Abstract
Carnitine deficiency contributes to developing various pathological conditions, such as cardiac dysfunction, muscle weakness, and erythropoietin-resistant anemia in patients undergoing hemodialysis. However, a conclusion has not been reached concerning the prevalence and the effect of carnitine deficiency in patients undergoing peritoneal dialysis (PD). In this study, the prevalence of carnitine deficiency and the clinical factors associated with carnitine deficiency were investigated in 60 patients undergoing PD. The median age of the patients was 62.5 years (52.5–72.5 years), the proportion of male sex was 44/60 (73.3%), and the median PD period was 24 months (12–45 months). Carnitine deficiency (acyl carnitine/free carnitine ratio >0.4) was detected in 56/60 (93%) patients. Multiple regression analysis showed that the erythropoietin resistance index was independently associated with carnitine deficiency (β = 0.283, p = 0.04). These results suggest that carnitine plays pivotal roles in hematogenesis in patients undergoing PD.
Keywords: Anemia, carnitine deficiency, erythropoietin resistance index, peritoneal dialysis
Introduction
Carnitine is an essential amino-acid derivative and plays pivotal roles in fatty-acid metabolism in skeletal muscle and cardiac muscle [1–3]. Carnitine is present in two forms in the body, acyl carnitine and free carnitine, and the sum of them is defined as total carnitine [4]. Free carnitine is converted to acylcarnitine by binding to an acyl residue. Additionally, acyl carnitine functions as a transporter of fatty acids to mitochondria and as a scavenger of excess and harmful acyl residues in cells [4]. A total of 75% of carnitine in the body is obtained by dietary intake, such as red meats, and the remaining 25% is biosynthesized by the kidney and liver [5,6]. In healthy individuals, most of the free carnitine is re-reabsorbed in the kidney, and acyl carnitine is preferentially excreted into urine. Carnitine homeostasis in the body is maintained by this mechanism.
Previous studies have been reported a high prevalence of carnitine deficiency in patients undergoing hemodialysis [1,5,7]. Carnitine deficiency in patients undergoing hemodialysis is defined as a low free carnitine level (<20 μmol/L) or imbalance between acyl carnitine and free carnitine (acyl carnitine/free carnitine >0.4) [7,8]. The causes of carnitine deficiency in patients undergoing hemodialysis are considered to be insufficient carnitine intake, decreased biosynthesis, removal by hemodialysis, and loss of preferential renal excretion of acyl carnitine [9,10].
Carnitine deficiency is associated with various pathological conditions, including anemia, cardiac dysfunction, and muscle weakness in patients undergoing hemodialysis [2,11–16]. The National Kidney Foundation has proposed that carnitine deficiency in patients undergoing hemodialysis is a dialysis-related carnitine disorder in a conference report [17]. They recommend carnitine supplementation for patients undergoing hemodialysis who have erythropoietin-resistant anemia, hypotension during hemodialysis sessions, cardiac dysfunction, and muscle weakness [17]. Therefore, detection of carnitine deficiency and appropriate carnitine supplementation are important for treating patients undergoing hemodialysis. There have also been several reports on carnitine deficiency and carnitine supplementation in patients undergoing peritoneal dialysis (PD) [18–22]. However, these studies had small sample sizes, leading to contradictory conclusions [18–22]. Although there appear to be similar risks for carnitine deficiency in patients undergoing PD as those undergoing hemodialysis, there are no established diagnostic criteria for carnitine deficiency and no guideline for carnitine supplementation in patients undergoing PD at this time. Therefore, studies on the prevalence and clinical effects of carnitine deficiency in patients undergoing PD are still required. In this study, we aimed to clarify the prevalence and clinical effect of carnitine deficiency in patients undergoing PD.
Materials and methods
Study design
We performed an observational, retrospective study. We examined patients’ characteristics and conducted a cross-sectional analysis of the prevalence of carnitine deficiency and factors associated with carnitine deficiency in patients undergoing PD.
Patients
This study was retrospectively conducted at Saitama Medical Center, Jichi Medical University. We investigated outpatients who underwent PD between January 2018 and December 2018. Inclusion criteria were as follows: (i) patients with end-stage renal failure undergoing PD; (ii) patients who had carnitine levels measured; and (iii) age > 18 years. Exclusion criteria were as follows: (i) patients who were receiving carnitine supplementation; (ii) acute kidney injury; (iii) patients who had undergone renal transplantation; and (iv) no consent to participate in this study. All of the patients were treated in accordance with the 2009 Japanese Society for Dialysis Therapy Guideline for PD and the 2015 Japanese Society for Dialysis Therapy Guideline for Renal Anemia in Chronic Kidney Disease [23,24]. This study was approved by the Saitama Medical Center, Jichi Medical University Ethics committee (DAI-RIN 15-34) and was conducted in accordance with the Declaration of Helsinki. Because this study was observational and retrospective, it was exempt from obtaining a consent form from individual patients and it was replaced with opt-out.
Data collection
All of the data were collected retrospectively from medical records: information of age, sex, body mass index, underlying diseases (diabetes mellitus, hepatic disease, cardiac diseases), PD period, hemodialysis combination, and PD modality. In this study, the modality of PD was initially classified as two types as follows. Continuous ambulatory PD (CAPD) is a method of changing PD fluids manually during the daytime with or without maintaining PD fluid retention during the nighttime. Automated PD (APD) is a method of changing PD fluids with automated systems [25–28]. APD was then further classified as nocturnal intermittent PD (NIPD) and continuous cyclic PD (CCPD). NIPD is APD during the nighttime without maintaining PD fluid retention during the daytime. CCPD is APD during the nighttime with maintenance of PD fluid retention during the daytime [25–28]. We also collected data on total weekly Kt/V urea, peritoneal weekly Kt/V urea, renal weekly Kt/V urea, 4-h dialysate/plasma creatinine, erythropoietin resistance index, ejection fraction, and laboratory markers, including carnitine levels. Diabetes mellitus was defined as hemoglobin A1c ≥ 6.5% or use of oral hypoglycemic agents and/or insulin therapy. Hepatic disease was defined as chronic liver injury, such as hepatitis and cirrhosis. Cardiac disease was defined as coronary artery disease or arrhythmia. Hemodialysis combination was defined as the combined treatment of hemodialysis once a week in addition to PD. Peritoneal weekly Kt/V urea was an indicator of efficacy of PD and renal weekly Kt/V was an indicator of residual renal function. Their summation was defined as total weekly Kt/V urea [29]. Four-hour dialysate/plasma creatinine is known as an indicator of peritoneal permeability [30].
Laboratory markers
Total carnitine and free carnitine levels were measured by a clinical chemistry laboratory (SRL, Tokyo, Japan) using the enzyme cycling method. This is an established method for accurately measuring carnitine levels [31]. Acyl carnitine levels were calculated by total carnitine minus free carnitine levels [4]. Other blood and PD dialysate fluid parameters were determined by the Department of Clinical Laboratory, Saitama Medical Center.
Definition of carnitine deficiency
In accordance with previous studies, the diagnostic criterion for carnitine deficiency in this study was a low free carnitine level (<20 μmol/L) or a high acyl carnitine/free carnitine ratio (>0.4) [7,8].
Definition of the erythropoietin resistance index
The resistance to the erythropoiesis-stimulating agent of renal anemia was evaluated by the erythropoietin resistance index [2,12]. The erythropoietin resistance index was defined as the average weekly dose of recombinant human erythropoietin (IU)/body weight (kg)/hemoglobin (g/dL) [2,12]. For dose conversion of recombinant human erythropoietin, the ratio of recombinant human erythropoietin darbepoetin alfa or epoetin beta pegol was converted to 200:1 [32].
Statistical analysis
The normality of distribution of the sample was determined by the Shapiro–Wilk test. For measurement data, quantitative variables are expressed as mean ± standard deviation for a normal distribution and as median and interquartile range for a non-normal distribution. Categorical variables are described as frequency and percentage. Fisher’s exact test was used for comparison of frequency. Continuous variables were compared using the Wilcoxon rank-sum test and one-way analysis of variance. Tukey’s multiple comparison test was used for multiple comparisons. Univariate and multiple regression analyses were performed to investigate the factors associated with the acyl carnitine/free carnitine ratio and erythropoietin resistance index. Multiple regression analysis was performed using the parameters that showed a significant correlation in univariate analysis. Patients were divided into two groups, with the median erythropoietin resistance index, and related parameters were compared. Statistical analysis was performed using JMP (SAS Institute Inc., Cary, NC, USA). Values of p < 0.05 were considered statistically significant.
Results
Clinical characteristics of the patients
Sixty patients met the criteria and were enrolled in this study. Table 1 shows the clinical characteristics of the study population (n = 60). The median age of the patients was 62.5 years (interquartile range: 52.5–72.5). Forty-four (73.3%) men were enrolled in the study. The median PD period was 24 months (12–45 months). Diabetes mellitus, hepatic disease, and cardiac disease were found in 23 (38.3%), two (3.3%) and 24 (40.0%) patients, respectively. Continuous ambulatory PD, nocturnal intermittent PD and continuous cyclic PD were selected as PD modalities for eight (13.3%), 21 (35.0%), and 31 (51.7) patients, respectively. The median total carnitine, free carnitine, and acyl carnitine levels were 45.2 µmol/L (36.6–52.7 µmol/L), 27.5 µmol/L (22.5–34.0 µmol/L), and 16.6 µmol/L (13.3–19.3 µmol/L), respectively. The median acyl carnitine/free carnitine ratio was 0.5 (0.5–0.7). The mean erythropoietin resistance index was 7.0 ± 4.4. Table 2 shows the prevalence of carnitine deficiency in patients undergoing PD. A total of 8% (5/60) of patients undergoing PD had low free carnitine level and 93% (56/60) of patients undergoing PD had a high acyl carnitine/free carnitine ratio. All patients with low free carnitine level had a high acyl carnitine/free carnitine ratio. The prevalence of carnitine deficiency was not different between the PD group and PD + HD combination group (Table 3). The prevalence of carnitine deficiency was also not different among different PD modalities (CAPD, NIPD, and CCPD) (Table 4).
Table 1.
Characteristics of the study population (n = 60).
| Variable | Mean ± SD or median [IQR] | Number (%) |
|---|---|---|
| Age (years) | 62.5 [52.5–72.5] | |
| Male sex | 44 (73.3) | |
| Body mass index (kg/m2) | 23.2 [20.8–25.3] | |
| Diabetes mellitus | 23 (38.3) | |
| Hepatic disease | 2 (3.3) | |
| Cardiac disease | 24 (40.0) | |
| PD period (months) | 24 [12–45] | |
| Hemodialysis combination | 13 (21.7) | |
| PD modality: CAPD | 8 (13.3) | |
| PD modality: APD (NIPD) | 21 (35.0) | |
| PD modality: APD (CCPD) | 31 (51.7) | |
| White blood cells (103/µL) | 6.24 [5.13–7.47] | |
| Red blood cells (104/µL) | 350.1 ± 46.2 | |
| Hemoglobin (g/dL) | 10.8 ± 1.3 | |
| Platelets (×104/µL) | 22.9 ± 7.5 | |
| Total protein (g/dL) | 6.3 ± 0.5 | |
| Albumin (g/dL) | 3.3 ± 0.5 | |
| Blood urea nitrogen (mg/dL) | 52.0 [48.0–62.8] | |
| Creatinine (mg/dL) | 10.9 ± 3.2 | |
| C-reactive protein (mg/dL) | 0.15 [0.04–0.48] | |
| Uric acid (mg/dL) | 5.9 [5.0–7.2] | |
| Sodium (mEq/L) | 138.2 ± 4.0 | |
| Potassium (mEq/L) | 4.4 [3.8–5.1] | |
| Chloride (mEq/L) | 99.8 ± 5.0 | |
| Corrected calcium (mg/dL) | 9.1 ± 0.6 | |
| Phosphorus (mg/dL) | 5.6 [4.6–6.0] | |
| Magnesium (mg/dL) | 2.1 [1.8–2.5] | |
| Zinc (µg/dL) | 60.5 [52.0–68.0] | |
| Copper (µg/dL) | 95.5 ± 20.8 | |
| Iron (µg/dL) | 74.5 [52.0–95.8] | |
| Ferritin (mg/mL) | 134 [85–254] | |
| Transferrin saturation (%) | 30.9 [21.2–39.2] | |
| Vitamin B12 (pg/mL) | 384 [275–734] | |
| Folate (ng/mL) | 4.5 [3.5–6.3] | |
| HbA1c (%) | 5.6 [5.0–6.1] | |
| Total carnitine (µmol/L) | 45.2 [36.6–52.7] | |
| Acyl carnitine (µmol/L) | 16.6 [13.3–19.3] | |
| Free carnitine (µmol/L) | 27.5 [22.5–34.0] | |
| Acyl carnitine/free carnitine ratio | 0.5 [0.5–0.7] | |
| Total cholesterol (mg/dL) | 178 ± 47 | |
| HDL cholesterol (mg/dL) | 46 [35–54] | |
| LDL cholesterol (mg/dL) | 97 [69–122] | |
| Triglycerides (mg/dL) | 124 [87–181] | |
| Intact PTH (pg/dL) | 231 [144–336] | |
| ERI (IU/week/kg/(g/dL)) | 7.0 ± 4.4 | |
| Total Kt/V urea | 1.61 [1.40–1.83] | |
| Peritoneal Kt/V urea | 1.07 [0.83–1.36] | |
| Renal Kt/V urea | 0.44 [0.25–0.82] | |
| D/P Cre | 0.66 ± 0.14 | |
| Ejection fraction (%) | 64 [59–68] |
SD: standard deviation; IQR: interquartile range; PD: peritoneal dialysis; CAPD: continuous ambulatory peritoneal dialysis; APD: automated peritoneal dialysis; NIPD: nocturnal intermittent peritoneal dialysis; CCPD: continuous cyclic peritoneal dialysis; HbA1c: hemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PTH: parathormone; ERI: erythropoietin resistance index; D/P Cre: 4-h dialysate/plasma creatinine.
Table 2.
Prevalence of carnitine deficiency in patients undergoing PD (n = 60).
| Carnitine levels | Number (%) |
|---|---|
| Free carnitine <20 μmol/L | 5 (8) |
| Acyl carnitine/free carnitine ratio >0.4 | 56 (93) |
PD: peritoneal dialysis.
Table 3.
Comparison of the prevalence of carnitine deficiency (HD combination) (n = 60).
| PD alone group (n = 47) | PD + HD combination group (n = 13) | ||
|---|---|---|---|
| Carnitine levels | Number (%) | p Value | |
| Free carnitine <20 μmol/L | 5 (11) | 0 (0) | 0.58 |
| Acyl carnitine/free carnitine ratio >0.4 | 43 (91) | 13 (100) | 0.57 |
HD: hemodialysis; PD: peritoneal dialysis.
Table 4.
Comparison of the prevalence of carnitine deficiency (PD modality) (n = 60).
| CAPD (n = 21) | APD (NIPD) (n = 8) | APD (CCPD) (n = 31) | ||
|---|---|---|---|---|
| Carnitine levels | Number (%) | p Value | ||
| Free carnitine <20 μmol/L | 3 (14) | 0 (0) | 2 (6) | 0.41 |
| Acyl carnitine/free carnitine ratio >0.4 | 20 (95) | 7 (88) | 29 (94) | 0.76 |
PD: peritoneal dialysis; CAPD: continuous ambulatory peritoneal dialysis; APD: automated peritoneal dialysis; NIPD: nocturnal intermittent peritoneal dialysis; CCPD: continuous cyclic peritoneal dialysis.
Factors associated with carnitine deficiency in patients who underwent PD
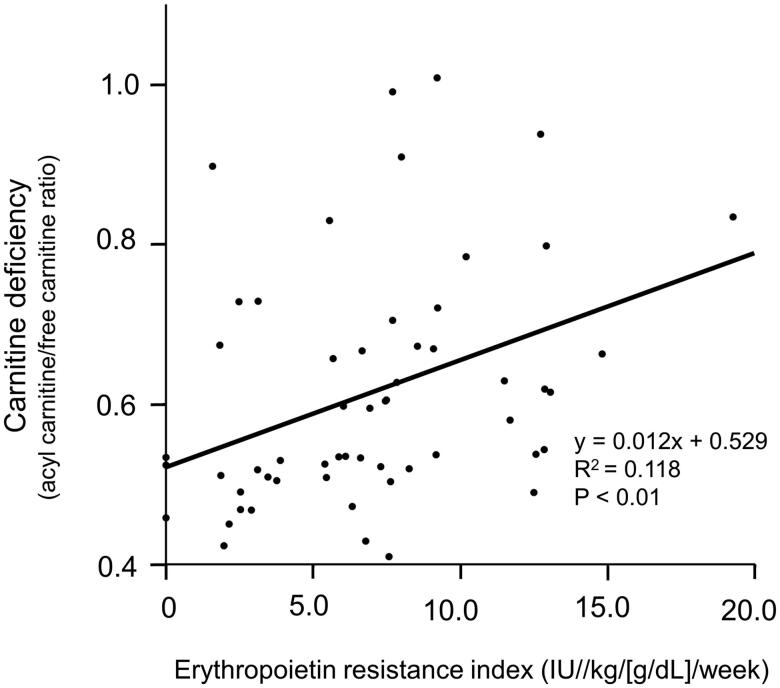
The correlations between the acyl carnitine/free carnitine ratio and the clinical factors of patients undergoing PD with carnitine deficiency (n = 56) are shown in Table 5. In univariate analysis, the PD period (p = 0.03, β = 0.294) and erythropoietin resistance index (p < 0.01, β = 0.343) were significantly correlated with carnitine deficiency. In multiple regression analysis, the erythropoietin resistance index (p = 0.04, β = 0.293) was significantly correlated with carnitine deficiency (Table 5 and Figure 1). The correlations between the erythropoietin resistance index and clinical factors, including free carnitine level and the acyl carnitine/free carnitine ratio, in patients who underwent PD with carnitine deficiency (n = 56) are shown in Table 6. Univariate and multivariate analyses showed that free carnitine level and the acyl carnitine/free carnitine ratio were significantly correlated with the erythropoietin resistance index in patients undergoing PD with carnitine deficiency. Additionally, we compared these factors between a group of patients who had a low erythropoietin resistance index and a group who had a high erythropoietin resistance index. No factors, except for free carnitine levels and the acyl carnitine/free carnitine ratio, were significantly different between these two groups (Table 7).
Table 5.
Factors associated with the acyl carnitine/free carnitine ratio in patients undergoing PD with carnitine deficiency (n = 56).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| β | p Value | β | p Value | |
| Age | −0.143 | 0.29 | ||
| Sex male | −0.095 | 0.49 | ||
| Body mass index | 0.045 | 0.74 | ||
| Diabetes mellitus | 0.019 | 0.89 | ||
| Liver disease | 0.100 | 0.47 | ||
| Heart disease | 0.151 | 0.26 | ||
| PD period | 0.294 | 0.03 | 0.213 | 0.11 |
| Hemodialysis combination | 0.102 | 0.46 | ||
| PD modality: CAPD | 0.143 | 0.28 | ||
| PD modality: APD (NIPD) | 0.179 | 0.17 | ||
| PD modality: APD (CCPD) | −0.014 | 0.91 | ||
| White blood cells | 0.182 | 0.18 | ||
| Red blood cells | −0.101 | 0.46 | ||
| Hemoglobin | −0.187 | 0.17 | ||
| Platelets | 0.192 | 0.16 | ||
| Total protein | 0.080 | 0.56 | ||
| Albumin | −0.006 | 0.96 | ||
| Blood urea nitrogen | 0.101 | 0.46 | ||
| Creatinine | 0.254 | 0.06 | ||
| C-reactive protein | 0.130 | 0.34 | ||
| Uric acid | 0.234 | 0.08 | ||
| Sodium | −0.125 | 0.36 | ||
| Potassium | 0.144 | 0.29 | ||
| Chloride | −0.151 | 0.27 | ||
| Corrected calcium | 0.136 | 0.32 | ||
| Phosphorus | −0.013 | 0.92 | ||
| Magnesium | 0.072 | 0.60 | ||
| Zinc | 0.164 | 0.23 | ||
| Copper | 0.091 | 0.50 | ||
| Iron | −0.164 | 0.23 | ||
| Ferritin | 0.236 | 0.08 | ||
| Transferrin saturation | −0.185 | 0.17 | ||
| Vitamin B12 | −0.352 | 0.13 | ||
| Folate | 0.111 | 0.65 | ||
| Blood glucose | −0.085 | 0.53 | ||
| HbA1c | −0.143 | 0.29 | ||
| Total cholesterol | 0.071 | 0.60 | ||
| HDL cholesterol | 0.051 | 0.71 | ||
| LDL cholesterol | 0.125 | 0.36 | ||
| Triglycerides | −0.133 | 0.33 | ||
| Intact PTH | −0.061 | 0.66 | ||
| ERI | 0.343 | <0.01 | 0.283 | 0.04 |
| Total Kt/V urea | −0.084 | 0.54 | ||
| Peritoneal Kt/V urea | 0.153 | 0.26 | ||
| Renal Kt/V urea | −0.106 | 0.43 | ||
| D/P urea | 0.111 | 0.42 | ||
| Ejection fraction | −0.062 | 0.65 | ||
PD: peritoneal dialysis; CAPD: continuous ambulatory peritoneal dialysis; APD: automated peritoneal dialysis; NIPD: nocturnal intermittent peritoneal dialysis; CCPD: continuous cyclic peritoneal dialysis; HbA1c: hemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PTH: parathormone; ERI: erythropoietin resistance index; D/P Cre: 4-h dialysate/plasma creatinine.
Figure 1.
Significant correlation between carnitine deficiency (acyl carnitine/free carnitine ratio) and the erythropoietin resistance index in patients who underwent peritoneal dialysis.
Table 6.
Factors associated with the ERI in patients undergoing PD with carnitine deficiency (n = 56).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| β | p Value | β | p Value | |
| Age | −0.235 | 0.08 | ||
| Sex male | 0.040 | 0.78 | ||
| Body mass index | −0.119 | 0.38 | ||
| Iron | −0.164 | 0.23 | ||
| Transferrin saturation | −0.196 | 0.15 | ||
| Ferritin | 0.251 | 0.06 | ||
| C-reactive protein | 0.171 | 0.21 | ||
| Corrected calcium | 0.238 | 0.08 | ||
| Phosphorus | −0.116 | 0.40 | ||
| Intact PTH | −0.089 | 0.52 | ||
| Vitamin B12 | −0.21 | 0.41 | ||
| Folate | 0.153 | 0.56 | ||
| Albumin | −0.245 | 0.07 | ||
| Zinc | 0.123 | 0.36 | ||
| Free carnitine | −0.341 | 0.01 | −0.268 | 0.04 |
| Acyl carnitine/free carnitine ratio | 0.343 | <0.01 | 0.271 | 0.04 |
ERI: erythropoietin resistance index; PD: peritoneal dialysis; Intact PTH: intact parathormone.
Table 7.
Comparison of clinical parameters between the low ERI group and the high ERI group in patients undergoing PD with carnitine deficiency (n = 56).
| Low ERI group (n = 28) | High ERI group (n = 28) | ||
|---|---|---|---|
| Carnitine status | Mean ± SD or median [IQR] or number (%) | p Value | |
| Age (years) | 61.5 ± 11.8 | 60.6 ± 16.4 | 0.90 |
| Sex male | 22 (79) | 18 (64) | 0.38 |
| Body mass index (kg/m2) | 23.6 [20.8–25.3] | 23.3 [21.0–25.3] | 0.89 |
| ERI (IU/week/kg/(g/dL)) | 3.2 [2.0–5.8] | 9.1 [7.7–12.7] | <0.01 |
| Iron (µg/dL) | 74.5 [56.5–96.0] | 74.0 [50.3–94.8] | 0.61 |
| Transferrin saturation (%) | 32.0 [21.5–40.1] | 30.5 [21.3–37.4] | 0.48 |
| Ferritin (mg/mL) | 141.9 [82.6–209.9] | 138 [90.5–288.7] | 0.53 |
| C-reactive protein (mg/dL) | 0.12 [0.05–0.34] | 0.21 [0.04–0.58] | 0.59 |
| Corrected calcium (mg/dL) | 9.0 ± 0.7 | 9.2 ± 0.6 | 0.32 |
| Phosphorus (mg/dL) | 5.5 ± 0.9 | 5.5 ± 1.4 | 0.71 |
| Intact PTH (pg/dL) | 273 ± 158 | 246 ± 147 | 0.52 |
| Vitamin B12 (pg/mL) | 558 ± 209 | 398 ± 216 | 0.13 |
| Folate (ng/mL) | 4.2 ± 2.1 | 6.1 ± 4.9 | 0.29 |
| Albumin (g/dL) | 3.4 ± 0.5 | 3.2 ± 0.4 | 0.18 |
| Zinc (µg/dL) | 59.5 [51.2–67.8] | 62.0 [52.3–69.8] | 0.64 |
| Free carnitine (µmol/L) | 29.8 [23.7–40.0] | 24.4 [21.7–30.2] | 0.03 |
| Acyl carnitine/free carnitine ratio | 0.5 [0.5–0.6] | 0.6 [0.5–0.8] | <0.01 |
ERI: erythropoietin resistance index; PD: peritoneal dialysis; SD: standard deviation; IQR: interquartile range; Intact PTH: intact parathormone.
Discussion
In this study, we found that carnitine deficiency was significantly correlated with the erythropoietin resistance index. To the best of our knowledge, this is the first report to show the correlation between carnitine deficiency and erythropoietin resistance in patients undergoing PD. In this study, 8% of patients who underwent PD had low free carnitine level (<20 μmol/L) and 93% of patients who underwent PD had a high acyl carnitine/free carnitine ratio (>0.4). Both of these indices are used for the definition of carnitine deficiency [7,8]. In our study, all patients with a low free carnitine level had a high acyl carnitine/free carnitine ratio. These results suggest that a high acylcarnitine/free carnitine ratio is more sensitive and has clinical utility in detecting carnitine deficiency compared with free carnitine level in patients undergoing PD. This could be because a high acylcarnitine/free carnitine ratio was independently associated with the erythropoietin resistance index in patients undergoing PD.
Several mechanisms can be considered for patients undergoing PD for developing carnitine deficiency. First, renal dysfunction leads to decreased biosynthesis and loss of preferential renal excretion of acyl carnitine [9,10]. Second, patients undergoing PD require restriction of foods containing carnitine owing to renal dysfunction [9,10]. Third, loss of free carnitine into PD fluid may also be involved [33]. Fourth, disproportionate clearance of carnitine before reaching the systemic circulation beyond the gastrointestinal compartment by PD fluid exchange may contribute to carnitine loss [34]. A previous study reported carnitine deficiency in infant patients undergoing PD [5], which is consistent with our results. Additionally, we found a significant correlation between carnitine deficiency and the PD period in univariate analysis. A previous study reported a high prevalence of carnitine deficiency in patients who underwent APD compared with those who underwent CAPD [35]. However, no correlations between the PD modality and carnitine deficiency were observed in our study. Further studies are required to investigate the correlation between PD modality and carnitine deficiency in patients undergoing PD.
Several studies have reported that carnitine deficiency is associated with anemia [2,36,37]. Carnitine contributes to stabilizing erythrocyte membranes, resulting in improvement of their deformability [2,36,37]. Previous studies have reported a significant correlation between carnitine deficiency and the erythropoietin resistance index, and improvement of erythropoiesis-resistant anemia by carnitine supplementation in patients undergoing hemodialysis [2,15,38]. Additionally, the erythropoietin resistance index is not only an indicator of erythropoietin-resistant renal anemia, but also a high mortality rate in patients undergoing hemodialysis [12]. Therefore, analyzing clinical factors associated with the erythropoietin resistance index in patients undergoing dialysis is important. Previous studies have reported that several factors, including iron deficiency, vitamin deficiency, chronic kidney disease-mineral and bone disorder (CKD-MBD), inflammation, and malnutrition are associated with erythropoietin resistance [23,39–52]. However, these associations were not observed in this study. This discrepancy between studies might be explained by the finding that the patients in our study had better control of levels of iron, transferrin saturation, ferritin, and vitamins (vitamin B12, folate), CKD-MBD, inflammation, and nutritional status compared with those in previous studies [23,39–52].
The National Kidney Foundation reported favorable effects of carnitine supplementation to patients undergoing hemodialysis [17]. The National Kidney Foundation recommends carnitine supplementation for 9 months to 1 year for erythropoietin-resistant anemia, hypotension during hemodialysis sessions, cardiac dysfunction, and muscle weakness [17]. However, carnitine supplementation for patients undergoing PD was not mentioned. A previous study reported showed that 12 patients who underwent PD showed improved erythropoietin-resistant anemia by carnitine supplementation [20]. However, another study reported that 12 infant patients who underwent PD did not have improved erythropoietin-resistant anemia by carnitine supplementation [19]. Additionally, the utility of using carnitine as an osmotic substance in PD fluid has been reported [53]. Furthermore, supplementation of carnitine by intravenous administration is more effective than oral carnitine intake in patients undergoing hemodialysis [54]. Another study showed that certain levels (80–199 μmol/L) of carnitine supplementation decreased clinical complications, such as hypotension and muscle cramps, during hemodialysis sessions compared with higher levels of free carnitine ( ≥ 300 μmol/L) in patients undergoing hemodialysis [6]. These results indicate that the supplementation route, monitoring of carnitine levels, and maintaining appropriate free carnitine levels by carnitine supplementation are important for decreasing clinical complications in patients undergoing hemodialysis and PD. Therefore, larger interventional studies are required to investigate the effects, including erythropoietin-resistant anemia, of carnitine supplementation for patients undergoing PD.
Our study has several limitations. First, because this study was conducted at a single institution, whether the results can be generalized at other institutions is unclear. Second, the design of this study was cross-sectional and observational. Therefore, we cannot suggest the importance of carnitine supplementation.
In conclusion, there is a high prevalence (93%) of carnitine deficiency (acyl carnitine/free carnitine ratio >0.4) in patients undergoing PD. Additionally, carnitine deficiency is significantly correlated with the erythropoietin resistance index. Clinicians should note this pathological condition in patients undergoing PD.
Acknowledgments
The authors thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Disclosure statement
The authors declare no conflicts of interest in this work.
References
- 1.Yang SK, Xiao L, Song PA, et al. Effect of L-carnitine therapy on patients in maintenance hemodialysis: a systematic review and meta-analysis. J Nephrol. 2014;27:317–329. [DOI] [PubMed] [Google Scholar]
- 2.Kamei D, Tsuchiya K, Nitta K, et al. Association between resistance to erythropoiesis-stimulating agents and carnitine profile in patients on maintenance haemodialysis. Nephrology (Carlton, Vic). 2018;23:737–743. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber BD. Debate forum: levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006;24:128–139. [DOI] [PubMed] [Google Scholar]
- 4.Rebouche CJ, Engel AG.. Carnitine metabolism and deficiency syndromes. Mayo Clin Proc. 1983;58:533–540. [PubMed] [Google Scholar]
- 5.Naseri M, Mottaghi Moghadam Shahri H, Horri M, et al. absolute and relative carnitine deficiency in patients on hemodialysis and peritoneal dialysis. Iran J Kidney Dis. 2016;10:36–43. [PubMed] [Google Scholar]
- 6.Zhang YM, Zhuo L, Hu J, et al. Clinical significance of different carnitine levels for improving the prognosis of patients undergoing hemodialysis. Ren Fail. 2016;38:1654–1658. [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka Y, Higuchi T, Akiya Y, et al. Prevalence of carnitine deficiency and decreased carnitine levels in patients on hemodialysis. Blood Purif. 2019;47 :38–44. [DOI] [PubMed] [Google Scholar]
- 8.Khositseth A, Jirasakpisarn S, Pakakasama S, et al. Carnitine levels and cardiac functions in children with solid malignancies receiving doxorubicin therapy. Indian J Med Paediatr Oncol. 2011;32:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans AM, Faull RJ, Nation RL, et al. Impact of hemodialysis on endogenous plasma and muscle carnitine levels in patients with end-stage renal disease. Kidney Int. 2004;66:1527–1534. [DOI] [PubMed] [Google Scholar]
- 10.Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am J Kidney Dis. 2003;41:S13–S26. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi T, Abe M, Yamazaki T, et al. Levocarnitine improves cardiac function in hemodialysis patients with left ventricular hypertrophy: a randomized controlled trial. Am J Kidney Dis. 2016;67:260–270. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Joki N, Tanaka Y, et al. Resistance to Erythropoiesis-Stimulating Agents in Pre-Dialysis and Post-Dialysis Mortality in Japanese Incident Hemodialysis Patients. Blood Purif. 2019;47 :31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siami G, Clinton ME, Mrak R, et al. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron. 1991;57:306–313. [DOI] [PubMed] [Google Scholar]
- 14.Fagher B, Cederblad G, Eriksson M, et al. L-carnitine and haemodialysis: double blind study on muscle function and metabolism and peripheral nerve function. Scand J Clin Laborat Investig. 1985;45:169–178. [DOI] [PubMed] [Google Scholar]
- 15.Hurot JM, Cucherat M, Haugh M, et al. Effects of L-carnitine supplementation in maintenance hemodialysis patients: a systematic review. J Am Soc Nephrol. 2002;13:708–714. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad S, Robertson HT, Golper TA, et al. Multicenter trial of L-carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects. Kidney Int. 1990;38:912–918. [DOI] [PubMed] [Google Scholar]
- 17.Eknoyan G, Latos DL, Lindberg J.. Practice recommendations for the use of L-carnitine in dialysis-related carnitine disorder. National Kidney Foundation Carnitine Consensus Conference. Am J Kidney Dis. 2003;41:868–876. [DOI] [PubMed] [Google Scholar]
- 18.Warady BA, Borum P, Stall C, et al. Carnitine status of pediatric patients on continuous ambulatory peritoneal dialysis. Am J Nephrol. 1990;10:109–114. [DOI] [PubMed] [Google Scholar]
- 19.Lilien MR, Duran M, Quak JM, et al. Oral L-carnitine does not decrease erythropoietin requirement in pediatric dialysis. Pediatric Nephrol (Berlin, Germany). 2000;15:17–20. [DOI] [PubMed] [Google Scholar]
- 20.Sotirakopoulos N, Athanasiou G, Tsitsios T, et al. The influence of l-carnitine supplementation on hematocrit and hemoglobin levels in patients with end stage renal failure on CAPD. Ren Fail. 2002;24:505–510. [DOI] [PubMed] [Google Scholar]
- 21.Kosan C, Sever L, Arisoy N, et al. Carnitine supplementation improves apolipoprotein B levels in pediatric peritoneal dialysis patients. Pediatric Nephrol (Berlin, Germany). 2003;18:1184–1188. [DOI] [PubMed] [Google Scholar]
- 22.Verrina E, Caruso U, Calevo MG, et al. ; on behalf of the Italian Registry of Pediatric Chronic Dialysis . Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr Nephrol. 2007;22:727–733. [DOI] [PubMed] [Google Scholar]
- 23.2009 Japanese Society for Dialysis Therapy guidelines for peritoneal dialysis. Ther Apher Dial. 2010;14:489–504. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3:36. [Google Scholar]
- 25.van Hoeck KJM, Rusthoven E, Vermeylen L, et al. Nutritional effects of increasing dialysis dose by adding an icodextrin daytime dwell to Nocturnal Intermittent Peritoneal Dialysis (NIPD) in children. Nephrol Dial Transplant. 2003;18:1383–1387. [DOI] [PubMed] [Google Scholar]
- 26.Badve SV, Zimmerman DL, Knoll GA, et al. Peritoneal phosphate clearance is influenced by peritoneal dialysis modality, independent of peritoneal transport characteristics. CJASN. 2008;3:1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Buxo JA, Walker PJ, Chandler JT, et al. Experience with intermittent peritoneal dialysis and continuous cyclic peritoneal dialysis. Am J Kidney Dis. 1984;4:242–248. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman V, Nolph KD.. Utilization of PD modalities: evolution. Semin Dial. 2002;15:380–384. [DOI] [PubMed] [Google Scholar]
- 29.Lo WK, Bargman JM, Burkart J, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Peritoneal Dial Int. 2006;26:520–522. [PubMed] [Google Scholar]
- 30.Teitelbaum I, Burkart J.. Peritoneal dialysis. Am J Kidney Dis. 2003;42:1082–1096. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi M, Ueda S, Misaki H, et al. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin Chem. 1994;40:817–821. [PubMed] [Google Scholar]
- 32.Aljama P, Bommer J, Canaud B, et al. Practical guidelines for the use of NESP in treating renal anaemia. Nephrol Dial Transplant. 2001;16:22–28. [DOI] [PubMed] [Google Scholar]
- 33.Grzegorzewska AE, Mariak I, Dobrowolska-Zachwieja A.. Continuous ambulatory peritoneal dialysis (CAPD) adequacy influences serum free carnitine level. Int Urol Nephrol. 1999;31:533–540. [DOI] [PubMed] [Google Scholar]
- 34.Fülöp T, Zsom L, Tapolyai MB, et al. Peritoneal dialysis: the unique features by compartmental delivery of renal replacement therapy. Med Hypotheses. 2017;108:128–132. [DOI] [PubMed] [Google Scholar]
- 35.Di Liberato L, Arduini A, Rossi C, et al. L-Carnitine status in end-stage renal disease patients on automated peritoneal dialysis. J Nephrol. 2014;27:699–706. [DOI] [PubMed] [Google Scholar]
- 36.Bonomini M, Zammit V, Pusey CD, et al. Pharmacological use of L-carnitine in uremic anemia: has its full potential been exploited? Pharmacol Res. 2011;63:157–164. [DOI] [PubMed] [Google Scholar]
- 37.Vlassopoulos DA, Hadjiyannakos DK, Anogiatis AG, et al. Carnitine action on red blood cell osmotic resistance in hemodialysis patients. J Nephrol. 2002;15:68–73. [PubMed] [Google Scholar]
- 38.Kudoh Y, Aoyama S, Torii T, et al. Long-term effects of oral L-carnitine supplementation on anemia in chronic hemodialysis. Cardiorenal Med. 2014;4:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CT, Chou FF, Chang HW, et al. Effects of parathyroidectomy on iron homeostasis and erythropoiesis in hemodialysis patients with severe hyperparathyroidism. Blood Purif. 2003;21:369–375. [DOI] [PubMed] [Google Scholar]
- 40.Akmal M, Telfer N, Ansari AN, et al. Erythrocyte survival in chronic renal failure. Role of secondary hyperparathyroidism. J Clin Invest. 1985;76:1695–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiffl H, Lang SM.. Folic acid deficiency modifies the haematopoietic response to recombinant human erythropoietin in maintenance dialysis patients. Nephrol Dial Transplant. 2006;21:133–137. [DOI] [PubMed] [Google Scholar]
- 42.Zachee P, Chew SL, Daelemans R, Lins RL.. Erythropoietin resistance due to vitamin B12 deficiency. Case report and retrospective analysis of B12 levels after erythropoietin treatment. Am J Nephrol. 1992;12:188–191. [DOI] [PubMed] [Google Scholar]
- 43.Barany P, Divino Filho JC, Bergstrom J.. High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis. 1997;29:565–568. [DOI] [PubMed] [Google Scholar]
- 44.Horl WH, Jacobs C, Macdougall IC, et al. European best practice guidelines 14-16: inadequate response to epoetin. Nephrol Dial Transplant. 2000;15:43–50. [PubMed] [Google Scholar]
- 45.Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19 :ii1–47. [DOI] [PubMed] [Google Scholar]
- 46.Johnson DW, Pollock CA, Macdougall IC.. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton, Vic). 2007;12:321–330. [DOI] [PubMed] [Google Scholar]
- 47.Macdougall IC, Cooper A.. The inflammatory response and epoetin sensitivity. Nephrol Dial Transplant. 2002;17:48–52. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Ortiz A, Correa-Rotter R, Vazquez-Rangel A, et al. Relationship between protein-energy wasting in adults with chronic hemodialysis and the response to treatment with erythropoietin. BMC Nephrol. 2019;20:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsubakihara Y, Nishi S, Akiba T, et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Therap Apheresis Dial. 2010;14:240–275. [DOI] [PubMed] [Google Scholar]
- 50.Sibbel SP, Koro CE, Brunelli SM, et al. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi H, Abe M, Okada K, et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients. 2015;7:3783–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int Suppl. 2012;2:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonomini M, Di Liberato L, Zammit V, et al. Current opinion on usage of L-carnitine in end-stage renal disease patients on peritoneal dialysis. Molecules (Basel, Switzerland). 2019;24:3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki A, Sakai Y, Hashimoto K, et al. Kinetics of carnitine concentration after switching from oral administration to intravenous injection in hemodialysis patients. Ren Fail. 2018;40:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]