Abstract
Many methods have been used to detect heavy metals in herbal medicines, while few are developed to remove them. In this study, a novel genetically engineered fusion protein composed of metallothionein (MT), cellulose binding module (CBM), and superfolder GFP (sfGFP) was designed to remove heavy metals. MT, a kind of cysteine-rich protein, was used to chelate heavy metals with high specific affinity. The CBM facilitated the fusion protein MT-CBM-sfGFP binding to cellulose specifically, which made the purification and immobilization in one step. The sfGFP was used to detect the fusion protein MT-CBM-sfGFP easily during the process of expression and immobilization. The MT from Cancer pagurus (MTCap) and the CBM from Cellulomonas fimi (CBMCef) were used as an example and the fusion protein (MTCap-CBMCef-sfGFP) was expressed in Escherichia coli. Then, the cell lysates were mechanically mixed with cellulose to create biosorbent MTCap-CBMCef-sfGFP@cellulose. The efficiency of the biosorbent MTCap-CBMCef-sfGFP@cellulose for Pb2+ removal was evaluated using the water decoction of Honeysuckle as a model. Results suggested that MTCap-CBMCef-sfGFP@cellulose had high efficiency for Pb2+ removal from the water decoction of Honeysuckle without affecting its active ingredients. The low-cost, easy production, and high efficiency of the biosorbent enable it to have many applications in heavy metal removal from aqueous solutions of herbal medicines and food.
Introduction
Traditional Chinese Medicine is widely used for treatment and health care throughout the world. However, it has been reported in many countries that some poisoning cases were associated with the presence of toxic heavy metals in medicinal plants.1−4 The main source of heavy metal contamination is always from the polluted air, water, and soil.5 Heavy metals may cause renal failure, symptoms of chronic toxicity, liver damage, and other health hazards.6−10 As a serious threat to human health, Chinese herb medicine with heavy metal contamination would lead to an enormous waste of resources and impaired economic benefits.
During past decades, much effort has been devoted to sensing,11−13 targeting,14−16 and removing17−19 heavy metals. Metallothioneins (MTs) are paid much attention in heavy metal removal attributing to their cysteine-rich molecular structures and thus the strong metal-binding affinity.20−24 Heterologous expression of MTs with a high yield in bacterial cells is regarded as a promising method of biosorption against heavy metals.15,25−27 Free bacteria involving cell surface display techniques are always applied to improve the adsorption efficiency of MTs against heavy metals. Nevertheless, the biggest shortcoming of using free bacteria to treat heavy metal contamination is the difficulty to separate them from the environment.28 Moreover, free bacteria immobilized to the matrix via whole cell immobilization technique can simplify the separation process.29 In addition, MTs being conjugated to bacterial cellulose through covalent interactions was also developed to enhance the metal-binding capacity of purified MT.14 Unfortunately, most active studies have been mainly focused on targeting and removal of heavy metals in waste water but not in polluted Chinese herb medicine. The main reason is that bacteria are restricted for use in drugs.
Cellulose binding modules (CBMs) are a class of protein modules which present a specific cellulose-binding activity.30,31 A reversible or irreversible attachment to the cellulose can be achieved with CBM from different families. For example, family I and IV CBMs bind reversibly to the cellulose while family II and III CBMs usually are irreversibly bound. These CBMs binding irreversibly to the cellulose can be used for applications involving immobilization.32 Moreover, cellulose is safe for use in food or pharmaceutical applications, which is an inexpensive, chemically inert material.
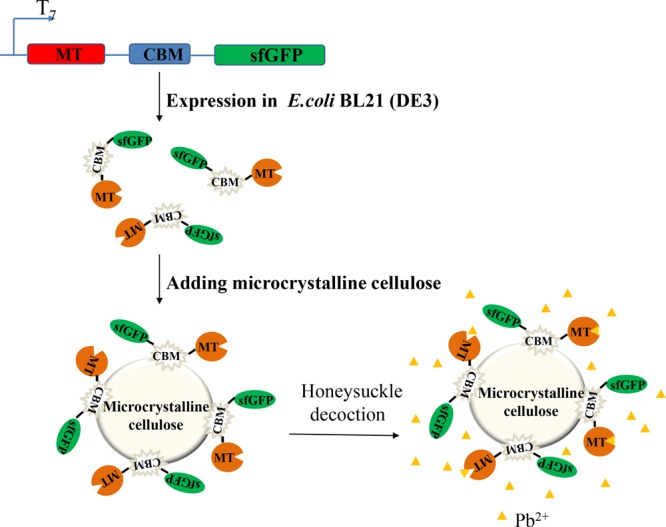
In the present study, the MT from cancer pagurus (MTCap) was expressed as a fusion protein with the CBM from Cellulomonas fimi (CBMCef) and superfolder GFP (sfGFP). The CBMCef in the fusion protein can bind to cellulose specifically and make the fusion protein be purified and immobilized in one-step. sfGFP in the fusion protein not only can improve the expression of MTCap but also make the process of expression and immobilization easier to check by taking advantage of its visible fluorescence under daylight. By mechanically immobilizing MTCap-CBMCef-sfGFP with cellulose, a highly efficient biosorbent was created. The efficiency of Pb2+ due to the biosorbent was evaluated using the water decoction of Honeysuckle as a model (Scheme 1).
Scheme 1. Schematic Image of the Expression of MTCap-CBMCef-sfGFP Fusion Protein; the Immobilization of the Fusion Protein to Microcrystalline Cellulose (MTCap-CBMCef-sfGFP@cellulose); the Removal of Pb2+ in Honeysuckle Water Decoction with MTCap-CBMCef-sfGFP@Cellulose.
Results and Discussion
Fusion Protein MTCap-CBMCef-sfGFP Expression and Binding to Cellulose
MTs, a class of low molecular mass and cysteine-rich proteins, are widely distributed in, particularly, the eukaryote.33 The MT from C. pagurus was chosen in this study because of its high affinity to Cd, Hg, and Cu.34,35 The low molecular mass of MTs makes the purification a hard process.36 Thus the fusion of MT with other protein tags such as GST or MBP simplifies the purification.37,38 In addition, CBMs that bind irreversibly to the cellulose are widely used for enzyme purification and immobilization. In this study, a family 2 CBM from the endoglucanase A of C. fimi was chosen to bind crystalline cellulose tightly which was widely used for enzyme immobilization.39,40 Meanwhile, sfGFP was used to make the process of expression and immobilization be easily checked attributing to its green fluorescence under daylight. The modules of MTCap, CBMCef, and sfGFP were connected by two flexible linkers to make their functions not be influenced by each other (Figure 1A). The gene that encodes the fusion protein MTCap-CBMCef-sfGFP was cloned into the expression vector pET28a and then transformed into the Escherichia coli BL21 (DE3). After being induced by isopropyl-β-d-thiogalactoside (IPTG) for 12 h, the cell turned green when compared to that of uninduced (Figure 1B), which suggested that the fusion protein has been expressed successfully. In other words, it is very convenient to check whether the recombinant fusion protein is expressed based on the green fluorescence. Then, the cells were collected and lysed with sonication. The microcrystalline cellulose mixed with the cell lysated turned green while those as the control became white (Figure 1C). These results suggested that the fusion protein MTCap-CBMCef-sfGFP binds to the microcrystalline quickly and tightly by using the CBMCef.
Figure 1.
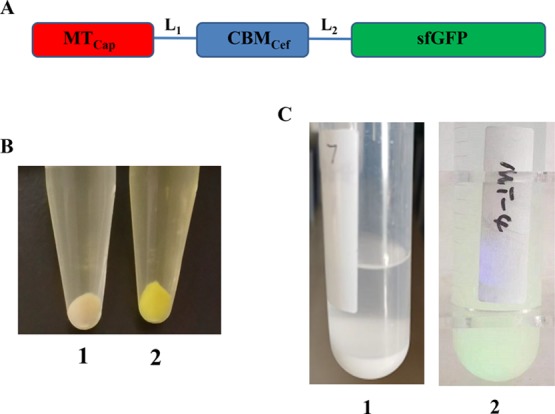
MTCap-CBMCef-sfGFP expression in E. coli and immobilized to microcrystalline cellulose. (A) Schematic representation of the fusion protein. MTCap, MT from C. pagurus (GenBank accession no. AM743087.1); CBMCef, family 2 CBM from the endoglucanase A of C. fimi (GenBank accession no. M15823.1); sfGFP, superfolder GFP. Two linkers L1 (GSGGAGGS) and L2 (GGGSPTGG) were used to connect the three modules. (B) Cell precipitate of an uninduced transformant harboring pET-MTCap-CBMCef-sfGFP (1) and IPTG-induced transformant harboring pET-MTCap-CBMCef-sfGFP (2) “Photograph courtesy of Guozeng Wang, Copyright 2019”. (C) Microcrystalline cellulose (1) and MTCap-CBMCef-sfGFP@cellulose complexes (2) “Photograph courtesy of Qing Xiao, Copyright 2019”.
Adsorption Capacity of MTCap-CBMCef-sfGFP@Cellulose against Pb2+
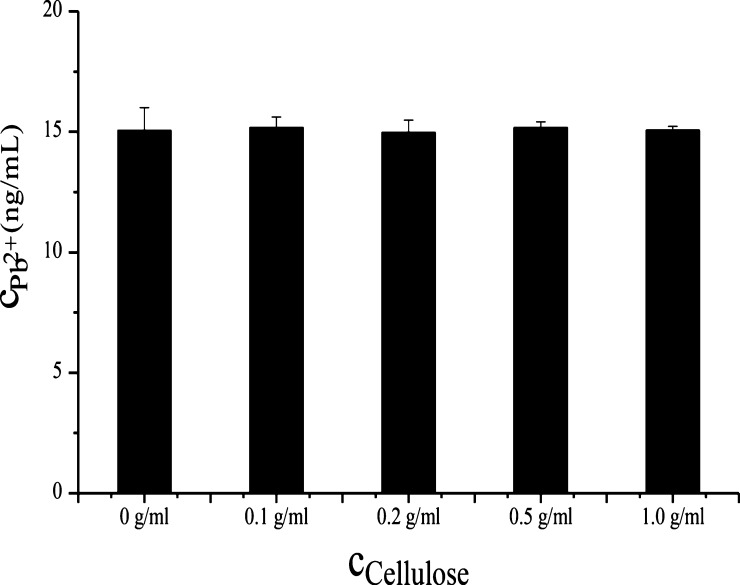
Water insoluble microcrystalline cellulose not only contributes to fast purification of MTCap-CBMCef-sfGFP but also easy separation of MTCap-CBMCef-sfGFP@cellulose from the liquid environment. It has been reported that natural and modified microcrystalline cellulose show the ability of adsorption against heavy metal ions.41,42 Thus, the adsorption capacity of the microcrystalline cellulose used in this study against heavy metals was evaluated. Almost all the Cd2+ (16.3 ng/mL) and Hg2+ (18.0 ng/mL) were adsorbed by 0.05 g/mL of cellulose. However, the adsorption of Pb2+ by cellulose was not obvious even at a concentration of 1 g/mL (Figure 2). It indicated that Pb2+ was barely adsorbed by the microcrystalline cellulose.
Figure 2.
Effect of cellulose with different concentrations on lead ions.
Because of strong affinity of microcrystalline cellulose against Cd2+ and Hg2+ but not Pb2+, the adsorption affinity of MTCap-CBMCef-sfGFP@cellulose against Pb2+ was further investigated. A sharp decrease in absorbance was observed obviously. As the concentration of lead ions was determined against the linearity curve plotted against the standard, it was found that the concentration of free lead ions fell from 15.05 to 0.39 ng/mL which was shown in Figure 3 (black part). It meant that 97.4% lead ions were chelated by MTCap-CBMCef-sfGFP@cellulose. The result revealed that MTCap-CBMCef-sfGFP@cellulose was the highly efficient biosorbent for Pb2+ removal.
Figure 3.
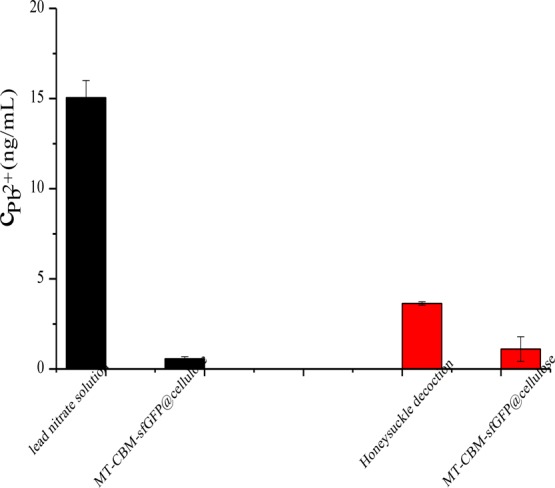
Chelating ability of MTCap-CBMCef-sfGFP@cellulose against lead ions in Pb(NO3)2 solution (black) and Honeysuckle water decoction (red).
Numerous reports had shown that MTs were the well-characterized proteins for heavy metal binding. However, there is little application of MT involved in the removal of heavy metals in Chinese herb medicine. Guo et al. studied and analyzed the papers about heavy metals in Chinese medical materials published in 2000–2016.5 They found that Honeysuckle was the only one in which its contents of arsenic, cadmium, lead, and mercury exceed the limits, especially for lead. In addition, decoction with water is the most widely used form of Chinese herb medicine. In the boiling process, most of active ingredients were extracted into the water solution. In addition, protein denaturation would lead to some of heavy metals being dissociated into the water solution too. That is to say, it is necessary to investigate the binding ability of MTCap-CBMCef-sfGFP@cellulose against Pb2+ in Honeysuckle water decoction. The water decoction obtained was treated with a 0.5 g/mL MTCap-CBMCef-sfGFP@cellulose suspension and subjected into atomic absorption spectrometer (AAS). A decrease of the concentration of Pb2+ from 3.637 to 1.111 ng/mL is seen in Figure 3 (red part), which meant that 69.5% lead in Honeysuckle water decoction was chelated by MTCap-CBMCef-sfGFP@cellulose. The efficiency of lead removal in Honeysuckle decoction was lower when compared with the lead nitrate solution. The reason for it might be that lead in the Honeysuckle water decoction determined by AAS was the total concentration of lead. However, divalent lead ions would be adsorbed by MTCap in MTCap-CBMCef-sfGFP@cellulose. That is to say, some nonfree lead was still left in the supernatant after being treated with MTCap-CBMCef-sfGFP@cellulose.
Effect of MTCap-CBMCef-sfGFP@Cellulose on Active Ingredients in Honeysuckle Water Decoction
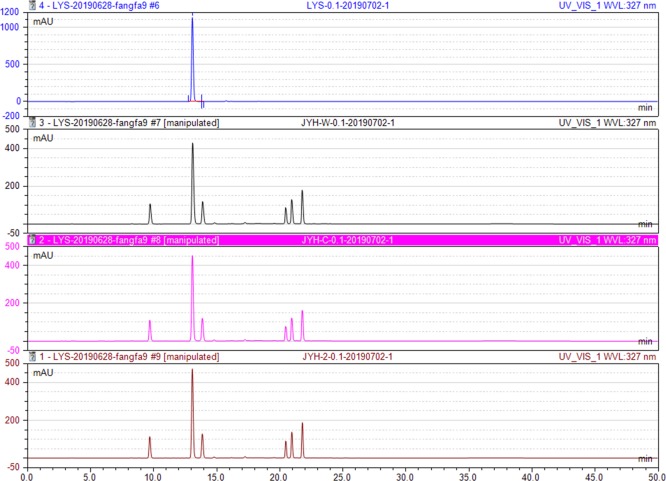
Honeysuckle, the dry flower bud or early blossoms of Lonicera japonica Thunb, possessing the effects of antisepsis and anti-inflammation, clearing heat antitoxicant, is the raw material of medicine, health products, cosmetics, and food. It is stipulated in Chinese Pharmacopoeia that chlorogenic acid is one of the most important quality indicators of Honeysuckle. Thus, the effect of MTCap-CBMCef-sfGFP@cellulose on the chlorogenic acid in Honeysuckle water decoction was investigated by HPLC. As shown in Figure 4 , the retention time of chlorogenic acid in the reference solution was 13.05 ± 0.05 min (blue). Moreover, the ones in the water decoctions after treatment with water (black), cellulose (red), and MTCap-CBMCef-sfGFP@cellulose (brown) were 13.09, 13.07, and 13.06 min separately. Besides, the peak areas in three water decoctions were 72.46, 72.17, and 72.14 mAu·min, respectively. Moreover, the retention time and peak area for other peaks were almost consistent. These results illustrated that MTCap-CBMCef-sfGFP@cellulose had no effect on the chlorogenic acid and other ingredients in the Honeysuckle water decoction. In addition to Honeysuckle, there are many Chinese herb medicine which also might be contaminated by heavy metals, such as Radix Pseudostellariae, Glycyrrhiza Uralensis, Astragalus Membranaceus, and so on. This method based on MTCap-CBMCef-sfGFP@cellulose was also used for the Pb2+ removal from their water decoctions.
Figure 4.
HPLC chromatograms of four solutions: (blue) standard solution of chlorogenic acid; (black) Honeysuckle water decoction treated with water; (red) Honeysuckle water decoction treated with cellulose suspension; (brown) Honeysuckle water decoction treated with MTCap-CBMCef-sfGFP@cellulose suspension.
Conclusions
In this study, a novel biosorbent MTCap-CBMCef-sfGFP@cellulose for heavy metal removal was designed and prepared. The advantages of CBMCef and sfGFP were combined to make the production of MTCap-CBMCef-sfGFP@cellulose visual, quick, and cheap. CBMCef facilitated the fusion protein bind to cellulose rapidly and specificity, which makes the MTCap purification and immobilization in one-step. The sfGFP, whose fluorescence can be seen under daylight, made it easier to check whether MTCap is expressed in the host and bonded to the cellulose. By mechanically mixing MTCap-CBMCef-sfGFP with cellulose, we created a highly efficient biosorbent for Pb2+ removal from the water decoction of Honeysuckle. In addition, the MTCap could be replaced with other sources to form new fusion proteins with high affinity to other heavy metal ions. The biosorbent can be used as an effective, renewable, and environmentally friendly method for heavy metal removal from decoctions of Traditional Chinese Medicine, Liquid Drugs, and Food.
Materials and Methods
Strains, Vectors, and Chemicals
Vector pET-28a (+) and E. coli BL21 (DE3) used for fusion gene expression was obtained from Novagen (San Diego, CA, USA). Kits for DNA purification and plasmid isolation were purchased from Omega (Norcross, GA, USA). Restriction endonucleases, T4 DNA ligase, DNA polymerase, and dNTPs were purchased from Thermo Fisher Scientific (Ipswich, MA, USA). IPTG and microcrystalline cellulose (Avicel; 50 μm) were purchased from Sigma (St. Louis, MO, USA). The standard substance of chlorogenic acid was purchased from National Institutes for Food and Drug Control (Beijing, China). The acetonitrile for HPLC was obtained from Merck (Darmstadt, Germany). Pb(NO3)2 and HgCl2 of analytical pure grade were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The single element standard solution of lead or mercury (1000 ppm in 1.0 M HNO3) was purchased from Guobiao Testing & Certification Co., Ltd (Beijing, China). All other chemicals were of analytical grade and commercially available.
Plasmid Construction and Fusion Protein Expression
The nucleotide sequence of family 2 CBM was obtained from the endoglucanase A of C. fimi (GenBank accession no. M15823.1). The nucleotide sequence of MT was from C. pagurus (GenBank accession no. AM743087.1). The nucleotide sequence of sfGFP was obtained from the plasmid pG1AK-sfGFP. The schematic representation of the fusion protein is illustrated in Figure 1A. The domains of MTCap, CBMCef, and sfGFP were connected by the linker of GSGGAGGS and GGGSPTGG to form the fusion protein MTCap-CBMCef-sfGFP. The nucleotide sequence of the fusion protein (see Supporting Information) was synthesized by Genscript (Nanjing, China). The full length gene of MTCap-CBMCef-sfGFP was amplified by PCR using the expression primers P-F: 5′-CTACCATGGATGCCGGACCCGTGCTGCAAG-3′ and P-R: 5′-CTCGAATTCTCATTTGTACAGTTCATCCATACCATGCGTG-3′. The PCR product was digested with restriction endonucleases NcoIand EcoRIand cloned into pET-28a (+).The recombinant plasmid, pET-MTCap-CBMCef-sfGFP, was verified by sequencing using the common sequencing primers T7 pro and T7 ter and then transformed into E. coli BL21 (DE3) competent cells. The transformant harboring the recombinant plasmid (pET-MTCap-CBMCef-sfGFP) was grown in LB medium containing 100 μg mL–1 of Kanamycin at 37 °C to an A600 of 0.6. Protein expression was induced by the addition of IPTG at a final concentration of 0.5 mM at 30 °C for 12 h.
Binding of the Fusion Protein MT-CBM-sfGFP to Cellulose
The cells were harvested by centrifugation (12,000g, 4 °C for 5 min) from 100 mL cultures and resuspended with 10 mL of Tris-HCl buffer (50 mM, pH 7.5). The cells were lysed by sonication (6 s, 200 W) on ice and then centrifuged at 12,000g for 5 min. The resulting supernatant was transferred to another tube containing 1 g of microcrystalline cellulose. After 1 h of gentle agitation at room temperature, MTCap-CBMCef-sfGFP@cellulose complexes were recovered through centrifugation at 10,000g for 2 min and rinsed with deionized water.
Adsorption Capacity of MTCap-CBMCef-sfGFP@Cellulose against Toxic Heavy Metals Using AAS
MTCap-CBMCef-sfGFP@cellulose was tested for absorbing heavy metals such as cadmium, lead, and mercury. Three solutions, including MTCap-CBMCef-sfGFP@cellulose suspension, cellulose suspension, and water, were mixed with different heavy metal samples (Pb(NO3)2, CdCl2, and HgCl2) in equal volume, respectively, and then incubated for 1 h at room temperature with a mild agitation of 200 rpm. The concentrations of Pb2+, Cd2+, and Hg2+ in heavy metal samples were 56.3, 16.3, and 18.0 ng/mL, separately. Next, the cellulose and MTCap-CBMCef-sfGFP@cellulose were removed by centrifuge. In addition, the supernatant was treated with 4% nitric acid in equal volume to obtain the samples. Then, these samples were subjected to AAS for estimating the concentrations of Pb2+, Cd2+, and Hg2+, which was determined against the linearity curve plotted against the standard. The amount of lead or cadmium was measured by the graphite furnace atomic absorption method. The amount of mercury was measured by the cold vapor generation absorption method. The operation condition for lead was as follows: drying temperature at 100 °C for 20 s; charring at 700 °C for 20 s; atomization at 2000 °C for 5 s; and the determined wavelength was 283.3 nm. The operation condition for cadmium was as follows: drying at 120 °C for 20 s; charring at 500 °C for 20 s; atomization at 1900 °C for 5 s; and the determined wavelength was 228.8 nm. The operation condition for mercury was as follows: 0.5% sodium borohydride and 0.1% sodium hydroxide (both fresh prepared) were used as the reductant in the hydride generator (LH-2A, General Research Institute for Nonferrous Metals, Beijing, China). Hydrochloric acid and nitrogen (0.36%) were used as the carrier liquid and gas, respectively. The determined wavelength was 253.6 nm.
MTCap-CBMCef-sfGFP@cellulose was also tested for adsorption of Pb2+ in the water decoction of Honeysuckle. The dry Honeysuckle was immersed for 4 h with 10-fold water, boiling refluxed for 1 h, cooled to room temperature, and filtered with gauze to obtain water decoction. Then, the water decoction was centrifuged at 12,000g for 10 min. The supernatant was treated with 4% nitric acid in equal volume and subjected to AAS for estimating the concentration of Pb2+.
Effect of MTCap-CBMCef-sfGFP@Cellulose on the Active Ingredients of Water Decoction of Honeysuckle by HPLC
Cellulose and MTCap-CBMCef-sfGFP@cellulose were mixed with the water decoction of Honeysuckle, respectively, with water as a control. After a mild agitation of 200 rpm for 1 h at room temperature, three solutions were centrifuged at 12,000g for 10 min. Then, the supernatants were filtered with a 0.22 μm filter membrane and sent for HPLC analysis. The reference solution and the samples were subjected to HPLC (DIONEX, ultimate 3000) to estimate the effect of MTCap-CBMCef-sfGFP@cellulose on the active ingredients of Honeysuckle. The standard substance of chlorogenic acid was resolved in water to prepare the reference solution (1 mg/mL). The chromatographic conditions were as follows: hypersil ODS2 C18 column (Elite, 250 mm × 4.6 mm, 5 μm) with a temperature of 30 °C, acetonitrile–0.1% phosphoric acid solution as the mobile phase with a gradient elution, and 0.5 mL/min flow rate with a detection wavelength of 327 nm.
Acknowledgments
This work was supported by the Fundamental Research Project for Social Commonweal Scientific Institutes in Fujian Province (no. 2019R1003-4).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03739.
Nucleotide sequence and amino acid sequence of fusion protein MTCap-CBMCef-sfGFP (PDF)
Author Contributions
Q.X. and J.H. contributed equally to the work. J.H., G.W., and Q.X. conceived and designed the experiments. Q.X., C.J., and Q.Z. performed the experiments. G.W. and J.H. analyzed the data. All authors contributed to the writing of the manuscript. All authors have given approval to the final version of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to be the accuracy or integrity.
The authors declare no competing financial interest.
Supplementary Material
References
- Xu J.; Liu M.; Xia Z. Asian medicine: call for more safety data. Nature 2012, 482, 35. 10.1038/482035d. [DOI] [PubMed] [Google Scholar]
- Ernst E. Toxic heavy metals and undeclared drugs in Asian herbral medicines. Trends Pharmacol. Sci. 2002, 23, 136–139. 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- Tang G.; Tu X.; Feng P. Lead poisoning caused by Traditional Chinese Medicine: a case report and literature review. Tohoku J. Exp. Med. 2017, 243, 127–131. 10.1620/tjem.243.127. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang Y.; Yang H.; Yu P.; Tang Y. Five heavy metals accumulation and health risk in a traditional Chinese medicine Cortex Moutan collected from different sites in China. Hum. Ecol. Risk Assess. 2018, 24, 2288–2298. 10.1080/10807039.2018.1459181. [DOI] [Google Scholar]
- Guo L. P.; Zhou L.; Wang S.; Kang C. Z.; Hao Q. X.; Yang W. Z.; Zhou L. Y.; Li Z. H.; Ma Z. H.; Huang L. Q. Statistic analysis of heavy metal residues in Chinese crude drugs with the international standards of Chinese medicine-Chinese herbal medicine heavy metal limit. Keji Daobao 2017, 35, 91–98. [Google Scholar]
- Bush A. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000, 4, 184–191. 10.1016/S1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Galanis A.; Karapetsas A.; Sandaltzopoulos R. Metal-induced carcinogenesis, oxidative stress and hypoxia signalling. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2009, 674, 31–35. 10.1016/j.mrgentox.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Leonard S. S.; Bower J. J.; Shi X. Metal-induced toxicity, carcinogenesis, mechanisms and cellular response. Mol. Cell. Biochem. 2004, 255, 3–10. 10.1023/B:MCBI.0000007255.72746.a6. [DOI] [PubMed] [Google Scholar]
- Menon A. V.; Chang J.; Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology 2016, 339, 58–72. 10.1016/j.tox.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urani C.; Melchioretto P.; Fabbri M.; Bowe G.; Maserati E.; Gribaldo L. Cadmium impairs p53 activity in HepG2 cells. ISRN Toxicol. 2014, 2014, 1–9. 10.1155/2014/976428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlina A. N.; Zherdev A. V.; Dzantiev B. B. Progress in rapid optical assays for heavy metal ions based on the use of nanoparticles and receptor molecules. Microchim. Acta 2019, 186, 172. 10.1007/s00604-018-3168-9. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Verma N.; Singh A. K. Development of cadmium specific recombinant biosensor and its application in milk samples. Sens. Actuators, B 2017, 240, 248–254. 10.1016/j.snb.2016.08.160. [DOI] [Google Scholar]
- Pawar N.; Gireesh-Babu P.; Sabnis S.; Rasal K.; Murthy R.; Zaidi S. G. S.; Sivasubbu S.; Chaudhari A. Development of a fluorescent transgenic zabrafish biosensor for sensing aquatic heavy metal pollution. Transgenic Res. 2016, 25, 617–627. 10.1007/s11248-016-9959-z. [DOI] [PubMed] [Google Scholar]
- Bari N. K.; Barua S.; Garg A.; Sannigrahi M. K.; Sinha S. Cellulose-metallothionein matrix for metal binding. Carbohydr. Polym. 2018, 192, 126–134. 10.1016/j.carbpol.2018.03.043. [DOI] [PubMed] [Google Scholar]
- Xu X.; Duan L.; Yu J.; Su C.; Li J.; Chen D.; Zhang X.; Song H.; Pan Y. Characterization analysis and heavy metal-binding properties of CsMTL3 in Escherichia coli. FEBS Open Bio 2018, 8, 1820–1829. 10.1002/2211-5463.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau A.; Bustamante P.; Haouz A.; Bourdineaud J. P.; Gonzalez-Rey M.; Lemouchi C.; Gautier-Luneau I.; Geertsen V.; Barruet E.; Rovezzi M.; Glatzel P.; Pin S. Mercury(II) binding to metallothionein in Mytilus edulis revealed by high energy-resolution XANES spectroscopy. Chem.—Eur. J. 2019, 25, 997–1009. 10.1002/chem.201804209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahpiri A.; Mohammadzadeh A. Mercury removal by engineered Escherichia coli cells expressing different rice metallothionein isoforms. Ann. Microbiol. 2018, 68, 145–152. 10.1007/s13213-018-1326-2. [DOI] [PubMed] [Google Scholar]
- Gupta D.; Satpati S.; Dixit A.; Ranjan R. Fabrication of biobeads expressing heavy metal-binding protein for removal of heavy metal from wastewater. Appl. Microbiol. Biotechnol. 2019, 103, 5411–5420. 10.1007/s00253-019-09852-6. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Bae W.; Mulchandani A.; Mehra R. K.; Chen W. Heavy metal removal by novel CBD-EC20 sorbents immobilized on cellulose. Biomacromolecules 2002, 3, 462–465. 10.1021/bm015631f. [DOI] [PubMed] [Google Scholar]
- Yang T.; Li Y.-K.; Chen M.-L.; Wang J.-H. Supported carbon dots decorated with metallothionein for selective cadmium adsorption and removal. Chin. Chem. Lett. 2015, 26, 1496–1501. 10.1016/j.cclet.2015.10.018. [DOI] [Google Scholar]
- Cabral A. C. S.; Jakovleska J.; Deb A.; Penner-Hahn J. E.; Pecoraro V. L.; Freisinger E. Further insights into the metal ion binding abilities and the metalation pathway of a plant metallothionein from Musa acuminata. J. Biol. Inorg Chem. 2018, 23, 91–107. 10.1007/s00775-017-1513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E. K.; Stillman M. J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. 10.1039/c0mt00102c. [DOI] [PubMed] [Google Scholar]
- Cismowski M. J.; Narula S. S.; Armitage I. M.; Chernaik M. L.; Huang P. C. Mutation of invariant cysteines of mammalian metallothionein alters metal capacity, cadmium resistance, and 113Cd NMR spectrum. J. Biol. Chem. 1991, 266, 24390–24397. [PubMed] [Google Scholar]
- Carpenè E.; Andreani G.; Isani G. Metallothionein functions and structural characteristics. J. Trace Elem. Med. Biol. 2007, 21, 35–39. 10.1016/j.jtemb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Pazirandeh M.; Chrisey L. A.; Mauro J. M.; Campbell J. R.; Gaber B. P. Expression of Neurospora crassa metallothionein gene in Escherichia coli and its effect on heavy-metal uptake. Appl. Microbiol. Biotechnol. 1995, 43, 1112–1117. 10.1007/BF00166934. [DOI] [PubMed] [Google Scholar]
- Ghanbarinia F.; Kheirbadi M.; Mollania N. Comamonas sp. Halotolerant bacterium from industrial zone of Jovein of Sabzevar introduced as good candidate to remove industrial pollution. Iran J. Microbiol. 2015, 7, 273. [PMC free article] [PubMed] [Google Scholar]
- Peña-Montenegro T. D.; Dussán J. Genome sequence and description of the heavy metal tolerant bacterium Lysinibacillus sphaericus strain OT4b. 31. Stand. Genomic Sci. 2013, 9, 42. 10.4056/sigs.4227894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmajeed A. N.; Khelil A. O.; Danial N. E. Immobilization technology for enhancing bio-products industry. Afr. J. Biotechnol. 2012, 11, 13528–13539. 10.5897/ajb12.547. [DOI] [Google Scholar]
- Shakya M.; Sharma P.; Meryem S. S.; Mahmood Q.; Kumar A. Heavy metal removal from industrial wastewater using fungi: uptake mechanism and biochemical aspects. J. Environ. Eng. 2016, 142, C6015001. 10.1061/(asce)ee.1943-7870.0000983. [DOI] [Google Scholar]
- Guillén D.; Sánchez S.; Rodríguez-Sanoja R. Carbohydrate-binding domains: multiplicity of biological roles. Appl. Microbiol. Biotechnol. 2010, 85, 1241–1249. 10.1007/s00253-009-2331-y. [DOI] [PubMed] [Google Scholar]
- Shoseyov O.; Shani Z.; Levy I. Carbohydrate binding modules: biochemical properties and novel applications. Microbiol. Mol. Biol. Rev. 2006, 70, 283–295. 10.1128/mmbr.00028-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.; Carvalho V.; Domingues L.; Gama F. M. Recombinant CBM-fusion technology – applications overview. Biotechnol. Adv. 2015, 33, 358–369. 10.1016/j.biotechadv.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Vašák M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. 10.1016/j.jtemb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Overnell J. Occurrence of cadmium in crabs (cancer pagurus) and the isolation and properties of cadmium metallothionein. Environ. Health Perspect. 1986, 65, 101–105. 10.1289/ehp.8665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leignel V.; Marchand J.; Moreau B.; Chénais B. Metallothionein genes from hydrothermal crabs (Bythograeidae, Decapoda): Characterization, sequence analysis, gene expression and comparison with coastal crabs. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2008, 148, 6–13. 10.1016/j.cbpc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Bourdineaud J.-P.; Baudrimont M.; Gonzalez P.; Moreau J.-L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. 10.1016/j.biochi.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Park H.; Ahn I.-Y.; Choi H. J.; Pyo S. H.; Lee H. E. Cloning, expression and characterization of metallothionein from the Antarctic clam Laternula elliptica. Protein Expression Purif. 2007, 52, 82–88. 10.1016/j.pep.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Terashima M.; Oka N.; Sei T.; Yoshida H. Adsorption of Cadmium Ion and Gallium Ion to Immobilized Metallothionein Fusion Protein. Biotechnol. Prog. 2002, 18, 1318–1323. 10.1021/bp0200550. [DOI] [PubMed] [Google Scholar]
- Myung S.; Zhang X.-Z.; Percival Zhang Y.-H. Ultra-stable phosphoglucose isomerase through immobilization of cellulose-binding module-tagged thermophilic enzyme on low-cost high-capacity cellulosic adsorbent. Biotechnol. Prog. 2011, 27, 969–975. 10.1002/btpr.606. [DOI] [PubMed] [Google Scholar]
- Singh S.; Hinkley T.; Nugen S. R.; Talbert J. N. Fusion of carbohydrate binding module to mutant alkaline phosphatase for immobilization on cellulose. Biocatal. Agric. Biotechnol. 2018, 13, 265–271. 10.1016/j.bcab.2018.01.003. [DOI] [Google Scholar]
- Cao J.; Fei D.; Tian X.; Zhu Y.; Wang S.; Zhang Y.; Mao Q.; Sun M. Novel modified microcrystalline cellulose-based porous material for fast and effective heavy-metal removal from aqueous solution. Cellulose 2017, 24, 5565–5577. 10.1007/s10570-017-1504-6. [DOI] [Google Scholar]
- Zhang N.; Zang G.-L.; Shi C.; Yu H.-Q.; Sheng G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. 10.1016/j.jhazmat.2016.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.