., editors. Protozoal Abortifacients in Farm Ruminants: Guidelines for Diagnosis and Control. CABI; Wallingford, UK: 2007. pp. 122–131. [Google Scholar]
- Buxton D., Gilmour J.S., Angus K.W., Blewett D.A., Miller J.K. Perinatal changes in lambs infected with Toxoplasma gondii. Res. Vet. Sci. 1982;32:170–176. [PubMed] [Google Scholar]
- Buxton D., Blewett D.A., Trees A.J., McColgan C., Finlayson J. Further studies in the use of monensin in the control of experimental ovine toxoplasmosis. J. Comp. Pathol. 1988;98:225–236. doi: 10.1016/0021-9975(88)90021-7. [DOI] [PubMed] [Google Scholar]
- Buzby J.C., Roberts T. Economic costs and trade impacts of microbial foodborne illness. World Health Stat. Q. 1997;50:57–66. [PubMed] [Google Scholar]
- Campo-Portacio D.M., Discuviche-Rebolledo M.A., Blanco-Tuirán P.J., Montero-Pérez Y.M., Orozco-Méndez K.E., Assia-Mercado Y.M. Toxoplasma gondii detection by gene B1 amplification in meat human consumption. Infectio. Asociación Colombiana de Infectología. 2014;18:93–99. [Google Scholar]
- Canada N., Meireles C.S., Rocha A., da Costa J.M., Erickson M.W., Dubey J.P. Isolation of viable Toxoplasma gondii from naturally infected aborted bovine fetuses. J. Parasitol. 2002;88:1247–1248. doi: 10.1645/0022-3395(2002)088[1247:IOVTGF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Carson A., Group, A.S.R.E Focus on ovine abortions. Vet. Rec. 2017;180:246–247. doi: 10.1136/vr.j1124. [DOI] [PubMed] [Google Scholar]
- Castano P., Fuertes M., Ferre I., Fernandez M., Ferreras Mdel C., Moreno-Gonzalo J., Gonzalez-Lanza C., Katzer F., Regidor-Cerrillo J., Ortega-Mora L.M., Perez V., Benavides J. Placental thrombosis in acute phase abortions during experimental Toxoplasma gondii infection in sheep. Vet. Res. 2014;45:9. doi: 10.1186/1297-9716-45-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano P., Fuertes M., Regidor-Cerrillo J., Ferre I., Fernandez M., Ferreras M.C., Moreno-Gonzalo J., Gonzalez-Lanza C., Pereira-Bueno J., Katzer F., Ortega-Mora L.M., Perez V., Benavides J. Experimental ovine toxoplasmosis: influence of the gestational stage on the clinical course, lesion development and parasite distribution. Vet. Res. 2016;47:43. doi: 10.1186/s13567-016-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catar G., Bergendi L., Holkova R. Isolation of Toxoplasma gondii from swine and cattle. J. Parasitol. 1969;55:952–955. [PubMed] [Google Scholar]
- Cazarotto C.J., Balzan A., Grosskopf R.K., Boito J.P., Portella L.P., Vogel F.F., Favero J.F., de C Cucco D., Biazus A.H., Machado G., Da Silva A.S. Horses seropositive for Toxoplasma gondii, Sarcocystis spp. and Neospora spp.: possible risk factors for infection in Brazil. Microb. Pathog. 2016;99:30–35. doi: 10.1016/j.micpath.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Cenci-Goga B.T., Ciampelli A., Sechi P., Veronesi F., Moretta I., Cambiotti V., Thompson P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013;9:25. doi: 10.1186/1746-6148-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaichan P., Mercier A., Galal L., Mahittikorn A., Ariey F., Morand S., Boumediene F., Udonsom R., Hamidovic A., Murat J.B., Sukthana Y., Darde M.L. Geographical distribution of Toxoplasma gondii genotypes in Asia: a link with neighboring continents. Infect. Genet. Evol. 2017;53:227–238. doi: 10.1016/j.meegid.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Chiari C. de A., Neves D.P. Human toxoplasmosis acquired by ingestion of goat's milk. Mem. Inst. Oswaldo Cruz. 1984;79:337–340. doi: 10.1590/s0074-02761984000300007. [DOI] [PubMed] [Google Scholar]
- Chikweto A., Kumthekar S., Tiwari K., Nyack B., Deokar M.S., Stratton G., Macpherson C.N., Sharma R.N., Dubey J.P. Seroprevalence of Toxoplasma gondii in pigs, sheep, goats, and cattle from Grenada and Carriacou, West Indies. J. Parasitol. 2011;97:950–951. doi: 10.1645/GE-2811.1. [DOI] [PubMed] [Google Scholar]
- Condoleo R., Musella V., Maurelli M.P., Bosco A., Cringoli G., Rinaldi L. Mapping, cluster detection and evaluation of risk factors of ovine toxoplasmosis in Southern Italy. Geospat. Health. 2016;11:432. doi: 10.4081/gh.2016.432. [DOI] [PubMed] [Google Scholar]
- Cong W., Huang S.Y., Zhou D.H., Xu M.J., Wu S.M., Yan C., Zhao Q., Song H.Q., Zhu X.Q. First report of Toxoplasma gondii infection in market-sold adult chickens, ducks and pigeons in northwest China. Parasit. Vectors. 2012;5:110. doi: 10.1186/1756-3305-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P.A., Barr B.C., Sverlow K.W., Anderson M., Draft B., Kinde H., Dubey J.P., Munson L., Ardans A. In vitro isolation and characterization of a Neospora sp. from aborted bovine foetuses. Parasitology. 1993;106:239–249. doi: 10.1017/s0031182000075065. [DOI] [PubMed] [Google Scholar]
- Consalter A., Silva A.F., Frazao-Teixeira E., Matos L.F., de Oliveira F.C.R., Leite J.S., Silva F.B.F., Ferreira A.M.R. Toxoplasma gondii transmission by artificial insemination in sheep with experimentally contaminated frozen semen. Theriogenology. 2017;90:169–174. doi: 10.1016/j.theriogenology.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Cook A.J.C., Gilbert R.E., Buffolano W., Zufferey J., Petersen E., Jenum P.A., Foulon W., Semprini A.E., Dunn D.T. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. Br. Med. J. 2000;321:142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosendey-KezenLeite R.I., de Oliveira F.C., Frazao-Teixeira E., Dubey J.P., de Souza G.N., Ferreira A.M., Lilenbaum W. Occurrence and risk factors associated to Toxoplasma gondii infection in sheep from Rio de Janeiro, Brazil. Trop. Anim. Health Prod. 2014;46:1463–1466. doi: 10.1007/s11250-014-0667-5. [DOI] [PubMed] [Google Scholar]
- Costa A.J., Araujo F.G., Costa J.O., Lima J.D., Nascimento E. Experimental infection of bovines with oocysts of Toxoplasma gondii. J. Parasitol. 1977;63:212–218. [PubMed] [Google Scholar]
- Costa G.H., da Costa A.J., Lopes W.D., Bresciani K.D., dos Santos T.R., Esper C.R., Santana A.E. Toxoplasma gondii: infection natural congenital in cattle and an experimental inoculation of gestating cows with oocysts. Exp. Parasitol. 2011;127:277–281. doi: 10.1016/j.exppara.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Costa D.G., Marvulo M.F., Silva J.S., Santana S.C., Magalhaes F.J., Filho C.D., Ribeiro V.O., Alves L.C., Mota R.A., Dubey J.P., Silva J.C. Seroprevalence of Toxoplasma gondii in domestic and wild animals from the Fernando de Noronha, Brazil. J. Parasitol. 2012;98:679–680. doi: 10.1645/GE-2910.1. [DOI] [PubMed] [Google Scholar]
- D'Alencar Mendonca C.E., Bomfim Barros S.L., Accioly Guimaraes V.A., Ferraudo A.S., Munhoz A.D. Prevalence and risk factors associated to ovine toxoplasmosis in northeastern Brazil. Rev. Bras. Parasitol. Vet. 2013;22:230–234. doi: 10.1590/S1984-29612013000200042. [DOI] [PubMed] [Google Scholar]
- Damriyasa I.M., Bauer C., Edelhofer R., Failing K., Lind P., Petersen E., Schares G., Tenter A.M., Volmer R., Zahner H. Cross-sectional survey in pig breeding farms in Hesse, Germany: seroprevalence and risk factors of infections with Toxoplasma gondii, Sarcocystis spp. and Neospora caninum in sows. Vet. Parasitol. 2004;126:271–286. doi: 10.1016/j.vetpar.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Danehchin L., Razmi G., Naghibi A. Isolation and genotyping of Toxoplasma gondii strains in ovine aborted fetuses in Khorasan Razavi Province, Iran. Korean J. Parasitol. 2016;54:15–20. doi: 10.3347/kjp.2016.54.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangoudoubiyam S., Oliveira J.B., Viquez C., Gomez-Garcia A., Gonzalez O., Romero J.J., Kwok O.C., Dubey J.P., Howe D.K. Detection of antibodies against Sarcocystis neurona, Neospora spp., and Toxoplasma gondii in horses from Costa Rica. J. Parasitol. 2011;97:522–524. doi: 10.1645/GE-2722.1. [DOI] [PubMed] [Google Scholar]
- de Barros Correia E.L., Feitosa T.F., dos Santos F.A., de Azevedo S.S., de Jesus Pena H.F., Gennari S.M., Mota R.A., Alves C.J. Prevalence and risk factors for Toxoplasma gondii in sheep in the State of Paraiba, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2015;24:383–386. doi: 10.1590/S1984-29612015043. [DOI] [PubMed] [Google Scholar]
- De Berardinis A., Paludi D., Pennisi L., Vergara A. Toxoplasma gondii, a foodborne pathogen in the swine production chain from a European perspective. Foodborne Pathog. Dis. 2017;14:637–648. doi: 10.1089/fpd.2017.2305. [DOI] [PubMed] [Google Scholar]
- de Macedo M.F., de Macedo C.A., Ewald M.P., Martins G.F., Zulpo D.L., da Cunha I.A., Taroda A., Cardim S.T., Su C., Garcia J.L. Isolation and genotyping of Toxoplasma gondii from pregnant dairy cows (Bos taurus) slaughtered. Rev. Bras. Parasitol. Vet. 2012;21:74–77. doi: 10.1590/s1984-29612012000100016. [DOI] [PubMed] [Google Scholar]
- de Moraes E.P., Batista A.M., Faria E.B., Freire R.L., Freitas A.C., Silva M.A., Braga V.A., Mota R.A. Experimental infection by Toxoplasma gondii using contaminated semen containing different doses of tachyzoites in sheep. Vet. Parasitol. 2010;170:318–322. doi: 10.1016/j.vetpar.2010.02.017. [DOI] [PubMed] [Google Scholar]
- de Moraes E.P., da Costa M.M., Dantas A.F., da Silva J.C., Mota R.A. Toxoplasma gondii diagnosis in ovine aborted fetuses and stillborns in the State of Pernambuco, Brazil. Vet. Parasitol. 2011;183:152–155. doi: 10.1016/j.vetpar.2011.06.023. [DOI] [PubMed] [Google Scholar]
- de Moura A.B., Ribeiro A., de Souza A.P., da Silva M.O., Machado G., Klauck V., Pazinato R., Da Silva A.S. Seroprevalence and risk factors for Toxoplasma gondii infection in goats in Southern Brazil. Acta Sci. Vet. 2016;44 [Google Scholar]
- de Oliveira F.C.R., da Coosta A.J., Bechara G.H., Sabatini G.A. Distribuicao e viabilidade de cistos de Toxoplasma gondii (Apicomplexa: Toxoplasmatinae) em tecidos de Bos indicus, Bos taurus e Bos bulbalis infectados com oocistos. Rev. Bras. Med. Vet. 2001;23:28–34. [Google Scholar]
- de Oliveira E., de Albuquerque P.P., de Souza Neto O.L., Faria E.B., Junior J.W., Mota R.A. Occurrence of antibodies to Toxoplasma gondii in mules and donkeys in the northeast of Brazil. J. Parasitol. 2013;99:343–345. doi: 10.1645/GE-3210.1. [DOI] [PubMed] [Google Scholar]
- de Santana Rocha D., de Sousa Moura R.L., Maciel B.M., Guimaraes L.A., O'Dwyer H.N., Munhoz A.D., Albuquerque G.R. Detection of Toxoplasma gondii DNA in naturally infected sheep's milk. Genet. Mol. Res. 2015;14:8658–8662. doi: 10.4238/2015.July.31.14. [DOI] [PubMed] [Google Scholar]
- de Sousa R.A., Lemos Jda F., Farias L.A., Lopes C.D., dos Santos K.R. Seroprevalence and risk factors for Toxoplasma gondii infection in pigs in southern Piaui. Rev. Bras. Parasitol. Vet. 2014;23:98–100. doi: 10.1590/s1984-29612014015. [DOI] [PubMed] [Google Scholar]
- de Souza J.B., Soares V.E., Maia M.O., Pereira C.M., Ferraudo A.S., Cruz B.C., Pires Teixeira W.F., Felippelli G., Maciel W.G., Goncalves W.A.J., da Costa A.J., Zanetti Lopes W.D. Spatial distribution and risk factors for Toxoplasma gondii seropositivity in cattle slaughtered for human consumption in Rondonia, north region, Brazil. Vet. Parasitol. 2016;226:145–149. doi: 10.1016/j.vetpar.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Dehkordi F.S., Borujeni M.R., Rahimi E., Abdizadeh R. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathog. Dis. 2013;10:120–125. doi: 10.1089/fpd.2012.1311. [DOI] [PubMed] [Google Scholar]
- Deksne G., Kirjusina M. Seroprevalence of Toxoplasma gondii in domestic pigs (Sus scrofa domestica) and wild boars (Sus scrofa) in Latvia. J. Parasitol. 2013;99:44–47. doi: 10.1645/GE-3187.1. [DOI] [PubMed] [Google Scholar]
- Deksne G., Ligere B., Sneidere A., Jokelainen P. Seroprevalence and factors associated with Toxoplasma gondii infections in sheep in Latvia: Latvian dark headed sheep breed associated with higher seroprevalence. Vector Borne Zoonotic Dis. 2017;17:478–482. doi: 10.1089/vbz.2016.2003. [DOI] [PubMed] [Google Scholar]
- Dempster R.P., Wilkins M., Green R.S., de Lisle G.W. Serological survey of Toxoplasma gondii and Campylobacter fetus fetus in sheep from New Zealand. N. Z. Vet. J. 2011;59:155–159. doi: 10.1080/00480169.2011.579240. [DOI] [PubMed] [Google Scholar]
- Deng H., Dam-Deisz C., Luttikholt S., Maas M., Nielen M., Swart A., Vellema P., van der Giessen J., Opsteegh M. Risk factors related to Toxoplasma gondii seroprevalence in indoor-housed Dutch dairy goats. Prev. Vet. Med. 2016;124:45–51. doi: 10.1016/j.prevetmed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Deng H., Devleesschauwer B., Liu M., Li J., Wu Y., van der Giessen J.W.B., Opsteegh M. Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of China: a systematic review and hierarchical meta-analysis. Sci. Rep. 2018;8:6218. doi: 10.1038/s41598-018-24361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakoua A., Papadopoulos E., Panousis N., Karatzias C., Giadinis N. Toxoplasma gondii and Neospora caninum seroprevalence in dairy sheep and goats mixed stock farming. Vet. Parasitol. 2013;198:387–390. doi: 10.1016/j.vetpar.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Diaz P., Cabanelas E., Diaz-Cao J.M., Vina M., Bejar J.P., Perez-Creo A., Prieto A., Lopez C.M., Panadero R., Fernandez G., Diez-Banos P., Morrondo P. Seroprevalence of Toxoplasma gondii and Neospora caninum in goats from north-western Spain. Ann. Agric. Environ. Med. 2016;23:587–590. doi: 10.5604/12321966.1226851. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen A.A., Morris R.S. Wageningen Pers.; Sydney: 1997. Animal Health Economics: Principles and Applications. [Google Scholar]
- Djokic V., Klun I., Musella V., Rinaldi L., Cringoli G., Sotiraki S., Djurkovic-Djakovic O. Spatial epidemiology of Toxoplasma gondii infection in goats in Serbia. Geospat. Health. 2014;8:479–488. doi: 10.4081/gh.2014.37. [DOI] [PubMed] [Google Scholar]
- Djokic V., Fablet C., Blaga R., Rose N., Perret C., Djurkovic-Djakovic O., Boireau P., Durand B. Factors associated with Toxoplasma gondii infection in confined farrow-to-finish pig herds in western France: an exploratory study in 60 herds. Parasit. Vectors. 2016;9:466. doi: 10.1186/s13071-016-1753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Su R., Lu Y., Wang M., Liu J., Jian F., Yang Y. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000–2017) from China. Front. Microbiol. 2018;9:2108. doi: 10.3389/fmicb.2018.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos T.R., Faria G.D., Guerreiro B.M., Dal Pietro N.H., Lopes W.D., da Silva H.M., Garcia J.L., Luvizotto M.C., Bresciani K.D., da Costa A.J. Congenital toxoplasmosis in chronically infected and subsequently challenged ewes. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0165124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Zhang Q., Yu Q., Hu M., Zhou Y., Zhao J. Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet. Parasitol. 2012;187:53–56. doi: 10.1016/j.vetpar.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Protective immunity against clinical toxoplasmosis in dairy goats vaccinated with Hammondia hammondi and Hammondia heydorni. Am. J. Vet. Res. 1981;42:2068–2070. [PubMed] [Google Scholar]
- Dubey J.P. Repeat transplacental transfer of Toxoplasma gondii in dairy goats. J. Am. Vet. Med. Assoc. 1982;180:1220–1221. [PubMed] [Google Scholar]
- Dubey J.P. Distribution of cysts and tachyzoites in calves and pregnant cows inoculated with Toxoplasma gondii oocysts. Vet. Parasitol. 1983;13:199–211. doi: 10.1016/0304-4017(83)90057-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Persistence of encysted Toxoplasma gondii in tissues of equids fed oocysts. Am. J. Vet. Res. 1985;46:1753–1754. [PubMed] [Google Scholar]
- Dubey J.P. A review of toxoplasmosis in cattle. Vet. Parasitol. 1986;22:177–202. doi: 10.1016/0304-4017(86)90106-8. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. A review of toxoplasmosis in pigs. Vet. Parasitol. 1986;19:181–223. doi: 10.1016/0304-4017(86)90070-1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Isolation of Toxoplasma gondii from a naturally infected beef cow. J. Parasitol. 1992;78:151–153. [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis in pigs-the last 20 years. Vet. Parasitol. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis in sheep—the last 20 years. Vet. Parasitol. 2009;163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57:60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. 2nd edition. CRC Press; Boca Raton: 2010. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Dubey J.P., Beattie C.P. CRC Press; Boca Raton, Florida, USA: 1988. Toxoplasmosis of Animals and Man; pp. 1–220. [Google Scholar]
- Dubey J.P., Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet. J. 1987;19:337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jones J.L. Comments on “detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran”. Foodborne Pathog. Dis. 2014;11:500–501. doi: 10.1089/fpd.2014.1786. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Streitel R.H. Prevalence of Toxoplasma infection in cattle slaughtered at an Ohio abattoir. J. Am. Vet. Med. Assoc. 1976;169:1197–1199. [PubMed] [Google Scholar]
- Dubey J.P., Thulliez P. Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. Am. J. Vet. Res. 1993;54:270–273. [PubMed] [Google Scholar]
- Dubey J.P., Urban J.F., Jr. Diagnosis of transplacentally induced toxoplasmosis in pigs. Am. J. Vet. Res. 1990;51:1295–1299. [PubMed] [Google Scholar]
- Dubey J.P., Camargo M.E., Ruff M.D., Wilkins G.C., Shen S.K., Kwok O.C., Thulliez P. Experimental toxoplasmosis in turkeys. J. Parasitol. 1993;79:949–952. [PubMed] [Google Scholar]
- Dubey J.P., Ruff M.D., Camargo M.E., Shen S.K., Wilkins G.L., Kwok O.C., Thulliez P. Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am. J. Vet. Res. 1993;54:1668–1672. [PubMed] [Google Scholar]
- Dubey J.P., Garner M.W., Willette M.M., Batey K.L., Gardiner C.H. Disseminated toxoplasmosis in magpie geese (Anseranas semipalmata) with large numbers of tissue cysts in livers. J. Parasitol. 2001;87:219–223. doi: 10.1645/0022-3395(2001)087[0219:DTIMGA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hill D.E., Jones J.L., Hightower A.W., Kirkland E., Roberts J.M., Marcet P.L., Lehmann T., Vianna M.C., Miska K., Sreekumar C., Kwok O.C., Shen S.K., Gamble H.R. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J. Parasitol. 2005;91:1082–1093. doi: 10.1645/GE-683.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Sundar N., Hill D., Velmurugan G.V., Bandini L.A., Kwok O.C., Majumdar D., Su C. High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. Int. J. Parasitol. 2008;38:999–1006. doi: 10.1016/j.ijpara.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Rajendran C., Ferreira L.R., Martins J., Kwok O.C., Hill D.E., Villena I., Zhou H., Su C., Jones J.L. High prevalence and genotypes of Toxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA. Int. J. Parasitol. 2011;41:827–833. doi: 10.1016/j.ijpara.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hotea I., Olariu T.R., Jones J.L., Darabus G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology. 2014;141:311–325. doi: 10.1017/S0031182013001509. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Ness S.L., Kwok O.C., Choudhary S., Mittel L.D., Divers T.J. Seropositivity of Toxoplasma gondii in domestic donkeys (Equus asinus) and isolation of T. gondii from farm cats. Vet. Parasitol. 2014;199:18–23. doi: 10.1016/j.vetpar.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Hemphill A., Calero-Bernal R., Schares G. CRC Press; Boca Rotan: 2017. Neosporosis in Animals. [Google Scholar]
- Elbez-Rubinstein A., Ajzenberg D., Darde M.L., Cohen R., Dumetre A., Yera H., Gondon E., Janaud J.C., Thulliez P. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 2009;199:280–285. doi: 10.1086/595793. [DOI] [PubMed] [Google Scholar]
- Ellis J.T. Polymerase chain reaction approaches for the detection of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 1998;28:1053–1060. doi: 10.1016/s0020-7519(98)00096-4. [DOI] [PubMed] [Google Scholar]
- El-Massry A., Mahdy O.A., El-Ghaysh A., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. J. Parasitol. 2000;86:627–628. doi: 10.1645/0022-3395(2000)086[0627:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ergin S., Ciftcioglu G., Midilli K., Issa G., Gargili A. Detection of Toxoplasma gondii from meat and meat products by the nested-PCR method and its relationship with seroprevalence in slaughtered animals. Bull. Vet. Inst. Pulawy. 2009;53:657–661. [Google Scholar]
- Esteban-Redondo I., Maley S.W., Thomson K., Nicoll S., Wright S., Buxton D., Innes E.A. Detection of T. gondii in tissues of sheep and cattle following oral infection. Vet. Parasitol. 1999;86:155–171. doi: 10.1016/s0304-4017(99)00138-7. [DOI] [PubMed] [Google Scholar]
- Esteves F., Aguiar D., Rosado J., Costa M.L., de Sousa B., Antunes F., Matos O. Toxoplasma gondii prevalence in cats from Lisbon and in pigs from centre and south of Portugal. Vet. Parasitol. 2014;200:8–12. doi: 10.1016/j.vetpar.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Evers F., Garcia J.L., Navarro I.T., Zulpo D.L., Nino Bde S., Ewald M.P., Pagliari S., Almeida J.C., Freire R.L. Diagnosis and isolation of Toxoplasma gondii in horses from Brazilian slaughterhouses. Rev. Bras. Parasitol. Vet. 2013;22:58–63. doi: 10.1590/s1984-29612013005000009. [DOI] [PubMed] [Google Scholar]
- Fajardo H.V., D'Avila S., Bastos R.R., Cyrino C.D., de Lima Detoni M., Garcia J.L., das Neves L.B., Nicolau J.L., Amendoeira M.R. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, Southern Brazil. Parasit. Vectors. 2013;6:191. doi: 10.1186/1756-3305-6-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger M.A., Villalobos E.M.C., Lara M.D.C.S.H., Cunha E.M.S., De Barros I.R., Deconto I., Dornbusch P.T., Ullmann L.S., Biondo A.W. Detection of anti-Toxoplasma gondii antibodies in carthorses in the metropolitan region of Curitiba, Parana, Brazil. Rev. Bras. Parasitol. Vet. 2013;22:179–181. doi: 10.1590/s1984-29612013005000001. [DOI] [PubMed] [Google Scholar]
- Fonseca de Araujo Valenca S.R., Barreto Valenca R.M., Pinheiro Junior J.W., Feitosa de Albuquerque P.P., Souza Neto O.L., Mota R.A. Risk factors of occurrence of Toxoplasma gondii among horses in the state of Alagoas, Brazil. Acta Parasitol. 2015;60:707–711. doi: 10.1515/ap-2015-0100. [DOI] [PubMed] [Google Scholar]
- Fortier B., Dealmeida E., Pinto I., Ajana F., Camus D. Prevalence de la toxoplasmose porcine et bovine a Porto. Med. Mal. Infect. 1990;20:551–554. [Google Scholar]
- Freyre A., Falcon J., Cedda C. El papel de la carne vacuna en la adquisicion de la infeccion toxoplasmica. Rev. Iber. Parasitol. 1990;50:15–24. [Google Scholar]
- Freyre A., Bonino J., Falcon J., Castells D., Correa O., Casaretto A. The incidence and economic significance of ovine toxoplasmosis in Uruguay. Vet. Parasitol. 1997;73:13–15. doi: 10.1016/s0304-4017(97)00069-1. [DOI] [PubMed] [Google Scholar]
- Garcia G., Sotomaior C., do Nascimento A.J., Navarro I.T., Soccol V.T. Toxoplasma gondii in goats from Curitiba, Parana, Brazil: risks factors and epidemiology. Rev. Bras. Parasitol. Vet. 2012;21:42–47. doi: 10.1590/s1984-29612012000100009. [DOI] [PubMed] [Google Scholar]
- Garcia J.L., Burrells A., Bartley P.M., Bartley K., Innes E.A., Katzer F. The use of ELISA, nPCR and qPCR for diagnosis of ocular toxoplasmosis in experimentally infected pigs. Res. Vet. Sci. 2017;115:490–495. doi: 10.1016/j.rvsc.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Garcia-Bocanegra I., Dubey J.P., Simon-Grife M., Cabezon O., Casal J., Allepuz A., Napp S., Almeria S. Seroprevalence and risk factors associated with Toxoplasma gondii infection in pig farms from Catalonia, north-eastern Spain. Res. Vet. Sci. 2010;89:85–87. doi: 10.1016/j.rvsc.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Garcia-Bocanegra I., Simon-Grife M., Dubey J.P., Casal J., Martin G.E., Cabezon O., Perea A., Almeria S. Seroprevalence and risk factors associated with Toxoplasma gondii in domestic pigs from Spain. Parasitol. Int. 2010;59:421–426. doi: 10.1016/j.parint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Bocanegra I., Cabezon O., Arenas-Montes A., Carbonero A., Dubey J.P., Perea A., Almeria S. Seroprevalence of Toxoplasma gondii in equids from Southern Spain. Parasitol. Int. 2012;61:421–424. doi: 10.1016/j.parint.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Bocanegra I., Cabezon O., Hernandez E., Martinez-Cruz M.S., Martinez-Moreno A., Martinez-Moreno J. Toxoplasma gondii in ruminant species (cattle, sheep, and goats) from southern Spain. J. Parasitol. 2013;99:438–440. doi: 10.1645/12-27.1. [DOI] [PubMed] [Google Scholar]
- Gazzonis A.L., Veronesi F., Di Cerbo A.R., Zanzani S.A., Molineri G., Moretta I., Moretti A., Piergili Fioretti D., Invernizzi A., Manfredi M.T. Toxoplasma gondii in small ruminants in Northern Italy - prevalence and risk factors. Ann. Agric. Environ. Med. 2015;22:62–68. doi: 10.5604/12321966.1141370. [DOI] [PubMed] [Google Scholar]
- Gebremedhin E.Z., Agonafir A., Tessema T.S., Tilahun G., Medhin G., Vitale M., Di Marco V., Cox E., Vercruysse J., Dorny P. Seroepidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet. Res. 2013;9:117. doi: 10.1186/1746-6148-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin E.Z., Abdurahaman M., Tessema T.S., Tilahun G., Cox E., Goddeeris B., Dorny P., De Craeye S., Darde M.L., Ajzenberg D. Isolation and genotyping of viable Toxoplasma gondii from sheep and goats in Ethiopia destined for human consumption. Parasit. Vectors. 2014;7:425. doi: 10.1186/1756-3305-7-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreyes W.A., Bahnson P.B., Funk J.A., McKean J., Patchanee P. Seroprevalence of Trichinella, Toxoplasma, and Salmonella in antimicrobial-free and conventional swine production systems. Foodborne Pathog. Dis. 2008;5:199–203. doi: 10.1089/fpd.2007.0071. [DOI] [PubMed] [Google Scholar]
- Genchi M., Vismarra A., Mangia C., Faccini S., Vicari N., Rigamonti S., Prati P., Marino A.M., Kramer L., Fabbi M. Lack of viable parasites in cured ‘Parma Ham’ (PDO), following experimental Toxoplasma gondii infection of pigs. Food Microbiol. 2017;66:157–164. doi: 10.1016/j.fm.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Gennari S.M., Esmerini P.D., Lopes M.G., Soares H.S., Vitaliano S.N., Cabral A.D., Pena H.F.J., Horta M.C., Cavalcante P.H., Fortes K.P., Villalobos E.M.C. Occurrence of antibodies against Toxoplasma gondii and its isolation and genotyping in donkeys, mules, and horses in Brazil. Vet. Parasitol. 2015;209:129–132. doi: 10.1016/j.vetpar.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Gilot-Fromont E., Aubert D., Belkilani S., Hermitte P., Gibout O., Geers R., Villena I. Landscape, herd management and within-herd seroprevalence of Toxoplasma gondii in beef cattle herds from Champagne-Ardenne, France. Vet. Parasitol. 2009;161:36–40. doi: 10.1016/j.vetpar.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Goerlich K. Tieraerztliche Hochschule Hannover; Hannover: 2011. Toxoplasma-Infektionen bei Schweinen: Semi-automatisiertes Testsystem für Surveillance und Monitoring, Querschnittsstudie in Niedersachsen. [Google Scholar]
- Gottstein B., Hentrich B., Wyss R., Thur B., Busato A., Stark K.D.C., Muller N. Molecular and immunodiagnostic investigations on bovine neosporosis in Switzerland. Int. J. Parasitol. 1998;28:679–691. doi: 10.1016/S0020-7519(98)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N.R., Almeida J.C., Silva E.L., Silva E.M., Santos J.A., Lepold R., Mota R.A., Alves L.C. Soroprevalência de Toxoplasma gondii em equídeos do Nordeste do Brasil. Pesqui. Vet. Bras. 2018;38:400–406. [Google Scholar]
- Guimaraes L.A., Bezerra R.A., Rocha Dde S., Albuquerque G.R. Prevalence and risk factors associated with anti-Toxoplasma gondii antibodies in sheep from Bahia state, Brazil. Rev. Bras. Parasitol. Vet. 2013;22:220–224. doi: 10.1590/S1984-29612013000200041. [DOI] [PubMed] [Google Scholar]
- Guo M., Dubey J.P., Hill D., Buchanan R.L., Gamble H.R., Jones J.L., Pradhan A.K. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 2015;78:457–476. doi: 10.4315/0362-028X.JFP-14-328. [DOI] [PubMed] [Google Scholar]
- Guo M., Mishra A., Buchanan R.L., Dubey J.P., Hill D.E., Gamble H.R., Jones J.L., Pradhan A.K. A systematic meta-analysis of Toxoplasma gondii prevalence in food animals in the United States. Foodborne Pathog. Dis. 2016;13:109–118. doi: 10.1089/fpd.2015.2070. [DOI] [PubMed] [Google Scholar]
- Gutierrez J., O'Donovan J., Williams E., Proctor A., Brady C., Marques P.X., Worrall S., Nally J.E., McElroy M., Bassett H., Sammin D., Buxton D., Maley S., Markey B.K. Detection and quantification of Toxoplasma gondii in ovine maternal and foetal tissues from experimentally infected pregnant ewes using real-time PCR. Vet. Parasitol. 2010;172:8–15. doi: 10.1016/j.vetpar.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Gutierrez J., O'Donovan J., Proctor A., Brady C., Marques P.X., Worrall S., Nally J.E., McElroy M., Bassett H., Fagan J., Maley S., Buxton D., Sammin D., Markey B.K. Application of quantitative real-time polymerase chain reaction for the diagnosis of toxoplasmosis and enzootic abortion of ewes. J. Vet. Diagn. Investig. 2012;24:846–854. doi: 10.1177/1040638712452730. [DOI] [PubMed] [Google Scholar]
- Habibi G., Imani A., Gholami M., Hablolvarid M., Behroozikhah A., Lotfi M., Kamalzade M., Najjar E., Esmaeil-Nia K., Bozorgi S. Detection and identification of Toxoplasma gondii type one infection in sheep aborted fetuses in Qazvin province of Iran. Iran. J. Parasitol. 2012;7:64–72. [PMC free article] [PubMed] [Google Scholar]
- Hajialilo E., Ziaali N., Harandi M.F., Saraei M., Hajialilo M. Prevalence of anti-Toxoplasma gondii antibodies in sport horses from Qazvin, Iran. Trop. Anim. Health Prod. 2010;42:1321–1322. doi: 10.1007/s11250-010-9576-4. [DOI] [PubMed] [Google Scholar]
- Halos L., Thebault A., Aubert D., Thomas M., Perret C., Geers R., Alliot A., Escotte-Binet S., Ajzenberg D., Darde M.L., Durand B., Boireau P., Villena I. An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int. J. Parasitol. 2010;40:193–200. doi: 10.1016/j.ijpara.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Hamilton C.M., Katzer F., Innes E.A., Kelly P.J. Seroprevalence of Toxoplasma gondii in small ruminants from four Caribbean islands. Parasit. Vectors. 2014;7:449. doi: 10.1186/1756-3305-7-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C.M., Kelly P.J., Boey K., Corey T.M., Huynh H., Metzler D., Villena I., Su C., Innes E.A., Katzer F. Predominance of atypical genotypes of Toxoplasma gondii in free-roaming chickens in St. Kitts, West Indies. Parasit. Vectors. 2017;10:104. doi: 10.1186/s13071-017-2019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Aryee K., Van Helden L.S., Van Helden P.D. The prevalence of antibodies to Toxoplasma gondii in sheep in the Western Cape, South Africa. Onderstepoort J. Vet. Res. 2015;82:e1–e5. doi: 10.4102/ojvr.v82i1.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfoush M., Tahoon Ael N. Seroprevalence of Toxoplasma gondii antibodies in domestic ducks, free-range chickens, turkeys and rabbits in Kafr El-Sheikh Governorate Egypt. J. Egypt. Soc. Parasitol. 2010;40:295–302. [PubMed] [Google Scholar]
- Haridy F.M., Saleh N.M., Khalil H.H., Morsy T.A. Anti-Toxoplasma gondii antibodies in working donkeys and donkey's milk in greater Cairo, Egypt. J. Egypt. Soc. Parasitol. 2010;40:459–464. [PubMed] [Google Scholar]
- Herrero L., Gracia M.J., Perez-Arquillue C., Lazaro R., Herrera M., Herrera A., Bayarri S. Toxoplasma gondii: pig seroprevalence, associated risk factors and viability in fresh pork meat. Vet. Parasitol. 2016;224:52–59. doi: 10.1016/j.vetpar.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Dubey J.P. Toxoplasma gondii prevalence in farm animals in the United States. Int. J. Parasitol. 2013;43:107–113. doi: 10.1016/j.ijpara.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Haley C., Wagner B., Gamble H.R., Dubey J.P. Seroprevalence of and risk factors for Toxoplasma gondii in the US swine herd using sera collected during the National Animal Health Monitoring Survey (swine 2006) Zoonoses Public Health. 2010;57:53–59. doi: 10.1111/j.1863-2378.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- Hill D., Coss C., Dubey J.P., Wroblewski K., Sautter M., Hosten T., Munoz-Zanzi C., Mui E., Withers S., Boyer K., Hermes G., Coyne J., Jagdis F., Burnett A., McLeod P., Morton H., Robinson D., McLeod R. Identification of a sporozoite-specific antigen from Toxoplasma gondii. J. Parasitol. 2011;97:328–337. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holec-Gasior L., Dominiak-Gorski B., Kur J. First report of seroprevalence of Toxoplasma gondii infection in sheep in Pomerania, northern Poland. Ann. Agric. Environ. Med. 2015;22:604–607. doi: 10.5604/12321966.1185761. [DOI] [PubMed] [Google Scholar]
- Hosein S., Limon G., Dadios N., Guitian J., Blake D.P. Toxoplasma gondii detection in cattle: a slaughterhouse survey. Vet. Parasitol. 2016;228:126–129. doi: 10.1016/j.vetpar.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Hou Z.F., Su S.J., Liu D.D., Wang L.L., Jia C.L., Zhao Z.X., Ma Y.F., Li Q.Q., Xu J.J., Tao J.P. Prevalence, risk factors and genetic characterization of Toxoplasma gondii in sick pigs and stray cats in Jiangsu Province, eastern China. Infect. Genet. Evol. 2018;60:17–25. doi: 10.1016/j.meegid.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Hutchinson J.P., Wear A.R., Lambton S.L., Smith R.P., Pritchard G.C. Survey to determine the seroprevalence of Toxoplasma gondii infection in British sheep flocks. Vet. Rec. 2011;169:582. doi: 10.1136/vr.d5764. [DOI] [PubMed] [Google Scholar]
- Innes E.A., Panton W.R., Thomson K.M., Maley S., Buxton D. Kinetics of interferon gamma production in vivo during infection with the S48 vaccine strain of Toxoplasma gondii. J. Comp. Pathol. 1995;113:89–94. doi: 10.1016/s0021-9975(05)80074-x. [DOI] [PubMed] [Google Scholar]
- Innes E.A., Bartley P.M., Buxton D., Katzer F. Ovine toxoplasmosis. Parasitology. 2009;136:1887–1894. doi: 10.1017/S0031182009991636. [DOI] [PubMed] [Google Scholar]
- Iovu A., Titilincu A., Mircean V., Junie M., Cozma V. Identification of Toxoplasma gondii and Hammondia hammondi in sow abortions. Int. J. Mol. Med. 2010;26:S71. [Google Scholar]
- Iovu A., Gyorke A., Mircean V., Gavrea R., Cozma V. Seroprevalence of Toxoplasma gondii and Neospora caninum in dairy goats from Romania. Vet. Parasitol. 2012;186:470–474. doi: 10.1016/j.vetpar.2011.11.062. [DOI] [PubMed] [Google Scholar]
- Isaac-Renton J., Bowie W.R., King A., Irwin G.S., Ong C.S., Fung C.P., Shokeir M.O., Dubey J.P. Detection of Toxoplasma gondii oocysts in drinking water. Appl. Environ. Microbiol. 1998;64:2278–2280. doi: 10.1128/aem.64.6.2278-2280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L., Moyle G.G. The prevalence of toxoplasmosis in New Zealand sheep and cattle. Am. J. Vet. Res. 1963;24:673–675. [PubMed] [Google Scholar]
- Jacobs L., Remington J.S., Melton M.L. A survey of meat samples from swine, cattle, and sheep for the presence of encysted Toxoplasma. J. Parasitol. 1960;46:23–28. [PubMed] [Google Scholar]
- James K.E., Smith W.A., Packham A.E., Conrad P.A., Pusterla N. Toxoplasma gondii seroprevalence and association with equine protozoal myeloencephalitis: a case-control study of Californian horses. Vet. J. 2017;224:38–43. doi: 10.1016/j.tvjl.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Jerome M.E., Radke J.R., Bohne W., Roos D.S., White M.W. Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development? Infect. Immun. 1998;66:4838–4844. doi: 10.1128/iai.66.10.4838-4844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.H., Huang S.Y., Zhou D.H., Zhang X.X., Su C., Deng S.Z., Zhu X.Q. Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasit. Vectors. 2013;6:227. doi: 10.1186/1756-3305-6-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen P., Nareaho A., Knaapi S., Oksanen A., Rikula U., Sukura A. Toxoplasma gondii in wild cervids and sheep in Finland: north-south gradient in seroprevalence. Vet. Parasitol. 2010;171:331–336. doi: 10.1016/j.vetpar.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Jokelainen P., Tagel M., Motus K., Viltrop A., Lassen B. Toxoplasma gondii seroprevalence in dairy and beef cattle: large-scale epidemiological study in Estonia. Vet. Parasitol. 2017;236:137–143. doi: 10.1016/j.vetpar.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Jungersen G., Bille-Hansen V., Jensen L., Lind P. Transplacental transmission of Toxoplasma gondii in minipigs infected with strains of different virulence. J. Parasitol. 2001;87:108–113. doi: 10.1645/0022-3395(2001)087[0108:TTOTGI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kantzoura V., Diakou A., Kouam M.K., Feidas H., Theodoropoulou H., Theodoropoulos G. Seroprevalence and risk factors associated with zoonotic parasitic infections in small ruminants in the Greek temperate environment. Parasitol. Int. 2013;62:554–560. doi: 10.1016/j.parint.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Jenum P.A., Stray-Pedersen B., Melby K.K., Eskild A., Eng J. Risk factors for Toxoplasma gondii infection in pregnancy: results of a prospective case-control study in Norway. Am. J. Epidemiol. 1996;144:405–412. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- Karatepe B., Babur C., Karatepe M., Kilic S. Seroprevalence of toxoplasmosis in horses in Nigde Province of Turkey. Trop. Anim. Health Prod. 2010;42:385–389. doi: 10.1007/s11250-009-9430-8. [DOI] [PubMed] [Google Scholar]
- Katzer F., Brulisauer F., Collantes-Fernandez E., Bartley P.M., Burrells A., Gunn G., Maley S.W., Cousens C., Innes E.A. Increased Toxoplasma gondii positivity relative to age in 125 Scottish sheep flocks; evidence of frequent acquired infection. Vet. Res. 2011;42:121. doi: 10.1186/1297-9716-42-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzer F., Canton G., Burrells A., Palarea-Albaladejo J., Horton B., Bartley P.M., Pang Y., Chianini F., Innes E.A., Benavides J. Immunization of lambs with the S48 strain of Toxoplasma gondii reduces tissue cyst burden following oral challenge with a complete strain of the parasite. Vet. Parasitol. 2014;205:46–56. doi: 10.1016/j.vetpar.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Khan M.U., Rashid I., Akbar H., Islam S., Riaz F., Nabi H., Ashraf K., Singla L.D. Seroprevalence of Toxoplasma gondii in South Asian countries. Rev. Sci. Tech. 2017;36:981–996. doi: 10.20506/rst.36.3.2730. [DOI] [PubMed] [Google Scholar]
- Kijlstra A., Eissen O.A., Cornelissen J., Munniksma K., Eijck I., Kortbeek T. Toxoplasma gondii infection in animal-friendly pig production systems. Invest. Ophthalmol. Vis. Sci. 2004;45:3165–3169. doi: 10.1167/iovs.04-0326. [DOI] [PubMed] [Google Scholar]
- Kijlstra A., Meerburg B., Cornelissen J., De Craeye S., Vereijken P., Jongert E. The role of rodents and shrews in the transmission of Toxoplasma gondii to pigs. Vet. Parasitol. 2008;156:183–190. doi: 10.1016/j.vetpar.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kang K.I., Kang W.C., Sohn H.J., Jean Y.H., Park B.K., Kim Y., Kim D.Y. Porcine abortion outbreak associated with Toxoplasma gondii in Jeju Island, Korea. J. Vet. Sci. 2009;10:147–151. doi: 10.4142/jvs.2009.10.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittas C., Henry L. Effect of sex hormones on the immune system of guinea-pigs and on the development of toxoplasmic lesions in non-lymphoid organs. Clin. Exp. Immunol. 1979;36:16–23. [PMC free article] [PubMed] [Google Scholar]
- Kittas C., Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br. J. Exp. Pathol. 1980;61:590–600. [PMC free article] [PubMed] [Google Scholar]
- Klauck V., Pazinato R., Radavelli W.M., Custodio E., Bianchi A.E., Camillo G., Cezar A.S., Vogel F.F., Tonin A.A., Ferreira R., Stefani L.M., Da Silva A.S. Toxoplasma gondii infection in dairy ewes: vertical transmission and influence on milk production and reproductive performance. Microb. Pathog. 2016;99:101–105. doi: 10.1016/j.micpath.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Klein S., Wendt M., Baumgartner W., Wohlsein P. Systemic toxoplasmosis and concurrent porcine circovirus-2 infection in a pig. J. Comp. Pathol. 2010;142:228–234. doi: 10.1016/j.jcpa.2009.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klun I., Djurkovic-Djakovic O., Katic-Radivojevic S., Nikolic A. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: seroprevalence and risk factors. Vet. Parasitol. 2006;135:121–131. doi: 10.1016/j.vetpar.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Klun I., Uzelac A., Villena I., Mercier A., Bobic B., Nikolic A., Rajnpreht I., Opsteegh M., Aubert D., Blaga R., van der Giessen J., Djurkovic-Djakovic O. The first isolation and molecular characterization of Toxoplasma gondii from horses in Serbia. Parasit. Vectors. 2017;10:167. doi: 10.1186/s13071-017-2104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe M., Pott S., Ludewig M., Bangoura B., Zoller B., Daugschies A., Tenter A.M., Spekker K., Bittame A., Mercier C., Fehlhaber K., Straubinger R.K. Prevalence of specific IgG-antibodies against Toxoplasma gondii in domestic turkeys determined by kinetic ELISA based on recombinant GRA7 and GRA8. Vet. Parasitol. 2011;180:179–190. doi: 10.1016/j.vetpar.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Koethe M., Straubinger R.K., Pott S., Bangoura B., Geuthner A.C., Daugschies A., Ludewig M. Quantitative detection of Toxoplasma gondii in tissues of experimentally infected turkeys and in retail turkey products by magnetic-capture PCR. Food Microbiol. 2015;52:11–17. doi: 10.1016/j.fm.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Kouam M.K., Diakou A., Kanzoura V., Papadopoulos E., Gajadhar A.A., Theodoropoulos G. A seroepidemiological study of exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in equids in Greece and analysis of risk factors. Vet. Parasitol. 2010;170:170–175. doi: 10.1016/j.vetpar.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lahmar I., Lachkhem A., Slama D., Sakly W., Haouas N., Gorcii M., Pfaff A.W., Candolfi E., Babba H. Prevalence of toxoplasmosis in sheep, goats and cattle in Southern Tunisia. J. Bacteriol. Parasitol. 2015;6 [Google Scholar]
- Lee S.H., Lee S.E., Seo M.G., Goo Y.K., Cho K.H., Cho G.J., Kwon O.D., Kwak D., Lee W.J. Evidence of Toxoplasma gondii exposure among horses in Korea. J. Vet. Med. Sci. 2014;76:1663–1665. doi: 10.1292/jvms.14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang Y., Yu F., Li T., Zhang D. An outbreak of lethal toxoplasmosis in pigs in the Gansu province of China. J. Vet. Diagn. Investig. 2010;22:442–444. doi: 10.1177/104063871002200318. [DOI] [PubMed] [Google Scholar]
- Limon G., Beauvais W., Dadios N., Villena I., Cockle C., Blaga R., Guitian J. Cross-sectional study of Toxoplasma gondii infection in pig farms in England. Foodborne Pathog. Dis. 2017;14:269–281. doi: 10.1089/fpd.2016.2197. [DOI] [PubMed] [Google Scholar]
- Liu Z.K., Li J.Y., Pan H. Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infections in small ruminants in China. Prev. Vet. Med. 2015;118:488–492. doi: 10.1016/j.prevetmed.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Lopes W.D., Santos T.R., da Silva Rdos S., Rossanese W.M., de Souza F.A., de Faria Rodrigues J.D., de Mendonca R.P., Soares V.E., Costa A.J. Seroprevalence of and risk factors for Toxoplasma gondii in sheep raised in the Jaboticabal microregion, Sao Paulo State, Brazil. Res. Vet. Sci. 2010;88:104–106. doi: 10.1016/j.rvsc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lopes W.D., Santos T.R., Luvizotto M.C., Sakamoto C.A., Oliveira G.P., Costa A.J. Histopathology of the reproductive system of male sheep experimentally infected with Toxoplasma gondii. Parasitol. Res. 2011;109:405–409. doi: 10.1007/s00436-011-2268-9. [DOI] [PubMed] [Google Scholar]
- Lopes A.P., Dubey J.P., Neto F., Rodrigues A., Martins T., Rodrigues M., Cardoso L. Seroprevalence of Toxoplasma gondii infection in cattle, sheep, goats and pigs from the north of Portugal for human consumption. Vet. Parasitol. 2013;193:266–269. doi: 10.1016/j.vetpar.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Lopes W.D., Rodriguez J.D., Souza F.A., dos Santos T.R., dos Santos R.S., Rosanese W.M., Lopes W.R., Sakamoto C.A., da Costa A.J. Sexual transmission of Toxoplasma gondii in sheep. Vet. Parasitol. 2013;195:47–56. doi: 10.1016/j.vetpar.2012.12.056. [DOI] [PubMed] [Google Scholar]
- Machacova T., Bartova E., Di Loria A., Sedlak K., Mariani U., Fusco G., Fulgione D., Veneziano V., Dubey J.P. Seroprevalence of Toxoplasma gondii in donkeys (Equus asinus) in Italy. J. Vet. Med. Sci. 2014;76:265–267. doi: 10.1292/jvms.13-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes F.J., Ribeiro-Andrade M., Alcantara A.M., Pinheiro J.W.J., Sena M.J., Porto W.J., Vieira R.F., Mota R.A. Risk factors for Toxoplasma gondii infection in sheep and cattle from Fernando de Noronha Island, Brazil. Rev. Bras. Parasitol. Vet. 2016;25:511–515. doi: 10.1590/S1984-29612016051. [DOI] [PubMed] [Google Scholar]
- Magalhães F.J.R., da Silva J.G., Ribeiro-Andrade M., Pinheiro J.W., Aparecido Mota R. High prevalence of toxoplasmosis in free-range chicken of the Fernando de Noronha Archipelago, Brazil. Acta Trop. 2016;159:58–61. doi: 10.1016/j.actatropica.2016.03.034. [DOI] [PubMed] [Google Scholar]
- Mahami-Oskouei M., Moradi M., Fallah E., Hamidi F., Asl Rahnamaye Akbari N. Molecular detection and genotyping of Toxoplasma gondii in chicken, beef, and lamb meat consumed in Northwestern Iran. Iran. J. Parasitol. 2017;12:38–45. [PMC free article] [PubMed] [Google Scholar]
- Mainar R.C., Delacruz C., Asensio A., Dominguez L., Vazquezboland J.A. Prevalence of agglutinating antibodies to Toxoplasma gondii in small ruminants of the Madrid region, Spain, and identification of factors influencing seropositivity by multivariate analysis. Vet. Res. Commun. 1996;20:153–159. doi: 10.1007/BF00385636. [DOI] [PubMed] [Google Scholar]
- Maksimov P., Buschtöns S., Herrmann D.C., Conraths F.J., Görlich K., Tenter A.M., Dubey J.P., Nagel-Kohl U., Thoms B., Bötcher L., Kühne M., Schares G. Serological survey and risk factors for Toxoplasma gondii in domestic ducks and geese in Lower Saxony, Germany. Vet. Parasitol. 2011;182:140–149. doi: 10.1016/j.vetpar.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Maksimov P., Basso W., Zerweck J., Schutkowski M., Reimer U., Maksimov A., Conraths F.J., Schares G. Analysis of Toxoplasma gondii clonal type-specific antibody reactions in experimentally infected turkeys and chickens. Int. J. Parasitol. 2018;48:845–856. doi: 10.1016/j.ijpara.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Mancianti F., Nardoni S., Papini R., Mugnaini L., Martini M., Altomonte I., Salari F., D'Ascenzi C., Dubey J.P. Detection and genotyping of Toxoplasma gondii DNA in the blood and milk of naturally infected donkeys (Equus asinus) Parasit. Vectors. 2014;7:165. doi: 10.1186/1756-3305-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques L.C., Costa A.J., Lopes C.W.G., de Moraes F.R., de Moraes J.R.E. Experimental toxoplasmosis in pregnant mares: a study of fetuses and placentas. Braz. J. Vet. Res. Anim. Sci. 1995;32:246–250. [Google Scholar]
- Marques P.X., O'Donovan J., Williams E.J., Gutierrez J., Worrall S., McElroy M., Proctor A., Brady C., Sammin D., Bassett H., Buxton D., Maley S., Markey B.K., Nally J.E. Detection of Toxoplasma gondii antigens reactive with antibodies from serum, amniotic, and allantoic fluids from experimentally infected pregnant ewes. Vet. Parasitol. 2012;185:91–100. doi: 10.1016/j.vetpar.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Masatani T., Takashima Y., Takasu M., Matsuu A., Amaya T. Prevalence of anti-Toxoplasma gondii antibody in domestic horses in Japan. Parasitol. Int. 2016;65:146–150. doi: 10.1016/j.parint.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Mateus-Pinilla N.E., Dubey J.P., Choromanski L., Weigel R.M. A field trial of the effectiveness of a feline Toxoplasma gondii vaccine in reducing T. gondii exposure for swine. J. Parasitol. 1999;85:855–860. [PubMed] [Google Scholar]
- Matsuo K., Kamai R., Uetsu H., Goto H., Takashima Y., Nagamune K. Seroprevalence of Toxoplasma gondii infection in cattle, horses, pigs and chickens in Japan. Parasitol. Int. 2014;63:638–639. doi: 10.1016/j.parint.2014.04.003. [DOI] [PubMed] [Google Scholar]
- McColgan C., Buxton D., Blewett D.A. Titration of Toxoplasma gondii oocysts in non-pregnant sheep and the effects of subsequent challenge during pregnancy. Vet. Rec. 1988;123:467–470. doi: 10.1136/vr.123.18.467. [DOI] [PubMed] [Google Scholar]
- Meerburg B.G., van Riel J.W., Cornelissen J.B., Kijlstra A., Mul M.F. Cats and goat whey associated with Toxoplasma gondii infection in pigs. Vector Borne Zoonotic Dis. 2006;6:266–274. doi: 10.1089/vbz.2006.6.266. [DOI] [PubMed] [Google Scholar]
- Mevelec M.N., Ducournau C., Bassuny Ismael A., Olivier M., Seche E., Lebrun M., Bout D., Dimier-Poisson I. Mic1-3 knockout Toxoplasma gondii is a good candidate for a vaccine against T. gondii-induced abortion in sheep. Vet. Res. 2010;41:49. doi: 10.1051/vetres/2010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q., Wang X., She L.N., Fan Y.T., Yuan F.Z., Yang J.F., Zhu X.Q., Zou F.C. Seroprevalence of Toxoplasma gondii in horses and donkeys in Yunnan Province, Southwestern China. Parasit. Vectors. 2013;6:168. doi: 10.1186/1756-3305-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T., Fennestad K.L., Eriksen L., Work K., Siim J.C. Experimental toxoplasmosis in pregnant sows. Acta Pathol. Microbiol. Scand. A. 1970;78:241–255. doi: 10.1111/j.1699-0463.1970.tb03299.x. [DOI] [PubMed] [Google Scholar]
- Moreno B., Collantes-Fernandez E., Villa A., Navarro A., Regidor-Cerrillo J., Ortega-Mora L.M. Occurrence of Neospora caninum and Toxoplasma gondii infections in ovine and caprine abortions. Vet. Parasitol. 2012;187:312–318. doi: 10.1016/j.vetpar.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Moskwa B., Kornacka A., Cybulska A., Cabaj W., Reiterova K., Bogdaszewski M., Steiner-Bogdaszewska Z., Bien J. Seroprevalence of Toxoplasma gondii and Neospora caninum infection in sheep, goats, and fallow deer farmed on the same area. J. Anim. Sci. 2018;96:2468–2473. doi: 10.1093/jas/sky122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday B.L. Bovine toxoplasmosis: experimental infections. Int. J. Parasitol. 1978;8:285–288. doi: 10.1016/0020-7519(78)90092-9. [DOI] [PubMed] [Google Scholar]
- Munoz-Zanzi C., Tamayo R., Balboa J., Hill D. Detection of oocyst-associated toxoplasmosis in swine from southern Chile. Zoonoses Public Health. 2012;59:389–392. doi: 10.1111/j.1863-2378.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- Murrell K.D., Djordjevic M., Cuperlovic K., Sofronic L., Savic M., Djordjevic M., Damjanovic S. Epidemiology of Trichinella infection in the horse: the risk from animal product feeding practices. Vet. Parasitol. 2004;123:223–233. doi: 10.1016/j.vetpar.2004.06.008. [DOI] [PubMed] [Google Scholar]
- O'Donovan J., Proctor A., Gutierrez J., Worrell S., Nally J., Marques P., Brady C., McElroy M., Sammin D., Buxton D., Maley S., Bassett H., Markey B. Distribution of lesions in fetal brains following experimental infection of pregnant sheep with Toxoplasma gondii. Vet. Pathol. 2012;49:462–469. doi: 10.1177/0300985811424732. [DOI] [PubMed] [Google Scholar]
- Okamoto I., Suzuki Y., Itho H., Fukuura H., Yokoyama I. A collective outbreak of porcine toxoplasmosis due to supplied feed supplements contaminated with toxoplasma oocysts. J. Jpn. Vet. Med. Assoc. 1989;42:729–732. [Google Scholar]
- Olinda R.G., Pena H.F., Frade M.T., Ferreira J.S., Maia L.A., Gennari S.M., Oliveira S., Dantas A.F., Riet-Correa F. Acute toxoplasmosis in pigs in Brazil caused by Toxoplasma gondii genotype Chinese 1. Parasitol. Res. 2016;115:2561–2566. doi: 10.1007/s00436-016-4999-0. [DOI] [PubMed] [Google Scholar]
- Opsteegh M., Teunis P., Zuchner L., Koets A., Langelaar M., van der Giessen J. Low predictive value of seroprevalence of Toxoplasma gondii in cattle for detection of parasite DNA. Int. J. Parasitol. 2011;41:343–354. doi: 10.1016/j.ijpara.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Opsteegh M., Maas M., Schares G., van der Giessen J. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01) an extensive literature review. Final report. EFSA Supporting Publications. 2016;13 (996E-n/a) [Google Scholar]
- Ortega-Pacheco A., Acosta Viana K.Y., Guzman-Marin E., Segura-Correa J.C., Alvarez-Fleites M., Jimenez-Coello M. Prevalence and risk factors of Toxoplasma gondii in fattening pigs farm from Yucatan, Mexico. Biomed. Res. Int. 2013;2013:231497. doi: 10.1155/2013/231497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M.R., Clarkson M.J., Trees A.J. Acute phase toxoplasma abortions in sheep. Vet. Rec. 1998;142:480–482. doi: 10.1136/vr.142.18.480. [DOI] [PubMed] [Google Scholar]
- Pan M., Lyu C., Zhao J., Shen B. Sixty years (1957–2017) of research on toxoplasmosis in China-an overview. Front. Microbiol. 2017;8:1825. doi: 10.3389/fmicb.2017.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini R.A., Buzzone G., Nardoni S., Rocchigiani G., Mancianti F. Seroprevalence and genotyping of Toxoplasma gondii in horses slaughtered for human consumption in Italy. J Equine Vet Sci. 2015;35:657–661. [Google Scholar]
- Passos L., Lima J., Figueiredo B. Determinacao da infeccao por Toxoplasma gondii em bovinos abatidos em Belo Horizonte (MG) através da frequencia de anticorps e tentativa de isoalmento a partir de musculatura diafragmantica. Arq. Bras. Med. Vet. Zoot. 1984;36:581–589. [Google Scholar]
- Pastiu A.I., Gyorke A., Blaga R., Mircean V., Rosenthal B.M., Cozma V. In Romania, exposure to Toxoplasma gondii occurs twice as often in swine raised for familial consumption as in hunted wild boar, but occurs rarely, if ever, among fattening pigs raised in confinement. Parasitol. Res. 2013;112:2403–2407. doi: 10.1007/s00436-013-3353-z. [DOI] [PubMed] [Google Scholar]
- Pastiu A.I., Gyorke A., Kalmar Z., Bolfa P., Rosenthal B.M., Oltean M., Villena I., Spinu M., Cozma V. Toxoplasma gondii in horse meat intended for human consumption in Romania. Vet. Parasitol. 2015;212:393–395. doi: 10.1016/j.vetpar.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Perry B.D., Randolph T.F. Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet. Parasitol. 1999;84:145–168. doi: 10.1016/s0304-4017(99)00040-0. [DOI] [PubMed] [Google Scholar]
- Pomares C., Ajzenberg D., Bornard L., Bernardin G., Hasseine L., Darde M.L., Marty P. Toxoplasmosis and horse meat, France. Emerg. Infect. Dis. 2011;17:1327–1328. doi: 10.3201/eid1707.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portella L.P., Cadore G.C., Sangioni L.A., Pellegrini L.F., Fighera R., Ramos F., Vogel F.S. Antibodies against apicomplexa protozoa and absence sarcocysts in heart tissues from horses in southern Brazil. Rev. Bras. Parasitol. Vet. 2017;26:100–103. doi: 10.1590/S1984-29612016068. [DOI] [PubMed] [Google Scholar]
- Puvanesuaran V.R., Noordin R., Balakrishnan V. Genotyping of Toxoplasma gondii isolates from wild boars in Peninsular Malaysia. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke J.R., Guerini M.N., Jerome M., White M.W. A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol. Biochem. Parasitol. 2003;131:119–127. doi: 10.1016/s0166-6851(03)00198-1. [DOI] [PubMed] [Google Scholar]
- Raeghi S., Akaberi A., Sedeghi S. Seroprevalence of Toxoplasma gondii in sheep, cattle and horses in Urmia North-West of Iran. Iran. J. Parasitol. 2011;6:90–94. [PMC free article] [PubMed] [Google Scholar]
- Rahdar M., Samarbaf-Zadeh A.R., Arab L. Evaluating the prevalence of Toxoplasma gondii in meat and meat products in Ahvaz by PCR method. Jundishapur J Microb. 2012;5:570–573. [Google Scholar]
- Rassouli M., Razmi G.R., Bassami M.R., Movassaghi A.R., Azizzadeh M. Study on ovine abortion associated with Toxoplasma gondii in affected herds of Khorasan Razavi Province, Iran based on PCR detection of fetal brains and maternal serology. Parasitology. 2011;138:691–697. doi: 10.1017/S0031182011000205. [DOI] [PubMed] [Google Scholar]
- Razmi G.R., Ghezi K., Mahooti A., Naseri Z. A serological study and subsequent isolation of Toxoplasma gondii from aborted ovine fetuses in Mashhad area, Iran. J. Parasitol. 2010;96:812–814. doi: 10.1645/GE-2428.1. [DOI] [PubMed] [Google Scholar]
- Razmi G.R., Abedi V., Yaghfoori S. Serological study of Toxoplasma gondii infection in Turkoman horses in the North Khorasan Province, Iran. J. Parasit. Dis. 2016;40:515–519. doi: 10.1007/s12639-014-0536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego W.M.F., Paula N.R.O., Vitor R.W.A., Silva R.A.B., Diniz B.L.M., Sousa M.M., Coelho W.A.C., Porfirio K.P., Pinheiro R.R., Alves F.S.F., Cavalcante A.C.R., Cardoso J.F.S. Risk factors for Toxoplasma gondii infection in goats and sheep raised in the State of Piaui in northeast Brazil. Small Rumin. Res. 2016;141:17–23. [Google Scholar]
- Ribeiro M.J., Rosa M.H., Bruhn F.R., Garcia Ade M., Rocha C.M., Guimaraes A.M. Seroepidemiology of Sarcocystis neurona, Toxoplasma gondii and Neospora spp. among horses in the south of the state of Minas Gerais, Brazil. Rev. Bras. Parasitol. Vet. 2016;25:142–150. doi: 10.1590/S1984-29612016029. [DOI] [PubMed] [Google Scholar]
- Rizzo H., Gaeta N.C., Hora J.H.C., Carvalho J.S., Pinheiro Júnior J.W., Gennari S.M., Pena H.F.J., Villalobos E.M.C., Gregory L. Risk factors for Toxoplasma gondii infection in sheep in the northeastern region of Brazil. Braz. J. Vet. Res. Anim. Sci. 2017;54:139–146. [Google Scholar]
- Roberts C.W., Cruickshank S.M., Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect. Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C.W., Walker W., Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli P.R., Freire R.L., Vidotto O., Marana E.R., Ogawa L., De Paula V.S., Garcia J.L., Navarro I.T. Prevalence of Neospora caninum and Toxoplasma gondii in sheep and dogs from Guarapuava farms, Parana State, Brazil. Res. Vet. Sci. 2007;82:202–207. doi: 10.1016/j.rvsc.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Rommel M., Sommer R., Janitschke K., Müller I. Experimentelle Toxoplasma-Infektion bei Kälbern. Berl. Muench. Tieraerztl. Wschr. 1966;79:41–45. [PubMed] [Google Scholar]
- Rosa C., Kasai N., Souza S.L.P., Guerra J.L., Rego A.A., Gennari S.M. Comparação das técnicas de imuno-histoquímica e bioensaio em camundongos para pesquisa de Toxoplasma gondii em tecidos de caprinos, experimentalmente inoculados. Arq. Inst. Biol. (São Paulo) 2001;68:13–17. [Google Scholar]
- Rougier S., Montoya J.G., Peyron F. Lifelong persistence of toxoplasma cysts: a questionable dogma? Trends Parasitol. 2017;33:93–101. doi: 10.1016/j.pt.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Ruiz A., Frenkel J.K. Intermediate and transport hosts of Toxoplasma gondii in Costa Rica. Am. J. Trop. Med. Hyg. 1980;29:1161–1166. doi: 10.4269/ajtmh.1980.29.1161. [DOI] [PubMed] [Google Scholar]
- Sa S.G., Lima D.C., Silva L.T., Pinheiro Junior J.W., Dubey J.P., Silva J.C., Mota R.A. Seroprevalence of Toxoplasma gondii among turkeys on family farms in the state of Northeastern Brazil. Acta Parasitol. 2016;61:401–405. doi: 10.1515/ap-2016-0053. [DOI] [PubMed] [Google Scholar]
- Sa S.G., Ribeiro-Andrade M., Silva L.T.R., Souza O.L.N., Lima D.C.V., Pedrosa C.M., Bezerra M.J.G., Mota R.A. Risk factors associated with Toxoplasma gondii infection in free-range chickens in the semiarid region of Brazil. Rev. Bras. Parasitol. Vet. 2017;26:221–225. doi: 10.1590/S1984-29612017033. [DOI] [PubMed] [Google Scholar]
- Saad N.M., Hussein A.A.A., Ewida R.M. Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in Upper Egypt. Vet World. 2018;11:1262–1265. doi: 10.14202/vetworld.2018.1262-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata F.B., Bellato V., Sartor A.A., de Moura A.B., de Souza A.P., Farias J.A. Toxoplasma gondii antibodies sheep in Lages, Santa Catarina, Brazil, and comparison using IFA and ELISA. Rev. Bras. Parasitol. Vet. 2012;21:196–200. doi: 10.1590/s1984-29612012000300004. [DOI] [PubMed] [Google Scholar]
- Samico-Fernandes E.F.T., Samico-Fernandes M.F.T., de Albuquerque P.P.F., de Almeida J.C., de Souza Santos A., da Rocha Mota A., de Souza Neto O.L., Mota R.A. Toxoplasma gondii in backyard pigs: seroepidemiology and mouse bioassay. Acta Parasitol. 2017;62:466–470. doi: 10.1515/ap-2017-0054. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sánchez R., Ferré I., Regidor-Cerrillo J., Gutiérrez-Expósito D., Ferrer L.M., Arteche-Villasol N., Moreno-Gonzalo J., Müller J., Aguado-Martínez A., Pérez V., Hemphill A., Ortega-Mora L.M., Benavides J. Virulence in mice of a Toxoplasma gondii type II isolate does not correlate with the outcome of experimental infection in pregnant sheep. Front. Cell. Infect. Microbiol. 2019;8:436. doi: 10.3389/fcimb.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger V.L., Cole C.R. Toxoplasmosis. VI. Isolation of toxoplasma from milk, placentas, and newborn pigs of asymptomatic carrier sows. Am. J. Vet. Res. 1955;16:536–539. [PubMed] [Google Scholar]
- Santana L.F., da Costa A.J., Pieroni J., Lopes W.D., Santos R.S., de Oliveira G.P., de Mendonca R.P., Sakamoto C.A. Detection of Toxoplasma gondii in the reproductive system of male goats. Rev. Bras. Parasitol. Vet. 2010;19:179–182. doi: 10.1590/s1984-29612010000300010. [DOI] [PubMed] [Google Scholar]
- Santoro A., Tagel M., Must K., Laine M., Lassen B., Jokelainen P. Toxoplasma gondii seroprevalence in breeding pigs in Estonia. Acta Vet. Scand. 2017;59:82. doi: 10.1186/s13028-017-0349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib M., Hussain M.H., Sajid M.S., Mansoor M.K., Asi M.N., Fadya A.A., Zohaib A., Sial A.U., Muhammad G., Ullah I. Sero-epidemiology of equine toxoplasmosis using a latex agglutination test in the three metropolises of Punjab, Pakistan. Trop. Biomed. 2015;32:276–285. [PubMed] [Google Scholar]
- Saraf P., Shwab E.K., Dubey J.P., Su C. On the determination of Toxoplasma gondii virulence in mice. Exp. Parasitol. 2017;174:25–30. doi: 10.1016/j.exppara.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Savvulidi F., Ptáček M., Stádník L. Pathogens in pocessed rm semen and aproaches for thir eimination. Acta Univ. Agric. Silvic. Mendel Brun. 2018;66:1065–1072. [Google Scholar]
- Schale S., Howe D., Yeargan M., Morrow J.K., Graves A., Johnson A.L. Protozoal coinfection in horses with equine protozoal myeloencephalitis in the eastern United States. J. Vet. Intern. Med. 2018;32:1210–1214. doi: 10.1111/jvim.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G., Bangoura B., Randau F., Goroll T., Ludewig M., Maksimov P., Matzkeit B., Sens M., Bärwald A., Conraths F.J., Opsteegh M., Van der Giessen J. High seroprevalence of Toxoplasma gondii and probability of detecting tissue cysts in backyard laying hens compared with hens from large free-range farms. Int. J. Parasitol. 2017;47:765–777. doi: 10.1016/j.ijpara.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Schares G., Herrmann D.C., Maksimov P., Matzkeit B., Conraths F.J., Moré G., Preisinger R., Weigend S. Chicken line-dependent mortality after experimental infection with three type IIxIII recombinant Toxoplasma gondii clones. Exp. Parasitol. 2017;180:101–111. doi: 10.1016/j.exppara.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Schlüter D., Däubener W., Schares G., Gross U., Pleyer U., Lüder C. Animals are key to human toxoplasmosis. Int. J. Med. Microbiol. 2014;304:917–929. doi: 10.1016/j.ijmm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Schnydrig P., Vidal S., Brodard I., Frey C., Posthaus H., Perreten V., Rodriguez-Campos S. Bacterial, fungal, parasitological and pathological analyses of abortions in small ruminants from 2012–2016. Schweiz. Arch. Tierheilkd. 2017;159:647–656. doi: 10.17236/sat00136. [DOI] [PubMed] [Google Scholar]
- Schoonman L.B., Wilsmore T., Swai E.S. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop. Anim. Health Prod. 2010;42:579–587. doi: 10.1007/s11250-009-9460-2. [DOI] [PubMed] [Google Scholar]
- Schulzig H.S., Fehlhaber K. Longitudinal study on the seroprevalence of Toxoplasma gondii infection in four German pig breeding and raising farms. Berl. Munch. Tierarztl. Wochenschr. 2005;118:399–403. [PubMed] [Google Scholar]
- Sechi P., Ciampelli A., Cambiotti V., Veronesi F., Cenci-Goga B.T. Seroepidemiological study of toxoplasmosis in sheep in rural areas of the Grosseto district, Tuscany, Italy. Ital. J. Anim. Sci. 2013;12 doi: 10.1186/1746-6148-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaapan R.M., Ghazy A.A. Isolation of Toxoplasma gondii from horse meat in Egypt. Pak. J. Biol. Sci. 2007;10:174–177. doi: 10.3923/pjbs.2007.174.177. [DOI] [PubMed] [Google Scholar]
- Shokri A., Sharif M., Teshnizi S.H., Sarvi S., Rahimi M.T., Mizani A., Ahmadpour E., Montazeri M., Daryani A. Birds and poultries toxoplasmosis in Iran: a systematic review and meta-analysis. Asian Pac J Trop Med. 2017;10:635–642. doi: 10.1016/j.apjtm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Shuralev E.A., Shamaev N.D., Mukminov M.N., Nagamune K., Taniguchi Y., Saito T., Kitoh K., Arleevskaya M.I., Fedotova A.Y., Abdulmanova D.R., Aleksandrova N.M., Efimova M.A., Yarullin A.I., Valeeva A.R., Khaertynov K.S., Takashima Y. Toxoplasma gondii seroprevalence in goats, cats and humans in Russia. Parasitol. Int. 2018;67:112–114. doi: 10.1016/j.parint.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Shwab E.K., Zhu X.Q., Majumdar D., Pena H.F., Gennari S.M., Dubey J.P., Su C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- Skjerve E., Waldeland H., Nesbakken T., Kapperud G. Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. Prev. Vet. Med. 1998;35:219–227. doi: 10.1016/s0167-5877(98)00057-9. [DOI] [PubMed] [Google Scholar]
- Sposito Filha E., Do Amaral V., Macruz R., Reboucas M.M., Santos S.M., Borgo F. Infeccao experimental de equinos com taquizoitos de Toxoplasma gondii. Rev. Bras. Parasitol. Vet. 1992;1:51–54. [Google Scholar]
- Stalheim O.H., Hubbert W.T., Boothe A.D., Zimmermann W.J., Hughes D.E., Barnett D., Riley J.L., Foley J. Experimental toxoplasmosis in calves and pregnant cows. Am. J. Vet. Res. 1980;41:10–13. [PubMed] [Google Scholar]
- Stormoen M., Tharaldsen J., Hopp P. Seroprevalence of Toxoplasma gondii infection in Norwegian dairy goats. Acta Vet. Scand. 2012;54:75. doi: 10.1186/1751-0147-54-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.W., Meng Q.F., Cong W., Shan X.F., Wang C.F., Qian A.D. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitol. Res. 2015;114:4211–4218. doi: 10.1007/s00436-015-4655-0. [DOI] [PubMed] [Google Scholar]
- Tan Q.D., Yang X.Y., Yin M.Y., Hu L.Y., Qin S.Y., Wang J.L., Zhou D.H., Zhu X.Q. Seroprevalence and correlates of Toxoplasma gondii infection in dairy cattle in northwest China. Acta Parasitol. 2015;60:618–621. doi: 10.1515/ap-2015-0087. [DOI] [PubMed] [Google Scholar]
- Tao Q., Wang Z., Feng H., Fang R., Nie H., Hu M., Zhou Y., Zhao J. Seroprevalence and risk factors for Toxoplasma gondii infection on pig farms in central China. J. Parasitol. 2011;97:262–264. doi: 10.1645/GE-2646.1. [DOI] [PubMed] [Google Scholar]
- Tassi P. Toxoplasma gondii infection in horses. A review. Parassitologia. 2007;49:7–15. [PubMed] [Google Scholar]
- Tavalla M., Sabaghan M., Abdizadeh R., Khademvatan S., Rafiei A., Razavi Piranshahi A. Seroprevalence of Toxoplasma gondii and Neospora spp. infections in Arab horses, Southwest of Iran. Jundishapur. J. Microbiol. 2015;8 doi: 10.5812/jjm.14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrusfield M.V. 3rd edition. Edition. Blackwell Science Ltd; Oxford: 2007. Veterinary Epidemiology. [Google Scholar]
- Tilahun B., Tolossa Y.H., Tilahun G., Ashenafi H., Shimelis S. Seroprevalence and risk factors of Toxoplasma gondii infection among domestic ruminants in East Hararghe zone of Oromia region, Ethiopia. Vet Med Int. 2018;2018:4263470. doi: 10.1155/2018/4263470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd E.C.D. Preliminary estimates of costs of foodborne disease in the United-States. J. Food Prot. 1989;52:595–601. doi: 10.4315/0362-028X-52.8.595. [DOI] [PubMed] [Google Scholar]
- Tonouhewa A.B., Akpo Y., Sessou P., Adoligbe C., Yessinou E., Hounmanou Y.G., Assogba M.N., Youssao I., Farougou S. Toxoplasma gondii infection in meat animals from Africa: systematic review and meta-analysis of sero-epidemiological studies. Vet World. 2017;10:194–208. doi: 10.14202/vetworld.2017.194-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.B., Savva D. Evidence of Toxoplasma gondii in an equine placenta. Vet. Rec. 1990;127:96. [PubMed] [Google Scholar]
- Turner C.B., Savva D. Detection of Toxoplasma gondii in equine eyes. Vet. Rec. 1991;129:128. doi: 10.1136/vr.129.6.128-a. [DOI] [PubMed] [Google Scholar]
- Turner C.B., Savva D. Transplacental infection of a foal with Toxoplasma gondii. Vet. Rec. 1992;131:179–180. doi: 10.1136/vr.131.8.179. [DOI] [PubMed] [Google Scholar]
- Tzanidakis N., Maksimov P., Conraths F.J., Kiossis E., Brozos C., Sotiraki S., Schares G. Toxoplasma gondii in sheep and goats: seroprevalence and potential risk factors under dairy husbandry practices. Vet. Parasitol. 2012;190:340–348. doi: 10.1016/j.vetpar.2012.07.020. [DOI] [PubMed] [Google Scholar]
- van den Brom R., Lievaart-Peterson K., Luttikholt S., Peperkamp K., Wouda W., Vellema P. Abortion in small ruminants in the Netherlands between 2006 and 2011. Tijdschr. Diergeneeskd. 2012;137:450–457. [PubMed] [Google Scholar]
- van der Giessen J., Fonville M., Bouwknegt M., Langelaar M., Vollema A. Seroprevalence of Trichinella spiralis and Toxoplasma gondii in pigs from different housing systems in The Netherlands. Vet. Parasitol. 2007;148:371–374. doi: 10.1016/j.vetpar.2007.06.009. [DOI] [PubMed] [Google Scholar]
- van Engelen E., Luttikholt S., Peperkamp K., Vellema P., Van den Brom R. Small ruminant abortions in The Netherlands during lambing season 2012–2013. Vet. Rec. 2014;174:506. doi: 10.1136/vr.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux J.D., Muccioli C., James E.R., Silveira C., Magargal S.L., Jung C., Dubey J.P., Jones J.L., Doymaz M.Z., Bruckner D.A., Belfort R., Jr., Holland G.N., Grigg M.E. Identification of an atypical strain of Toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. J. Infect. Dis. 2010;202:1226–1233. doi: 10.1086/656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi S.S., da Silva A.F., Frazao-Teixeira E., de Oliveira F.C.R., Consalter A., Padilha F.G.F., Fonseca A.B.M., Ferreira A.M.R. Characterization of the zoonotic potential of Toxoplasma gondii in horses from Rio de Janeiro State. Acta Trop. 2017;171:159–162. doi: 10.1016/j.actatropica.2017.03.036. [DOI] [PubMed] [Google Scholar]
- Verhelst D., De Craeye S., Vanrobaeys M., Czaplicki G., Dorny P., Cox E. Seroprevalence of Toxoplasma gondii in domestic sheep in Belgium. Vet. Parasitol. 2014;205:57–61. doi: 10.1016/j.vetpar.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Verhelst D., De Craeye S., Jennes M., Dorny P., Goddeeris B., Cox E. Interferon-gamma expression and infectivity of toxoplasma infected tissues in experimentally infected sheep in comparison with pigs. Vet. Parasitol. 2015;207:7–16. doi: 10.1016/j.vetpar.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Ranucci D., Branciari R., Miraglia D., Mammoli R., Fioretti D.P. Seroprevalence and risk factors for Toxoplasma gondii infection on finishing swine reared in the Umbria region, central Italy. Zoonoses Public Health. 2011;58:178–184. doi: 10.1111/j.1863-2378.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- Vesco G., Buffolano W., La Chiusa S., Mancuso G., Caracappa S., Chianca A., Villari S., Curro V., Liga F., Petersen E. Toxoplasma gondii infections in sheep in Sicily, southern Italy. Vet. Parasitol. 2007;146:3–8. doi: 10.1016/j.vetpar.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Villari S., Vesco G., Petersen E., Crispo A., Buffolano W. Risk factors for toxoplasmosis in pigs bred in Sicily, Southern Italy. Vet. Parasitol. 2009;161:1–8. doi: 10.1016/j.vetpar.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. Experimental transmission of Toxoplasma gondii by filth-flies. Am. J. Trop. Med. Hyg. 1971;20:411–413. doi: 10.4269/ajtmh.1971.20.411. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. Experimental transmission of Toxoplasma gondii by cockroaches. J. Infect. Dis. 1972;126:545–547. doi: 10.1093/infdis/126.5.545. [DOI] [PubMed] [Google Scholar]
- Wallander C., Frossling J., Dorea F.C., Uggla A., Vagsholm I., Lunden A. Pasture is a risk factor for Toxoplasma gondii infection in fattening pigs. Vet. Parasitol. 2016;224:27–32. doi: 10.1016/j.vetpar.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wanderley F.S., Porto W.J., Camara D.R., da Cruz N.L., Feitosa B.C., Freire R.L., de Moraes E.P., Mota R.A. Experimental vaginal infection of goats with semen contaminated with the “CPG” strain of Toxoplasma gondii. J. Parasitol. 2013;99:610–613. doi: 10.1645/12-126.1. [DOI] [PubMed] [Google Scholar]
- Wang J.L., Zhou D.H., Chen J., Liu G.X., Pu W.B., Liu T.Y., Qin S.Y., Yin M.Y., Zhu X.Q. The prevalence of antibodies to Toxoplasma gondii in horses in Changji Hui Autonomous Prefecture, Xinjiang, northwestern China. Rev. Bras. Parasitol. Vet. 2015;24:298–302. doi: 10.1590/S1984-29612015050. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhao G.W., Wang W., Zhang Z.C., Shen B., Hassan I.A., Xie Q., Yan R.F., Song X.K., Xu L.X., Li X.R. Pathogenicity of five strains of Toxoplasma gondii from different animals to chickens. Korean J. Parasitol. 2015;53:155–162. doi: 10.3347/kjp.2015.53.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang L., Ren Q., Yu F., Yang Y. Diagnosis of swine toxoplasmosis by PCR and genotyping of Toxoplasma gondii from pigs in Henan, Central China. BMC Vet. Res. 2017;13:152. doi: 10.1186/s12917-017-1079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts E., Zhao Y., Dhara A., Eller B., Patwardhan A., Sinai A.P. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. MBio. 2015;6 doi: 10.1128/mBio.01155-15. (e01155-01115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R.M., Dubey J.P., Siegel A.M., Kitron U.D., Mannelli A., Mitchell M.A., Mateus-Pinilla N.E., Thulliez P., Shen S.K., Kwok O.C.H., Todd K.S. Risk factors for transmission of Toxoplasma gondii on swine farms in Illinois. J. Parasitol. 1995;81:736–741. [PubMed] [Google Scholar]
- Weissenbock H., Dubey J.P. Toxoplasmosis epizootic in a fattening swine herd. Dtsch. Tierarztl. Wochenschr. 1993;100:370–374. [PubMed] [Google Scholar]
- Wiengcharoen J., Thompson R.C., Nakthong C., Rattanakorn P., Sukthana Y. Transplacental transmission in cattle: is Toxoplasma gondii less potent than Neospora caninum? Parasitol. Res. 2011;108:1235–1241. doi: 10.1007/s00436-010-2172-8. [DOI] [PubMed] [Google Scholar]
- Williams R.H., Morley E.K., Hughes J.M., Duncanson P., Terry R.S., Smith J.E., Hide G. High levels of congenital transmission of Toxoplasma gondii in longitudinal and cross-sectional studies on sheep farms provides evidence of vertical transmission in ovine hosts. Parasitology. 2005;130:301–307. doi: 10.1017/s0031182004006614. [DOI] [PubMed] [Google Scholar]
- Work K., Eriksen L., Fennestad K.L., Moller T., Siim J.C. Experimental toxoplasmosis in pregnant sows. I. Clinical, parasitological and serological observations. Acta Pathol. Microbiol. Scand. B: Microbiol. Immunol. 1970;78:129–139. [PubMed] [Google Scholar]
- Work T.M., Dagenais J., Rameyer R., Breeden R. Mortality patterns in endangered Hawaiian geese (Nene; Branta Sandvicensis) J. Wildl. Dis. 2015;51:688–695. doi: 10.7589/2014-11-256. [DOI] [PubMed] [Google Scholar]
- Wyss R., Sager H., Muller N., Inderbitzin F., Konig M., Audige L., Gottstein B. Distribution of Toxoplasma gondii and Neospora caninum under aspects of meat hygiene. Schweiz. Arch. Tierheilkd. 2000;142:95–108. [PubMed] [Google Scholar]
- Xu P., Song X., Wang W., Wang F., Cao L., Liu Q. Seroprevalence of Toxoplasma gondii infection in chickens in Jinzhou, northeastern China. J. Parasitol. 2012;98:1300–1301. doi: 10.1645/GE-3164.1. [DOI] [PubMed] [Google Scholar]
- Xu P., Li X., Tang F., Liu Y.H., Kou X., Zhao M.L., Li B., Guo L., Liu X.G., Zhao Q. Seroprevalence and risk factors for Toxoplasma gondii in sheep and goats in Jinzhou, Northeastern China. Trop. Biomed. 2015;32:563–567. [PubMed] [Google Scholar]
- Yang N., Mu M.Y., Li H.K., Long M., He J.B. Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasit. Vectors. 2012;5:237. doi: 10.1186/1756-3305-5-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Mu M.Y., Yuan G.M., Zhang G.X., Li H.K., He J.B. Seroprevalence of Toxoplasma gondii in slaughtered horses and donkeys in Liaoning province, northeastern China. Parasit. Vectors. 2013;6:140. doi: 10.1186/1756-3305-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M.Y., Wang J.L., Huang S.Y., Qin S.Y., Zhou D.H., Liu G.X., Tan Q.D., Zhu X.Q. Seroprevalence and risk factors of Toxoplasma gondii in Tibetan sheep in Gansu province, Northwestern China. BMC Vet. Res. 2015;11:41. doi: 10.1186/s12917-015-0358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wang S., Wang D., Li C., Zhang Z., Yao Z., Li T., Xie Q., Liu S., Zhang H. Seroprevalence of Toxoplasma gondii infection and risk factors in domestic sheep in Henan province, central China. Parasite. 2016;23:53. doi: 10.1051/parasite/2016064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Cao S., Sevinc F., Sevinc M., Ceylan O., Liu M., Wang G., Moumouni P.F., Jirapattharasate C., Suzuki H., Nishikawa Y., Xuan X. Enzyme-linked immunosorbent assays using recombinant TgSAG2 and NcSAG1 to detect Toxoplasma gondii and Neospora caninum-specific antibodies in domestic animals in Turkey. J. Vet. Med. Sci. 2017;78:1877–1881. doi: 10.1292/jvms.16-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Yin J., Xiao Y., Jiang N., Ankarlev J., Lindh J., Chen Q. A sero-epidemiological survey of Toxoplasma gondii infection in free-range and caged chickens in northeast China. Vet. Parasitol. 2008;158:360–363. doi: 10.1016/j.vetpar.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Zöller B., Koethe M., Ludewig M., Pott S., Daugschies A., Straubinger R.K., Fehlhaber K., Bangoura B. Tissue tropism of Toxoplasma gondii in turkeys (Meleagris gallopavo) after parenteral infection. Parasitol. Res. 2013;112:1841–1847. doi: 10.1007/s00436-013-3337-z. [DOI] [PubMed] [Google Scholar]
- Zou F., Yu X., Yang Y., Hu S., Chang H., Yang J., Duan G. Seroprevalence and risk factors of Toxoplasma gondii infection in buffaloes, sheep and goats in Yunnan Province, Southwestern China. Iran. J. Parasitol. 2015;10:648–651. [PMC free article] [PubMed] [Google Scholar]