Abstract
Fishborne heterophyid trematodes infecting humans are at least 29 species worldwide and belong to 13 genera. Its global burden is much more than 7 million infected people. They include Metagonimus (M. yokogawai, M. takahashii, M. miyatai, M. minutus, and M. katsuradai), Heterophyes (H. heterophyes, H. nocens, H. dispar, and H. aequalis), Haplorchis (H. taichui, H. pumilio, H. yokogawai, and H. vanissimus), Pygidiopsis (P. summa and P. genata), Heterophyopsis (H. continua), Stellantchasmus (S. falcatus), Centrocestus (C. formosanus, C. armatus, C. cuspidatus, and C. kurokawai), Stictodora (S. fuscata and S. lari), Procerovum (P. varium and P. calderoni), Acanthotrema (A. felis), Apophallus (A. donicus), Ascocotyle (A. longa), and Cryptocotyle (C. lingua). Human infections are scattered around the world but the major endemic areas are located in Southeast Asia. The source of human infection is ingestion of raw or improperly cooked fish. The pathogenicity, host-parasite relationships, and clinical manifestations in each species infection are poorly understood; these should be elucidated particularly in immunocompromised hosts. Problems exist in the differential diagnosis of these parasitic infections because of close morphological similarity of eggs in feces and unavailability of alternative methods such as serology. Molecular diagnostic techniques are promising but they are still at an infant stage. Praziquantel has been proved to be highly effective against most of the patients infected with heterophyid flukes. Epidemiological surveys and detection of human infections are required for better understanding of the geographical distribution and global burden of each heterophyid species. In this review, the most updated knowledge on the morphology, biology, epidemiology, pathogenesis and pathology, immunology, clinical manifestations, diagnosis and treatment, and prevention and control of fishborne zoonotic heterophyid infections is provided.
Keywords: Fishborne trematode, Zoonotic trematode, Heterophyid, Metagonimus, Heterophyes, Haplorchis
Highlights
-
•
Fishborne intestinal flukes are diverse and heterophyids are an important group.
-
•
Thirteen heterophyid genera and 29 species are known to infect humans.
-
•
Metagonimus, Heterophyes, and Haplorchis are the most important genera.
-
•
Infections are significant due to the potential for morbidity and mortality.
-
•
Epidemiological surveys and detection of human infections are urgently required.
1. Introduction
Foodborne zoonotic trematodes are highly diverse in terms of the species of parasites involved and the kinds and types of foods concerned. They can be largely divided into liver flukes, lung flukes, and intestinal flukes (Fürst et al., 2012, Chai, 2014a). The global burden of foodborne trematodes was estimated at about 50–60 million people (Fried et al., 2004, Fürst et al., 2012). However, this certainly is a far underestimate of the true number of infected people because of difficulty in case detection and diagnosis (Chai et al., 2009a).
Among them, intestinal flukes are the most neglected group despite a wide geographical distribution, a high prevalence in some endemic countries, and potentially significant morbidity and mortality. Intestinal trematodes are taxonomically diverse, and consist of more than 60 species worldwide (Chai et al., 2009a). They include heterophyids (including Metagonimus yokogawai, Heterophyes nocens, and Haplorchis taichui), echinostomes (including Echinostoma revolutum, Echinostoma ilocanum, Echinochasmus japonicus, Artyfechinostomum malayanum, and Acanthoparyphium tyosenense), fasciolids (Fasciolopsis buski), gymnophallids (Gymnophalloides seoi), microphallids (Gynaecotyla squatarolae), neodiplostomes/diplostomes/strigeids (Neodiplostomum seoulense), lecithodendriids (Prosthodendrium molenkampi and Phaneropsolus bonnei), and plagiorchiids (Plagiorchis muris) (Chai, 2007, Chai, 2014a, Chai et al., 2009a). Among them, the heterophyids have seldom been the subject of extensive reviews. Thus, in this article, the authors would like to review on the heterophyid group of intestinal flukes.
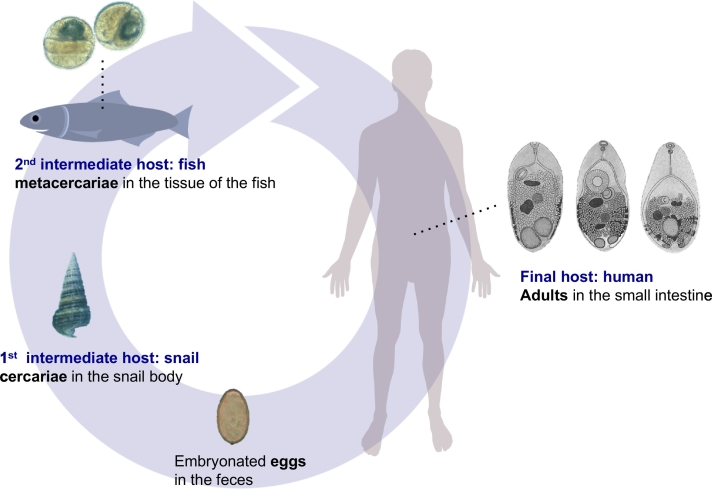
Heterophyids (= family Heterophyidae Leiper, 1909) are a group of minute-sized (1–2 mm in length) trematodes infecting vertebrate animals, including mammals and birds (Yamaguti, 1958). At least 36 genera are known within this family (Pearson, 2008), and among them, 13 genera are known to be zoonotic (Chai, 2007); Metagonimus, Heterophyes, Haplorchis, Pygidiopsis, Heterophyopsis, Stellantchasmus, Centrocestus, Stictodora, Procerovum, Acanthotrema, Apophallus, Ascocotyle, and Cryptocotyle. They are exclusively fishborne and contracted to humans by ingesting raw or improperly cooked freshwater or brackish water fish (Chai, 2007, Chai, 2014a) (Fig. 1). Most of the infected people live in Asian countries, including Korea, China, Taiwan, Vietnam, Laos, Thailand, Malaysia, Indonesia, the Philippines, and India.
Fig. 1.
Common life cycle of fishborne zoonotic heterophyid trematodes. Human infection occurs when the infected fish are consumed raw or under improperly cooked conditions. Some images were adapted from Google.
In view of the wide geographical distribution and the number of infected people around the world, Metagonimus, Heterophyes, and Haplorchis are the three most important genera (Chai, 2015). Metagonimus morphologically differs from Heterophyes and Heterophyopsis in that the former has a smaller, submedian-located ventral sucker and no genital sucker, whereas the latter two have a bigger and almost median-located ventral sucker and a prominent genital sucker (Chai, 2007, Yu and Chai, 2010, Yu and Chai, 2013). Heterophyopsis has an elongated body unlike Heterophyes (Chai, 2007). Metagonimus has two testes but Haplorchis and Procerovum have only one testis (Chai, 2015). Haplorchis, Procerovum, and Stictodora have prominent gonotyls armed with several to numerous rodlets, superimposed on the submedially located ventral sucker (Chai, 2015). Pygidiopsis has a small and median-located ventral sucker with the presence of a ventrogenital apparatus armed with 12–15 minute spines (Chai et al., 1986a). Stellantchasmus has a small, laterally deviated ventral sucker and an elongated sac-like seminal vesicle with a muscular expulsor at the opposite side of the ventral sucker (Yu and Chai, 2010, Yu and Chai, 2013, Chai, 2015). Centrocestus has minute circumoral spines on the oral sucker (Yu and Chai, 2010, Yu and Chai, 2013, Chai, 2015). Stitodora has a gonotyl armed with spines and a separate opening of the ejaculatory duct and metraterm, but lacks a muscular bulb in the genital atrium (Chai et al., 1988). Acanthotrema is similar to Stictodora but differs in having fewer than 12 sclerotizations on the ventral sucker; more than 12 spines in the latter (Sohn et al., 2003a, Sohn et al., 2003b).
The purpose of this review is to provide the most updated knowledge on the morphology, biology, epidemiology, pathogenesis and pathology, immunology, clinical manifestations, diagnosis and treatment, and prevention and control of fishborne zoonotic heterophyid infections. In this review, those species naturally occurring in humans, as well as those in which experimental human infection was successful, were included.
2. Morphology, biology, and epidemiology
2.1. Metagonimus
Flukes of Metagonimus are characterized by the presence of a small submedian and unarmed ventral sucker; they differ from Heterophyes in lacking a genital sucker and gonotyl and from Haplorchis in having two testes (Chai, 2007, Chai, 2017). The genus Metagonimus was erected with M. yokogawai as the type species (Katsurada, 1912, Yokogawa, 1913a, Ito, 1964a), and 8 more species (total 9 species) have been described to date. They include M. ovatus (Yokogawa, 1913b); M. takahashii (Suzuki, 1930), M. minutus (Katsuta, 1932a), M. katsuradai (Izumi, 1935), M. otsurui (Saito and Shimizu, 1968), M. miyatai (Saito et al., 1997), M. hakubaensis (Shimazu, 1999), and M. suifunensis (Shumenko et al., 2017) (Table 1). Among them, M. yokogawai, M. takahashii, and M. miyatai are the 3 major human-infecting species in Japan and Korea (Chai et al., 2005a, Chai et al., 2009a, Chai et al., 2015a). An experimental human infection was reported to be successful in M. katsuradai (Izumi, 1935), and M. minutus was included among the list of human-infecting species in Taiwan without literature background (Yu and Mott, 1994). Hence, 5 species, namely, M. yokogawai, M. takahashii, M. miyatai, M. minutus, and M. katsuradai, are regarded as zoonotic or potentially zoonotic species. It should be reminded that some of old literature on M. yokogawai is actually referring to M. takahashii or M. miyatai, and caution is required when reviewing M. yokogawai in strict sense (Chai et al., 2009a, Chai, 2015, Chai, 2017).
Table 1.
Species of Metagonimus reported in the literature.
| Species | Human infection | Country/region | First reporter |
|---|---|---|---|
| Metagonimus yokogawai | Yes | Korea, Japan, China, Taiwan, Russia, India, Europe | Katsurada (1912) |
| Metagonimus takahashii | Yes | Korea, Japan | Takahashi (1929a), Suzuki (1930) |
| Metagonimus miyatai | Yes | Korea, Japan | Katsurada (1912), Saito et al. (1997) |
| Metagonimus minutus | Yesa | Taiwan | Katsuta (1932a) |
| Metagonimus katsuradai | Yesb | Japan, Russia | Izumi (1935) |
| Metagonimus ovatus | No | Japan | Yokogawa (1913b) |
| Metagonimus otsurui | No | Japan | Saito and Shimizu (1968) |
| Metagonimus hakubaensis | No | Japan | Shimazu (1999) |
| Metagonimus suifunensis | No | Russia | Shumenko et al. (2017) |
Listed as a human-infecting species (Yu and Mott, 1994) without adequate documentation.
Experimental human infection was reported.
2.1.1. Metagonimus yokogawai (Katsurada, 1912) Katsurada, 1912
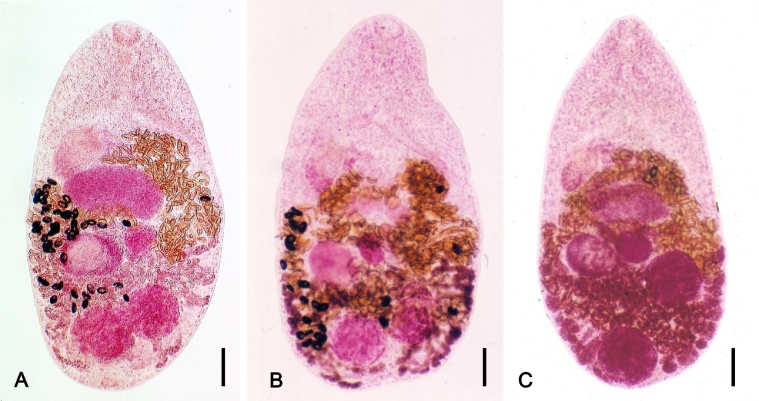
The original description of this species is based on adult specimens recovered from an experimental dog fed the metacercariae in the sweetfish (Plecoglossus altivelis) from Taiwan (Katsurada, 1912, Shimazu and Kino, 2015). It is now known to distribute mainly in the Far Eastern countries (Chai, 2017). Its characteristic morphology include a minute body, a small laterally deviated ventral sucker with no ventrogenital apparatus and no genital sucker, a medially-located ovary, and two testes located almost side-by-side near the posterior end of body (Ito, 1964a, Chai et al., 2009a, Yu and Chai, 2013) (Fig. 2). M. takahashii and M. miyatai have two testes separated from each other (Chai et al., 2009a). In addition, the vitelline follicles of M. yokogawai extend in lateral fields from the level of the ovary down to the posterior end of the posterior testis, but not beyond the posterior testis (Saito et al., 1997, Chai, 2015). In M. takahashii, the vitelline follicles distribute abundantly beyond the posterior testis level (Chai et al., 2009a). The uterine tubules of M. yokogawai never overlap or cross over the middle portion of the anterior testis, whereas M. takahashii and M. miyatai have the uterine tubules which overlap the whole anterior testis (Chai et al., 1993a, Saito et al., 1997).
Fig. 2.
Morphology of Metagonimus spp. infecting humans. (A) Metagonimus yokogawai, (B) Metagonimus takahashii, and (C) Metagonimus miyatai. Note that M. yokogawai has two adjacent testes, whereas M. takahashii and M. miyatai have more or less two separated testes. The uterine tubule of M. yokogawai does not overlap with the anterior testis, but the tubules of M. takahashii and M. miyatai do overlap with the anterior testis. Vitelline follicles are distributed diffusely beyond the post-testicular areas in M. takahashii but not in M. yokogawai and M. miyatai. Scale bars; (A) = 100 μm, (B) = 100 μm, (C) = 100 μm.
Freshwater snails (including Semisulcospira libertina and S. coreana) have been reported to be the molluscan intermediate host (Ito, 1964b, Cho et al., 1984). The most important fish host is the sweetfish (Plecoglossus altivelis) in Korea and Japan (Ito, 1964a, Chai and Lee, 2002, Sohn, 2009, Chai, 2007, Chai, 2015). The big-scaled redfin (Tribolodon hokonensis), Pacific redfin (T. taczanowskii), and the perch (Lateolabrax japonicus) have also been reported as the fish host (Chai, 2007, Chai et al., 2009a, Yu and Chai, 2013, Chai, 2015). The natural definitive hosts are humans (Chai et al., 2009a), dogs (Komiya, 1965, Cho et al., 1981), rats (Seo et al., 1981a), cats (Huh et al., 1993), foxes (Miyamoto, 1985), boars (Komiya, 1965), and kites (bird) (Miyamoto, 1985). Mice, rats, cats, dogs, gerbils, hamsters, and ducks are experimental definitive hosts (Guk et al., 2005, Chai et al., 2009a, Li et al., 2010, Li et al., 2013). Guk et al. (2005) studied on the growth and development of M. yokogawai in different strains of mice (BALB/c, ddY, C57BL/6J, C3H/HeN, and A/J) and found that ddY mice were the most highly suitable strain.
The principal mode of human infection is consumption of raw or improperly cooked freshwater fish, notably the sweetfish (P. altivelis) and the big-scaled redfin (T. hakonensis) (under the name T. taczanowskii) (Yu and Chai, 2013, Chai, 2015). Pickled, salted, or fermented fish, as well as cooking knife and chopping board contaminated with the metacercariae may also cause human infections (Chai, 2015). Major endemic areas are scattered in Far Eastern countries, including the Republic of Korea (= Korea), Japan, China, and Russia (Chai, 2015). In Korea, numerous endemic foci have been found, and almost all small streams to large rivers in eastern and southern coastal areas were confirmed to be endemic areas (Chai and Lee, 2002, Chai, 2007, Chai, 2015, Chai, 2017, Chai et al., 2009a). High endemic areas are the Seomjin, Tamjin, Boseong Rivers, Geoje Island, and Osip Stream in Samcheok-shi (Gangwon-do) which revealed 20–70% egg positive rates of the riparian residents (Chai and Lee, 2002, Chai et al., 1977, Chai et al., 2000, Chai et al., 2015a). Of considerable interest in Korea is that the eggs of M. yokogawai were detected from mummies of the 17th century, Joseon Dynasty (Seo et al., 2008, Shin et al., 2009). It is thus presumed that the life cycle of M. yokogawai has been actively maintained at least more than 400 years in Korea (Seo et al., 2008).
In Japan, it was originally thought that M. yokogawai infection is distributed nationwide with the exception of Hokkaido (Ito, 1964a). However, the presence of its life cycle in Hokkaido was also recognized (Miyamoto, 1985). Until the 1960s, the prevalence in humans ranged 0.5–35.1% depending on the locality surveyed (Ito, 1964a). Kagei and Kihata (1970) surveyed 26 areas of Japan and reported M. yokogawai egg positive rates as 0–73.9%; three areas of Shimane Prefecture showed the highest prevalence (73.9%, 71.9%, and 57.1%, respectively) followed by areas of Hiroshima (38.9%), Kochi (33.2%), Kagoshima (28.0%), and Saga Prefecture (27.0%). The last available literature on the prevalence (Ito et al., 1991) reported 13.2% egg positive rate among 4524 riparian people examined in Hamamatsu Lake, Shizuoka Prefecture during 1982–1988. It is presumed that the prevalence of M. yokogawai in Japan has been decreasing until now. In China, 8 human infections were confirmed by genetic analysis of fecal eggs in Guangxi Province (Jeon et al., 2012). It was stated that human infections exist in Guangdong, Anhui, Hubei, and Zhejiang Province (Yu and Mott, 1994). In Taiwan, the metacercariae of M. yokogawai were first found from the sweetfish in 1912 (Yokogawa, 1912, Ito, 1964a), and after then, human infections were occasionally reported (Chen, 1991). However, little surveys have been undertaken on the prevalence among humans (Li et al., 2013).
In Russia, the Amur and Ussuri valleys of the Khabarovsk territory have been known to be endemic areas of M. yokogawai (Yu and Mott, 1994). In this territory, the prevalence among the total population was 1–2% but the prevalence among the ethnic minority was 20–70% (Yu and Mott, 1994). Also in the north of Sakhalin Island, the infection rate was 10% among ethnic minorities and 1.5% among Russians (Yu and Mott, 1994). Sporadic cases were also reported in the Amur district and the Primorye territory (Yu and Mott, 1994, Chai, 2015). In the Khabarovsk territory, the population at risk was once estimated at 859,000, which is 14.7% of the total population in this territory (Yu and Mott, 1994). In India, the presence of human infection was suspected because two heterophyid egg positive cases (designated as M. yokogawai infection) were detected (Mahanta et al., 1995, Uppal and Wadhwa, 2005). In Europe, the existence of M. yokogawai infection (in some references described as Metagonimus sp.) have been reported in fish hosts and wild animals of Ukraine (Davydov et al., 2011), Serbia (Djikanovic et al., 2011), Bulgaria (Nachev and Sures, 2009, Ondračkova et al., 2012), and Czech Republic (Francová et al., 2011). However, human infections have not yet been confirmed.
2.1.2. Metagonimus takahashii (Takahashi, 1929) Suzuki, 1930
This species was originally found from the small intestine of mice and dogs fed the metacercariae encysted in freshwater fish in Japan (Takahashi, 1929a). A year later, it was proposed as a new species M. takahashii admitting that the large egg size is enough to be a specific character (Suzuki, 1930). Its taxonomic validity had long been debated by Japanese parasitologists. However, Saito, 1972, Saito, 1973 strongly supported the validity of M. takahashii based not only on its remarkably larger egg size but also on differential morphologies of larval and adult stages and also by the different host specificities at experimental infection with the cercariae. Thereafter, the name M. takahashii has been settled. M. takahashii differs morphologically from M. yokogawai and M. miyatai in the position of the two testes, distribution of vitelline follicles, and size of their eggs (Saito, 1984, Chai et al., 1993a, Chai, 2015) (Fig. 2). It also differs from M. katsuradai, M. otsurui, and M. hakubaensis in that the latter three species have a smaller ventral sucker than the oral sucker (Saito, 1984, Shimazu, 1999, Chai, 2015). M. minutus, having a larger ventral sucker than the oral sucker, differs from M. takahashii and M. yokogawai in having a smaller body and eggs, larger seminal vesicle, and different fish host (Katsuta, 1932a, Chai et al., 1993a).
The snail hosts are Semisulcospira spp. (including S. libertina and S. coreana) (Saito, 1972, Cho et al., 1984). The fish hosts include the crussian carp (Carassius auratus), carp (Cyprinus carpio), big-scaled redfin (T. hakonensis), and perch (L. japonicus) (Takahashi, 1929a, Saito, 1984, Sohn, 2009, Chai, 2015). The natural definitive hosts are mice, rats, dogs, cats, pelicans, kites, and other avian species (Takahashi, 1929a, Yamaguti, 1958, Ahn, 1993). The experimental definitive hosts include mice, rats and dogs, cats, hamsters, and rabbits (Takahashi, 1929a, Saito, 1972, Chai et al., 1991, Rim et al., 1996, Guk et al., 2005, Chai, 2017). The growth and development of M. takahashii were studied in different strains of mice (BALB/c, ddY, C57BL/6J, C3H/HeN, and A/J), that were generally not a good experimental host (Guk et al., 2005).
This species is known to distribute only in Korea and Japan. However, it is possibly distributed in other countries. In Korea, M. takahashii was first found in experimental rabbits fed the metacercariae from carps (Chun, 1960a). The presence of human infections (mixed with Metagonimus sp., presumably M. miyatai) was first demonstrated by adult worm recovery from riparian people along the Hongcheon River, Gangwon-do (Ahn and Ryang, 1988). An endemic area was subsequently discovered along the upper reaches of the Namhan River, mixed-infected with M. miyatai, with an egg positive rate of 9.7% for both species (Chai et al., 1993a). A recent survey performed along the Boseong River, Jeollanam-do (M. yokogawai endemic area) detected 3293 specimens of M. takahashii from 11 riparian residents out of a total of 70,223 intestinal fluke specimens (mostly M. yokogawai) (Chai et al., 2015a). In Japan, articles regarding its existence have been published (Ito, 1964a, Saito, 1972, Saito, 1973). However, because of taxonomic debates and confusion between M. takahashii and M. yokogawai, its precise epidemiologic status, including the prevalence and geographical distribution of human infections, has not been clearly defined (Chai, 2015). An earlier study in Okayama City reported infection of M. takahashii in 43 (0.64%) of 6680 residents examined, whereas infection of M. yokogawai was found in 54 (0.81%) residents (Takahashi, 1929a). Later, in Fuchu City, Hiroshima Prefecture, 11 (4.8%) of 231 residents examined were infected with M. takahashii, whereas 81 (35.1) were infected with M. yokogawai (Asada et al., 1957).
2.1.3. Metagonimus miyatai Saito, Chai, Kim, Lee, and Rim, 1997
Saito et al. (1997) described M. miyatai as a new species based on adult flukes collected from dogs and hamsters experimentally fed the metacercariae from the sweetfish (P. altivelis), dace (T. hakonensis and T. taczanowskii), Amur fat-minnow (Phoxinus steindachneri) (under the name Morocco steindachneri), pale chub (Zacco platypus), and dark chub (Zacco temminckii) in Korea and Japan. This species was actually first found by Katsurada (1912) (shown only by a figure drawing) together with M. yokogawai in Taiwan, but at that time it was regarded as a paratype specimen of M. yokogawai (Saito et al., 1997). Human infections have been reported from Korea and Japan (Saito et al., 1997, Chai et al., 1993a, Chai et al., 2015a). M. miyatai is morphologically characterized by two markedly separated testes from each other, with the posterior one located very close to the posterior body wall, vitelline follicles never distributing beyond the posterior testis, and the egg size which is intermediate between those of M. yokogawai and M. takahashii (Chai et al., 1993a, Saito et al., 1997, Yu and Chai, 2013, Chai, 2015) (Fig. 2). It differs from M. minutus in its larger body and egg size (Katsuta, 1932a, Chai et al., 1993a). M. miyatai is also genetically distinct from M. yokogawai and M. takahashii (Yu et al., 1997a, Yu et al., 1997b, Lee et al., 1999, Yang et al., 2000).
The snail host includes S. libertina, S. dolorosa, S. globus, and Koreanomelania nodifila (Kim, 1980, Kim et al., 1987, Shimazu, 2002). The metacercariae have been detected in freshwater fish (including Z. platypus, Z. temminckii, P. altivelis, T. hakonensis, T. taczanowskii, Opsariichthys bidens, and Phoxinus steindachneri) (Saito et al., 1997, Shimazu, 2002, Sohn, 2009). Natural definitive hosts include the dog, red fox, raccoon dog, and black-eared kite (Saito et al., 1997, Chai, 2015). Experimental definitive hosts are the mouse, rat, hamster, and dog (Kim, 1980, Kim et al., 1987, Chai et al., 1991, Ahn and Ryang, 1995, Saito et al., 1997, Shimazu, 2002, Chai, 2017). The worm growth and development was studied in different strains of mice (BALB/c, ddY, C57BL/6J, C3H/HeN, and A/J); mice were generally not a susceptible animal host (Guk et al., 2005).
The major source of human infection is raw or improperly cooked freshwater fish, in particular, the pale chub (Z. platypus) in Korea and Japan (Chai, 2015). In Korea, the presence of human infections (under the name Metagonimus sp.) was first demonstrated by Kim (1980) in Geum River by detecting eggs in the feces and recovery of adult flukes from several cases. Kim et al. (1987) performed another survey around the Lake Daecheong and its upper reaches (designated the worms as Metagonimus Miyata type of Saito, 1984) and reported Z. platypus and Opsariichthys bidens as the most heavily infected fish species. Adult specimens were recovered from 32 people living along the Namhan River in Eumseong-gun (9.7% in egg positive rate) and Yeongwol-gun (48.1% in egg positive rate) (Chai et al., 1993a). A most recent survey performed along the Boseong River, Jeollanam-do (M. yokogawai endemic area) detected 343 specimens of M. miyatai from 11 riparian residents out of a total of 70,223 intestinal fluke specimens (mostly M. yokogawai) (Chai et al., 2015a). In Japan, epidemiological studies, particularly on human infections, are scarce. With regard to animal definitive hosts, Saito et al. (1997) listed dogs, foxes, raccoon dogs, and black-eared kites in Shimane, Kochi, and Yamagata Prefectures. Metacercariae were detected in a fish species (P. steindachneri) in Hiroi River basin, Nagano Prefecture (Shimazu, 2002). Small rivers of Shizuoka Prefecture were also found to have M. miyatai metacercariae-infected fish (Kino et al., 2006).
2.1.4. Metagonimus minutus Katsuta, 1932
The original description of this species is based on adult flukes recovered from cats and mice experimentally fed the metacercariae in the brackish water mullet in Taiwan (Katsuta, 1932a). It is smaller than M. yokogawai, M. takahashii, and M. miyatai in its egg and adult worm size (Katsuta, 1932a, Ito, 1964a, Saito et al., 1997). Its body size is slightly larger than M. katsuradai but its egg size is smaller than M. katsuradai (Katsuta, 1932a, Izumi, 1935). Its oral sucker is smaller than the ventral sucker, whereas, in M. katsuradai, the oral sucker is bigger than the ventral sucker (Katsuta, 1932a, Izumi, 1935, Chai, 2015). This species has been listed as a human-infecting intestinal fluke species in Taiwan but the literature background is not available (Yu and Mott, 1994).
The molluscan host is unknown. The metacercariae are found in the scales, gills, and fins of the mullets (Mugil cephalus) (Katsuta, 1932a). Natural definitive hosts have not been discovered. Cats and mice were used as experimental definitive hosts (Katsuta, 1932a). Eating raw or improperly cooked mullets in endemic areas is a risk factor. Distribution of this species in other countries is unknown.
2.1.5. Metagonimus katsuradai Izumi, 1935
The original description of this species is based on adult flukes recovered from rats, mice, rabbits, dogs, and cats experimentally infected with the metacercariae obtained from freshwater fish (including Pseudorasbora parva, Z. platypus, and Tanakia lanceolata) in Japan (Izumi, 1935). The possibility of human infection was experimentally proven through infection to author himself and family (Izumi, 1935). The body of M. katsuradai is slightly smaller than M. minutus but its eggs are larger than M. katsuradai (Katsuta, 1932a, Izumi, 1935). It differs from M. yokogawai, M. takahashii, M. miyatai, and M. minutus in having a smaller ventral sucker than the oral sucker (Yu and Chai, 2013, Chai, 2015). It also differs from M. otsurui in the position of the seminal receptacle (Saito and Shimizu, 1968). It differs from M. hakubaensis in its long ceca that enter the post-testicular region (Shimazu, 1999).
The molluscan host is S. libertina in Japan (Kurokawa, 1939) and Juga tegulata in Russia (Besprozvannykh et al., 1987). The second host is freshwater fish (including Tanakia lanceolata, T. oryzae, T. limbata, Acheilognathus rhombeus, T. moriokae, P. parva, Z. platypus, and Gnathopogon elongatus) (Izumi, 1935, Ito, 1964a, Shimazu, 2003). Dogs are the only found natural definitive host (Yoshikawa et al., 1940). Experimental definitive hosts include humans, mice, white mice, rats, rabbits, puppies, kittens, ducks, and golden hamsters (Izumi, 1935, Kurokawa, 1939, Shimazu, 2003, Chai, 2017). Consumption of raw or improperly cooked freshwater fish (Z. platypus and others) is a risk factor (Ito, 1964a).
In Japan, its presence has been reported in several localities (Kurokawa, 1939, Ito, 1964a, Urabe, 2003). However, natural human cases have never been documented. In Russia, the existence of its life cycle was reported by Besprozvannykh et al. (1987) in the southern Primorye region through discovery of cercariae in Juga snails and metacercariae in 6 species of freshwater fish. Without firm evidence, it is listed among the human-infecting trematodes in the Primorye Territory (Emolenko et al., 2015).
2.2. Heterophyes
Flukes of Heterophyes are characterized by the presence of a genital sucker and armed gonotyl; they differ from Metagonimus in having a large median located ventral sucker and from Haplorchis in having only one testis (Chai, 2007). Heterophyes flukes were first discovered by Bilharz in 1851 at an autopsy of an Egyptian and named as Distomum heterophyes by von Siebold in 1852 (Chai, 2007, Chai, 2014b). The genus Heterophyes was raised by Cobbold in 1866 with Heterophyes aegyptiaca as the type; later this was synonymized with H. heterophyes (Witenberg, 1929). A total of 18 species or subspecies had been described in the genus Heterophyes (Chai, 2014b). However, three species were moved to another genus Alloheterophyes by Pearson (1999) and nine species were synonymized with H. heterophyes or other pre-existing species (Chai, 2014b). Now, the number of valid species of Heterophyes is only six (Pearson, 1999, Chai, 2014b), namely, H. heterophyes (by von Siebold in 1852), H. nocens (Onji and Nishio, 1916), H. dispar (Looss, 1902), H. aequalis (Looss, 1902), H. indica (Rao and Ayyar, 1931), and H. pleomorphis (Bwangamoi and Ojok, 1977) (Table 2). Four species, H. heterophyes, H. nocens, H. dispar, and H. aequalis, are known to infect humans (Ashford and Crewe, 2003, Chai, 2014b). Caution is needed to refer to some old literature which dealt with H. nocens as a synonym of H. heterophyes.
Table 2.
Species of Heterophyes reported in the literature.
| Species | Human infection | Country/region | First reporter |
|---|---|---|---|
| Heterophyes heterophyes | Yes | Egypt, Sudan, Palestine, Turkey, India, Middle East, Japana, Koreaa | von Siebold in 1852 (Ransom, 1920) |
| Heterophyes nocens | Yes | Korea, Japan | Onji and Nishio (1916) |
| Heterophyes dispar | Yes | Egypt, Middle East, Koreab | Looss (1902) |
| Heterophyes aequalis | Yes | Egypt, Middle East | Looss (1902) |
| Heterophyes indica | No | India | Rao and Ayyar (1931) |
| Heterophyes pleomorphis | No | Uganda | Bwangamoi and Ojok (1977) |
| Twelve other species (subspecies)c | No |
Imported human cases were reported in Japan (Kagei et al., 1980) and Korea (Eom et al., 1985a, Chai et al., 1986b).
Imported human cases were reported in Korea (Eom et al., 1985a, Eom et al., 1985b, Chai et al., 1986a, Chai et al., 1986b).
Six of them, including H. aegyptiaca, H. fraternus, H. persicus, H. heterophyes sentus, H. inops, and H. palidus, were synonymized with H. heterophyes (Witenberg, 1929). H. dispar limatus was synonymized with H. dispar (Witenberg, 1929). The validity of H. elliptica has been questioned (Waikagul and Pearson, 1989). H. katsuradai was synonymized with H. nocens (Witenberg, 1929). H. superspinata (syn. H. bitorquatus) and H. chini have been transferred to another genus Alloheterophyes (Pearson, 1999).
2.2.1. Heterophyes heterophyes (v. Siebold, 1852) Stiles and Hassal, 1900
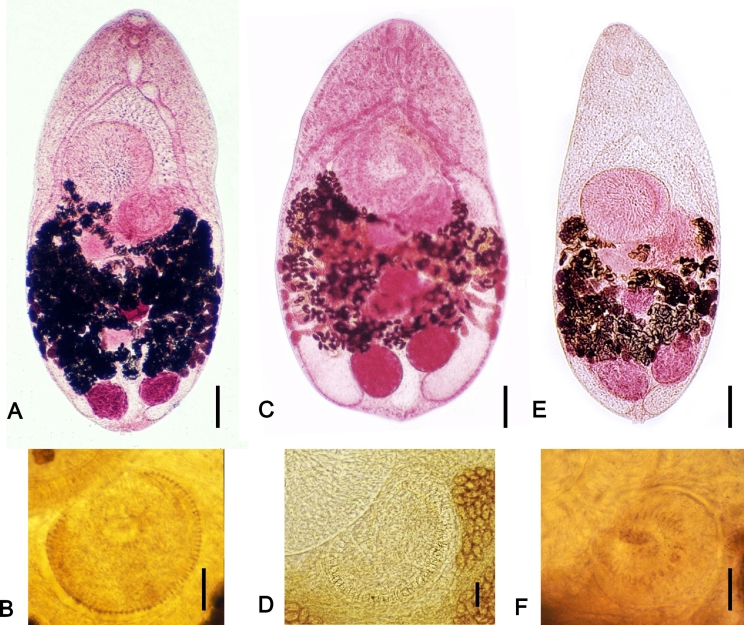
This species was first discovered by Bilharz in 1851 at autopsy of an Egyptian in Cairo (Chai, 2007, Chai, 2014b). It is now known to cause human infections along the Nile Delta of Egypt and Sudan, the Middle East, southeastern Europe, and India (Yu and Mott, 1994, Mahanta et al., 1995, Pica et al., 2003, Chai et al., 2005a). The adult flukes are minute, ovoid to elliptical, elongate, or pyriform in shape (Witenberg, 1929). Their unique morphologies include the presence of two side-by-side testes near the posterior extremity of the body, a large ventral sucker which is located median, and a large submedian genital sucker armed with 70–85 chitinous rodlets on the gonotyl (Chai, 2007, Chai, 2014b) (Fig. 3). Adults of H. heterophyes differ from those of H. nocens mainly in the number of rodlets on the gonotyl; 50–62 in H. nocens and 70–85 in H. heterophyes (Witenberg, 1929, Chai et al., 1994b, Chai et al., 2009a). Adults of H. dispar are slightly smaller in body size and have smaller sizes of the genital sucker and smaller numbers of rodlets on the gonotyl (22–35) compared with those of H. heterophyes and H. nocens (Witenberg, 1929, Chai et al., 1986a, Chai et al., 2009a).
Fig. 3.
Morphology of Heterophyes spp. infecting humans. (A, B) Heterophyes heterophyes, (C, D) Heterophyes nocens, and (E, F) Heterophyes dispar. Note that H. heterophyes has a gonotyl armed with more than 70 chitinous spines (rodlets) around the genital sucker (B), whereas H. nocens and H. dispar have about 60 (D) and 30 rodlets (F), respectively. Scale bars; (A) = 100 μm, (B) = 100 μm, (C) = 100 μm, (D) = 10 μm, (E) = 10 μm, (F) = 10 μm. The photo (A) has been reproduced, with permission, from the black and white photo of the same specimen reported by Chai et al. (1986b) in Korean J. Parasitol. 24, 82–88.
The eggs contain a fully developed miracidium and hatch after ingestion by an appropriate freshwater snail (for example, Pirenella conica) (Taraschewski, 1984). The cercariae enter between the scales of freshwater or brackish water fish, including mullets (Mugil cephalus, Mugil capito, Mugil auratus, Mugil saliens, and Mugil chelo), Tilapia fish (Tilapia nilotica and Tilapia zilli), and gobies/other fish (Aphanius fasciatus, Barbus canis, Sciaena aquilla, Solea vulgaris, and Acanthogobius sp.), and encyst chiefly in the muscle of these fish host (Paperna and Overstreet, 1981, Velasquez, 1982). Various species of mammals and birds, including dogs, cats, wolves, bats, rats, foxes, sea gulls, and pelicans, act as the natural definitive hosts and take the role of reservoir hosts (Yamaguti, 1958, Chai, 2014b). Rats, dogs, cats, foxes, badgers, pigs, macaques, and gulls can be experimental definitive hosts (Chai, 2014b).
The principal mode of human infections is consuming raw or improperly cooked fish, notably mullets and gobies (Chai, 2014b). Human and/or animal infections have been reported in Egypt, Sudan, Greece, Turkey, Palestine, Italy, Tunisia, India, and the Middle East, including Saudi Arabia, Iran, Iraq, United Arab Emirates, Kuwait, and Yemen (Yu and Mott, 1994, Chai, 2014b, Tandon et al., 2015). About 30 million people are estimated to be infected with this fluke (Mehlhorn, 2015). In Egypt, human infections are commonly found in the northern part of the Nile Delta, particularly around Lakes Manzala, Burullus, and Edku, where fishermen and domestic animals frequently consume fish (Yu and Mott, 1994, Youssef and Uga, 2014). In an earlier report, an extremely high prevalence (88%) was found among schoolchildren at Mataria on Lake Manzala (Khalil, 1933). In Dakahlia Governorate, the disease was common in both urban and rural localities owing to the habit of consuming salted or insufficiently baked fish (Sheir and Aboul-Enein, 1970). After 1960s–1980s, the prevalence began to decline down to 2.5–10.0% in these areas (Rifaat et al., 1980, Youssef et al., 1987a). However, near the Lake Edku, the prevalence among 2219 individuals was 33.8% demonstrating still considerably high endemicity in northern parts of Nile Delta (Abou-Basha et al., 2000). Interestingly, some Japanese immigrants living in Egypt were found positive for heterophyid eggs (including H. heterophyes); the prevalence was 11% in 2005, 11% in 2006, 15% in 2007, and 14% in 2008 (Okuzawa et al., 2010).
In Sudan, few epidemiological surveys have been undertaken, although some humans (Koreans) who lived in Sudan and returned home were found infected with H. heterophyes and H. dispar (Eom et al., 1985a). In Iran, the prevalence of heterophyid (including H. heterophyes) infections in humans and animals was documented by Massoud et al. (1981); in 13 villages of Khuzestan, the prevalence ranged 2–24%. In Saudi Arabia, Kuwait, Dubai, and United Arab Emirates, no reports are available on human infections. However, the existence of H. heterophyes has been documented in fish intermediate hosts or animal reservoir hosts (Abdul-Salam and Baker, 1990, Khalil et al., 2014, El-Azazy et al., 2015). Sporadic H. heterophyes infection has been reported from Greece, Turkey, Italy, Spain, Tunisia, Yemen, and Sri Lanka (Taraschewski and Nicolaidou, 1987, Yu and Mott, 1994, Martínez-Alonso et al., 1999). Imported human cases were reported in France (Rousset and Pasticier, 1972), Korea (Eom et al., 1985a, Chai et al., 1986a), and Japan (Kagei et al., 1980); the patients returned from Egypt, Saudi Arabia, or Sudan. In USA, a woman patient was found to be infected with H. heterophyes (the species is uncertain because only eggs were detected in her stool) after eating ‘sushi’ in a local Japanese restaurant (Adams et al., 1986).
2.2.2. Heterophyes nocens Onji and Nishio, 1916
The original description of this species is based on adult flukes recovered from experimental dogs and cats fed the metacercariae encysted in mullets Mugil cephalus in Japan (Onji and Nishio, 1916). Later, Heterophyes katsuradai was recovered from a man in Kobe, Japan and described as a new species (Ozaki and Asada, 1926). However, it was synonymized with H. nocens (Witenberg, 1929, Waikagul and Pearson, 1989). It is now known to occur in Korea, Japan, China, Taiwan (in China and Taiwan, the name was written as H. heterophyes probably by an error), and Thailand (Yokogawa et al., 1965, Seo et al., 1981b, Chai et al., 1984, Chai et al., 1985, Yu and Mott, 1994, Namchote et al., 2015). Its adult flukes are elongate, elliptical, or pyriform, and morphologically close to H. heterophyes (Chai et al., 1986b, Chai et al., 1994b). The only recognizable difference is the smaller number of rodlets on the gonotyl of the genital sucker in H. nocens (50–62) compared to that in H. heterophyes (70–85) (Taraschewski, 1984, Chai et al., 1986b, Chai et al., 1994b, Chai and Lee, 2002) (Fig. 3). H. dispar has a slightly smaller body, a smaller-sized genital sucker, and a smaller number (22–35) of rodlets on the gonotyl compared to H. nocens and H. heterophyes (Witenberg, 1929, Chai et al., 1986b). H. aequalis is also smaller in body size than H. nocens and H. heterophyes, has relatively short ceca, and has a smaller number (14–25) of rodlets on the gonotyl (Witenberg, 1929, Chai et al., 1986b).
The eggs hatch after ingestion by an appropriate brackish water snail, for example, Cerithidea cingulata (= Tympanotonus microptera) or Cerithidea fluviatilis (Ito, 1964b). The cercariae enter between the scales of brackish water fish, including mullets and gobies (Mugil cephalus, Liza menada, Tridentiger obscurus, Glossogobius brunnaeus, Therapon oxyrhynchus, and Acanthogobius flavimanus) (Ito, 1964a, Komiya, 1965, Sohn, 2009). Two species of brackish water gobies (Boleophthalmus pectinirostris and Scartelaos sp.) can also host H. nocens (Sohn et al., 2005). Cats, dogs, and rats are natural definitive hosts (Yoshikawa et al., 1940, Ito, 1964a, Seo et al., 1981a, Eom et al., 1985b, Sohn and Chai, 2005, Shin et al., 2015). Mice and rats can be used as experimental definitive hosts (Chai et al., 2009a, Chai, 2014b).
Raw or improperly cooked mullets and gobies are the major source of human infections (Chai, 2014b). Human infections have been reported in Korea, Japan, China, and Taiwan (Yu and Mott, 1994). In Korea, about 50,000 people are estimated to be infected with this fluke (Chai and Lee, 2002). In Korea, human infection was first identified in a man residing in a seashore village along the Western Sea (= Yellow Sea) (Seo et al., 1981b). Thereafter, cases were reported from western and southern coastal areas (Chai et al., 1984, Chai et al., 1985). Subsequently, numerous endemic areas with 10–40% prevalences were detected in southwestern coastal areas, i.e., Jeollanam-do and Gyeongsangnam-do Province (Chai et al., 1994b, Chai et al., 1997, Chai et al., 1998, Chai et al., 2004, Park et al., 2007, Guk et al., 2007). In addition, in an inland area of Boseong-gun (along the Boseong River) where M. yokogawai is highly endemic, a few adult specimens of H. nocens were also recovered from some riparian residents (Chai et al., 2015a).
In Japan, an earlier study reported 18.5% prevalence among inhabitants in Yamaguchi Prefecture (Onji, 1915). Later, in Chiba Prefecture, the heterophyid egg positive rate was 8.0% among residents in Goi-Machi village and 9.0% in Misaki-Machi village (Yokogawa et al., 1965). In Shizuoka Prefecture, the prevalence of H. nocens was 20.7% in Harai village of Izu peninsula but six years after a mass treatment, inhibition of raw fish eating, and health education, the prevalence decreased to 3.4% (Ito et al., 1967). Human H. nocens infection was also reported from Chugoku and Hiroshima Prefectures (Suzuki et al., 1982). Two lakeside villages of Mikkabi-cho, north end of Hamana Lake, Shizuoka Prefecture were also found to have 7.5% and 10.5% prevalences of H. nocens eggs (Kino et al., 2002). In China, human infections (under the name H. heterophyes) were reported in provinces of mainland China (Guangdong, Hubei, and Beijing) and Taiwan (Yu and Mott, 1994). However, the parasite may have been actually H. nocens (Chai, 2014b). The life cycle of H. nocens may be present in Thailand, since cercariae of Heterophyes sp. were recovered from brackish water snails (Namchote et al., 2015). A French professor who visited Japan and consumed raw fish and aquatic plants there was found to be infected with H. nocens (under the name H. heterophyes) (Lamy et al., 1976).
2.2.3. Heterophyes dispar Looss, 1902
The original description is based on specimens discovered in the intestines of dogs and cats in Egypt (Looss, 1902). It was found in other mammals, including foxes and wolves, in the northern Africa and eastern Mediterranean, including Greece and Palestine (Witenberg, 1929, Taraschewski and Nicolaidou, 1987, Yu and Mott, 1994, Chai, 2014b). Human infections were first reported from 2 Korean men returning from Saudi Arabia (Chai et al., 1986a) and then also found in Kalasin Province, Thailand (Yu and Mott, 1994). There were taxonomic debates on H. dispar and H. aequalis, both of which were described by Looss (1902) in Egypt. However, H. dispar is distinct from H. aequalis in the larger number of rodlets (22–35) on the gonotyl compared with H. aequalis (14–25) and long ceca extending down to the posterior margin of two testes in H. dispar, whereas they are ending before the anterior margin of two testes in H. aequalis (Looss, 1902, Witenberg, 1929). Based on several differential characters, Taraschewski, 1985a, Taraschewski, 1985b, Taraschewski, 1987 acknowledged the validity of both species. Adults of H. dispar are elliptical or pyriform, and slightly smaller than those of H. heterophyes and H. nocens (Chai et al., 1986a, Chai, 2014b). A remarkable difference is the smaller number of rodlets on the gonotyl of the genital sucker in H. dispar (22–35) compared to that in H. heterophyes (70–85) and H. nocens (50–62) (Chai et al., 1986a, Chai et al., 1994b, Chai, 2014b) (Fig. 3).
Freshwater or brackish water snails (P. conica) serve as the first intermediate host, and various species of freshwater or brackish water fish (including Mugil spp., Epinephelus enaeus, Tilapia spp., Lichia spp., Barbus canis, Solea vulgaris, and Sciaena aquilla) are the second intermediate hosts (Witenberg, 1929, Paperna and Overstreet, 1981, Taraschewski, 1984). Natural definitive hosts include dogs, cats, wolves, jackals, foxes, and kites (Witenberg, 1929, Chai, 2014b). Pups, rabbits, rats, cats, and red foxes are experimental definitive hosts (Taraschewski, 1985b).
Human infections may occur when Mugil spp. fish are eaten raw or under inadequately cooked conditions (Chai, 2014b). However, human infections were unknown before 1985–1986 when some Korean men were found infected with this fluke together with H. heterophyes who returned from Sudan (Eom et al., 1985a) and Saudi Arabia (Chai et al., 1986a). Human infectins were also confirmed in Thailand (Yu and Mott, 1994).
2.2.4. Heterophyes aequalis Looss, 1902
The original description is based on specimens discovered in the intestines of dogs and cats in Egypt (Looss, 1902). It infects various other mammals too (Witenberg, 1929, Taraschewski, 1985b). The presence of human infections was first mentioned by Kahlil in Egypt in 1991 (Ashford and Crewe, 2003), although the literature background is difficult to obtain. Morphologically H. aequalis is most similar to H. dispar but distinct from it in the smaller number of rodlets (14–25) on the gonotyl in comparison with H. dispar (22–35) and short ceca ending extending before the anterior margin of two testes in H. aequalis, whereas they reach down to the posterior margin of two testes in H. dispar (Looss, 1902, Witenberg, 1929).
Brackish water snails (P. conica) serve as the first intermediate host (Taraschewski, 1984). Freshwater or brackish water fish (including M. cephalus, M. capito, M. auratus, E. enaeus, T. simonis, L. amia, L. glauca, and B. canis) are the second intermediate hosts (Witenberg, 1929). Natural definitive hosts include cats, dogs, Persian wolves, ref. foxes, rats, pigs, pelicans, kites, and herons (Witenberg, 1929, Taraschewski, 1985b). Pups, rabbits, rats, cats, and red foxes are experimental definitive hosts (Taraschewski, 1985b). Human infections may occur when Mugil spp. fish are eaten raw or under inadequately cooked conditions (Chai, 2014b). Human infections seem to occur in Egypt (Ashford and Crewe, 2003).
2.3. Haplorchis
Flukes of Haplorchis are characterized by the presence of only one testis and a small armed ventral sucker lacking a gonotyl; they differ from Metagonimus and Heterophyes in having only one testis (Chai, 2007). They lack the expulsor-style distal part of the seminal vesicle which is present in genera Procerovum and Stellantchasmus (Pearson and Ow-Yang, 1982). The genus Haplorchis was erected with Haplorchis pumilio as the type and Haplorchis cahirinus as another species (Looss, 1899). However, Witenberg (1929) transferred H. cahirinus to another family because it is an intestinal parasite of fish in its adult stage, whereas H. pumilio is an intestinal parasite of birds and mammals. At present, 9 species can be recognized as valid species in the genus Haplorchis. They include H. pumilio (Looss, 1899), H. taichui (Nishigori, 1924a), H. yokogawai (Katsuta, 1932b), H. vanissimus (Africa, 1938), H. parataichui (Pearson, 1964), H. sprenti (Pearson, 1964), H. wellsi (Pearson, 1964), H. parapumilio (Pearson and Ow-Yang, 1982), and H. paravanissimus (Pearson and Ow-Yang, 1982) (Table 3). Among them, H. taichui, H. pumilio, H. yokogawai, and H. vanissimus are human-infecting species. Human infections with Haplorchis spp. are prevalent in Southeast Asia, including countries located in Indo-China Peninsula, Taiwan, the Philippines, and also probably in Egypt (Chai, 2007).
Table 3.
Species of Haplorchis reported in the literature.
| Species | Human infection | Country/region | First reporter |
|---|---|---|---|
| Haplorchis taichui | Yes | Thailand, Laos, Malaysia, Philippines, India, Bangladesh, Vietnam, China, Taiwan, Egypt, Palestine | Nishigori (1924a) |
| Haplorchis pumilio | Yes | Thailand, Laos, Egypt, Palestine, India, Bangladesh, China, Taiwan, Cambodia, Philippines, Malaysia, Vietnam, Koreaa | Looss in 1886 (Looss, 1902) |
| Haplorchis yokogawai | Yes | Thailand, Laos, Malaysia, Philippines, India, Indonesia, China, Taiwan, Cambodia, Vietnam, Australia, Egypt | Katsuta (1932b) |
| Haplorchis vanissimus | Yes | Philippines, Australia | Africa (1938) |
| Haplorchis sprenti | No | Australia | Pearson (1964) |
| Haplorchis wellsi | No | Taiwan | Pearson (1964) |
| Haplorchis parataichui | No | Australia | Pearson (1964) |
| Haplorchis parapumilio | No | Indonesia | Pearson and Ow-Yang (1982) |
| Haplorchis paravanissimus | No | Australia | Pearson and Ow-Yang (1982) |
A natural human case co-infected with H. pumilio, Gymnophalloides seoi, and Gynaecotyla squatarolae has been reported in Korea (Chung et al., 2011). However, Korea is tentatively not regarded as an endemic area for Haplorchis spp.
2.3.1. Haplorchis taichui (Nishigori, 1924) Chen, 1936
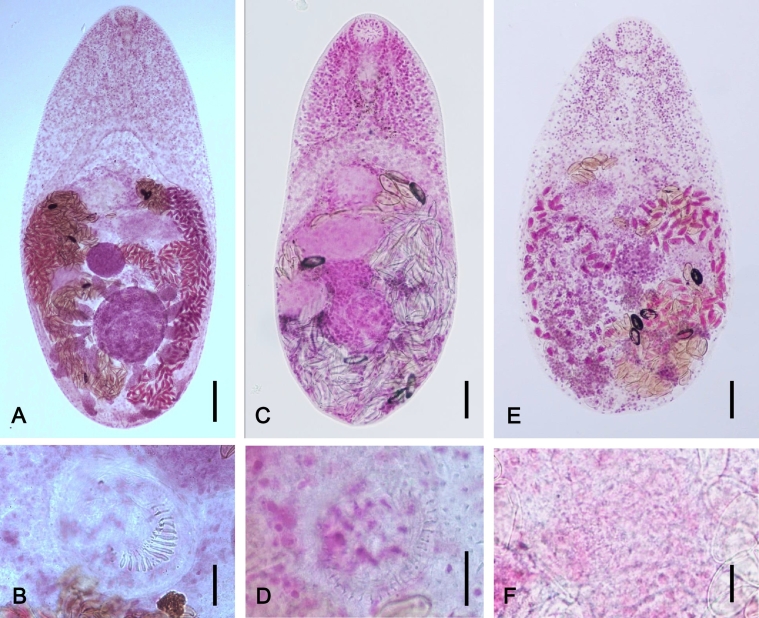
The original description of this species was based on specimens recovered from birds and mammals caught in the middle part of Taiwan (Nishigori, 1924a). Its characteristic morphology (Fig. 4) includes a minute and oval body with flattened dorsal and ventral sides (Faust and Nishigori, 1926). The most specific morphological feature for differentiation from other Haplorchis species is the size, shape, and number of spines on the ventral sucker (Chen, 1936, Pearson and Ow-Yang, 1982). H. taichui has a semi-lunar group of 12–16 long, crescentic, and hollow spines (Fig. 4) and a sinistral patch of very minute solid spines (Pearson and Ow-Yang, 1982). Other species can be differentiated by the presence of minute sclerites on the ventral sucker (H. pumilio, H. parapumilio, H. vanissimus, and H. paravanissimus), remarkably large ventral sucker (H. wellsi), or a ventral sucker smaller than the oral sucker (H. yokogawai and H. sprenti) (Pearson and Ow-Yang, 1982).
Fig. 4.
Morphology of Haplorchis spp. infecting humans. (A, B) Haplorchis taichui, (C, D) Haplorchis pumilio, and (E, F) Haplorchis yokogawai. Note that H. taichui has a gonotyl armed with 12–16 long and crescentic spines (B), whereas H. pumilio and H. yokogawai have about 32–40 minute sclerites (D) and numerous (uncountable) tiny spines (F), respectively. Scale bars; (A) = 100 μm, (B) = 100 μm, (C) = 100 μm, (D) = 10 μm, (E) = 10 μm, (F) = 10 μm.
The snail intermediate host is freshwater snails; Melania obliquegranosa in Taiwan (experimental) (Faust and Nishigori, 1926), Melanoides tuberculata and Melania juncea in the Philippines (Velasquez, 1973a), and Tarebia granifera in Hawaii (Martin, 1958). The cercariae enter between the scales of various species of freshwater fish (including Barbodes gonionotus, Cirrhinus molitorella, Cyclocheilichthys spp., Hampala spp., Labiobarbus leptocheila, Mystacoleucus marginatus, Onychostoma elongatum, Puntius spp., and Rhodeus ocellatus) (Velasquez, 1973b, Velasquez, 1982, Scholz et al., 1990, Rim et al., 2008). Birds and mammals, including dogs, cats, and humans, have been reported as the natural definitive hosts (Yamaguti, 1958). Experimental definitive hosts include mice, rats, hamsters, guinea pgs, rabbits, cats, and dogs (Faust and Nishigori, 1926, Sukontason et al., 2001). A human was also successfully infected with this fluke after swallowing the fish flesh (Faust and Nishigori, 1926).
In Thailand and Laos, fermentation is a traditional procedure for preservation of freshwater fish, and consumption of semi-fermented fish shortly after preparation increases the risk of human infections with Haplorchis spp. (Sukontason et al., 1998). Local fish dishes, notably ‘koi-pla’, ‘pla-som’, ‘pla-ra’, and ‘lab-pla’ are the major source of human infections (Sukontason et al., 1998). In the Philippines, a local fish dish called ‘kinilaw’ (freshwater fish seasoned only with salt and vinegar) is popularly consumed (Belizario et al., 2004). Other types of local fish dish in the Philippines include ‘sabaw’ (boiling fish for several minutes) and ‘sugba’ (grilling over charcoal) (Belizario et al., 2004). In Vietnam, raw or pickeld fish, for example, slices of silver carp, sold in Vietnamese restaurants are popularly consumed (Dung et al., 2007). H. taichui is known to distribute in Thailand, Laos, Cambodia, Vietnam, South China, Taiwan, the Philippines, Hawaii, Egypt, Palestine, Bangladesh, India, and Malaysia (Velasquez, 1982, Yu and Mott, 1994, Chai et al., 2005a, Chai et al., 2009a, Chai et al., 2014a).
In Thailand, northern parts, including Chiang Rai, Chiang Mai, Mae Hong Son, and Lamphun Provinces, were found to be endemic (Radomyos et al., 1998). In Phrae Province, the prevalence of H. taichui worms among 87 worm-recovery cases was 64.4% (Pungpak et al., 1998). In Nan Province, 37 of 50 praziquantel-treated patients were positive for H. taichui worms in their diarrheic stools, whereas in Lampang Province, 69 of 100 patients revealed H. taichui worms (Wijit et al., 2013). In northeastern parts, such as Khon Kaen Province, heterophyid trematodes are common among village people (Srisawangwong et al., 1997). In Laos, human H. taichui infection was first reported from 5 Laotians (Giboda et al., 1991). Thereafter, in fecal examinations of 29,846 Loatian people from 17 provinces and Vientine Municipality, the overall positive rate of small trematode eggs (O. viverrini, H. taichui, or other minute intestinal flukes) was 10.9% (3263 peole) (Rim et al., 2003). Chai et al. (2005b) performed worm recovery of O. viverrini, H. taichui, or other minute intestinal flukes from small trematode egg positive cases in two areas; Vientiane Municipality was highly prevalent with O. viverrini worms with a small number of intestinal flukes, including H. taichui, whereas Saravane Province was severely infected with H. taichui worms with a small number of O. viverrini specimens. Hyperendemicity of H. taichui, with high prevalence and heavy worm burden, was again documented in Saravane Province (Chai et al., 2013a). In Savannakhet Province, the prevalence of small trematode eggs among the riparian people was 67.1% (658/981), and the worms recovered after chemotherapy and purging of 29 egg-positive people consisted of similar numbers of O. viverrini (3347 worms) and H. taichui (2977 worms) (Chai et al., 2007). In Khammouane Province, the prevalence of small trematode eggs among the riparian people was higher than that in Savannakhet Province, 81.1% (1007/1242) (Chai et al., 2009b). An interesting finding in Phongsali Province was that the high prevalence (18.4%) of small trematode eggs notified (Rim et al., 2003) was confirmed to be exclusively due to H. taichui and H. yokogawai infections but not to O. viverrini infection (Chai et al., 2010). Also in Champasak and Luang Prabang Provinces, the infection was mostly due to intestinal flukes, in particular, H. taichui, H. yokogawai, and H. pumilio (Chai et al., 2013a, Chai et al., 2013b, Chai et al., 2013c, Sohn et al., 2014).
In Vietnam, a high prevalence (64.9%; 399/615) of minute intestinal trematodes, including H. taichui, H. pumilio (dominant species), and H. yokogawai, has been confirmed in human infections in two communes of Nam Dinh Province, a northern part of Vietnam (Dung et al., 2007). Later, in another commune in Nam Dinh Province, 22.7% (92/405) of household members were positive for small trematode eggs (De and Le, 2011). In Southern China, the presence of human H. taichui infection was first documented from Guangxi Province in 2004 (T. Li et al., 2010). Later, in several different areas of Guangxi Province, 28.0–70.6% prevalence was obtained by fecal examination for small trematode eggs; PCR analysis of the feces revealed that 29 of 46 egg positive cases were due to H. taichui (Jeon et al., 2012). In Taiwan, where H. taichui was originally discovered (Nishigori, 1924a, Faust and Nishigori, 1926), little study has been available on human infections with this fluke. In the Philippines, the Lake Lanao, Marawi City, Mindanao Island and U.P. rice paddies, Diliman, Quezon City, and Luzon Island have been listed as endemic areas of H. taichui (Velasquez, 1973b). In southern Mindanao, 36.0% (87/242) egg positive rate was reported from residents; H. taichui adult flukes were recovered from some of these people (Belizario et al., 2004). In Egypt and Kuwait, little has been reported regarding human infections; however, the presence of H. taichui was documented from animals (Kuntz and Chandler, 1956, El-Azazy et al., 2015).
2.3.2. Haplorchis pumilio (Looss, 1896) Looss, 1899
The original description of this species is based on adult specimens recovered from the small intestine of birds and mammals in Egypt (Looss, 1899). Its specific morphological feature (Fig. 4) for differentiation from other Haplorchis species is the size, shape, and number of spines on the ventral sucker (Chen, 1936, Pearson and Ow-Yang, 1982). H. pumilio has a circle of 32–40 I- or Λ-shaped sclerites (Fig. 4), 2.5–5.9 μm long, interrupted dorsally between latero-dorsal lobes of the ventral sucker, and a few to 9 small simple spines of various lengths on latero-dorsal and mid-dorsal lobes, respectively (Pearson, 1964, Pearson and Ow-Yang, 1982). H. taichui is differed from H. pumilio by the presence of a semi-lunar group of 12–16 long, crescentic, and hollow spines and a sinistral patch of very minute solid spines. Other species can be differed from H. pumilio by the presence of 15–21 hollow spines (H. parataichui), ventral sucker with four lobes and armed with spines (H. vanissimus), ventral sucker without lobes and spines interrupted mid-ventrally (H. paravanissimus), remarkably large ventral sucker (H. wellsi), or a ventral sucker smaller than the oral sucker (H. yokogawai and H. sprenti) (Pearson and Ow-Yang, 1982).
The snail host is Melania reiniana var. hitachiens (experimental) in Taiwan (Faust and Nishigori, 1926, Velasquez, 1982) and Melanoides (= Thiara) tuberculata (natural) in Taiwan (Lo and Lee, 1996, Wang et al., 2002), India (Umadevi and Madhavi, 2006), and Egypt (Khalifa et al., 1977). The second intermediate host is various species of freshwater or brackish water fish which belong to the Cyprinidae, Siluridae, and Cobitidae (Acanthogobius spp., Ambassis buruensis, Anabas spp., Astatotilapia desfontainesi, Barbus spp., Carrssius spp., Cyprinus spp., Esomus longimana, Gerris filamentosus, Glossogobius giurus, Hampala macrolepidota, Mugil capito, Ophicephalus striatus, Puntius binotatus, Therapon plumbeus, Teuthis javus, and Tilapia spp.) (Faust and Nishigori, 1926, Velasquez, 1982, Scholz et al., 1990, Scholz, 1991). Fish-eating birds and mammals, including dogs, cats, and humans, have been reported as the natural definitive hosts (Yamaguti, 1958, Yu and Mott, 1994). Experimental definitive hosts include mice, rats, hamsters, guinea pgs, rabbits, cats, and dogs (Faust and Nishigori, 1926, Chai et al., 2012, Nissen et al., 2013).
In Vietnam, where this parasite is highly endemic, raw or pickled fish, for example, slices of silver carp, sold in Vietnamese restaurants is a major source of infection (Dung et al., 2007). In Thailand and Laos, where fermentation is a traditional preservation method for freshwater fish, semi-fermented fish shortly after preparation is the major source of human infections with liver (Opisthorchis viverrini) and intestinal flukes (H. pumilio, H. taichui, and H. yokogawai) (Sukontason et al., 1998). Practically, local fish dishes, namely ‘lab-pla’ and ‘pla-som’ are important sources of infection (Radomyos et al., 1998, Sukontason et al., 1998, Onsurathum et al., 2016). H. pumilio is distributed in Vietnam, Thailand, Laos, Cambodia, South China, Taiwan, the Philippines, Egypt, Palestine, Iraq, India, Sri Lanka, Bangladesh, and Malaysia (Witenberg, 1929, Pearson and Ow-Yang, 1982, Chai et al., 2005a, Chai et al., 2009a, Chai et al., 2015b).
In Vietnam, the presence of H. pumilio, H. taichui, and H. yokogawai, has been confirmed in human infections in two communes of Nam Dinh Province, a northern part of Vietnam (Dung et al., 2007). The prevalence of small trematode eggs among riparian people in this area was 64.9% (399/615) (Dung et al., 2007). Later, in another commune in Nam Dinh Province, 22.7% (92/405) of household members were positive for small trematode eggs (De and Le, 2011). In Ninh Binh Province, 20.5% (381/1857) of commune people were positive for small trematode eggs (Hung et al., 2015). In Thailand, 12 human cases were first reported by Radomyos et al., 1983, Radomyos et al., 1984 through recovery of adult flukes. Thereafter, northern parts (including Chiang Rai and Chiang Mai Provinces) were found to be endemic areas of this fluke (Radomyos et al., 1998). In Laos, the presence of human H. pumilio infection was first documented by Chai et al. (2005b) based on recovery of adult flukes after praziquantel treatment and purging in Saravane Province and Vientiane Municipality. Later, in Saravane and Champasak Province, 796 and 247 adult specimens of H. pumilio were recovered in 5 and 5 patients, respectively (Chai et al., 2013a). In Savannakhet Province, a small proportion of recovered worms from 3 patients (80 among 7693 fluke specimens) were H. pumilio (Chai et al., 2007). In Luang Prabang Province, total 41 specimens of H. pumilio were recovered in 4 patients (Sohn et al., 2014). In Xieng Khouang Province, 2268 specimens of H. pumilio were harvested from 8 egg positive people (Chai et al., 2015b).
In Cambodia, no human cases have been detected until present; however, in 2014, a fish survey in Pursat Province, near the Lake Tonlesap, revealed the infection of freshwater fish with the metacercariae of H. pumilio (Chai et al., 2014a). In Southern China, the presence of human H. pumilio infection was documented in Guangdong Province in 1964; however, the background literature was not provided (T. Li et al., 2010). In Taiwan, H. pumilio was described in earlier times (Nishigori, 1924a, Faust and Nishigori, 1926); however, there have been no reports on human infections with this fluke. In the Philippines, human infections have never been documented. In Egypt, human H. pumilio infection was first documented by Khalifa et al. (1977) in a 9-year-old child passing diarrheic stools. In Korea, a 5-year old patient was found to be naturally infected with one specimen of H. pumilio, together with 841 Gymnophalloides seoi and 3 Gynaecotyla squatarolae specimens (Chung et al., 2011).
2.3.3. Haplorchis yokogawai (Katsuta, 1932) Chen, 1936
The original description of this species, Monorchotrema yokogawai, is based on adult flukes obtained in the small intestine of dogs and cats experimentally fed the metacercariae encysted in the mullet Mugil cephalus in Taiwan (Katsuta, 1932b). Its characteristic morphology (Fig. 4) differentiated from other Haplorchis species is the size, shape, and number of spines on the ventral sucker (Chen, 1936, Pearson and Ow-Yang, 1982). H. yokogawai has a small ventral sucker with its apex comprising a large ventral lobe armed with numerous tiny spines (Fig. 4) and a pair of large variable-sized sclerites ventro-dextrally, and 3 small lobes armed with tiny spines (Chen, 1936, Pearson and Ow-Yang, 1982). The total number of tiny spines on the ventral sucker was reported to be 70–74 (Katsuta, 1932b) but difficult to count. Ceca of H. yokogawai exceed the middle level of the testis (Pearson and Ow-Yang, 1982). H. pumilio is morphologically similar to H. yokogawai but the latter has 23–32 I- or Λ-shaped sclerites on the ventral sucker (Pearson and Ow-Yang, 1982). Other species can be differentiated by the presence of 12–16 or 15–21 large spines (H. taichui or H. parataichui, respectively), ventral sucker with four lobes and armed dorsal pocket (H. vanissimus), ventral sucker without lobes but with armed dorsal pocket with tiny spines interrupted mid-ventrally (H. paravanissimus), or remarkably large ventral sucker (H. wellsi) (Pearson and Ow-Yang, 1982).
The snail host is Melanoides (= Thiara) tuberculata or Stenomelania newcombi (Ito, 1964b, Velasquez, 1982). The second intermediate host is freshwater fish (Ambassis burunensis, Amphacanthus javus, Anabas testudineus, Boleophthalmus spp., Carassius auratus, Cirrhinus jullieni, Clarias fuscus, Cyclocheilichthys spp., Gambusia affinis, Gerris kapas, Hampala dispar, Hemiramphus georgii, Misgurnus spp. Mugil affinis, Mullus spp., Ophicephalus stratus, Pectinirostris spp., Puntioplites proctozysron, Puntius spp., and Tilapia nilotica) in Taiwan, the Philippines, Laos, Cambodia, Vietnam, South China, Malaysia, northern Thailand, India, Australia, and Egypt (Velasquez, 1982, Rim et al., 2008, Rim et al., 2013, Lobna et al., 2010, Chai et al., 2012, Chai et al., 2014a). Fish-eating birds and mammals, including dogs, cats, cattle, and humans, have been reported as the natural definitive hosts (Yamaguti, 1958, Yu and Mott, 1994). Experimental definitive hosts include mice, cats, dogs, and humans (Katsuta, 1932b).
In Thailand and Laos, consumption of semi-fermented fish shortly after preparation is the major risk for human infections with liver and intestinal flukes, including H. yokogawai (Sukontason et al., 1998). Notably, local fish dishes, ‘lab-pla’ and ‘pla-som’ are important sources of infection (Radomyos et al., 1998, Sukontason et al., 1998, Onsurathum et al., 2016). In the Philippines, local fish dishes called ‘kinilaw’ (freshwater fish seasoned only with salt and vinegar), ‘sabaw’ (boiling fish for several minutes), and ‘sugba’ (grilling over charcoal) are popularly consumed and seem to be the source of infection with Haplorchis spp. (Belizario et al., 2004). In Vietnam, raw or pickeld fish, for example, slices of silver carp, is an example of the infection source (Dung et al., 2007). H. yokogawai is now known to distribute in Thailand, Laos, Cambodia, Malaysia, Vietnam, South China, Taiwan, the Philippines, Hawaii, Egypt, India, Indonesia, and Australia (Velasquez, 1982, Yu and Mott, 1994, Chai et al., 2005a, Chai et al., 2009a, Chai et al., 2014a). Human infections are known in Thailand, Laos, Vietnam, the Philippines, and China.
In Thailand, human infection was first recorded by Manning and Lertprasert (1971), Manning et al. (1971), and then by Kliks and Tantachamrun (1974) and Radomyos et al. (1984). Subsequently, northern parts of Thailand (including Chiang Rai and Chiang Mai Provinces) were found to have human cases infected with H. yokogawai (Radomyos et al., 1998). In another study performed in Phrae Province, the prevalence of H. yokogawai among 87 worm-recovery cases was 2.3%, much lower than 64.4% of H. taichui (Pungpak et al., 1998). In Laos, the presence of human infection was first documented by Chai et al. (2005a) based on adult flukes recovered after praziquantel treatment and purging. Low grade infections were subsequently detected in Savannakhet Province (Chai et al., 2007). In another study in Savannakhet and Saravane Province, adult specimens were recovered from a small number of patients (Sayasone et al., 2009). In Phongsali Province, Haplorchis spp., predominantly H. taichui and a small number of H. yokogawai were recovered from local people (Chai et al., 2010). Similarly, in Luang Prabang Province, adults were recovered in 5 of 10 patients treated with praziquantel (Sohn et al., 2014). In Vietnam, the presence of H. yokogawai, together with H. pumilio, H. taichui, and other intestinal flukes, has been first confirmed in human infections in two communes of Nam Dinh Province (Dung et al., 2007).
In the Philippines, human infections were described by Africa et al. (1940) from 16 of 33 human autopsies. Later, however, the presence of this parasite has seldom been documented. In Southern China, the presence of human H. pumilio infection was first documented in Guangdong Province in 1979; however, the literature background was not provided (T. Li et al., 2010). In Taiwan where H. yokogawai was originally discovered, the possibility of human infection was proved by a human experimental infection (Katsuta, 1932b); however, no reports are available on natural human infections. In Cambodia, a recent fish survey in Pursat Province, near the Lake Tonlesap, revealed the infection of freshwater fish (Puntioplites falcifer) with the metacercariae of H. yokogawai (Chai et al., 2014a); however, no human infection cases have been detected.
2.3.4. Haplorchis vanissimus (Africa, 1938) Yamaguti, 1958
The original description of this species is based on adult flukes recovered at an autopsy of a Filipino (Africa, 1938). It was morphologically redescribed by Pearson (1964) in Australia. Its specific morphology includes the presence of four armed lobes and an armed dorsal pocket on the ventral sucker with spines continuous across the width of ventral face (Pearson, 1964, Pearson and Ow-Yang, 1982). It is readily distinguished from other species of Haplorchis (H. pumilio, H. parapumilio, H. taichui, H. parataichui, H. yokogawai, H. wellsi, and H. sprenti) by the form and spination of the ventral sucker (Pearson, 1964, Pearson and Ow-Yang, 1982). The most closely resembled species is H. paravanissimus which has a simple ventral sucker without obvious lobes and the presence of minute spines interrupted mid-ventrally (Pearson and Ow-Yang, 1982).
The snail host has not been confirmed. Freshwater fish probably play the role of the second intermediate hosts (Yu and Mott, 1994). In Australia, fish-eating birds were found to be natural definitive hosts (Pearson, 1964). No further human infections have been documented. The practical mode of human infection or the type of fish dish involved is unknown. The geographical distribution of this fluke is confined to the Philippines and Australia.
2.4. Pygidiopsis
2.4.1. Pygidiopsis summa Onji and Nishio, 1916
The original description of this species is based on adult flukes recovered from dogs fed brackish water fish infected with the metacercariae in Japan (Onji and Nishio, 1916). Its characteristic morphologies include a small concave body, median location of the ventral sucker, unique morphology of the ventrogenital apparatus (having two groups of spines on the gonotyl; 5–6 right side and 7–9 left side), side-by-side location of the two testes (Chai et al., 1986a), and small pyriform eggs with no distinct muskmelon patterns on the shell (Lee et al., 1984a). In P. genata, the ventral sucker is median and globular in shape, whereas in P. summa, it is slightly submedian and transversely elliptical; the former has only one group of spines on the gonotyl whereas the latter has two groups of spines (Chai et al., 1986a).
The life cycle of P. summa has been elucidated by Ochi (1931) in Yamaguti, Hiroshima, and Okayama Prefectures, Japan. The snail host is the brackish water snail Tympanotonus microptera (= Cerithidea fluviatilis) in Korea (Chai, unpublished observation) and in Japan (Ochi, 1931). The metacercariae are detected in the gill and muscle of the mullets (Mugil cephalus and Liza menada), redlip mullets (Chelon haematocheilus), and gobies (Acanthogobius flavimanus) (Onji and Nishio, 1916, Chun, 1963, Sohn et al., 1994a, Kim et al., 2006, Sohn, 2009, Cho et al., 2012). Natural infections of domestic or feral cats (Eom et al., 1985b, Sohn and Chai, 2005, Shin et al., 2015) and raccoon dogs (E.H. Shin et al., 2009) have been reported. Experimental hosts include rats, cats, and dogs (Ochi, 1931, Chun, 1963).
Humans are contracted by this fluke through consumption of raw or undercooked brackish water fish, in particular, the mullet, goby, and perch. It is now known to distribute in Japan, Korea, and Vietnam (Yokogawa et al., 1965, Seo et al., 1981b, Vo et al., 2008, Sohn et al., 2016) (Table 4). Human infections with P. summa were first reported by detection of eggs in human feces in Japan (Takahashi, 1929b). Subsequently, adult flukes were identified from humans in Japan (Yokogawa et al., 1965) and Korea (Seo et al., 1981b). In Korea, eight people residing in a salt-farm village of Okku-gun, Jeollabuk-do who habitually ate the raw flesh of the mullet were proved to be infected with P. summa through recovery of adult flukes after bithionol treatment and purging (Seo et al., 1981b). In another coastal area of South Korea, 18 heterophyid egg-positive people were found to be mixed-infected with P. summa and Heterophyes nocens (Chai et al., 1994b). Eighteen more cases were detected in another coastal area, Muan-gun, Jeollanam-do, from whom total 703 adult specimens of P. summa were recovered (Chai et al., 1997). Five more cases were detected in Buan-gun, Jeollabuk-do, and total 1732 adult specimens were collected (Chai et al., 1998). Subsequently, more wide distribution of this parasite has been found along the western and southern coastal islands of South Korea, although the egg positive rate was remarkably higher for Heterophyes nocens (11.0%) than that for P. summa (1.2%) (Chai et al., 2004).
Table 4.
Other heterophyid species infecting humans around the world.
| Species | Human infection | Country/region | First reporter |
|---|---|---|---|
| Pygidiopsis summa | Yes | Korea, Japan, Vietnam | Onji and Nishio (1916) |
| Pygidiopsis genata | Yes | Egypt, Palestine, Rumania, Iran, Ukraine, Tunisia, Israel, Kuwait, Philippines | Looss (1907) |
| Heterophyopsis continua | Yes | Korea, Japan, China, Vietnam, Saudi Arabia, United Arab Emirate | Onji and Nishio (1916) |
| Stellantchasmus falcatus | Yes | Korea, Japan, Philippines, USA (Hawaii), Thailand, Vietnam, China, Taiwan, Laos, Australia, India, Iran, Palestine | Onji and Nishio (1916) |
| Centrocestus formosanus | Yes | Taiwan, China, Japan, Philippines, India, Vietnam, Laos, Thailand, Turkey, Croatia, USA, Mexico, Costa Rica, Colombia, Brazil | Nishigori (1924b) |
| Centrocestus armatus | Ye | Japan, Korea | Tanabe (1922) |
| Centrocestus cuspidatus | Yes | Egypt, Taiwan, China, Kuwait, Tunisia | Looss in 1896 (Looss, 1902) |
| Centrocestus kurokawai | Yes | Japan | Kurokawa (1935) |
| Stictodora fuscata | Yes | Korea, Japan, Kuwait | Onji and Nishio (1916) |
| Stictodora lari | Yes | Korea, Japan, Australia, Russia, Vietnam | Yamaguti (1939) |
| Procerovum varium | Yes | Japan, China, Philippines, Cambodia, Laos, Vietnam, Australia, India, Korea | Onji and Nishio (1916) |
| Procerovum calderoni | Yes | Philippines, China, Egypt | Africa and Garcia (1935) |
| Acanthotrema felis | Yes | Korea | Sohn et al., 2003a, Sohn et al., 2003b |
| Apophallus donicus | Yes | USA, Canada, Europe | Skrjabin and Lindtrop in 1919 (Price, 1931) |
| Ascocotyle longa | Yes | USA, Brazil, Mexico, Colombia, Panama, Peru, Czech Republic, Germany, Greece, Romania, Egypt, Georgia, Israel, Turkey | Ransom (1920) |
| Cryptocotyle lingua | Yes | UK, Denmark, Iceland, Norway, Russia, Russia, North America, Japan, Greenland | Creplin in 1825 (Stunkard, 1930) |
2.4.2. Pygidiopsis genata Onji and Nishio, 1916
The original description of this species is based on adult flukes recovered from the small intestine of a pelican in Cairo, Egypt (Looss, 1907, Ransom, 1920). Its characteristic morphologies include the presence of 16 small spines on the oral sucker which are seen only in fresh preparations, pear-shape or triangular body, short ceca terminating at the level of the ovary, and a small oval gonotyl on the ventrogenital sac (Witenberg, 1929). In P. summa, the body is ovoid to oval, with relatively long ceca terminating near the anterior border of testes, and two small oval gonotyl armed with spines (Chai et al., 1986a).
The snail host is Melanoides tuberculata (Youssef et al., 1987b) and Melanopsis costata (Dzikowski et al., 2004). The second intermediate host is brackish water fish (including Barbus canis, Mugil capito, and Tilapia spp.) (Witenberg, 1929, Mahdy and Shaheed, 2001, Ibrahim and Soliman, 2010, Lobna et al., 2010), and experimentally Gambusia affinis and Tilapia nilotica (Youssef et al., 1987b). Natural definitive hosts include wolves, cats, dogs, foxes, shrews, rats, pelicans, kites, ducks, and cormorants (Looss, 1907, Witenberg, 1929, Kuntz and Chandler, 1956, Dzikowski et al., 2004). Experimental hosts include mice, rats, and hamsters (Mansour et al., 1981).
The mode of human infection is probably the consumption of raw or undercooked brackish water fish. Human infections were unknown before 1987 when eggs were detected in human fecal examinations from Maryut area on Manzala Lake, Egypt; the egg positive rate was 2.7% (2/73) (Youssef et al., 1987a). Although the adult flukes were not identified, the eggs of P. genata were morphologically well discriminated from those of Heterophyes heterophyes (Youssef et al., 1987a). The existence of human infections in other countries, including Rumania, Iran, Palestine, Ukraine, Tunisia, Israel, and Kuwait should be determined. After its discovery, P. genata was found in pelicans in Romania (Ciurea, 1924), dogs in China (it should be verified whether the species was actually P. genata or a related species P. summa) (Faust and Nishigori, 1926), and dogs and cats (both hosts were experimentally infected) in the Philippines (Africa and Garcia, 1935, Africa et al., 1940), and a Persian wolf, dogs, and cats in Palestine (Witenberg, 1929). In Egypt, this parasite has been found from cats, dogs, foxes, shrews, rats, kites, and ducks (Kuntz and Chandler, 1956). Recently, this parasite was recovered from birds in Israel (Dzikowski et al., 2004) and stray cats in Kuwait (El-Azazy et al., 2015).
2.5. Heterophyopsis
2.5.1. Heterophyopsis continua (Onji and Nishio, 1916) Price, 1940
The original description of this parasite is based on specimens recovered from experimental cats fed the mullet harboring the metacercariae in Japan (described under the name Heterophyes continuus) (Onji and Nishio, 1916). Tubangui and Africa (1938) created a new genus Heterophyopsis. Yamaguti (1939) also created a new genus Pseudoheterophyes and assigned H. continuus to this genus. However, Africa et al. (1940) and Price (1940) synonymized Pseudoheterophyes with Heterophyopsis and thereafter, the status of the genus Heterophyopsis has been settled. Its characteristic morphology includes the elongate body, genital sucker located separately at a slightly postero-sinistral position from the ventral sucker, and two obliquely tandem testes (Seo et al., 1984a, Chai and Lee, 2002). The most similar genus Heterophyes is different from Heterophyopsis in having a genital sucker closely adjacent with the ventral sucker, whereas the genital sucker is much separated from the ventral sucker; the former has a ovoid to elliptical or pyriform body but the latter has an elongated body (Chai, 2007).
The first intermediate host is yet unknown. Metacercariae encyst in the perch Lateolabrax japonicas, goby Acanthogobius flavimanus, Konosirus (= Clupanodon) punctatus, and Conger myriaster (Chun, 1960b, Seo et al., 1984a, Sohn et al., 1994a, Sohn et al., 2009a, Chai et al., 2005a, Kim et al., 2006, Cho et al., 2012). Metacercariae were also detected in the sweetfish (P. altivelis), gobies (Boleophthalmus pectinirostris and Scartelaos sp.), mullets (M. cephalus), and groupers (Epinephelus bleekeri and E. coioides) (Sohn et al., 2005, Kim et al., 2006, Vo et al., 2008, Vo et al., 2011, Thanh et al., 2009, Chai et al., 2009a). Natural definitive hosts are domestic or feral cats (Eom et al., 1985b, Sohn and Chai, 2005, Schuster et al., 2009, Chai et al., 2013c), dogs (Yamaguti, 1958), ducks (Onji and Nishio, 1916), and sea-gulls (Yamaguti, 1939). Experimental definitive hosts include cats (Onji and Nishio, 1916), dogs (Chun, 1960b, Seo et al., 1984a) and domestic chicks (Hong et al., 1990, Shin et al., 2015).
The presence of human infections was mentioned in Japan (Yamaguti, 1958, Komiya and Suzuki, 1966); however, no proper literature background is available for this. Subsequently, in Korea, two natural human infections were first discovered (Seo et al., 1984a). To date, total 17 human cases, including the first two cases, have been confirmed by recovery of adult flukes in Korea (Hong et al., 1996, Guk et al., 2006, Park et al., 2007, Chai et al., 1997, Chai et al., 1998, Chai et al., 2004, Chai et al., 2014a, Chai et al., 2015b). It is now distributed in Korea (Seo et al., 1984a, Chai et al., 2009b), China (Yu and Mott, 1994), Japan (Yamaguti, 1958, Komiya and Suzuki, 1966), Vietnam (Vo et al., 2008, Chai et al., 2012), Saudi Arabia (under the name Heterophyopsis sp.; Kalantan et al., 2000), and United Arab Emirates (Schuster et al., 2009).
2.6. Stellantchasmus
2.6.1. Stellantchasmus falcatus Onji and Nishio, 1916
The original description of this species was based on adult flukes obtained from cats, dogs, and a bird experimentally fed the mullet harboring the metacercariae in Japan (Onji and Nishio, 1916). Witenberg (1929) created a new genus Diorchitrema for the flukes obtained from the intestine of dogs and cats from Palestine, reporting them as D. pseudocirrata. However, Alicata and Schattenberg (1938) and Price (1940) synonymized Diorchitrema with Stellantchasmus, placing D. pseudocirrata as a synonym of S. falcatus. In Taiwan, Stellantchasmus formosanus and Stellantchamus amplicaecalis were reported as new species; however, both species were synonymized with S. falcatus (Pearson, 1964). The adult flukes are morphologically characterized by its pyriform shape with the presence of a small submedian ventral sucker and an expulsor type elongated sac-like seminal vesicle (Seo et al., 1984b, Chai and Lee, 2002).
The snail host has been confirmed to be the brackish water snail (Melanoides tuberculata, Stenomelania newcombi, Thiara granifera, and T. granifera mauiensis) (Martin, 1958, Noda, 1959). The second intermediate host was shown to be brackish water fish, in particular, the mullets (M. cephalus, other Mugil spp., Liza menada, and L. haematocheila) and gobies (Acanthogobius flavimanus) (Alicata and Schattenburg, 1938, Martin, 1958, Chai and Sohn, 1988, Chai et al., 2009a). However, freshwater fish, in particular, the half-beaked fish (Dermogenus pusillus) and climbing perch (Anabas testudineus) also serve as the second intermediate host in the northern part of Thailand (Wongsawad et al., 2004, Wongsawad and Wongsawad, 2010). Analysis of the genomic DNA revealed that the two types (the freshwater type and the brackish water type) of S. falcatus are strongly suggested to be different species (Wongsawad and Wongsawad, 2010). Further stdies are warranted to verify this point. Natural definitive hosts are stray cats, dogs, pigs, rats, humans, and birds (Takahashi, 1929b, Seo et al., 1984b, Sohn and Chai, 2005, Anh et al., 2009, Shin et al., 2015). Experimental hosts include chicks, mice, rats, cats, dogs, and humans (Katsuta, 1931, Alicata and Schattenburg, 1938, Wongsawad et al., 1998, Saenphet et al., 2003, Ramsuk et al., 2004).
Human infections with S. falcatus were first detected by recovery of eggs in human feces (105 of 6680 inhabitants) in Japan, but the adult flukes were not confirmed (Takahashi, 1929b). Katsuta (1931) succeeded in infecting himself with S. formosanus (a synonym of S. falcatus), and Africa and Garcia (1935) detected people infected with S. falcatus (under the name D. pseudocirrata) by adult worm recovery in the Philippines. Thereafter, a few human cases were found by recovery of adult flukes in Hawaii (Alicata and Schattenburg, 1938, Glover and Alicata, 1957), Japan (Kagei et al., 1964, Ito, 1964a), Thailand (Kliks and Tantachamrun, 1974, Tantachamrun and Kliks, 1978, Radomyos et al., 1990), Korea (Seo et al., 1984b, Hong et al., 1986, Chai et al., 1998), and Vietnam (Dung et al., 2007). Now this fluke is known to distribute in Japan, the Philippines, Hawaii, Korea, China, Taiwan, Laos, Vietnam, Thailand, Cambodia, Australia, India, Iran, and Palestine (Pearson, 1964, Ditrich et al., 1990, Radomyos et al., 1990, Wongsawad et al., 1997, Wongsawad et al., 1998, Farahnak et al., 2004, Chai et al., 2009a, Chai et al., 2016).
2.7. Centrocestus
2.7.1. Centrocestus formosanus (Nishigori, 1924) Price, 1932
The original description of this species was based on worms obtained from experimental dogs, cats, mice, rats, and ducks fed freshwater fish containing the metacercariae and found also in a naturally infected night herons (Nycticorax nycticorax) in Taiwan (Nishigori, 1924a, Nishigori, 1924b). Thereafter, numerous species of this genus have been described. However, Chai et al. (2013a) proposed the recognition of only six species, namely, C. armatus, C. cuspidatus, C. formosanus, C. kurokawai, C. polyspinosus, and C. asadai in the genus Centrocestus. The adult flukes are morphologically characterized by having a body rounded posteriorly and tapering anteriorly, no caudal appendage of the oral sucker, and 30–36 circumoral spines around the oral sucker (Witenberg, 1929, Chen, 1942). The number of oral spines is the most useful key to differentiate the species; C. armatus has 42–48 circumoral spines, C. cuspidatus 36 spines, C. kurokawai 38–40 spines, C. polyspinosus 50–60 spines, and C. asadai 26–30 spines (Waikagul et al., 1997, Chai, 2007).
The snail host is S. libertina in Taiwan (Nishigori, 1924a, Nishigori, 1924b, Ito, 1964b), Stenomelania newcombi in Hawaii (Martin, 1958), and Melanoides tuberculata in Taiwan (Lo and Lee, 1996), USA, and Mexico (Scholz and Salgado-Maldonado, 1999, Mitchell et al., 2000). Various species of fish, including Anabas testudineus, Astyanax fasciatus, Carassius auratus, Cirrhinus molitorella, Cyclocheilichthys armatus, C. repasson, Cyprinus carpio, Etheostoma fonticola, Gambusia affinis, Hampala dispar, Hypsibarbus pierrei, Misgurnus anguillicaudatus, Mystacoeleucus atridorsalis, M. greenwayi, Ophicephalus striatus, Oreochromis aureus, Osteochilus hasselti, Physoschistura meridionalis, P. parva, Puntioplites proctozysron, Puntius brevis, P. gonionotus, P. leiacanthus, Tilapia nilotica, T. zillii, and Zacco platypus, serve as the second intermediate host (Chen, 1942, Martin, 1958, Waikagul et al., 1997, Srisawangwong et al., 1997, Mitchell et al., 2000, Han et al., 2008, Rim et al., 2008, Rim et al., 2013). Natural definitive hosts are fish-eating birds and mammals, such as the green heron (Butorides vitrescens), heron (Butorides striatus), pond heron (Ardeola grayii), night heron (N. nycticorax), great egret (Ardea alba), and dog (Chen, 1942, Premvati and Pande, 1974, Scholz and Salgado-Maldonado, 1999, Mitchell et al., 2000). Experimental definitive hosts include mice, rats, dogs, cats, rabbits, and ducks (Nishigori, 1924a, Nishigori, 1924b, Chen, 1942, Waikagul et al., 1997, Saenphet et al., 2006a, Han et al., 2008, Pinto et al., 2015).
Regarding human infections, an experimental infection was performed successfully when this species was first reported as a new species (Nishigori, 1924a, Nishigori, 1924b). After then, possible occurrence of natural human infections was suggested by several authors (Ito, 1964a, Premvati and Pande, 1974). T. Li et al. (2010) listed C. formosanus as one of the human infecting helminths found in 1979 in Guangdong Province, China, but there is no related literature provided. Yu and Mott (1994) described the presence of human C. formosanus infections in China, Taiwan, and the Philippines without proper literatures. It is unclear whether these reports were truly based on identification of adult worms. Waikagul et al. (1997) reported two human cases infected with C. formosanus (under the name C. caninus) in Thailand. This can be regarded as the first adult-proven human C. formosanus infections. The next report on natural human C. formosanus infections (3 cases) was published from Vietnam (De and Le, 2011). Although there are no detailed worm descriptions, the figures of the whole worm and of the 32 circumoral spines on the oral sucker apparently indicate that the worms are C. formosanus (De and Le, 2011). Thereafter, ten additional human cases were reported from Lao PDR by recovery of adult flukes after chemotherapy and purging (Chai et al., 2013a, Chai et al., 2013b, Chai et al., 2015a). This species is now known to distribute in Taiwan, China, Japan, the Philippines, India, Vietnam, Lao PDR, Thailand, Turkey, Croatia, USA (Hawaii, Florida, Texas, and Utah), Mexico, Colombia, and Brazil (Chen, 1942, Martin, 1958, Yamaguti, 1975, Yu and Mott, 1994, Waikagul et al., 1997, Scholz and Salgado-Maldonado, 1999, Mitchell et al., 2000, Velásquez et al., 2006, Gjurčevic et al., 2007, De and Le, 2011, Pinto et al., 2013, Chai et al., 2013a, Chai et al., 2013b). Interestingly, C. formosanus became an exotic trematode species in the New World (USA, Mexico, Costa Rica, and Brazil) that originated from Southeast Asia from the late 1950s (Scholz and Salgado-Maldonado, 1999, Mitchell et al., 2000, Cortés et al., 2010, Pinto and de Melo, 2010, Johnson et al., 2012). Human infections are unknown in USA, Mexico, and Brazil (Chai et al., 2013a).
2.7.2. Centrocestus armatus (Tanabe, 1922) Price, 1932
The original description of this species was based on worms recovered from dogs, cats, rabbits, rats, and mice experimentally fed cyprinoid fish harboring the metacercariae in Japan (Tanabe, 1922). Morphological characteristics of C. armatus include the presence of 42–48 circumoral spines on the oral sucker, small number of intrauterine eggs, median location of the ovary, and side-by-side location of two testes (Hong et al., 1988, Chai, 2007). Regarding human infections, a successful experimental infection was reported in Japan when this fluke was first reported as a new species (Tanabe, 1922). Later, a naturally infected human case was reported in Korea (Hong et al., 1988). To date, the geographical distribution of this species is limited to Korea and Japan.
The first intermediate host is the fresh water snail, S. libertina (Takahashi, 1929c, Komatsu et al., 2014). The second intermediate hosts are various species of freshwater fish, such as, Zacco platypus, Nipponocypris temminckii (= Zacco temminckii), Rhodeus ocellatus, Gobius similis, P. parva, and Pelteobagrus fulvidraco (Chai et al., 2009a, Komatsu et al., 2014). The large egret Egretta alba modesta (Ryang et al., 1991), kites, herons, and three unidentified avian species (Komatsu et al., 2014), and the cat (Sohn and Chai, 2005, Shin et al., 2015) have been reported to be the natural definitive hosts. Rats and hamsters were useful experimental definitive hosts that allowed full development of worms than rats, mice, and chicks (Hong et al., 1989, Kimura et al., 2007).
2.7.3. Centrocestus cuspidatus (Looss, 1896) Looss, 1899
The original description of this species was based on adult flukes recovered from a naturally infected dog in Egypt (Ransom, 1920). The adult fluke has 36 circumoral spines (Ransom, 1920), which is a unique feature differed from other Centrocestus spp. The snail intermediate host is yet to be determined. The second intermediate host is freshwater fish, including Gambusia sp. (Yamaguti 1975). The reservoir hosts include dogs, cats, foxes, rats, and chickens (Yu and Mott, 1994, El-Azazy et al., 2015). Adult flukes were obtained experimentally from rats fed the fish Astatotilapia desfontainesi (Yamaguti, 1958) and chicks fed the metacercariae from Gambusia (Yamaguti, 1975). Human infections were found in Egypt, Taiwan, and mainland China (Yu and Mott, 1994, Li et al., 2010); however, the precise literature background is unavailable for this. Now, this fluke is known to distribute in Egypt (Ransom, 1920, Martin, 1959), Kuwait (El-Azazy et al., 2015), Tunisia (Yamaguti, 1958), Taiwan (Yu and Mott 1994), and China (Li et al., 2010, Li et al., 2010).
2.7.4. Centrocestus kurokawai (Kurokawa, 1935) Yamaguti, 1958
The original description of this species was based on worms recovered from a naturally infected human in Hiroshima Prefecture, Japan (under the name C. formosanus var. kurokawai) (Kurokawa, 1935). The adult worm has 38–40 circumoral spines (Kurokawa, 1935), and it was treated as a distinct species by Yamaguti (1958). Ito (1964a) and Yu and Mott (1994) continued to use C. formosanus var. kurokawai or C. kurokawai for this species. On the other hand, Waikagul et al. (1997) synonymized them and used the name C. armatus for C. kurokawai. However, Chai et al. (2013b) retained the name C. kurokawai in consideration that the number of circumoral spines (38–40) may be a specific feature. No information is available on the intermediate hosts and reservoir hosts. The second intermediate host may be some species of freshwater fish (Yu and Mott, 1994).
2.8. Stictodora
2.8.1. Stictodora fuscata (Onji and Nishio, 1916) Yamaguti, 1958
The original description of this species was based on specimens recovered from cats experimentally fed infected mullets in Japan (Onji and Nishio, 1916). Its characteristic morphology includes the presence of a gonotyl superimposed on the ventral sucker and armed with 12 chitinous spines, a metraterm, and two testes located obliquely in the middle field of the body (Chai et al., 1988). The snail intermediate host is not yet determined (Komiya, 1965, Chai et al., 2009a). The second intermediate host is brackish water fish, including mullets (M. cephalus and Liza macrolepis), redlip mullets (Chelon haematocheilus), and gobies (A. flavimanus) (Onji and Nishio, 1916, Sohn et al., 1994a, Sohn et al., 1994b, Abdul-Salam et al., 2000, Cho et al., 2012). Natural definitive hosts include cats, dogs, humans (Onji and Nishio, 1916, Yamaguti, 1958, Sohn and Chai, 2005, Chai et al., 2013c), and birds (Komiya, 1965, Yamaguti, 1971). Cats can be an experimental definitive host (Onji and Nishio, 1916, Sohn et al., 1994b, Abdul-Salam et al., 2000).
The presence of human infection with this fluke was briefly mentioned by Yamaguti, 1958, Yamaguti, 1971 but without literature background. Later, a human infection was discovered from a Korean young man (under the name Stictodora sp.), who enjoyed eating raw mullets and gobies (Chai et al., 1988). Thereafter, about 35 human infection cases have been detected by recovery of adult flukes in seashore villages in Korea (Chai et al., 1994b, Chai et al., 1997, Chai et al., 1998, Chai et al., 2004, Chai et al., 2014c, Chai et al., 2015a, Guk et al., 2006, Park et al., 2007). The existence of S. fuscata life cycle has been reported in Korea (Chai et al., 2009a), Japan (Komiya, 1965), and Kuwait (Abdul-Salam et al., 2000).
2.8.2. Stictodora lari Yamaguti, 1939
The original description of this fluke was based on worms discovered in the small intestine of the sea gull Larus crassirostris in Japan (Yamaguti, 1939). Its morphological characters include a gonotyl armed with 70–80 minute spines arranged in two groups; one densely crowded group of 30–40 spines and the other linearly-arranged group containing 30–40 spines, which make a C-form or a comma- or reversed comma-shape (Chai et al., 1989a). The first intermediate host is the brackish water gastropod (Velacumantus australis) (Bearup, 1961). A number of estuarine fish species (including Flavingobius lateralis obliquus, Atherinosoma microstoma, Urocampus carinorostris, Waiteopsis paludis, Mugil sp., and Gambusia affinis) have been identified as the fish hosts (Bearup, 1961). Natural definitive hosts include feral cats (Sohn and Chai, 2005, Chai et al., 2013c, Shin et al., 2015), humans (Chai et al., 2002), and dogs (Anh et al., 2009). Cats, dogs, and seagulls were used as experimental definitive hosts (Belogurov et al., 1968, Bearup, 1961, Chai et al., 1989a, Chai et al., 1989b).
S. lari has been found in Australia (Bearup, 1961), Russia (Belogurov et al., 1968), Korea (Chai et al., 1989a), and Vietnam (Anh et al., 2009). Human infections were first documented by Chai et al. (2002b) in Korea; 6 human patients were found infected with 1–10 specimens of S. lari in southern coastal areas. Subsequently, five additional cases were detected in Korea (Chai et al., 2004, Cho et al., 2010).
2.9. Procerovum
2.9.1. Procerovum varium Onji and Nishio, 1916
The original description of this parasite was based on adult flukes recovered from experimental dogs infected with the metacercariae encysted in the mullet Mugil cephalus in Japan (Onji and Nishio, 1916). It can be discriminated from Procerovum calderoni in having a shorter expulsor on the ventrogenital sac (Pearson, 1964). The first intermediate host is the brackish water snail, Melanoides (= Thiara) tuberculata (Umadevi and Madhavi, 2000). The second intermediate hosts are various kinds of freshwater fish (Velasquez, 1973b, Yu and Mott, 1994, Umadevi and Madhavi, 2000, Vo et al., 2008, Thanh et al., 2009, Skov et al., 2009, Chai et al., 2012, Chai et al., 2014a). Natural definitive hosts include cats (Sohn and Chai, 2005, Chai et al., 2013c, Shin et al., 2015) and birds (Umadevi and Madhavi, 2000). Adult flukes were experimentally reared in dogs, chicks, ducklings, and mice (Onji and Nishio, 1916, Umadevi and Madhavi, 2000). Experimental human infections were reported in Japan (Komiya and Suzuki, 1966); however, there have been no reports on natural human infections. It is now known to be distributed in Japan, China, the Philippines, Cambodia, Laos, Vietnam, Australia, India, and Korea (Umadevi and Madhavi, 2000, Sohn and Chai, 2005, Vo et al., 2008, Thanh et al., 2009, Chai et al., 2014a, Eom et al., 2015, Hung et al., 2015).
2.9.2. Procerovum calderoni (Africa and Garcia, 1935) Price, 1940
The original description of this species was based on worms recovered in dogs, cats, and two humans in the Philippines (under the name Monorchotrema calderoni) (Africa and Garcia, 1935). It was later renamed as Haplorchis calderoni (Africa 1938); however, it was subsequently assigned to Procerovum Onji and Nishio 1916 by Price (1940). It can be discriminated from P. varium in that the former has an extensively long expulsor, whereas the latter has a comparatively shorter expulsor (Pearson, 1964). The first intermediate host is the brackish water snail (Thiara riquetti) (Velasquez, 1973c). The second intermediate hosts are various kinds of freshwater fish (Velasquez, 1973b, Velasquez, 1973c, Yu and Mott, 1994). Natural reservoir hosts as we all as experimental hosts are dogs and cats (Velasquez, 1973c). This fluke has been reported from the Philippines (Africa and Garcia, 1935, Velasquez, 1973b, Velasquez, 1973c), China (Kobayasi, 1968, Yu and Mott, 1994), and Egypt (Tawfik et al., 2000).
2.10. Acanthotrema
2.10.1. Acanthotrema felis Sohn, Han and Chai, 2003
The original description of this species was based on worms recovered from the small intestine of stray cats in Korea (Sohn et al., 2003a). Its characteristic morphology includes the presence of a ventrogenital sac armed with three sclerites (two long and pointed and one short and thumblike) (Sohn et al., 2003a). The snail host has not been reported. The metacercariae were discovered in the goby (Acanthogobius flavimanus), a brackish water fish species; they were experimentally fed to kittens, and adult flukes were harvested 7 days later (Sohn et al., 2003b). Only cats were reported to be the natural definitive host (Sohn and Chai, 2005, Shin et al., 2015). Human infection was first found in a 70-year-old Korean woman residing in a coastal area of Korea (Cho et al., 2010). Thereafter, four more human infections were detected in Korea (Chai et al., 2014c). There have been no reports on the presence of this fluke in other countries.
2.11. Apophallus
2.11.1. Apophallus donicus (Skrjabin and Lindtrop, 1919) Price, 1931
The original description of this species was based on specimens recovered from the small intestine of dogs, cats, rats, foxes, and rabbits (experimental) in Europe and North America (Yamaguti, 1958, Yamaguti, 1971). Its morphological details were described by Niemi and Macy (1974). Experimental infection of a human with this species was successful in the United States (USA) (Niemi and Macy, 1974). Other reports of human infections were available in USA (Schell, 1985). Cercariae were shed by the stream snail (Flumenicola virens) (Niemi and Macy, 1974). Various kinds of fish, including blackside dace, suckers, squawfish, redside shiners, salmon, and rainbow trout were found naturally infected with the metacercariae (Niemi and Macy, 1974). Reservoir hosts include dogs, cats, rats, foxes, and species of birds (Yamaguti, 1958, Yu and Mott, 1994).
2.12. Ascocotyle
2.12.1. Ascocotyle (Phagicola) longa Ransom 1920
The original description of this species was based on specimens from an Alaskan fox from National Zoological Park in Washington DC, USA (Ransom, 1920). The morphological details of this species in relation to other species were given by Scholz (1999). The natural and experimental first intermediate host is the cochliopid snail (Heleobia australis) in Brazil (Simões et al., 2010). Freshwater fish, in particular, various species of mullets serve as the second intermediate host (Chieffi et al., 1992, Oliveira et al., 2007, Simões et al., 2010). Various kinds of birds, including the pelican and eagle, and mammals, particularly dogs, are reservoir hosts of this fluke (Chieffi et al., 1992, Simões et al., 2010). Hamsters were an experimental definitive host (Simões et al. 2010). Total 10 cases of human infections presumably due to this species (described as Phagicola sp.) were first reported in Brazil (Chieffi et al., 1992). Subsequently, Antunes and Almeida-Dias (1994) added 10 more human patients who consumed raw mullets in previous four months in Brazil. The distribution of this species is now known to be worldwide; North (Mexico and USA) and South America (Brazil, Colombia, Panama, and Peru), Europe (Czech Republic, Germany, Greece, and Romania), Africa (Egypt), and the Middle East (Georgia, Israel, and Turkey) (Scholz, 1999).
2.13. Cryptocotyle
2.13.1. Cryptocotyle lingua (Creplin, 1825) Fischoeder, 1903
The original description of this species was based on specimens recovered in the intestine of an avian species (Larus marinus) (under the name Distoma lingua by Creplin in 1825) (Stunkard, 1930). It was renamed as Cryptocotyle lingua by Fischoeder in 1903 (Ransom, 1920). Its characteristic morphology includes the presence of a genital sucker connected with the posterior part of the ventral sucker and obliquely located two testes (Ransom, 1920). Cercariae develop in littorina snails (Littorina littorea) and metacercariae encyst in freshwater fish (Gobius ruthensparri and Labrus bergylta) and mullet (Chelon labrosus) (Yamaguti, 1958, Matthews and Matthews, 1993). Various fish-eating birds and mammals were reported as reservoir hosts, which included cats, dogs, rats, foxes, gulls, terns, and herons in Europe (including UK, Denmark, Iceland, and Norway), Russia, North America, and Japan (Stunkard, 1930, Yamaguti, 1958, Kristoffersen, 1991, Saeed et al., 2006, Christensen et al., 2015). Adults can be experimentally grown in gulls by feeding metacercarial cysts (Yamaguti, 1958). Human infection with this fluke was reported only one time in Greenland (Babbott et al., 1961). Although adult flukes were not identified, it seemed that the egg size of 40–50 by 24–28 μm (significantly larger than the other members of heterophyids) strongly suggested them to be C. lingua (Babbott et al., 1961).
3. Genomics and molecular studies
Molecular studies using PCR-RFLP (ITS1 and CO1), random amplification of polymorphic DNA (RAPD), and simple sequence repeat anchored PCR (SSR-PCR) have shown that M. yokogawai, M. takahashii, and M. miyatai are genetically distinct from each other (Yu et al., 1997a, Yu et al., 1997b, Yang et al., 2000, Yu and Chai, 2010, Yu and Chai, 2013). Chromosomes and karyotypes were also used to differentiate M. yokogawai, M. takahashii, and M. miyatai (Lee et al., 1999). Gene sequence studies were also performed on 28S ribosomal DNA (rRNA) and CO1 of three Metagonimus species, with helpful results (Lee et al., 2004). Nucleotide sequence differences were 23.0% (92/400 bp) between M. miyatai and M. takahashii, 16.2% (65/400 bp) between M. miyatai and M. yokogawai, and 13.2% (53/400 bp) between M. takahashii and M. yokogawai (Lee et al., 2004). In addition, by the neighbor-joining and parsimony methods, M. takahashii and M. yokogawai were placed in the same clade, whereas M. miyatai was placed in a different clade (Lee et al., 2004). This result was agreed by another research group (Thaenkham et al., 2012). In a numerical taxonomy study, interestingly, M. miyatai was classified as a subspecies level of M. takahashii, whereas M. yokogawai and M. takahashii were distinct taxa (Kim et al., 1991).
Using six species available in Japan, a phylogenetic study was recently performed on the genus Metagonimus; M. yokogawai, M. takahashii, M. miyatai, M. hakubaensis, M. katsuradai, and M. otsurui (Pornruseetairatn et al., 2016). It was revealed that the former four species were grouped into one big clade, and the latter two species formed another clade, based on a combined 28S rRNA, ITS2, and cox1 sequence dataset (Pornruseetairatn et al., 2016). M. suifunensis was recently reported as a new species based on molecular analysis of ITS1–5.8S–ITS2 region and 28S nuclear rRNA of adult worms; it formed a separate group from M. yokogawai, M. takahashii, M. miyatai, M. hakubaensis, M. katsuradai, and M. otsurui (Shumenko et al., 2017). No information is available regarding proteomics of Metagonimus flukes.
Genomics and molecular characteristics of Heterophyes spp. have seldom been the subject of study. Before the study of Masala et al. (2016), the only molecular data available for Heterophyes spp. were mitochondrial cytochrome c oxidase 1 (CO1) and nuclear ribosomal gene (28S rRNA) of H. nocens (Korea) deposited in GenBank with accession numbers AF188119 for CO1 and AF181890 for 28S rRNA. The sequences of internal transcribed spacer 2 (ITS2) and 28S rRNA were recently analyzed in H. heterophyes (adults from a hamster infected with metacercariae in mullets from Sardinia), H. nocens (adults from a cat in Korea), H. cf. nocens (an adult from a hamster infected with metacercariae in mullets from Sardinia, having a smaller number of rodlets on the gonotyl than H. heterophyes), and H. dispar (small-sized metacercariae in mullets from Sardinia) (Masala et al., 2016). The results revealed that H. heterophyes (GenBank no. KU674951 for ITS2 and KU559553 and KU559556 for 28S) and H. cf. nocens (no. KU674951 for ITS2 and KU559559 for 28S) from Sardinia formed one clade, whereas H. nocens (no. KU674959 and KU674960 for ITS2) from Korea and H. dispar (no. KU674953 for ITS2 and KU559560 for 28S rRNA) from Sardinia formed other two distinct clusters (Masala et al., 2016). By this data, it was suggested that H. cf. nocens may be conspecific with H. heterophyes, and the number of rodlets on the gonotyl of H. heterophyes may be wider than previously considered (Masala et al., 2016).
The complete mitochondrial genome of H. taichui was obtained and comparatively analyzed with other trematodes (Lee et al., 2013). It has been shown that Lao, Thai, and Vietnamese populations of H. taichui are genetically distant from one another (Thaenkham et al., 2016). In Vietnam, Dung et al. (2013) studied on genetic variations of three Vietnamese isolates; they attributed the low gene flow among the isolates to topographic features that isolated them geographically, including mountainous and wetland areas. However, based on mitochondrial CO1 gene analysis, another hypothesis was made; Thailand, Laos, and Vietnam population structures of H. taichui are affected by the national border rather than environmental factors such as common river basins and distribution of intermediate hosts (Thaenkham et al., 2016). Further studies are required to elucidate this point.
The taxonomic relationships of P. summa with other trematode parasites were studied by Lee et al. (2007). Using 28S rDNA D1 gene analysis, P. summa was shown to be located in the same clade as Paragonimus westermani and the next clade to M. yokogawai and M. takahashii, whereas Clonorchis sinensis and Plagiorchis muris were put in the same clade (Lee et al., 2007). However, analysis of mitochondrial CO1 showed more proximity of P. summa to C. sinensis than to P. westermani, M. yokogawai, and M. takahashii (Lee et al., 2007). A numerical taxonomy study reported that P. summa were more closely related to Stellantchasmus falcatus rather than to Heterophyes or Metagonimus (Kim et al., 1991). A molecular analysis using rRNA ITS1 and ITS2 revealed that P. genata is genetically more close to Ascocotyle longa and Ascocotyle pindoramensis than Metagonimus yokogawai and Haplorchis pumilio (Al-Kandari et al., 2015).
Sequences of nuclear rRNA genes, including 18S rRNA, 28S rRNA, and ITS2 region, were used for analysis of the phylogenetic relationships of S. falcatus with other heterophyid and opisthorchiid trematodes (Thaenkham et al., 2010, Sripalwit et al., 2015). S. falcatus was phylogenetically distinct from Haplorchis spp., and Procerovum spp., and it was indicated that S. falcatus might have diverged first from a common ancestor of Haplorchiinae species (Thaenkham et al., 2010). S. falcatus was also far from Haplorchoides sp., Centrocestus spp., M. yokogawai, and O. viverrini (Sripalwit et al., 2015). A numerical taxonomy study reported that S. falcatus was more closely related to Pygidiopsis summa rather than to Heterophyes or Metagonimus (Kim et al., 1991).
4. Pathogenesis and pathology
Two principal factors are generally related to the pathogenicity and virulence of intestinal parasites; they include mechanical and chemical irritations by the flukes (Chai, 2014b). Mechanical irritation is chiefly caused by movement of worms which can give harmful effects to the mucosa (= villous and crypt layers) of the small intestine (Chai, 2014b). Chemical substances produced by the flukes, which include excretory-secretory proteins (ESP), can play a role for not only antigens but also toxins to the host (Chai, 2014b).
The main habitat of adult Metagonimus spp. is the mucosa (villi and crypt) of the small intestine, and worms give mechanical and chemical/immunological stimuli to the host intestinal mucosa. In immunocompetent hosts, worms never invade deeper layers of the submucosa, muscularis mucosa, or serosa (Chai, 2015). However, they may invade deeper levels beyond the mucosa in immunocompromised hosts (Chai et al., 1995a, Chai, 2015). Pathological changes in the mucosa lead to difficulty in nutrient absoption from the intestine; increase in permeability in the intestinal mucosa was reported in mice experimentally infected with M. yokogawai (Ohnishi and Taufen, 1984). The resulting watery diarrhea seems to be due to poor absorption of intestinal secretions from secretory crypt cells (Cho et al., 1985). Decreased enzyme activities were suggested to be associated with malabsorption and diarrhea in acute M. yokogawai infection (Hong et al., 1991).
Experimental studies on the intestinal histopathology were performed by Chai (1979), Lee et al. (1981), and Kang et al. (1983) using rats, cats, and dogs as the host, respectively. The major histopathological findings were villous atrophy and crypt hyperplasia, with variable degrees of inflammatory reactions (Chai and Lee, 2002, Chai, 2015, Chai, 2017). The infected mucosa showed blunting and fusion of the villi, edema of the villus tips, congestion, goblet cell hyperplasia, mastocytosis, and inflammatory cell infiltrations in the villous stroma, with decreased villus/crypt height ratios (Chai, 1979, Chai et al., 1993b). In a human metagonimiasis patient, a histopathological study of the small intestine was possible, and almost similar intestinal histopathology was observed (Chi et al., 1988). In animals, the intestinal histopathology was normalized at 3–4 weeks after the infection (Chai, 1979, Chai et al., 1995a). In M. miyatai-infected mice, similar intestinal histopathology was observed; although the degree of mucosal damage was less severe than in M. yokogawai-infected mice (Yu et al., 1997c).
The intestinal histopathology in Heterophyes spp. and Haplorchis spp. infection is essentially the same as that seen in M. yokogawai infection. The adult flukes of H. heterophyes (Hamdy and Nicola, 1980) or H. nocens (Ryang et al., 1999) parasitize the middle part of the small intestines; within the crypt of Lieberkuhn in early stages of infection (by day 2–3 post-infection), and the intervillous space in later stages (Chai, 2014b). They can elicit mild inflammatory reactions, together with ulcers, irritation, and superficial necrosis of the site of their attachment to the host intestinal mucosa (Yu and Mott, 1994, Fried et al., 2004, Toledo et al., 2014). In experimental dogs and cats infected with H. heterophyes, involvement of Peyer's patches and mesenteric lymph nodes by adult flukes were frequently seen (Hamdy and Nicola, 1980). In avian hosts, like sea gulls, the flukes frequently invade extraintestinal or somatic tissues and organs, in particular, the liver, pancreas, and bile duct (Chai, 2014b). Immune responses of the host against the flukes or their ESP may be too strong (hypersensitive) that the host immunity can damage the host itself (Chai, 2014b). The affected mucosa may undergo hypersensitive and allergic reactions, including severe catarrhal inflammation and loss of villi (Chai, 2014b).
In Thailand, a human autopsy case infected with H. taichui and two cases infected with H. yokogawai were reported from Udonthani Province (Manning and Lertprasert, 1971, Manning et al., 1971). Another human case was subsequently reported in Chiang Mai, Thailand; worm sections were found deep in the crypt of the ileum (Kliks and Tantachamrun, 1974). Later, three more human cases infected with H. taichui were reported with evidence of this infection as a pathogenic parasite (Sukontason et al., 2005). Surgical resection of the small intestines revealed the pathological features of mucosal ulceration, mucosal and submucosal hemorrhages, fusion and shortening of villi, chronic inflammation, and fibrosis of the submucosa; H. taichui worms were found with their mouth-parts attached to the mucosal epithelium and distorting the epithelial lining (Sukontason et al., 2005). Moreover, H. taichui was suggested to be an etiological agent of irritable bowel syndrome-like symptoms in humans (Watthanakulpanich et al., 2010).
The pathogenicity and pathology as well as mucosal immune responses of the host against P. summa infection were studied in experimental rats and mice (Seo et al., 1986, Chai et al., 2014b). The mucosal pathology was severe in rodent intestines and characterized by villous atrophy accompanied by crypt hyperplasia and severe stromal inflammation (Seo et al., 1986). However, no deep invasion of worms beyond the submucosal level was found, and the intestinal lesion was restored from three weeks after the infection (Seo et al., 1986). Immune effectors in the intestinal mucosa against P. summa infection included IEL, goblet cells, mucosal mast cells, and IgA (Chai et al., 2014b).
Six species of heterophyid flukes, including 3 Haplorchis spp. (H. taichui, H. yokogawai, and H. vanissimus), Stellantchasmus falcatus, Procerovum calderoni, and Carneophallus brevicaeca (syn. Heterophyes brevicaeca Africa and Garcia 1935; Spelotrema brevicaeca Tubangui and Africa 1938), were reported to have caused erratic extraintestinal parasitism in humans, which was often fatal (Africa et al., 1940). The most frequently affected site was the heart (valve), brain, and spinal cord, where eggs and adult flukes originating from the small intestinal mucosa (Africa et al., 1940). Yokogawa (1940) suggested that these patients may have been immunocompromised to become susceptible to this type of extraintestinal heterophyid infections.
Metagonimus spp. and Heterophyes spp. are also highly suggested to be able to cause such erratic parasitism in immunocompromised patients. It is of note that a patient infected with Metagonimus sp. underwent intracerebral hemorrhage and diabetes mellitus (Yamada et al., 2008). The eggs of H. heterophyes (Gallais et al., 1955, Collomb and Bert, 1959, Collomb et al., 1960) and H. nocens (under the name H. heterophyes) (Zhang et al., 1990) were found encapsulated in the brain of patients with neurological symptoms. In H. nocens infection, the eggs were also detected within an intestinal tumor near the appendix in a 10-year old girl in Japan (Nakano and Inuoe, 1955). An experimental background was provided in H. heterophyes using rats in Egypt; in four of 20 rats, possibly due to immunosuppression as well as malnutrition and malabsorption, the eggs and/or immature worms were found in the intestinal wall, lymph nodes, liver, and spleen which evidently demonstrated the potential to cause extraintestinal spreading of the infection (Elsheikha, 2007). Another histopathological study in H. heterophyes infection demonstrated deposition of antigens or immune complexes in the kidneys and brain of experimental mice; these deposits were thought to play important roles in histopathological changes in the kidneys and brain (Daoud, 2012).
Despite that S. falcatus was involved in causing erratic parasitisms in the heart, brain, and spinal cord of humans (Africa et al., 1940), the pathogenicity and pathology as well as mucosal immune responses of the host has seldom been studied. However, innate immunity against S. falcatus infection was demonstrated in mice and rats; in the former, the worms spontaneously expelled within a week after infection, and in the latter, the worms expelled after 28 days (Saenphet et al., 2003). The blood eosinophils were increased from the first week of infection and remained relatively high until the worms are expelled from these rodent hosts (Saenphet et al., 2003).
5. Immunology
Considering that the intestinal histopathology caused by M. yokogawai was normalized around 3–4 weeks after the infection (Chai, 1979, Chai et al., 1995a), host protective mechanisms (including innate resistance) against worms seem to be significantly operating. The possible immune effectors for the spontaneous recovery include at least 4–5 components. They include intestinal intraepithelial lymphocytes (IELs) (Chai et al., 1994a), lamina propria lymhocytes (LPLs) (Chai et al., 1994a), mucosal mast cells, and goblet cells (Chai et al., 1993b, Chai et al., 2009a). Eosinophils were also increased in the peripheral blood of M. yokogawai-infected mice (Ohnishi, 1987), although their roles in worm expulsion are unclear. Hong et al. (1993) infected rats with Neodiplostomum seoulense (under the name Fibricola seoulensis) first and thereafter challenged M. yokogawai and observed the worm recovery and intestinal histopathology; interestingly, the pre-established N. seoulense in the duodenum of the rats affected adversely the settlement of M. yokogawai flukes in the jejunum or ileum.
Immunogold studies revealed that the antigenicity of M. yokogawai originates from the syncytial tegument, tegumental cell cytoplasms, vitelline cells, and epithelial lamellae of the cecum (Ahn et al., 1991, Rim et al., 1992). SDS-PAGE and immunoblot analyses on crude extracts of metacercariae showed that out of 14 protein bands found 66 kDa and 22 kDa proteins were the parasite-specific antigens (Lee et al., 1993). Han et al. (2014) detected an interesting somatic antigen, 100 kDa in size, from the tegumental layer of M. yokogawai adults, which commonly reacts against different kinds of trematodes, including Gymnophalloides seoi (intestinal fluke), Paragonimus westermani (lung fluke), and Clonorchis sinensis and Fasciola hepatica (liver flukes).
Also, in Heterophyes spp. infection, the fact that intestinal histopathology induced is spontaneously restored indicates the development of strong host protective immunity (Toledo et al., 2014). In sera of humans infected with H. heterophyes, elevated levels of IgG, IgM, and IgE have been detected (El-Ganayni et al., 1989, Martínez-Alonso et al., 1999, Toledo et al., 2014). In the intestine of infected humans, elevated levels of IgG, IgM, and IgA were also reported (El-Ganayni et al., 1989). In Haplorchis spp. infection, Sukontason et al. (2001), Kumchoo et al. (2003), and Saenphet et al. (2008) demonstrated spontaneous expulsion of worms from the intestine of mice, chicks, and rats experimentally infected with H. taichui within 9, 18, and 28 days post infection, respectively, which demonstrated the development of innate and/or acquired immunity of the host. MMCs were significantly increased in number in infected rats peaking at day 21 post infection (Saenphet et al., 2008). Eosinophil counts and serum IgE levels were elevated after infection which peaked at day 14 post infection (Saenphet et al., 2008). In humans infected with H. taichui, serum antibodies (probably IgG) against H. taichui were increased as measured by ELISA; enzyme-linked immunoelectron transfer (EITB) assay was useful to discriminate H. taichui infection (10 kDa band) from O. viverrini infection (70 kDa band) (Ditrich et al., 1991).
Saenphet et al. (2006b) studied on mucosal mast cell responses in rats experimentally infected with C. formosanus (under the name C. caninus); their possible role in worm expulsion was suggested. Pinto et al. (2015) studied on the worm burden, morphology, and fecundity of C. formosanus in immunosuppressed mice; the worm burden in dexamethasone-treated mice was significantly greater than that in the control (immunocompetent) mice.
6. Clinical manifestations
Clinical symptoms due to Metagonimus spp. and other heterophyid infections are generally mild and transient, unless the patients are heavily infected, complicated with other diseases, or immunocompromised (Chai and Lee, 2002, Chai et al., 2009a, Yu and Chai, 2013). The most frequent clinical symptoms in M. yokogawai infection include abdominal pain, diarrhea, lethargy, anorexia, malabsorption, and weight loss (Cho et al., 1984, Seo et al., 1985). The severity of clinical symptoms is closely related to individual worm burdens (Chai, 2015). In immunocompromised individuals, M. yokogawai infection may cause severe clinical manifestations, possibly including erratic parasitism in vital organs as reported in other heterophyid fluke infections (Africa et al., 1940, Chai, 2007, Yu and Chai, 2013).
Major clinical manifestations in heterophyiasis patients are mild to moderate degrees of abdominal pain, diarrhea, lethargy, anorexia, and weight loss (Chai, 2014b). However, the severity of symptoms may vary and depend on host-side factors such as the intensity of infection, immune status of the patient, and previous history of infection with these flukes (Chai, 2014b). Immunocompromised patients may undergo severer clinical course, including erratic parasitism (= extraintestinal heterophyiasis) in the heart, brain, and spinal cord, as reported by Africa et al. (1940). Both H. heterophyes and H. nocens are suspected to be the causes of cerebral involvement, including epilepsy, brain abscess, or brain cyst (Gallais et al., 1955, Collomb and Bert, 1959, Collomb et al., 1960, Zhang and Fan, 1990). In an acute appendicitis case in a 10-year old girl in Japan, H. nocens eggs were demonstrated within the tumor formed near the appendix (Nakano and Inuoe, 1955).
Few reports regarding the clinical symptoms and signs of human Haplorchis spp. infection are available. This is largely because humans in endemic areas are usually mixed-infected with other foodborne trematodes, namely, O. viverrini, other heterophyids, or lecithodendriids. Thus, the clinical manifestations solely due to Haplorchis spp. infection are difficult to assume. Africa et al. (1940) assumed that the symptoms due to Haplorchis spp. infection may be intestinal disturbances such as colicky pain and mucous diarrhea, and the degree of such symptoms would depend upon the number of worms, the extent of penetration, or the destruction or necrosis of the epithelium occasioned by the presence of worms. Recently, it has been reported that H. taichui infection may cause irritable bowel syndrome-like symptoms, and the patients may complain of dyspepsia, nausea, vomiting, lassitude, abdominal pain, flatulence, loose fecal excretion, fever, pallor, abdominal distension, and jaundice with enlarged liver (Watthanakulpanich et al., 2010).
The symptoms and signs due solely to P. summa infection have not been elucidated. One of the reasons is mixed-infection of the majority of human cases with other heterophyid flukes such as H. nocens (Chai et al., 1994b, Chai et al., 1997, Chai et al., 1998, Chai et al., 2004). The most heavily infected person ever documented was a 52-year-old Korean man, with 4045 specimens recovered after chemotherapy and purging (Seo et al., 1981b). In this patient, no significant symptoms or signs were presented, and the only abnormal laboratory finding was 7% peripheral blood eosinophilia (Seo et al., 1981b).
7. Diagnosis and treatment
Heterophyid fluke infections can be diagnosed by recovery of eggs in fecal examinations (Chai, 2007). A confirmatory diagnosis can be made when adult flukes are detected during gastroduodenoscopy, surgical procedures in the intestine, or at autopsy (Chai, 2007). A tedious but practical way in the field or laboratory is recovery of adult flukes from diarrheic stools following anthelmintic treatment and purging (Chai, 2007).
An important drawback in the diagnosis of Metagonimus spp., Heterophyes spp., and Haplorchis spp. and other heterophyid infection is close similarity of their eggs to other heterophyid species as well as small liver flukes (Clonorchis sinensis, Opisthorchis viverrini, and Opisthorchis felineus) and lecithodendriid flukes (Prosthodendrium molenkampi and Phaneropsolus bonnei) (Chai and Lee, 2002, Lee et al., 2012, Chai, 2015). Therefore, in areas of mixed infections, specific diagnosis is usually difficult unless the adult flukes are recovered (Chai, 2007). The eggs found in fecal examinations should be expressed as a broad term, i.e., small trematode eggs (STE), minute intestinal fluke eggs (MIF eggs), or at least heterophyid fluke eggs (Lee et al., 1984a, Lee et al., 2012). Potassium permanganate staining was introduced to distinguish the eggs of H. taichui from those of O. viverrini; the staining made the muskmelon patterns on the egg shell surface of O. viverrini more clearly visible (Sukontason et al., 1999).
Among light infection cases with heterophyid flukes (lesss than 100 specimens), there could be false egg negative cases (Chai and Lee, 2002). The number of eggs produced per day per worm (EPDPW) for M. yokogawai in the human host was reported to be only 14–64 eggs (Seo et al., 1985). The EPDPW of H. taichui in humans was 82, only slightly higher than that of M. yokogawai (Sato et al., 2009a). Therefore, the detectability of eggs in feces from such low worm burden cases is negligible (Chai and Lee, 2002). In these cases, serological tests, in particular, ELISA, against M. yokogawai may be helpful (Chai et al., 1989b, Cho et al., 1987). In H. heterophyes infection, three immunodiagnostic methods (counter current immunoelectrophoresis, intradermal test, and indirect fluorescent immunoassay) were tried to detect serum antibodies in experimental dogs (Elshazly et al., 2008). However, the diagnosis of erratic parasitism in the heart, brain, or spinal cord is practically impossible unless biopsy or necropsy is performed on the affected lesion (Chai, 2014b).
The specific diagnosis of human P. summa or S. falcatus infection is problematic because it is difficult to discriminate these eggs from other heterophyid or opisthorchiid eggs in fecal examinations (Lee et al., 1984a, Lee et al., 2012). However, a favorable point is that P. summa eggs are very small in size and S. falcatus eggs are slightly elongated (Lee et al., 1984a, Chai et al., 2004, Lee et al., 2012). The small egg size is also applicable to P. genata infection in Egypt (Youssef et al., 1987a).
Recently, molecular techniques, including PCR or PCR-RFLP, were introduced to detect heterophyid infections in human feces (Jeon et al., 2012) and food materials (Pyo et al., 2013). The PCR technique could differentiate M. yokogawai infection from C. sinensis or Haplorchis taichui infection with detection of mixed-infection cases also (Jeon et al., 2012). The PCR targeting 18S rRNA could also detect M. yokogawai infection in sweetfish and Gymnophalloides seoi infection in oysters (Pyo et al., 2013). Thaenkham et al. (2007) developed a rapid and sensitive tool for detecting H. taichui with low DNA concentrations and for distinguishing H. taichui from Opisthorchis viverrini, H. pumilio, and H. yokogawai. They further suggested that PCR-RFLP profiles would be useful for diagnosing mixed H. taichui and O. viverrini infection. PCR diagnosis was also applied to detect low-intensity H. taichui and O. viverrini infections in field surveys (Lovis et al., 2009). HAT-RAPD technique was developed to discriminate H. taichui infection from various other infections, including O. viverrini and S. falcatus which generated a 256 bp amplicon and showed a positive result only for H. taichui (Wongsawad et al., 2009). A multiplex PCR assay based on HAT-RAPD results was also developed for detecting H. taichui (Wongsawad and Wongsawad, 2012). PCR targeting ITS1 and ITS2 regions of ribosomal DNA revealed useful results; particularly in ITS2 region, the amplicon size for H. taichui was significantly different from that of O. viverrini, C. sinensis, and H. pumilio (Sato et al., 2009b). However, Sato et al. (2010) reported that PCR for copro-DNA targeting ITS1 and ITS2 regions could detect lower number of H. taichui cases than those with H. taichui worms expelled after treatment, whereas the same DNA technique was excellent for copro-detection of O. viverrini infection. Molecular techniques were also used in Vietnam (De and Le, 2011), China (Jeon et al., 2012), and Thailand (Wongsawad et al., 2012, Sato et al., 2015) to detect human infections with H. pumilio and H. taichui (Vietnam, China, and Thailand). However, the diagnosis of erratic parasitism in the heart, brain, or spinal cord is difficult unless biopsy or necropsy is done on the affected lesion (Chai, 2014b). Detection of serum antibodies or parasite genetic markers cannot discriminate intestinal versus visceral infections. Cardiac ultrasonography combined with serum antibody and molecular tests would help the diagnosis of possible cardiac infections with heterophyid fluke infections.
The drug of choice for heterophyid fluke infections, including Metagonimus spp., Heterophyes spp., and Haplorchis spp., is praziquantel (Chai, 2007, Chai, 2013). A single oral dose of 10–20 mg/kg is usually satisfactory (Chai, 2013). However, in areas of mixed infection with the liver fluke, a single oral dose of 40 mg/kg praziquantel can be used in mass treatment (Pungpak et al., 1998, Radomyos et al., 1998, Sayasone et al., 2009, Chai et al., 2010, Chai et al., 2013a, Sohn et al., 2014). However, reduced doses, for example, 20–30 mg/kg, were also useful to treat individual patients in endemic areas (Chai et al., 2005a, Chai et al., 2007, Chai et al., 2009b). The cure rate of 95–100% was reported for M. yokogawai infection (Rim et al., 1978, Lee et al., 1984b), and over 95% cure rate for H. heterophyes spp. infection (Ata et al., 1988, El-Hawy et al., 1988). Praziquantel is highly safe at this dose even in children and pregnant women (Chai, 2013). Bithionol (Seo et al., 1981b) or niclosamide (El-Hawy et al., 1988, Ramsuk et al., 2004, Kumchoo et al., 2007) can also be used as an alternative drug.
8. Prevention and control
The prevention and control measures for heterophyid infections include control of the snail host, control of the fish host, mass chemotherapy of residents in endemic areas, and health education (Chai, 2015). However, snail control and fish control are difficult to perform successfully (Chai, 2015). Mass drug administration (MDA) can temporarily reduce the prevalence and infection intensity (worm load) but reinfection steadily occurs in endemic areas (Chai, 2015). Health education to not consume raw or undercooked fish will help prevention of heterophyid fluke infection; however, the old tradition of enjoying raw fish dish is practically hard to change (Chai, 2014b, Chai, 2015). The infectivity of M. yokogawai metacercariae in fish can be controlled by gamma-irradiation at 200 Gy (Chai et al., 1995b). However, this method is not feasible in the field due to various reasons, including the necessity of an irradiator, high cost, and low preference of irradiated fish by the consumers (Chai et al., 1995b, Chai, 2015).
The metacercariae of heterophyids can survive only 1 day in pickled fish dish; therefore, consuming ‘pla-som’ after 3 days of preparation would be safe from infection (Sukontason et al., 1998). However, the metacercariae of heterophyids can survive longer than 3 h in ‘lab-pla’ which is usually consumed immediately after preparation; therefore, this type of fish dish may be dangerous for parasite infection (Sukontason et al., 1998). Smoking to a temperature of 65 °C can be considered for parasitic nematodes in fish, but little is known about the effectiveness of this process for intestinal trematodes, including Metagonimus, Heterophyes, and Haplorchis (Chai, 2014b).
9. Conclusions
Fishborne zoonotic heterophyid infections are strongly linked to deeply embeded cultural traits, for example, consumption of raw or improperly cooked fish in traditional ways, in each endemic area, which is located mostly in Southeast Asia. Thus, the viscious cycle of reinfection continues in this area, and control effort often results in failure. Nevertheless, national as well as international health policies seldom put foodborne trematode infections, including intestinal heterophyid infections, on high health priorities; thus, these infections have become a long-time neglected group of diseases. In order to provide a better understanding of the global disease prevalence and geographical distribution of these infections, improved diagnostic tools are urgently needed, especially those that can differentiate the various species of parasites involved. Finally, long-term pilot projects with risk assessment studies are needed to provide proper control strategies at local as well as national and international levels.
Conflict of interest
We do not have any conflict of interest related to this work.
Acknowledgments
Acknowledgments
We would like to sincerely thank our colleagues who cooperated with us but not listed among the authors.
References
- Abdul-Salam J.M., Baker K. Prevalence of intestinal helminths in stray cats in Kuwait. Pak. Vet. J. 1990;10:17–21. [Google Scholar]
- Abdul-Salam A., Nair S.B., Ashkanani H. Surface ultrastructure of Stictodora fuscatum (Trematoda: Heterophyidae) from Kuwait Bay. Parasitol. al. Día. 2000;24(1-2) doi: 10.1016/s1383-5769(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Abou-Basha L.M., Abdel-Fattah M., Orecchia P., Di Cave D., Zaki A. Epidemiological study of heterophyiasis among humans in an area of Egypt. East Mediterr. Health J. 2000;6:932–938. (in Arabic) [PubMed] [Google Scholar]
- Adams K.O., Jungkind D.L., Bergquist E.J., Wirts C.W. Intestinal fluke infection as a result of eating sushi. Am. J. Clin. Pathol. 1986;86:688–689. doi: 10.1093/ajcp/86.5.688. [DOI] [PubMed] [Google Scholar]
- Africa C.M. Description of three trematodes of the genus Haplorchis (Heterophyidae) with notes on two other Philippine members of this genus. Philipp. J. Sci. 1938;66:299–307. [Google Scholar]
- Africa C.M., Garcia E.Y. Two more new heterophyid trematodes from the Philippines. Philipp. J. Sci. 1935;57:443–450. [Google Scholar]
- Africa C.M., de Leon W., Garcia E.Y. Visceral complications in intestinal heterophyidiasis of man. Acta Med. Philipp. 1940;1:1–132. (Monographic Series No) [Google Scholar]
- Ahn Y.K. Intestinal flukes of genus Metagonimus and their second intermediate hosts in Kangwon-do. Korean J. Parasitol. 1993;31:331–340. doi: 10.3347/kjp.1993.31.4.331. (in Korean) [DOI] [PubMed] [Google Scholar]
- Ahn Y.K., Ryang Y.S. Epidemiological studies on Metagonimus infection along the Hongcheon river, Kangwon Province. Korean J. Parasitol. 1988;26:207–213. doi: 10.3347/kjp.1988.26.3.207. (in Korean) [DOI] [PubMed] [Google Scholar]
- Ahn Y.K., Ryang Y.S. Growth, egg-laying and life span of Miyata type of genus Metagonimus (Trematoda: Heterophyidae) in the final hosts. J. Wonju Med. Coll. 1995;8:1–12. (in Korean) [Google Scholar]
- Ahn H., Rim H.J., Kim S.J. Antigenic localities in the tissues of Metagonimus yokogawai observed by immunogold labeling method. Korean J. Parasitol. 1991;29:245–257. doi: 10.3347/kjp.1991.29.3.245. (in Korean) [DOI] [PubMed] [Google Scholar]
- Alicata J.E., Schattenburg O.L. A case of intestinal heterophyidiasis of man in Hawaii. J. Am. Med. Assoc. 1938;110:1100–1101. [Google Scholar]
- Al-Kandari W.Y., Alnaqeeb M.A., Isaac A.M., Al-Bustan S.A. Molecular characterization of Stictodora tridactyla (Trematode: Heterophyidae) from Kuwait Bay using rDNA ITS and mtCO1. Parasitol. Res. 2015;114:4259–4266. doi: 10.1007/s00436-015-4665-y. [DOI] [PubMed] [Google Scholar]
- Anh N.T.L., Phuong N.T., Johansen M.V., Murrell K.D., Phan T.V., Dalsgaard A., Luong T.T., Thamsborg S.M. Prevalence and risks for fishborne zoonotic trematode infections in domestic animals in a highly endemic area of North Vietnam. Acta Trop. 2009;112:198–203. doi: 10.1016/j.actatropica.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Antunes S.A., Almeida-Dias E.R. Phagicola longa (Trematoda: Heterophyidae) em mugilídeos estocados resfriados e seu consume cru em São Paulo, SP. Hig. Alim. 1994;8:41–42. [Google Scholar]
- Asada J., Kaji F., Ochi G., Murakami G., Kaji T. On the prevalent occurrence of metagonimiasis caused by Plecoglossus altivelis of the Ashida River, Hiroshima Prefecture. Tokyo Iji Shinshi. 1957;74:325–330. (in Japanese) [Google Scholar]
- Ashford R.W., Crewe W. An Annotated Checklist of the Protozoa, Helminths and Arthropods for Which We Are Home. 2nd ed. Taylor & Francis Group; London and New York: 2003. The parasites of Homo sapiens. [Google Scholar]
- Ata A.A., el-Khashab M.N., Mourad A.A., Soliman H.M. Effect of praziquantel on Heterophyes heterophyes. J. Egypt. Soc. Parasitol. 1988;18:515–518. [PubMed] [Google Scholar]
- Babbott F.L., Frye W.W., Gordon J.E. Intestinal parasites of man in Arctic Greenland. Am. J. Trop. Med. Hyg. 1961;10:185–190. doi: 10.4269/ajtmh.1961.10.185. [DOI] [PubMed] [Google Scholar]
- Bearup A.J. Observations on the life cycle of Stictodora lari (Trematoda: Heterophyidae) Proc. Linnean Soc. NSW. 1961;86:251–257. [Google Scholar]
- Belizario V.Y., Jr., de Leon W.U., Bersabe M.J.J., Purnomo, Baird J.K., Bangs M.J. A focus of human infection by Haplorchis taichui (Trematoda: Heterophyidae) in the southern Philippines. J. Parasitol. 2004;90:1165–1169. doi: 10.1645/GE-3304RN. [DOI] [PubMed] [Google Scholar]
- Belogurov O.I., Leonov V.A., Zueva L.S. Helminth fauna of fish eating birds (gulls and guillemots) from the coast of the Sea of Okhotsk. In: Skryabin K.I., Mamaev Y.L., editors. Helminth of Animals of the Pacific Ocean. 1968. pp. 105–124. [Google Scholar]
- Besprozvannykh V.V., Ermolenko A.V., Dvoryadkin V.A. Occurrence of Metagonimus katsuradai Izumi, 1935 (Trematoda: Heterophyidae) in the southern Primor'e region. In: Mamaev Y.L., editor. Gel'minty I vyzyvaemye imi zabollevaniya. Vladivostock; Russia: 1987. pp. 47–52. [Google Scholar]
- Bwangamoi O., Ojok L. Helminthiasis in dogs in Uganda. IV. Heterophyes pleomorphis sp. n. (Trematoda: Heterophyidae) from the small intestine of dogs in Kampala. Bull. Anim. Health Prod. Afr. 1977:427–430. [Google Scholar]
- Chai J.Y. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea V. Intestinal pathology in experimentally infected albino rats. Seoul J. Med. 1979;20:104–117. [Google Scholar]
- Chai J.Y. Intestinal flukes. In: Murrell K.D., Fried B., editors. Food-borne Parasitic Zoonoses, Fish and Plant-borne Parasites. Springer; New York: 2007. pp. 53–115. [Google Scholar]
- Chai J.Y. Praziquantel in treatment of trematode and cestode infections. J. Infect. Chemother. 2013;45:32–43. doi: 10.3947/ic.2013.45.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y. Epidemiology of trematode infections. In: Toledo R., Fried B., editors. Digenetic Trematodes. Vol. 766. Springer; 2014. pp. 241–292. (Adv. Exp. Med. Biol). (Chapter 8) [Google Scholar]
- Chai J.Y. Encyclopedia of Food Safety. Vol. 2. Elsevier; 2014. Helminth-trematode. Heterophyes heterophyes; pp. 158–163. [Google Scholar]
- Chai J.Y. Metagonimus. In: Xiao L., Ryan U., Feng Y., editors. Biology of Foodborne Parasites. CRC Press, Taylor & Francis Group; Boca Raton, Florida: 2015. pp. 427–443. [Google Scholar]
- Chai J.Y. Metagonimus. In: Liu D., editor. Laboratory Models for Foodborne Infections. CRC Press, Taylor & Francis Group; Boca Raton, Florida: 2017. pp. 743–763. (Food Microbiology Series). [Google Scholar]
- Chai J.Y., Lee S.H. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol. Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Sohn W.M. Identification of Stellantchasmus falcatus metacercariae encysted in mullets in Korea. Korean J. Parasitol. 1988;26:65–68. doi: 10.3347/kjp.1988.26.1.65. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Cho S.Y., Seo B.S. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. IV. An epidemiological investigation along Tamjin River basin, South Cholla Do. Korean J. Parasitol. 1977;15:115–120. doi: 10.3347/kjp.1977.15.2.115. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Seo B.S., Lee S.H. Studies on intestinal trematodes in Korea XI. Two cases of human infection by Heterophyes heterophyes nocens. Korean J. Parasitol. 1984;22:37–42. doi: 10.3347/kjp.1984.22.1.37. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Hong S.J., Sohn W.M., Seo B.S. Further cases of human Heterophyes heterophyes nocens infection in Korea. Seoul J. Med. 1985;26:197–200. [Google Scholar]
- Chai J.Y., Seo B.S., Lee S.H., Hong S.T. Growth and development of Pygidiopsis summa in rats and mice with a supplementary note on its morphological characters. Korean J. Parasitol. 1986;24:55–62. doi: 10.3347/kjp.1986.24.1.55. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Seo B.S., Lee S.H., Hong S.J., Sohn W.M. Human infections by Heterophyes heterophyes and H. dispar imported from Saudi Arabia. Korean J. Parasitol. 1986;24:82–88. doi: 10.3347/kjp.1986.24.1.82. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Hong S.J., Lee S.H., Seo B.S. Stictodora sp. (Trematoda: Heterophyidae) recovered from a man in Korea. Korean J. Parasitol. 1988;26:127–132. doi: 10.3347/kjp.1988.26.2.127. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Park S.K., Hong S.J., Choi M.H., Lee S.H. Identification of Stictodora lari (Heterophyidae) metacercariae encysted in the brackish water fish, Acanthogobius flavimanus. Korean J. Parasitol. 1989;27:253–259. doi: 10.3347/kjp.1989.27.4.253. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Yu J.R., Lee S.H., Jung H.C., Song I.S., Cho S.Y. An egg-negative patient of acute metagonimiasis diagnosed serologically by ELISA. Seoul J. Med. 1989;30:139–142. [Google Scholar]
- Chai J.Y., Sohn W.M., Kim M.H., Hong S.T., Lee S.H. Three morphological types of the genus Metagonimus encysted in the dace, Tribolodon taczanowskii, caught from the Sumjin River. Korean J. Parasitol. 1991;29:217–225. doi: 10.3347/kjp.1991.29.3.217. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Huh S., Yu J.R., Kook J., Jung K.C., Park E.C., Sohn W.M., Hong S.T., Lee S.H. An epidemiological study of metagonimiasis along the upper reaches of the Namhan River. Korean J. Parasitol. 1993;31:99–108. doi: 10.3347/kjp.1993.31.2.99. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Kim T.H., Kho W.G., Chung S.W., Hong S.T., Lee S.H. Mucosal mast cell responses to experimental Metagonimus yokogawai infection in rats. Korean J. Parasitol. 1993;31:129–134. doi: 10.3347/kjp.1993.31.2.129. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Yun T.Y., Kim J., Huh S., Choi M.H., Lee S.H. Chronological observation on intestinal histopathology and intraepithelial lymphocytes in the intestine of rats infected with Metagonimus yokogawai. Korean J. Parasitol. 1994;32:215–221. doi: 10.3347/kjp.1994.32.4.215. (in Korean) [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Nam H.K., Kook J., Lee S.H. The first discovery of an endemic focus of Heterophyes nocens (Heterophyidae) infection in Korea. Korean J. Parasitol. 1994;32:157–161. doi: 10.3347/kjp.1994.32.3.157. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Kim J., Lee S.H. Invasion of Metagonimus yokogawai into the submucosal layer of the small intestine of immunosuppressed mice. Korean J. Parasitol. 1995;33:313–321. doi: 10.3347/kjp.1995.33.4.313. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Kim S.J., Kook J., Lee S.H. Effects of gamma-irradiation on the survival and development of Metagonimus yokogawai metacercariae in rats. Korean J. Parasitol. 1995;33:297–303. doi: 10.3347/kjp.1995.33.4.297. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Kim I.M., Seo M., Guk S.M., Kim J.L., Sohn W.M., Lee S.H. A new focus of Heterophyes nocens, Pygidiopsis summa, and other intestinal flukes in a coastal area of Muan-gun, Chollanam-do. Korean J. Parasitol. 1997;35:233–238. doi: 10.3347/kjp.1997.35.4.233. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Song T.E., Han E.T., Guk S.M., Park Y.K., Choi M.H., Lee S.H. Two endemic foci of heterophyids and other intestinal fluke infections in southern and western coastal areas in Korea. Korean J. Parasitol. 1998;36:155–161. doi: 10.3347/kjp.1998.36.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Han E.T., Park Y.K., Guk S.M., Kim J.L., Lee S.H. High endemicity of Metagonimus yokogawai infection along residents of Samcheok-shi, Kangwon-do. Korean J. Parasitol. 2000;38:33–36. doi: 10.3347/kjp.2000.38.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Han E.T., Park Y.K., Guk S.M., Park J.H., Lee S.H. Stictodora lari (Digenea: Heterophyidae): the discovery of the first human infections. J. Parasitol. 2002;88:627–629. doi: 10.1645/0022-3395(2002)088[0627:SLDHTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Park J.H., Han E.T., Shin E.H., Kim J.L., Guk S.M., Hong K.S., Lee S.H., Rim H.J. Prevalence of Heterophyes nocens and Pygidiopsis summa infections among residents of the western and southern coastal islands of the Republic of Korea. Am. J. Trop. Med. Hyg. 2004;71:617–622. [PubMed] [Google Scholar]
- Chai J.Y., Murrell K.D., Lymbery A.J. Fish-borne parasitic zoonoses: status and issues. Int. J. Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Park J.H., Han E.T., Guk S.M., Shin E.H., Lin A., Kim J.L., Sohn W.M., Yong T.S., Eom K.S., Min D.Y., Hwang E.H., Phommmasack B., Insisiengmay B., Rim H.J. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane municipality and Saravane Province in Laos. J. Helminthol. 2005;79:283–289. doi: 10.1079/joh2005302. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Han E.T., Guk S.M., Shin E.H., Sohn W.M., Yong T.S., Eom K.S., Lee K.H., Jeong H.G., Ryang Y.S., Hoang E.H., Phommasack B., Insisiengmay B., Lee S.H., Rim H.J. High prevalence of liver and intestinal fluke infections among residents of Savannakhet Province, Laos. Korean J. Parasitol. 2007;45:213–218. doi: 10.3347/kjp.2007.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Shin E.H., Lee S.H., Rim H.J. Foodborne intestinal flukes in Southeast Asia. Korean J. Parasitol. 2009;47:S69–S102. doi: 10.3347/kjp.2009.47.S.S69. (suppl.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Han E.T., Shin E.H., Sohn W.M., Yong T.S., Eom K.S., Min D.Y., Um J.Y., Park M.S., Hoang E.H., Phommasack B., Insisiengmay B., Lee S.H., Rim H.J. High prevalence of Haplorchis taichui, Prosthodendrium molenkampi, and other helminth infections among people in Khammouane province, Lao PDR. Korean J. Parasitol. 2009;47:243–247. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Yong T.S., Eom K.S., Min D.Y., Shin E.H., Banouvong V., Insisiengmay B., Insisiengmay S., Phommasack B., Rim H.J. Prevalence of the intestinal flukes Haplorchis taichui and H. yokogawai in a mountainous area of Phongsaly Province, Lao PDR. Korean J. Parasitol. 2010;48:339–342. doi: 10.3347/kjp.2010.48.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., De N.V., Sohn W.M. Foodborne trematode metacercariae in fish from northern Vietnam and their adults recovered from experimental hamsters. Korean J. Parasitol. 2012;50:317–325. doi: 10.3347/kjp.2012.50.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Yong T.S., Eom K.S., Min D.Y., Jeon H.K., Kim T.Y., Jung B.K., Sisabath L., Insisiengmay B., Phommasack B., Rim H.J. Hyperendemicity of Haplorchis taichui infection among riparian people in Saravane and Champasak Province, Lao PDR. Korean J. Parasitol. 2013;51:305–311. doi: 10.3347/kjp.2013.51.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Sohn W.M., Yong T.S., Eom K.S., Min D.Y., Lee M.Y., Lim H., Insisiengmay B., Phommasack B., Rim H.J. Centrocestus formosanus (Heterophyidae): human infections and the infection source in Lao PDR. J. Parasitol. 2013;99:531–536. doi: 10.1645/12-37.1. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Bahk Y.Y., Sohn W.M. Trematodes recovered in the small intestine of stray cats in the Republic of Korea. Korean J. Parasitol. 2013;51:99–106. doi: 10.3347/kjp.2013.51.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Sohn W.M., Na B.K., Yong T.S., Eom K.S., Yoon C.H., Hoang E.H., Jeoung H.G., Socheat D. Zoonotic trematode metacercariae in fish from Phnom Penh and Pursat, Cambodia. Korean J. Parasitol. 2014;52:35–40. doi: 10.3347/kjp.2014.52.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Park Y.J., Park J.H., Jung B.K., Shin E.H. Mucosal immune responses of mice experimentally infected with Pygidiopsis summa (Trematoda: Heterophyidae) Korean J. Parasitol. 2014;52:27–33. doi: 10.3347/kjp.2014.52.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Kim L.J., Seo M. Four human cases of Acanthotrema felis (Digenea: Heterophyidae) infection in Korea. Korean J. Parasitol. 2014;52:291–294. doi: 10.3347/kjp.2014.52.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Jung B.K., Kim D.G., Kim J.L., Lim H., Shin E.H., Lee K.H., Kim M.R., Han S.J., Yeom J.H., Park S.M., Hwang J.S. Heterophyid trematodes recovered from people residing along the Boseong River, South Korea. Acta Trop. 2015;148:142–146. doi: 10.1016/j.actatropica.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Sohn W.M., Jung B.K., Yong T.S., Eom K.S., Min D.Y., Insisiengmay B., Insisiengmay S., Phommasack B., Rim H.J. Intestinal helminths recovered from humans in Xieng Khouang Province, Lao PDR with a particular note on Haplorchis pumilio infection. Korean J. Parasitol. 2015;53:439–445. doi: 10.3347/kjp.2015.53.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.Y., Sohn W.M., Na B.K., Jeoung H.G., Sinuon M., Socheat D. Stellantchasmus falcatus (Digenea: Heterophyidae) in Cambodia: discovery of metacercariae in mullets and recovery of adult flukes in an experimental hamster. Korean J. Parasitol. 2016;54:537–541. doi: 10.3347/kjp.2016.54.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.T. A study of the Haplorchinae (Looss, 1899) Poche, 1926 (Trematoda: Heterophyidae) Parasitology. 1936;28:40–55. [Google Scholar]
- Chen H.T. The metacercaria and adult of Centrocestus formosanus (Nishigori, 1924), with notes on the natural infection of rats and cats with C. armatus (Tanabe, 1922) J. Parasitol. 1942;28:285–298. [Google Scholar]
- Chen E.R. Current status of food-borne parasitic zoonoses in Taiwan. Southeast Asian J. Trop. Med. Public Health. 1991;22:62–64. (suppl) [PubMed] [Google Scholar]
- Chi J.G., Kim C.W., Kim J.R., Hong S.T., Lee S.H. Intestinal histopathology in human metagonimiasis with ultrastructural observations of parasites. J. Korean Med. Sci. 1988;3:171–177. doi: 10.3346/jkms.1988.3.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieffi P.P., Gorla M.C.O., Torres D.M., Dias R.M., Mangini A.C., Monteiro A.V., Woiciechovski E. Human infection by Phagicola sp. (Trematoda, Heterophyidae) in the municipality of Registro, São Paulo State, Brazil. J. Trop. Med. Hyg. 1992;95:346–348. [PubMed] [Google Scholar]
- Cho S.Y., Kang S.Y., Ryang Y.S. Helminthes infections in the small intestine of stray dogs in Ejungbu City, Kyunggi Do, Korea. Korean J. Parasitol. 1981;19:55–59. doi: 10.3347/kjp.1981.19.1.55. [DOI] [PubMed] [Google Scholar]
- Cho S.Y., Kang S.Y., Lee J.B. Metagonimiasis in Korea. Arzneim.-Forsch. 1984;34(9B):1211–1213. [PubMed] [Google Scholar]
- Cho S.Y., Kim S.I., Earm Y.E., Ho W.K. A preliminary observation on water content of small intestine in Metagonimus yokogawai infected dog. Korean J. Parasitol. 1985;23:175–177. doi: 10.3347/kjp.1985.23.1.175. [DOI] [PubMed] [Google Scholar]
- Cho S.Y., Kim S.Y., Kang S.Y. Specific IgG antibody response in experimental cat metagonimiasis. Korean J. Parasitol. 1987;25:149–153. doi: 10.3347/kjp.1987.25.2.149. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Cho P.Y., Lee D.M., Kim T.S., Kim I.S., Hwang E.J., Na B.K., Sohn W.M. Epidemiological survey on the infection of intestinal flukes in residents of Muan-gun, Jeollanam-do, the Republic of Korea. Korean J. Parasitol. 2010;48:133–138. doi: 10.3347/kjp.2010.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.H., Kim I.S., Hwang E.J., Kim T.S., Na B.K., Sohn W.M. Infection status of esturine fish and oysters with intestinal fluke metacercariae in Muan-gun, Jeollanam-do, Korea. Korean J. Parasitol. 2012;50:215–220. doi: 10.3347/kjp.2012.50.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N.D., Skirnisson K., Nielsen O.K. The parasite fauna of the gyrfalcon (Falco rusticolus) in Iceland. J. Wildl. Dis. 2015;51:929–933. doi: 10.7589/2015-01-022. [DOI] [PubMed] [Google Scholar]
- Chun S.K. A study on the metacercaria of Metagonimus takahashii and Exorchis oviformis from Carassius carassius. Bull. Pusan Fish. Coll. 1960;3:31–39. (in Korean) [Google Scholar]
- Chun S.K. A study on some trematodes whose intermediate hosts are brackish water fish (I). The life history of Heterophyes continus, the intermediate host of which is Lateolabrax japonicus. Bull. Pusan Fish. Coll. 1960;3:40–44. (in Korean) [Google Scholar]
- Chun S.K. Bull. Fish. Coll. Pusan Nat. Univ. Vol. 5. 1963. On some trrematodes whose intermediate hosts are brackish water fish (II). The life history of Pygidiopsis summus the intermediate host of which is Mugil cephalus; pp. 1–6. (in Korean) [Google Scholar]
- Chung O.S., Lee H.J., Kim Y.M., Sohn W.M., Kwak S.J., Seo M. First report of human infection with Gynaecotyla squatarolae and first Korean record of Haplorchis pumilio in a patient. Parasitol. Int. 2011;60:227–229. doi: 10.1016/j.parint.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Ciurea P.J. Heterophyidae de la faune Parasitaire de Roumanie. Parasitology. 1924;16:1–25. (in French) [Google Scholar]
- Collomb H., Bert J. Parasite epilepsy of unusual origin: distomiasis (due to Heterophyes heterophyes) Neurochirurgie. 1959;5:330–333. [PubMed] [Google Scholar]
- Collomb H., Deschiens R., Demarchi J. On 2 cases of cerebral distomiasis caused by Heterophyes heterophyes. Bull. Soc. Pathol. Exot. Filiales. 1960;53:144–147. [PubMed] [Google Scholar]
- Cortés D.A., Dolz G., Zúñiga J.J.R., Rocha A.E.J., Aláan D.L. Centrocestus formosanus (Opisthorchiida: Heterophyidae) como causa de muerte de alevines de tilapia gris Oreochromis niloticus (Perciforme: Cichlidae) en el Pacifico seco de Costa Rica. Rev. Biol. Trop. 2010;58:1453–1465. [PubMed] [Google Scholar]
- Daoud A. Experimental heterophyiasis: histopathological and immunological study. Int. Symp. Front. Immunol. Inflam. World Allergy Organ. J. 2012;(254):S100. (abstracts) [Google Scholar]
- Davydov O.N., Lysenko V.N., Kurovskaya L.Y. Species diversity of carp, Cyprinus carpio (Cypriniformes, Cyprinidae), parasites in some cultivation regions. Vestnik. Zoologii. 2011;45 [Google Scholar]
- De N.V., Le T.H. Human infections of fish-borne trematodes in Vietnam: prevalence and molecular specific identification at an endemic commune in Nam Dinh province. Exp. Parasitol. 2011;129:355–361. doi: 10.1016/j.exppara.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ditrich O., Scholz T., Giboda M. Occurrence of some medically important flukes (Trematoda: Opisthorchiidae and Heterophyidae) in Nam Ngum Water Reservoir, Laos. Southeast Asian J. Trop. Med. Public Health. 1990;21:482–488. [PubMed] [Google Scholar]
- Ditrich O., Kopacek P., Giboda M., Gutvirth J., Scholz T. Serological differentiation of human small fluke infections using Opisthorchis viverrini and Haplorchis taichui antigens. Southeast Asian J. Trop. Med. Public Health. 1991;22:174–178. (suppl) [PubMed] [Google Scholar]
- Djikanovic V., Paunovic M., Nikolic V., Simonovic P., Cakic P. Parasitofauna of freshwater fishes in the Serbian open waters: a checklist of parasites of freshwater fishes in Serbian open waters. Rev. Fish Biol. Fish. 2011;22:297–324. [Google Scholar]
- Dung D.T., De N.V., Waikagul J., Dalsgaard A., Chai J.Y., Sohn W.M., Murrell K.D. Fishborne zoonotic intestinal trematodes, Vietnam. Emerg. Infect. Dis. 2007;13:1828–1833. doi: 10.3201/eid1312.070554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dung D.T., Hop N.T., Thaenkham U., Waikagul J. Genetic difference among Vietnamese Haplorchis taichui populations using the COI genetic marker. J. Helminthol. 2013;87:66–70. doi: 10.1017/S0022149X12000041. [DOI] [PubMed] [Google Scholar]
- Dzikowski R., Levy M.G., Poore M.F., Flowers J.R., Paperna I. Use of rDNA polymorphism for identification of Heterophyidae infecting freshwater fishes. Dis. Aquat. Org. 2004;59:35–41. doi: 10.3354/dao059035. [DOI] [PubMed] [Google Scholar]
- El-Azazy O.M.E., Ibrahim Abdou N.E.M., Khalil A.I., Al-Batel M.K., Majeed Q.A., Henedi A.A., Tahrani L.M. Potential zoonotic trematodes recovered in stray cats from Kuwait municipality, Kuwait. Korean J. Parasitol. 2015;53:279–287. doi: 10.3347/kjp.2015.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ganayni G.A., Youssef M.E., Handousa A.E., Bou-Zakham A.A., Hegazi M.M. Serum and intestinal immunoglobulin levels in heterophyiasis. J. Egypt. Soc. Parasitol. 1989;19:219–223. [PubMed] [Google Scholar]
- El-Hawy A.M., El-Nasr M.S.S., Abdel Rahman M.M. The cure rates of patients infected with heterophyiasis and treated with praziquantel versus niclosamide. J. Egypt. Soc. Parasitol. 1988;18:437–442. [PubMed] [Google Scholar]
- Elshazly A.M., Elsheikha H.M., Rahbar M.H., Awad S.I. Comparison of three immunodiagnostic tests for experimental Heterophyes heterophyes infection in dogs. Vet. Parasitol. 2008;151:196–202. doi: 10.1016/j.vetpar.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Elsheikha H.M. Heterophyosis: risk of ectopic infection. Vet. Parasitol. 2007;147:341–342. doi: 10.1016/j.vetpar.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Emolenko A.V., Bespozvannykh V.V., Rumyantseva E.E., Voronok V.M. Pathogens of human trematodiases in the Primorye Terrotory. Med. Parazitol. (Mosk) 2015;2:6–10. (in Russian) [PubMed] [Google Scholar]
- Eom K.S., Yoo H.W., Chung M.S. Heterophyid trematodes (Heterophyes heterophyes and H. dispar) human infections imported from Sudan to Korea. Korean J. Parasitol. 1985;23:360–361. (abstract in Korean) [Google Scholar]
- Eom K.S., Son S.Y., Lee J.S., Rim H.J. Heterophyid trematodes (Heterophyopsis continua, Pygidiopsis summa and Heterophyes heterophyes nocens) from domestic cats in Korea. Korean J. Parasitol. 1985;23:197–202. doi: 10.3347/kjp.1985.23.2.197. [DOI] [PubMed] [Google Scholar]
- Eom K.S., Park H.S., Lee D., Sohn W.M., Yong T.S., Chai J.Y., Min D.Y., Rim H.J., Insisiengmay B., Phommasack B. Infection status of zoonotic trematode metacercariae in fishes from Vientiane municipality and Champasak Province in Lao PDR. Korean J. Parasitol. 2015;53:447–453. doi: 10.3347/kjp.2015.53.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahnak A., Shiekhian R., Mobedi I. A faunistic survey on the bird helminth parasites and their medical importance. Iran. J. Public Health. 2004;33:40–46. [Google Scholar]
- Faust E.C., Nishigori M. The life cycles of two new species of Heterophyidae parasitic in mammals and birds. J. Parasitol. 1926;13:91–131. [Google Scholar]
- Francová K., Ondračková M., Polačik M., Jurajda P. Parasite fauna of native and non-native populations of Neogobius melanostomus (Pallas, 1814) (Gobiidae) in the longitudinal profile of the Danube River. J. Appl. Ichthyol. 2011;27:879–886. [Google Scholar]
- Fried B., Graczyk T.K., Tamang L. Food-borne intestinal trematodiases in humans. Parasitol. Res. 2004;93:159–170. doi: 10.1007/s00436-004-1112-x. [DOI] [PubMed] [Google Scholar]
- Fürst T., Keiser J., Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- Gallais P., Paillas P., Collomb H., Luigi D.M., Demarchi J., Deschiens R. Anatomo-pathological study of a parasitic cerebral cyst in man. Bull. Soc. Pathol. Exot. Filiales. 1955;48:830–832. (in French) [PubMed] [Google Scholar]
- Giboda M., Ditrich O., Scholz T., Viengsay T., Bouaphanh S. Human Opisthorchis and Haplorchis infection in Laos. Trans. R. Soc. Trop. Med. Hyg. 1991;85:538–540. doi: 10.1016/0035-9203(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Gjurčevic E., Petrinec Z., Kozaric Z., Kužir S., Kantura V.G., Kantura M., Vučemilo M., Džaja P. Metacercariae of Centrocestus formosanus in goldfish (Carassius auratus L.) imported into Croatia. Helminthologia. 2007;44:214–216. [Google Scholar]
- Glover M.A., Alicata J.E. Intestinal heterophyidiasis. Hawaii Med. J. 1957;16:636–688. [PubMed] [Google Scholar]
- Guk S.M., Park J.Y., Seo M., Han E.T., Kim J.L., Chai J.Y. Susceptibility of inbred mouse strains to infection with three species of Metagonimus prevalent in the Republic of Korea. J. Parasitol. 2005;91:12–16. doi: 10.1645/GE-367R. [DOI] [PubMed] [Google Scholar]
- Guk S.M., Park J.H., Shin E.H., Kim J.L., Lin A., Chai J.Y. Prevalence of Gymnophalloides seoi infection in coastal villages of Haenam-gun and Yeongam-gun, Republic of Korea. Korean J. Parasitol. 2006;44:1–5. doi: 10.3347/kjp.2006.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guk S.M., Shin E.H., Kim J.L., Sohn W.M., Hong K.S., Yoon C.H., Lee S.H., Rim H.J., Chai J.Y. A survey of Heterophyes nocens and Pygidiopsis summa metacercariae in mullets and gobies along the coastal areas of the Republic of Korea. Korean J. Parasitol. 2007;45:205–211. doi: 10.3347/kjp.2007.45.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy E.I., Nicola E. On the histopathology of the small intestine in animals experimentally infected with H. heterophyes. J. Egypt. Med. Assoc. 1980;63:179–184. [Google Scholar]
- Han E.T., Shin E.H., Phommakorn S., Sengvilaykham B., Kim J.L., Rim H.J., Chai J.Y. Centrocestus formosanus (Digenea: Heterophyidae) encysted in the freshwater fish, Puntius brevis, from Lao PDR. Korean J. Parasitol. 2008;46:49–53. doi: 10.3347/kjp.2008.46.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E.T., Yang H.J., Park Y.J., Park J.H., Chai J.Y. Metagonimus yokogawai: a 100-kDa somatic antigen commonly reacting with other trematodes. Korean J. Parasitol. 2014;52:201–204. doi: 10.3347/kjp.2014.52.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.T., Chai J.Y., Lee S.H. Ten human cases of Fibricola seoulensis infection and mixed one with Stellantchasmus and Metagonimus. Korean J. Parasitol. 1986;24:94–96. doi: 10.3347/kjp.1986.24.1.95. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Seo B.S., Lee S.H., Chai J.Y. A human case of Centrocestus armatus infection in Korea. Korean J. Parasitol. 1988;26:55–60. doi: 10.3347/kjp.1988.26.1.55. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Woo H.C., Kim I.T. Study on Centrocestus armatus in Korea II. Recovery rate, growth and development of worms in albino rats. Korean J. Parasitol. 1989;27:47–56. doi: 10.3347/kjp.1989.27.1.47. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Lee S.H., Chai J.Y., Seo B.S. Recovery rate, growth and development of Heterophyopsis continua in experimental chicks. Korean J. Parasitol. 1990;28:53–62. doi: 10.3347/kjp.1990.28.1.53. [DOI] [PubMed] [Google Scholar]
- Hong S.T., Yu J.R., Myong N.H., Chai J.Y., Lee S.H. Activities of brush border membrane bound enzymes of the small intestine in Metagonimus yokogawai infection in mice. Korean J. Parasitol. 1991;29:9–20. doi: 10.3347/kjp.1991.29.1.9. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Woo H.C., Lee S.Y., Ahn J.H., Park C.K., Chai J.Y., Lee S.H. Worm recovery and small intestinal lesions of albino rats coinfected with Fibricola seoulensis and Metagonimus yokogawai. Korean J. Parasitol. 1993;31:109–116. doi: 10.3347/kjp.1993.31.2.109. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Chang C.K., Lee D.H., Woo H.C. One human case of natural infection by Heterophyopsis continua and three other species of intestinal trematodes. Korean J. Parasitol. 1996;34:87–90. doi: 10.3347/kjp.1996.34.1.87. [DOI] [PubMed] [Google Scholar]
- Huh S., Sohn W.M., Chai J.Y. Intestinal parasites of cats purchased in Seoul. Korean J. Parasitol. 1993;31:371–373. doi: 10.3347/kjp.1993.31.4.371. [DOI] [PubMed] [Google Scholar]
- Hung N.M., Dung D.T., Anh N.T.L., Van P.T., Thanh B.N., Van H.N., Van Hien H., Canh Le X. Current status of fish-borne zoonotic trematode infection in Gia Vien district, Ninh Binh Province, Vietnam. Parasit. Vectors. 2015;8:21. doi: 10.1186/s13071-015-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.M., Soliman M.F.M. Prevalence and site preferences of heterophyid metacercariae in Tilapia zilli from Ismalia fresh water canal, Egypt. Parasite. 2010;17:233–239. doi: 10.1051/parasite/2010173233. [DOI] [PubMed] [Google Scholar]
- Ito J. Progr. Med. Parasitol. Japan. Vol. 1. 1964. Metagonimus and other human heterophyid trematodes; pp. 314–393. [Google Scholar]
- Ito J. A monograph of cercariae in Japan and adjacent territories. Progr. Med. Parasitol. Japan. 1964;1:395–550. [Google Scholar]
- Ito J., Mochizuki H., Noguchi M. An epidemiological study of human helminths in rural areas of Shizuoka Prefecture. IV. Three human trematodes. Jpn. J. Parasitol. 1967;16:134–138. (in Japanese) [Google Scholar]
- Ito J., Mochizuki H., Ohno Y., Ishiguro M. On the prevalence of Metagonimus sp. among the inhabitants at Hamamatsu basin in Shizuoka Prefecture, Japan. Jpn. J. Parasitol. 1991;40:274–278. (in Japanese) [Google Scholar]
- Izumi M. Studies on a new species of Metagonimus and its life cycle. Tokyo Iji Shinshi. 1935;2929:1224–1236. (in Japanese) [Google Scholar]
- Jeon H.K., Lee D., Park H., Min D.Y., Rim H.J., Zhang H., Yang Y., Li X., Eom K.S. Human infections with liver and minute intestinal flukes in Guangxi, China: analysis by DNA sequencing, ultrasonography, and immunoaffinity chromatography. Korean J. Parasitol. 2012;50:391–394. doi: 10.3347/kjp.2012.50.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.S., Bolick A., Alexander M., Huffman D., Oborny E., Monroe A. Fluctuation in densities of the invasive gill parasite Centrocestus formosanus (Trematoda: Heterophyidae) in the Comal River, Comal County, Texas, U.S.A. J. Parasitol. 2012;98:111–116. doi: 10.1645/GE-2841.1. [DOI] [PubMed] [Google Scholar]
- Kagei N., Kihata M. Report of Nat. Instit. Public Hyg. (Japan) Vol. 19. 1970. On the development of Metagonimus yokogawai (Katsurada, 1912) (Heterophyidae, Trematoda) in laboratory animals; pp. 48–63. (in Japanese) [Google Scholar]
- Kagei N., Oshima T., Ishikawa K., Kihata M. Two cases of human infection with Stellantchasmus falcatus Onji et Nishio 1915 (Heterophyidae) in Kochi Prefecture. Jpn. J. Parasitol. 1964;13:472–478. (in Japanese) [Google Scholar]
- Kagei N., Hayashi S., Kato K. On the heterophyid trematode (Heterophyes heterophyes) infection cases imported from Egypt to Japan. Jpn. J. Trop. Med. 1980;8:1–7. (in Japanese) [Google Scholar]
- Kalantan A.M.N., Arfin M., Mosjen A.A.A. Digenetic trematodes of Larus genei (Lariformes: Laridae) caught from Al-Rames Coast of Al-Qateef in Eastern Province of Saudi Arabia. Pak. J. Biol. Sci. 2000;3:1708–1711. [Google Scholar]
- Kang S.Y., Cho S.Y., Chai J.Y., Lee J.B., Jang D.H. A study on intestinal lesions of experimentally reinfected dogs with Metagonimus yokogawai. Korean J. Parasitol. 1983;21:58–73. doi: 10.3347/kjp.1983.21.1.58. [DOI] [PubMed] [Google Scholar]
- Katsurada F. Heterophyes in Japan. II. Creation of a new genus Metagonimus. Okayama Igakkai zasshi. 1912;(273):768–778. (in Japanese) [Google Scholar]
- Katsuta I. Studies on trematodes that take brackish water fish as the second intermediate host in Taiwan. I. On a new trematode Stellantchasmus formosanus which takes Mugil cephalus as its intermediate host. Taiwan Igakkai Zasshi. 1931;30:1404–1417. (in Japanese) [Google Scholar]
- Katsuta I. Studies on the metacercariae of Formosan brackish water fishes. 2. On a new species, Metagonimus minutus n. sp. parasitic in Mugil cephalus. Taiwan Iggakai Zasshi. 1932;31:26–39. (in Japanese) [Google Scholar]
- Katsuta I. Studies on trematodes that take brackish water fish as the second intermediate host in Taiwan (4th report). Monorchotrema yokogawai which takes Mugil cephalus as its intermediate host. Taiwan Igakkai Zasshi. 1932;31:253–265. (in Japanese) [Google Scholar]
- Khalifa R., El-Naffar M.K., Arafa M.S. Studies on heterophyid cercariae from Assiut Province, Egypt. I. Notes on the life cycle of Haplorchis pumilio (Looss, 1896) with a discussion on previously described species. Acta Parasitol. Pol. 1977;25:25–38. [Google Scholar]
- Khalil M. The life history of the human trematode parasite Heterophyes heterophyes in Egypt. Lancet. 1933;222:537. [Google Scholar]
- Khalil M.I., El-Shahawy I.S., Abdelkader H.S. Studies on some fish parasites of public health importance in the southern area of Saudi Arabia. Braz. J. Vet. Parasitol. 2014;23:435–442. doi: 10.1590/S1984-29612014082. [DOI] [PubMed] [Google Scholar]
- Kim C.H. Study on the Metagonimus sp. in Gum River basin, Chungchung-nam Do, Korea. Korean J. Parasitol. 1980;18:215–228. doi: 10.3347/kjp.1980.18.2.215. (in Korean) [DOI] [PubMed] [Google Scholar]
- Kim C.H., Kim N.M., Lee C.H., Park J.S. Studies on the Metagonimus fluke in the Daecheong reservoir and the upper stream of Geum River, Korea. Korean J. Parasitol. 1987;25:69–82. doi: 10.3347/kjp.1987.25.1.69. (in Korean) [DOI] [PubMed] [Google Scholar]
- Kim K.H., Yoon Y.M., Lee J.S., Rim H.J. A numerical taxonomic study on heterophyid trematodes. Korean J. Parasitol. 1991;29:55–65. doi: 10.3347/kjp.1991.29.1.55. (in Korean) [DOI] [PubMed] [Google Scholar]
- Kim D.G., Kim T.S., Cho S.H., Song H.J., Sohn W.M. Heterophyid metacercarial infections in brackish water fishes from Jinju-man (Bay), Kyongsangnam-do, Korea. Korean J. Parasitol. 2006;44:7–13. doi: 10.3347/kjp.2006.44.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D., Paller V.G., Uga S. Development of Centrocestus armatus in different final hosts. Vet. Parasitol. 2007;146:367–371. doi: 10.1016/j.vetpar.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Kino H., Oishi H., Ohno Y., Ishiguro M. An endemic human infection with Heterophyes nocens Onji et Nishio, 1916 at Mikkabi-cho, Shizuoka, Japan. Jpn. J. Trop. Med. Hyg. 2002;30:301–304. [Google Scholar]
- Kino H., Suzuki T., Oishi H., Suzuki S., Yamagiwa S., Ishiguro M. Geographical distribution of Metagonimus yokogawai and M. miyatai in Shizuoka Prefecture, Japan, and their site preferences in the sweetfish, Plecoglossus altivelis, and hamsters. Parasitol. Int. 2006;55:201–206. doi: 10.1016/j.parint.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kliks M., Tantachamrun T. Heterophyid (Trematoda) parasites of cats in north Thailand, with note on a human case found at necropsy. Southeast Asian J. Trop. Med. Public Health. 1974;5:547–555. [PubMed] [Google Scholar]
- Kobayasi H. Studies on Trematode in Hainan island, South China and Viet-Nam (French Indo-China). Reports of Scientifical Works by H. Kobayasi. 1968. Reports on scientifical works; pp. 155–251. [Google Scholar]
- Komatsu S., Kimura D., Paller V.G.V., Uga S. Dynamics of Centrocestus armatus transmission in endemic river in Hyogo Prefecture, Japan. Trop. Med. Health. 2014;42:35–42. doi: 10.2149/tmh.2013-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y. Metacercariae in Japan and adjacent territories. Progr. Med. Parasitol. Japan. 1965;2:1–328. [Google Scholar]
- Komiya Y., Suzuki N. The metacercariae of trematodes belonging to the family Heterophyidae from Japan and adjacent territories. Jpn. J. Parasitol. 1966;15:208–214. (in Japanese) [Google Scholar]
- Kristoffersen R. Occurrence of the digenean Cryptocotyle lingua in farmed Arctic charr Salvelinus alpinus and periwinkles Littorina littorea sampled close to charr farms in northern Norway. Dis. Aquat. Org. 1991;12:59–65. [Google Scholar]
- Kumchoo K., Wongsawad C., Chai J.Y., Vanittanakom P., Rojanapaibul A. Recovery and growth of Haplorchis taichui (Trematoda: Heterophyidae) in chicks. Southeast Asian J. Trop. Med. Public Health. 2003;34:718–722. [PubMed] [Google Scholar]
- Kumchoo K., Wongsawad C., Vanittanakom P., Chai J.Y., Rojanapaibul A. Effect of niclosamide on the tegumental surface of Haplorchis taichui using scanning electron microscopy. J. Helminthol. 2007;81:329–337. doi: 10.1017/S0022149X07381108. [DOI] [PubMed] [Google Scholar]
- Kuntz R.E., Chandler A.C. Studies on Egyptian trematodes with special reference to the heterophyid of mammals. I. Adult flukes, with descriptions of Phagicola longicollis n. sp., Cynodiplostomum namrui n. sp., and a Stephanoprora from cats. J. Parasitol. 1956;42:445–459. [PubMed] [Google Scholar]
- Kurokawa T. On a new species of Stamnosoma found from a man. Tokyo Iji Shinshi no. 1935;2915:293–298. (in Japanese) [Google Scholar]
- Kurokawa T. Studies on the genus Metagonimus, especially on the life history of M. katsuradai Izumi, 1935 and determination of its first intermediate host. Tokyo Iji Shinshi. 1939;3161:2877–2885. (in Japanese) [Google Scholar]
- Lamy C., Duhamel C., Morel C., Valla A. A case of intestinal fluke infection. Nouv. Press. Med. 1976;5:1005–1006. (in French) [PubMed] [Google Scholar]
- Lee J.B., Chi J.G., Lee S.K., Cho S.Y. Study on pathology of metagonimiasis in experimentally infected cat intestine. Korean J. Parasitol. 1981;19:109–129. doi: 10.3347/kjp.1981.19.2.109. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Hwang S.W., Chai J.Y., Seo B.S. Comparative morphology of eggs of heterophyids and Clonorchis sinensis causing human infections in Korea. Korean J. Parasitol. 1984;22:171–180. doi: 10.3347/kjp.1984.22.2.171. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Chai J.Y., Hong S.J., Chon Y.S., Seo B.S. Anthelmintic effect of praziquantel (Distocide®) against Metagonimus yokogawai infection. J. Korean Soc. Chemother. 1984;2:167–171. (in Korean) [Google Scholar]
- Lee S.C., Chung Y.B., Kong Y., Kang S.Y., Cho S.Y. Antigenic protein fractions of Metagonimus yokogawai reacting with patient sera. Korean J. Parasitol. 1993;31:43–48. doi: 10.3347/kjp.1993.31.1.43. [DOI] [PubMed] [Google Scholar]
- Lee S.U., Huh S., Park G.M., Chai J.Y. A cytogenetic study on human intestinal trematodes of the genus Metagonimus (Digenea: Heterophyidae) in Korea. Korean J. Parasitol. 1999;37:237–241. doi: 10.3347/kjp.1999.37.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.U., Huh S., Sohn W.M., Chai J.Y. Sequence comparisons of 28S ribosomal DNA and mitochondrial cytochrome c oxidase subunit I of Metagonimus yokogawai, M. takahashii and M. miyatai. Korean J. Parasitol. 2004;42:129–134. doi: 10.3347/kjp.2004.42.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.U., Chun H.C., Huh S. Molecular phylogeny of parasitic Platyhelminthes based on sequences of partical 28S rDNA D1 and mitochondrial cytochrome c oxidase subunit 1. Korean J. Parasitol. 2007;45:181–189. doi: 10.3347/kjp.2007.45.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Jung B.K., Lim H., Lee M.Y., Choi S.Y., Shin E.H., Chai J.Y. Comparative morphology of minute intestinal fluke eggs that can occur in human stools in the Republic of Korea. Korean J. Parasitol. 2012;50:207–213. doi: 10.3347/kjp.2012.50.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Choe S., Park H., Jeon H.K., Chai J.Y., Sohn W.M., Yong T.S., Min D.Y., Rim H.J., Eom K.S. Complete mitochondrial genome of Haplorchis taichui and comparative analysis with other trematodes. Korean J. Parasitol. 2013;51:719–726. doi: 10.3347/kjp.2013.51.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.H., Liao C.W., Lee Y.L., Ooi H.K., Du W.Y., Lu S.C., Huang H.I., Su K.E., Fan C.K. Infectivity and development of Metagonimus yokogawai in experimentally infected domestic ducks (Cairina muschata) Vet. Parasitol. 2010;168:45–50. doi: 10.1016/j.vetpar.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Li T., He S., Zhao H., Zhao G., Zhu X.Q. Major trends in human parasitic diseases in China. Trends Parasitol. 2010;26:264–270. doi: 10.1016/j.pt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Li M.H., Juang H.I., Chen P.L., Huang C.H., Chen Y.H., Ooi H.K. Metagonimus yokogawai: metacercariae survey in fishes and its development to adult worms in various rodents. Parasitol. Res. 2013;112:1647–1653. doi: 10.1007/s00436-013-3320-8. [DOI] [PubMed] [Google Scholar]
- Lo C.T., Lee K.M. Pattern of emergence and the effects of temperature and light on the emergence and survival of heterophyid cercariae (Centrocestus formosanus and Haplorchis pumilio) J. Parasitol. 1996;82:347–350. [PubMed] [Google Scholar]
- Lobna S.M.A., Metawea Y.F., Elsheikha H.M. Prevalence of heterophyiosis in Tilapia fish and humans in northern Egypt. Parasitol. Res. 2010;107:1029–1034. doi: 10.1007/s00436-010-1976-x. [DOI] [PubMed] [Google Scholar]
- Looss A. Weitere Beitrage zur Kentniss der Trematoden-Fauna Aegyptens, zugleich Versuch einer naturalischen Gliedrung des Genus Distomum Retzius. Zool. Yb. 1899;12:521–784. (in Germany) [Google Scholar]
- Looss A. Notizen zur Helminthologie Aegyptens. V. Eine Revision der Fasciolidengattung Heterophyes Cobb. Centralbl. Bakteriol. 1902;32:886–891. (in Germany) [Google Scholar]
- Looss A. Notizen zur Helminthologie Aegyptens. VII. Ueber einige neue Trematoden der äegyptischen Fauna. Centralbl. Bakteriol. 1907;43:478–490. (in Germany) [Google Scholar]
- Lovis L., Mak T.K., Phongluxa K., Soukhathammavong P., Sayasone S., Akkhavong K., Odermatt P., Keiser J., Felger I. PCR diagnosis of Opisthorchis viverrini and Haplorchis taichui infections in a Lao community in an area of endemicity and comparison of diagnostic methods for parasitological field surveys. J. Clin. Microbiol. 2009;47:1517–1523. doi: 10.1128/JCM.02011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanta J., Narain K., Srivastava V.K. Heterophyid eggs in human stool samples in Assam: first report for India. J. Commun. Dis. 1995;27:142–145. [PubMed] [Google Scholar]
- Mahdy O.A., Shaheed I.B. Studies on metacercarial infection among Tilapia species in Egypt. Helminthologia. 2001;38:35–42. [Google Scholar]
- Manning G.S., Lertprasert P. Four new trematodes of man from Thailand. Trans. R. Soc. Trop. Med. Hyg. 1971;65:101–102. doi: 10.1016/0035-9203(71)90193-3. [DOI] [PubMed] [Google Scholar]
- Manning G.S., Lertprasert P., Watanasirmkit K., Chetty C. A description of newly discovered intestinal parasites endemic to northern Thailand. J. Med. Assoc. Thail. 1971;54:466–474. [PubMed] [Google Scholar]
- Mansour N.S., Youssef M., Awadalla H.N., Hammouda N.H., Boulos L.M. Susceptibility of small laboratory animals to Pygidiopsis genata (Trematoda: Heterophyidae) J. Egypt. Soc. Parasitol. 1981;11:225–234. [PubMed] [Google Scholar]
- Martin W.E. The life histories of some Hawaiian heterophyid trematodes. J. Parasitol. 1958;44:305–318. [PubMed] [Google Scholar]
- Martin W.E. Egyptian heterophyid trematodes. Trans. Am. Microsc. Soc. 1959;78:172–181. [Google Scholar]
- Martínez-Alonso J.C., Armentia A., Vega J.M., Gómez C.A. Anaphylactic reaction concurrent with Heterophyes heterophyes infestation. Rev. Exp. Alergol. Immunol. Clin. 1999;14:37–39. (in Spanish) [Google Scholar]
- Masala S., Piras M.C., Sanna D., Chai J.Y., Jung B.K., Sohn W.M., Garippa G., Merella P. Epidemiological and molecular data of zoonotic trematode metacercariae found in the muscle of grey mullets (Osteichthys: Mugilidae) from Sardinia (western Mediterranean Sea) Parasitol. Res. 2016;115:3409–3417. doi: 10.1007/s00436-016-5101-7. [DOI] [PubMed] [Google Scholar]
- Massoud J., Jalali H., Reza M. Studies on trematodes of the family Heterophyidae (Odhner, 1914) in Iran. 1. Preliminary epidemiological surveys in man and carnivores in Khuzestan. J. Helminthol. 1981;55:255–260. doi: 10.1017/s0022149x00027851. [DOI] [PubMed] [Google Scholar]
- Matthews R.A., Matthews B.F. Cryptocotyle lingua in mullet, Chelon labrosus; significance of metacercarial excretory proteins in the stimulation of the immune response. J. Helminthol. 1993;67:1–9. doi: 10.1017/s0022149x00012785. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H. Encyclop. Parasitol. 2015. Heterophyes heterophyes; pp. 1–2. [Google Scholar]
- Mitchell A.J., Salmon M.J., Huffman D.G., Goodwin A.E., Brandt T.M. Prevalence and pathogenicity of a heterophyid trematode infecting the gills of an endangered fish, the fountain darter, in two central Texas spring-fed rivers. J. Aquat. Anim. Health. 2000;12:283–289. [Google Scholar]
- Miyamoto K. Studies on zoonoses in Hokkaido 7. Survey of natural definitive hosts of Metagonimus yokogawai. Jpn. J. Parasitol. 1985;34:371–376. (in Japanese) [Google Scholar]
- Nachev N., Sures B. The endohelminth fauna of barbel (Barbus barbus) correlates with water quality of the Danube River in Bugaria. Parasitology. 2009;136:545–552. doi: 10.1017/S003118200900571X. [DOI] [PubMed] [Google Scholar]
- Nakano T., Inoue M. A human case of intestinal tumor caused by deposited eggs of Heterophyes heterophyes nocens. Gekkano Ryoiki. 1955;3:272–274. (in Japanese) [Google Scholar]
- Namchote S., Sritongtae S., Butnin S., Chai J.Y., Jung B.K., Sohn W.M., Garippa G., Merella P. Larval stage of trematodes obtained from brackish water snails in the central and east coast of the gulf of Thailand. Sci. Res. Essays. 2015;10:386–401. [Google Scholar]
- Niemi D.R., Macy R.W. The life cycle and infectivity to man of Apophallus donicus (Skrjabin and Lindtrop, 1919) (Trematoda: Heterophyidae) in Oregon. Proc. Helminthol. Soc. Wash. 1974;41:223–229. [Google Scholar]
- Nishigori M. Two new trematodes of the family Heterophyidae found in Formosa. Taiwan Igakkai Zasshi. 1924;(237):569–570. (in Japanese) [Google Scholar]
- Nishigori M. On a new trematode, Stamnosoma formosanum n. sp., and its life history. Taiwan Igakkai Zasshi. 1924;(234):181–228. (in Japanese) [Google Scholar]
- Nissen S., Nguyen L.A.T., Dalsgaard A., Thamsborg S.M., Johansen M.V. Experimental infection with the small intestinal trematode, Haplorchis pumilio, in young dogs. Vet. Parasitol. 2013;191:138–142. doi: 10.1016/j.vetpar.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Noda K. The larval development of Stellantchasmus falcatus (Trematoda: Heterophyidae) in the first intermediate host. J. Parasitol. 1959;45:635–642. [PubMed] [Google Scholar]
- Ochi S. Studies on the trematodes whose intermediate hosts are brackish water fishes-on the life cycle of Pygidiopsis summus. Tokyo Iji Shinshi. 1931;2712:346–353. (in Japanese) [Google Scholar]
- Ohnishi Y. Eosinophil responses in the mice infected with Metagonimus yokogawai. Jpn. J. Parasitol. 1987;36:271–275. [Google Scholar]
- Ohnishi Y., Taufen M. Increase of permeability in the intestinal mucosa of mice infected with Metagonimus yokogawai. Jpn. J. Vet. Sci. 1984;46:885–887. doi: 10.1292/jvms1939.46.885. [DOI] [PubMed] [Google Scholar]
- Okuzawa E., Hamada A., Fukushima S., Kino H., Sakurai1 Y. Prevalence of heterophyid infection among Japanese residents of Egypt (2005–2008) and its association with length of stay. Trop. Med. Health. 2010;38:143–146. [Google Scholar]
- Oliveira S.A., Blazquez F.J.H., Antunes S.A., Maria A.A.M. Ascocotyle (Phagicola) longa ransom, 1920 (Digenea: Heterophyidae) metacercariae, in Mugil platanus in estuarine of Cananéia, SP, Brazil. Ciencia Rural Santa Maria. 2007;37:1056–1059. (in Portuguese) [Google Scholar]
- Ondračkova M., Slovačkova I., Trichkova T., Polačik M., Jurajda P. Shoreline distribution and parasitic infection of black-striped pipefish Syngnathus abaster Risso, 1827 in the low River Danube. J. Appl. Ichthyol. 2012;28:590–596. [Google Scholar]
- Onji Y. Studies on the heterophyid metacercaria in Mugil cephalus (I) Tokyo Iji Shinshi. 1915;1918:875–883. (in Japanese) [Google Scholar]
- Onji Y., Nishio T. On the trematodes whose intermediate host is brackish water fishes. Chiba Igaku Semmon Gakko Zasshi. 1916;81&82:229–249. (in Japanese) [Google Scholar]
- Onsurathum S., Pinlaor P., Charoensuk L., Haonon O., Chaidee A., Intuyod K., Laummaunwai P., Boonmars T., Kaewkes W., Pinlaor S. Contamination of Opisthorchis viverrini and Haplorchis taichui metacercariae in fermented fish products in northeastern Thailand markets. Food Control. 2016;59:493–498. [Google Scholar]
- Ozaki Y., Asada J. A new human trematode, Heterophyes katsuradai n. sp. J. Parasitol. 1926;12:215–218. [Google Scholar]
- Paperna I., Overstreet R.M. Parasites and diseases of mullets (Mugilidae) In: Oren O.H., editor. Aquaculture of Grey Mullets. Cambridge University Press; Cambridge, UK: 1981. pp. 411–493. [Google Scholar]
- Park J.H., Kim J.L., Shin E.H., Guk S.M., Park Y.K., Chai J.Y. A new endemic focus of Heterophyes nocens and other heterophyid infections in a coastal area of Gangjin-gun, Jeollanam-do. Korean J. Parasitol. 2007;45:33–38. doi: 10.3347/kjp.2007.45.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.C. A revision of the subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae). I. The Haplorchis group. Parasitology. 1964;54:601–676. doi: 10.1017/s003118200008269x. [DOI] [PubMed] [Google Scholar]
- Pearson J.C. Proposed division of the genus Heterophyes Cobbold, 1865 (Trematoda: Heterophyidae) Syst. Parasitol. 1999;43:11–15. doi: 10.1023/a:1006160813129. [DOI] [PubMed] [Google Scholar]
- Pearson J.C. Family Heterophyidae Leiper, 1909. In: Bray, editor. Key to the Trematodes. Vol. 3. Natural History Museum; London, U.K.: 2008. pp. 113–141. [Google Scholar]
- Pearson J.C., Ow-Yang C.K. New species of Haplorchis from Southeast Asia, together with keys to the Haplorchis-group of heterophyid trematodes of the region. Southeast Asian J. Trop. Med. Public Health. 1982;13:35–60. [PubMed] [Google Scholar]
- Pica R., Castellano C., Cilia C., Errico F.P. Intestinal fluke infections: the heterophyids. Clin. Ter. 2003;154:61–63. [PubMed] [Google Scholar]
- Pinto H.A., de Melo A.L. Vol. 52. 2010. Melanoides tuberculata (Mollusca: Thiaridae) As an Intermediate Host of Centrocestus formosanus (Trematoda: Heterophyidae) in Brazil. Rev. Instit. Med. Trop. São Paulo; pp. 207–210. [DOI] [PubMed] [Google Scholar]
- Pinto H.A., Mati A.L.T., de Melo A.L. New records and a checklist of trematodes from Butorides striata (AvesL Ardeidae) Rev. Mexicana Biodiversidad. 2013;84:1100–1110. [Google Scholar]
- Pinto H.A., Mati V.L.T., de Melo A.L. Experimental centrocestiasis: worm burden, morphology and fecundity of Centrocestus formosanus (Trematoda: Heterophyidae) in dexamethasone immunosuppressed mice. Parasitol. Int. 2015;64:236–239. doi: 10.1016/j.parint.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Pornruseetairatn S., Kino H., Shimazu T., Nawa Y., Scholz T., Ruangsittichai J., Saralamba N.T., Thaenkham U. A molecular phylogeny of Asian species of the genus Metagonimus (Digenea)-small intestinal flukes-based on representative Japanese population. Parasitol. Res. 2016;115:1123–1130. doi: 10.1007/s00436-015-4843-y. [DOI] [PubMed] [Google Scholar]
- Premvati G., Pande V. On Centrocestus formosanus (Nishigori, 1924) Price, 1932 and its experimental infections in white leghorn chicks. Jpn. J. Parasitol. 1974;23:79–84. [Google Scholar]
- Price E.W. Proceed. US Nat. Mus. Vol. 79. 1931. New species of trematode of the family Heterophyidae, with a note on the genus Apophallus and related genera; pp. 1–6. [Google Scholar]
- Price E.W. A review of the trematode superfamily Opisthorchioidea. Proc. Helminthol. Soc. Wash. 1940;7:1–13. [Google Scholar]
- Pungpak S., Radomyos P., Radomyos B., Schelp F.P., Jongsuksuntigul P., Bunnag D. Treatment of Opisthorchis viverrini and intestinal fluke infections with praziquantel. Southeast Asian J. Trop. Med. Public Health. 1998;29:246–249. [PubMed] [Google Scholar]
- Pyo K.H., Kang E.Y., Hwang Y.S., Jun H.C., Sohn W.M., Cho S.H., Lee W.J., Chai J.Y., Shin E.H. Species identification of medically important trematodes in aquatic food samples using PCR-RFLP targeting 18S rRNA. Foodborne Pathog. Dis. 2013;10:1–3. doi: 10.1089/fpd.2012.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomyos P., Bunnag D., Harinasuta T. Haplorchis pumilio (Looss) infection in man in northeastern Thailand. Southeast Asian J. Trop. Med. Public Health. 1983;14:223–227. [PubMed] [Google Scholar]
- Radomyos P., Bunnag D., Harinasuta T. Worms recovered in stool following praziquantel treatment. Arzneim.-Forsch. 1984;34:1186–1188. [Google Scholar]
- Radomyos P., Charoenlarp P., Radomyos B., Tungtrongchitr A. Two human cases of Stellantchasmus falcatus (Trematoda: Heterophyidae) infection in northeastern Thailand. Jpn. J. Parasitol. 1990;39:7–11. [Google Scholar]
- Radomyos P., Wongsaroj T., Wilairatana P., Radomyos P., Praevanich R., Meesomboon V., Jongsuksuntikul P. Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J. Trop. Med. Public Health. 1998;29:123–127. [PubMed] [Google Scholar]
- Ramsuk A., Wongsawad C., Wongsawad P., Saenphet S. Treatment of Stellantchasmus falcatus in Gallus gallus domesticus by niclosamide and some anthelminthic plants. Southeast Asian J. Trop. Med. Public Health. 2004;35(Suppl. 1):296–298. [Google Scholar]
- Ransom B.H. Proc. US Nat. Mus. 57(2322) 1920. Synopsis of the trematode family Heterophyidae with descriptions of a new genus and five new species; pp. 527–573. [Google Scholar]
- Rao M.A.N., Ayyar L.S.P. Heterophyes species from dogs in Madras. Indian Vet. J. 1931;8:251–253. [Google Scholar]
- Rifaat M.M., Salem S.A.M., El Kholy S.I., Hegazi M.M., Yousef M. El-M. Studies on the incidence of Heterophyes heterophyes in Dakahlia governorate. J. Egypt. Soc. Parasitol. 1980;10:369–373. [Google Scholar]
- Rim H.J., Chu D.S., Lee J.S., Joo K.H., Won C.Y. Anthelmintic effects of various drugs against metagonimiasis. Korean J. Parasitol. 1978;16:117–122. doi: 10.3347/kjp.1978.16.2.117. (in Korean) [DOI] [PubMed] [Google Scholar]
- Rim H.J., Kim S.J., Yang M.G. Antigenic localities in the tissues of Metagonimus yokogawai in the period of growth. Korean J. Parasitol. 1992;30:309–321. doi: 10.3347/kjp.1992.30.4.309. (in Korean) [DOI] [PubMed] [Google Scholar]
- Rim H.J., Kim K.H., Joo K.H. Classification of host specificity of Metagonimus spp. from Korean freshwater fish. Korean J. Parasitol. 1996;34:7–14. doi: 10.3347/kjp.1996.34.1.7. [DOI] [PubMed] [Google Scholar]
- Rim H.J., Chai J.Y., Min D.Y., Cho S.Y., Eom K.S., Hong S.J., Sohn W.M., Yong T.S., Deodato G., Standgaard H., Phommasack B., Yun C.H., Hoang E.H. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol. Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- Rim H.J., Sohn W.M., Yong T.S., Eom K.S., Chai J.Y., Min D.Y., Lee S.H., Hoang E.H., Phommasack B., Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane municipality and Savannakhet Province, Lao PDR. Korean J. Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim H.J., Sohn W.M., Yong T.S., Eom K.S., Chai J.Y., Min D.Y., Lee S.H., Hoang E.H., Phommasack B., Insisiengmay S. Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. Korean J. Parasitol. 2013;51:107–114. doi: 10.3347/kjp.2013.51.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset J.J., Pasticier A. Two cases of heterophyid infection. Epidemiological and clinical aspects and importance of opsopathology. Ann. Parasitol. Hum. Comp. 1972;47:465–474. (in French) [PubMed] [Google Scholar]
- Ryang Y.S., Ahn Y.K., Yoon M.B. Trematode infections in the small intestine of Egretta alba medesta in Kangwon-do. Korean J. Parasitol. 1991;29:227–233. doi: 10.3347/kjp.1991.29.3.227. (in Korean) [DOI] [PubMed] [Google Scholar]
- Ryang Y.S., Lee C.Y., Lee K.J., Lee S.H., Chai J.Y. An incidental case of human Heterophyes nocens infection diagnosed by sectional morphology in a biopsy specimen of the small intestine. Korean J. Parasitol. 1999;37:189–194. doi: 10.3347/kjp.1999.37.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Saenphet S., Wongsawad C., Saenphet K., Chai J.Y. Susceptibility of rodents to Stellantchasmus falcatus infection. Southeast Asian J. Trop. Med. Public Health. 2003;34(Suppl. 2):123–127. [PubMed] [Google Scholar]
- Saenphet S., Wongsawad C., Saenphet K., Rojanapaibul A., Vanittanakom P., Chai J.Y. Chronological observations of intestinal histopathology in rats (Rattus novegicus) infected with Centrocestus caninus. Southeast Asian J. Trop. Med. Public Health. 2006;37(Suppl. 3):69–73. [PubMed] [Google Scholar]
- Saenphet S., Wongsawad C., Saenphet K., Chai J.Y. Mucosal mast cell responses in rats (Rattus novegicus) experimentally infected with Centrocestus caninus. Southeast Asian J. Trop. Med. Public Health. 2006;37:446–451. [PubMed] [Google Scholar]
- Saenphet S., Wongsawad C., Saenphet K., Rojanapaibul A., Vanittanakom P., Chai J.Y. Haplorchis taichui: worm recovery rate and immune responses in infected rats (Ratus norvegicus) Exp. Parasitol. 2008;120:175–179. doi: 10.1016/j.exppara.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Saito S. On the differences between Metagonimus yokogawai and Metagonimus takahashii. I. The morphological comparisons. Jpn. J. Parasitol. 1972;21:449–458. (in Japanese) [Google Scholar]
- Saito S. On the differences between Metagonimus yokogawai and Metagonimus takahashii. II. The experimental infections to the second intermediate hosts. Jpn. J. Parasitol. 1973;22:39–44. (in Japanese) [Google Scholar]
- Saito S. Report of Parasite Classification and Morphology Seminar (Japan) Vol. 2. 1984. On the synonymy of the trematodes of the genus Metagonimus; pp. 1–5. (in Japanese) [Google Scholar]
- Saito S., Shimizu T. A new trematode, Metagonimus otsurui sp. nov. from the fresh-water fishes (Trematoda: Heterophyidae) Jpn. J. Parasitol. 1968;17:167–174. [Google Scholar]
- Saito S., Chai J.Y., Kim K.H., Lee S.H., Rim H.J. Metagonimus miyatai sp. nov. (Digenea: Heterophyidae), a new intestinal trematode transmitted by freshwater fishes in Japan and Korea. Korean J. Parasitol. 1997;35:223–232. doi: 10.3347/kjp.1997.35.4.223. [DOI] [PubMed] [Google Scholar]
- Sato M., Sanguankiat S., Pubampen S., Kusolsuk T., Maipanich W., Waikagul J. Egg paying capacity of Haplorchis taichui (Digenea: Heterophyidae) in humans. Korean J. Parasitol. 2009;47:315–318. doi: 10.3347/kjp.2009.47.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Thaengkham U., Dekumyoy P., Waikagul J. Discrimination of O. viverrini, C. sinensis, H. pumilio and H. taichui using nuclear DNA-based PCR targeting ribosomal DNA ITS regions. Acta Trop. 2009;109:81–83. doi: 10.1016/j.actatropica.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Sato M., Pongvongsa T., Sanguankiat S., Yoonuan T., Dekumyoy P., Kalambaheti T., Keomoungkhoun M., Phimmayoi I., Boupha B., Moji K., Waikagul J. Copro-DNA diagnosis of Opisthorchis viverrini and Haplorchis taichui infection in an endemic area of Lao PDR. Southeast Asian J. Trop. Med. Public Health. 2010;41:28–35. [PubMed] [Google Scholar]
- Sato M., Pongvongsa T., Sanguankiat S., Yoonuan T., Kobayashi J., Boupha B., Nishimoto F., Moji K., Sato M.O., Waikagul J. Patterns of trematode infections of Opisthorchis viverrini (Opisthorchiidae) and Haplorchis taichui (Heterophyidae) in human populations from two villages in Savannakhet Province, Lao PDR. J. Helminthol. 2015;89:439–445. doi: 10.1017/S0022149X14000261. [DOI] [PubMed] [Google Scholar]
- Sayasone S., Vonghajack Y., Vanmany M., Rasphone O., Tesana S., Utzinger J., Akkhavong K., Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 2009;103:247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Schell S.C. University Press of Idaho; Moscow, Idaho, USA: 1985. Handbook of Trematodes of North America North of Mexico; pp. 1–263. [Google Scholar]
- Scholz T. Metacercariae of trematodes from fish in Vientina Province, Laos. Acta Soc. Zool. Bohemoslov. 1991;55:130–145. [Google Scholar]
- Scholz T. Taxonomic study of Ascocotyle (Phagicola) longa Ransom, 1920 (Digenea: Heterophyidae) and related taxa. Syst. Parasitol. 1999;43:147–158. doi: 10.1023/a:1006120500518. [DOI] [PubMed] [Google Scholar]
- Scholz T., Salgado-Maldonado G. The introduction and dispersal of Centrocestus formosanus (Nishigori, 1924) (Digenea: Heterophyidae) in Mexico: a review. Am. Midl. Nat. 1999;143:185–200. [Google Scholar]
- Scholz T., Ditrich O., Giboda M. Larval stages of medically important flukes (Trematoda) from Vientiane Province, Laos, Part I. Metacercariae. Ann. Parasitol. Hum. Comp. 1990;65:238–243. doi: 10.1051/parasite/199267375. [DOI] [PubMed] [Google Scholar]
- Schuster R.K., Thomas K., Sivakumar S., O'Donovan D. The parasite fauna of stray cats (Felis catus) in Dubai, United Arab Emirates. Parasitol. Res. 2009;105:125–134. doi: 10.1007/s00436-009-1372-6. [DOI] [PubMed] [Google Scholar]
- Seo B.S., Cho S.Y., Hong S.T., Hong S.J., Lee S.H. Studies on parasitic helminths of Korea. V. Survey on intestinal trematodes of house rats. Korean J. Parasitol. 1981;19:131–136. doi: 10.3347/kjp.1981.19.2.131. [DOI] [PubMed] [Google Scholar]
- Seo B.S., Hong S.T., Chai J.Y. Studies on intestinal trematodes in Korea. III. Natural human infections of Pygidiopsis summa and Heterophyes heterophyes nocens. Seoul J. Med. 1981;22:228–235. [Google Scholar]
- Seo B.S., Lee S.H., Chai J.Y., Hong S.J. Studies on intestinal trematodes in Korea. XIII. Two cases of natural human infection by Heterophyopsis continua and the status of metacercarial infection in brackish water fishes. Korean J. Parasitol. 1984;22:51–60. doi: 10.3347/kjp.1984.22.1.51. [DOI] [PubMed] [Google Scholar]
- Seo B.S., Lee S.H., Chai J.Y., Hong S.J. Studies on intestinal trematodes in Korea, XII. Two cases of human infection by Stellantchasmus falcatus. Korean J. Parasitol. 1984;22:43–50. doi: 10.3347/kjp.1984.22.1.43. [DOI] [PubMed] [Google Scholar]
- Seo B.S., Lee S.H., Chai J.Y., Lee S.H. Intensity of Metagonimus yokogawai infection among inhabitants in Tamjin River basin with reference to its egg laying capacity in human host. Seoul J. Med. 1985;26:207–212. [Google Scholar]
- Seo B.S., Cheong S.K., Chai J.Y., Lee S.H., Jung B. Histopathology of small intestine of rats and mice experimentally infected with Pygidiopsis summa. Seoul J. Med. 1986;27:125–134. [Google Scholar]
- Seo M., Shin D.H., Guk S.M., Oh C.S., Lee E.J., Shin M.H., Kim M.J., Lee S.D., Kim Y.S., Yi Y.S., Spigelman M., Chai J.Y. Gymnophalloides seoi eggs from the stool of a 17th century female mummy found in Hadong, Republic of Korea. J. Parasitol. 2008;94:467–472. doi: 10.1645/GE-1365.1. [DOI] [PubMed] [Google Scholar]
- Sheir Z.M., Aboul-Enein M.E. Demographic, clinical and therapeutic appraisal of heterophyiasis. J. Trop. Med. Hyg. 1970;73:148–152. [PubMed] [Google Scholar]
- Shimazu T. Metagonimus hakubaensis sp. n. (Digenea, heterophyidae) from Nagano, Japan: morphology and life cycle. Bull. Nat. Sci. Mus. Tokyo, Ser. A. 1999;25:87–99. [Google Scholar]
- Shimazu T. Life cycle and morphology of Metagonimus miyatai (Digenea: Heterophyidae) from Nagano, Japan. Parasitol. Int. 2002;51:271–280. doi: 10.1016/s1383-5769(02)00038-7. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Morphology of metacercariae and adults of Metagonimus katsuradai Izumi (Digenea, Heterophyidae) from Shiga, Japan. Bull. Nat. Sci. Mus. Tokyo, Ser. A. 2003;29:47–51. [Google Scholar]
- Shimazu T., Kino Metagonimus yokogawai (Trematoda: Heterophyidae): from discovery to designation of a neotype. Korean J. Parasitol. 2015;53:627–639. doi: 10.3347/kjp.2015.53.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.H., Oh C.S., Chung T., Yi Y.S., Chai J.Y., Seo M. Detection of parasite eggs from a moat encircling the royal palace of Silla, the ancient Korean Kingdom. J. Archaeol. Sci. 2009;36:2534–2539. [Google Scholar]
- Shin E.H., Park J.H., Guk S.M., Kim J.L., Chai J.Y. Intestinal helminth infections in feral cats and a raccoon dog on Aphaedo Island, Shinan-gun, with a special note on Gymnophalloides seoi infection in cats. Korean J. Parasitol. 2009;47:189–191. doi: 10.3347/kjp.2009.47.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.S., Oh D.S., Ahn K.S., Cho S.H., Lee W.J., Na B.K., Sohn W.M. Zoonotic intestinal trematodes in stray cats (Felis catus) from riverside areas of the Republic of Korea. Korean J. Parasitol. 2015;53:209–213. doi: 10.3347/kjp.2015.53.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumenko P.G., Tatonova Y.V., Besprozvannkh V.V. Metagonimus suifunensis sp. n. (Trematoda: Heterophyidae) from the Russian Southern Far East: morphology, life cycle, and molecular data. Parasitol. Int. 2017;66:982–991. doi: 10.1016/j.parint.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Simões S.B.E., Barbosa H.S., Santos C.P. The life cycle of Ascocotyle (Phagicola) longa (Digenea: Heterophyidae), a causative agent of fish-borne trematodes. Acta Trop. 2010;113:226–233. doi: 10.1016/j.actatropica.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Skov J., Kania P.W., Dalsgaard A., Jørgensen T.R., Buchmann K. Life cycle stages of heterophyid trematodes in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet. Parasitol. 2009;160:66–75. doi: 10.1016/j.vetpar.2008.10.088. [DOI] [PubMed] [Google Scholar]
- Sohn W.M. Fish-borne zoonotic trematode metacercariae in the Republic of Korea. Korean J. Parasitol. 2009;47:S103–S113. doi: 10.3347/kjp.2009.47.S.S103. (Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Chai J.Y. Infection status with helminthes in feral cats purchased from a market in Busan, Republic of Korea. Korean J. Parasitol. 2005;43:93–100. doi: 10.3347/kjp.2005.43.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Han G.G., Kho W.G., Chai J.Y., Lee S.H. Infection status with the metacercariae of heterophyid flukes in the brackish water fish from Haenam-gun, Chollanam-do. Korean J. Parasitol. 1994;32:163–169. doi: 10.3347/kjp.1994.32.3.163. (in Korean) [DOI] [PubMed] [Google Scholar]
- Sohn W.M., Chai J.Y., Lee S.H. Stictodora fuscatum (Heterophyidae) metacercariae encysted in gobies, Acanthogobius flavimanus. Korean J. Parasitol. 1994;32:143–148. doi: 10.3347/kjp.1994.32.3.143. [DOI] [PubMed] [Google Scholar]
- Sohn W.M., Han E.T., Chai J.Y. Acanthotrema felis n. sp. (Digenea: Heterophyidae) from the small intestine of stray cats in the Republic of Korea. J. Parasitol. 2003;89:154–158. doi: 10.1645/0022-3395(2003)089[0154:AFNSDH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sohn W.M., Jan E.T., Seo M., Chai J.Y. Identification of Acanthotrema felis (Digenea: Heterophyidae) metacercariae encysted in the brackish water fish Acanthogobius flavimanus. Korean J. Parasitol. 2003;41:101–105. doi: 10.3347/kjp.2003.41.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Kim J.A., Chai J.Y. Two species of goby, Boleophthalmus pectinirostris and Scartelaos sp., as the new second intermediate hosts of heterophyid fluke in Korea. Korean J. Parasitol. 2005;43:161–164. doi: 10.3347/kjp.2005.43.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Na B.K., Cho S.H. Echinostoma hortense and heterophyid metacercariae encysted in yellowfin goby, Acanthogobius flavimanus, from Shinan-gun and Muan-gun (Jellanam-do), Korea. Korean J. Parasitol. 2009;47:307–310. doi: 10.3347/kjp.2009.47.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Na B.K., Cho S.H., Lee W.J., Park M.Y., Lee S.W., Choi S.B., Huh B.N., Seok W.S. Pygidiopsis summa (Digenea: Heterophyiae): status of metacercarial infection in mullets from coastal areas in the Republic of Korea. Korean J. Parasitol. 2016;54:497–502. doi: 10.3347/kjp.2016.54.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Yong T.S., Eom K.S., Min D.Y., Lee D., Jung B.K., Banouvong V., Insisiengmay B., Phommasack B., Rim H.J., Chai J.Y. Prevalence of Haplorchis taichui among humans and fish in Luang Prabang Province, Lao PDR. Acta Trop. 2014;136:74–80. doi: 10.1016/j.actatropica.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Sripalwit P., Wongsawad C., Chontananarth T., Anuntalabhochai S., Wongsawad P., Chai J.Y. Development and phylogenetic characteristics of Stellantchasmus falcatus (Trematoda: Heterophyidae) from Thailand. Korean J. Parasitol. 2015;53:201–207. doi: 10.3347/kjp.2015.53.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawangwong T., Sithithaworn P., Tesana S. Metacercariae isolated from cyprinoid fishes in Khon Kaen district by digestion technic. Southeast Asian J. Trop. Med. Public Health. 1997;28:224–226. [PubMed] [Google Scholar]
- Stunkard H.W. The life history of Cryptocotyle lingua (Creplin), with notes on the physiology of the metacercariae. J. Morphol. 1930;50:143–191. [Google Scholar]
- Sukontason K., Methanitikorn R., Sukontason K., Piangjai S., Choochote W. Viability of metacercariae in northern Thai traditional foods. Southeast Asian J. Trop. Med. Public Health. 1998;29:714–716. [PubMed] [Google Scholar]
- Sukontason K., Piangjai S., Sukontason K., Chaithong U. Potassium permanganate staining for differentiation of the surface morphology of Opisthorchis viverrini, Haplorchis taichui, and Phaneropsolus bonnei eggs. Southeast Asian J. Trop. Med. Public Health. 1999;30:371–374. [PubMed] [Google Scholar]
- Sukontason K., Sukontason K.L., Boonsriwong N., Chaithong U., Piangjai S., Choochote W. Development of Haplorchis taichui (Trematoda: Heterophyidae) in Mus musculus mice. Southeast Asian J. Trop. Med. Public Health. 2001;32(Suppl. 2):43–47. [PubMed] [Google Scholar]
- Sukontason K., Unpunyo P., Sukontason K.L., Piangjai S. Evidence of Haplorchis taichui infection as a pathogenic parasite: three case reports. Scand. J. Infect. Dis. 2005;37:388–390. doi: 10.1080/00365540510034473. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Okayama Prefectural Report. Vol. 146. 1930. Yokogawa's Metagonimus. List of publications on special animals in Okayama prefecture. (in Japanese) [Google Scholar]
- Suzuki N., Kumazawa H., Araki T., Hara M., Morita H., Segawa T., Shiina T., Yamada S., Tsumoto S. Occurrence of Heterophyes heterophyes nocens Onji et Nishio, 1916 in man in Kochi Prefecture. Jpn. J. Parasitol. 1982;31:271–274. (in Japanese) [Google Scholar]
- Takahashi S. On the life-history of Metagonimus yokogawai, a new species of Metagonimus and Exorchis major. Okayama Igakkai zasshi. 1929;479:2687–2755. (in Japanese) [Google Scholar]
- Takahashi S. On the eggs of Stellantchasmus falcatus and Pygidiopsis summus found in human stools which are similar to those of the liver fluke. Okayama Igakkai zasshi. 1929;41:1502–1513. (in Japanese) [Google Scholar]
- Takahashi S. A contribution to the life-history of Stamnosoma armatum Tanabe. Okayama Igakkai zasshi. 1929;41:1759–1771. (in Japanese) [Google Scholar]
- Tanabe H. Studien uber die Trematoden mit Susswasser-fischen als Zwischenwirt. I. Stamnosoma armatum n. g., n. sp. Kyoto Igaku Zasshi. 1922;19:239–252. (in Japanese) [Google Scholar]
- Tandon V., Shylla J.A., Ghatani S., Athokpam V.D. Neglected tropical diseases: trematodiases-the Indian scenario. Proc. Natl. Acad. Sci., India, Sect. B. 2015;85:901–907. [Google Scholar]
- Tantachamrun T., Kliks M. Heterophyid infection in human ileum: report of three cases. Southeast Asian J. Trop. Med. Public Health. 1978;9:228–231. [PubMed] [Google Scholar]
- Taraschewski H. Vol. 1984. Universität Hohenheim; Germany: 1984. Die Trematoden der gattung Heterophyes Taxonomie, Biologie, Epidemiologie; pp. 1–169. (Ph.D. Dissertation) [Google Scholar]
- Taraschewski H. Investigations on the prevalence of Heterophyes species in twelve populations of the first intermediate host in Egypt and Sudan. J. Trop. Med. Hyg. 1985;88:265–271. [PubMed] [Google Scholar]
- Taraschewski H. Transmission experiments on the host specificity of Heterophyes species in 16 potential definitive hosts. Z. Parasitenkd. 1985;71:505–518. doi: 10.1007/BF00928353. [DOI] [PubMed] [Google Scholar]
- Taraschewski H. Experiments on habitat selection of Heterophyes species in different definitive hosts. J. Helminthol. 1987;61:33–42. doi: 10.1017/s0022149x00009688. [DOI] [PubMed] [Google Scholar]
- Taraschewski H., Nicolaidou A. Heterophyes in Greece: record of H. heterophyes, H. aequalis and H. dispar from the first intermediate host, Pirenella conica. J. Helminthol. 1987;61:28–32. doi: 10.1017/s0022149x00009676. [DOI] [PubMed] [Google Scholar]
- Tawfik M.A.A., Elnawawi F.A., Shaapan R.M. Studies on some fish-borne trematodes in Egypt. Egypt. J. Vet. Sci. 2000;34:39–48. [Google Scholar]
- Thaenkham U., Visetsuk K., Dung D.T., Waikagul J. Discrimination of Opisthorchis viverrini and Haplorchis taichui using COI sequence marker. Acta Trop. 2007;103:26–32. doi: 10.1016/j.actatropica.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Thaenkham U., Dekumyoy P., Komalamisra C., Sato M., Dung do T., Waikagul J. Systematics of the subfamily Haplorchiinae (Trematoda: Heterophyidae), based on nuclear ribosomal DNA genes and ITS2 region. Parasitol. Int. 2010;59:460–465. doi: 10.1016/j.parint.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Thaenkham U., Blair D., Nawa Y., Waikagul J. Families Opisthorchiidae and Heterophyidae: are they distinct? Parasitol. Int. 2012;61:90–93. doi: 10.1016/j.parint.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Thaenkham U., Phuphisut O., Nuamtanong S., Yoonuan T., Sa-Nguankiat S., Vonghachack Y., Belizario V.Y., Dung D.T., Dekumyoy P., Waikagul J. Genetic differences among Haplorchis taichui populations in Indochina revealed by mitochondrial COX1 sequences. J. Helminthol. 2016;14:1–8. doi: 10.1017/S0022149X1600050X. [DOI] [PubMed] [Google Scholar]
- Thanh B.N., Dalsgaard A., Evenson Ǿ., Murrell K.D. Survey for fishborne zoonotic metacercariae in farmed grouper in Vietnam. Foodborne Pathog. Dis. 2009;6:1037–1039. doi: 10.1089/fpd.2008.0230. [DOI] [PubMed] [Google Scholar]
- Toledo R., Munoz-Antoli C., Esteban J.G. Intestinal trematode infections. In: Toledo R., Fried B., editors. Digenetic Trematodes. Vol. 766. Springer; New York, USA: 2014. pp. 201–240. (Adv. Exp. Med. Biol). [Google Scholar]
- Tubangui M.A., Africa C.M. The systematic position of some trematodes-reported from the Philippines. Philipp. J. Sci. 1938;67:117–125. [Google Scholar]
- Umadevi K., Madhavi R. Observations on the morphology and life-cycle of Procerovum varium (Onji & Nishio, 1916) (Trematoda: Heterophyidae) Syst. Parasitol. 2000;46:215–225. doi: 10.1023/a:1006398205390. [DOI] [PubMed] [Google Scholar]
- Umadevi K., Madhavi R. The life cycle of Haplorchis pumilio (Trematoda: Heterophyidae) from the Indian region. J. Helminthol. 2006;80:327–332. doi: 10.1017/joh2006359. [DOI] [PubMed] [Google Scholar]
- Uppal B., Wadhwa V. Rare case of Metagonimus yokogawai. Indian J. Med. Microbiol. 2005;23:61–62. doi: 10.4103/0255-0857.13878. [DOI] [PubMed] [Google Scholar]
- Urabe M. Trematode fauna of prosobranch snails of the genus Semisulcospira in Lake Biwa and the connected drainage system. Parasitol. Int. 2003;52:21–34. doi: 10.1016/s1383-5769(02)00083-1. [DOI] [PubMed] [Google Scholar]
- Velasquez C.C. Molluscan stages of Haplorchis taichui (Nishigori) in Melania juncea Lea in the Philippines. J. Parasitol. 1973;59:281. [PubMed] [Google Scholar]
- Velasquez C.C. Observations on some Heterophyidae (Trematoda: Digenea) encysted in Philippine fishes. J. Parasitol. 1973;59:77–84. [PubMed] [Google Scholar]
- Velasquez C.C. Life cycle of Procerovum calderoni (Africa and garcia, 1935), Price, 1940 (Trematoda: Digenea: Heterophyidae) J. Parasitol. 1973;59:813–816. [Google Scholar]
- Velasquez C.C. Heterophyidiasis. In: Steele J.H., editor. CRC Handbook Series in Zoonoses, Section C: Parasitic Zoonoses. vol. III. CRC Press; Boca Raton, Florida, USA: 1982. pp. 99–107. (Trematode Zoonoses) [Google Scholar]
- Velásquez L.E., Bedoya J.C., Areiza A., Vález I. First record of Centrocestus formosanus (Digenea: Heterophyidae) in Colombia. Rev. Mexicana Biodiversidad. 2006;77:119–121. [Google Scholar]
- Vo D.T., Murrell D., Dalsgaard A., Bristow G., Nguyen D.H., Bui T.N., Vo D.T. Prevalence of zoonotic metacercariae in two species of groupers, Epinephelus coioides and Epinephelus bleekeri, and flathead mullet, Mugil cephalus, in Vietnam. Korean J. Parasitol. 2008;46:77–82. doi: 10.3347/kjp.2008.46.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo D.T., Bristow G.A., Vo D.T., Nguyen D.H., Nguyen T.N.N., Tran T.C. Digenean trematodes of cultured grouper (Epinephelus coioides and E. bleekeri) in Khanh Hoa Province, Vietnam. Dis. Asian Aquacult. 2011;VII:39–52. [Google Scholar]
- Waikagul J., Pearson J.C. Heterophyes nocens Onji and Nishio, 1916 (Digenea: Heterophyidae) from the water rat, Hydromys chrysogaster Geoffroy, 1804 in Australia. Syst. Parasitol. 1989;13:53–61. [Google Scholar]
- Waikagul J., Wongsaroj T., Radomyos P., Meesomboon V., Praewanich R., Jongsuksuntikul P. Human infection of Centrocestus caninus in Thailand. Southeast Asian J. Trop. Med. Public Health. 1997;28:831–835. [PubMed] [Google Scholar]
- Wang J.J., Chung L.Y., Lee J.D., Chang E.E., Chen E.R., Chao D., Yen C.M. Haplorchis infections in intermediate hosts from a clonorchiasis endemic area in Meinung, Taiwan, Republic of China. J. Helminthol. 2002;76:185–188. doi: 10.1079/JOH2002114. [DOI] [PubMed] [Google Scholar]
- Watthanakulpanich D., Waikagul J., Maipanich W., Nuamtanong S., Sanguankiat S., Pubampen S., Praevanit R., Mongkhonmu S., Nawa Y. Haplorchis taichui as a possible etiologic agent of irritable bowel syndrome-like symptoms. Korean J. Parasitol. 2010;48:225–229. doi: 10.3347/kjp.2010.48.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijit A., Morakote N., Klinchid J. High prevalence of haplorchiasis in Nan and Lampang Provinces, Thailand, proven by adult worm recovery from suspected opisthorchiasis cases. Korean J. Parasitol. 2013;51:767–769. doi: 10.3347/kjp.2013.51.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witenberg G. Studies on the trematode – family Heterophyidae. Ann. Trop. Med. Parasitol. 1929;23:131–268. [Google Scholar]
- Wongsawad C., Wongsawad P. Molecular markers for identification of Stellantchasmus falcatus and a phylogenetic study using the HAT-RAPD method. Korean J. Parasitol. 2010;48:303–307. doi: 10.3347/kjp.2010.48.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsawad C., Wongsawad P. Opisthorchis viverrini and Haplorchis taichui: development of a multiplex PCR assay for their detection and differentiation using specific primers derived from HAT-RAPD. Exp. Parasitol. 2012;132:237–242. doi: 10.1016/j.exppara.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Wongsawad C., Rojanapaibul A., Vanittanakom P. Surface ultrastructure of encysted metacercariae and of adult Stellantchasmus sp. (Trematoda: Heterophyidae) Southeast Asian J. Trop. Med. Public Health. 1997;11:19–26. [PubMed] [Google Scholar]
- Wongsawad C., Chariyahpongpun P., Namue C. Experimental host of Stellantchasmus falcatus. Southeast Asian J. Trop. Med. Public Health. 1998;29:406–409. [PubMed] [Google Scholar]
- Wongsawad C., Rojtinnakorn J., Wongsawad P., Rojanapaibul A., Marayong T., Suwattanacoupt S., Sirikanchana P., Sey O., Jadhav B.V. Helminths of vertebrates from Mae Sa stream, Chiang Mai, Thailand. Southeast Asian J. Trop. Med. Public Health. 2004;35(Suppl. 1):140–146. [PubMed] [Google Scholar]
- Wongsawad C., Wonsawad P., Chai J.Y., Anuntalabhochaia S. Haplorchis taichui, Witenberg, 1930: development of a HAT-RAPD marker for the detection of minute intestinal fluke infection. Exp. Parasitol. 2009;123:158–161. doi: 10.1016/j.exppara.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Wongsawad C., Phalee A., Noikong W., Chuboon S., Nithikathkul C. Co-infection with Opisthorchis viverrini and Haplorchis taichui detected by human fecal examination in Chomtong district, Chiang Mai Province, Thailand. Parasitol. Int. 2012;61:56–59. doi: 10.1016/j.parint.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Yamada S.M., Yamada S., Takada H., Hoshiai Y.C., Yamada S. A case of metagonimiasis complicated with multiple intracerebral hemorrhages and diabetes mellitus. J. Nippon Med. Sch. 2008;75:32–35. doi: 10.1272/jnms.75.32. [DOI] [PubMed] [Google Scholar]
- Yamaguti S. Studies on the helminth fauna of Japan. Part 25. Trematodes of birds, IV. Jpn. J. Zool. 1939;8:131–210. [Google Scholar]
- Yamaguti S. The Digenetic Trematodes of Vertebrates (Part I) vol. I. Interscience Publishers Inc.; New York, USA: 1958. Systema helminthum; pp. 1–979. [Google Scholar]
- Yamaguti S. vol. I. Keigaku Publishing Co.; Tokyo, Japan: 1971. Synopsis of Digenetic Trematodes of Vertebrates; pp. 1–1074. [Google Scholar]
- Yamaguti S. Keigaku Publishing Co.; Tokyo, Japan: 1975. Synoptical Review of Life Histories of Digenetic Trematodes of Vertebrates; pp. 1–590. [Google Scholar]
- Yang H.J., Guk S.M., Han E.T., Chai J.Y. Molecular differentiation of three species of Metagonimus by simple sequence repeat anchored polymerase chain reaction (SSR-PCR) amplification. J. Parasitol. 2000;86:1170–1172. doi: 10.1645/0022-3395(2000)086[1170:MDOTSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yokogawa S. Reports on visceral parasites on Taiwan, II. Taiwan Igakkai Zasshi. 1912;114:366–370. (in Japanese) [Google Scholar]
- Yokogawa S. On a new trematode which takes Plecoglossus altivelis as second intermediate host-Metagonimus. Tokyo Igakkai Zashii. 1913;27:685–709. (in Japanese) [Google Scholar]
- Yokogawa S. On a new species of the genus Metagonimus, Metagonimus ovatus n. sp. Tokyo Igakkai Zasshi. 1913;27:1705–1709. (in Japanese) [Google Scholar]
- Yokogawa S. On the visceral heterophyidiasis, especially on the etiology of heart, brain and spinal cord heterophyidiasis. Taiwan Igakkai Zasshi. 1940;39:1729–1730. (in Japanese) [Google Scholar]
- Yokogawa M., Sano M., Itabashi T., Kachi S. Studies on the intestinal flukes. II. Epidemiological studies on heterophyid trematodes of man in Chiba Prefecture. Jpn. J. Parasitol. 1965;14:577–585. [Google Scholar]
- Yoshikawa M., Miyata I., Uesugi S. Contributions to the knowledge of dog parasites in Kobe City. Nippon Juigakkai Zasshi. 1940;2:450–464. (in Japanese) [Google Scholar]
- Youssef A.I., Uga S. Review of parasitic zoonoses in Egypt. Trop. Med. Health. 2014;42:3–14. doi: 10.2149/tmh.2013-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef M.M., Mansour N.S., Awadalla H.N., Hammouda N.A., Khalifa R., Boulos L.M. Heterophyid parasites of man from Idki, Maryut and Manzala Lakes areas in Egypt. J. Egypt. Soc. Parasitol. 1987;17:475–479. [PubMed] [Google Scholar]
- Youssef M.M., Mansour N.S., Hammouda N.A., Awadalla H.N., Khalifa R., Boulos L.M. Studies on some developmental stages in the life cycle of Pygidiopsis genata Looss, 1907 (Trematoda: Heterophyidae) from Egypt. J. Egypt. Soc. Parasitol. 1987;17:463–474. [PubMed] [Google Scholar]
- Yu J.R., Chai J.Y. Metagonimus. In: Liu D., editor. Molecular Detection of Foodborne Pathogens. CRC Press (Taylor & Francis Group); Boca Raton, Florida, USA: 2010. pp. 805–811. (Chapter 59) [Google Scholar]
- Yu J.R., Chai J.Y. Metagonimus. In: Liu D., editor. Molecular Detection of Human Parasitic Pathogens. CRC Press (Taylor & Francis Group); Boca Raton, Florida, USA: 2013. pp. 389–398. (Chapter 36) [Google Scholar]
- Yu S.H., Mott K.E. Epidemiology and morbidity of food-borne intestinal trematode infections. Trop. Dis. Bull. 1994;91:R125–R152. [Google Scholar]
- Yu J.R., Chung J.S., Huh S., Lee S.H., Chai J.Y. PCR-RFLP pattern of three kinds of Metagonimus in Korea. Korean J. Parasitol. 1997;35:271–276. doi: 10.3347/kjp.1997.35.4.271. [DOI] [PubMed] [Google Scholar]
- Yu J.R., Chung J.S., Chai J.Y. Different RAPD patterns between Metagonimus yokogawai and Metagonimus Miyata type. Korean J. Parasitol. 1997;35:295–298. doi: 10.3347/kjp.1997.35.4.295. [DOI] [PubMed] [Google Scholar]
- Yu J.R., Myong N., Chai J.Y. Expression patterns of proliferating cell nuclear antigen in the small intestine of mice infected with Metagonimus yokogawai and Metagonimus Miyata type. Korean J. Parasitol. 1997;35:239–244. doi: 10.3347/kjp.1997.35.4.239. [DOI] [PubMed] [Google Scholar]
- Zhang S.L., Fan G.H. Case report: brain abscess caused by heterophyid eggs. Chin. J. Parasitol. Parasit. Dis. 1990;8:178. (in Chinese) [Google Scholar]