Abstract
In a time of increasing threats and decreasing financial resources, monitoring and controlling all possible foodborne hazards at the same time and to the same extent has become more challenging than ever. Therefore, attention is increasingly being paid to the so-called “risk ranking” methods that enable decision makers to focus on the most important foodborne hazards — even when time is limited and knowledge incomplete. In this review paper, we provide an overview of the most common quantitative methods and metrics used for ranking the risks associated with foodborne parasites and present the state of the art on risk ranking exercises for foodborne parasites.
A number of risk ranking metrics and methods are available, ranging from simple approaches that can be used to assess the health or economic impact of a foodborne parasitic disease, to more complicated but more comprehensive multi-criteria assessments. For health impact assessment, measures of population health such as disease occurrence and number of deaths; Disability-Adjusted Life Years (DALYs) measuring the healthy life years lost; and Quality-Adjusted Life Years (QALYs) measuring the number of life years lived in optimal health, are described. For economic impact assessment, applied approaches that measure the cost-of-illness from a societal perspective and stated preference methods are outlined. Finally, Multi-Criteria Decision Analysis (MCDA), which can be used to integrate multiple metrics and criteria into a single ranking, is described.
These risk ranking methods for foodborne parasites are increasingly performed to aid priority setting at global, regional, and national levels. As different stakeholders have their own prioritization objectives and beliefs, the outcome of such exercises is necessarily context-dependent. Therefore, when designing a risk ranking exercise for foodborne parasites, it is important to choose the metrics and methods, as well as what to rank, in the light of the predefined context of the question being addressed and the target audience.
Abbreviations: DALY, Disability-Adjusted Life Year; FAO, Food and Agriculture Organization of the United Nations; GBD, Global Burden of Disease; MCDA, Multi-Criteria Decision Analysis; QALY, Quality-Adjusted Life Year; SMPH, Summary Measure of Population Health; WHO, World Health Organization; WTA, Willingness-to-accept; WTP, Willingness-to-pay; YLD, Year Lived with Disability; YLL, Year of Life Lost
Keywords: Cost-of-illness, Disability-adjusted life years, Foodborne parasites, Multi-criteria decision analysis, Priority setting
Highlights
-
•
Risk ranking of foodborne parasites is increasingly used to set priorities.
-
•
Methods range from simple metrics to comprehensive multi-criteria assessments.
-
•
The choice of methods and the prior definition of what to rank define the outcomes.
-
•
The design of risk ranking exercises critically depends on the target audience.
1. Introduction
In a time of increasing threats (or recognition, c.q., perception thereof) and decreasing financial resources, it has become more challenging than ever to monitor and control all possible foodborne hazards at the same time and to the same extent (Speybroeck et al., 2015). Consequently, attention is being increasingly directed on methods that enable decision makers to focus on the most important foodborne hazards — even when time is limited and knowledge incomplete (Stella et al., 2013). These exercises are often labeled “risk ranking”, but may differ widely in their intention, scope and methodology. According to the Codex Alimentarius, risk is defined as “a function of the probability of an adverse health effect and the severity of that effect, consequential to a hazard(s) in food” (CAC (Codex Alimentarius Commission), 1999). However, severity can be quantified in different ways — it may, for instance, be defined as the health or economic impact of the adverse health effects. Furthermore, the function can take many different shapes — ranging from a mere sum to complicated weighted averages. As a result, the concept of “risk”, and thus “risk ranking”, is not as standardized as it should be. However, the same goal, which is to accomplish an internally consistent and comparable set of risk estimates allowing ranking, and thus prioritization among a given number of hazards, is shared in all risk ranking exercises.
Foodborne parasitic diseases present some unique challenges, including their often prolonged incubation period and association with chronic sequelae. Furthermore, as most foodborne parasitic diseases are not notifiable, their true importance is often underreported and under-recognized (Torgerson et al., 2015). In this review paper, we aim to provide an overview of the most common quantitative methods and metrics used for ranking foodborne parasites according to their associated risks. We also provide the state of the art on risk ranking exercises for foodborne parasites. For further information on risk ranking, readers are kindly referred to Brookes et al. (2015), who discuss risk ranking in the context of decision science, and to O'Brien et al. (2016) and Van der Fels-Klerx et al. (2016), who discuss risk ranking methods for infectious and foodborne diseases, respectively.
2. Health impact
2.1. Methods and metrics
Quantifying health impacts may be based on disease occurrence (prevalence or incidence) or on the number of deaths (mortality). However, these unidimensional or simple measures of population health do not provide a complete picture of the impact of foodborne parasites on human health as they do not combine the impacts of morbidity and mortality, thus precluding a comparative ranking of diseases with high morbidity, but low case-fatality, such as chorioretinitis due to toxoplasmosis, and highly lethal diseases such as alveolar echinococcosis (Batz et al., 2012, Devleesschauwer et al., 2015a). Furthermore, disease severity, defined by the impact on quality of life and the duration of the symptoms, as well as the expected residual life expectancy at the age of death, should be accounted for when quantifying burden of disease. Indeed, certain parasitic infections may be very common, but their clinical impact may be minimal. For instance, infections with a highly prevalent parasite such as the pinworm, Enterobius vermicularis, have a very low burden because most of the cases are mild to asymptomatic and self-limiting (Knopp et al., 2012).
In order to overcome the limitations of simple measures such as incidence and mortality, summary measures of population health (SMPHs) have been developed as an additional way of expressing information for quantifying disease burden. The Disability-Adjusted Life Year (DALY) is currently the most widely used SMPH in public health research. Originally developed to quantify and compare the burden of diseases, injuries, and risk factors within and across countries, the DALY summarizes the occurrence and impact of morbidity and mortality in a single metric (Devleesschauwer et al., 2014a). The DALY is the key measure in the Global Burden of Disease (GBD) studies and has been officially adopted by the World Health Organization (WHO) for reporting on health information (Murray et al., 2012; WHO (World Health Organization), 2017).
The DALY is a health gap measure, measuring the quantity of healthy life years lost due to a disease or injury against some idealized health profile. DALYs are calculated by adding the number of years lived with disability adjusted for the severity of the disease (YLDs) and the number of years of life lost due to premature mortality (YLLs):
YLD = Number of incident cases × Duration until remission or death × Disability Weight.
YLL = Number of deaths × Residual life expectancy at the age of death.
An alternative formula for calculating YLDs was introduced by the GBD 2010 study (Murray et al., 2012):
YLD = Number of prevalent cases × Disability Weight.
This formula reflects a prevalence perspective instead of an incidence perspective. The incidence perspective assigns all health outcomes, including those in future years, to the initial event (e.g., exposure to a certain foodborne parasite). This approach therefore reflects the future burden of disease resulting from current events. In the prevalence perspective, on the other hand, the health status of a population is assessed at a specific point in time, and prevalent diseases are attributed to initial events that happened in the past. This approach thus reflects the current burden of disease resulting from previous events. Although both perspectives are valid, the incidence perspective is more appropriate for foodborne parasites, as it is more sensitive to current epidemiological trends, including the effects of intervention measures (Murray, 1994, Devleesschauwer et al., 2015a).
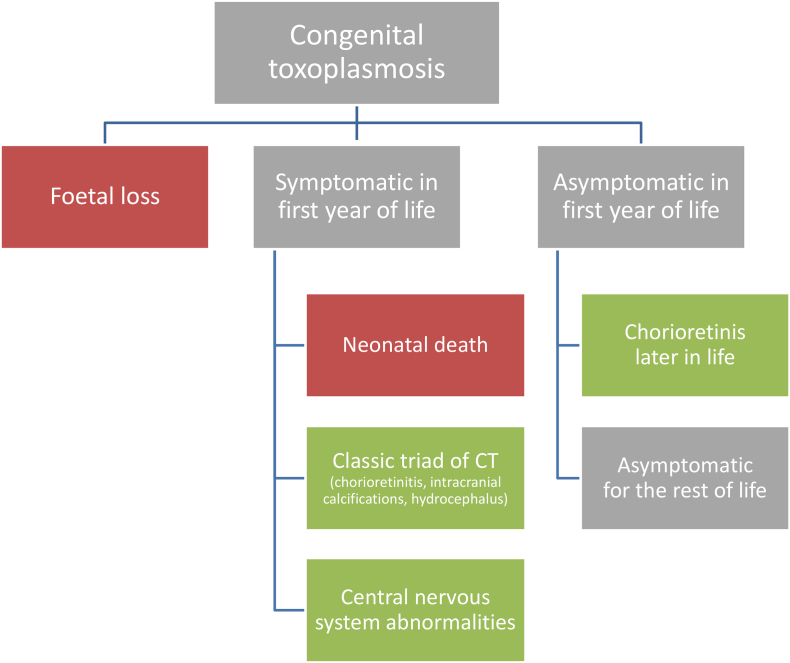
Different approaches can be used for calculating DALYs, depending on whether the interest lies in quantifying the burden of a health outcome, hazard, or risk factor (Devleesschauwer et al., 2014b). An obvious choice for quantifying the health impact of foodborne parasites is the hazard-based approach. This approach defines the burden of a specific foodborne parasites as that resulting from the health states, i.e., acute symptoms, chronic sequelae, and death, which are causally related to the concerned parasite transmitted through food, and which may become manifest at different time scales or have different severity levels (Mangen et al., 2013). The starting point for quantifying DALYs is therefore typically the construction of a disease model or outcome tree, which is a schematic representation of the various health states associated with the concerned hazard, and the possible transitions between these states (Devleesschauwer et al., 2014b). Fig. 1 presents an example disease model for congenital toxoplasmosis, but excludes the potential for long-term psychiatric outcomes or the potential for reactivation of infection in people who develop immunosuppressive disorders.
Fig. 1.
Disease model for congenital toxoplasmosis (CT), adapted from Nissen et al. (2017).
Green boxes accrue years lived with disability, red boxes accrue years of life lost. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The key epidemiological data required by DALYs are the number of cases for all considered health states, including death, which are associated with the foodborne parasite under study. This requires that an association has to be established, and quantified, between the health state and the foodborne parasite. Two complementary approaches may be defined for quantifying foodborne parasite attributable cases (Stella et al., 2013, Devleesschauwer et al., 2015a). The top-down approach starts from available epidemiological data, and associates health states with the concerned hazard at an individual level (i.e., categorical attribution) or at a population level (i.e., comparative risk assessment based on the calculation of population attributable fractions). The bottom-up approach starts from exposure and dose-response data, and predicts the expected number of cases through risk assessment (see, for instance, Anantanawat et al., 2012). Although both methods build on solid methodological foundations, they have been shown to provide differing estimates for chemical (Jakobsen et al., 2015) and microbial hazards (Evers and Bouwknegt, 2016), and there are no indications that this would not be the case for foodborne parasites.
In addition to the DALY metric, other SMPHs may be used to quantify the health impact of foodborne parasites, such as the Quality-Adjusted Life Year (QALY) metric. QALYs are a measure of the number of life years lived in optimal health, obtained by integrating quantity and quality of life:
QALY = Duration × Health-related quality of life weight.
Although the QALY is a key metric in cost-effectiveness analyses (Sanders et al., 2016), its use in burden of disease assessments has so far remained limited. Batz et al. (2012), for instance, compared QALYs in presence versus in absence of a certain foodborne parasitic illness, thereby obtaining an estimate of the QALY losses due to the foodborne parasite, and thus a measure of health impact.
Although SMPHs provide a clear advantage over unidimensional measures of population health, they come at a cost of being more data-demanding, making them more prone to biases and uncertainties. Furthermore, the integration of morbidity and mortality necessitates normative assumptions, for instance regarding death being the worst health state to experience. Such assumptions result in methodological differences for calculating a given SMPH, as well as sparking continuous debate among experts (Gold et al., 2002). Moreover, SMPHs per definition only quantify tangible health impact, thus ignoring economic impact and perceived burden. Finally, the aggregated nature of SMPHs also makes it difficult to understand which factors drive a high burden (incidence, severity, mortality, age) and estimates should always be accompanied by disaggregated data.
2.2. State of the art
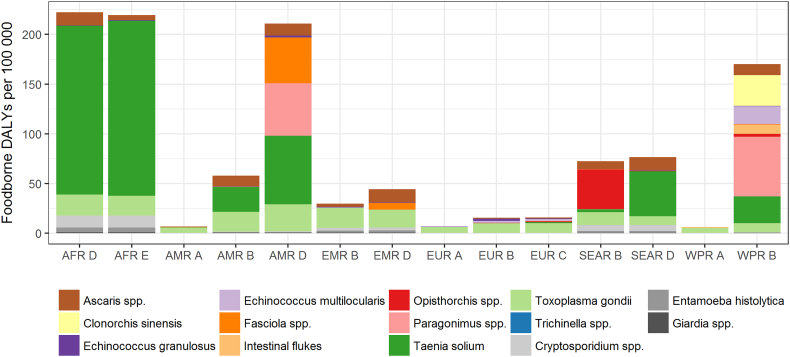
To date, the most comprehensive risk ranking of foodborne parasites based on health impact has been achieved by the Foodborne Disease Burden Epidemiology Reference Group (FERG) of the World Health Organization. FERG quantified the global and regional burden of 31 foodborne hazards (Havelaar et al., 2015), including four protozoa (Cryptosporidium spp., Entamoeba histolytica, Giardia spp., and Toxoplasma gondii) and ten helminths (including two nematodes: Ascaris spp., Trichinella spp.; three cestodes: Echinococcus granulosus, Echinococcus multilocularis, Taenia solium; and five trematodes: Clonorchis sinensis, Fasciola spp., intestinal flukes, Opisthorchis spp., Paragonimus spp.) (Kirk et al., 2015, Torgerson et al., 2015). Data were abstracted from systematic reviews, disease databases, and reports from national surveillance systems, and used to estimate the number of infections, sequelae, deaths, and DALYs, by age and region for 2010. A Bayesian random effects model was used to impute data gaps, while expert elicitation was used to attribute disease burden to different exposure routes and food items. Together, the considered parasitic diseases caused more than 400 million illnesses, resulting in nearly 100,000 deaths and 12 million DALYs. Intestinal protozoa were responsible for nearly 90% of illnesses, while helminths were responsible for the majority (60%) of deaths and DALYs. Across all parasites being considered, 22% of illnesses, 55% of deaths, and 61% of DALYs were estimated to be due to foodborne transmission. The highest numbers of global foodborne deaths were due to T. solium, E. multilocularis, and C. sinensis; while the highest numbers of global foodborne DALYs were due to T. solium, Paragonimus spp., and T. gondii (Table 1). The largest burden of foodborne parasitic disease occurred in the African sub-regions and the developing sub-regions of the Americas and Southeast Asia (Fig. 2).
Table 1.
World Health Organization Foodborne Disease Burden Epidemiology Reference Group (WHO/FERG) estimates of the global burden of fourteen foodborne parasitic hazards, 2010 (Torgerson et al., 2015, Kirk et al., 2015).
| Cases (‘000) | Deaths | Disability-adjusted life years (‘000) |
|---|---|---|
| Giardia spp. (28236) | Taenia solium (28114) | Taenia solium (2788) |
| Entamoeba histolytica (28024) | Echinococcus multilocularis (7771) | Paragonimus spp. (1049) |
| Toxoplasma gondii (10280) | Clonorchis sinensis (5770) | Toxoplasma gondii (829) |
| Ascaris spp. (12281) | Cryptosporidium spp. (3759) | Ascaris spp. (605) |
| Cryptosporidium spp. (8585) | Opisthorchis spp. (1498) | Clonorchis sinensis (523) |
| Taenia solium (370) | Entamoeba histolytica (1470) | Echinococcus multilocularis (312) |
| Paragonimus spp. (139) | Ascaris spp. (1008) | Cryptosporidium spp. (296) |
| Echinococcus granulosus (43) | Toxoplasma gondii (684) | Opisthorchis spp. (188) |
| Clonorchis sinensis (32) | Echinococcus granulosus (482) | Intestinal flukes (155) |
| Intestinal flukes (19) | Paragonimus spp. (250) | Entamoeba histolytica (139) |
| Opisthorchis spp. (16) | Trichinella spp. (4) | Fasciola spp. (90) |
| Fasciola spp. (11) | Fasciola spp. (0) | Echinococcus granulosus (40) |
| Echinococcus multilocularis (8) | Giardia spp. (0) | Giardia spp. (26) |
| Trichinella spp. (4) | Intestinal flukes (0) | Trichinella spp. (0.6) |
Fig. 2.
Foodborne Disability-Adjusted Life Years (DALYs) per 100,000 population, per World Health Organization sub-region (Torgerson et al., 2015, Kirk et al., 2015).
AFR: African Region, AMR: Region of the Americas, EMR: Eastern Mediterranean Region, EUR: European Region, SEAR: South-East Asia Region, WPR; Western Pacific Region; Stratum A: very low child and adult mortality, Stratum B: low child mortality and very low adult mortality, Stratum C: low child mortality and high adult mortality, Stratum D: high child and adult mortality, Stratum E: high child mortality and very high adult mortality.
In addition to the FERG estimates, internally comparable estimates of the global, regional and national health burden of foodborne parasites have been generated by the Institute for Health Metrics and Evaluation (GBD 2016 DALYs and HALE Collaborators, 2017). In the Global Burden of Disease 2016 study, five foodborne parasitic diseases were included – i.e., cysticercosis, cystic echinococcosis, foodborne trematodoses, cryptosporidiosis, and amoebosis. Table 2 shows the estimated number of YLDs, deaths and DALYs for the year 2016.
Table 2.
Institute for Health Metrics and Evaluation (IHME) estimates of the global burden of five foodborne parasitic diseases (GBD 2016 DALYs and HALE Collaborators, 2017; IHME (Institute for Health Metrics and Evaluation), 2016).
| Years lived with disability (‘000) | Deaths | Disability-adjusted life years (‘000) |
|---|---|---|
| Foodborne trematodoses (1771) | Cryptosporidiosis (57203) | Cryptosporidiosis (4610) |
| Cysticercosis (421) | Amoebosis (26748) | Foodborne trematodoses (1771) |
| Amoebosis (207) | Cystic echinococcosis (1012) | Amoebosis (1277) |
| Cryptosporidiosis (117) | Cysticercosis (999) | Cysticercosis (468) |
| Cystic echinococcosis (90) | Foodborne trematodoses (0) | Cystic echinococcosis (137) |
In addition to global and regional risk ranking exercises, several authors have estimated the burden of foodborne parasites at country level to support national decision making (Haagsma et al., 2013). Table 3 provides an overview of comparative burden of disease studies conducted at national level. These studies were mainly set in developed countries (i.e., the Netherlands, United States, Canada, Greece), while two were set in Nepal and Kyrgyzstan. Most included T. gondii, Cryptosporidium spp., Giardia spp., and a few included E. granulosus, E. multilocularis, Entamoeba spp., and Cyclospora cayetanensis.
Table 3.
National risk ranking of foodborne parasites based on summary measures of population health.
| Reference | Country | Reference period | Scope | Foodborne parasite (disease); ranked from highest to lowest estimated burden |
|---|---|---|---|---|
| Gkogka et al., 2011 | Greece | 1996–2006 | 19 foodborne diseases |
Echinococcus granulosus (cystic echinococcosis) Toxoplasma gondii (congenital toxoplasmosis) N/A (“other helminthoses”) Cryptosporidium spp. (cryptosporidiosis) Giardia spp. (giardiosis) Entamoeba spp. (amoebosis) |
| Hoffmann et al., 2012 | United States of America | 2009 | 14 foodborne pathogens |
Toxoplasma gondii (acquired and congenital toxoplasmosis) Cryptosporidium spp. (cryptosporidiosis) Cyclospora cayetanensis (cyclosporosis) |
| Havelaar et al., 2012 | The Netherlands | 2009 | 14 foodborne pathogens |
Toxoplasma gondii (acquired and congenital toxoplasmosis) Giardia spp. (giardiosis) Cryptosporidium spp. (cryptosporidiosis) |
| Kwong et al., 2012 | Ontario, Canada | 2006 | 51 infectious diseases |
Giardia spp. (giardiosis) Cryptosporidium spp. (cryptosporidiosis) Cyclospora cayetanensis (cyclosporosis) |
| Devleesschauwer et al., 2014c | Nepal | 2000–2012 | 3 parasitic zoonoses |
Taenia solium (neurocysticercosis) Toxoplasma gondii (congenital toxoplasmosis) Echinococcus granulosus (cystic echinococcosis) |
| Mangen et al., 2015 | The Netherlands | 2011 | 14 foodborne pathogens |
Toxoplasma gondii (acquired and congenital toxoplasmosis) Giardia spp. (giardiosis) Cryptosporidium spp. (cryptosporidiosis) |
| Counotte et al., 2016 | Kyrgyzstan | 2013 | 7 zoonoses |
Echinococcus multilocularis (alveolar echinococcosis) Echinococcus granulosus (cystic echinococcosis) Toxoplasma gondii (toxoplasmosis) |
| van Lier et al., 2016 | The Netherlands | 2007–2011 | 32 infectious diseases |
Toxoplasma gondii (acquired and congenital toxoplasmosis) Giardia spp. (giardiosis) Cryptosporidium spp. (cryptosporidiosis) |
3. Economic impact
3.1. Methods and metrics
As for health impact, different methods exist for estimating the economic impact of foodborne parasites. The most commonly applied approach measures the cost-of-illness from a societal perspective, taking into account that foodborne parasites have an impact on several stakeholders within the society (Mangen et al., 2015). In cost-of-illness studies, three broad families of cost items are typically defined (Mangen et al., 2010). First, direct healthcare costs defined as the resources provided by the healthcare sector, such as healthcare provider consultations, diagnostic testing, medication, and hospitalization. Second, patient costs (or direct non-healthcare costs) defined as the resources used for healthcare that are not borne by the healthcare system, such as over-the-counter medications and other patient co-payments, and travel expenses to visit a healthcare provider. Third, productivity losses (or indirect non-healthcare costs) defined as the losses due to absenteeism or job loss of patients and their caregivers. A fourth category, the future savings in healthcare costs due to premature death (or indirect healthcare costs), is increasingly being discussed, but not yet routinely included in cost-of-illness studies.
An alternative to cost-of-illness studies are stated preference methods, which elicit general population estimates on the amount people would be willing to pay to prevent (willingness-to-pay; WTP) or be willing to receive to compensate the presence of (willingness-to-accept; WTA) a certain foodborne illness. Estimates are typically derived using discrete choice (or contingent valuation) experiments, in which respondents are asked to choose between two mutually exclusive scenarios, such as the purchase of non-labeled chicken at current market prices, versus the purchase of Campylobacter-free chicken at a higher price (Van der Fels-Klerx et al., 2016). Health economists consider stated preferences as the most complete and correct economic welfare measures, as they are not limited to tangible costs but also allow incorporation of changes in consumer welfare associated with pain, distress and inconvenience. Furthermore, they quantify societal preferences, instead of relying solely on technical grounds. Nonetheless, their use has been very limited to date, as the technique is complicated, resource-intensive, and known to suffer from significant between-respondent variability — reflecting differential consumer behavior, which, to some extent, is associated with the respondents' differential ability to pay. Comparability of stated preferences across regions may therefore also be difficult.
On top of the costs linked to the health impact of foodborne parasites (quantified through cost-of-illness or stated preferences), these hazards may also incur an economic impact due to surveillance and other regulatory activities in place to monitor and prevent infection. In the EU, for instance, inspection of pigs at slaughterhouse level for Trichinella spp. induces an estimated annual cost of € 25 million (Torgerson, 2013), while the health impact of trichinellosis is negligible (Devleesschauwer et al., 2015b). As many foodborne parasites are zoonotic, livestock losses due to clinical or subclinical infection may further add to the economic burden. In Tanzania, the impact of lower prices for T. solium infected pigs was estimated at US$ 2.8 million, accounting for 35% of the total economic impact of T. solium in the country (Trevisan et al., 2017). At a global level, Budke et al. (2006) estimated up to US$ 2 billion livestock production losses due to cystic echinococcosis as a result of liver condemnation, reduction in carcass weight, decrease in hide value, decrease in milk production, and decreased fecundity. Market access (or the lack thereof) may also have significant economic impacts. Furthermore, these knock-on economic effects are not only limited to the population affected by the outbreak. For instance, the first foodborne outbreaks of cyclosporosis in the United States that were associated with raspberries imported from South America resulted in huge economic losses and unemployment in the already marginal economic area of Guatemala where the raspberries originated (Pratdesaba et al., 2001).
Risk ranking based on economic impact may be more tangible and appealing to certain risk managers. It also allows taking multiple dimensions into account, ranging from medical costs, to trade impacts and livestock losses; this however comes at a cost of requiring an even larger amount of data than health impact measures. As for SMPHs, different methodologies and normative values exist for estimating economic impact, leading to limited comparability between studies. Finally, economic impact assessments do not always capture the costs of pain and suffering, yielding cost estimates that are strongly dependent on the economic development level of the study area.
3.2. State of the art
Although foodborne parasites are of global concern, there are so far no global risk rankings of foodborne parasites based solely on economic impact. Murrell (1991), Roberts et al. (1994) and Torgerson and Macpherson (2011) aimed at providing a global perspective by reviewing the economic impact of foodborne parasites in multiple countries; however, given the methodological differences between different studies, such reviews do not provide accurate rankings. Furthermore, there are relatively few assessments of the global economic impact of individual foodborne parasites. Budke et al. (2006) estimated global monetary losses resulting from human and livestock cystic echinococcosis. Human-associated direct and indirect costs resulted in a global loss of US$ 764 million, while livestock-associated losses due to liver condemnation and reductions in carcass weight, hide value, milk production, and fecundity resulted in a global loss of US$ 2 billion.
More efforts have been made to conduct risk ranking of foodborne parasites based on economic impact at a national level, in particular in the United States and in the Netherlands. Hoffmann et al. (2012) estimated the annual cost-of-illness in the United States of 14 foodborne pathogens for the year 2009, including that of T. gondii (US$ 2973 million), Cryptosporidium parvum (US$ 47 million), and C. cayetanensis (US$ 2 million). Jointly, these three foodborne parasites accounted for 21% of the cost-of-illness of all 14 considered pathogens. In a more comprehensive study including 31 foodborne pathogens and a broad category of unspecified agents, Scharff (2012) estimated the cost of foodborne illness in the United States for the year 2010, based on medical costs, monetized QALY losses, and illness-related mortality. The total economic impact was estimated at US$ 78 billion, of which 5% was due to the five included foodborne parasites – i.e., T. gondii (US$ 3456 million), Giardia duodenalis (US$ 282 million), Cryptosporidium spp. (US$ 168 million), C. cayetanensis (US$ 17 million) and Trichinella spp. (US$ 2 million). The cost per case was significantly higher for T. gondii and Trichinella spp. (US$ 40,000 and US$ 15,000, respectively), than for the three other included foodborne parasites (< US$ 4000). In the Netherlands, the cost-of-illness of Cryptosporidium spp., Giardia spp. and T. gondii was € 8 million, € 11 million, and € 55 million, respectively, accounting for 16% of the economic impact of all considered foodborne pathogens (Mangen et al., 2015). Whereas direct healthcare costs were found to be the dominant component of the cost-of-illness of T. gondii, productivity losses were the most important component of Cryptosporidium spp. and Giardia spp. cost-of-illness. This was also noted in the cost estimate for the enormous waterborne outbreak of cryptosporidiosis in Milwaukee in 1993, in which the total cost of outbreak-associated illness was estimated at 96.2 million US dollars, of which 31.7 million US dollars were medical costs and 64.6 million US dollars were productivity losses (Corso et al., 2003).
4. Integrating multiple criteria
4.1. Methods and metrics
Using a single criterion to rank risks may be insufficient as diseases vary greatly in incidence, clinical manifestations, control measures, transmission potential, and socio-economic impact in animals and humans. Trichinella spp., for instance, have a near negligible health impact in Europe, but their economic impact remains important due to continued monitoring and trade implications (Devleesschauwer et al., 2015b). Likewise, Taenia saginata mainly poses an economic burden to farmers and society, while the health impact of T. saginata taeniosis is limited (Laranjo-González et al., 2016). To explore this limitation of single criterion based risk rankings, it may be useful to rank risks according to multiple criteria (Mangen et al., 2010). Mangen et al. (2015), for instance, quantified the burden of foodborne disease in the Netherlands in 2011 using DALYs and cost-of-illness estimates, both at a population and individual level. These different criteria led to four different rankings, with some hazards, most notably T. gondii, scoring high on multiple rankings.
Several authors have gone beyond the simple comparison of different rankings and proposed methods for combining multiple criteria into a single ranking. The most basic methods rely on the qualitative or semi-quantitative integration of two dimensions, for instance through the construction of a risk matrix – i.e., a two-dimensional combination of two criteria, such as an exposure and a consequence measure, with results ranging from low/low to high/high — but see Cox (2008) for a review of limitations of this method. A specific example of combining two criteria is the translation of DALYs (or QALY losses) into economic impact estimates, and vice versa. Indeed, some authors combined DALYs and economic impact estimates by assuming one DALY to correspond to an economic loss equal to the per capita gross national product (Torgerson et al., 2008). This however implies that the relative value of health loss depends on the wealth of the nation, which may raise equity issues, especially when performing cross-country or global rankings. To address this limitation, Torgerson et al. (2017) introduced the zDALY metric, which is the sum of the DALY metric for human health losses and the equivalent time losses associated with animal losses, defined as the economic impact of the animal losses divided by the per capita gross national product.
More advanced methods make use of the Multi-Criteria Decision Analysis (MCDA) framework. In MCDA, an overall importance measure is constructed based on different criteria, which are assigned weights reflecting their perceived contribution (Cardoen et al., 2009, Havelaar et al., 2010; FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2014; Robertson et al., 2015). A typical MCDA exercise thus contains the following steps: identification of pathogens to be ranked; definition of criteria and description of scoring methods (e.g., economic impact, health impact, impact on trade); elicitation of weights for the different criteria (e.g., economic impact may be perceived as less important than health impact, and morbidity or mortality may vary in importance depending on circumstance and perception); and scoring of the criteria, in a quantitative, semi-quantitative, or qualitative way, based on existing data or on expert elicitation. The final ranking is then given by the weighted sum of the different criteria. In addition to this simple additive ranking approach, more advanced MCDA approaches based on utility theory or outranking have been proposed (Geldermann and Schöbel, 2011). Their application to rank foodborne parasites has so far remained limited — but see Ng and Sargeant (2012) for an application of conjoint analysis and Kadohira et al. (2015) for an application of the analytic hierarchy process method, both examples of utility based methods. Despite potential subjective elements, MCDA provides a reproducible, standardized and transparent framework for ranking risks, and is consequently used in multiple sectors (Cardoen et al., 2009, Behzadian et al., 2010, Anderson et al., 2011) — the European Centre for Disease Prevention and Control, for instance, prepared an MCDA toolkit for prioritizing infectious disease threats (ECDC (European Centre for Disease Prevention and Control), 2017). MCDA also allows the simultaneous consideration of criteria that are quantitative (e.g., incidence, case-fatality ratio) with others for which only semi-quantitative measures are available (e.g., trade relevance scored as “no relevance”, “some relevance”, and “high relevance”) (Ruzante et al., 2010; FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2014). MCDA as such does not strive for an absolute numerical reflection of the actual situation; rather, it strives to compare the relative importance of foodborne parasites using a comprehensive consideration of all components that are deemed relevant for that importance.
4.2. State of the art
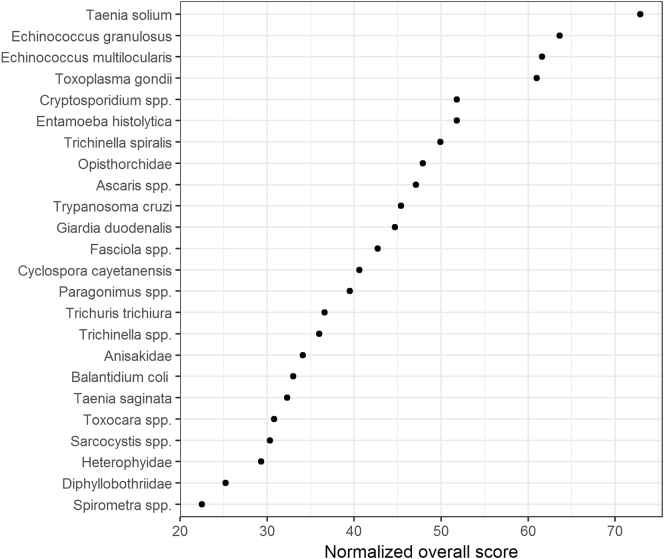
In 2012, the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) jointly organized a multicriteria-based ranking of foodborne parasites at a global level. The exercise considered 24 foodborne parasites, narrowed down from an initial list of 93, which were scored according to seven criteria, including criteria related to health impact, trade relevance, and impacts on economically vulnerable communities. Fig. 3 presents the obtained global ranking of foodborne parasites, confirming the importance of T. solium at a global level, closely followed by E. granulosus, E. multilocularis, and T. gondii. Recently, the FAO/WHO MCDA exercise has been repeated at a regional level. Robertson et al. (2015) used the MCDA approach to perform a risk ranking of foodborne parasites in India, while Bouwknegt et al. (2017) used the approach to generate European risk rankings. As expected, the results differed from those of the global ranking, as the epidemiology and impact of foodborne parasites is known to vary considerably between countries and regions, while perhaps also the perception of different criteria may vary across cultures. Specifically, in India T. solium was ranked highest, followed by Cryptosporidium spp. and E. granulosus, while in Europe, E. multilocularis ranked first, followed by T. gondii and Trichinella spiralis. The criteria weights obtained in the global and European exercises were relatively similar, while the Indian exercise elicited a lower weight for morbidity severity but a higher weight for the number of illnesses (Table 4).
Fig. 3.
Global ranking of foodborne parasites using multi-criteria decision analysis (FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2014).
The normalized overall scores are weighted sums of normalized criteria scores and weights elicited from expert meeting participants.
Table 4.
Criterion weights obtained in three multi-criteria decision analyses of foodborne parasites.
| Scoring criterion | Globala | Indiab | Europec |
|---|---|---|---|
| Number of global food-borne illnesses | 0.22 | 0.30 | 0.23 |
| Global distribution | 0.14 | 0.13 | 0.13 |
| Morbidity severity | 0.22 | 0.14 | 0.23 |
| Case-fatality ratio | 0.15 | 0.15 | 0.15 |
| Increasing illness potential | 0.07 | 0.12 | 0.10 |
| Trade relevance | 0.10 | 0.07 | 0.09 |
| Impacts on economically vulnerable communities | 0.10 | 0.08 | 0.07 |
Several other MCDA exercises have been conducted to rank foodborne parasites at national or regional level (Table 5). These studies had a broad scope, focusing for instance on zoonotic diseases or, even more broadly, on communicable diseases. Foodborne parasites generally scored relatively low, although it should be noted that the resulting rankings are not necessarily comparable, given the differences in pathogens to rank, methodologies, and criteria. Inclusion of different pathogens to rank can for instance change the relative ranking of the other pathogens that are the same. Likewise, different criteria will result in different values being expressed.
Table 5.
National and regional risk ranking of foodborne parasites and other pathogens based on multi-criteria decision analysis.
| Reference | Location | Scope | Criteria | Ranking of foodborne parasites |
|---|---|---|---|---|
| Cardoen et al., 2009 | Belgium | 51 zoonotic pathogens |
|
|
| Havelaar et al., 2010 | The Netherlands | 86 zoonotic pathogens |
|
|
| Balabanova et al., 2011 | Germany | 127 pathogens |
|
|
| Ng and Sargeant, 2012 | Canada and US | 62 zoonoses |
|
|
| Humblet et al., 2012 | Europe | 100 animal diseases and zoonoses | 57 criteria, including 17 for epidemiology, 8 for prevention/control, 16 for economy/trade, 12 for public health, and 4 for society |
|
| Dahl et al., 2015 | Sweden | 106 pathogens |
|
|
| Kadohira et al., 2015 | Japan | 98 zoonoses |
|
Echinococcosis: 16/20 most important zoonoses |
5. General considerations
To conclude, we want to present three general considerations when designing a risk ranking exercise of foodborne parasites.
-
•
Whose ranking perspective? The aim of risk ranking exercises is to prioritize for decision making certain hazards, hazard-commodity pairs, or exposure routes for a given hazard, based on their perceived importance. As different stakeholders have their own prioritization objectives, the outcome of such exercises is necessarily context-dependent. Consequently, there is no unique or intrinsically correct ranking of risks. Furthermore, when designing a risk ranking exercise, it is important that the precise question and goal being addressed are defined explicitly, and from which perspective, along with the intended group who will use the ranking and act upon it. When opting for MCDA, quantifying the criteria weights should be conducted along this line by considering whose perception on relative importance among criteria needs to be reflected in the ranking.
-
•
Which risk metric? As outlined in the review, there are different metrics, and methods, that can be used to define and rank risks. Different metrics have different philosophical implications, and result in different rankings. It is thus important to give considerable thought to the definition of metrics, and to ensure that the chosen metric and method fulfill the needs of the target audience. When multiple criteria are of interest in defining a ranking, methods relying on SMPHs or MCDA provide the most appropriate quantifications.
-
•
What to rank? Obviously, the prior definition of included foodborne parasites will have an impact on the final ranking. Furthermore, specific foodborne parasites may have multiple appearances, such as congenital versus acquired toxoplasmosis; whether or not to consider these as one or multiple entities is an important prior choice. A further major consideration is whether or not the included food-related parasites should be ranked solely according to their foodborne transmission; and if so, whether drinking water is considered in the definition of food.
6. Conclusion
Risk ranking of foodborne parasites is increasingly performed to aid priority setting at global, regional, and national levels. Different risk ranking metrics and methods are available, ranging from single measures of health or economic impact, to complicated, but more complete, multi-criteria assessments. When designing a risk ranking exercise of foodborne parasites, it is important to consider the target audience and the reason for which the ranking is done, the choice of metrics and methods, and the prior definition of what to rank.
Acknowledgments
Acknowledgments
This work was a collaboration within the framework of EURO-FBP, A European Network for Foodborne Parasites, COST Action FA1408.
Funding information
BD recognizes travel funding from the International Association for Food and Waterborne Parasitology, enabling him to present this work at the IAFWP International Symposium, Kuala Lumpur, Malaysia.
Conflict of interests
The authors state that they have no competing interests.
References
- Anantanawat S., Kiermeier A., McLeod C., Sumner J. South Australian Research & Development Institute; 2012. A Semi-Quantitative Risk Assessment of Harmful Parasites in Australian Finfish. [Google Scholar]
- Anderson M., Jaykus L.A., Beaulieu S., Dennis S. Pathogen-produce pair attribution risk ranking tool to prioritize fresh produce commodity and pathogen combinations for further evaluation (P3ARRT) Food Control. 2011;22:1865–1872. [Google Scholar]
- Balabanova Y., Gilsdorf A., Buda S., Burger R., Eckmanns T., Gärtner B. Communicable diseases prioritized for surveillance and epidemiological research: results of a standardized prioritization procedure in Germany, 2011. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz M.B., Hoffmann S., Morris J.G. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012;75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- Behzadian M., Kazemzadeh R.B., Albadvi A., Aghdasi M. PROMETHEE: a comprehensive literature review on methodologies and applications. Eur. J. Oper. Res. 2010;200:198–215. [Google Scholar]
- Bouwknegt M., Devleesschauwer B., Graham H., Robertson L.J., van der Giessen J. On behalf of the COST action participants. Prioritization of foodborne parasites in Europe. Euro Surveill. 2017 doi: 10.2807/1560-7917.ES.2018.23.9.17-00161. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes V.J., Vilas V.D.R., Ward M.P. Disease prioritization: what is the state of the art? Epidemiol. Infect. 2015;143:2911–2922. doi: 10.1017/S0950268815000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke C.M., Deplazes P., Torgerson P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAC (Codex Alimentarius Commission) FAO; Rome: 1999. Principles and Guidelines for the Conduct of a Microbiological Risk Assessment. (CAC/GL-30) [Google Scholar]
- Cardoen S., Van Huffel X., Berkvens D., Quoilin S., Ducoffre G., Saegerman C. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog. Dis. 2009;6:1083–1096. doi: 10.1089/fpd.2009.0291. [DOI] [PubMed] [Google Scholar]
- Corso P.S., Kramer M.H., Blair K.A., Addiss D.G., Davis J.P., Haddix A.C. Costs of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 2003;9:426. doi: 10.3201/eid0904.020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte M.J., Minbaeva G., Usubalieva J., Abdykerimov K., Torgerson P.R. The burden of Zoonoses in Kyrgyzstan: a systematic review. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A.T.L. What's wrong with risk matrices? Risk Anal. 2008;28:497–512. doi: 10.1111/j.1539-6924.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- Dahl V., Tegnell A., Wallensten A. Communicable diseases prioritized according to their public health relevance, Sweden, 2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devleesschauwer B., Havelaar A.H., Maertens de Noordhout C., Haagsma J.A., Praet N., Dorny P. Calculating disability-adjusted life years to quantify burden of disease. Int. J. Public Health. 2014;59:565–569. doi: 10.1007/s00038-014-0552-z. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Havelaar A.H., Maertens de Noordhout C., Haagsma J.A., Praet N., Dorny P. DALY calculation in practice: a stepwise approach. Int. J. Public Health. 2014;59:571–574. doi: 10.1007/s00038-014-0553-y. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Ale A., Torgerson P., Praet N., Maertens de Noordhout C., Pandey B.D. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devleesschauwer B., Haagsma J.A., Angulo F.J., Bellinger D.C., Cole D., Döpfer D. Methodological framework for World Health Organization estimates of the global burden of foodborne disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devleesschauwer B., Praet N., Speybroeck N., Torgerson P.R., Haagsma J.A., De Smet K. The low global burden of trichinellosis: evidence and implications. Int. J. Parasitol. 2015;45:95–99. doi: 10.1016/j.ijpara.2014.05.006. [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) ECDC Tool for the Prioritisation of Infectious Disease Threats – Handbook and Manual. 2017. https://ecdc.europa.eu/en/publications-data/ecdc-tool-prioritisation-infectious-disease-threats Available from. (accessed 17/11/2017)
- Evers E.G., Bouwknegt M. Combining QMRA and epidemiology to estimate campylobacteriosis incidence. Risk Anal. 2016;36:1959–1968. doi: 10.1111/risa.12538. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization) FAO Headquarters; Rome Italy: 2014. Multicriteria-Based Ranking for Risk Management of Food-borne Parasites.http://www.fao.org/3/a-i3649e.pdf Report of a Joint FAO/WHO Expert Meeting, 3–7 September 2012. Available from. (accessed 17/11/2017) [Google Scholar]
- GBD 2016 DALYs, HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldermann J., Schöbel A. On the similarities of some multi-criteria decision analysis methods. J. Multi-Crit. Dec. Anal. 2011;18:219–230. [Google Scholar]
- Gkogka E., Reij M.W., Havelaar A.H., Zwietering M.H., Gorris L.G. Risk-based estimate of effect of foodborne diseases on public health, Greece. Emerg. Infect. Dis. 2011;17:1581. doi: 10.3201/eid1709.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M.R., Stevenson D., Fryback D.G. HALYS and QALYS and DALYS, oh my: similarities and differences in summary measures of population health. Annu. Rev. Public Health. 2002;23:115–134. doi: 10.1146/annurev.publhealth.23.100901.140513. [DOI] [PubMed] [Google Scholar]
- Haagsma J.A., Polinder S., Stein C.E., Havelaar A.H. Systematic review of foodborne burden of disease studies: quality assessment of data and methodology. Int. J. Food Microbiol. 2013;166:34–47. doi: 10.1016/j.ijfoodmicro.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Havelaar A.H., van Rosse F., Bucura C., Toetenel M.A., Haagsma J.A., Kurowicka D. Prioritizing emerging zoonoses in the Netherlands. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Haagsma J.A., Mangen M.J.J., Kemmeren J.M., Verhoef L.P., Vijgen S.M. Disease burden of foodborne pathogens in the Netherlands, 2009. Int. J. Food Microbiol. 2012;156:231–238. doi: 10.1016/j.ijfoodmicro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Batz M.B., Morris Jr J.G. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012;75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- Humblet M.-F., Vandeputte S., Albert A., Gosset C., Kirschvink N., Haubruge E. Multidisciplinary and evidence-based method for prioritizing diseases of food-producing animals and zoonoses. Emerg. Infect. Dis. 2012;18 doi: 10.3201/eid1804.111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHME (Institute for Health Metrics and Evaluation) IHME, University of Washington; Seattle, WA: 2016. GBD Compare Data Visualization.http://vizhub.healthdata.org/gbd-compare Available from. (accessed 17/11/2017) [Google Scholar]
- Jakobsen L.S., Nauta M., Knudsen V.K., Pires S.M., Poulsen M. Burden of disease estimates of cancer caused by dietary exposure to acrylamide: how methodological choices affect the outcome. Toxicol. Lett. 2015;238:115. [Google Scholar]
- Kadohira M., Hill G., Yoshizaki R., Ota S., Yoshikawa Y. Stakeholder prioritization of zoonoses in Japan with analytic hierarchy process method. Epidemiol. Infect. 2015;143:1477–1485. doi: 10.1017/S0950268814002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Steinmann P., Keiser J., Utzinger J. Nematode infections: soil-transmitted helminths and Trichinella. Infect. Dis. Clin. N. Am. 2012;26:341–358. doi: 10.1016/j.idc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kwong J.C., Ratnasingham S., Campitelli M.A., Daneman N., Deeks S.L., Manuel D.G. The impact of infection on population health: results of the Ontario burden of infectious diseases study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjo-González M., Devleesschauwer B., Gabriël S., Dorny P., Allepuz A. Epidemiology, impact and control of bovine cysticercosis in Europe: a systematic review. Parasit. Vectors. 2016;9:81. doi: 10.1186/s13071-016-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen M.J., Batz M.B., Käsbohrer A., Hald T., Morris J.G., Taylor M. Integrated approaches for the public health prioritization of foodborne and zoonotic pathogens. Risk Anal. 2010;30:782–797. doi: 10.1111/j.1539-6924.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- Mangen M.J., Plass D., Havelaar A.H., Gibbons C.L., Cassini A., Mühlberger N. The pathogen- and incidence-based DALY approach: an appropriate [corrected] methodology for estimating the burden of infectious diseases. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen M.J., Bouwknegt M., Friesema I.H., Haagsma J.A., Kortbeek L.M., Tariq L. Cost-of-illness and disease burden of food-related pathogens in the Netherlands, 2011. Int. J. Food Microbiol. 2015;196:84–93. doi: 10.1016/j.ijfoodmicro.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Murray C.J. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull. World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Ezzati M., Flaxman A.D., Lim S., Lozano R., Michaud C. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- Murrell K.D. Economic losses resulting from food-borne parasitic zoonoses. Southeast Asian J. Trop. Med. Public Health. 1991;22:377–381. Suppl. [PubMed] [Google Scholar]
- Ng V., Sargeant J.M. A quantitative and novel approach to the prioritization of zoonotic diseases in North America: a public perspective. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen J., Jokelainen P., Stensvold C.R., Trevisan C., Fuchs J., Burgdorf K.S. The disease burden of congenital toxoplasmosis in Denmark, 2014. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E.C., Taft R., Geary K., Ciotti M., Suk J.E. Best practices in ranking communicable disease threats: a literature review, 2015. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.17.30212. [DOI] [PubMed] [Google Scholar]
- Pratdesaba R.A., González M., Piedrasanta E., Mérida C., Contreras K., Vela C. Cyclospora cayetanensis in three populations at risk in Guatemala. J. Clin. Microbiol. 2001;39:2951–2953. doi: 10.1128/JCM.39.8.2951-2953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T., Murrell K.D., Marks S. Economic losses caused by foodborne parasitic diseases. Parasitol. Today. 1994;10:419–423. doi: 10.1016/0169-4758(94)90171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L.J., Sehgal R., Goyal K. An Indian multicriteria-based risk ranking of foodborne parasites. Food Res. Int. 2015;77:315–319. [Google Scholar]
- Ruzante J.M., Davidson V.J., Caswell J., Fazil A., Cranfield J.A., Henson S.J. A multifactorial risk prioritization framework for foodborne pathogens. Risk Anal. 2010;30:724–742. doi: 10.1111/j.1539-6924.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- Sanders G.D., Neumann P.J., Basu A., Brock D.W., Feeny D., Krahn M. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- Scharff R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012;75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- Speybroeck N., Devleesschauwer B., Depoorter P., Dewulf J., Berkvens D., Van Huffel X. Needs and expectations regarding risk ranking in the food chain: a pilot survey amongst decision makers and stakeholders. Food Control. 2015;54:135–143. [Google Scholar]
- Stella P., Cerf O., Hugas M., Koutsoumanis K.P., Nguyen-The C., Sofos J.N. Ranking the microbiological safety of foods: a new tool and its application to composite products. Trends Food Sci. Technol. 2013;33:124–138. [Google Scholar]
- Torgerson P.R. One world health: socioeconomic burden and parasitic disease control priorities. Vet. Parasitol. 2013;195:223–232. doi: 10.1016/j.vetpar.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Macpherson C.N. The socioeconomic burden of parasitic zoonoses: global trends. Vet. Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Schweiger A., Deplazes P., Pohar M., Reichen J., Ammann R.W. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008;49:72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson P.R., Rüegg S., Devleesschauwer B., Abela-Ridder B., Havelaar A.H., Shaw A.P.M. zDALY: an adjusted indicator to estimate the burden of zoonotic diseases. One Health. 2017 doi: 10.1016/j.onehlt.2017.11.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan C., Devleesschauwer B., Schmidt V., Winkler A.S., Harrison W., Johansen M.V. The societal cost of Taenia solium cysticercosis in Tanzania. Acta Trop. 2017;165:141–154. doi: 10.1016/j.actatropica.2015.12.021. [DOI] [PubMed] [Google Scholar]
- van Lier A., McDonald S.A., Bouwknegt M., Kretzschmar M.E., Havelaar A.H., Mangen M.J.J. Disease burden of 32 infectious diseases in the Netherlands, 2007–2011. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Fels-Klerx H.J., Van Asselt E.D., Raley M., Poulsen M., Korsgaard H., Bredsdorff L. Critical review of methods for risk ranking of food related hazards, based on risks for human health. Crit. Rev. Food Sci. Nutr. 2016 doi: 10.1080/10408398.2016.1141165. (in press) [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Global Health Estimates Technical Paper. WHO/HIS/IER/GHE/2017.1.; 2017. WHO methods and data sources for global burden of disease estimates 2000–2015.http://www.who.int/healthinfo/global_burden_disease/GlobalDALYmethods_2000_2015.pdf Available from. (accessed 17/11/2017) [Google Scholar]